Abstract
Objectives:
Electroencephalography (EEG) allows monitoring of generalized seizures induced during electroconvulsive therapy (ECT). Scalp EEG recordings show different phases of electroencephalographic ictal activity during ECT seizures, documenting a pattern of seizures that may vary within and across individuals. In this case series, we used 64-electrode high-density EEG recording to detect topographic electroencephalographic changes not typically evident with conventional limited montage commonly used during ECT.
Methods:
EEG recordings were acquired from 5 participants (24 ECT sessions) during index courses for treatment-resistant depression. Using previously proposed staging criteria, the ictal EEG and simultaneously acquired video were interpreted by an expert reviewer blinded to study treatment parameters.
Results:
EEG recordings of all seizures showed generalized, high-amplitude, central-positive complexes (CPCs), which emerged at the beginning of Phase III (polyspike and slow wave activity), with median duration of 47 seconds (IQR 77 seconds), ranging from 14–203 seconds. While individuals showed variability in frequency and amplitude of CPCs, CPCs typically evolved from 4.0 Hz to 1.5 Hz in frequency and decreased in amplitude as the seizure progressed. Elaborating on previously described phases of ECT-induced electrographic seizures, we describe variability in morphology at seizure termination. Initiation of CPCs typically corresponded with clonic movements, but often terminated after motor signs ceased.
Conclusions:
Generalized, high-amplitude, CPCs during ECT are a previously uncharacterized ictal waveform during ECT, which may have important scientific and clinical value. These complexes offer a specific marker for correlating clinical outcomes in ECT and greater understanding of generalized tonic-clonic seizures.
Keywords: Electroconvulsive therapy, electroencephalography, seizures
Introduction
The induction of generalized seizure activity by electroconvulsive therapy (ECT) has been utilized as treatment for depression1 but the ictal electroencephalographic (EEG) markers of these seizures have not been fully characterized. While 15 seconds2 has been proposed as a threshold for adequate seizure duration, this measure alone does not predict therapeutic efficacy.3 Past analyses of peri-ictal EEG correlate successful ECT clinical outcomes with either the duration of postictal generalized EEG suppression (PGES)4–6 or the degree of associated postictal delta range activity.7–9 However, other investigations of ictal EEG measures have not revealed significant clinical correlations with ECT treatment outcomes.5;10;11
Common ECT practice employs bilateral frontomastoid EEG to assess the onset and resolution of ictal EEG changes. Systematic investigations of these ictal patterns were generally limited to coverage by one to four EEG channels over the frontal-polar or temporal regions.5;12 Weiner described variable durations in ictal patterns, with initial high frequency oscillations analogous to epileptic recruiting, followed by an early polyspike and subsequent period of polyspike and slow waves.13 These generally correspond to three proposed phases of ictal EEG progression based on full 10–20 montage EEG recordings from a series of publications14;15 and a description of a series of 80 ECT-induced seizures.16 Phase I was described as initial rhythmic beta (14–22 Hz) activity, followed by arrhythmic polyspike activity of Phase II and rhythmic 2.5–3.5 Hz spike/polyspike-wave activity (Phase III). Multichannel 10–20 EEG montage recordings, which are the clinical standard for characterization of epileptic seizures, have correlated ECT outcomes to regional quantitative EEG measures17 or topographic patterns for different frequency bands.18 Nevertheless, the spatiotemporal properties of these patterns remain incompletely characterized.
High-density EEG entails the application of 64 to 256 electrodes to the scalp, face and neck. The dense array of electrodes and coverage of regions over the entire head offers several benefits over standard clinical 10–20 scalp montage EEG recordings. Perhaps the greatest advantage is polarity and dipole localization beyond the spatial coverage of standard 10–20 EEG montages.19 Given the previous reports of spatiotemporal evolution in ictal discharges during ECT-induced seizures, particularly during the mid-ictal period,20 high-density scalp EEG presents a promising avenue that could better describe and quantitate ECT-induced ictal changes.
In this case series, we describe analyses of twenty-four 64-channel EEG recordings acquired from five patients during the initial course of ECT for treatment-resistant depression. We used standard 10–20 EEG montage displays to assess general morphology and phases. High-density EEG signals were then evaluated to further assess global EEG changes. Using synchronized video, we correlated morphology of the ictal complexes with the clinical characteristics of the seizures in all subjects.
Materials and Methods:
Study Design and Intervention
Patients were enrolled in a randomized clinical trial designed to evaluate cognitive function and EEG patterns during the recovery from ECT and concurrent general anesthesia (Trial Registration: Clinicaltrials.gov NCT02761330). This trial was approved by the Human Research Protection Office (HRPO) at Washington University School of Medicine in St. Louis. Written informed consent was obtained from all participants prior to participation, with adherence to the Declaration of Helsinki. There were no known serious unexpected adverse events related to study involvement. From this study population, we present a case series of five subjects to characterize the clinical and ictal evolution of ECT-induced seizures, using high-density EEG recordings to display both standard 10–20 EEG montage recordings and topographic mapping during seizures.
A Thymatron System IV (Somatics, LLC, Venice, FL, USA) was used to deliver the electrical stimulus and record frontomastoid EEG, allowing direct comparison to high-density EEG recordings. Right unilateral electrode placement with ultra-brief pulse stimulation (0.3 milliseconds, 0.9 amperes) was used in all sessions. During the dose-charge titration sessions of all 5 participants, an initial charge of 25 millicoulombs (10 Hz) was sufficient to induce a generalized seizure (Seizure 1). For subsequent stimulations, the charge was 150 millicoulombs in all cases, given local practice of administering a six-fold increase in dose for treatments consistent with the work of Sackheim and colleagues.21 Concurrent general anesthesia was induced through either etomidate (0.2 mg/kg) or ketamine (2 mg/kg).
Data Acquisition
Patients wore an appropriately sized 64-channel EEG Geodesics Sensor Net (Electrical Geodesics, Inc./Phillips, Eugene, OR, USA) modified for EEG acquisition before, during, and after ECT stimulation. The recording equipment was powered through a grounded medical isolation transformer (ISB 060M model, Toroid Corporation, Salisbury, MD) to reduce line noise artifacts and patient risk of electrical shock. Cap modification allowed placement of stimulation electrodes for right-unilateral ECT (FIGURE 1A). These changes required exclusion of two central electrodes right of vertex (E4 and E54, approximate locations FCz and FC2) and one right facial electrode (E1, approximately F10). A 4–6 cm diameter window was made just right anterior of the vertex electrode. Elefix electrode paste (Nihon Kohden America, Inc., Irvine, CA, USA) was injected into the silver/silver-chloride electrodes. Efforts were made to maintain electrode impedances of each channel to be less than 100 kOhms/channel. EEG recordings were acquired using a Net Amps 400 amplifier (500 Hz sampling rate) and Net Station version 5.3.0.1 software (Electrical Geodesics, Inc./Phillips, Eugene, OR, USA). Synchronized video was acquired using an Axis P3364LV network camera (Axis Communications, Lund, Sweden). For all seizures, the EEG amplifier was transiently disconnected and then reconnected from the sensor cap during ECT electrical stimulus delivery.
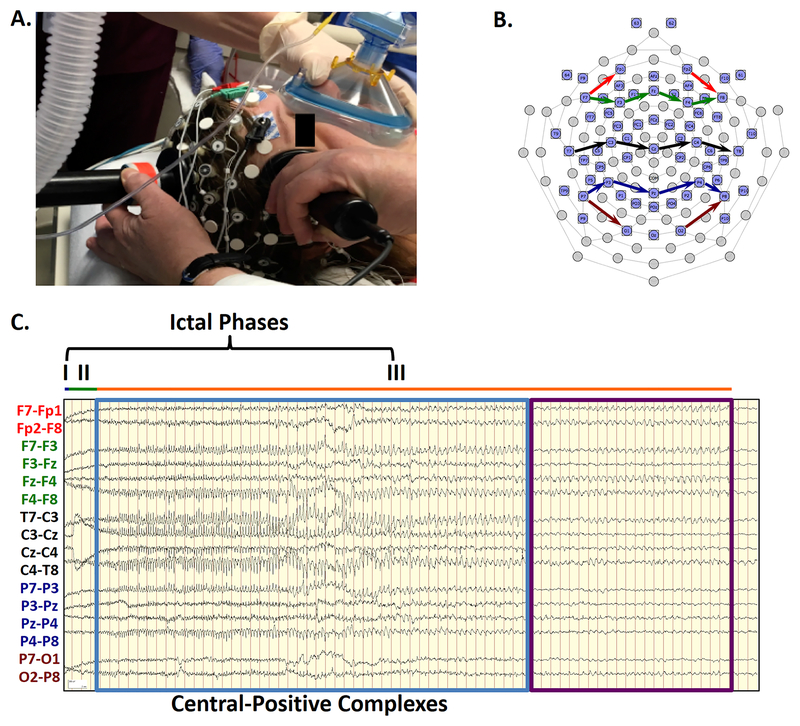
FIGURE 1: Ictal Phases Following ECT Charge Delivery.
1A. Modified 64-channel EEG sensor net allows high-density EEG recording without interference with ECT stimulation electrode placement. 1B. The sensor net diagram shows the localization of the standard 10–20 electrode locations. Arrows indicate the locations of the recording chains of the bipolar transverse montage in FIGURE 1C. 1C. High-amplitude complexes are demonstrated in a bipolar transverse montage. The electrodes contributing to each EEG channel in a chain are connected by color-coded arrows on the electrode map. Intervals for the different ictal phases are depicted (blue, very short Phase I; green, Phase II; orange, Phase III). As each hash mark represents one second, clearly-formed, rhythmic complexes (blue box) arise by the 8th second of the record, with the highest differential amplitudes over the midline chain (black). The polarity of the complexes shifts from relatively negative polarity (upward deflection) to relatively positive (downward deflection) following the chain from the left (T7) to the right (T8), identifying localization at the vertex electrode (Cz). These complexes were initially at a frequency of 4.0 Hz but gradually decreased to 2.0 Hz. At second 50, these complexes abruptly stop, while the seizure continues for another 21 seconds of Phase III (purple box). Signals have been bandpass filtered from 1–70 Hz.
EEG Preprocessing
Data underwent the following pre-processing steps in Net Station Tools: high-pass filtering at 1 Hz to remove drift and low frequency motion artifact, replacement of bad channels marked with excessive noise, and re-referencing to the average signal among all channels. Signals at E1, E4, E54, E65 (reference, Cz), and channels with substantial artifact were reconstructed using spherical spline interpolation. Processed data were then exported and reviewed using Persyst software version 12 (Persyst, Solana Beach, CA, USA), Net Station Review EEG visualization tools, and Brainstorm.22
EEG Interpretation of Epileptiform Complexes and Correlation With Seizure Motor Activity
A neurologist board-certified in epilepsy (REH) evaluated ictal EEG changes using standard 10–20 EEG montages and high-density EEG tracings. The following seizure phase staging (Phases I-III) as described by Brumback and Staton16 were employed: Phase I was identified as 14–22 Hz rhythmic beta activity at the onset of recording after cessation of the ECT stimulus. Polyspike activity that begins in the right (stimulated hemisphere) and spreads to homologous regions in the contralateral hemisphere was used to define Phase II. Phase III was reported as emergence of rhythmic 2–3 Hz spike/polyspike-wave complexes that simultaneously replaced Phase II activity. In our final classification of seizures phases, we followed the description of Brumback and Stanton, with the exception of using CPC frequencies up to 4 Hz. as the marker for the beginning of Phase III.
EEG tracings were correlated with patient movement in all subjects using synchronized video recordings. Motor patterns were characterized as stimulus/immediate post-stimulus-related tonic movements and subsequent myoclonic/clonic movements.
The duration of postictal generalized electroencephalography suppression (PGES) was also determined for each seizure. PGES was defined as generalized EEG attenuation below 10 microvolts for any duration longer than 1 second or within 30 seconds after an ictal EEG pattern had terminated, allowing for muscle, movement, breathing, and electrode artifacts.23;24
Results:
Overall, Phases I and II were relatively short for all seizures, ranging in length from 0–5 seconds for Phase I and 0–12 seconds for Phase II. Of the 24 seizures, Phase I was observed in 3 (12.5%) while Phase II was observed in 23 (95.8%) recordings. In contrast, Phase III was observed in all recordings, ranging from 30–205 seconds (median 65 seconds).
Supplementary Digital Content 1 includes detailed descriptions of all seizures from the first patient in the series (subject 1), while FIGURE 1 and FIGURE 2 show EEG of seizures from the same subject.
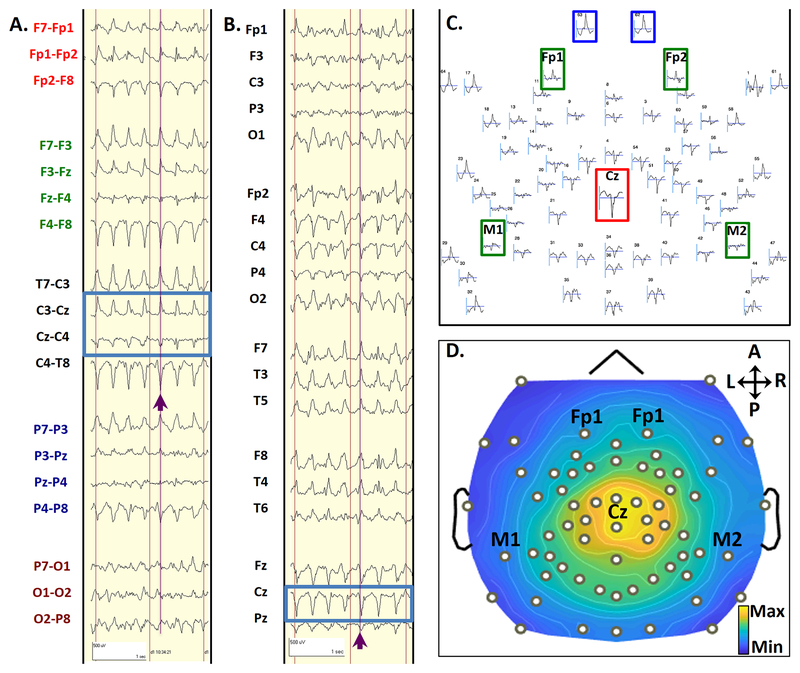
FIGURE 2: Topology of CPCs.
A typical CPC from seizure 2 in subject 1, in a bipolar transverse montage (2A) and an average referenced montage (2B). Purple lines and arrows highlight a single CPC, which occurred 13 seconds after seizure onset during Phase III of Seizure 2. Blue boxes highlight the phase reversal in bipolar montages (2A) and electrodes with maximal amplitude referenced to the average signal (2B). The scalp topography layout for this single CPC is shown in 2C. The topographic plot layout shows all 64 electrodes in relative space as they are distributed over the head, using an average reference. The standard electrode numbers for 64-channel high-density recordings are marked above each waveform. This complex showed a maximal positive voltage over the vertex (Cz, red inset) electrode and maximal negative voltage over sub-ocular electrodes 62 and 63 (blue insets). Electrode locations approximating those for conventional frontomastoid EEG are inset in green. Duration of display (represented by the horizontal gray bar below each waveform) is 300 ms. 2D shows a topographic voltage map of the peak amplitude of the CPC over a 2-ms time window, using an average reference. This voltage map displays the maximal positive voltages over the vertex and surrounding regions (red), and maximal negative voltages over the sub-ocular electrodes (blue). Black dots represent electrode locations, with selected 10–20 locations annotated. Signals have been bandpass filtered from 1–70 Hz.
Novel Generalized, High-amplitude, Central-positive Complexes (CPCs) and Phase III: Correspondence at Initiation but Not Termination
Ictal morphology is apparent in the 10–20 montage EEG derived from the 64-channel EEG sensor net. Chains of a standard transverse bipolar montage were generated through re-referencing denoted by colored arrows on the EEG sensor map (FIGURE 1B). FIGURE 1C shows the EEG of the entire ictal recording of one seizure, presented in a standard transverse bipolar montage. This visual arrangement of EEG channels allows assessment of differential signal amplitude across nearby electrode pairs linked in a series of transverse chains from left to right. For this seizure, Phase I is short (horizontal blue line) while Phase II lasts approximately 3 seconds (green line). The amplitude of EEG signals is relatively low across all channels compared to Phase III (orange line). In particular, high differential amplitude sharp wave complexes (blue box) constitute the bulk of Phase III, with a subsequent period of non-stereotyped ictal slowing (purple box). These complexes have their largest amplitude differentials in the central chain of EEG channels (T7-C3, C3-Cz, Cz-C4, C4-T8). In particular, the differential amplitude changes from a large, relatively negative (upward) to a large, relatively positive (downward) component as the chain crosses midline (Cz). This phenomenon, known as a phase reversal, is consistent with localization of the positive field over the midline given that the change occurred in channels with Cz as the common electrode. Inspection of the frontal transverse chain (F7-F3, F3-Fz, Fz-F4, F4-F8), and parietal transverse chain (P7-P3, P3-Pz, Pz-P4, P4-P8), shows a similar pattern of differential amplitude and polarity change over the midline electrodes (i.e. phase reversal at Fz and Pz), but at relatively lower differential amplitude in comparison to the vertex electrode (Cz).
Topology of Generalized, High-amplitude CPCs
CPCs are shown in greater temporal and spatial detail in FIGURE 2. FIGURE 2A shows a typical CPC during Phase III using a standard bipolar 10–20 EEG montage. Localization is based on phase reversals (inversions of polarity) between contiguous EEG channels in the chain (FIGURE 2A, blue box). These phase reversals occur across the vertex electrode (Cz). Re-referencing to the average facilitates localization of these sharp wave complexes across the scalp. In the referential 10–20 EEG montage (FIGURE 2B), the highest amplitude, best-formed sharp wave complex is localized to the vertex electrode (Cz).
The use of high-density EEG allows for improved qualitative spatial characterization of CPCs compared to the 10–20 montage. Spatial distribution of the average-referenced EEG signals was assessed for a 300 ms interval centered on a single CPC (e.g. purple arrows in FIGURE 2A–B). Peak positive amplitude is maximal at the top of the head (FIGURE 2C, Cz electrode highlighted by the red box), with maximal negative potentials in subocular electrodes (62, and 63, highlighted by the blue box). Electrodes with close proximity to clinical frontomastoid electrodes (green) also deserved inspection. Among these electrodes, signals at Fp1 and Fp2 demonstrate comparatively lower amplitude negative polarity sharp waves and both mastoids show markedly damped deflections of positive polarity sharp waves). The distributions of these potentials interpolated across the scalp are further represented as a topographic voltage map (FIGURE 2D). This descriptive voltage map shows the distribution of voltage amplitude corresponding with the peak of the sharp wave. The maximal positive voltages are over the vertex and surrounding regions (yellow), and maximal negative voltages over the sub-ocular electrodes (61–64, dark blue). The EEG and topographic maps highlight the prominent positive polarity of waveforms near the central head regions, which we describe as central-positive complexes (CPCs).
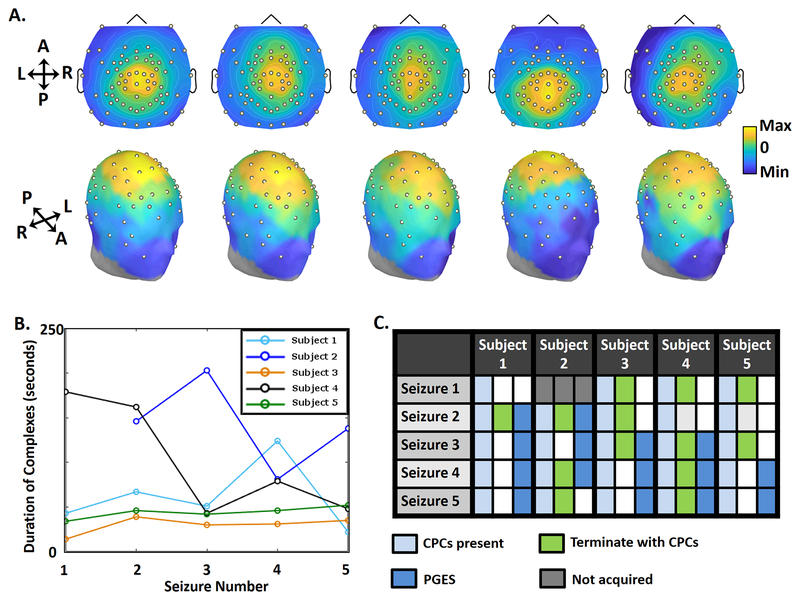
High-density scalp EEG voltage topologies were similar for CPCs recorded across five patients and twenty-four seizures. FIGURE 3A shows topographic maps for representative CPCs across patients. As in the FIGURE 2D (subject 1), the maximum positive amplitude is located near the vertex in central regions with maximal negative voltage in the suborbital EEG electrodes. (FIGURE 3B).
FIGURE 3. Consistency of CPCs.
3A. Topograms showing distribution of EEG power across the scalp during a CPC from one seizure for each participant over a 2 ms window. Each complex was taken during the first 5 seconds of Phase III in seizure 2, which was the first seizure after the dose-charge titration. The topology of CPCs remains consistent across all patients. 3B. Duration of CPCs in all seizures of the 5 subjects. The duration of CPCs for all seizures varied across patients, but showed relative consistency in two study participants (Subjects 3 and 5). 3C. The table shows characteristics of seizures, with three columns for each subject. The first column shows that CPCs were present during Phase III of all 24 seizures (light blue). The second column shows seizures that terminated with CPCs (green), and those that did not (white). The third column marks seizures with postictal generalized EEG suppression (PGES, dark blue), and those without PGES (white).
Within Phase III of individual seizures, CPCs began in a rhythmic 3.5–4.0 Hz pattern and progressively slowed in frequency to 1.5–2.0 Hz prior to termination, as illustrated in FIGURE 1B. As the complexes progressed, there were occasional short periods of discontinuity (typically lasting 0.5–2 seconds) of the rhythmic pattern. Termination of the central maximal positive waveforms was visually distinct in all seizures. Amplitude of complexes qualitatively diminished during seizure progression.
Duration of Ictal Phases, CPCs, and Postictal Suppression
CPCs were present in all 24 seizures but subsequent signals varied following termination (FIGURE 3C). The median duration of CPC expression was 47 seconds (IQR 77 seconds), ranging from 14–203 seconds. These CPCs persisted until the end of the seizure in 13 recordings, with abrupt and simultaneous termination of the complexes and the seizure. In contrast, CPCs ended before the seizure in 11/24 (45.8%) recordings with subsequent ongoing ictal activity of such seizures lasting from 3–62 seconds (median duration 23 seconds, IQR 19 seconds). This Phase III activity post-CPCs (purple box, FIGURE 1C) typically manifested as diffuse 1–1.5 Hz slow waves, decreasing in amplitude until the end of the seizure. Thus, high-amplitude CPCs typically constitute the bulk but not entirety of Phase III.
Postictal generalized EEG suppression (PGES) was observed in 58% of our seizures, with durations varying from 0–57 seconds after seizure termination, but with no clear relationship to the presence of CPCs.
Correspondence Between Clinical and High-Density EEG Signals
Clinical standard of care dual-channel EEG Thymatron IV recordings and concurrent EEG from a high-density array of electrodes (high-density EEG) were acquired without compromising data quality or clinical care. Comparison of high-density EEG recordings with Thymatron IV recordings showed similar ictal recording durations.
Ictal Motoric Activity
Review of video and associated motor signs during seizures showed consistency among recordings. Bilateral facial tonic movements coincided with ECT charge delivery. Rarely, these tonic movements of the face were accompanied by bilateral upper extremity flexion. Bilateral clonic movements of the extremities, typically most pronounced in the lower extremities, accompanied the progression of seizures into Phase III. The clonic movements were synchronous with CPCs, especially at the beginning of Phase III. However, clonic movements usually subsided before CPCs resolved.
Discussion:
Generalized, High-amplitude, Central-positive Complexes (CPCs)
The most prominent finding from the high-density EEG recordings was the characterization of generalized, high-amplitude CPCs. High-density EEG allowed broad spatial coverage over the entire head, including the scalp and face. Topographic maps show a reproducible spatial pattern of the CPCs on both the intra-subject and inter-subject level, with a pattern of maximal positivity over the central regions, and maximal negativity over the suborbital region. CPCs occurred irrespective of whether etomidate or ketamine was used as the anesthetic, demonstrating independence from potential influence on seizure threshold.25 These findings extend the previous work describing phases of EEG activity for ECT-induced seizures.
Ictal Phases
Our EEG findings are comparable with Brumback and Staton’s descriptions of ECT-induced seizures using full 10–20 scalp montage recordings.14–16 The duration of Phase I activity lasted 0–5 seconds in our series, with the predominant EEG finding of diffuse rhythmic beta activity. Phase II activity persisted for 0–12 seconds, showing focal EEG findings over the right hemisphere before progressing to generalized EEG discharges found in Phase III. Phase III activity also showed previously described patterns of generalized, high-amplitude sharp-wave activity that typically persisted for the majority of the seizure.
Alternative descriptions exist for the classification of ictal EEG changes during ECT. Weiner described five phases of ECT-induced ictal EEG: pre-ictal; epileptic recruiting activity, polyspike activity, polyspike and slow wave activity, and termination.13 Comparing the descriptions of Brumback and Staton to the descriptions of Weiner, Phase I correlates with pre-ictal and epileptic recruiting activity, Phase II corresponds with polyspike and slow wave activity, and Phase III matches with polyspike and slow wave activity and termination. Interestingly, Weiner describes the frequency of polyspike and slow-wave discharges that progress from 4–6 Hz to 1–2 Hz during the polyspike and slow wave phase. This agrees with our finding of generalized, high-amplitude, CPCs that evolve at similar frequencies. Additionally, Weiner describes the termination phase at the end of the seizure, which corresponds well with the ongoing ictal activity we observe after resolution of generalized, high-amplitude CPCs in many of our recorded seizures. There is well-documented variability of seizures within subjects, with some seizures showing prolonged Phase I activity16 while other seizures show no progression to a slow spike-wave pattern.12 Thus, the seizures described for our participants show a degree of variability for duration and pattern, which is typical according to multiple previous descriptions of ECT-induced seizures.
Evolution of EEG Complexes in Phase III
The qualitative progression of frequency for these generalized CPCs is consistent with previous work on high amplitude ECT-induced seizure discharges. The complexes in our data typically followed a rhythmic 3.5–4.0 Hz pattern that slowly declined to 1.5–2.0 Hz. The overall significance of the evolving pattern from faster to slower frequencies of the CPCs remains open for further study, but previous investigators have described temporal evolution of complexes during the “mid-ictal” portion of ECT-induced seizures. Zoldi et al.20 evaluated 10 patients during ECT-induced generalized tonic-clonic (GTC) seizures. Using visual and quantitative analysis, they evaluated evolution of statistical stationarity (which theoretically could be due to repetitive stereotypical CPCs) during seizures. Their findings demonstrated that while stationarity varied between seizures, this property was most prominent in the middle of the GTC seizure, during repetitive sharp wave complexes. Redundancy analysis showed posterior-to-anterior time delays of the sharp wave complexes in the mid-ictal region of GTC seizures. The fronto-central regional distribution of most seizures sustained relatively higher redundancies with the other leads. In one of their examples with high mid-ictal stationarity, a standard 10–20 montage EEG showed repetitive CPCs which evolved from 4 to 3 Hz. over a 10 second period. Therefore, it is possible that the phenomenon of CPCs documented by high-density EEG in our case series corresponds with the mid-seizure redundancy findings documented by Zoldi and colleagues. Further work will quantitatively evaluate the evolution of these epileptiform complexes over the spatial and temporal domains.
Postictal Suppression
Our case series outlines a quantifiable ictal EEG finding of generalized, high-amplitude CPCs, consistently present in each seizure during the course of ECT. There are multiple potentially important clinical associations of this quantifiable EEG change, including the relationship with outcomes after ECT as well as other pathophysiological changes in epilepsy. For example, postictal generalized electroencephalography suppression (PGES) occurs in both epileptic and ECT-induced seizures.23 Further studies of ECT-induced seizures with high-density EEG may further our understanding of the relationship of ictal EEG changes, PGES, and other important clinical factors.
Potential of High-density EEG and Methodological Considerations
From the basic principles of EEG, all waveforms by definition have both a positive and negative field. However, the three-dimensional representation of the electrical field, especially on scalp EEG, typically is very complex and therefore often precludes meaningful definition with standard recording techniques.19 The major exception to this general rule, however, is localization of high amplitude sharp wave discharges with well-defined regions of positive and negative polarity over the surface of the scalp, neck and face (known as dipole localization). The CPCs we describe are well-defined high-amplitude, localized complexes, which show well-defined regions of positive and negative polarity. Three-dimensional localization to specific brain regions may be possible in future studies.
High-density scalp EEG offers several advantages over standard montage scalp EEG recordings, including uncovering characteristics of polarity and dipole localization in higher amplitude EEG waveforms19, such as the high-amplitude, CPCs we describe in this series. Recording of EEG potentials over the entire head, as with high-density EEG, allows for better localization of electrical fields in three-dimensional space. This “picture” of EEG activity during the peak of the CPCs was consistently localized, possibly indicative of a specific region of activation during ECT-induced seizures and may prove to be a useful marker to understand variance in therapeutic response to ECT. There are past descriptions of the importance of standard 10–20 montage scalp EEG recordings with nasopharyngeal electrodes recording during ECT, with non-scalp electrodes adding important information, especially during Phase II of ECT-induced seizures.16 Our high-density EEG sensor net includes suborbital electrodes that allow for better characterization of EEG signals that project to inferior-oriented brain regions (such as the temporal lobe). Therefore, as with nasopharyngeal electrodes, high-density EEG recording may offer important additional information in characterizing ECT-induced seizures.
Safety and signal quality considerations are important. Electrodes in the sensor net were displaced to prevent potential burns related to conduction of current between stimulating and recording electrodes. The use of a medical isolation transformer with grounding both reduced the chance of electrical shock and addressed sources of electrical artifact from nearby equipment. During recording sessions, amplifiers were also disconnected from the sensor net during ECT stimulation. Data loss arising from this disconnection and reconnection of the amplifier could have contributed to an underestimation of time in Phase I and II. Finally, even despite the administration of the intravenous depolarizing muscle paralytic, succinylcholine, muscle-related artifacts may still contaminate EEG at higher frequencies (> 20 Hz).
Comparison with generalized epileptiform discharges in spontaneous seizures
Our findings of CPCs during ECT seizures represent a new characterization of ictal EEG phenomena. Most ictal waveforms during spontaneous epileptic seizures show a negative polarity during scalp surface EEG recordings.19 One advantage of scalp ictal recordings of ECT-induced seizures is the relative lack of movement artifact, given the use of paralytic agents during ECT treatments. Better quality scalp recordings during ECT-induced seizures may in part explain the finding of well-characterized CPCs. However, further comparison of ECT-induced seizures and spontaneous epileptic seizures will be necessary to characterize their pathophysiological differences.
Conclusion
We report a case series of high-density ictal EEG recordings in five subjects undergoing multiple sessions of ECT. High-density EEG aids mapping of global EEG signal changes and therefore offers the possibility of evaluating different aspects of the previously described seizure phases and CPCs during ECT seizures. Our current results show newly-described, reproducible generalized, high-amplitude CPCs across seizures induced by ECT in multiple patients. Further study using high-density EEG will be necessary to quantitatively compare intra- and inter-subject variance of the CPCs. Metrics based on these specific EEG markers of generalized, high-amplitude CPCs may offer insight into therapeutic effects of ECT-induced seizures across psychiatric indications and better define the pathophysiology of GTC seizures.
Supplementary Material
Key findings:
During generalized seizures induced by ECT, generalized, high-amplitude, central-positive complexes (CPCs) were present in all seizures in this series.
The onset of clonic movements were synchronized to the appearance of generalized CPCs, but ended before cessation of these seizure complexes.
These CPCs need further evaluation in relation to ECT efficacy and pathophysiology of spontaneous generalized tonic clonic epileptic seizures.
Funding:
The study was funded by the James S. McDonnell Foundation (US), Recipient: Michael S. Avidan, M.B.B.Ch.
Footnotes
Conflicts of interest: The study was funded by the James S. McDonnell Foundation (US), Recipient: Michael S. Avidan, M.B.B.Ch. There are no other relevant conflicts of interest.
Contributor Information
R. Edward Hogan, Email: hoganre@wustl.edu.
Emma R. Trammel, Email: etrammel@umich.edu.
Nuri B. Farber, Email: farbern@wustl.edu.
Michael S. Avidan, Email: avidanm@wustl.edu.
Ben Julian A. Palanca, Email: palancab@wustl.edu.
Reference List
- 1.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med 2007;357:1939–45. [DOI] [PubMed] [Google Scholar]
- 2.Weiner RD. Indications for use of electroconvulsive therapy In: Weiner RD, ed. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging (A Task Force Report of the American Psychiatric Association). Washington, D.C.: American Psychiatric Publishing, Inc.; 2000:5–26. [Google Scholar]
- 3.Abrams R Electroconvulsive Therapy. 4th edition New York, NY: Oxford University Press; 2002. [Google Scholar]
- 4.Krystal AD, Weiner RD. EEG correlates of the response to ECT: a possible antidepressant role of brain-derived neurotrophic factor. J ECT 1999;15:27–38. [PubMed] [Google Scholar]
- 5.Mayur P. Ictal electroencephalographic characteristics during electroconvulsive therapy: a review of determination and clinical relevance. J ECT 2006;22:213–7. [DOI] [PubMed] [Google Scholar]
- 6.Azuma H, Fujita A, Sato K et al. Postictal suppression correlates with therapeutic efficacy for depression in bilateral sine and pulse wave electroconvulsive therapy. Psychiatry Clin Neurosci 2007;61:168–73. [DOI] [PubMed] [Google Scholar]
- 7.Fink M. Nonconvulsive status epilepticus and electroconvulsive therapy. J ECT 2004;20:131–32. [DOI] [PubMed] [Google Scholar]
- 8.Sackeim HA, Luber B, Katzman GP et al. The Effects of Electroconvulsive Therapy on Quantitative Electroencephalograms: Relationship to Clinical Outcome. Arch Gen Psychiatry 1996;53:814–24. [DOI] [PubMed] [Google Scholar]
- 9.Fink M, Kahn RL. Relation of electroencephalographic delta activity to behavioral response in electroshock; quantitative serial studies. AMA Arch Neurol Psychiatry 1957;78:516–25. [DOI] [PubMed] [Google Scholar]
- 10.Nobler MS, Sackeim HA, Solomou M, Luber B, Devanand DP, Prudic J. EEG manifestations during ECT: effects of electrode placement and stimulus intensity. Biol Psychiatry 1993;34:321–30. [DOI] [PubMed] [Google Scholar]
- 11.Dinwiddie SH, Glick DB, Goldman MB. The effect of propofol-remifentanil anesthesia on selected seizure quality indices in electroconvulsive therapy. Brain Stimul 2012;5:402–7. [DOI] [PubMed] [Google Scholar]
- 12.Krystal AD, Weiner RD, Coffey CE. The ictal EEG as a marker of adequate stimulus intensity with unilateral ECT. J Neuropsychiatry Clin Neurosci 1995;7:295–303. [DOI] [PubMed] [Google Scholar]
- 13.Weiner RD. Electroencephalographic correlates of ECT. Psychopharmacol Bull 1982;18:78–81. [PubMed] [Google Scholar]
- 14.Staton RD, Hass PJ, Brumback RA. EEG--monitored ECT. J Clin Psychiatry 1980;41:68–9. [PubMed] [Google Scholar]
- 15.Staton RD, Hass PJ, Brumback RA. Electroencephalographic recording during bitemporal and unilateral non-dominant hemisphere (Lancaster Position) electroconvulsive therapy. J Clin Psychiatry 1981;42:264–9. [PubMed] [Google Scholar]
- 16.Brumback RA, Staton RD. The electroencephalographic pattern during electroconvulsive therapy. Clin Electroencephalogr 1982;13:148–53. [DOI] [PubMed] [Google Scholar]
- 17.Nobler MS, Luber B, Moeller JR et al. Quantitative EEG during seizures induced by electroconvulsive therapy: relations to treatment modality and clinical features. I. Global analyses. J ECT 2000;16:211–28. [DOI] [PubMed] [Google Scholar]
- 18.Luber B, Nobler MS, Moeller JR et al. Quantitative EEG during seizures induced by electroconvulsive therapy: relations to treatment modality and clinical features. II. Topographic analyses. J ECT 2000;16:229–43. [DOI] [PubMed] [Google Scholar]
- 19.Kaiboriboon K, Luders HO, Hamaneh M, Turnbull J, Lhatoo SD. EEG source imaging in epilepsy--practicalities and pitfalls. Nat Rev Neurol 2012;8:498–507. [DOI] [PubMed] [Google Scholar]
- 20.Zoldi SM, Krystal A, Greenside HS. Stationarity and redundancy of multichannel EEG data recorded during generalized tonic-clonic seizures. Brain Topogr 2000;12:187–200. [DOI] [PubMed] [Google Scholar]
- 21.Sackeim HA, Prudic J, Nobler MS et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 2008;1:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011;2011:879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010;68:787–96. [DOI] [PubMed] [Google Scholar]
- 24.Tao JX, Yung I, Lee A, Rose S, Jacobsen J, Ebersole JS. Tonic phase of a generalized convulsive seizure is an independent predictor of postictal generalized EEG suppression. Epilepsia 2013;54:858–65. [DOI] [PubMed] [Google Scholar]
- 25.Zavorotnyy M, Kluge I, Ahrens K et al. S-ketamine compared to etomidate during electroconvulsive therapy in major depression. Eur Arch Psychiatry Clin Neurosci 2017;267:803–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.