Abstract
The development and regulatory approval of chimeric antigen receptor T cell (CAR-T) therapies targeting the B-lineage surface antigen CD19 represents a major milestone in cancer immunotherapy. This treatment also results in depletion of normal CD19+ B cells and is associated with hypogammaglobulinemia. These on-target, off-tumor toxicities may result in an increased risk for infection, particularly for encapsulated bacteria. Data regarding the efficacy and cost-effectiveness of prophylactic IgG replacement in CD19-targeted CAR-T cell therapy recipients is lacking, and current expert recommendations are extrapolated from the data for individuals with primary immune deficiencies. This article reviews CAR-T cell therapies targeting B-lineage lymphocytes, associated side effects, and considerations for the approach to management of hypogamaglobulinemia in this patient population. Studies are needed to establish evidence-based approaches to prophylactic immunoglobulin administration in this context, and strategies may differ by patient and CAR-T cell product characteristics.
Keywords: CAR, CD19, IVIG, IgG, immunoglobulin, prophylaxis, infection
1. INTRODUCTION
Harnessing the body’s own immune system to target and specifically kill malignant cells while ignoring normal cells has long been a dream for cancer therapy. With the recent approval by the United States Food and Drug Administration (FDA) of two chimeric antigen receptor (CAR-) T cell products targeting CD 19+ B-lineage neoplasms, we have come much closer to achieving this goal. Approvals have been granted for pediatric and young adult B cell acute lymphocytic leukemia (ALL; approval for tisagenlecleucel) and adult lymphomas (including diffuse large B cell lymphoma, high grade B cell lymphoma, primary mediastinal B cell lymphoma, and transformed follicular lymphoma; approval for tisagenlecleucel and axicabtagene ciloleucel). However, the current therapeutic agents still do not distinguish between malignant B-lineage cells and normal B cells. One result is the potential for long term dysfunction of the immune system, which may lead to hypogammaglobulinemia and a need to consider antibody replacement therapy. Understanding why normal B cells are affected by current CAR-T cell therapies, identifying which patients would benefit from antibody replacement therapy, and how to safely and effectively administer immunoglobulins are the focus of this review. This journal recently published a review of immunoglobulin use in patients with hematologic malignancies and hematopoietic cell transplants (HCTs),[1] but the rapidly increasing utilization of CAR-T cell therapies warrants this additional focused review and recommendations.
1.1. Background and Historic Perspective
A major step toward adapting T cells to specifically target tumor cells was reported in the sentinel 1989 paper by Gross, Waks, and Eshhar, who described creating a gene for a chimeric T cell receptor (TCR) and introducing it into cytotoxic T cells.[2] The chimeric TCR consisted of the variable regions (VH + VL) of an antibody recognizing the hapten, 2,4,6-trinitrophenyl (TNP), fused to the C-region segments of the alpha or beta TCR chains. The resulting T cell lines demonstrated TNP antigen-specific, non-MHC-restricted cytotoxicity and interleukin-2 production.[2] This observation served as the foundation for creating cells with the potential to be used as specific anti-cancer immune therapies. Over the next 25 years, other investigators built upon this approach, substituting chimeric receptors specific for cancer cell surface antigens for the hapten-specific receptor.[3] Subsequent versions of antigen-specific T cells incorporated engineered intracellular stimulatory domains to improve efficacy. These studies have culminated in the development of second and third-generation chimeric antigen receptors (CAR) in which a gene segment encoding the variable region of an antibody directed at a tumor cell surface antigen such as CD19 is used to provide the extracellular targeting domain. This is fused to the gene segment for the transmembrane domain of CD8 or another T cell surface molecule, then one or more gene segments encoding intracellular co-stimulatory domains are added to the intracellular tail of the CD3-ζ chain.[4] Consequently, when expressed in T cells, this CAR provides both “signal 1” and “signal 2” for robust stimulation of the T cells whenever CD19 bearing targets (such as B-lineage lymphomas or normal B cells) are encountered. Anti-CD19 CAR-T cell therapy is an extremely effective, albeit potentialy toxic therapy, which has been shown to eradicate disease in some patients with relapsed, treatment-resistant (R/R) B-lineage neoplasms. Ideally, the target antigen would be stably expressed at relatively high concentrations on the malignant cells but absent on normal cells. Unfortunately, for the approved agents targeting CD19, normal CD19+ B cells also become a target
1.2. Clinical Implementation
Development and clinical administration of CAR-T cells is complex and requires multiple steps, each of which is associated with some risk to the patient.[5,6] First, adequate numbers of patient (autologous) T cells must be collected via an apheresis procedure, and the cells must be transduced with a viral vector encoding the CAR molecule so it can be efficiently expressed.[5] Further, the genetically modified cells must be expanded many-fold in vitro for therapeutic use.[5] After IV administration, the cells must continue to express the chimeric receptor, proliferate in vivo, and persist long enough to reach and kill tumor targets, ultimately resulting in a clinically-significant and durable anti-tumor response.[5-7] Because the cells are highly activated and primed to aggressively attack their targets, administration of CAR-T cells is associated with the potential for unique toxicities including cytokine release syndrome (CRS) and encephalopathy.[6-8]
A recently published international, multi-center “real world” trial of the first anti-CD19 CAR-T cell product to be licensed, tisagenlecleucel,[9] illustrates many of the major risks associated with the use of B cell targeted CAR-T cell therapies.[10] First, the extent of pre-existing humoral immune deficits from prior therapy was evident with low peripheral blood CD19+ B cell counts in all but one patient before CAR-T cell infusion; and immunogloblin (Ig) G (IgG), IgM, and IgA levels that were below normal in 74%, 63%, and 49% of patients pre-CAR-T cell infusion. Second, while 165 patients were enrolled in the trial, only 67% (111) actually received their gene-modified cells. In 12 patients (7%), the CAR-T cells could not be manufactured. Another 30% of the patients discontinued participation in the study without receiving CAR-T cells, primarly due to disease progression and death, during the interval between collection of patient cells and administration of the CAR-T cells (median, 54 days). Third, of the 111 patients that received the CAR-T cells, 58% had some degree of cytokine release syndrome (CRS), with a median time to onset of 3-4 days after infusion. Grade 3 or 4 CRS occurred in 22% of subjects. Neurologic events occurred within the first 8 weeks after infusion in 21% of patients and were severe in 12%; most neurotoxicity occurred concurrently with CRS. Lastly, approximately one third of patients had infections early after treatment, with 20% reported as severe Grade 3 or 4 events. Prophylactic IVIG was administered to 30% of subjects at local investigator’s discretion prior to CAR-T infusion.[10] There was no analysis of an association between IVIG pre-CAR-T infusion and subsequent infections.
Despite the complexities and complications, there have been stunning successes reported with CD19 targeting CAR-T cell therapies. Initial studies were undertaken in children and young adults up to age 25 who were suffering from R/R CD19+ B cell precursor ALL. [11] The FDA granted regulatory approval to this product (tisagenlecleucel, Novartis) in this population, as well as in adults with R/R lymphomas, because response rates have been highly encouraging in this setting where outcomes are otherwise extremely poor.[9] However, severe adverse events due to CRS, neurotoxicity, prolonged and severe B cell aplasia, opportunistic infection, and other treatment-related toxicities have been considerable.[6-8, 10-12] Furthermore, this ‘personalized’ therapeutic modality has several logistical barriers including significant financial cost, extended time required to generate the gene-modified cells, and failure to generate a CAR-T cell product in some patients.[5,6,10,13] This has hindered its widespread use and will need to be overcome to further extend its practicability.[4-6,13] The required technical and clinical expertise, cost, and potential adverse effects of CAR-T cell therapy have thus far limited its use to highly-specialized and specifically accredited tertiary care centers treating patients in which other therapies, including hematopoietic cell transplant (HCT), have failed. Thus, many patients who receive CAR-T cell therapy have already experienced significant toxicity and morbidity from their underlying disease and prior treatments.[5-8, 10-12]
1.3. Extending Results Beyond ALL
The initial use of CD19 CAR-T cell therapy to treat ALL in children and young adults has been expanded to include adult lymphomas (including diffuse large B cell lymphoma, high grade B cell lymphoma, primary mediastinal B cell lymphoma, and transformed follicular lymphoma) with R/R disease after at least two types of conventional therapies. These trials have also been successful, and the FDA subsequently approved both tisagenlecleucel and axicabtagene ciloleucel (Kite Pharmaceuticals) for use in this group of diseases.[14,15] At present, many investigators are developing other CAR constructs which extend the target beyond CD19+ lymphoid malignancies to include other specific epitopes such as CD22 and CD30 for Hodgkin lymphoma[16,17] and B cell maturation antigen (BCMA) for R/R multiple myeloma.[18,19] BCMA is expressed on essentially all myeloma cells,[18] and the early reports of BCMA-targeted CAR-T cell therapy for refractory multiple myeloma have been promising. While CD19 is expressed on earlier lineage B cells and declines on fully differentiated plasma cells,[20] BCMA is selectively expressed by plasma cells. Thus, BCMA-targetd therapies may result in substantial deficits in pre-existing pathogen-specific immunity through the destruction of long-lived plasma cells.[20-25] Other groups have begun forays into treating advanced, acute myeloid leukemias by targeting CD33, CD123, CLL-1 and other antigens.[26,27] The future is encouraging given the numerous single- and multi-institutional trials designed to refine the ideal cell dose and product composition. This work includes targeting immune therapy to simultaneously recognize multiple epitope targets (dual specificity), methodologies dedicated to regulate in vivo CAR-T cell activity (i.e. “on” and “off” signals) to minimize off-target toxicities, and the design and execution of combination trials that incorporate checkpoint inhibitors and/or other novel therapies (e.g. ibrutinib) to minimize resistance to CAR-T cell mediated killing.[28,29] Finally, investigators are endeavoring to develop “off-the-shelf products that may significantly overcome many of the logistical drawbacks of present CAR-T cell therapies.[30,31] These efforts suggest that CAR-T cell therapy will continue to dramatically improve in efficacy and implementation, but it is likely that normal cells sharing expression of the targeted tumor antigen will continue to be victims of ‘collateral damage’ referred to as on-target, off-tumor toxicity.
This review focuses on CAR-T cells targeting B-lineage malignancies. Newer CAR-T cell constructs recognizing solid tumors and other types of malignant cells are unlikely to have the same toxicity to normal B cells and effects on antibody production as the currently licensed products.
2. CD19 CAR-T CELL AND CD19+ B CELL KINETICS, HYPOGAMMAGLOBULINEMIA, AND INFECTIONS FOLLOWING CAR-T CELL THERAPY
2.1. CD19 CAR-T Cell and CD19+ B Cell Kinetics after CD19 CAR-T Cell Therapy:
Current CD19 CAR-T cell therapies for B cell ALL and lymphomas also target normal CD19+ B lymphocytes through on-target, off-tumor effects. CAR-T cells are a ‘living’ drug, and as such, can survive in treated patients for years after infusion.[11,32-36] While this is likely beneficial for maintaining durable responses, it may result in persistent CD19+ B cell aplasia with possible risk for reduced humoral immunocompetence and infection. In the ALL cohort reported by Maude et al in 2014 including 25 children and 5 adults, all had prolonged CD19+ B cell aplasia after CD19-CAR-T cell therapy.[11] In a subsequent report from the University of Pennsylvania and Children’s Hospital of Philadelphia, Bhoj et al reported that 16 adults and children had CD19+ B cell aplasia persisting for a mean of 571 days following CAR-T cell therapy.[37] In the series reported by Hill et al, 116 of 118 evaluated adult patients with ALL, CLL, or lymphoma had endogenous B cell depletion (< 0.01% CD19+B cells in peripheral blood) within 28 days.[12] However, CD19+B cells can recover, and 17 (21%) of 82 evaluable patients in this study had CD19+B cell detection in peripheral blood by day 90.[12] In a series of adults with DLBCL treated with tisagenlecleucel, polyclonal B-cell recovery was sustained in 8 (50%) of 16 patients with complete responses, and the median time to onset of sustained B-cell recovery was 6.7 months (range, 0.3 to 12 months).[15] In a trial of adults treated with axicabtagene for large B cell lymphomas, 6 (17%) of the 35 assessable patients with ongoing responses had detectable B cells in their blood by 3 months after infusion, 20 (61%) of 33 assessable patients had detectable B cells at 9 months, and 24 (75%) of 32 assessable patients had detectable B cells at 24 months.[38] Similar findings have been reported in patients with even longer sustained complete responses of up to 4 years.[39] These data suggest that durable responses in adults with lymphoma do not require long-term persistence of functional CAR-T cells, which may allow for recovery of CD19+ B cells.
2.2. Hypogammaglobulinemia after CD19 CAR-T Cell Therapy:
Reported rates of hypogammaglobulinemia vary widely after CD19 CAR-T cell therapies in part due to variable definitions, replacement strategies, follow up duration, age, and underlying diseases. These data also do not account for the rate of hypogammablobulinemia that preceded CD19 CAR-T cell therapy. Children with fewer established plasma cell clones producing specific antibodies might be more susceptible to developing hypogammaglobulinemia and/or specific antibody deficiency following CAR-T cell therapy than adults (who may have been ‘boosted’ by re-infection or re-vaccination).[40,41] In the registration trial of tisagenlecleucel for children with ALL, 43% developed hypogammaglobulinemia. In contrast, the incidence of post-CAR-T cell hypogammaglobulinemia was 14% and 15% in studies of tisagenlecleucel and axicabtagene in adults with DLBCL, respectively. This discrepancy is consistent with the understanding of plasma cell development in young vs mature animals and on antibody titers in children vs adults with HIV infection. [41-43] In a study of adults with CLL treated with tisagenlecleucel, all 8 (57%) of 14 participants with a complete response developed hypogammaglobulinemia managed with supplemental IgG.[44] A summary of the proportion of patients receiving IgG replacement in pivotal CAR-T cell therapy trials for tisagenlecleucel and axicabtagene is presented in Table 1.
Table 1.
Summary of IgG replacement and infections in CAR-T cell therapy trials for tisagenlecleucel and axicabtagene.
| CAR-T cell therapy pivotal trials |
Product | IgG replacement recommendations |
Proportion of patients receiving any IgG replacement |
Infections, ≥ Grade 3 |
|---|---|---|---|---|
| Pediatric and young adult ALL | ||||
| •Maude et al, 2014 [11] Single center, N = 30a | Kymriah (CTL019/tisagenlecleucel) | To maintain IgG levels > 500 mg/dL | 100% | None reported with up to 12 months follow up |
| • Maude et al, 2018 [9] Multi-center, N = 75 | Kymriah (CTL019/ tisagenlecleucel) |
According to local guidelines. | ‘Most’ | Data not reported |
| Adult lymphomas | ||||
| • Schuster et al, 2017 [15] Single center, N = 28 | Kymriah (CTL019/tisagenlecleucel) | For clinically significant hypogammaglobulinemia, defined as systemic infection plus low IgG (level not stated) | 64% | 8 events by a median follow up of 28.6 months |
| • Neelapu et al, 2017 [52] Locke et all, 2019 [38] Multi-center, N = 108 | Yescarta (axicabtagene ciloleucel) | Maintain trough IgG level >400 mg/dL, especially in the setting of infection. | 31% overall; 8% between CAR-T cell infusion and first hospital discharge; 17 (44%) of 39 patients with ongoing response | 28% of patients by 2 years |
| • Shuster et al, 2019 [10] Multi-center, N = 111 |
Kymriah (CTL019/tisagenlecleucel) | According to local guidelines. | Not reported | 20% of patients by a median of 14 months (20% of patients within ≤8 weeks, 18% after >8 weeks) |
25 pediatric and young adult patients plus 5 adult patients.
It is important to note that although CD19 expression begins on early pre-B cells and persists throughout B cell selection and maturation, CD19 expression declines as B cells terminally differentiate into CD138+ CD38+ plasma cells.[45] These findings suggest that some memory antibody responses may persist even if all CD19+ cells are eradicated. It is likely that these cells reside in the spleen, lymph nodes and other tissues, and thus would not be evaluable in the peripheral circulation.[37,45,46] In patients treated with anti-CD20 (rituximab) therapy, pre-existing specific IgG titers to vaccine antigens may be maintained with little change after B cell depletion, although de novo responses to vaccinations are reduced.[47] It has been proposed that humoral immunity relies on heterogeneous populations of plasma cells with complementary functions and different dynamics-one set representing plasmablasts which have recently differentiated from memory B cells and which have transient survival, and another subset which are resistant to apoptosis and survive in the bone marrow, providing a long-term baseline level of specific antibody.[46,48,49] These CD19-negative plasma cells may provide a varying degree of long-term humoral immunity and are likely resistant to killing by anti-CD19 CAR-T cells.
As proof-of-concept, data from one study of pathogen-specific IgG levels after treatment with CD19 CAR-T cells demonstrate stable levels of pathogen-specific IgG in 2 adults despite development of prolonged B cell aplasia as determined by flow cytometry of peripheral blood and decreasing total IgG levels after CAR-T therapy.[37] Other studies have demonstrated that terminally differentiated B cells can produce substantial total IgG without generating antibodies to specific pathogens.[48,49] Interestingly, in 10 adults with durable responses who were not receiving supplemental IgG after treatement with tisagenlecleucel, increased levels of IgG, IgM, and IgA were demonstrated in 4, 6, and 3 patients, respectively, by ≥ 6 months after CAR-T cell infusion.[15] This occured despite molecular evidence of CAR-T cell persistence. Thus, the direct role of CD19-CAR-T cell therapy on the development of subsequent hypogammaglobulinemia remains incompletely understood, and measurement of peripheral blood B cell counts should not be used as the sole determinant of humoral immune competence or the need for immunoglobulin replacement therapy. It is also important to note that up to half of patients with hematologic malignancies who are treated with CD19 CAR-T cells have had prior HCT without completion of subsequent vaccinations, which are required to reestablish immunity to vaccine-preventable infections and generate a stable, diverse population of antigen-specific plasma cells.[50,51] As such, they may begin CAR-T cell therapy with a limited plasma cell and antibody repertoire.
2.3. Infections and IgG Supplementation Following CD19 CAR-T Cell Therapy
A summary of infectious adverse events in CAR-T cell therapy trials for for tisagenlecleucel and axicabtagene is presented in Table 1. In the first year of follow up post-CAR-T cell infusion in the pivotal trial using axicabtagene in adults with lymphoma, any infection occurred in 41 (38%) of 108 patients, and 23% of these were ≥ Grade 3.[52] Grade 3 or higher infections with an unspecified pathogen occurred in 16% of patients, bacterial infections in 9%, and viral infections in 4%. Febrile neutropenia was observed in 36% of patients but overlapped with the presentation of CRS. In the second year of followup, an additional three Grade 3 infections were reported.[38] In the pivotal trial for tisagenlecleucel for children or young adults with ALL, Grade 3 and 4 infections occurred in 21% and 3% of patients, respectively, within 8 weeks postinfusion.[9] Grade 3 and 4 febrile neutropenia occurred in 32% and 4% of patients, respectively, within 8 weeks postinfusion. Among all patients treated with tisagenlecleucel for ALL and lymphomas, infections occurred in 95 (55%) of 174 patients, and 58 (33%) patients experienced Grade ≥3 infections. Fatal infections occurred in 2 (3%) patients with ALL. The package inserts for both products comment on the risk for reactivation of hepaptitis B virus, which can be severe in patients treated with B cell targeted drugs,[53] although there are no reported cases to date after CD19 CAR-T cell therapy.
Three studies have performed more in-depth analyses of specific infection events and risk factors in patients receiving other CD19 CAR-T cell products. Hill et al reported data from 133 adults with a median age of 54 years who had R/R ALL, CLL or NHL.[12] Before initiating CD19 CAR-T cell therapy, patients had received a median of 4 treatment regimens and 38% had received an autologous or allogeneic HCT. Even prior to lymphodepletion preparative therapy, most of the patients already had significant immunosuppression: 26% had serum IgG < 400 mg/dL, 13% had absolute neutrophil count (ANC) < 500/μL, and 80% had < 200 lymphocytes/μL. In the first 28 days after CAR-T treatment, 22 patients (17%) developed 24 bacterial infections, including 12 bacteremias, of which 4 were resistant gram negatives. Thirteen viral infections (primarily respiratory viral infections) occurred in 11 patients and 6 invasive fungal infections occurred in 4 patients. Infections were more common in subjects with ALL. Between days 29 and 90, infections were less frequent overall, with 23 infections in 17 (14%) of 119 evaluable patients, including 13 viral infections and 8 bacterial infections, 4 with bacteremia. Overall, 41% of infections in the first 90 days after CD19 CAR-T therapy were considered severe, and 6% life-threatening.[12] In a survey of infectious complications in 53 adults with ALL who received CD19CAR-T cell therapy at Memorial Sloan Kettering Cancer Center, Park et al reported that 22 (42%) experienced 26 infections within 30 days of receiving the CAR-T cells. Seventeen of these were bacterial and 5 were viral, the remainder were fungal.[54] Three patients (5.7%) died of infection-related causes. In 10 (31%) of 32 patients in whom complete remission was achieved, 15 infections developed between days 31 and 180; the majority of these late infections were due to respiratory viruses. In both of these studies, higher severity CRS was associated with an increased risk of infection (hazard ratio [HR] of 2.67, P=0.05 in patients with CRS ≥ grade 3), and in particular, bacteremia (HR= 19.97, P<0.001).[12,54] In a study of 83 children and young adults treated with CD19 CAR-T cells for ALL, 33 patients (40%) had 37 infections within 28 days after CAR-T cell infusion.[55] Infections consisted of 20 bacterial infections, 16 viral infections (primarily due to respiratory viruses), and 1 fungal infection with invasive pulmonary Mucormycosis. Similar to other studies, infections were less frequent beyond the first 28 days after CAR-T cell infusion.
The cause of infections after CD19 CAR-T cell therapy is almost certainly multifactorial. In the above studies, infections were more frequent in the early period after CAR-T cell therapy, which likely reflects factors including antecedent immunodeficiencies due to the effects of the underlying malignancies and prior cytotoxic treatments. The lymphodepletion chemotherapy administered immediately before CAR-T cell infusion may also cause cytopenias and damage mucosal barriers. Furthermore, the specific lymphodepletion regimen (e.g. inclusion of fludarabine versus non-fludarabine based regimens) may affect the duration of cytopenias and the incidence of infections and hypogammaglobulinemia. The development of CRS and neurotoxicity often requires management in the intensive care unit, which is associated with its own risk of infection. Patients with higher grade CRS may require treatment with corticosteroids and/or tocilizumab, a humanized IL-6 receptor monoclonal antibody, both of which may increase infection risk.[38,56] A study by Hay and colleagues demonstrated that severe CRS is characterized by endothelial activation with increased angiopoietin-2 and von Willebrand factor in the blood.[57] Thus, it is plausible that CRS-induced endothelial damage might initiate or facilitate infectious processes.[58] Whether depletion of normal CD19+ B cells and hypogammaglobulinemia contribute to the early-onset infections is not clear, but invasive bacterial infections, bacteremia and an increased incidence of viral respiratory infections are hallmarks of primary antibody deficiencies.[59,60] Systematic studies of total and pathogen-specific IgG before and after CAR-T cell therapies, placebo-controlled trials of IgG replacement in this population, and vaccination strategies have not been performed to date. Antimicrobial prophylaxis regimens may also have a role in preventing infections after CD19 CAR-T cell therapy. American and European guidelines, adapted from the autologous HCT setting, were recently published.[6]
3. INDICATIONS FOR AND ADMINISTRATION OF IgG REPLACEMENT THERAPY
The indications for prophylactically replacing IgG in the context of CAR-T therapies are controversial given the absence of data from randomized clinical trials. Although the available data are limited and difficult to compare given differences in patient popultions and CAR-T cell products, there are no striking differences in the incidence and spectrum of infections despite a variety of institutional or clinical trial guidelines for IgG replacement therapy (Table 1). In light of this, there remains equipoise in IgG replacement strategies, ranging from replacement only in patients with severe or recurrent infections to replacement in any patient with IgG <400 mg/dL.[61,62] The recent ASBMT Choosing Wisely initiative recommends against routine IVIG after HCT based on the conclusions of systematic reviews and meta-analyses suggesting limited benefit and the possibility of an increased risk of sinusoidal obstructive syndrome.[62] Other informative resources published within the last 5 years include several expert consensus guidelines for the diagnosis and management of primary humoral immune deficiencies [59,60,63] and a review of the use of IgG in children with ALL in a large health system.[64] A recent article in Blood Reviews applies the principles of management of primary immune deficiencies to secondary immune deficiencies occurring in the context of hematologic malignancies and/or HCT.[1] We suggest that these can also be adapted for patients who are undergoing CAR-T cell therapy targeting B cell antigens. Since data are lacking and evidence-based guidelines are not yet available, the goal of this manuscript is to provide pragmatic recommendations given the current state of knowledge in the field.
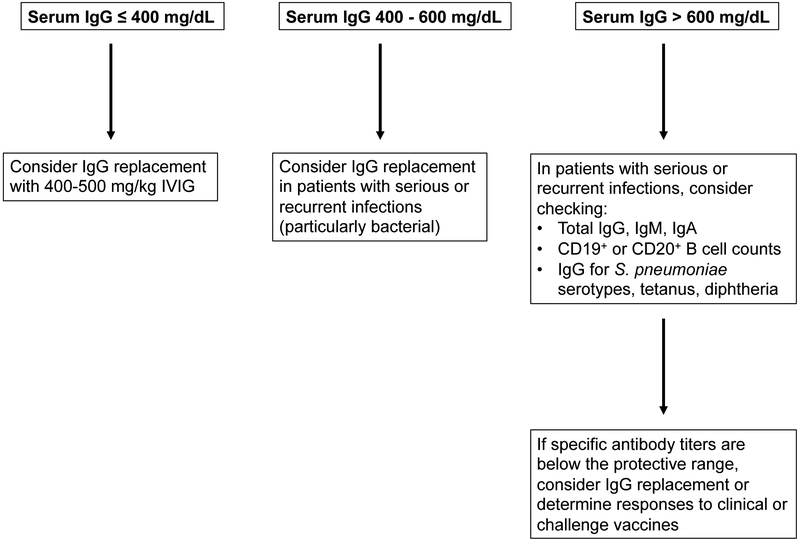
A practical algorithm based on readily available lab tests and the patient’s history is presented in Figure 1. At a minimum, we recommend screening for serum IgG prior to, and in the first 3 months post-CAR-T cell therapy for B cell malignancies. In the absence of data for the efficacy and cost-effectiveness of prophylactic IgG after CD19-targeted CAR-T cell therapies, we recommend consideration of prophylactic IgG in patients with IgG ≤400 mg/dl immediately prior to and during the first 3 months after CAR-T cell infusion. Beyond that time frame, we recommend consideration of IgG replacement in patients with continued severe hypogammaglobulinemia (IgG ≤400 mg/dL) AND serious, persistent, or recurrent bacterial infections, particularly of the sinopulmonary tract. These recommendations are based on data supporting the efficacy of IgG replacement in patients with severe hypogammaglobulinemia in other contexts (e.g. primary immunodeficiencies, after solid organ or hematopoietic cell transplant, and other B cell malignancies).[59-66]. In patients with moderate hypogammaglobulinemia, defined as serum IgG > 400 mg/dL but < 600 mg/dL (or the lower limit of normal for age), AND serious or recurrent bacterial or viral infections plus additional risk factors such as cytopenias, IgG replacement may be considered given that antibody deficiency is more readily correctable than other immune deficits. Specific antibody titers and vaccine responses may help risk-stratify patients and the need for prophylactic IgG.[1,47,59,60,63]
Figure 1. Algorithm for determining need to initiate IgG replacement therapy in the first 3 months after receiving anti-CD19 CAR-T cell therapy.
Beyond the first 3 months after CAR-T cell infusion, we recommend IgG supplementation in patients with IgG ≤400 mg/dL AND serious, persistent, or recurrent bacterial infections.
3.1. IgG Preparations
Although patients with B cell lymphopenia due to targeted depletion therapy and/or B cell aplasia due to CD19 CAR-T therapy may be deficient in any or all immunoglobulin classes, only IgG can be practically replaced. All IgG preparations available in the U.S. are made from 10,000 to 50,000 units of plasma obtained only from U.S. sources supervised by the FDA.[67] Donors are carefully screened and must have negative serologic and nucleic acid-based tests for potential blood-borne infectious agents including Hepatitis A, B and C, HIV and Parvovirus B19. Each unit of plasma is then held until the same individual gives a subsequent donation which also must test negatively before the previous unit can be used for manufacturing. Intermediate pools are also subjected to nucleic acid-based testing to verify the absence of potential viral pathogens. Although processes used by different manufacturers differ, all begin with an initial step of precipitation with cold ethanol, which itself can disrupt and/or partition many viruses.[67,68] This step is followed by additional maneuvers of precipitation with alcohols or fatty acids, or treatment with solvent-detergent mixtures, which disrupt the lipids of enveloped viruses. The coat proteins of non-enveloped viruses are disrupted by treatment at pH4 or pasteurization (prolonged heating to 60°C). At least two distinct viral inactivation and/or removal steps are used by all manufacturers. The efficacy of viral and prion removal/inactivation by these dedicated safety steps, as well as the purification procedures, are validated by studies with model animal viruses,[67-69] and all preparations are subjected to nanofiltration (pore size 15 nm). Viral safety steps in current usage have been shown to remove/inactivate recently emergent viruses such as Zika and West Nile,[70] and there are no known transmission of HIV or any other blood-borne infection by any currently marketed product.
Although all products currently marketed in the U.S. contained highly purified (≥ 95%) IgG, the content of IgA, other trace proteins, excipients and stabilizers varies in different products and may change over time. Some products contain particular sugars or specific amino acids as stabilizers, which must be avoided by certain individual patients. The prescribing information for the individual product to be used for any given patient should be carefully reviewed before it is actually administered. Readers are directed to frequently updated compendia for comparisons of the characteristics and contents of the various IgG products available.[71,72]
3.2. Adverse effects of IgG and precautions during infusion
Most adverse effects (AEs) of IgG infusions are transient, infusion-related symptoms which do not have long-term sequelae, but a few potentially serious AEs do stand out.[73-75] The only absolute contradictions to IgG therapy are a history of anaphylactic or severe systemic reaction to the administration of human immune globulin, and IgA-deficiency with known antibodies to IgA and a history of hypersensitivity reactions to IgA containing products. IgA deficiency by itself is not a contraindication to the use of intravenous (IV) or subcutaneous (SC) IgG, but caution should be used.[76] All IgG products in the US carry a “black box” warning about risks of thrombosis, renal dysfunction, and acute renal failure. For patients at risk of thrombosis, renal dysfunction, or renal failure, IVIG should be administered at the minimum dose and infusion rate that is feasible. Further, one must ensure adequate hydration in patients before administration, and the recipient should be monitored for signs and symptoms of thrombosis. Blood viscosity should be assessed in patients at risk for hyperviscosity, for example due to paraproteinemia. Renal dysfunction and acute renal failure have occurred more commonly with IVIG products containing sucrose; however, this sugar has been eliminated from most currently available products in the U.S.[77] Glycine or proline are currently the most commonly used stabilizers, but some preparations still contain glucose, maltose or sorbitol.[71,72] Some patients, particularly those receiving high dose IVIG for autoimmune disease, and with blood groups other than O, may develop transient, mild hemolytic anemia due to the actions of isoagglutinins present in the normal plasma from which the pooled IgG products are produced.[78,79] This is rarely clinically significant, but if necessary, can be avoided by the use of preparations from which the isoagglutinins have been reduced by immunoaffinity chromatography.[80]
In contrast to these rare, but potentially life-threatening AEs, IV infusion-related AEs are relatively common, accompanying up to 40% of infusions.[73-75] Headache is the most common but other symptoms may include musculoskeletal pain, chest tightness, nausea, flushing, tachycardia, dyspnea and a sense of anxiety. Mild pyrexia is also common. These symptoms are more common when a patient is first started on IVIG therapy and/or first receives a new brand of IVIG.[74,81] Although the combination of tachycardia, flushing, and dyspnea often suggest anaphylaxis, true IgE mediated hypersensitivity is extremely rare. Careful monitoring of the vital signs in patients experiencing these symptoms often reveals elevation of the blood pressure rather than hypotension which would accompany true anaphylaxis. These reactions are therefore termed anaphylactoid. They are frequently related to the rate of IgG infusion and often ameliorated by temporarily stopping or slowing the infusion rate. In most cases, reactions can be avoided by beginning the infusions slowly (0.01 mg/kg/min of 10% IVIG, 1 mg/kg/min) and increasing the rate stepwise at 15-30 minute intervals as tolerated, with frequent monitoring of the patient’s status and vital signs. Different products may have different maximum infusion rates recommended in their labeling, but in general 0.08 ml of 10% IgG solution /kg/min (8 mg/kg/min of IgG) should not be exceeded. Once this rate is achieved, the average infusion of 400 mg/kg can usually be completed within an hour. Infusion-related symptoms such as those described here can be prevented or treated with NSAIDs, anti-histamines and/or corticosteroids. Given the possibility that corticosteroids may affect the function of CAR-T cells, corticosteroids should be avoided when possible in this setting. An important distinction between these infusion rate-related symptoms and true anaphylaxis or other forms of immunologic hypersensitivity is that they frequently become less severe with repeated exposure to the same product.
3.3. Practical Considerations
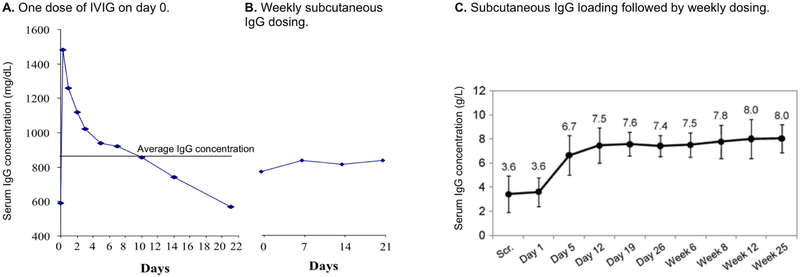
Practical considerations and recommendations for safe delivery of IgG therapy are discussed in expert consensus statements and other recent publications.[59,60,63,81] Usual starting doses for IgG replacement therapy are in the range of 400-800 mg/kg every 3-4 weeks intravenously or 100-200 mg/kg/week subcutaneously. IV dosing, which is usually given by trained medical professionals, is repeated every 3-4 weeks in most cases. Subcutaneous dosing is usually repeated weekly, although the total dose may be divided into smaller fractions which are given more frequently.[82,83] The IV route generally results in very high peak serum IgG levels, which drop rapidly as the IgG distributes into the total extracellular fluid space, then more slowly with a catabolic half-life of 22-28 days (Figure 2A).[83] Headache and other infusion-related adverse events described above may accompany the high post-infusion peak, and inadequate serum IgG levels may occur toward the end of long dosing intervals. In contrast, fractionating the monthly dose into biweekly, weekly or more frequent increments given subcutaneously results in more even, steady-state IgG levels (Figure 2B).[82,83] When IgG is infused into the subcutaneous tissue, it is slowly absorbed into the circulation over 2-3 days. This slow rate of systemic absorption is associated with decreased infusion-related systemic adverse events.[82-85] Swelling and redness at the infusion sites, sometimes with an itching or burning sensation, is common-especially when patients are begun on SCIG, but rarely are sufficiently bothersome to interfere with therapy. Patients may be given an initial dose of IVIG in the hospital, then switched to self-administered SC therapy at home, if desired. If initiating therapy by the SC route, a “loading regimen” may be considered in which the weekly dose is given five times on consecutive days. This strategy may be used to rapidly bring the patient’s IgG level to > 400 mg/dLd (Figure 2C).[86]
Figure 2. Serum IgG concentrations over time by intravenous versus subcutaneous routes of administration.
A) Serum IgG concentrations in a patient with X-linked agammaglobulinemia who received a single infusion of ~400 mg/kg of 5% IVIG. The horizontal line shows the average concentration. Adapted from Berger et al, Clin Immunol (2004) 112(1):1-7.
B) Serum IgG concentration of the same patient in panel A while receiving ~160 mg/kg of 16% IgG subcutaneously every 7 days. Adapted from Berger et al, Clin Immunol (2004) 112(1):1-7.
C) Mean serum IgG levels (+/− the standard deviation of median individual levels) in 18 previously untreated patients with primary immunodeficiencies given 100 mg/kg subcutaneous IgG for 5 days (days 1-5) followed by 100 mg/kg/week. 7 patients had IgG >500 mg/dL by day 7. Adapted from Borte et al, J Clin Immunol (2011) 31:952-961.
Pooled analyses of data on hundreds of patients from multiple primary immune deficiency licensing trials shows that higher IgG doses result in higher serum levels and increased freedom from infection.[87,88] However, studies of individual patients suggest that each may require individualized adjustment of level to remain free from infection.[89,90] The patient’s clinical condition, rather than an arbitrary “target” blood level, should be used to guide therapy and judge the adequacy of the dose/frequency of administration. Nevertheless, a target level of 400 mg/dL is widely considered a minimal “trough” level which should be maintained. Many patients receiving IVIG at 4 week intervals complain that they feel the effects of their IVIG “wearing off” before the next dose is due.[91] Additionally, studies have shown that patients’ overall feeling of wellness decreases and the probability of infection increases towards the end of their IVIG treatment cycles.[92] Patients who experience increased fatigue, flu-like symptoms and/or frank infections toward the end of their dosing interval may benefit from a higher total IgG dose, a shorter dosing interval, or a switch to subcutaneous administration.
Finally, cost and access are additional important consideration when administering IgG products. Monthly doses of IVIG may cost as much as $5,000-$10,000 per infusion in North America.[93,94] Insurance coverage is not always guaranteed, and clinicians in other countries may have difficulty accessing IgG products.
3.4. Monitoring and Duration of IgG Replacement Therapy
The primary indicator of adequate dosing of IgG replacement therapy is a reduction in the signs and symptoms of serious and/or recurrent infection. The patient’s exposure history, particularly to school-aged children and/or dormitory type settings, should be considered in distinguishing repeated simple infections (i.e. of the upper respiratory tract) from chronic, more deep-seated infections such as chronic sinusitis. Similarly, recurrent cough due to multiple minor respiratory infections should be distinguished from chronic bronchitis and/or bronchiectasis. Examination of the sputum and radiography, including CT scans of the sinuses and high resolution (thin slice) CT scans of the chest, may be helpful.[95]
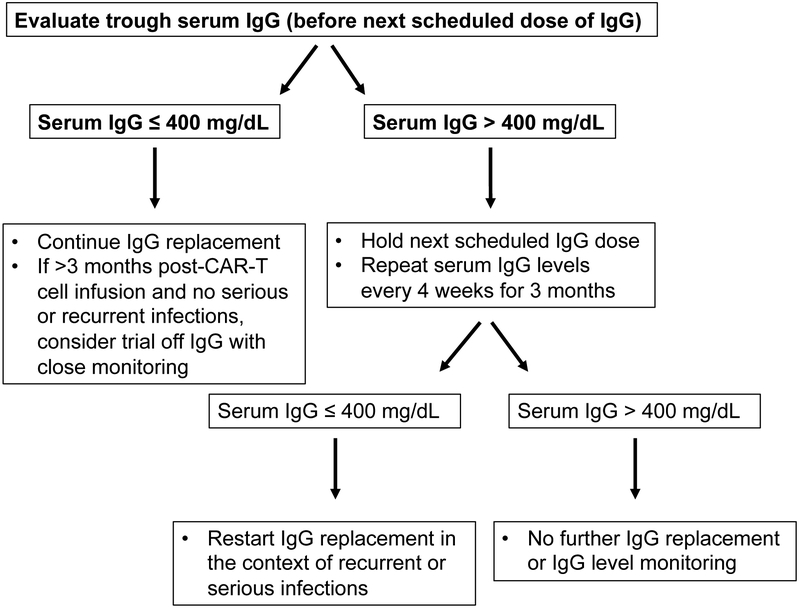
In patients who are free from signs and symptoms of persistent or recurrent infections, laboratory studies and vaccine responses may help determine if the patient has sufficient B cell function to warrant a trial off IgG replacement therapy, as illustrated in Figure 3. Recovery of peripheral blood CD19+ or CD20+ B cell counts, as assessed by flow cytometry, is likely an important metric. Normalization of serum IgG, IgA, and IgM will also help to determine if normal B cell function is present/returning. When considering a transition off supplemental IgG, the treatment intervals can be lengthened with continued monitoring of serum IgG to confirm maintenance above 400 mg/dL. If IgG levels remain > 400 mg/dL for more than 3 months, further testing could include measurement of specific antibody titers to previously administered vaccines or newly administered vaccines. In patients continuing IgG replacement, challenge with test antigens to which normal human IgG preparations lack antibodies, such as bacteriophage ΦX-174 or Salmonella typhimurium polysaccharide vaccine, may be used to assess humoral immune competence,[47,96,97] although these may not be broadly available to many providers.
Fig 3.
Algorithm for evaluating continuing need for IgG replacement.
4. SUMMARY AND FUTURE CONSIDERATIONS
CAR-T cell immunotherapy has proven remarkably effective in patients with R/R B cell malignancies. Despite the technical obstacles in producing and administering individualized cellular therapies, and the toxicities experienced by many patients, regulatory approval and commercialization are likely to lead to a rapid increase in the use of this treatment modality. Currently available CAR-T cell therapies have on-target off-tumor activity against normal B cells that results in CD19+ B cell depletion, and many of these patients have pre- and post-CAR-T cell therapy hypogammaglobulinemia. Because the CAR-T cells may proliferate and survive in vivo, this B cell aplasia and gaps in humoral immunity may persist as a long-term problem. The incidence of infection, especially in the first 28 days after CAR-T cell therapy, is relatively high. This is unquestionably a multifactorial problem, but antibody deficiency may be an important contributor. We recommend that IgG replacement be considered pre- and post-CAR-T cell therapy in patients with severe hypogammaglobulinemia, particularly in the context of serious or recurrent bacterial infections. However, there are no data supporting the benefit of IgG after CAR-T cell therapies, and IgG products are costly and have side effects that warrant consideration. As a conservative approach, we recommend consideration of prophylactic IgG prior to and for 3 months after CAR-T cell infusion in patients with severe hypogammaglobulinemia (IgG ≤ 400 mg/dL). In patients with moderate hypogammaglobulinemia (IgG > 400 mg/dL to 600 mg/dL or the lower-limit of normal for age), we recommend considering IgG replacement in the setting of severe or recurrent infections. In patients with prolonged B cell aplasia but normal IgG levels, IgG supplementation could be considered if there are repeated or severe infections and evidence of functional humoral immune dysfunction (i.e. poor responses to vaccines). In many cases, it may be possible to safely discontinue prophylactic IgG as pre-existing plasma cells may persist and some B cell recovery is likely. Monitoring for infections and laboratory correlates of humoral immune competence, including total IgG, IgM, and IgA; pathogen-specific antibody levels; B cell recovery; and vaccine responses will help identify patients who may benefit from IgG replacement. Randomized controlled studies of IgG replacement strategies, as well as studies of immune reconstitution, seroprotection to vaccine-preventable infections, and vaccine responsiveness after CAR-T cell immunotherapies will be critical to refine infection prevention strategies in this rapidly growing patient population.
5. PRACTICE POINTS.
Anti-CD19 CAR-T cell therapy is often associated with B cell aplasia and hypogammaglobulinemia, which may persist for years. However, polyclonal B cell recovery has been demonstrated in patients with durable complete remissions. [Data Based]
The serum total IgG level should be checked prior to and at monthly intervals after CAR-T cell therapy for at least 3 months. If the serum IgG is ≤ 400 mg/dL, IgG replacement should be considered, particularly in patients with severe or recurrent bacterial infections. Beyond the first 3 months after CAR-T cell infusion, we recommend IgG supplementation in patients with IgG ≤400 mg/dL AND serious, persistent, or recurrent bacterial infections. [Expert Opinion]
Severe cytokine release syndrome (≥ grade 3) is associated with increased risk for subsequent infections. [Data Based]
Peripheral blood B cell counts, quantitative immunoglobulins, specific antibody titers, history of infection, and vaccine responses may be helpful in determining how long IgG replacement therapy should be continued. [Expert Opinion]
6. RESEARCH AGENDA.
Measurement of serum IgG levels at the time infection is diagnosed in patients who are candidates for or recipients of CAR-T cells will help define the contribution of hypogammaglobulinemia to the risk of infection in these patients.
Randomized, placebo-controlled, blinded trials of IgG therapy will be important to determine whether prophylactic IgG is beneficial and cost-effective in CAR-T cell therapy recipients.
Determination of quantitative immunoglobulins, specific antibody titers, responses to vaccines and test antigens such as the polysaccharide S. typhimurium vaccine and capsid proteins of bacteriophage ΦX-174 in patients with prolonged peripheral blood B cell depletion may guide criteria for discontinuing IgG replacement therapy.
Footnotes
DISCLOSURES
JAH consults for Nohla Therapeutics and Amplyx, receives research support from Nohla Therapeutics, Shire, and Karius, and is a DSMB member for the HIV Vaccines Trials Network, all unrelated to the current work. TRT is a DSMB member for Shire and consultant for Shire, CSL Behring, Grifols, and UCB Pharma. HML is a consultant and DSMB member for Celgene, a promotional speaker for Novartis, and has consulted for CSL Behring.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ueda M, Berger M, Gale RP, Lazarus HM. Immunoglobulin therapy in hematologic neoplasms and after hematopoietic cell transplantation. Blood Rev 2018;32:106–15. doi: 10.1016/j.blre.2017.09.003. [DOI] [PubMed] [Google Scholar]
- [2].Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gill S, Maus MV, Porter DL Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev 2016;30:157–67. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- [4].Maus MV, Grupp SA, Porter DL, June CH. Anti body-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perica K, Curran KJ, Brentjens RJ, Giralt SA. Building a CAR Garage: Preparing for the Delivery of Commercial CAR T Cell Products at Memorial Sloan Kettering Cancer Center. Biol Blood Marrow Transplant 2018;24:1135–41. doi: 10.1016/j.bbmt.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical Utilization of Chimeric Antigen Receptor T Cells in B Cell Acute Lymphoblastic Leukemia: An Expert Opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2019;25:e76–85. doi: 10.1016/j.bbmt.2018.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2019;25:625–38. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- [8].Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- [11].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018;131:121–30. doi: 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chabannon C, Kuball J, Mcgrath E, Bader P, Dufour C, Lankester A, et al. CAR-T cells: the narrow path between hope and bankruptcy? Bone Marrow Transplant 2017;52:1588–9. doi: 10.1038/bmt.2017.241. [DOI] [PubMed] [Google Scholar]
- [14].Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med 2017;377:2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–8. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang C-M, Wu Z-Q, Wang Y, Guo Y-L, Dai H-R, Wang X-H, et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin Cancer Res 2017;23:1156–66. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- [18].Brudno JN, Marie I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 2018;36:2267–80. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ghosh A, Mailankody S, Giralt SA, Landgren CO, Smith EL, Brentjens RJ. CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leuk Lymphoma 2018;59:2056–67. doi: 10.1080/10428194.2017.1393668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-Lived Plasma Cells Are Contained within the CD19−CD38hiCD138+Subset in Human Bone Marrow. Immunity 2015;43:132–45. doi: 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997;388:133–4. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- [22].Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med 1998;188:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Robillard N, Wuillème S, Moreau P, Béné MC. Immunophenotype of normal and myelomatous plasmacell subsets. Front Immunol 2014;5:137. doi: 10.3389/fimmu.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perez-Andres M, Paiva B, Nieto WG, Caraux A, Schmitz A, Almeida J, et al. Human peripheral blood B-Cell compartments: A crossroad in B-cell traffic. Cytom Part B - Clin Cytom 2010;78:47–60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- [25].Pihlgren M, Schallert N, Tougne C, Bozzotti P, Kovarik J, Fulurija A, et al. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol 2001;31:939–46. doi: . [DOI] [PubMed] [Google Scholar]
- [26].Petrov JC, Wada M, Pinz KG, Yan LE, Chen KH, Shuai X, et al. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia 2018;32:1317–26. doi: 10.1038/S41375-018-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol 2018; 11:7. doi: 10.1186/s13045-017-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guedan S, Calderon H, Posey AD, Maus MV. Engineering and Design of Chimeric Antigen Receptors. Mol Ther - Methods Clin Dev 2019; 12:145–56. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016;127:1117–27. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Torikai H, Cooper LJ. Translational Implications for Off-the-shelf Immune Cells Expressing Chimeric Antigen Receptors. Mol Ther 2016;24:1178–86. doi: 10.1038/mt.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. SciTransI Med 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- [32].Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Turtle CJ, Hanafi L, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Marie I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016;128:360–70. doi: 10.1182/blood-2016-01-694356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA, et al. Long-Duration Complete Remissions of Diffuse Large B Cell Lymphoma after Anti-CD 19 Chimeric Antigen Receptor T Cell Therapy. Mol Ther 2017;25:2245–53. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pihlgren M, Schallert N, Tougne C, Bozzotti P, Kovarik J, Fulurija A, et al. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol 2001;31:939–46. doi:. [DOI] [PubMed] [Google Scholar]
- [42].Yap PL. Does intravenous immune globulin have a role in HIV-infected patients? Clin Exp Immunol 1994;97 Suppl 1:59–67. [PMC free article] [PubMed] [Google Scholar]
- [43].Mofenson L, Moye JJ. Intravenous immune globulin for the prevention of infections in children with symptomatic human immunodeficiency virus infection. Pediatr Res 1993;22:S80–87. [DOI] [PubMed] [Google Scholar]
- [44].Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-Lived Plasma Cells Are Contained within the CD19-CD38hiCD138+ Subset in Human Bone Marrow. Immunity 2015;43:132–45. doi: 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mei HE, Wirries I, Frölich D, Brisslert M, Giesecke C, Grün JR, et al. A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood 2015;125:1739–48. doi: 10.1182/blood-2014-02-555169. [DOI] [PubMed] [Google Scholar]
- [47].Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, et al. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol 2011; 128:1295–1302.e5. doi: 10.1016/j.jaci.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tiburzy B, Kulkarni U, Hauser AE, Abram M, Manz RA. Plasma cells in immunopathology: concepts and therapeutic strategies. Semin Immunopathol 2014;36:277–88. doi: 10.1007/s00281-014-0426-8. [DOI] [PubMed] [Google Scholar]
- [49].Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol 2005;23:367–86. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- [50].Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Krüger W, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol 2016;95:1435–55. doi: 10.1007/s00277-016-2711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chong PP, Avery RK. A Comprehensive Review of Immunization Practices in Solid Organ Transplant and Hematopoietic Stem Cell Transplant Recipients. Clin Ther 2017;39:1581–98. doi: 10.1016/j.clinthera.2017.07.005. [DOI] [PubMed] [Google Scholar]
- [52].Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531–44. doi: 10.1056/NEJMoal707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shih C-A, Chen W-C, Yu H-C, Cheng J-S, Lai K-H, Hsu J-T, et al. Risk of Severe Acute Exacerbation of Chronic HBV Infection Cancer Patients Who Underwent Chemotherapy and Did Not Receive Anti-Viral Prophylaxis. PLoS One 2015; 10:e0132426. doi: 10.1371/journal.pone.0132426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis 2018; 67:533–40. doi: 10.1093/cid/ciy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vora S, Waghmare A, Englund J, Hill J, Gardner R Infectious complications following CD19CAR T cell immunotherapy for children and young adults with refractory ALL. Biol Blood Marrow Transplant 2019;25:S355–S356. [Google Scholar]
- [56].Navarro G, Taroumian S, Barroso N, Duan L, Furst D. Tocilizumab in rheumatoid arthritis: A meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum 2014;43:458–69. doi: 10.1016/j.semarthrit.2013.08.001. [DOI] [PubMed] [Google Scholar]
- [57].Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017; 130:2295–306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Budde LE, Zaia JA. CD19CAR-T therapy and sepsis: dancing with the devil. Blood 2018;131:7–8. doi: 10.1182/blood-2017-11-812982. [DOI] [PubMed] [Google Scholar]
- [59].Buckley RH. Diagnostic & Clinical Care Guidelines for Primary Immunodeficiency Diseases, 3rd Edition. 2015; The Immune Deficiency Foundation. [Google Scholar]
- [60].Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 2015; 136:1186–1205.e78. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
- [61].Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 2019; 16:45–63. doi: 10.1038/s41571-018-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bhella S, Majhail NS, Betcher J, Costa LJ, Daly A, Dandoy CE, et al. Choosing Wisely BMT: American Society for Blood and Marrow Transplantation and Canadian Blood and Marrow Transplant Group’s List of 5 Tests and Treatments to Question in Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2018;24:909–13. doi: 10.1016/j.bbmt.2018.01.017. [DOI] [PubMed] [Google Scholar]
- [63].Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol 2017;139:S1–46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- [64].Van Winkle P, Burchette R, Kim R, Raghunathan R, Qureshi N. Prevalence and Safety of Intravenous Immunoglobulin Administration During Maintenance Chemotherapy in Children with Acute Lymphoblastic Leukemia in First Complete Remission: A Health Maintenance Organization Perspective. Perm J 2018;22:17–141. doi: 10.7812/TPP/17-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Norlin A-C, Sairafi D, Mattsson J, Ljungman P, Ringdén O, Remberger M. Allogeneic stem cell transplantation: low immunoglobulin levels associated with decreased survival. Bone Marrow Transplant 2008;41:267–73. doi: 10.1038/sj.bmt.1705892. [DOI] [PubMed] [Google Scholar]
- [66].Florescu DF, Kalil AC, Qiu F, Schmidt CM, Sandkovsky U. What is the impact of hypogammaglobulinemia on the rate of infections and survival in solid organ transplantation? A meta-analysis. Am J Transplant 2013;13:2601–10. doi: 10.1111/ajt.12401. [DOI] [PubMed] [Google Scholar]
- [67].Berger M Immunoglobulin products Ross. Princ. Transfus. Med, Chichester, WestSussex: John Wiley &Sons, Ltd.; 2016, p. 358–70. doi: 10.1002/9781119013020.ch31. [DOI] [Google Scholar]
- [68].FARSHID M, TAFFS R, SCOTT D, ASHER D, BRORSON K The clearance of viruses and transmissible spongiform encephalopathy agents from biologicals. CurrOpin Biotechnol 2005;16:561–7. doi: 10.1016/j.copbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [69].Cai K, Gierman TM, Hotta J, Stenland CJ, Lee DC, Pifat DY, et al. Ensuring the biologic safety of plasma-derived therapeutic proteins: detection, inactivation, and removal of pathogens. BioDrugs 2005; 19:79–96. doi: 10.2165/00063030-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Roth NJ, Schäfer W, Popp B, Stucki M, Fang R. Verification of effective Zika virus reduction by production steps used in the manufacture of plasma-derived medicinal products. Transfusion 2017;57:720–1. doi: 10.1111/trf.14038. [DOI] [PubMed] [Google Scholar]
- [71].Immune Deficiency Foundation. Characteristics of Ig Products Used to Treat PI in the US. 2018. https://primaryimmune.org/publication/characteristics-ig-products-used-treat-pi-licensed-use-us
- [72].Ballow M, Shehata N. Overview of intravenous immune globulin (IVIG) therapy - UpToDate 2019. https://www.uptodate.com/contents/overview-of-intravenous-immune-globulin-ivig-therapy [Google Scholar]
- [73].Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfus Med Rev 2003;17:241–51. [DOI] [PubMed] [Google Scholar]
- [74].Berger M Adverse Effects of IgG Therapy. J Allergy Clin Immunol Pract 2013; 1:558–66. doi: 10.1016/j.jaip.2013.09.012. [DOI] [PubMed] [Google Scholar]
- [75].Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev 2013;27:171–8. doi: 10.1016/j.tmrv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- [76].Rachid R, Bonilla FA. The role of anti-IgA antibodies in causing adverse reactions to gammaglobulin infusion in immunodeficient patients: a comprehensive review of the literature. J Allergy Clin Immunol 2012;129:628–34. doi: 10.1016/j.jaci.2011.06.047. [DOI] [PubMed] [Google Scholar]
- [77].Centers for Disease Control and Prevention (CDC). Renal insufficiency and failure associated with immune globulin intravenous therapy-United States, 1985-1998. MMWR Morb Mortal Wkly Rep 1999;48:518–21. [PubMed] [Google Scholar]
- [78].Daw Z, Padmore R, Neurath D, Cober N, Tokessy M, Desjardins D, et al. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion 2008;48:1598–601. doi: 10.1111/j.1537-2995.2008.01721.x. [DOI] [PubMed] [Google Scholar]
- [79].Scott DE, Epstein JS. Safeguarding immune globulin recipients against hemolysis: what do we know and where do we go? Transfusion 2015;55 Suppl 2:S122–6. doi: 10.1111/trf.13196. [DOI] [PubMed] [Google Scholar]
- [80].Hoefferer L, Glauser I, Gaida A, Willimann K, Marques Antunes A, Siani B, et al. Isoagglutinin reduction by a dedicated immunoaffinity chromatography step in the manufacturing process of human immunoglobulin products. Transfusion 2015;55 Suppl 2:S117–21. doi: 10.1111/trf.13088. [DOI] [PubMed] [Google Scholar]
- [81].AAAAI. Eight Guiding Principles for Effective Use of IVIG for Patients with Primary Immunodeficiency - American Academy of Allergy, Asthma & Immunology. https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20Resources/IVIG-guiding-principles.pdf [Google Scholar]
- [82].Berger M Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol 2004;112:1–7. doi: 10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- [83].Bonilla FA. Pharmacokinetics of Immunoglobulin Administered via Intravenous or Subcutaneous Routes. Immunol Allergy Clin North Am 2008;28:803–19. doi: 10.1016/j.iac.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [84].Windegger TM, Lambooy CA, Hollis L, Morwood K, Weston H, Fung YL. Subcutaneous Immunoglobulin Therapy for Hypogammaglobulinemia Secondary to Malignancy or Related Drug Therapy. Transfus Med Rev 2017;31:45–50. doi: 10.1016/j.tmrv.2016.06.006. [DOI] [PubMed] [Google Scholar]
- [85].Compagno N, Cinetto F, Semenzato G, Agostini C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: a single-center experience in 61 patients. Haematologica 2014;99:1101–6. doi: 10.3324/haematol.2013.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Borte M, Quinti I, Soresina A, Fernández-Cruz E, Ritchie B, Schmidt DS, et al. Efficacy and Safety of Subcutaneous Vivaglobin® Replacement Therapy in Previously Untreated Patients with Primary Immunodeficiency: A Prospective, Multicenter Study. J Clin Immunol 2011;31:952–61. doi: 10.1007/s10875-011-9588-5. [DOI] [PubMed] [Google Scholar]
- [87].Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- [88].Orange JS, Belohradsky BH, Berger M, Borte M, Hagan J, Jolles S, et al. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin Exp Immunol 2012; 169:172–81. doi: 10.1111/j.1365-2249.2012.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol 2008;122:210–2. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- [90].Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol 2010; 125:1354–1360.e4. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- [91].Immune Deficiency Foundation. Treatment Experiences and Preferences of Patients with Primary Immune Deficiency Diseases: First National Survey (2003). 2003. https://primaryimmune.org/publication/surveys/treatment-experiences-and-preferences-patients-primary-immune-deficiency
- [92].Rojavin MA, Hubsch A, Lawo J-P. Quantitative Evidence of Wear-Off Effect at the End of the Intravenous IgG (IVIG) Dosing Cycle in Primary Immunodeficiency. J Clin Immunol 2016;36:210–9. doi: 10.1007/s10875-016-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C Jr., Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barre syndrome. BMC Health Serv Res 2011;11:101. doi: 10.1186/1472-6963-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Blackhouse G, Gaebel K, Xie F, Campbell K, Assasi N, Tarride J-E, et al. Cost-utility of Intravenous Immunoglobulin (IVIG) compared with corticosteroids for the treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Canada. Cost Eff Resour Alloc 2010;8:14. doi: 10.1186/1478-7547-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schütz K, Alecsandru D, Grimbacher B, Haddock J, Bruining A, Driessen G, et al. Imaging of Bronchial Pathology in Antibody Deficiency: Data from the European Chest CT Group. J Clin Immunol 2019;39:45–54. doi: 10.1007/s10875-018-0577-9. [DOI] [PubMed] [Google Scholar]
- [96].Smith LL, Buckley R, Lugar P. Diagnostic Immunization with Bacteriophage ΦX 174 in Patients with Common Variable Immunodeficiency/Hypogammaglobulinemia. Front Immunol 2014;5:410. doi: 10.3389/fimmu.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bausch-Jurken MT, Verbsky JW, Gonzaga KA, Elms NP, Hintermeyer MK, Gauld SB, et al. The Use of Salmonella Typhim Vaccine to Diagnose Antibody Deficiency. J Clin Immunol 2017;37:427–33. doi: 10.1007/s10875-017-0406-6. [DOI] [PubMed] [Google Scholar]