Abstract
The human CYP2C locus harbors the polymorphic CYP2C18, CYP2C19, CYP2C9 and CYP2C8 genes, and of these, CYP2C19 and CYP2C9 are directly involved in the metabolism of ~15% of all medications. All variant CYP2C19 and CYP2C9 star (*) allele haplotypes currently catalogued by the Pharmacogene Variation (PharmVar) Consortium are defined by sequence variants. To determine if structural variation also occurs at the CYP2C locus, the 10q23.33 region was interrogated across deidentified clinical chromosomal microarray (CMA) data from 20,642 patients tested at two academic medical centers. Fourteen copy number variants that affected the coding region of CYP2C genes were detected in the clinical CMA cohorts, which ranged in size from 39.2–1,043.3 kb. Selected deletions and duplications were confirmed by MLPA or ddPCR. Analysis of the clinical CMA and an additional 78,839 cases from the Database of Genomic Variants (DGV) and ClinGen (total n=99,481) indicated that the carrier frequency of a CYP2C structural variant is ~1 in 1000, with ~1 in 2,000 being a CYP2C19 full-gene or partial-gene deletion carrier, designated by PharmVar as CYP2C19*36 and *37, respectively. Although these structural variants are rare in the general population, their detection will likely improve metabolizer phenotype prediction when interrogated for research and/or clinical testing.
Keywords: CYP2C, CYP2C19, CYP2C9, copy number variation, deletion, duplication, pharmacogenomics, chromosomal microarray, database
INTRODUCTION
The human cytochrome P450 (CYP) enzyme superfamily is responsible for the oxidative metabolism of many drugs, xenobiotics, and other endogenous substances. The polymorphic CYP2C locus at chromosome 10q23.33 is comprised of the CYP2C18, CYP2C19, CYP2C9 and CYP2C8 genes, which encode enzymes that together are involved in the hepatic metabolism of ~25% of commonly prescribed drugs. Moreover, CYP2C19 and CYP2C9 metabolize ~15% of drugs currently listed on the U.S. Food and Drug Administration (FDA) Pharmacogenomic Biomarkers in Drug Labeling table (www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm, which includes medications commonly used in neurology, rheumatology, psychiatry, cardiology, gastroenterology, gynecology, and infectious disease.
Currently 35 CYP2C19 and 60 CYP2C9 variant star (*) allele haplotypes are catalogued in the Pharmacogene Variation Consortium (PharmVar) database (www.pharmvar.org) (Gaedigk et al., 2018; Gaedigk et al., 2019). Without exception, all are defined by sequence variants. As such, no CYP2C19 or CYP2C9 star (*) alleles currently include structural variation (e.g., copy number variants (CNV)), which is consistent with our previously reported pilot study that employed multiplex ligation-dependent probe amplification (MLPA) screening of this gene region across a multi-ethnic cohort of ~500 individuals (Martis et al., 2013). However, pharmacogenomic CNV alleles can play important roles in enzyme activity and drug response variability (He, Hoskins, & McLeod, 2011), which have been characterized among some cytochrome P450 (CYP2B6, CYP2D6), glutathione S-transferase (GSTT1, GSTM1), and sulfotransferase (SULT1A1, SULT2A1) genes (Gaedigk, Gaedigk, & Leeder, 2010; Gaedigk, Twist, & Leeder, 2012; Gjerde et al., 2008; Martis et al., 2013; Schulze et al., 2013; Vijzelaar et al., 2018). Notably, interrogation of publicly available sequencing data has recently indicated that some populations harbor CNV alleles at the CYP2C gene region (Santos et al., 2018). The potential clinical significance of low frequency structural variation at the CYP2C gene region in the general population prompted our interrogation of chromosomal microarray (CMA) data from multiple database sources for pharmacogenomic CNV discovery.
DATA SPECIFICATIONS
IMPACT OF DATA
In contrast to the well-described pharmacogenomic alleles that are relatively common in the general population (e.g., CYP2C9*2, CYP2C19*2), it is increasingly appreciated that the majority of human genetic variation is actually rare [minor allele frequency (MAF) <1%] (Genomes Project et al., 2010; Tennessen et al., 2012), making association studies between these variants and drug response phenotypes challenging (Verma et al., 2018). To facilitate the discovery of low frequency variants that potentially influence drug response, recent studies have interrogated high-throughput sequencing data across drug target genes (Nelson et al., 2012), CYP450 genes (Gordon et al., 2014), and selected drug absorption, distribution, metabolism and excretion (ADME) and other candidate pharmacogenes (Bush et al., 2016; Li et al., 2014; Santos et al., 2018), all indicating that rare and potentially functional pharmacogenomic variants are prevalent in diverse populations. These studies highlight the importance of interrogating large datasets for rare pharmacogenomic variation discovery, which prompted our interrogation of deidentified CMA data from large databases to further study low frequency pharmacogenomic structural variation at the clinically relevant CYP2C gene cluster region.
MATERIALS AND METHODS
Experimental Design
The design of this study included interrogation of multiple databases of CMA data, including both clinical cytogenomic laboratory CMA data (discovery and replication cohorts), as well as publicly available CMA data from the general population (research and clinical cohorts). Selected CYP2C gene deletion and duplication samples from the discovery and replication cohorts were analytically confirmed by orthogonal copy number methods.
Clinical Cytogenomic Testing Cohort – Discovery
Individuals in the discovery cohort were referred to the Cytogenetics and Cytogenomics Laboratory at Mount Sinai Genomics Inc. (DBA Sema4), New York, from 2010 to 2017 for pre- or postnatal clinical CMA testing. All patients were tested with informed consent and deidentified CMA data were stored in an internal database that enabled interrogation and CNV frequency analyses. A total of 11,096 unique samples were analyzed, which included 6,083 prenatal (amniotic fluid, chorionic villus specimens, fetal blood) and 5,013 postnatal (peripheral blood, products of conception) samples. Although race and ethnicity were not commonly available, the prenatal cohort self-reported as white (82.5%), Asian (8.0%), black (4.7%), Hispanic/Latino (4.5%) and American Indian (0.36%), and the postnatal cohort self-reported as white (59.1%), Hispanic/Latino (19.1%), black (11.8%), Asian (6.3%), and American Indian (3.8%).
Clinical Cytogenomic Testing Cohort – Replication
Individuals in the replication cohort were referred to the Clinical Genetics and Genomics Laboratory at Children’s Mercy, Kansas City, from 2009 to 2018 for pre- or postnatal clinical CMA testing. All patients were tested with informed consent and deidentified CMA data were stored in an internal database that enabled interrogation and CNV frequency analyses. A total of 9,760 unique peripheral blood samples were analyzed as a replication cohort for structural variation at the CYP2C locus. Race and ethnicity demographics of the patient cohort were not available.
Chromosomal Microarray (CMA) Analysis – Discovery
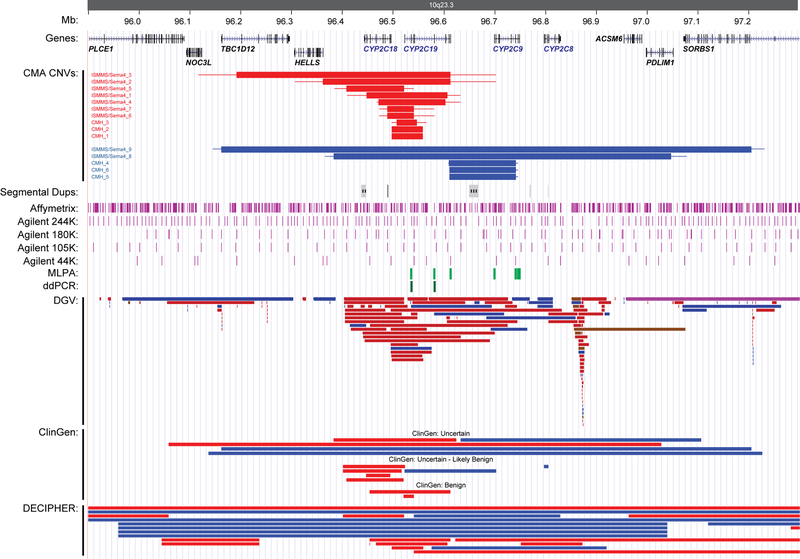
CMA was performed on the discovery cohort using the Agilent Technologies platform (Santa Clara, CA, USA) according to the manufacturer’s instructions and as previously reported (Reiner et al., 2017; Scott et al., 2010). Throughout the period of clinical CMA testing and data analysis (2010–2017), three commercial microarrays were used that had increasing probe density and resolution [44K (design 015141), 105K (design 031750), and 180K (design 029830); Agilent Technologies]; however, all three microarray designs had adequate probe coverage across the CYP2C gene cluster region to detect multi-exon CNVs within CYP2C18 (NG_008373.1), CYP2C19 (NG_008384.3), CYP2C9 (NG_008385.1), and/or CYP2C8 (NG_007972.1) (Figure 1). All genomic coordinates are reported using NCBI human genome reference Build 37 (GRCh37/hg19).
Figure 1.
Illustration of the chromosome 10q23.33 region with the identified CYP2C copy number variants (CNVs) noted in relation to the location of known human genes and transcripts from the UCSC Genes track, segmental duplications, microarray (purple), MLPA (light green), and ddPCR (dark green) probe locations, structural variants from the Database of Genomic Variants (DGV), ClinGen, and DECIPHER. Blue bars represent copy number gains (duplications), red bars represent copy number losses (deletions). The minimum and maximum CNV sizes in the clinical CMA cohorts are denoted by thick and thin horizontal bars, respectively.
Chromosomal Microarray (CMA) Analysis – Replication
CMA was performed on the replication cohort using the Affymetrix Cytoscan® HD CNV+SNP array platform (Santa Clara, CA, USA) or the Agilent 244K whole genome oligonucleotide microarray (design 014693; Santa Clara, CA, USA) according to the manufacturer’s instructions. The CytoScan® HD microarray contains 1,953,246 non-polymorphic and 743,304 single nucleotide polymorphism (SNP) markers, which are enriched in disease gene areas, and the Agilent 244K microarray contains ~244,000 oligonucleotide probes spaced at a median distance of 6.4 kb across the human genome (Figure 1). Data were analyzed by ChAS 3.2 (Affymetrix) or Genomic Workbench (Agilent Technologies) software as appropriate. As above, all genomic coordinates are reported using NCBI human genome reference Build 37 (GRCh37/hg19).
Copy Number Variation (CNV) Confirmation
Multiplex Ligation-dependent Probe Amplification (MLPA)
Copy number results from CMA testing were validated by multiplex ligation-dependent probe amplification (MLPA) testing on samples from the discovery cohort with available DNA. MLPA was performed using the Cytochrome P-450 MLPA kit (P128-B1; MRC-Holland, Amsterdam, The Netherlands) according to the manufacturer’s instructions and as previously reported (Martis et al., 2013; Vijzelaar et al., 2018). This commercial MLPA probe mix includes three CYP2C19 probes (exons 2, 6, and 9) and five CYP2C9 probes (exons 2, 7, 8 [2 probes], and 9) (Figure 1), plus an additional 34 probes that interrogate 12 other pharmacogenetic genes (CYP2D6, CYP1B1, CYP3A4, CYP3A5, CYP2E1, CYP1A1, CYP1A2, CYP2A6, CYP2B6, GSTP1, GSTT1 and GSTM1) (Martis et al., 2013). Amplified products were separated by capillary gel electrophoresis and analyzed using GeneMarker v1.90 software (SoftGenetics, State College, PA). After quality control and data normalization, copy number was determined according to the following peak ratio ranges: one copy >0.25 and <0.75; two copies >0.75 and <1.25; three copies >1.25 and <1.7; four copies >1.7 and <2.0.
Droplet Digital PCR (ddPCR)
Copy number results from CMA testing were also validated by droplet digital PCR (ddPCR) testing on samples from the replication cohort with available DNA. TaqMan™ copy number assays targeting CYP2C19 exon 2 (Hs05148033_cn) and intron 6 (Hs02932336_cn) were employed and signals normalized against the TERT gene (Cat# 4403316; Thermo Fisher, Waltham, MA) (Figure 1), and analysis was performed using the Bio-Rad QX-200 Droplet Digital PCR System (Bio-Rad, Hercules, CA). Genomic DNA were digested with EcoRI-HF (New England BioLabs, Ipswich, MA) and inactivated at 65ºC. Digested DNA were subsequently combined with 1X ddPCR Supermix for Probes (Bio–Rad, Hercules, CA), TaqMan™ and TERT reference assays. Droplets were generated with the Auto Droplet Generator and cycled in a C1000 Touch Thermocycler using recommended parameters. Droplets were analyzed with the QX200 Droplet Reader instrument and data analysis performed with the Quantasoft™ Software (Bio-Rad, Hercules, CA).
Cytogenomic Copy Number Variation (CNV) Population Cohorts
Structural variation databases were also interrogated to identify CYP2C region CNVs in healthy and clinical cohorts. Three independent sources were utilized: (1) the Database of Genomic Variants (DGV) (http://dgv.tcag.ca), which catalogues structural variation (>50 bp) in healthy population samples; (2) Clinical Genome Consortium (ClinGen) (http://dbsearch.clinicalgenome.org/search/), which catalogues genomic variation in patient population samples; and (3) DECIPHER (https://decipher.sanger.ac.uk), which also catalogues genomic variation in patient population samples. A 1 Mb genomic region was queried across all public databases [chr10:96100000_97100000 (GRCh37/hg19)], and only CNVs that included coding regions of any CYP2C gene were included in the study. Larger overlapping chromosome 10q23.33-q24.1 deletions and duplications (>2 Mb) in the clinical databases were excluded from the CYP2C CNV allele and carrier frequency analyses, as these likely pathogenic aberrations would be more consistent with a syndromic Mendelian phenotype.
DATA:
Chromosomal Microarray (CMA) Detection of CYP2C Deletions and Duplications
The CMA probe coverage across the CYP2C region for all clinical microarrays used in the study are illustrated in Figure 1. In the discovery cohort (ISMMS/Sema4; n=11,096), CYP2C gene region CNVs were detected in nine unrelated patients, including seven deletions and two duplications (Table 1 and Table 2). The identified deletions ranged in size from 52.0 kb (exons 8 to 9 of CYP2C18 and exons 1 to 4 of CYP2C19) to 421.0 kb (including TBC1D12, HELLS, CYP2C18, and CYP2C19) (Table 1 and Figure 1). The identified duplications were larger, 663.8 kb and 1.0 Mb, and included all CYP2C (CYP2C18, CYP2C19, CYP2C9, CYP2C8) and the neighboring ACMS6, PDLIM1 and SORBS1 genes (Table 1 and Figure 1). In the replication cohort (CMH; n=9,760), CYP2C region CNVs were detected in six unrelated patients, including three CYP2C deletions and three CYP2C duplications (Table 1 and Table 2). The deletions ranged in size from 39.2 kb to 61.5 kb (exons 1 to 5 of CYP2C19) (Table 1 and Figure 1), whereas the duplications (130.8 and 131.9 kb) included the 3’ region of CYP2C19 (exon 9 with or without exon 8) and exons 1 to 7 of CYP2C9 (Table 1 and Figure 1). All CYP2C CNV alleles identified in the discovery and replication CMA cohorts were submitted to the Leiden Open Variation Database (LOVD) (https://databases.lovd.nl) (Fokkema et al., 2011), and their unique variant IDs are listed in Table 1.
Table 1.
CYP2C gene region copy number variants identified by clinical chromosomal microarray (CMA) testing.
| Sample | ISCN 2016 Nomenclature | Microarray | Size (kb) | Genes Included | LOVD Variant ID |
|---|---|---|---|---|---|
| Discovery Cohort | |||||
| ISMMS/Sema4_6 | arr[GRCh37] 10q23.33(96488443_96540495)x1 | Agilent 180K | 52.1 | CYP2C18 (exons 8–9), CYP2C19 (exons 1–4) | 0000484257 |
| ISMMS/Sema4_7 | arr[GRCh37] 10q23.33(96488443_96540495)x1 | Agilent 180K | 52.1 | CYP2C18 (exons 8–9), CYP2C19 (exons 1–4) | 0000484258 |
| ISMMS/Sema4_5 | arr[GRCh37] 10q23.33(96407740_96521658)x1 | Agilent 180K | 113.9 | CYP2C18 (exons 1–8), CYP2C19 (exon 1) | 0000484256 |
| ISMMS/Sema4_4† | arr[GRCh37] 10q23.33(96470997_96602860)x1 | Agilent 180K | 131.9 | CYP2C18 (exons 5–8), CYP2C19 (exons 1–7) | 0000484255 |
| ISMMS/Sema4_1 | arr[GRCh37] 10q23.33(96447479_96606715)x1 | Agilent 105K | 159.2 | CYP2C18 (exons 2–8), CYP2C19 (exons 1–7) | 0000484252 |
| ISMMS/Sema4_2† | arr[GRCh37] 10q23.33(96361629_96612764)x1 | Agilent 44K | 251.5 | HELLS, CYP2C18, CYP2C19 | 0000484253 |
| ISMMS/Sema4_3 | arr[GRCh37] 10q23.33(96192063_96612764)x1 | Agilent 44K | 421.1 | TBC1D12, HELLS, CYP2C18, CYP2C19 | 0000484254 |
| ISMMS/Sema4_8† | arr[GRCh37] 10q23.33q24.1(96383288_97047098)x3 | Agilent 180K | 663.8 | CYP2C18, CYP2C19, CYP2C9, CYP2C8, ACMS6, PDLIM1 | 0000484259 |
| ISMMS/Sema4_9 | arr[GRCh37] 10q23.33q24.1(96161314_97204585)x3 | Agilent 180K | 1043.3 | CYP2C18, CYP2C19, CYP2C9, CYP2C8, ACMS6, PDLIM1, SORBS1 (exons 3–32) | 0000484260 |
| Replication Cohort | |||||
| CMH_3 | arr[GRCh37] 10q23.33(96507212_96546422)x1 | Agilent 244K | 39.2 | CYP2C19 (exons 1–5) | 0000484261 |
| CMH_1 | arr[GRCh37] 10q23.33(96497260_96558710)x1 | Affymetrix | 61.5 | CYP2C19 (exons 1–5) | 0000484262 |
| CMH_2 | arr[GRCh37] 10q23.33(96497260_96558710)x1 | Affymetrix | 61.5 | CYP2C19 (exons 1–5) | 0000484263 |
| CMH_5 | arr[GRCh37] 10q23.33(96610653_96741497)x3 | Affymetrix | 130.8 | CYP2C19 (exon 9), CYP2C9 (exons 1–7) | 0000484264 |
| CMH_6 | arr[GRCh37] 10q23.33(96610653_96741497)x3 | Affymetrix | 130.8 | CYP2C19 (exon 9), CYP2C9 (exons 1–7) | 0000484265 |
| CMH_4 | arr[GRCh37] 10q23.33(96609567_96741497)x3 | Affymetrix | 131.9 | CYP2C19 (exons 8–9), CYP2C9 (exons 1–7) | 0000484266 |
Ethnicity for individuals ISMMS/Sema4_2, ISMMS/Sema4_4, and ISMMS/Sema4_8 is Hispanic/Latino, White, and Asian, respectively. Ethnicity was not available for all other reported subjects.
Affymetrix: refers to the Cytoscan® microarray; CMH: Children’s Mercy Hospital; ISMMS: Icahn School of Medicine at Mount Sinai; LOVD: Leiden Open Variation Database (https://databases.lovd.nl).
Table 2.
CYP2C copy number variant (CNV) confirmation by multiplex ligation-dependent probe amplification (MLPA).
| SAMPLE ID | CYP2C19 | CYP2C9 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exon 2 | Exon 6 | Exon 9 | Exon 1 | Exon 7 | Exon 8A | Exon 8B | Exon 9 | ||
| ISMMS/Sema4_4 | 0.499 | 0.458 | 0.517 | 0.928 | 0.962 | 0.953 | 0.989 | 0.961 | |
| ISMMS/Sema4_5 * | 0.977 | 1.030 | 1.004 | 1.016 | 0.995 | 0.982 | 0.949 | 1.075 | |
| ISMMS/Sema4_6 | 0.508 | 0.903 | 0.968 | 0.953 | 0.959 | 1.005 | 1.041 | 1.014 | |
| ISMMS/Sema4_7 | 0.481 | 0.854 | 0.992 | 1.004 | 1.010 | 1.027 | 1.064 | 0.984 | |
| ISMMS/Sema4_9 | 1.440 | 1.665 | 1.297 | 1.209 | 1.561 | 1.368 | 1.487 | 1.577 | |
A 113.9 kb deletion was detected by CMA in this sample that included exons 1–8 of CYP2C18 and only exon 1 of CYP2C19.
Light gray shaded cells indicate heterozygous deletion or duplication by CMA testing. MLPA ratios: one copy >0.25 and <0.75; two copies: >0.75 and <1.25; three copies >1.25 and <1.7.
Confirmation of CYP2C Copy Number Variants (CNVs)
Among all subjects with CYP2C CNVs identified by CMA testing, five samples from the discovery cohort had available DNA for confirmation by MLPA testing. The locations of the MLPA probes in relation to the CMA probes are illustrated in Figure 1. All MLPA results were consistent with the CNVs detected by CMA testing (Table 2). Of note, given that the CMA and MLPA platforms have unique probe locations to interrogate copy number across the CYP2C region, deletions that affected only CYP2C18 and/or only exon 1 of CYP2C19 were not detected by MLPA. As noted in the Materials and Methods, the MLPA probe mix only interrogates exons 2, 6, and 9 of CYP2C19 and exons 2, 7, 8, and 9 of CYP2C9 (Figure 1). In addition, all subjects with CYP2C19 CNVs identified by CMA testing in the replication cohort were confirmed by ddPCR. The locations of the ddPCR probes in relation to the CMA probes are illustrated in Figure 1. All ddPCR results at exon 2 and intron 6 of CYP2C19 were consistent with the partial gene deletions and duplications detected by CMA testing.
Database Detection of CYP2C Copy Number Variants (CNVs)
Structural variants within a 1 Mb region at the CYP2C locus (chr10:96100000_97100000) were also identified in the DGV, which is commonly considered to be a CMA database representative of the general population. These CYP2C CNVs were consistent with those detected in our clinical cohorts and are illustrated in Figure 1 and detailed in the Appendix. A total of 36 CNVs (from 9 independent studies) overlapping the CYP2C18, CYP2C19, CYP2C9, and/or CYP2C8 genes were catalogued in the DGV. The majority (n=27; 75%) were deletions, including 17 and 4 deletions that overlapped coding regions of CYP2C19 and/or CYP2C9, respectively (Table 3).
Table 3.
CYP2C copy number variant (CNV) allele and carrier frequencies.
| CYP2C18 | CYP2C19 | CYP2C9 | CYP2C8 | Any CYP2C gene | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Carriers/ Cohort |
Allele Frequency (%) |
Carrier Frequency (%) |
Carriers/ Cohort |
Allele Frequency (%) |
Carrier Frequency (%) |
Carriers/ Cohort |
Allele Frequency (%) |
Carrier Frequency (%) |
Carriers/ Cohort |
Allele Frequency (%) |
Carrier Frequency (%) |
Carriers/ Cohort |
Allele Frequency (%) |
Carrier Frequency (%) |
| Deletion | |||||||||||||||
| ISMMS/Sema4 | 7/11096 | 0.032 | 0.063 | 7/11096 | 0.032 | 0.063 | 0/11096 | - | - | 0/11096 | - | - | 7/11096 | 0.032 | 0.063 |
| CMH | 0/9760 | - | - | 3/9760 | 0.015 | 0.031 | 0/9760 | - | - | 0/9760 | - | - | 3/9760 | 0.015 | 0.031 |
| DGV | 38/41392 | 0.046 | 0.092 | 31/41392 | 0.037 | 0.075 | 4/41392 | 0.005 | 0.010 | 3/41392 | 0.004 | 0.007 | 50/41392 | 0.060 | 0.121 |
| ClinGen | 7/37447 | 0.009 | 0.019 | 5/37447 | 0.007 | 0.013 | 1/37447 | 0.001 | 0.003 | 1/37447 | 0.001 | 0.003 | 8/37447 | 0.011 | 0.021 |
| Total: | 52/99695 | 0.026 | 0.052 | 46/99695 | 0.023 | 0.046 | 5/99695 | 0.003 | 0.005 | 4/99695 | 0.002 | 0.004 | 68/99695 | 0.034 | 0.068 |
| Duplication | |||||||||||||||
| ISMMS/Sema4 | 2/11096 | 0.009 | 0.018 | 2/11096 | 0.009 | 0.018 | 2/11096 | 0.009 | 0.018 | 2/11096 | 0.009 | 0.018 | 2/11096 | 0.009 | 0.018 |
| CMH | 0/9760 | - | - | 3/9760 | 0.015 | 0.031 | 3/9760 | 0.015 | 0.031 | 0/9760 | - | - | 3/9760 | 0.015 | 0.031 |
| DGV | 1/41392 | 0.001 | 0.002 | 3/41392 | 0.004 | 0.007 | 4/41392 | 0.005 | 0.010 | 4/41392 | 0.005 | 0.010 | 9/41392 | 0.016 | 0.022 |
| ClinGen | 2/37447 | 0.003 | 0.005 | 3/37447 | 0.004 | 0.003 | 4/37447 | 0.005 | 0.011 | 4/37447 | 0.005 | 0.011 | 5/37447 | 0.007 | 0.013 |
| Total: | 5/99695 | 0.002 | 0.005 | 10/99695 | 0.005 | 0.010 | 12/99695 | 0.006 | 0.012 | 10/99695 | 0.005 | 0.010 | 18/99695 | 0.009 | 0.018 |
| Deletion/Duplication | |||||||||||||||
| ISMMS/Sema4 | 9/11096 | 0.041 | 0.081 | 9/11096 | 0.041 | 0.081 | 2/11096 | 0.009 | 0.018 | 2/11096 | 0.009 | 0.018 | 9/11096 | 0.041 | 0.081 |
| CMH | 0/9760 | - | - | 6/9760 | 0.031 | 0.061 | 3/9760 | 0.015 | 0.031 | 0/9760 | - | - | 6/9760 | 0.031 | 0.061 |
| DGV | 39/41392 | 0.047 | 0.094 | 34/41392 | 0.041 | 0.082 | 8/41392 | 0.010 | 0.019 | 7/41392 | 0.008 | 0.017 | 59/41392 | 0.076 | 0.143 |
| ClinGen | 9/37447 | 0.012 | 0.024 | 8/37447 | 0.011 | 0.021 | 5/37447 | 0.007 | 0.013 | 5/37447 | 0.007 | 0.013 | 13/37447 | 0.016 | 0.033 |
| Total: | 57/99695 | 0.029 | 0.057 | 57/99695 | 0.029 | 0.057 | 18/99695 | 0.009 | 0.018 | 14/99695 | 0.007 | 0.014 | 87/99695 | 0.044 | 0.087 |
Structural variants at the CYP2C gene region were also identified in the ClinGen and DECIPHER databases. Given that these databases are comprised of CMA data from patient cohorts with variable phenotypes, larger deletions and duplications of the chromosome 10q23.33-q24.1 region were present; however, aberrations >2 Mb were not included in the CYP2C CNV analyses as detailed in the Materials and Methods (Appendix). Consistent with the DGV CYP2C CNVs, 22 CNVs were present in ClinGen and DECIPHER that overlapped CYP2C18, CYP2C19, CYP2C9 and/or CYP2C8, including 13 deletions (8.6 kb to 969.7 kb), and eight duplications (8.9 kb to 1.1 Mb) (Figure 1, Table 3 and Appendix). Notably, the most common CYP2C CNVs in all three population databases were deletions that overlapped CYP2C19 (n=26; 44.8% of all CYP2C CNVs).
CYP2C Deletion and Duplication Frequencies and Allele Nomenclature
The CYP2C CNV frequency data from our clinical CMA cohorts and the CNV population databases are summarized in Table 3. The CYP2C CNV data from DECIPHER was not incorporated into the population frequencies given the difficulty with determining an accurate size of this dynamic clinical cohort. Taken together, the overall carrier frequency of a CYP2C CNV is ~1 in 1000 [0.085% (95% CI: 0.067–0.104%)] (Table 3). The data for these CNV alleles were reviewed by the PharmVar Consortium (Gaedigk et al., 2018; Gaedigk et al., 2019), which subsequently classified a full gene CYP2C19 deletion as CYP2C19*36 and a partial gene CYP2C19 deletion (that includes at least exon 1) as CYP2C19*37 [combined carrier frequency of ~1 in 2000; 0.046% (95% CI: 0.033–0.059%)]. The full gene and partial gene CYP2C9 deletion alleles identified in the DGV and ClinGen had a carrier frequency of ~1 in 20,000; 0.005% (95% CI: 0.001–0.009%)] (Table 3 and Figure 1). The CYP2C19, CYP2C9, and CYP2C8 duplications did not receive designated star (*) alleles as PharmVar recommends classifying gene duplications based on their haplotype sequence and total detected copy number (e.g., CYP2C9*1/*1x2), consistent with established CYP2D6 duplication nomenclature (Gaedigk et al., 2018) (www.pharmvar.org). Notably, one sample in the clinical CMA cohort with the largest chromosome 10q23.33 duplication (1043.3 kb) that included multiple CYP2C genes was genotyped for a panel of CYP2C star (*) allele variants, which resulted in the following diplotypes: CYP2C19*17/*17x2, CYP2C9*1/*1x2, and CYP2C8*1/*1x2.
DISCUSSION
Recent studies that utilized large high-throughput sequencing datasets for pharmacogenomic variant discovery prompted our interrogation of deidentified CMA data from 99,695 individuals for pharmacogenomic CNV discovery at the clinically relevant CYP2C gene cluster region. Consistent with the sequencing studies that identified novel coding variants in pharmacogenomic genes (Bush et al., 2016; Gordon et al., 2014; Li et al., 2014; Nelson et al., 2012), our large CNV study resulted in the discovery of pharmacogenomic deletion and duplication alleles at the CYP2C region. The full gene and partial gene CYP2C19 deletions have been designated by the PharmVar Consortium as CYP2C19*36 and CYP2C19*37, respectively (www.pharmvar.org). Of note, the CYP2C19*37 partial deletion allele is consistent with the CYP2C19 partial gene deletions recently identified in the Exome Aggregation Consortium (ExAC) database (Ruderfer et al., 2016; Santos et al., 2018). Dissemination of these newly defined CYP2C19 alleles through the widely utilized PharmVar database will likely increase awareness of these low frequency structural variants across the pharmacogenomics community.
The significance of structural variation in human disease and phenotypic diversity is increasingly being recognized, and several genomic studies have generated catalogs of CNVs to facilitate a better understanding of their clinical relevance (Johansson & Feuk, 2011; Sudmant et al., 2015). It is estimated that up to 60% of the human genome may contain structural variants in the general population, which typically range in size from 100 bp to 50 kb (Escaramis, Docampo, & Rabionet, 2015), and clinical interpretation of these aberrations when identified by CMA testing is facilitated by professional medical genetics practice guidelines (South et al., 2013). Larger gene-dense aberrations are more likely to result in penetrant syndromic phenotypes; however, some smaller CNVs have increasingly been implicated as susceptibility alleles for several phenotypes, including neurodegenerative disorders, cancer, autism, and psychiatric diseases (Cook & Scherer, 2008; Gonzalez et al., 2005; Han et al., 2017; Nishioka et al., 2006; Rovelet-Lecrux et al., 2006; Sebat et al., 2007). CNVs can influence these human traits by altering the copy number of dosage-sensitive genes (Douglas et al., 2005; Roa, Garcia, & Lupski, 1991) and/or modulating local gene expression (Cahan, Li, Izumi, & Graubert, 2009; Henrichsen, Chaignat, & Reymond, 2009).
Pharmacogenomic structural variation has been previously characterized at several clinically relevant regions (Santos et al., 2018), including CYP450 genes (CYP2A6, CYP2B6, CYP2C cluster, CYP2D6), glutathione S-transferases (GSTT1, GSTM1), and sulfotransferases (SULT1A1, SULT2A1) (Gaedigk et al., 2010; Gjerde et al., 2008; Martis et al., 2013; Schulze et al., 2013; Vijzelaar et al., 2018). Notably, individuals with greater than two functional CYP2D6 copies (e.g. *1xN, *2xN, *35xN) have higher enzyme activity, whereas CYP2D6*5 deletion alleles do not encode a functional CYP2D6 protein. Moreover, common full gene GSTT1 and GSTM1 deletions have been associated with increased risk for chemotherapy toxicity among lymphoma patients (Cho et al., 2010) and susceptibility to tacrine hepatotoxicity among Alzheimer’s patients (Simon et al., 2000).
Loss-of-function CYP2C19 and CYP2C9 alleles have extensive evidence for important roles in clopidogrel, voriconazole, antidepressant, warfarin and/or phenytoin response, which prompted recent Clinical Pharmacogenetics Implementation Consortium (CPIC) and Dutch Pharmacogenetics Working Group (DPWG) practice guidelines for CYP2C19 and CYP2C9 pharmacogenetic-guided prescribing (Caudle et al., 2014; Hicks et al., 2015; Hicks et al., 2017; Johnson et al., 2017; Moriyama et al., 2017; Scott et al., 2013; Swen et al., 2011). Given that the newly defined CYP2C19*36 and *37 deletion alleles are presumed to be nonfunctional, it is expected that they would have the same effects on drug response as the well-known alleles defined by sequence variants (e.g., CYP2C19*2, *3). Interestingly, CYP2C19 deletions have previously been reported in a Northern Finnish case-control study, which identified an association between CYP2C19 deletion and triple-negative breast cancer (ER/PR/HER2 negative), implicating CYP2C19 in estrogen catabolism (Tervasmaki, Winqvist, Jukkola-Vuorinen, & Pylkas, 2014). Our combined analysis of clinical CMA data from two academic medical centers and publicly available databases indicate that the carrier frequency of a CYP2C structural variant is ~1 in 1000, with ~1 in 2000 being a CYP2C19 deletion carrier; however, these aberrations may have higher allele frequencies in specific subpopulations (Santos et al., 2018; Tervasmaki et al., 2014).
Recurrent CNVs are often flanked by segmental duplications, which can act as substrates for non-allelic homologous recombination (NAHR) and the meiotic formation of both deletion and duplication alleles (Carvalho & Lupski, 2016). Importantly, two pairs of segmental duplications are nested within the CYP2C gene cluster region, one directly oriented ~10–20 kb element (~92% identical) that flank CYP2C18 and CYP2C19, and a smaller element (~1.6 kb) directly oriented on the negative strand that are located at the 3’ region of CYP2C8 (Figure 1). In addition to these segmental duplications, the CYP2C subfamily genes have a high degree of sequence homology. This homology and the segmental duplications flanking CYP2C18 and CYP2C19 are likely driving the formation of the more common and recurrent deletions at this region, which is consistent with the NAHR mechanism generally favoring deletions over duplications (Liu, Carvalho, Hastings, & Lupski, 2012).
Notably, the resolution of microarrays used for CMA testing was different across platforms and laboratories. As such, our reported CNV sizes are based on the minimum number of probes that detected gains or losses by a specific microarray; however, based on both our data and those from public databases there are recurrent deletions that affect both CYP2C18 and CYP2C19, as well as larger CNVs that can include multiple CYP2C genes. Unfortunately, precise breakpoints were not feasible to determine given the multiple CMA platforms used across studies, as well as the paucity of available DNA for follow up sequencing.
In conclusion, our interrogation of CMA data from almost 100,000 individuals identified low frequency pharmacogenomic CNVs at the clinically relevant CYP2C region in the general population. These results are consistent with previously reported pharmacogenomic sequencing studies, which identified a spectrum of rare pharmacogenomic variants that are likely to be functional (Bush et al., 2016; Gordon et al., 2014; Li et al., 2014; Nelson et al., 2012). Although the identified CYP2C deletion and duplication alleles have low frequencies in the studied populations, their contribution to an individual’s CYP2C19 and CYP2C9 metabolizer phenotype status is most likely clinically relevant. The nonfunctional deletion alleles can lead to either intermediate or poor metabolizer phenotypes, and the larger CYP2C gene region duplications discovered in our study could lead to an ultrarapid metabolizer phenotype across CYP2C19, CYP2C19, CYPC9 and CYP2C8 if the duplication alleles do not harbor sequence variants that obliterate function. These rare individuals would likely be at risk for atypical CYP2C-mediated metabolism across multiple drugs and drug classes (e.g., voriconazole, clopidogrel, phenytoin, fosphenytoin, phenobarbital, amytriptiline, torsemide). Although the technical infrastructure and additional cost of interrogating copy number at the CYP2C gene region may not currently be feasible for clinical laboratories that offer pharmacogenetic testing, the clinical relevance of these low frequency CNV alleles indicates that future iterations of clinical pharmacogenomic sequencing assays that incorporate computational copy number detection pipelines should include this gene family in addition to the pharmacogenes with more common CNV alleles.
ACKNOWLEDGEMENTS
The study was supported, in part, by Sema4, a Mount Sinai venture, Stamford, CT. This study makes use of data generated by the DECIPHER community. A full list of centers who contributed to the generation of the data is available from http://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. Funding for the project was provided by the Wellcome Trust.
SOURCES OF SUPPORT:
This research was supported, in part, by Sema4, a Mount Sinai venture, Stamford, CT, and by the National Institutes of Health through grant R24 GM123930 (A.G.; Pharmacogene Variation (PharmVar) Consortium).
APPENDIX
Appendix Table 1.
CYP2C copy number variants (CNVs) identified in the Database of Genomic Variants (DGV), ClinGen, and DECIPHER.
| CNV ID | Genes | CNV type | Region size | Frequency | Study population | Ref. |
|---|---|---|---|---|---|---|
| DGV | ||||||
| esv3624253 | CYP2C18 | Deletion | 118,150 | 1/2504 | Healthy | (Genomes Project et al., 2015) |
| esv2672446 | CYP2C18 | Deletion | 118,136 | 1/1092 | Healthy | (Genomes Project et al., 2012) |
| esv3624254 | CYP2C18 | Deletion | 79,667 | 3/2504 | Healthy | (Genomes Project et al., 2015) |
| dgv955n100 | CYP2C18 | Deletion | 111,509 | 6/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| nsv551954 | CYP2C18 | Deletion | 115,921 | 2/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| dgv956n100 | CYP2C18 | Deletion | 69,696 | 2/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| esv2657299 | CYP2C18 | Deletion | 8,618 | 1/1092 | Healthy | (Genomes Project et al., 2012) |
| nsv522538 | CYP2C18, CYP2C19 | Deletion | 142,135 | 1/2026 | Healthy | (Shaikh et al., 2009) |
| dgv1355n54 | CYP2C18, CYP2C19 | Deletion | 246,123 | 3/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| esv2674280 | CYP2C18, CYP2C19 | Deletion | 155,809 | 1/1092 | Healthy | (Genomes Project et al., 2012) |
| nsv1046308 | CYP2C18, CYP2C19 | Deletion | 191,713 | 1/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| dgv1355n54 | CYP2C18, CYP2C19 | Deletion | 246,123 | 3/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| dgv957n100 | CYP2C18, CYP2C19 | Deletion | 70,440 | 5/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| esv3624256 | CYP2C18, CYP2C19 | Deletion | 44,223 | 2/2504 | Healthy | (Genomes Project et al., 2015) |
| esv2761617 | CYP2C18, CYP2C19 | Deletion | 53,597 | 1/1109 | Healthy | (Vogler et al., 2010) |
| esv3624258 | CYP2C18, CYP2C19 | Duplication | 80,151 | 1/2504 | Healthy | (Genomes Project et al., 2015) |
| dgv160e214 | CYP2C18, CYP2C19 | Deletion | 80,151 | 1/2504 | Healthy | (Genomes Project et al., 2015) |
| dgv1356n54 | CYP2C18, CYP2C19 | Deletion | 61,544 | 6/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| esv3891886 | CYP2C18, CYP2C19, CYP2C9 | Deletion | 271,380 | 1/3017 | Infectious diseases, Thyrotoxic Hypokalemic Periodic Paralysis (THPP) and Hb E/b-thalassemia | (Suktitipat et al., 2014) |
| nsv1035409 | CYP2C18, CYP2C19, CYP2C9, CYP2C8 | Deletion | 336,881 | 1/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| nsv551962 | CYP2C19 | Deletion | 54,075 | 1/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| esv2659638 | CYP2C19 | Deletion | 61,848 | 1/1092 | Healthy | (Genomes Project et al., 2012) |
| esv3624260 | CYP2C19 | Deletion | 39,678 | 2/2504 | Healthy | (Genomes Project et al., 2015) |
| nsv516555 | CYP2C19 | Deletion | 12,880 | 2/2026 | Healthy | (Shaikh et al., 2009) |
| nsv523259 | CYP2C19 | Duplication | 159,144 | 1/2026 | Healthy | (Shaikh et al., 2009) |
| nsv1052578 | CYP2C19 | Deletion | 103,868 | 1/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| nsv1047782 | CYP2C19, CYP2C9 | Duplication | 137,386 | 1/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| esv2741937 | CYP2C19, CYP2C9 | Deletion | 138,826 | 1/96 | Healthy | (Wong et al., 2013) |
| esv3624265 | CYP2C9 | Duplication | 72,590 | 1/2504 | Healthy | (Genomes Project et al., 2015) |
| nsv551963 | CYP2C9 | Deletion | 23,682 | 1/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| nsv7497 | CYP2C9 | Duplication | 34,308 | 1/8 | Healthy | (Kidd et al., 2008) |
| esv3624264 | CYP2C9, CYP2C8 | Duplication | 175,419 | 1/2504 | Healthy | (Genomes Project et al., 2015) |
| nsv1050544 | CYP2C8 | Duplication | 47,756 | 1/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| nsv1044182 | CYP2C8 | Duplication | 30,694 | 1/29084 | Intellectual disability and/or developmental delay | (Coe et al., 2014) |
| esv2761618 | CYP2C8 | Duplication | 30,682 | 1/1109 | Healthy | (Vogler et al., 2010) |
| nsv551964 | CYP2C8 | Deletion | 56,916 | 1/15767 | Intellectual disability and/or developmental delay | (Cooper et al., 2011) |
| nsv467436 | CYP2C8 | Deletion | 56,916 | 1/2493 | Healthy | (Itsara et al., 2009) |
| ClinGen | ||||||
| Pathogenic | ||||||
| nssv13651550_unk | 150 genes | Deletion | 7,901,553 | Behavioral abnormality, global development delay, microcephaly | (Miller et al., 2010) | |
| nssv13646178_unk | 2,161 genes | Duplication | 135,285,622 | Development delay and/or other significant development or morphological phenotypes | (Miller et al., 2010) | |
| nssv13638976_unk, nssv13640749_unk | 2,161 genes | Duplication | 135,327,117 | Abnormal facial shape, Intrauterine growth retardation, Micrognathia, Syndactyly, Ventricular septal defect | (Miller et al., 2010) | |
| nssv13655969_unk | 122 genes | Deletion | 6,302,504 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| nssv13655409_unk | 928 genes | Deletion | 53,839,386 | Global developmental delay | (Miller et al., 2010) | |
| nssv13653237_unk | 717 genes | Duplication | 42,143,651 | Patent ductus arteriosus | (Miller et al., 2010) | |
| nssv577306_dnovo | 109 genes | Deletion | 5,128,423 | Dilatation, Hydronephrosis | (Kaminsky et al., 2011) | |
| nssv577307_dnovo | 176 genes | Deletion | 8,175,579 | Abnormal facial shape | (Kaminsky et al., 2011) | |
| nssv1494941_unk | 54 genes | Deletion | 2,827,219 | Autism, Failure to thrive | (Miller et al., 2010) | |
| Benign | ||||||
| nssv581618_unk | CYP2C18, CYP2C19 | Deletion | 158,935 | Intellectual disability | (Miller et al., 2010) | |
| nssv1608783_unk | CYP2C19 | Deletion | 20,211 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| Likely Benign | ||||||
| nssv13650654_unk | CYP2C18, CYP2C19 | Deletion | 121,822 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| nssv13655256_unk | CYP2C18 | Deletion | 114,674 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| nssv13647268_unk | CYP2C19, CYP2C9 | Duplication | 180,257 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| nssv13650288_unk | CYP2C18 | Deletion | 48,162 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| nssv585041_unk | CYP2C18 | Deletion | 112,487 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| nssv13655998_unk | CYP2C8 | Duplication | 8,867 | Developmental delay and/or other significant developmental or morphological phenotypes | (Miller et al., 2010) | |
| Uncertain | ||||||
| nssv13650363_unk | CYP2C18, CYP2C19 | Deletion | 240,911 | Seizures | (Miller et al., 2010) | |
| nssv583937_pat | PLCE1, NOC3L, TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLM1, ACSM6 | Deletion | 969,724 | Low-set ears | (Miller et al., 2010) | |
| nssv580748_unk | CYP2C9, CYP2C8, PDLIM1, SORBS1 | Duplication | 473,822 | Global developmental delay | (Kaminsky et al., 2011) | |
| nssv1495397_unk | TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLIM1, SORBS1, ACSM6 | Duplication | 1,043,272 | Autistic behavior, Global developmental delay | (Miller et al., 2010) | |
| nssv3395040_unk | TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLIM1, SORBS1, ACSM6 | Duplication | 1,089,626 | Seizures | (Miller et al., 2010) | |
| DECIPHER | ||||||
| 184 | 175 genes | Deletion | 12,434,019 | Anterior creases of earlobe, capillary hemangiomas, hypoglycemia, intellectual disability, microcephaly | (Firth et al., 2009) | |
| 341717 | 122 genes | Duplication | 8,517,656 | Emotional lability, growth delay, intellectual disability | (Firth et al., 2009) | |
| 2578 | 232 genes | Duplication | 17,187,727 | Behavioral abnormality, constipation, deeply set eye, delayed speech and language development, intellectual disability, macrocephaly, pectus excavatum, plagiocephaly, short stature, ventricular septal defect | (Firth et al., 2009) | |
| 337109 | PLCE1, NOC3L, TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLIM1 | Duplication | 1,080,102 | Intellectual disability - moderate, oromotor apraxia, severe expressive language delay | (Firth et al., 2009) | |
| 292398 | PLCE1, NOC3L, TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLIM1 | Duplication | 1,080,102 | Anxiety, cognitive impairment | (Firth et al., 2009) | |
| 265438 | PLCE1, NOC3L, TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLIM1 | Duplication | 1,080,102 | - | (Firth et al., 2009) | |
| 260874 | PLCE1, NOC3L, TBC1D12, HELLS, CYP2C18, CYP2C19, CYP2C9, CYP2C8, PDLIM1 | Duplication | 1,080,102 | Delayed speech and language development, incomprehensible speech, intellectual disability - moderate, oromotor apraxia | (Firth et al., 2009) | |
| 318690 | CYP2C18, CYP2C19 | Deletion | 120,242 | Delayed speech and language development, EEG abnormality, moderate global developmental delay | (Firth et al., 2009) | |
| 300098 | CYP2C18, CYP2C19 | Deletion | 158,935 | Cognitive impairment | (Firth et al., 2009) | |
| 283724 | CYP2C18, CYP2C19 | Deletion | 140,797 | Abnormal facial shape, intellectual disability | (Firth et al., 2009) | |
| 278463 | CYP2C19 | Deletion | 61,682 | Behavioral abnormality | (Firth et al., 2009) | |
| 270359 | CYP2C19, CYP2C8, CYP2C9 | Duplication | 288,534 | Hypospadias, rudimentary fibula | (Firth et al., 2009) | |
| 256495 | 34 genes (CYP2C18 is not included) | Deletion | 2,093,635 | - | (Firth et al., 2009) | |
| 305972 | CYP2C19, CYP2C8, CYP2C9 | Duplication | 344,191 | - | (Firth et al., 2009) | |
| 272275 | CYP2C8, CYP2C9, PDLIM1, SORBS1 | Deletion | 694,656 | - | (Firth et al., 2009) | |
Footnotes
DATA REPOSITORY INFORMATION:
Leiden Open Variation Database (LOVD) (https://databases.lovd.nl; DOI: 10.1002/humu.21438) cases:
https://databases.lovd.nl/shared/individuals/00239123
https://databases.lovd.nl/shared/individuals/00239124
https://databases.lovd.nl/shared/individuals/00239125
https://databases.lovd.nl/shared/individuals/00239126
https://databases.lovd.nl/shared/individuals/00239127
https://databases.lovd.nl/shared/individuals/00239128
https://databases.lovd.nl/shared/individuals/00239129
https://databases.lovd.nl/shared/individuals/00239130
https://databases.lovd.nl/shared/individuals/00239131
https://databases.lovd.nl/shared/individuals/00239132
https://databases.lovd.nl/shared/individuals/00239133
https://databases.lovd.nl/shared/individuals/00239134
https://databases.lovd.nl/shared/individuals/00239135
https://databases.lovd.nl/shared/individuals/00239136
https://databases.lovd.nl/shared/individuals/00239137
Clinical Genome Consortium (http://dbsearch.clinicalgenome.org/search/; DOI: 10.1056/NEJMsr1406261)
Database of Genomic Variants (http://dgv.tcag.ca; DOI: 10.1093/nar/gkt958)
DECIPHER (https://decipher.sanger.ac.uk; DOI: 10.1016/j.ajhg.2009.03.010)
Pharmacogene Variation Consortium (PharmVar) database (www.pharmvar.org; DOI: 10.1002/cpt.1268)
CONFLICT OF INTEREST
X.L., G.Z., Y.S., E.E.S., L.E., and S.A.S. are paid employees of Sema4, a Mount Sinai venture, Stamford, CT.
REFERENCES
- Bush WS, Crosslin DR, Owusu-Obeng A, Wallace J, Almoguera B, Basford MA, … Ritchie MD (2016). Genetic variation among 82 pharmacogenes: The PGRNseq data from the eMERGE network. Clin Pharmacol Ther, 100(2), 160–169. doi: 10.1002/cpt.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahan P, Li Y, Izumi M, & Graubert TA (2009). The impact of copy number variation on local gene expression in mouse hematopoietic stem and progenitor cells. Nat Genet, 41(4), 430–437. doi: 10.1038/ng.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, & Lupski JR (2016). Mechanisms underlying structural variant formation in genomic disorders. Nat Rev Genet, 17(4), 224–238. doi: 10.1038/nrg.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, … Clinical Pharmacogenetics Implementation, C. (2014). Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther, 96(5), 542–548. doi: 10.1038/clpt.2014.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Eom HS, Kim HJ, Kim IS, Lee GW, & Kong SY (2010). Glutathione-S-transferase genotypes influence the risk of chemotherapy-related toxicities and prognosis in Korean patients with diffuse large B-cell lymphoma. Cancer Genet Cytogenet, 198(1), 40–46. doi: 10.1016/j.cancergencyto.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P, … Eichler EE (2014). Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet, 46(10), 1063–1071. doi: 10.1038/ng.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH Jr., & Scherer SW (2008). Copy-number variations associated with neuropsychiatric conditions. Nature, 455(7215), 919–923. doi: 10.1038/nature07458 [DOI] [PubMed] [Google Scholar]
- Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, … Eichler EE (2011). A copy number variation morbidity map of developmental delay. Nat Genet, 43(9), 838–846. doi: 10.1038/ng.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J, Tatton-Brown K, Coleman K, Guerrero S, Berg J, Cole TR, … Rahman N (2005). Partial NSD1 deletions cause 5% of Sotos syndrome and are readily identifiable by multiplex ligation dependent probe amplification. J Med Genet, 42(9), e56. doi: 10.1136/jmg.2005.031930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escaramis G, Docampo E, & Rabionet R (2015). A decade of structural variants: description, history and methods to detect structural variation. Brief Funct Genomics, 14(5), 305–314. doi: 10.1093/bfgp/elv014 [DOI] [PubMed] [Google Scholar]
- Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D, … Carter NP (2009). DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet, 84(4), 524–533. doi: 10.1016/j.ajhg.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, & den Dunnen JT (2011). LOVD v.2.0: the next generation in gene variant databases. Hum Mutat, 32(5), 557–563. doi: 10.1002/humu.21438 [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Gaedigk R, & Leeder JS (2010). UGT2B17 and SULT1A1 gene copy number variation (CNV) detection by LabChip microfluidic technology. Clin Chem Lab Med, 48(5), 627–633. doi: 10.1515/CCLM.2010.128 [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Ingelman-Sundberg M, Miller NA, Leeder JS, Whirl-Carrillo M, Klein TE, & PharmVar Steering C (2018). The Pharmacogene Variation (PharmVar) Consortium: Incorporation of the Human Cytochrome P450 (CYP) Allele Nomenclature Database. Clin Pharmacol Ther, 103(3), 399–401. doi: 10.1002/cpt.910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Twist GP, Klein TE, Miller NA, & PharmVar Steering C (2019). The Evolution of PharmVar. Clin Pharmacol Ther, 105(1), 29–32. doi: 10.1002/cpt.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A, Twist GP, & Leeder JS (2012). CYP2D6, SULT1A1 and UGT2B17 copy number variation: quantitative detection by multiplex PCR. Pharmacogenomics, 13(1), 91–111. doi: 10.2217/pgs.11.135 [DOI] [PubMed] [Google Scholar]
- Genomes Project C, Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, … McVean GA (2010). A map of human genome variation from population-scale sequencing. Nature, 467(7319), 1061–1073. doi: 10.1038/nature09534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, … McVean GA (2012). An integrated map of genetic variation from 1,092 human genomes. Nature, 491(7422), 56–65. doi: 10.1038/nature11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, … Abecasis GR (2015). A global reference for human genetic variation. Nature, 526(7571), 68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE, Kisanga ER, … Lien EA (2008). Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol, 19(1), 56–61. doi: 10.1093/annonc/mdm434 [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, … Ahuja SK (2005). The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science, 307(5714), 1434–1440. doi: 10.1126/science.1101160 [DOI] [PubMed] [Google Scholar]
- Gordon AS, Tabor HK, Johnson AD, Snively BM, Assimes TL, Auer PL, … Project, N. G. E. S. (2014). Quantifying rare, deleterious variation in 12 human cytochrome P450 drug-metabolism genes in a large-scale exome dataset. Hum Mol Genet, 23(8), 1957–1963. doi: 10.1093/hmg/ddt588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Jin X, Li H, Wang K, Gao J, Song L, & Lv Y (2017). Microarray analysis of copy-number variations and gene expression profiles in prostate cancer. Medicine (Baltimore), 96(28), e7264. doi: 10.1097/MD.0000000000007264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hoskins JM, & McLeod HL (2011). Copy number variants in pharmacogenetic genes. Trends Mol Med, 17(5), 244–251. doi: 10.1016/j.molmed.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichsen CN, Chaignat E, & Reymond A (2009). Copy number variants, diseases and gene expression. Hum Mol Genet, 18(R1), R1–8. doi: 10.1093/hmg/ddp011 [DOI] [PubMed] [Google Scholar]
- Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, … Clinical Pharmacogenetics Implementation, C. (2015). Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther, 98(2), 127–134. doi: 10.1002/cpt.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Muller DJ, Shimoda K, … Stingl JC (2017). Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther, 102(1), 37–44. doi: 10.1002/cpt.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, … Eichler EE (2009). Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet, 84(2), 148–161. doi: 10.1016/j.ajhg.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AC, & Feuk L (2011). Characterization of copy number-stable regions in the human genome. Hum Mutat, 32(8), 947–955. doi: 10.1002/humu.21524 [DOI] [PubMed] [Google Scholar]
- Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, … Wadelius M (2017). Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin Pharmacol Ther, 102(3), 397–404. doi: 10.1002/cpt.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D, … Martin CL (2011). An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med, 13(9), 777–784. doi: 10.1097/GIM.0b013e31822c79f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd JM, Cooper GM, Donahue WF, Hayden HS, Sampas N, Graves T, … Eichler EE (2008). Mapping and sequencing of structural variation from eight human genomes. Nature, 453(7191), 56–64. doi: 10.1038/nature06862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lao X, Zhang C, Tian L, Lu D, & Xu S (2014). Increased genetic diversity of ADME genes in African Americans compared with their putative ancestral source populations and implications for pharmacogenomics. BMC Genet, 15, 52. doi: 10.1186/1471-2156-15-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Carvalho CM, Hastings PJ, & Lupski JR (2012). Mechanisms for recurrent and complex human genomic rearrangements. Curr Opin Genet Dev, 22(3), 211–220. doi: 10.1016/j.gde.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martis S, Mei H, Vijzelaar R, Edelmann L, Desnick RJ, & Scott SA (2013). Multi-ethnic cytochrome-P450 copy number profiling: novel pharmacogenetic alleles and mechanism of copy number variation formation. Pharmacogenomics J, 13(6), 558–566. doi: 10.1038/tpj.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, … Ledbetter DH (2010). Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet, 86(5), 749–764. doi: 10.1016/j.ajhg.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, … Walsh TJ (2017). Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin Pharmacol Ther, 102(1), 45–51. doi: 10.1002/cpt.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MR, Wegmann D, Ehm MG, Kessner D, St Jean P, Verzilli C, … Mooser V (2012). An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science, 337(6090), 100–104. doi: 10.1126/science.1217876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, … Hattori N (2006). Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol, 59(2), 298–309. doi: 10.1002/ana.20753 [DOI] [PubMed] [Google Scholar]
- Reiner J, Karger L, Cohen N, Mehta L, Edelmann L, & Scott SA (2017). Chromosomal Microarray Detection of Constitutional Copy Number Variation Using Saliva DNA. J Mol Diagn, 19(3), 397–403. doi: 10.1016/j.jmoldx.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa BB, Garcia CA, & Lupski JR (1991). Charcot-Marie-Tooth disease type 1A: molecular mechanisms of gene dosage and point mutation underlying a common inherited peripheral neuropathy. Int J Neurol, 25–26, 97–107 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11980069 [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, … Campion D (2006). APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet, 38(1), 24–26. doi: 10.1038/ng1718 [DOI] [PubMed] [Google Scholar]
- Ruderfer DM, Hamamsy T, Lek M, Karczewski KJ, Kavanagh D, Samocha KE, … Purcell SM (2016). Patterns of genic intolerance of rare copy number variation in 59,898 human exomes. Nat Genet, 48(10), 1107–1111. doi: 10.1038/ng.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Niemi M, Hiratsuka M, Kumondai M, Ingelman-Sundberg M, Lauschke VM, & Rodriguez-Antona C (2018). Novel copy-number variations in pharmacogenes contribute to interindividual differences in drug pharmacokinetics. Genet Med, 20(6), 622–629. doi: 10.1038/gim.2017.156 [DOI] [PubMed] [Google Scholar]
- Schulze J, Johansson M, Thorngren JO, Garle M, Rane A, & Ekstrom L (2013). SULT2A1 Gene Copy Number Variation is Associated with Urinary Excretion Rate of Steroid Sulfates. Front Endocrinol (Lausanne), 4, 88. doi: 10.3389/fendo.2013.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Cohen N, Brandt T, Toruner G, Desnick RJ, & Edelmann L (2010). Detection of low-level mosaicism and placental mosaicism by oligonucleotide array comparative genomic hybridization. Genet Med, 12(2), 85–92. doi: 10.1097/GIM.0b013e3181cc75d0 [DOI] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, … Clinical Pharmacogenetics Implementation, C. (2013). Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther, 94(3), 317–323. doi: 10.1038/clpt.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, … Wigler M (2007). Strong association of de novo copy number mutations with autism. Science, 316(5823), 445–449. doi: 10.1126/science.1138659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, … Hakonarson H (2009). High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res, 19(9), 1682–1690. doi: 10.1101/gr.083501.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Becquemont L, Mary-Krause M, de Waziers I, Beaune P, Funck-Brentano C, & Jaillon P (2000). Combined glutathione-S-transferase M1 and T1 genetic polymorphism and tacrine hepatotoxicity. Clin Pharmacol Ther, 67(4), 432–437. doi: 10.1067/mcp.2000.104944 [DOI] [PubMed] [Google Scholar]
- South ST, Lee C, Lamb AN, Higgins AW, Kearney HM, Working Group for the American College of Medical, G., & Genomics Laboratory Quality Assurance, C. (2013). ACMG Standards and Guidelines for constitutional cytogenomic microarray analysis, including postnatal and prenatal applications: revision 2013. Genet Med, 15(11), 901–909. doi: 10.1038/gim.2013.129 [DOI] [PubMed] [Google Scholar]
- Sudmant PH, Rausch T, Gardner EJ, Handsaker RE, Abyzov A, Huddleston J, … Korbel JO (2015). An integrated map of structural variation in 2,504 human genomes. Nature, 526(7571), 75–81. doi: 10.1038/nature15394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suktitipat B, Naktang C, Mhuantong W, Tularak T, Artiwet P, Pasomsap E, … Jinawath N (2014). Copy number variation in Thai population. PLoS One, 9(8), e104355. doi: 10.1371/journal.pone.0104355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, … Guchelaar HJ (2011). Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther, 89(5), 662–673. doi: 10.1038/clpt.2011.34 [DOI] [PubMed] [Google Scholar]
- Tennessen JA, Bigham AW, O’Connor TD, Fu W, Kenny EE, Gravel S, … Project, N. E. S. (2012). Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science, 337(6090), 64–69. doi: 10.1126/science.1219240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervasmaki A, Winqvist R, Jukkola-Vuorinen A, & Pylkas K (2014). Recurrent CYP2C19 deletion allele is associated with triple-negative breast cancer. BMC Cancer, 14, 902. doi: 10.1186/1471-2407-14-902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SS, Josyula N, Verma A, Zhang X, Veturi Y, Dewey FE, … Pendergrass SA (2018). Rare variants in drug target genes contributing to complex diseases, phenome-wide. Sci Rep, 8(1), 4624. doi: 10.1038/s41598-018-22834-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijzelaar R, Botton MR, Stolk L, Martis S, Desnick RJ, & Scott SA (2018). Multi-ethnic SULT1A1 copy number profiling with multiplex ligation-dependent probe amplification. Pharmacogenomics, 19(9), 761–770. doi: 10.2217/pgs-2018-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler C, Gschwind L, Rothlisberger B, Huber A, Filges I, Miny P, … Papassotiropoulos A (2010). Microarray-based maps of copy-number variant regions in European and sub-Saharan populations. PLoS One, 5(12), e15246. doi: 10.1371/journal.pone.0015246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LP, Ong RT, Poh WT, Liu X, Chen P, Li R, … Teo YY (2013). Deep whole-genome sequencing of 100 southeast Asian Malays. Am J Hum Genet, 92(1), 52–66. doi: 10.1016/j.ajhg.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]