Abstract
Wilson’s disease patients with neurological symptoms have motor symptoms and cognitive deficits, including frontal executive, visuospatial processing, and memory impairments. Although the brain structural abnormalities associated with Wilson’s disease have been documented, it remains largely unknown how Wilson’s disease affects large-scale functional brain networks. In this study, we investigated functional brain networks in Wilson’s disease. Particularly, we analyzed resting state functional magnetic resonance images of 30 Wilson’s disease patients and 26 healthy controls. First, functional brain networks for each participant were extracted using an independent component analysis method. Then, a computationally efficient pattern classification method was developed to identify discriminative brain functional networks associated with Wilson’s disease. Experimental results indicated that Wilson’s disease patients, compared with healthy controls, had altered large-scale functional brain networks, including the dorsal anterior cingulate cortex and basal ganglia network, the middle frontal gyrus, the dorsal striatum, the inferior parietal lobule, the precuneus, the temporal pole, and the posterior lobe of cerebellum. Classification models built upon these networks distinguished between neurological WD patients and HCs with accuracy up to 86.9% (specificity: 86.7%, sensitivity: 89.7%). The classification scores were correlated with the United Wilson’s Disease Rating Scale measures and durations of disease of the patients. These results suggest that Wilson’s disease patients have multiple aberrant brain functional networks, and classification scores derived from these networks are associated with severity of clinical symptoms.
Keywords: Wilson’s disease, large-scale functional brain networks, functional magnetic resonance images, machine learning, biomarkers
Introduction
Wilson’s disease (WD) is a rare autosomal recessive genetic disorder affecting copper metabolism, and WD patients have excessive copper deposition in the body, mainly the liver and cornea, or the brain (Hegde et al. 2010; Ala et al. 2007; Algin et al. 2010). Clinically, WD has a wide variety of hepatic, psychiatric, and neurological symptoms (Leinweber et al. 2008), with the latter including deficits in motor and cognitive abilities, such as frontal executive functions, visuospatial processing, and memory due to excessive copper deposition in the brain (Hegde et al. 2010; Ala et al. 2007; Frota et al. 2009; Wenisch et al. 2013; Portala et al. 2001).
Recent neuroimaging studies have investigated structural and other abnormalities in neurological WD using a variety of techniques, including structural magnetic resonance imaging (sMRI) (Hegde et al. 2010; Algin et al. 2010; Mochizuki et al. 1997; Magalhaes et al. 1994), diffusion tensor imaging (DTI) (Jadav et al. 2013; Sener 2003), positron emission tomography (PET) (Westermark et al. 1995; Schlaug et al. 1996), and magnetic resonance spectroscopy (MRS) (Juan et al. 2005). The structural abnormalities of neurological WD patients have been found in the midbrain, brainstem, cerebellum, and subcortical gray matter structures of basal ganglia and thalamus using sMRI (Hegde et al. 2010; Algin et al. 2010; Mochizuki et al. 1997; Jadav et al. 2013; Sener 2003; Magalhaes et al. 1994; Aikath et al. 2006; Sinha et al. 2006; Starosta-Rubinstein et al. 1987) and DTI (Algin et al. 2010; Jadav et al. 2013; Sener 2003). The abnormalities also include white matter lesions in brain regions such as frontal lobe, temporal lobe, occipital lobe, internal capsule, midbrain, brainstem, and cerebellar peduncle (Aikath et al. 2006; Magalhaes et al. 1994; Starosta-Rubinstein et al. 1987; Jadav et al. 2013). Metabolic abnormalities have been reported in the structurally-abnormal brain regions of neurological WD patients, including the basal ganglia, in imaging studies using PET (Schlaug et al. 1996; Westermark et al. 1995; Hawkins et al. 1987) or MRS (Juan et al. 2005).
Overall, these studies have revealed structural and functional abnormalities in neurological WD that might contribute to clinical symptoms. Our recent study found that neurological WD patients had altered default model network (DMN) connectivity compared with healthy controls (Han et al. 2016). However, it is largely unknown how the neurological WD alters this and other large-scale intrinsic functional brain networks (Bressler and Menon 2010; Mesulam 2009).
In this study, we investigated large-scale intrinsic functional brain networks of neurological WD patients using resting state functional magnetic resonance imaging (rsfMRI). In particular, we adopted independent component analysis (ICA) to compute large-scale intrinsic functional brain networks of the neurological WD patients and healthy controls (HCs) and then used a pattern classification method to identify functional brain networks that distinguished between the neurological WD patients and the HCs (Y. Fan et al. 2011; Yong Fan et al. 2010; Li et al. 2017). Furthermore, we explored whether the altered functional brain networks correlate with clinical symptom measures, including United Wilson’s Disease Rating Scale (UWDRS) score(Leinweber et al. 2008) and duration of disease, to examine the predictive power of the altered brain networks for clinical symptoms in neurological WD.
Methods
Subjects
This study recruited 39 neurological WD patients and 34 HCs at the Institute of Neurology, Anhui University of Chinese Medicine in China. The diagnosis criteria for neurological WD patients included the presentation of extrapyramidal symptoms, a slit lamp of corneal Kayser-Fleischer rings, serum ceruloplasmin lower than 20 mg/dL, and a 24-h urinary copper concentration higher than 100 μg (Roberts et al. 2008). The exclusion criteria for neurological WD patients were as follows: (1) patients with mental retardation (a score on the Wechsler Adult Intelligence Scale-Revised Chinese Version [WAIS-RC]-IQ<70 points), (2) patients with dysaudia and lalopathy, (3) patients with significant impairment of liver function (alanine aminotransferase>100 U or patients with liver cirrhosis), (4) patients with possible anxiety and depression (Hamilton Anxiety Scale [HAMA] and Hamilton Depression Scale [HAMD]>7 points), and (5) patients taking levodopa or other drugs that affect cognitive function. All neurological WD patients had no visual acuity or field deficits. The entire spectrum of clinical features for patients was evaluated by the United Wilson’s Disease Rating Scale (UWDRS) (Leinweber et al. 2008). Every patient received regular copper chelation therapy.
At the same institute, HCs were recruited from a local volunteer group, who were matched to neurological WD patients on education level, intellectual level, age and sex. All HCs had no deficits in visual acuity, linguistic and comprehension skills, and no history of psychiatric or mental illness. All participants were right-handed. All participants were right-handed.
The work described has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments. All participants provided written informed consent. The research protocol was approved by the Ethics Committee of the Institute of Neurology, Anhui University of Chinese Medicine, Hefei, China.
Instead of using frame censoring to reduce the effect of the head motion, which may result in loss of temporal degrees of freedom and make different subjects to have different numbers of time points (Ciric et al. 2017), we excluded subjects with large head motion. Particularly, 9 neurological WD patients and 8 HCs were excluded due to large head motion when their rsfMRI scans were collected (the maximum change [delta] in displacement of brain voxels between 2 successive time points of fMRI scans was>1 mm, or the root-mean-square (RMS) value of the delta frame-wise displacement was>0.15mm). The final sample comprised 30 neurological WD patients and 26 HCs (Table 1).
Table 1.
Clinical and demographic data and Neuropsychological task performances of WD patients and HCs
| Characteristics | WD patients (N=30; Mean ± SD) | HCs (N=26; Mean ± SD) | p |
|---|---|---|---|
| Sex (Male / Female) | 18 /12 | 18/8 | 0.47 |
| Age (years) | 22.53 (3.72) | 22.77 (3.49) | 0.54 |
| Years of school education | 11.27 (2.86) | 10.58 (2.45) | 0.52 |
| Duration of disease (years) | 4.26 (3.18) | -- | -- |
| UWDRS | 20.40 (9.32) | -- | -- |
| WAIS-RC | 99.93 (11.39) | 99.38 (11.38) | 0.76 |
| Verbal fluency | 11.41 (1.96) | 11.27 (2.34) | 0.92 |
 |
7.83 (0.46) | 7.73 (0.60) | 0.53 |
| 5.13 (1.14) | 4.92 (1.20) | 0.73 | |
| HAMA | 2.20 (2.01) | 2.54 (1.96) | 0.40 |
| HAMD | 2.00 (1.64) | 2.77 (2.18) | 0.20 |
WD = Wilson’s disease; NCs = Normal controls; SD = standard deviation; UWDRS = United Wilson’s Disease Rating Scale; WAIS-RC = Wechsler Adult Intelligence Scale-Revised Chinese version; DT = Digit span test; HAMA = Hamilton Anxiety Scale; HAMD = Hamilton Depression Scale.
All subjects underwent neuropsychological and psychiatric evaluation. The tests or instruments included the following items: (1) intelligence level was estimated by the WAIS-RC (Gong 1992), (2) anxiety and depressive states were estimated by the HAMA (Gjerris et al. 1983) and the HAMD (Miller et al. 1985) respectively, (3) frontal lobe functions (Hanlly et al. 1990; Henry and Crawford 2004) was estimated by a verbal fluency (animals/min) test, and (4) attention and working memory were measured by the digit span tests, including forward and backward digits (Blackburn and Benton 1957). The scores of neuropsychological tests were compared between neurological WD patients and HCs. Two-tailed nonparametric tests (Mann-Whitney U-test) using SPSS13 (SPSS Inc., IL, USA) was performed, and the level of statistical significance was set at p<0.05. Sex difference was accessed using Chi-square test.
Imaging data acquisition
The magnetic resonance imaging data was scanned using a standard birdcage head coil with a 3.0 T GE Signa scanner (HDxt; GE Medical Systems) at the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, China. Head movement was minimized with restraining foam pads which also helped diminish scanner noise. Resting state blood oxygenation level dependent (BOLD) images of the whole brain using a gradient echo EPI sequence were acquired in 39 transverse slices (repetition time [TR]=2000ms, echo time [TE]=30ms, flip angle=90°, in-plane matrix=64×64, field of view [FOV]=240×240mm2, voxel size=3.75×3.75×3.8mm3, slice thickness=3mm). For each subject, the rsfMRI scanning lasted 8 min. Structural axial images were obtained using a magnetization prepared rapid acquisition spoiled gradient echo 3D T1-weighted sequence for each subject (TR=7.01ms, TE=2.88ms, flip angle=8°, in-plane matrix=256×256, slices=166, FOV=240×240mm2, voxel size=0.94×0.94×1.2mm3 ).
structural MRI and Resting state fMRI data preprocessing
The imaging data were preprocessed using Statistical Parametric Mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), Analysis of Functional NeuroImage (AFNI) software (http://afni.nimh.nih.gov/afni), and the FMRIB Software Library (FSL, http://fsl.fmrib.ox.ac.uk/fsl).
The T1-weighted images were segmented into gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), and other non-brain tissues using SPM8’s new segmentation approach. Then, the GM and WM maps were registered to a group template using DARTEL, and warped to Montreal Neurological Institute (MNI) space with affine transformation. Finally, modulated GM maps were generated. The T1-weighted images were co-registered with their corresponding fMRI scans, and WM and CSF maps were resampled to have the same spatial resolution of fMRI scans.
The first 6 volumes of the functional images, collected before equilibrium magnetization was reached, were discarded. Then, the fMRI scans were pre-processed as follows: (1) De-spiking of the remaining volumes, (2) slice-timing correction and head motion correction, (3) intensity scaling of each fMRI scan to yield a whole brain mean value of 1000, (4) regressing out 6 affine motion parameters and mean signals of the co-registered WM and CSF regions, (5) temporal band-pass filtering (0.009<f<0.08Hz) to reduce the effects of low-frequency drifts and high-frequency noise, (6) normalizing single-subject images nonlinearly to Montreal Neurological Institute (MNI) space with the deformation field obtained with their co-registered T1 scans using DARTEL within SPM8, and resampling images to 3×3 ×3mm3, (7) spatially smoothing with a 6mm full width at half-maximum (FWHM) Gaussian kernel. For head motion correction, the rsfMRI volumes were realigned to the mean of all volumes using 3dvolreg of AFNI. The head motion of fMRI scans was measured by the maximum and the RMS value of the frame-wise displacement of brain voxels between different time points. No significant difference was found between neurological WD (maximum: 0.43mm±0.24, RMS value: 0.078mm±0.034) and HC (maximum: 0.43mm±0.23, RMS value: 0.088mm±0.030) groups (maximum: p=0.90, RMS value: p=0.29).
Extracting functional networks (FNs) and identifying altered FNs due to the neurological WD
We adopted independent component analysis (ICA) to extract functional brain networks from rsfMRI data. The ICA method was a widely used data-driven technique for extracting spatially independent components (ICs), referred to as functional networks (FNs) (Beckmann et al. 2005). Particularly, we used group information-guided ICA (GIG-ICA) to compute subject-specific ICs in a fully unbiased setting (Du and Fan 2013). A gray matter mask was adopted in the ICA computing in order to minimize the CSF partial volume effects and spurious effects on connectivity measures, and to increase sensitivity to BOLD signal changes (Tuovinen et al. 2016; Bodurka et al. 2007). The number of components was estimated automatically to be 72 using 40 healthy controls from an independent dataset (see supplemental material for details) by Group ICA of fMRI Toolbox (GIFT, http://icatb.sourceforge.net/). These group level ICs were then used as guidance information to compute ICs of the 30 WD patients and 26 HC using GIG-ICA, and each rs-fMRI scan was characterized with 72 FNs (supplemental Figure).
A computationally efficient pattern classification method was then applied to FNs of all neurological WD patients and HCs to identify the most discriminative combination of FNs and build Support Vector Machine (SVM) classifiers for distinguishing neurological WD patients from HC subjects (Li et al. 2017; Y. Fan et al. 2011; Yong Fan et al. 2010). Particularly, we proposed a simplified forward component selection technique to select FNs for constructing the most discriminative set of FNs based Riemannian distance on the Grassmann manifold (Y. Fan et al. 2011; Li et al. 2017). First, the forward component selection algorithm built a classifier upon each individual FN, the classifier’s performance was then estimated with a leave-one-out cross-validation (LOOCV) so that each FN could be evaluated for its classification performance. We chose the area under a receiver operating characteristic curve (AUROC) and classification accuracy as the measurements of the classification performance. Thus, all FNs were sorted by AUROC (first rank) and classification accuracy (second rank) numerically from the largest to the smallest. Then, by combining the first k (k=1, 2, …, 72) ordered components, a classifier could be built and a function of classification performance of k components was obtained. This procedure was repeated to include more components in the classification one by one until one single classifier was built upon all ordered 72 ICs. Finally, across these 72 classifiers with different k (k=1, 2, …, 72) components, the combination of ICs with the overall best classification performance was chosen as the most discriminative FNs. The simplified forward component selection procedure was carried out based on training data.
Specifically, a repeated hold-out cross-validation procedure was used to build classifiers and evaluate their performance. The repeated hold-out cross-validation procedure carried out a total of 30 training and testing runs with five neurological WD patients and one HC selected randomly as validation sample in each run. In each run, we applied LOOCV procedure to build Sigmoid kernel SVM classifiers for classifying the left-one-out testing subject upon the remaining 25 WD patients and 25 HCs. Finally, the overall classification performance was evaluated based on the 30 training and testing runs.
To avoid any bias in the classification, the classifiers were built with a nested leave-one-out procedure based on the training data to optimize their performance by tuning SVM parameters (the trade-off parameter C) and choosing the most discriminative combination of FNs with a simplified forward component selection algorithm, yielding a number of SVM classifiers, referred to as classification models (Li et al. 2017). The classification model, when applied to its corresponding test sample’s FNs, generated a classification score which was a median probability value to characterize its brain status with positive value indicating neurological WD state and negative value indicating healthy state. One repeated hold-out cross-validation experiment yielded 50 classification models. Since different FNs might be selected in the training and testing runs due to inter-subject variability, aberrant FNs of neurological WD were identified as those that were selected as the most discriminative ones with higher frequency.
Nonparametric permutation tests were adopted to estimate statistical significance of the classification results (Nichols and Holmes 2002). Particularly, all neurological WD patients and HC subjects were divided into two groups randomly with their proportion unchanged. Then, 10-fold cross-validation was adopted to estimate classification performance of SVM classification models build upon training data with aberrant FNs of neurological WD. This procedure was repeated for 10000 times. Finally, a histogram of classification accuracy measures estimated based on permuted samples was obtained.
Voxel-wise whole brain functional connectivity (FC) measures of each aberrant FN were computed as Pearson correlation coefficients between time courses of the FN and the whole brain voxels for each individual subject. The correlation coefficients were Fisher z-transformed to obtain a FC map for each FN. Then, one sample t-tests were applied to each FN of neurological WD patients and HC subjects separately to identify brain regions with significant FC measures. Finally, the FC maps were compared between neurological WD patients and HC subjects with pseudo two sample t-test (p=0.05, permutation test n=10000, age, sex, years of education and head motions as covariates) using Statistical Nonparametric Mapping (SnPM) software (http://warwick.ac.uk/snpm).
In order to explore if functional changes are associated with structural alterations, a voxel based morphometry (VBM) analysis was carried out to compare GM maps of neurological WD patients and HC subjects. Particularly, two sample t-tests were adopted to compare gray matter maps between WD patients and HC subjects with age, sex, education level, and total intracranial volume as covariates. Statistically significant group differences were identified based on a statistical significance threshold at p-value=0.001 (FWE corrected) and an extent threshold of 50 adjacent voxels.
Exploring the relationship between classification scores and clinical scores
In order to explore the relationship between the classification scores and clinical scores, a correlation analysis was performed. Particularly, the classification scores were firstly obtained by the median values of SVM classifiers. Then, the correlations between the scores and clinical scores, including UWDRS score and duration of disease, were estimated using a general linear model with age, sex, years of school education and head motions as covariates in the neurological WD patients. In addition, the subjects incorrectly classified were discarded for observations.
Results
Demographics
The demographic and clinical data were summarized in Table 1. No significant group difference was observed in age, sex, years of education, WAIS-RC, HAMA, HAMD, DT, and VFT scores. For the neurological WD patients, the duration of disease and UWDRS scores were 4.26±3.18 years and 20.4±9.32.
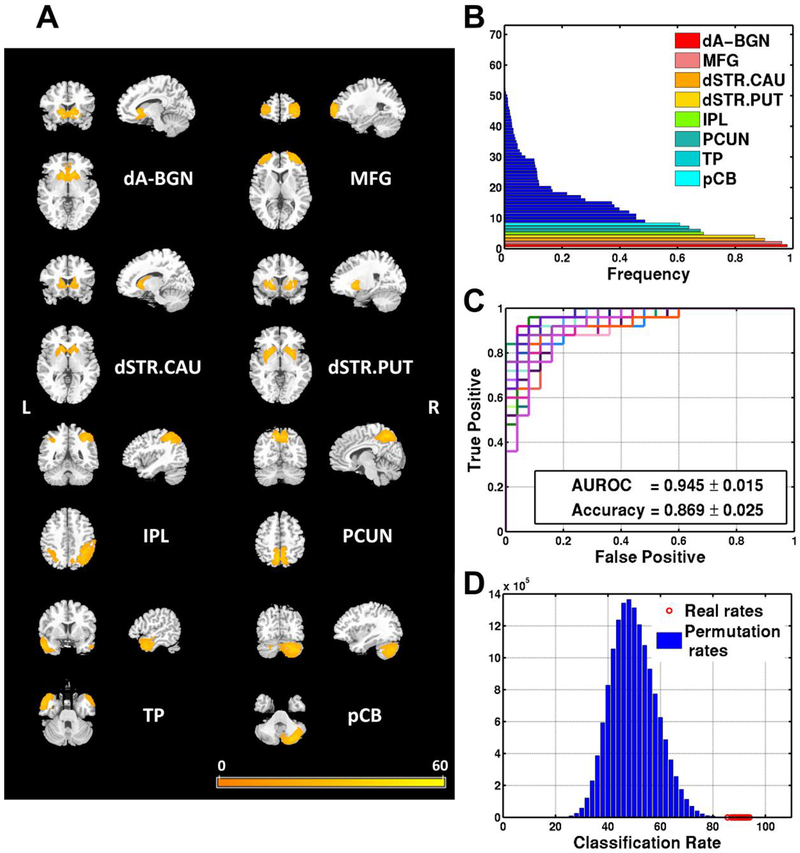
Aberrant FNs of neurological WD patients identified by pattern classification method
The aberrant FNs (Figure 1B, frequency>0.5) included the dorsal anterior cingulate cortex and basal ganglia network (dA-BGN), the middle frontal gyrus (MFG), the dorsal striatum (centered at caudate, dSTR.CAU), the dorsal striatum (centered at putamen, dSTR.PUT), the inferior parietal lobule (IPL), the precuneus (PCUN), the temporal pole (TP), and the posterior lobe of cerebellum (pCB) as shown in Figure 1A. The pattern classification models built upon these aberrant FNs obtained an average AUROC of 0.945 and an average classification accuracy of 86.9% (specificity: 86.7%, sensitivity: 89.7%) in Figure 1C. Nonparametric permutation test results also demonstrated that the classification results were statistically significant (p<0.0001), as indicated by the histogram of permuted classification rates shown in Figure 1D. The classification results on validation dataset showed that the aberrant FNs achieved good classification performance with AUROC of 0.94 and a correct classification rate of 89.4% (specificity: 90.0%, sensitivity: 89.3%).
Figure 1.
The left panel (A) shows spatial maps of the eight selected resting state functional networks (FNs) for building classification models that yielded the best discrimination performance for distinguishing the neurological Wilson’s disease patients from the controls. The top right graph (B) shows the frequency of FNs selected in the cross-validation experiment in ascending order. Networks selected with the highest frequencies (>0.5) were deemed as the most discriminative FNs. The middle right graph (C) shows receiver operating characteristic (ROC) curves of 30 repetitions of nested leave-one-out cross-validation results(average area under the ROC curve [AUROC]=0.945) of the classification models built upon the most discriminative FNs. The bottom right graph (D) shows distribution histogram of the classification rates of permutation tests and real classification rates of 30 repetitions of the cross-validation results. Brain regions are shown as a voxel-wise map of t statistics of one sample t-tests across all subjects with threshold p<0.02 (t value>16) and cluster size>100. The colorbar indicates t values of one sample t-tests. dA-BGN = dorsal anterior cingulate cortex and basal ganglia network; MFG = middle frontal gyrus; dSTR.CAU = dorsal striatum (centered at caudate); dSTR.PUT = dorsal striatum (centered at putamen); IPL = inferior parietal lobule; PCUN = precuneus; TP = temporal pole; pCB = posterior lobe of cerebellum.
Statistical comparisons of FCs between WD patients and HCs
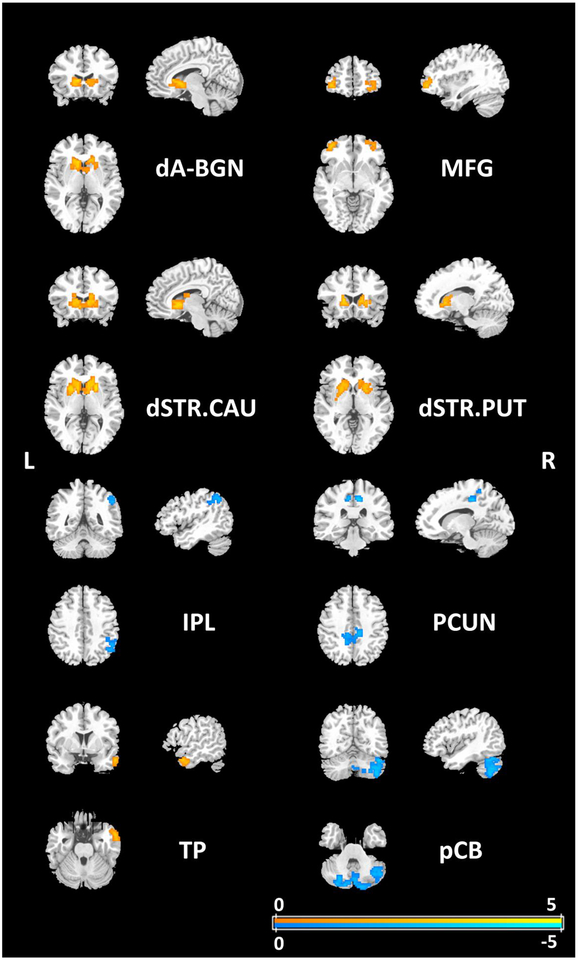
Pseudo voxel-wise two sample t-tests (p=0.05, n=10000, age, sex, years of school education and head motions as covariates) were carried out to compare differences between patients and HCs (Figure 2). For the altered FNs including dA-BGN (caudate), MFG, dSTR.CAU, dSTR.PUT, and TP (right middle temporal gyrus), the neurological WD patients had weaker connectivity than HCs. Meanwhile, the neurological WD patients exhibited increase in FC in IPL (right angular gyrus), PCUN and pCB when compared to HCs.
Figure 2.
Voxelwise differences in FC of the altered FNs between the WD patients and the HC are shown with t values of pseudo two sample t-tests as indicated by the colorbar (p=0.05, permutation test n=10000, age, sex, years of education and head motions as covariates). For hypothesis testing, two alternative hypotheses are specified as, positive: HC > WD, negative: HC < WD. dA-BGN = dorsal anterior cingulate cortex and basal ganglia network; MFG = middle frontal gyrus; dSTR.CAU = dorsal striatum (centered at caudate); dSTR.PUT = dorsal striatum (centered at putamen); IPL = inferior parietal lobule; PCUN = precuneus; TP = temporal pole; pCB = posterior lobe of cerebellum.
Significant group differences in GM maps between the neurological WD patients and HC subjects are shown in Supplementary Figure S2. The VBM results showed that the WD patients and HC subjects had significant differences mainly in the basal ganglia and the thalamus.
Relationship between classification scores and clinical response
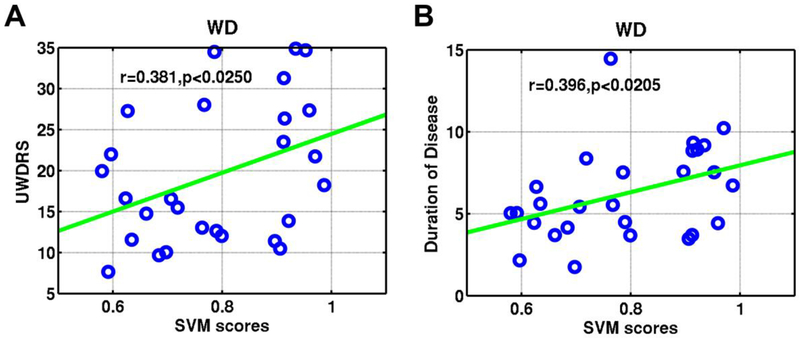
A general linear model was built to model relationship between UWDRS, duration of disease and the classification scores of neurological WD patients. The classification scores of patients were positively correlated with their UWDRS (Pearson correlation: r=0.381, p<0.0250; Spearman’s rank correlation: r=0.377, p<0.0205) and duration of disease (Pearson correlation: r=0.396, p<0.0205; Spearman’s rank correlation: r=0.427, p<0.0109) (Figure 3).
Figure 3.
Association between the classification scores and clinical symptom measures for Wilson’s disease patients with age, sex, years of education and head motions as covariates. Scatterplots show that classification scores of patients were positively correlated with UWDRS (A), and duration of disease (B). UWDRS = United Wilson’s Disease Rating Scale.
Discussion
The present study investigated the functional brain networks of neurological WD patients based on rsfMRI data using machine learning techniques and statistical analysis (Y. Fan et al. 2011; Li et al. 2017; Yong Fan et al. 2010; Wetherill et al. 2018). To our knowledge, this is the first study to explore the functional abnormalities in large-scale functional brain networks in neurological WD.
The classification models built upon discriminative FNs distinguished the patients from the HCs in a data driven way with an average accuracy of 86.9% (specificity: 86.7%, sensitivity: 89.7%). The most frequently selected discriminative FNs included 8 FNs, namely the dorsal anterior cingulate cortex and basal ganglia network, the middle frontal gyrus, the dorsal striatum (centered at caudate), the dorsal striatum (centered at putamen), the inferior parietal lobule, the precuneus, the temporal pole, and the posterior lobe of cerebellum. The receiver operating characteristic (ROC) curves (average AUROC=0.945±0.015) suggested that the classifiers had promising performance for distinguishing the neurological WD patients from HCs in this study, and the validation dataset demonstrated that the aberrant FNs identified were generalizable.
This study adopted an unbiased design for computing group level FNs and estimating the number of FNs based on imaging data of 40 subjects that were not involved in the pattern classification procedure. We used a group information-guided ICA technique in this study to extract the subject-specific FNs with stronger independence and better spatial correspondence across subjects (Du and Fan 2013). In contrast to traditional ICA techniques for group inference using PCA-based back-reconstruction approaches, or regression-based methods (e.g., dual regression) (Calhoun et al. 2009), group information-guided ICA estimate the components based on a multiple-objective optimization framework, which simultaneously optimized the independence of subject-specific FNs as well as enforced the correspondence across FNs of different subjects (Du and Fan 2013).
A simplified forward component selection technique was proposed to select the most informative FNs. Although the subject-specific FNs may contain noisy components due to CSF or head motion, none of them were selected by our pattern classification method as informative FNs, indicating that our method is robust to imaging noise. In the present study, we adopted the Sigmoid kernel SVM following our previous classification setting (Y. Fan et al. 2011). Other kernels could also be adopted to build classifiers. However, nonlinear SVM classifiers are more difficult to interpret compared with their linear counterpart. Source codes of the classification method and GIG-ICA are included as supplementary material and will be also available at https://www.nitrc.org/.
Frame censoring has been an effective means to reduce the effect of the head motion, which however often results in loss of temporal degrees of freedom and makes different subjects to have different numbers of time points (Ciric et al. 2017). Since it remains unclear how different numbers of time points of rsfMRI affect comparability of their functional connectivity measures, we excluded subjects with large head motion. Furthermore, ICA has been adopted to effectively remove head motion effect (Pruim et al. 2015). Our classification results have also demonstrated that noisy components were automatically excluded in a data driven way by our classification algorithm.
The discriminative FNs identified with the highest frequencies were dA-BGN, MFG, dSTR.CAU, dSTR.PUT, IPL, PCUN, TP, and pCB, largely consistent with the existing findings (Starosta-Rubinstein et al. 1987; Hawkins et al. 1987). For distinguishing the neurological WD patients from the HCs, the functional networks of basal ganglia and dorsal striatum, centered in the caudate and the putamen, played an important role. Lesions were also found in these brain regions in neurological WD patients (Starosta-Rubinstein et al. 1987; Hawkins et al. 1987; Wenisch et al. 2013; Mironov 1993; Alexander and Crutcher 1990; Han et al. 2014). These results suggested that structural abnormalities might be associated with aberrant functional brain networks in neurological WD patients. Although few studies evaluated the relationship between neurological WD patients and dorsal ACC (mainly involved in cognitive component), Huntington’s disease (HD) patients with basal ganglia disorder presented altered habituation in the associated network (Gray et al. 2013). Among these 8 FNs, PCUN, as a functional hub of the default mode network (DMN) (Andrews-Hanna et al. 2014), was discriminative for neurological WD. Neurological WD patients had altered DMN’s functional brain connectivity (Han et al. 2016), and other existing findings also demonstrated that Parkinson’s disease (PD), HD patients and other basal ganglia disorders have disrupted FC in the DMN (Tessitore et al. 2012; Delaveau et al. 2010; Wolf et al. 2012; Quarantelli et al. 2013). The PCUN was involved in visual, sensorimotor, and attentional information. For the middle frontal gyrus, the right part was reported to play a role in reorienting of attention (Japee et al. 2015), and WD patients showed attention deficits in previous studies (Han et al. 2016; Han et al. 2014). The inferior parietal lobule, including angular gyrus, a functional cross-modal hub of DMN (Andrews-Hanna et al. 2014) and the supramarginal gyrus, forms a multimodal complex that receives somatosensory, visual, and auditory inputs from the brain. Abnormalities in the supramarginal gyrus have also been found in PD patients (Kim et al. 2013; Nobili et al. 2009). The seventh discriminative FN we identified was the temporal pole that was connected with the amygadala, the hippocampus, the superior temporal gyrus, and the occipitobasal cortex, as well as the orbitary gyrus and the insula (Chabardès et al. 2002). It played a key role in various functions, including autobiographical memory, face and visual pattern recognition, linguistic integration and the processing of emotional language (Dupont 2002). Similarly, abnormalities in the temporal pole have been found in PD and HD patients (Muhlau et al. 2009; Rosas et al. 2005; Potgieser et al. 2014). The posterior lobe of the cerebellum, or neocerebellum, is located below the primary fissure receiving the cerebellar connections from the cerebrum (Center ; Webb and Adler 2016). The cerebellum played an important role in emotion, language, working memory, cognitive and executive functions (Habas et al. 2009; Stoodley and Schmahmann 2009; Levisohn et al. 2000), and brain abnormalities were found in the cerebellum widely in patients with neurological WD (Jadav et al. 2013; Han et al. 2014; Starosta-Rubinstein et al. 1987). The right neocerebellum (particularly in the right crus I-II) we found in this study has been reported to contribute to the left executive control network (Habas et al. 2009), and canonical resting-state networks of PD patients exhibited decreased FC in left executive control networks (Yoneyama et al. 2018).
Functional connectivity analysis of neurological WD demonstrated that the aberrant FNs identified by the pattern classification method were coupled with disrupted FC changes. Neurological WD patients had weaker functional connectivity than HCs in the dA-BGN (caudate), MFG, dSTR.CAU, dSTR.PUT, and TP (right middle temporal gyrus). At the same time, compared with HCs, Neurological WD patients exhibited increased FC in IPL (right angular gyrus), PCUN and pCB. WD patients and HC might exhibit abnormal FC in other unselected FNs, while these eight aberrant FNs were the best informative for distinguishing two groups.
In this study, the classification scores reflected the likelihood of fMRI scans to be neurological WD (positive value) or a healthy state (negative value). The classification scores may be a valuable predictor reflecting functional brain alternations in neurological WD patients as revealed in the correlation analyses with age, sex years of school education and head motions as covariates. In particular, neurological WD patients with higher classification scores had higher UWDRS and longer duration of disease. An increase in classification scores suggested that their corresponding functional network patterns might shift from a healthy-specific state to a disease-specific state. Thus, the positive correlation might indicate that the clinical symptoms were significantly correlated with the altered FNs that were discriminative for distinguishing the neurological WD patients from the HCs.
Significant group differences in GM maps between the neurological WD patients and HC subjects were identified mainly in the basal ganglia and the thalamus, consistent with findings reported in the literature (Mironov 1993; Zaheryany et al. 2012). The structural alternation was partially overlapped with the dorsal anterior cingulate cortex and basal ganglia network and the dorsal striatum (centered at caudate) network. However, no statistically significant structural alternation was observed at other functional networks. These results indicated that functional network changes were partially related to structural alternation in the WD patients.
The present study has several limitations. One limitation of the present study is that we compared healthy controls and neurological WD patients in their large-scale functional networks. Since some of the functional networks affected by WD might be also altered in HD and PD patients, it would be interesting to directly compare large-scale functional networks of WD, HD, and PD patients to identify common and specific functional networks affected by these diseases. This study mainly investigated the resting state functional brain connectivity patterns. A study of structural brain networks of neurological WD patients may provide complementary information to the present study. The sample size of the present study is moderate. A larger sample is needed to yield more representative findings although it is difficult due to the rareness of WD.
In conclusion, this is the first study to investigate the large-scale functional brain networks utilizing a multivariate pattern recognition technique in neurological WD patients. The large-scale FNs, including dorsal anterior cingulate cortex and basal ganglia network, the middle frontal gyrus, the dorsal striatum (centered at caudate), the dorsal striatum (centered at putamen), the inferior parietal lobule, the precuneus, the temporal pole, and the posterior lobe of cerebellum, might be altered due to neurological WD. The present results suggest that aberrant functional networks are predictive in neurological WD patients, and may help open up novel avenues for clinical interventions for neurological WD.
Supplementary Material
Acknowledgments
This work was supported in part by the National Basic Research Program of China (grant number 2015CB856404), National Natural Science Foundation of China (grant number 61473296), the Clinical Research Key Project of Anhui University of Chinese Medicine (grant number 2014lckf02006), the Anhui Provincial Science and Technology Project (grant number 15011d04009), and NIH grants (EB022573, DA039215, and DA039002).
Footnotes
Relevant conflicts of interests/financial disclosures: All authors declare that they have no conflict of interests.
Financial Disclosures of all authors
None.
References
- Aikath D, Gupta A, Chattopadhyay I, Hashmi MA, Gangopadhyay PK, Das SK, et al. (2006). Subcortical white matter abnormalities related to drug resistance in Wilson disease. Neurology, 67(67), 878–880. [DOI] [PubMed] [Google Scholar]
- Ala A, Walker AP, Ashkan K, Dooley JS, & Schilsky ML (2007). Wilson’s disease. The Lancet, 369(9559), 397–408, doi: 10.1016/s0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- Alexander GE, & Crutcher MD (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in neurosciences, 13(7), 266–271. [DOI] [PubMed] [Google Scholar]
- Algin O, Taskapilioglu O, Hakyemez B, Ocakoglu G, Yurtogullari S, Erer S, et al. (2010). Structural and neurochemical evaluation of the brain and pons in patients with Wilson’s disease. Jpn J Radiol, 28(9), 663–671, doi: 10.1007/s11604-010-0491-4. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci, 1316, 29–52, doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, & Smith SM (2005). Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci, 360(1457), 1001–1013, doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn HL, & Benton AL (1957). Revised administration and scoring of the digit span test. Journal of consulting psychology, 21(2), 139. [DOI] [PubMed] [Google Scholar]
- Bodurka J, Ye F, Petridou N, Murphy K, & Bandettini PA (2007). Mapping the MRI Voxel Volume in Which Thermal Noise Matches Physiological Noise-Implications for fMRI. Neuroimage, 34(2), 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, & Menon V (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290, doi: [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, & Adali T (2009). A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage, 45(1 Suppl), S163–172, doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center, U. O. U. H. S. Hyperbrain glossary of terms. University of Utah Health Sciences Center. [Google Scholar]
- Chabardès S, Kahane P, Minotti L, Hoffmann D, & Benabid A-L (2002). Anatomy of the temporal pole region. Epileptic disorders, 4(1), 9–16. [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage, 154, 174–187, doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, & Blin O (2010). Dopaminergic modulation of the default mode network in Parkinson’s disease. European Neuropsychopharmacology the Journal of the European College of Neuropsychopharmacology, 20(11), 784. [DOI] [PubMed] [Google Scholar]
- Du Y, & Fan Y (2013). Group information guided ICA for fMRI data analysis. Neuroimage, 69, 157–197, doi: 10.1016/j.neuroimage.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Dupont S (2002). Investigating temporal pole function by functional imaging. Epileptic Disorders International Epilepsy Journal with Videotape, 4 Suppl 1(Suppl 1), S17. [PubMed] [Google Scholar]
- Fan Y, Liu Y, Jiang T, Liu Z, Hao Y, & Liu H (2010). Discriminant analysis of resting-state functional connectivity patterns on the Grassmann manifold. 7623, 76231J, doi: 10.1117/12.844495. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu Y, Wu H, Hao Y, Liu H, Liu Z, et al. (2011). Discriminant analysis of functional connectivity patterns on Grassmann manifold. Neuroimage, 56(4), 2058–2067, doi: 10.1016/j.neuroimage.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Frota NAF, Caramelli P, & Barbosa ER (2009). Cognitive impairment in Wilson’s disease. Dementia & Neuropsychologia, 3(1), 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerris A, Bech P, Bøjholm S, Bolwig T, Kramp P, Clemmesen L, et al. (1983). The Hamilton Anxiety Scale: evaluation of homogeneity and inter-observer reliability in patients with depressive disorders. Journal of affective disorders, 5(2), 163–170. [DOI] [PubMed] [Google Scholar]
- Gong Y (1992). Wechsler adult intelligence scale-revised in China Version Hunan Medical College, Changsha, Hunan/China. [Google Scholar]
- Gray MA, Egan GF, Ando A, Churchyard A, Chua P, Stout JC, et al. (2013). Prefrontal activity in Huntington’s disease reflects cognitive and neuropsychiatric disturbances: the IMAGE-HD study. Exp Neurol, 239, 218–228, doi: 10.1016/j.expneurol.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. (2009). Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci, 29(26), 8586–8594, doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Cheng H, Toledo JB, Wang X, Li B, Wang K, et al. (2016). Impaired functional default mode network in patients with mild neurological Wilson’s disease. Parkinsonism Relat Disord, 30, 46–51, doi: 10.1016/j.parkreldis.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang F, Tian Y, Hu P, Li B, & Wang K (2014). Selective impairment of attentional networks of alerting in Wilson’s disease. PLoS One, 9(6), e100454, doi: 10.1371/journal.pone.0100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlly J, Dewick H, Davies A, Playeer J, & Turnbull C (1990). Verbal fluency in Parkinson’s disease. Neuropsychologia, 28(7), 737–741. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Mazziotta JC, & Phelps ME (1987). Wilson’s disease studied with FDG and positron emission tomography. Neurology, 37(11), 1707. [DOI] [PubMed] [Google Scholar]
- Hegde S, Sinha S, Rao SL, Taly AB, & Vasudev M (2010). Cognitive profile and structural findings in Wilson’s disease: A neuropsychological and MRI-based study. Neurology India, 58(5), 708. [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2004). Verbal fluency deficits in Parkinson’s disease: a meta-analysis. Journal of the International Neuropsychological Society, 10(04), 608–622. [DOI] [PubMed] [Google Scholar]
- Jadav R, Saini J, Sinha S, Bagepally B, Rao S, & Taly AB (2013). Diffusion tensor imaging (DTI) and its clinical correlates in drug naive Wilson’s disease. Metab Brain Dis, 28(3), 455–462, doi: 10.1007/s11011-013-9407-1. [DOI] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, & Ungerleider LG (2015). A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci, 9, 23, doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C-J, Chen C-Y, Liu Y-J, Chung H-W, Chin S-C, Hsueh C-J, et al. (2005). Acute putaminal necrosis and white matter demyelination in a child with subnormal copper metabolism in Wilson disease: MR imaging and spectroscopic findings. Neuroradiology, 47(6), 401–405. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim SJ, Kim HS, Choi CG, Kim N, Han S, et al. (2013). Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neurosci Lett, 550, 64–68, doi: 10.1016/j.neulet.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Leinweber B, Möller JC, Scherag A, Reuner U, Günther P, Lang CJ, et al. (2008). Evaluation of the Unified Wilson’s Disease Rating Scale (UWDRS) in German patients with treated Wilson’s disease. Movement disorders, 23(1), 54–62. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Croningolomb A, & Schmahmann JD (2000). Neuropsychological consequences of cerebellar tumour resection in children: Cerebellar cognitive affective syndrome in a paediatric population. Brain A Journal of Neurology, 123 ( Pt 5)(5), 1041. [DOI] [PubMed] [Google Scholar]
- Li P, Jing RX, Zhao RJ, Ding ZB, Shi L, Sun HQ, et al. (2017). Electroconvulsive therapy-induced brain functional connectivity predicts therapeutic efficacy in patients with schizophrenia: a multivariate pattern recognition study. NPJ Schizophr, 3, 21, doi: 10.1038/s41537-017-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes ACA, Caramelli P, Menezes JR, Lo LS, Bacheschi LA, Barbosa ER, et al. (1994). Wilson’s disease: MRI with clinical correlation. [journal article]. Neuroradiology, 36(2), 97–100, doi: 10.1007/bf00588068. [DOI] [PubMed] [Google Scholar]
- Mesulam M (2009). Defining Neurocognitive Networks in the BOLD New World of Computed Connectivity. Neuron, 62(1), 1–3, doi: [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, & Maddever H (1985). The modified Hamilton rating scale for depression: reliability and validity. Psychiatry research, 14(2), 131–142. [DOI] [PubMed] [Google Scholar]
- Mironov A (1993). Decreased signal intensity of the putamen and the caudate nucleus in Wilson disease of the brain. Neuroradiology, 35(2), 166–166. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Kamakura K, Masaki T, Okano M, Nagata N, Inui A, et al. (1997). Atypical MRI features of Wilson’s disease: high signal in globus pallidus on T1-weighted images. Neuroradiology, 39(3), 171–174. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Wohlschlager AM, Gaser C, Valet M, Weindl A, Nunnemann S, et al. (2009). Voxel-based morphometry in individual patients: a pilot study in early Huntington disease. [Controlled Clinical Trial]. AJNR Am J Neuroradiol, 30(3), 539–543, doi: 10.3174/ajnr.A1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, & Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili F, Abbruzzese G, Morbelli S, Marchese R, Girtler N, Dessi B, et al. (2009). Amnestic mild cognitive impairment in Parkinson’s disease: a brain perfusion SPECT study. Movement Disorders Official Journal of the Movement Disorder Society, 24(3), 414–421. [DOI] [PubMed] [Google Scholar]
- Portala K, Levander S, Westermark K, Ekselius L, & von Knorring L (2001). Pattern of neuropsychological deficits in patients with treated Wilson’s disease. European archives of psychiatry and clinical neuroscience, 251(6), 262–268. [DOI] [PubMed] [Google Scholar]
- Potgieser AR, Van d. H. A., Meppelink AM, Teune LK, Koerts J, & de Jong BM (2014). Anterior temporal atrophy and posterior progression in patients with Parkinson’s disease. Neuro-degenerative diseases, 14(3), 125–132. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, & Beckmann CF (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage, 112, 267–277, doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Quarantelli M, Salvatore E, Giorgio SM, Filla A, Cervo A, Russo CV, et al. (2013). Default-mode network changes in Huntington’s disease: an integrated MRI study of functional connectivity and morphometry. PLoS One, 8(8), e72159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EA, Schilsky ML, & American Association for Study of Liver, D. (2008). Diagnosis and treatment of Wilson disease: an update. [Practice Guideline]. Hepatology, 47(6), 2089–2111, doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, & Fischl B (2005). Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology, 65(5), 745–747, doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Hefter H, Engelbrecht V, Kuwert T, Arnold S, Stöcklin G, et al. (1996). Neurological impairment and recovery in Wilson’s disease: evidence from PET and MRI. Journal of the neurological sciences, 136(1), 129–139. [DOI] [PubMed] [Google Scholar]
- Sener RN (2003). Diffusion MR imaging changes associated with Wilson disease. American Journal of Neuroradiology, 24(5), 965–967. [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Taly AB, Ravishankar S, Prashanth LK, Venugopal KS, Arunodaya GR, et al. (2006). Wilson’s disease: cranial MRI observations and clinical correlation. Neuroradiology, 48(9), 613–621, doi: 10.1007/s00234-006-0101-4. [DOI] [PubMed] [Google Scholar]
- Starosta-Rubinstein S, Young AB, Kluin K, Hill G, Aisen AM, Gabrielsen T, et al. (1987). Clinical assessment of 31 patients with Wilson’s disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol, 44(4), 365–370. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage, 44(2), 489–501. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, et al. (2012). Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology, 79(23), 2226–2232. [DOI] [PubMed] [Google Scholar]
- Tuovinen T, Rytty R, Moilanen V, Abou Elseoud A, Veijola J, Remes AM, et al. (2016). The Effect of Gray Matter ICA and Coefficient of Variation Mapping of BOLD Data on the Detection of Functional Connectivity Changes in Alzheimer’s Disease and bvFTD. Front Hum Neurosci, 10, 680, doi: 10.3389/fnhum.2016.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb W, & Adler RK (2016). Neurology for the Speech-Language Pathologist-E-Book: Elsevier Health Sciences. [Google Scholar]
- Wenisch E, De Tassigny A, Trocello J-M, Beretti J, Girardot-Tinant N, & Woimant F (2013). Cognitive profile in Wilson’s disease: A case series of 31 patients. Revue neurologique, 169(12), 944–949. [DOI] [PubMed] [Google Scholar]
- Westermark K, Tedroff J, Thuomas KÅ, Hartvig P, Långström B, Andersson Y, et al. (1995). Neurological Wilson’s disease studied with magnetic resonance imaging and with positron emission tomography using dopaminergic markers. Movement disorders, 10(5), 596–603. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Rao H, Hager N, Wang J, Franklin TR, & Fan Y (2018). Classifying and characterizing nicotine use disorder with high accuracy using machine learning and resting-state fMRI. Addiction Biology, 0(0), doi:doi: 10.1111/adb.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Wolf ND, Thomann PA, Saft C, et al. (2012). Default-mode network changes in preclinical Huntington’s disease. Exp Neurol, 237(1), 191. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Watanabe H, Kawabata K, Bagarinao E, Hara K, Tsuboi T, et al. (2018). Severe hyposmia and aberrant functional connectivity in cognitively normal Parkinson’s disease. PLoS One, 13(1), e0190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheryany SMS, Bidaki R, Brujeni NH, Rezvani M, & Shooshtari MH (2012). Idiopathic thrombocytopenia and neurologic manifestations in a young female leading to the diagnosis of Wilson’s disease. Iranian Journal of Psychiatry and Behavioral Sciences, 6(2), 96. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.