Abstract
Insulin-like peptides (ILPs) and the insulin/insulin-like growth factor 1 signaling (IIS) cascade regulate numerous physiological functions, including lifespan, reproduction, immunity, and metabolism, in diverse eukaryotes. We previously demonstrated that in female Anopheles stephensi and Aedes aegypti mosquitoes, activation of the IIS cascade in the fat body led to a significant increase in lifespan. In this work, we elucidated two putative mechanisms in A. stephensi behind the observed lifespan extension and assessed whether this lifespan extension confers an overall fitness advantage to the mosquito. Specifically, we demonstrated that increased Akt signaling in the mosquito fat body following a blood meal significantly suppressed the expression of ILP2 in the head. Moreover, overexpression of active Akt in the fat body altered the expression of a putative insulin binding protein ortholog, Imaginal morphogenesis protein-Late 2 (Imp-L2), in response to transgene expression. Combined, these two factors may act to reduce overall levels of circulating ILP2 or other ILPs in the mosquito, in turn conferring increased survival. We also examined the impact increased fat body IIS had on lifetime fecundity and demonstrated that transgenic female mosquito populations had higher lifetime fecundity relative to non-transgenic sibling controls. These studies provide new insights into the complex hormonal and molecular mechanisms regulating the interplay between IIS, aging, and reproduction in this important vector of human malaria parasites.
Keywords: Insulin-like peptide, Imaginal morphogenesis protein-Late 2, Imp-L2, insulin binding protein, insulin signaling, mosquito
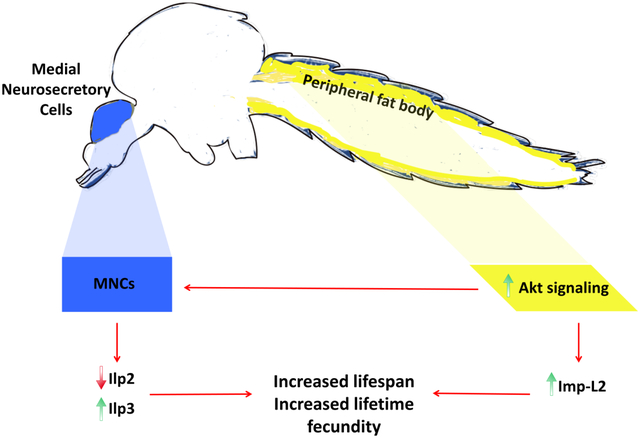
Graphical Abstract
1. Introduction
Malaria is a devastating disease afflicting over 200 million people annually and is a leading cause of death among children under the age of five in sub-Saharan Africa (World Health Organization 2018). Plasmodium parasites, the causative agent of malaria, are transmitted by Anopheles spp. mosquitoes including Anopheles stephensi, a key malaria vector in the Indian subcontinent that recently expanded its geographic range into East Africa (Faulde et al., 2014). In an effort to combat malaria, a combination of vector control and anti-malarial therapies resulted in a dramatic reduction in malaria cases and malaria-related mortality. Globally, from 2000 and 2015, there was a 37% decrease in malaria cases and a 60% decline in malaria-related deaths, with approximately 440,000 deaths in 2015 as compared to 839,000 in 2004 (Bhatt et al., 2015). Despite these successes, insecticide-based tools are threatened by the rapid spread of resistance to critical insecticides such as pyrethroids and Plasmodium parasites have developed resistance against the most effective anti-malarial drugs (Thomsen et al., 2014; Toe et al., 2014). These issues highlight the urgent need for novel malaria control strategies. One strategy is to genetically modify key Anopheles spp. vectors and render them unable to transmit human Plasmodium parasites (Marshall and Taylor 2009). While numerous anti-Plasmodium effector molecules have been identified, the majority of these incur fitness costs when engineered into the mosquito vector. Genetic drive strategies such as maternal effect dominant embryonic arrest (MEDEA) and clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) may be able to overcome some of these fitness costs, but other approaches to minimize fitness load should be explored. An alternative approach is to manipulate key signaling cascades, such as the insulin/insulin growth factor 1 signaling (IIS) cascade, to optimize critical life history traits that impact both fitness and pathogen resistance.
The IIS cascade is an evolutionarily conserved pathway that regulates diverse physiologies in a broad range of eukaryotes including insects, nematodes, and mammals (Antonova-Koch et al., 2013; Okamoto and Yamanaka 2015). IIS functions in a tissue-specific manner, with important signaling centers located in the midgut, brain and fat body affecting both local and systemic signaling networks (Antonova-Koch et al., 2013). Fat body IIS in insects, including mosquitoes, has been implicated in the regulation of lifespan, reproduction, and innate immunity (Antonova-Koch et al., 2013). In this work, we explored how fat body IIS influences the balance between lifespan and fecundity, two key life history traits critical to mosquito transmission of pathogens.
Lifespan is one of the most complex physiologies regulated by the IIS cascade (Antonova-Koch et al., 2013; Okamoto and Yamanaka 2015). Numerous studies in a variety of organisms implicate both insulin-like peptides (ILPs) and IIS as important regulators of senescence (Okamoto and Yamanaka 2015). However, the molecular mechanisms of lifespan control are not yet fully elucidated. In D. melanogaster, suppression of the IIS cascade, either through mutation of the insulin receptor (InR), knockdown of the insulin receptor substrate ortholog chico or ectopic expression of phosphatase and tensin homolog (PTEN) resulted in increased lifespan (Tatar et al., 2003; Slack et al., 2011; Tu et al., 2002; Clancy et al., 2001; Hwangbo et al., 2004). In C. elegans, genetic changes in daf-2 (InR), age-1 (phosphatidylinositol 3-kinase) and daf-16 (forkhead box protein O) all led to dramatic changes in nematode lifespan. In D. melanogaster, ablation of the medial neurosecretory cells (MNCs) reduced levels of ILPs, the activating ligands for the IIS cascade, and increased fly survival (Wessels and Perez-Pomares 2004; Broughton et al., 2005). Identifying the impact of individual ILPs on lifespan has been challenging due to that fact that many invertebrates have multiple ILPs that have both discreet and overlapping roles and can exhibit compensatory expression (Broughton et al., 2008b; Min et al., 2008). Success in knocking out each of the seven individual dilp genes in D. melanogaster, however, using homologous recombination revealed that the absence of dilp2 was sufficient to significantly increase survival (Grönke et al., 2010). A subsequent study demonstrated that overexpression of dilp6 in the adult fat body suppressed dilp2 and dilp5 mRNA in the brain, which in turn, also extended lifespan (Bai et al., 2012).
The IIS cascade also plays a critical role in the hormonal regulation of reproduction during both steroidogenesis in the ovary and vitellogenesis in the fat body. In mosquitoes, ILPs synthesized by the brain activate IIS in the follicle cells surrounding the developing oocyte to stimulate the synthesis of 20-hydroxyecdysone (Brown et al., 2008). In the fat body of Ae. aegypti, the IIS cascade, along with amino acids and ecdysteroids, is essential for the complete activation of vitellogenin synthesis (Hansen et al., 2014). Notably, systemic activation of Ae. aegypti IIS through knockdown of the IIS inhibitor PTEN via RNAi significantly increased egg production (Arik et al., 2009). Further, both AaegILP3 and AaegILP4 activate ovarian steroidogenesis and yolk uptake by the developing oocytes (Brown et al., 2008; Wen et al., 2010) and disruption of AaegILP7 and 8 by CRISPR-Cas9 resulted in defects in the development of primary oocytes (Ling et al., 2017). In D. melanogaster, reduced IIS activity can decrease or even prevent female reproduction. Specifically, ablation of insulin-producing cells (IPCs) in the female brain (Broughton et al., 2005) and knockout of dilp2, dilp3, and dilp6 led to significant reductions in fecundity (Grönke et al., 2010). Inhibition of IIS through overexpression of forkhead transcription factor (dFOXO) in the fruit fly peripheral fat body resulted in reduced fecundity, while dFOXO overexpression in the pericerebral fat body showed normal fecundity (Hwangbo et al., 2004), suggesting tissue-specific regulation of the balance between lifespan and reproduction. Finally, loss-of-function mutations in InR and the insulin receptor substrate chico resulted in increased female sterility in D. melanogaster, primarily due to the failure of vitellogenesis (Tatar et al., 2001; Drummond-Barbosa and Spradling 2001).
In vertebrates, the activities of circulating insulin and insulin growth factor 1 (IGF-1) are titrated through binding to IGF-1 binding proteins (IGFBPs) that prevent receptor activation by IGFBP-bound hormones. An insulin binding protein was recently characterized in the fruit fly and is known as Imaginal morphogenesis protein-Late 2 (Imp-L2) (Flatt et al., 2008; Honegger et al., 2008). Imp-L2 is a putative ortholog of human IGFBP7, which unlike most IGFBPs, binds insulin with high affinity and IGF-1 with low affinity (Yamanaka et al., 1997). In D. melanogaster, Imp-L2 acts as a negative regulator of IIS during development, controlling cell growth and antagonizing the activity of dilp2 (Honegger et al., 2008). Notably, removal of the germline late in fruit fly development resulted in extended lifespan and increased Imp-L2 transcript levels (Flatt et al., 2008), suggesting that Imp-L2 reduces IIS activity by binding to circulating dilps. In this context, D. melanogaster Imp-L2 has been shown to bind dilp2 and dilp5 in the adult fly and enhanced expression of Imp-L2 resulted in increased dilp2, dilp3, and dilp5 transcript levels. More importantly, induced expression of Imp-L2 in the midgut/fat body increased dilp2 mRNA levels, suggesting that a feedback loop occurs between the fly’s head and fat body. Finally, overexpression of Imp-L2 in both dilp-producing cells and in the fat body resulted in extended lifespan (Alic et al., 2011). Collectively, these data suggest that Imp-L2 negatively regulates IIS in insects by binding and inactivating circulating ILPs, which extends lifespan.
Previously we demonstrated that overexpression of Akt in the fat body of A. stephensi resulted in a 20% increase in the survival of female transgenic (TG) mosquitoes as compared to non-transgenic (NTG) sibling controls reared under identical conditions (Arik et al., 2014). We also observed a significant increase in the expression of vitellogenin proteins in the fat bodies of TG females relative to controls, although we did not observe changes in overall egg production or viability during the first or second reproductive cycles (Arik et al., 2014). The extension of lifespan through increased fat body IIS was surprising and the mechanism that contributed to the extended lifespan was unclear. Thus, in this study, we have attempted to elucidate possible mechanisms for the extended lifespan phenotype that we observed in TG A. stephensi. In addition, we examined the impact of increased fat body IIS on vitellogenesis and lifetime fecundity to define the balance or tradeoffs between lifespan and fecundity in this important malaria vector species.
2. Materials and Methods
2.1. Mosquito rearing
A. stephensi (Indian) mosquitoes were reared at 27°C and 75% relative humidity in a 16/8 h light/dark photoperiod as previously described (Arik et al., 2014). During each generation, the Vg-myrAsteAkt-HA TG line was outcrossed with wild type (WT) colony mosquitoes to maximize genetic diversity and minimize fitness effects. For experiments, TG mosquitoes were crossed with WT mosquitoes resulting in a 50:50 mix of TG and NTG sibling mosquitoes reared in the same pan (identical crowding, food availability, etc). TG and NTG mosquitoes were separated based on eye fluorescence at the pupal stage using an Olympus SZX10 fluorescent stereomicroscope with EGFP filters and allowed to emerge as adults into two separate 3.8 L cartons. For line maintenance and experiments, female mosquitoes were provided human blood (American Red Cross; IBC protocol #2010–014) via artificial membrane feeders.
2.2. RNA extraction and cDNA synthesis
A total of five A. stephensi females were dissected per biological replicate to obtain various tissues, including the head, midgut, and abdominal wall for total RNA isolation. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD) per manufacturer’s instructions and the concentration of total RNA in each sample determined using a NanoDrop 2000 Spectrophotometer (Thermo Scientific, West Palm Beach, FL). Total RNA was DNase-treated (Fermentas, Thermo Scientific, West Palm Beach, FL) to eliminate genomic DNA contamination and cDNA was prepared from the DNase-treated RNA using the High-Capacity cDNA Reverse Transcription Kit per manufacturer’s instructions (Applied Biosystems, Wobum, MA).
2.3. Identification of A. stephensi Imp-L2
To identify the A. stephensi Imp-L2 ortholog, sequences of conserved regions of the immunoglobulin (Ig) superfamily containing two Ig C2-like domains of Imp-L2 from Anopheles gambiae (CP2953) and D. melanogaster (CG15009-RB) were used to search all six reading frames of the A. stephensi genome and predicted transcript database (http://vectorbase.org) using tblastn. The upstream and downstream sequences of significant matches were translated and examined for additional encoded conserved regions of Imp-L2 to identify ASTE008413-RA as the A. stephensi Imp-L2 ortholog.
2.4. A. stephensi ILPs, vitellogenin and Imp-L2 transcript analysis
A total of 100 female TG and NTG A. stephensi (3–5 days post-emergence) from a single generational cohort were provided with a single blood meal and 10% sucrose ad libitum through soaked cotton pads that were replaced every other day. Mosquito heads, midguts and abdominal body walls (after removal of other tissues) were dissected from five females at various time points prior to and following a blood meal (0 hour (h) or non-bloodfed (NBF), 6h, 24h, 36h, and 48h) for total RNA extraction and cDNA synthesis. Optimal annealing temperatures for each primer set were determined using gradient PCR with an annealing range of 55°C to 65°C. Transcript expression patterns for the five A. stephensi ILPs, vitellogenin (this primer set amplifies both AsteVg1 and AsteVg2, but the high sequence identity makes it impossible to differentiate between the two) and Imp-L2 were determined by qPCR using a Bio-Rad CFX Real-Time System Thermocycler (Bio-Rad, Hercules, CA). Each sample was assayed in triplicate using Maxima SYBR Green/ROX qPCR Master Mix (2X; Thermo Scientific, Waltham, MA). Expression levels of these transcripts were compared between TG and NTG mosquitoes to determine the effect of increased fat body IIS on expression. Transcript levels were normalized to ribosomal protein S7 control, with fold changes in mRNA expression between TGs and NTGs calculated using the 2^(−ΔΔCt) method described by Livak et al 2001. The qPCR assays were performed using a minimum of three separate cohorts (biological replicates) of A. stephensi at each time point.
2.5. Lifetime fecundity assay
Approximately 120 to 130 TG and NTG adult female A. stephensi mosquitoes from a heterozygous TG and WT cross were separated into two cages. Mosquitoes were provided with human blood daily via membrane feeders and allowed to feed on 10% dextrose ad libitum. Oviposition substrates were placed into each cage 48 h after the initial blood meal and changed daily until the final female mosquito perished. Eggs were counted using Image-J 1.42 software (NIH) following the protocol described in (Mains et al., 2008). In addition, dead females were counted and removed daily to establish survivorship curves as previously described(Arik et al., 2014)These experiments were replicated a minimum of three times using unique biological cohorts.
2.6. Statistical analysis
For lifespan studies, the survival curves were analyzed in JMP 13 software using Log-rank (Mantel-Cox) analysis. To assess differences between survivorship and egg clutch size we performed linear regression. For all other data sets, significance was determined by Student’s t-test (p<0.05) using Graphpad Prism 7.
3. Results
3.1. Overexpression of myr-AsteAkt-HA in the mosquito fat body suppressed ILP2 and induced ILP3 transcript expression in the head
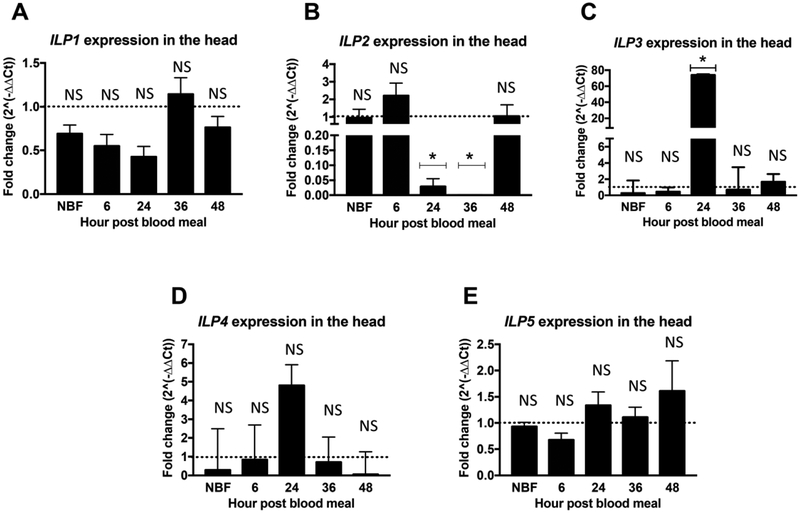
Transcript expression of all five ILPs prior to and throughout the reproductive cycle (NBF, 6h, 24h, 36h, 48h) was measured to establish relative expression between TG mosquitoes and NTG sibling controls (Figures 1, 2 and S1). While expression levels of ILP1 in the head were unchanged (Figure 1A), expression of myr-AsteAkt-HA in the fat body significantly reduced ILP2 transcript expression in the head of female TG A. stephensi relative to NTG siblings. Specifically, suppression of ILP2 expression in the head was observed at 24h and 36h post blood meals (Figure1B; p=0.014 and p=0.004 respectively). These timepoints are consistent with myr-AsteAkt-HA expression that begins at 12–24h post feeding and rises to maximal levels in the fat body at 36–48h after the blood meal (Arik et al., 2014). In contrast to ILP2, expression of ILP3 in the head was significantly increased at 24h post blood meal (Figure 1C; p=0.002) relative to NTG controls and returned to comparable NTG control levels by 36h post blood meal. We did not observe significant differences in the expression of ILP4 in the head between TG and NTG siblings, although a modest increase was observed at 24h (Figure 1D). Moreover, as with ILP1 (Figure 1A), we detected only minimal expression of ILP5 in the heads of female TG and NTG A. stephensi mosquitoes (Figures 1E and S4).
Figure 1: Overexpression of myr-AsteAkt-HA in the abdominal fat body suppressed ILP2 but increased ILP3 transcript expression in the head.
Heads from 3 to 5 day old TG and NTG A. stephensi females were dissected to assess mRNA expression of (A) ILP1, (B) ILP2, (C) ILP3, (D) ILP4, and (E) ILP5, at 0h (non-blood fed, NBF), 6h, 24h, 36h, and 48h post blood meal. ILP expression was quantified by qPCR. NTG samples were used as a control 2for each time points for the relative transcript expression determination. The dotted line on the graphs represents normalized transcript levels in the NTG samples (control). The Y-axis represents the log2 of fold changes which were calculated by the 2^−ΔΔCt method in which the Ct values of TG gene were normalized to the level of NTG control within each time point. Each value is the mean ± SEM of three independent experiments from five pooled mosquito heads. Results were analyzed using Student’s paired t-test (* = p<0.05; Graphpad Prism 7) to determine differences between TG mosquitoes and NTG sibling controls. All mRNA expression studies were replicated a minimum of three times.
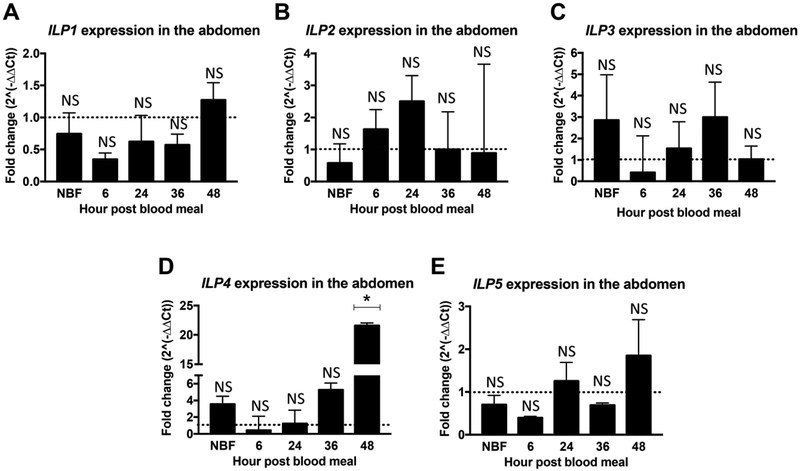
Figure 2: Overexpression of myr-AsteAkt-HA in the abdominal fat body induced ILP4 transcript expression in the abdominal body wall.
Abdominal body walls from 3 to 5 day old TG and NTG A. stephensi females were dissected to assess the transcript expression of (A) ILP1, (B) ILP2, (C) ILP3, (D) ILP4, and (E) ILP5, at 0h (non-blood fed, NBF), 6h, 24h, 36h, and 48h post blood meal. ILP expression was quantified by qPCR. NTG samples were used as a control for each time points for the relative transcript expression determination. The dotted line on the graphs represents transcript levels in the NTG control samples normalized to one. The Y-axis represents the log2 of fold changes which were calculated by the 2^−ΔΔCt method in which the Ct values of TG gene were normalized to the level of NTG control within each time point. Each value is the mean ± SEM of three independent experiments from five pooled mosquito abdominal body walls. Results were analyzed using Student’s paired t-test (* = p<0.05) to determine differences between TG mosquitoes and NTG sibling controls. All mRNA expression studies were replicated a minimum of three times.
In contrast to the head samples, we observed that overexpression of myr-AsteAkt-HA in the fat body had no impact on abdominal ILP1, ILP2, ILP3 and ILP5 transcript levels at the five time points examined (Figures 2A, B, C and E). We did observe, however, significantly increased levels of ILP4 transcript at 48h post blood meal (Figure1D; p=0.011), but not during earlier time points. Similar to the head, we detected only minimal expression of ILP1 and ILP5 in the abdominal body wall at the five different time points. Only minimal expression levels of ILPs were observed in midgut samples from both TG and their NTG siblings (Supplemental Figures S1A–E).
3.2. Activation of Akt signaling in the fat body induced Imp-L2 transcript expression
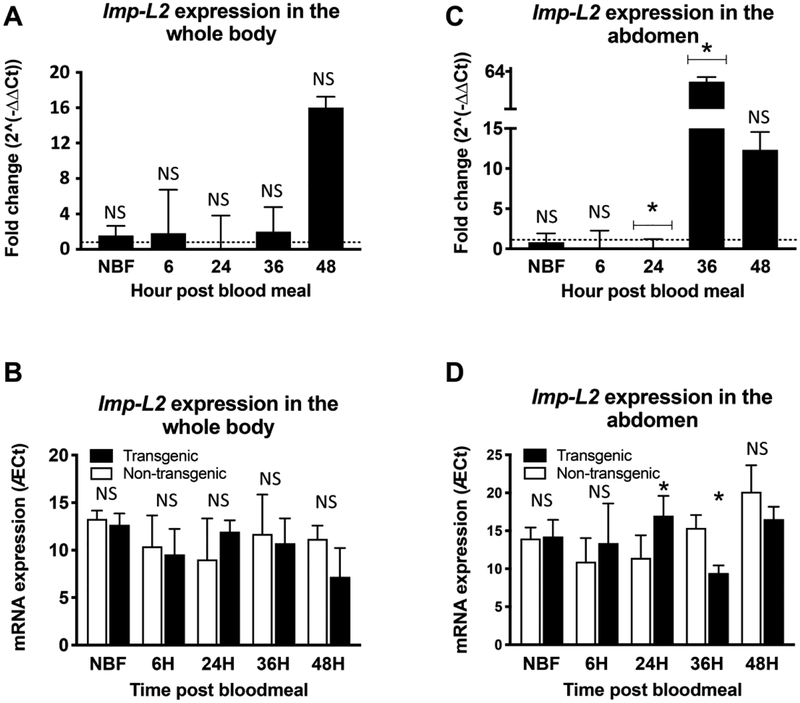
Prior to myr-AsteAkt-HA transgene expression (NBF and 6h) we observed no significant differences between expression of Imp-L2 in the whole body or abdominal body wall of TG and NTG mosquitoes (Figures 3A–B and C–D). However, following expression of myr-AsteAkt-HA and activation of the Akt signaling pathway (24h and 36h) we observed a significant decrease in Imp-L2 expression (~5 fold, p=0.03) in TG relative to NTG A. stephensi mosquitoes at 24h, buta large significant increase (~60 fold, p<0.001) at 36h. We observed a similar, but non-significant, trend of Imp-L2 mRNA expression in the whole body between TG and NTG mosquitoes (Figure 3A–B). Combined, these data suggest that Imp-L2 could moderate systemic IIS through the binding and inactivation of circulating ILPs, resulting in compensatory transcription, in a manner similar to that observed in D. melanogaster and C. elegans (Alic et al., 2011).
Figure 3: Overexpression of myr-AsteAkt-HA in the abdominal fat body impacts Imp-L2 transcript expression.
Abdominal body walls from 3 to 5 days old TG and NTG A. stephensi females were dissected to assess the mRNA expression of Imp-L2 at 0h (non-blood fed, NBF), 6h, 24h, 36h, and 48h after a blood meal. Imp-L2 mRNA expression was quantified by qPCR. NTG samples were used as a control for each time points for the relative transcript expression determination. The dotted line on the upper graphs (A and C) represents transcript levels in the NTG control samples normalized to one. The Y-axis represents the log2 of fold changes which were calculated by the 2^−ΔΔCt method in which the Ct values of TG gene were normalized to the level of NTG control within each time point. Each value is the mean ± SEM of three independent experiments from five pooled mosquito (A and B = whole mosquito; C and D = abdominal body wall). The lower graphs (B and D) represent the ΔCt of Imp-L2 relative to the RP17 loading control for both TG and NTG mosquitoes. Results were analyzed using Student’s paired t-test (* = p<0.05) to determine differences between TG mosquitoes and NTG sibling controls. (Note: while Imp-L2 TG expression showed a large fold increase at 48 h, this change was marginally insignificant (p=0.07 (A) and 0.127 (C)) due to variation in Ct values (see B and D) between replicates. It should also be noted that ΔCT values in Fig. 3B and D are the inverse of the 2^−ΔΔCt values presented in A and C.) All expression studies were replicated a minimum of three times with distinct cohorts of mosquitoes.
3.3. Effect of myr-AsteAkt-HA expression on vitellogenin transcript expression
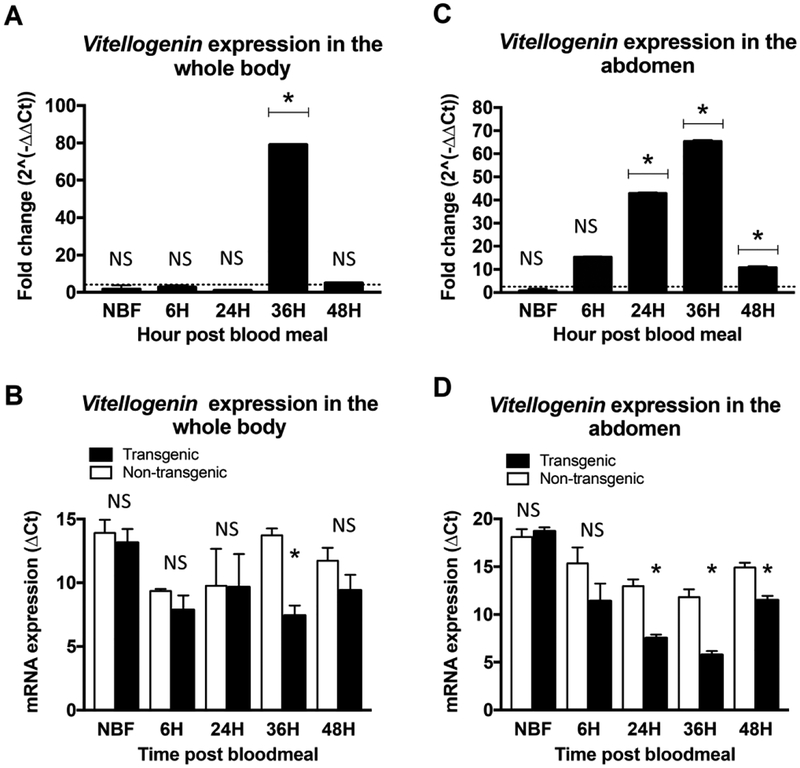
IIS in the fat body plays a critical role in vitellogenin synthesis and ultimately egg production. We previously demonstrated that increased fat body IIS led to enhanced vitellogenin protein levels in both A. stephensi and Ae. aegypti (Arik et al., 2014). To confirm whether overexpression of Akt signaling in the fat body also impacts Vg1/2 mRNA expression in A. stephensi, we examined fat body Vg1/2 transcript expression in both TG and NTG A. stephensi at various time points (NBF, 6h, 24h, 36h and 48h) after the blood meal. In TG A. stephensi we observed a significant increase in Vg1/2 expression in the abdominal wall body at 24, 36, and 48h (10 to 65 fold; p<0.05) after the blood meal relative to NTG mosquitoes (Figure 4C and D), consistent with myr-AsteAkt-HA transgene expression and our previous vitellogenin protein expression results (Arik et al., 2014). In assays of whole body samples, we observed a significant increase in Vg1/2 expression in TG mosquitoes at 36h post-blood meal (~70 fold; p<0.05), but and no significant change in the absence of a blood meal (NBF) or at 6h, 24h, or 48h after a blood meal (Figure 4A and B).
Figure 4: Overexpression of myr-AsteAkt-HA expression in the abdominal fat body induced vitellogenin 1/2 (Vg1/2) transcript expression.
Whole bodies (A and B) and Abdominal body walls (C and D) from 3 to 5 days old TG and NTG A. stephensi females were used to assess transcript expression of Vg1/2 at 0h (non-blood fed, NBF), 6h, 24h, 36h and 48h after a blood meal. Vg1/2 expression was quantified by qPCR. NTG samples were used as a control for each time points for the relative transcript expression determination. The dotted line on the graphs represents transcript levels in the NTG control samples normalized to one. The Y-axis represents the log2 of fold changes, which were calculated by the 2^−ΔΔCt method in which the Ct values of TG gene were normalized to the level of NTG control within each time point. Each value is the mean ± SEM of three independent experiments from five pooled mosquito heads. Results were analyzed using Student’s paired t-test (* = p<0.05) to determine differences between TG mosquitoes and NTG sibling controls. All expression studies were replicated a minimum of three times.
3.4. Impact of myr-AsteAkt-HA on lifetime fecundity
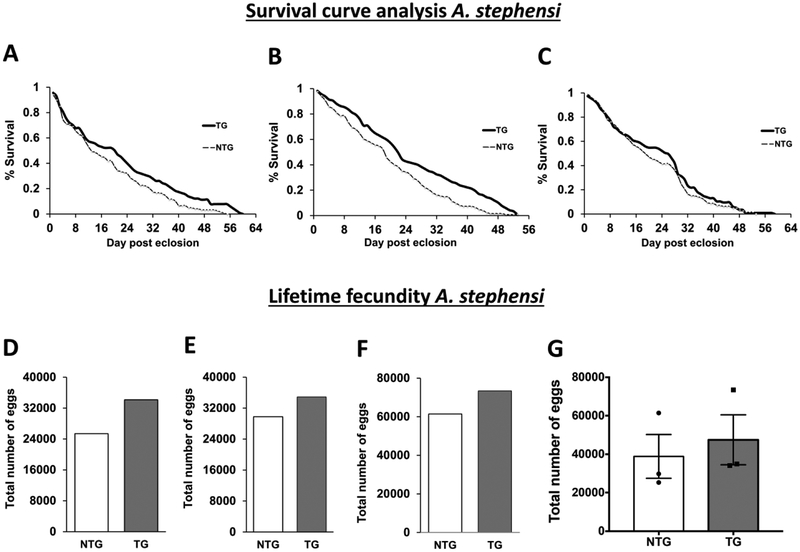
Even though vitellogenin transcript and protein levels were significantly increased in TG A. stephensi, we did not observe significantly increased egg production during the first or second reproductive cycles (Arik et al., 2014). The lack of effect on the first reproductive cycle was not surprising, however, since myr-AsteAkt-HA transgene expression and the corresponding increase in IIS occurred late in the reproductive cycle after the critical window of IIS regulation of reproduction had passed (Arik et al., 2014). However, the observed increases in vitellogenin protein (Arik et al., 2014) and transcript levels (Figure 4) coupled with a lack of effect of transgene expression on fecundity during the second reproductive cycle was unexpected. To examine this surprising result in more detail, we examined the lifetime fecundity of TG and NTG mosquitoes provided access to daily blood meals, which ensured ample opportunities for acquiring blood meals and sustained expression of the transgene. Consistent with our previous study (Arik et al., 2014) survival curves from our three new lifetime fecundity assays with distinct cohorts of TG and NTG mosquitoes again revealed a significant lifespan extension in two of the three replicates (Figures 5A–C). In replicates represented in Figures 5A and 5B, TG females survived for an average of 22 and 21 days as compared to 19 and 13 days respectively for the NTG controls, resulting in a significant increase in survivorship of 15% (p=0.013) to 47% (p=0.0067). In the replicate represented in Figure 5C, TG mosquitoes displayed a similar trend of increased survivorship relative to NTG controls (26 days TG vs 19 days NTG), but the difference in survival curves was not significant (p=0.432). These survival assays, in combination with all four significant assays in our previous work (Arik et al., 2014), reconfirm that increased fat body IIS improves adult survival, although some variation is observed. Analyses of three independent lifetime fecundity experiments, we found that caged populations of TG A. stephensi laid more eggs relative to NTG siblings (Figures 5D–F). We then compared the three combined lifetime fecundity assays using paired t-test and found that caged populations of TG A. stephensi laid significantly more eggs relative to NTG siblings (p=0.049; Figure 5G). However, the number of eggs laid by TG and NTG mosquitoes within individual gonotrophic cycles were not significantly different, even during later cycles when longer lived mosquitoes might be expected to have reduced egg production to offset their extended lifespans (Figures S2A–C). Notably, cumulative egg production from the caged TG and NTG populations revealed that TG mosquitoes start producing more eggs by approximately the fifth reproductive cycle when survival curve separation is taking place (Figures S2D–F). This observation suggests that the increased lifetime fecundity observed in TG mosquitoes was due to the increased survivorship of the TG line and not to an increase in egg production per mosquito. In fact, when total egg production is divided by the number of surviving mosquitoes in the caged population (to estimate average eggs produced per female) we did not observe a significant difference in egg production between TG and NTG mosquitoes (Figures S3A–C), again suggesting that the observed increases in the lifetime fecundity of TG caged populations were due to the increased survival of the TG line. Finally, when we compared egg production per reproductive cycle against percent survivorship (Figure S3D) we did not see a significant difference in the TG and NTG linear regression slopes of two of the three slopes (replicate 2 (p=0.806) and 3 (p=0.148)), and only a modestly significant difference in the slope of replicate 1 (0.0473), again indicating that increased survival was responsible for the observed increase in lifetime fecundity. Importantly, these data also demonstrate that a tradeoff between lifespan and fecundity is not absolute and that these two physiologies can be uncoupled.
Figure 5: Impact of myr-AsteAkt-HA overexpression in the abdominal fat body on lifetime fecundity.
A-C) Survivorship curves of A. stephensi NTG and TG mosquitoes reared under identical conditions and provided with a blood meal daily in addition to 10% dextrose solution ad libitum. Each survivorship curve represents the data from a single biological replicate using a minimum of 100 TG and NTG female mosquitoes. Significant differences between TG and NTG survival curves were assessed using Log-rank (Mantel-Cox) analysis and were observed in replicates 1 (A p=0.013) and 2 (B p=0.0067). Replicate 3 was not significant (C = =0.3161) D-F) Graphs represent total egg production of each cage of TG and NTG sibling mosquitoes throughout their lifespan and are matched to the survival curves in A-C. G) Graph represent total egg production from all three independent experiments between TG and NTG sibling mosquitoes throughout their lifespan. Significant differences were observed in the lifetime fecundity between TG and NTG using Student’s paired t-test (p<0.05; Graphpad Prism 7). Each graph represents the total number of eggs collected from an independent experimental caged population.
4. Discussion
In our previous work, we demonstrated that overexpression of active Akt in the fat body of A. stephensi and Ae. aegypti significantly extended mosquito lifespan (Arik et al., 2014). However, the mechanism regulating this increased lifespan was unknown, as was the impact that lifespan extension had on overall reproductive capacity. For the lifespan effects we observed, studies in D. melanogaster suggested a possible explanation. D. melanogaster encodes dilps that have distinct temporal and tissue specific expression patterns (Brogiolo et al., 2001; Grönke et al., 2010; Garelli et al., 2012; Broughton et al., 2005; Grönke et al., 2010; Garelli et al., 2012). Of these, only dilp2 has been shown to have a marked impact on lifespan when knocked out via ends-out homologous recombination (Grönke et al., 2010). Knockout of dilp2 led to significant lifespan extensions in both male and female flies, although partial knockdown via RNAi did not elicit this phenotype (Broughton et al., 2008a). More recently it was demonstrated that increased IIS in the fat body of D. melanogaster, induced through the fat body specific overexpression of dilp6, led to a significant decrease in dilp2 expression in the head (Bai et al., 2012). These results led us to explore the impact of increased fat body Akt signaling on ILPs expression in A. stephensi.
The five ILPs are predominantly expressed in the A. stephensi brain, but can also be found in various other tissues such as the midgut and fat body (Marquez et al., 2011). In this work, we demonstrated that when myr-AsteAkt-HA transgene expression in the fat body was maximal (24–36h post blood meal) and a corresponding increase in fat body IIS was observed (Arik et al., 2014) transcript expression of ILP2 was significantly downregulated in the head (Figure 1B). Based on phylogenetic analysis of the complete ILP coding regions, A. stephensi ILP2 is most closely related to dilp3, but is also quite similar to dilp2. A closer examination of the biologically active domains (i.e. the A and B chains) of A. stephensi ILP2/3 and dilp2/3, A-chain homology suggests that A. stephensi ILP2 is at least as similar to dilp2, if not more so, than dilp3 (Figure 5S). This makes it is difficult to infer physiological functions of the A. stephensi ILPs simply by phylogenetic relatedness to the dilps. With that said it is certainly feasible, considering the extended lifespan and decreased expression of ILP2 in the TG line, that A. stephensi ILP2 shares many of the lifespan regulatory functions attributed to dilp2. We also observed a significant increase in ILP3 transcript levels in the head when fat body Akt signaling was increased (24h post blood feeding; Figure 1C), but not at other time points. In D. melanogaster, when individual dilps were knocked out, compensatory expression of other dilps has been observed (Grönke et al., 2010). For example, the knockout of dilp2 led to compensatory expression of dilp3 in the brain, which may explain the observed increase in ILP3 expression. Until a functional analysis of A. stephensi ILP2 is conducted it will be difficult to attribute the observed lifespan extension in this line directly to the reduced expression of ILP2.
In addition to directly regulating expression of A. stephensi ILPs in the head, increased IIS in the fat body may indirectly regulate ILP activity via the expression of the putative insulin/IGF-1 binding protein ortholog Imp-L2. In D. melanogaster, overexpression of Imp-L2, either ubiquitously or in specific tissues (fat body or dilp-producing cells) led to an extension of lifespan, (Alic et al., 2011) and Imp-L2 directly bound and inactivated dilp2 (Honegger et al., 2008; Alic et al., 2011; Grönke et al., 2010). In this work, we identified the Imp-L2 ortholog in A. stephensi and demonstrated that increased fat body IIS through myr-AsteAkt-HA expression influenced Imp-L2 expression in A. stephensi fat body. Importantly, we observed a nearly 60-fold increase in Imp-L2 transcript expression in the abdominal body wall at 36h post blood meal in TG mosquitoes relative to NTG controls (Figure 3C–D) corresponding to high levels of fat body IIS in TG A. stephensi. This suggests a second mechanism for the unexpected lifespan extension in which circulating ILP levels are reduced due to increased binding and inactivation by ImpL2, which would dampen systemic IIS and extend A. stephensi lifespan. We recognize that transcript expression may not necessarily correlate with biological function of ILPs, which are stored as peptides in the MNCs and secreted as needed. At this time, however, experimental tools to address ILP regulation of IIS in A. stephensi are not available. Nevertheless, the dramatic changes in the transcript levels of ILP2 (~20–100 fold reduction), ILP3 (~70 fold increase) and Imp-L2 (~60 fold increase) in response to increased Akt signaling in the A. stephensi fat body are strongly suggestive of a role in lifespan regulation. Collectively, results from our studies in mosquitoes and others in D. melanogaster suggest that lifespan regulation by fat body IIS is likely controlled systemically by at least two mechanisms: the suppression of ILPs, particularly ILP2, in the brain and the enhanced expression of the IGF binding protein Imp-L2 to inactivate circulating ILPs.
The evolutionary theory of aging suggests that a selective advantage exists for species capable of responding to reproductive opportunities by increasing reproductive output, even when this increased fecundity shortens lifespan (Partridge and Harvey 1988). This tradeoff between reproduction and lifespan has been observed in organisms ranging from nematodes to mammals (Nasiri Moghadam et al., 2015). However, this tradeoff is not absolute and there are multiple examples where these physiologies are uncoupled. For example, C. elegans age-1 mutants are long-lived, yet do not have a corresponding loss of fecundity (Johnson et al., 1994). Similarly, certain long-lived C. elegans daf-2 mutants do not exhibit reduced reproduction (Gems et al., 1998; Kenyon et al., 1993). The influence of IIS on the balance between lifespan and reproduction, however, is both tissue- and stage-specific. For example, silencing daf-2 with RNAi in pre-adult C. elegans extends lifespan but decreases fertility, while knockdown of daf-2 in adults promotes longevity without impairing reproduction (Dillin et al., 2002). In D. melanogaster, overexpression of dFOXO in the pericerebral fat body extends life span but does not reduce fecundity (Hwangbo et al., 2004), in some cases, lifespan extension is even associated with increased reproductive output (Partridge and Fowler 1992). Fruit flies with mutations in Indy, a gene that encodes a transporter protein for Krebs cycle intermediates, are long-lived and exhibit increased fecundity relative to wild type flies (Marden et al., 2003). Similarly, female D. melanogaster with a mutation in the ecdysone receptor (EcR) show increased lifespan as well as greater age-specific fecundity and fertility relative to control flies (Simon et al., 2003).
In this study, we compared lifetime fecundity of both TG and NTG mosquitoes and found that even though TG mosquitoes survived longer than NTG mosquitoes, egg production did not differ significantly during each reproductive cycle (Figures 2SA–C and 3SA–D). In other words, TG mosquitoes do not appear to compensate for their increased lifespans by reducing egg production. Because of this, we found that the lifetime fecundity of caged populations of TG mosquitoes was significantly higher than their NTG counterparts due to the fact that their increased survivorship increased their potential for additional reproductive cycles later in life (Figures 5D–G). These results suggest that, under certain conditions and manipulations, lifespan and reproduction can be uncoupled in A. stephensi. It is important to note that most studies demonstrating uncoupling of lifespan and reproduction, including ours, were conducted under optimal conditions with abundant resources. Under suboptimal conditions, the physiological tradeoff between lifespan and reproduction may be more intractable. For example, long-lived C. elegans age-1 mutants had lower fitness than wild type nematodes when they were nutritionally stressed (Walker et al., 2000). Similarly, under optimal nutritional conditions long-lived D. melanogaster Indy mutants produced more eggs than control flies, however Indy mutant females produced fewer eggs on a decreased calorie diet, suggesting that Indy mediates a conditional tradeoff between survival and reproduction (Marden et al., 2003).
In summary, we identified two possible mechanisms to explain the unexpected lifespan extensions we observed in A. stephensi and Ae. aegypti engineered for increased abdominal fat body IIS. First, we established that overexpression of Akt in the A. stephensi fat body following blood feeding led to the suppression of ILP2, a potential ortholog for D. melanogaster dilp2 that regulates lifespan (Grönke et al., 2010). Furthermore, we found that fat body specific expression of Imp-L2 was increased in long-lived TG A. stephensi, suggesting further suppression of ILP2 activity in these mosquitoes. We also demonstrated that sustained overexpression of Akt in the A. stephensi fat body resulted in increased lifetime fecundity in caged populations. Importantly, this was not due to an increase in egg production during any individual reproductive cycle, but rather sustained reproduction throughout the extended lifespan of TG populations. Thus, TG A. stephensi did not display the typical tradeoff in reproduction associated with extended lifespan. These findings open new avenues to explore the impact of Akt signaling on lifespan and reproduction, allowing us to further elucidate the molecular mechanisms regulating fitness in mosquitoes. Furthermore, we have developed a strategy that may be useful in offsetting fitness costs associated with the engineering of pathogen-resistant mosquitoes for use in population replacement strategies to combat vector-borne pathogen transmission.
Supplementary Material
Supplemental Figure 1: Overexpression of myr-AsteAkt-HA in the abdominal fat body had no impact on ILP1-5 mRNA levels in the midgut. Midguts from 3 to 5 days old TG and NTG A. stephensi females were dissected to assess mRNA expression of (A) ILP1, (B) ILP2, (C) ILP3, (D) ILP4, and (E) ILP5, at 0h (non-blood fed, NBF), 6h, 24h, 36h, and 48h post blood meal. ILP expression was quantified by qPCR. NTG samples were used as a control for each time points for the relative transcript expression determination. The horizontal line on the graphs represents transcript levels in the NTG control samples normalized to one. The vertical bars represent the levels of ILPs in TG samples relative to the control. Graphs represents mean ± SEMs of fold change from five pooled mosquito midguts. Results were analyzed using student-paired t-test (* = p<0.05) to determine differences between TG mosquitoes and NTG sibling controls. All expression studies were replicated a minimum of three times.
Supplemental Figure 2: Fecundity and cumulative egg production of caged A. stephensi populations per reproductive cycle. Egg production during successive reproductive cycles in TG and NTG mosquitoes. A-C) Total number of eggs per reproductive cycle for TG and NTG mosquitoes. D-E) Cumulative egg production after each reproductive cycle for caged TG and NTG mosquito populations. This experiment was replicated three times using different mosquito cohorts.
Supplemental Figure 3: Cumulative egg production of A. stephensi per individual mosquito and relative to population survival. Cumulative egg production for individual mosquitoes was calculated by dividing the number of eggs produced in each cycle by the number of surviving TG or NTG mosquitoes. A-C) Cumulative numbers of eggs per individual mosquito per reproductive cycle for TG and NTG mosquitoes. D) Egg production was compared relative to percent population survival in the cage populations to assess whether a difference in egg production due to the transgene was observed. TG lines are denoted by solid symbols and NTG by open symbols. Each symbol represents successive reproductive cycle and represents the amount of eggs produced during that clutch relative to the percentage of mosquitoes still alive in the cage population. Linear regression slopes between TG and NTG mosquitoes in a replicate were found to not be significantly different in two of the replicates (Rep. 2 p=0.806; Rep. 3 p=0.148) and only modestly significant in the third (Rep. 3 p=0.0473) indicating that egg production per mosquito was not significantly impacted by transgene expression.
Supplemental Figure 4. Relative expression of ILP transcripts in TG and NTG A. stephensi. Graph represents means (ΔCt) transcript expression of five ILP genes in RNA samples prepared from pooled mosquito tissues at different time points: ILP expression in mosquito heads (A-E), abdominal body walls (F-J), and midguts (K-O) are shown. ΔCt values great than 20 indicate minimal expression of the ILP in that tissue. Data were analyzed by Student’s paired t-test; significant p-values are shown, n = 5.
Supplemental Figure 5. Alignment of A. stephensi ILP2/3 and dilp2/3. Amino acid sequences were aligned using Clustal Omega. The more highly conserved A-chain of A. stephensi ILP2 is more similar to dilp2 and, likewise, for A. stephensi ILP3 and dilp3. Unique amino acid conservation between A. stephensi ILP2/dilp2 are highlighted in green and A. stephensi ILP3/dilp3 in yellow. In contrast, the B-chain shows slightly more conservation between A. stephensi ILP2/dilp3 (green) and A. stephensi ILP3/dilp2 (yellow).
Supplemental Table 1: List of primer sequences used for qPCR in this study.
Highlights.
Akt signaling was increased in the Anopheles stephensi fat body using the vitellogenin promoter.
Increased fat body Akt activity increased ILP2 and decreased ILP3 transcript levels in the head.
Increased fat body Akt activity greatly increased transcript levels of the putative insulin binding protein Imp-L2.
Akt transgenic mosquitoes had a significantly higher lifetime fecundity than non-transgenic sibling controls
Acknowledgements:
We would like to thank Jenet Soto-Shoumaker for the maintenance of the wild type, non-transgenic and transgenic mosquito lines. We would also like to thank Drs. Kathleen Walker, Roger Miesfeld and John Jewitt for their critical review of the manuscript.
Funding: This work was supported by the National Institutes of Health (Grant numbers R21AI1225823, R56AI118926 and R56AI129420).
List of Abbreviations
- DILPs
DROSOPHILA INSULIN-LIKE PEPTIDES
- EGFP
ENHANCED GREEN FLUORESCENT PROTEIN
- FOXO
FORKHEAD BOX O
- IGF-1
INSULIN-LIKE GROWTH FACTOR-1
- INR
INSULIN RECEPTOR
- ILP
INSULIN-LIKE PEPTIDE
- IIS
INSULIN/INSULIN GROWTH FACTOR 1 SIGNALING
- Myr
MYRISTOYLATION
- NTG
NON-TRANSGENIC
- PI3K
PHOSPHOINOSITIDE 3-KINASE
- PTEN
PHOSPHATASE AND TENSIN HOMOLOG
- PTTH
PROTHORACICOTROPIC HORMONE
- TG
TRANSGENIC
- VG
VITELLOGENIN
- WHO
WORLD HEALTH ORGANIZATION
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alic N, Hoddinott MP, Vinti G, Partridge L, 2011. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 10, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova-Koch Y, Riehle MA, Arik AJ, Brown MR, 2013. Insulin-Like Peptides Handbook of Biologically Active Peptides, 267. [Google Scholar]
- Arik AJ, Hun LV, Quicke K, Piatt M, Ziegler R, Scaraffia PY, Badgandi H, Riehle MA, 2014. Increased Akt signaling in the mosquito fat body increases adult survivorship. Faseb J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arik AJ, Rasgon JL, Quicke KM, Riehle MA, 2009. Manipulating insulin signaling to enhance mosquito reproduction. BMC Physiol 9, 15 6793-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Kang P, Tatar M, 2012. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 11, 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle K, Moyes C, Henry A, Eckhoff P, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E, 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Current Biology 11, 213–221. [DOI] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L, 2008a. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PloS One 3, e3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L, 2008b. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One 3, e3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L, 2005. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U. S. A 102, 3105–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR, 2008. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A 105, 5716–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L, 2001. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106. [DOI] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C, 2002. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298, 830–834. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC, 2001. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol 231, 265–278. [DOI] [PubMed] [Google Scholar]
- Faulde MK, Rueda LM, Khaireh BA, 2014. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop 139, 39–43. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M, 2008. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. U. S. A 105, 6368–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M, 2012. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–582. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL, 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150, 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S, Clarke D, Broughton S, Andrews TD, Partridge L, 2010. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genetics 6, e1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Rodriguez SD, Drake LL, 2014. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Frontiers in Physiology 5, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger B, Galic M, Köhler K, Wittwer F, Brogiolo W, Hafen E, Stocker H, 2008. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. Journal of Biology 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo DS, Gersham B, Tu M, Palmer M, Tatar M, 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Tedesco PM, Lithgow GJ, 1994. Comparing mutants, selective breeding, and transgenics in the dissection of aging processes of Caenorhabditis elegans In: Genetics and Evolution of Aging Springer, pp. 83–95. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Ling L, Kokoza VA, Zhang C, Aksoy E, Raikhel AS, 2017. MicroRNA-277 targets insulin-like peptides 7 and 8 to control lipid metabolism and reproduction in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U. S. A 114, E8017–E8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains JW, Mercer DR, Dobson SL, 2008. Digital image analysis to estimate numbers of Aedes eggs oviposited in containers. J. Am. Mosq. Control Assoc 24, 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JH, Rogina B, Montooth KL, Helfand SL, 2003. Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies. Proc. Natl. Acad. Sci. U. S. A 100, 3369–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S, 2011. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol 173, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Taylor CE, 2009. Malaria control with transgenic mosquitoes. PLoS Med 6, e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K, Yamamoto R, Buch S, Pankratz M, Tatar M, 2008. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell 7, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiri Moghadam N, Holmstrup M, Manenti T, Brandt Mouridsen M, Pertoldi C, Loeschcke V, 2015. The role of storage lipids in the relation between fecundity, locomotor activity, and lifespan of Drosophila melanogaster longevity-selected and control lines. PLoS One 10, e0130334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Yamanaka N, 2015. Nutrition-dependent control of insect development by insulin-like peptides. Current Opinion in Insect Science 11, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Fowler K, 1992. Direct and correlated responses to selection on age at reproduction in Drosophila melanogaster. Evolution 46, 76–91. [DOI] [PubMed] [Google Scholar]
- Partridge L, Harvey PH, 1988. The ecological context of life history evolution. Science 241, 1449–1455. [DOI] [PubMed] [Google Scholar]
- Simon AF, Shih C, Mack A, Benzer S, 2003. Steroid control of longevity in Drosophila melanogaster. Science 299, 1407–1410. [DOI] [PubMed] [Google Scholar]
- Slack C, Giannakou ME, Foley A, Goss M, Partridge L, 2011. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 10, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A, 2003. The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS, 2001. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110. [DOI] [PubMed] [Google Scholar]
- Thomsen EK, Strode C, Hemmings K, Hughes AJ, Chanda E, Musapa M, Kamuliwo M, Phiri FN, Muzia L, Chanda J, 2014. Underpinning sustainable vector control through informed insecticide resistance management. PLoS One 9, e99822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toe KH, Jones CM, N’Fale S, Ismail HM, Dabire RK, Ranson H, 2014. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis 20, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M, Epstein D, Tatar M, 2002. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell 1, 75–80. [DOI] [PubMed] [Google Scholar]
- Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ, 2000. Natural selection: evolution of lifespan in C. elegans. Nature 405, 296–297. [DOI] [PubMed] [Google Scholar]
- Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR, Brown MR, 2010. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol. Cell. Endocrinol 328, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A, Perez-Pomares J, 2004. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 276, 43–57. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2018. World Malaria Report 2018 (World Health Organization, Geneva: ). [Google Scholar]
- Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y, 1997. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J. Biol. Chem 272, 30729–30734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Overexpression of myr-AsteAkt-HA in the abdominal fat body had no impact on ILP1-5 mRNA levels in the midgut. Midguts from 3 to 5 days old TG and NTG A. stephensi females were dissected to assess mRNA expression of (A) ILP1, (B) ILP2, (C) ILP3, (D) ILP4, and (E) ILP5, at 0h (non-blood fed, NBF), 6h, 24h, 36h, and 48h post blood meal. ILP expression was quantified by qPCR. NTG samples were used as a control for each time points for the relative transcript expression determination. The horizontal line on the graphs represents transcript levels in the NTG control samples normalized to one. The vertical bars represent the levels of ILPs in TG samples relative to the control. Graphs represents mean ± SEMs of fold change from five pooled mosquito midguts. Results were analyzed using student-paired t-test (* = p<0.05) to determine differences between TG mosquitoes and NTG sibling controls. All expression studies were replicated a minimum of three times.
Supplemental Figure 2: Fecundity and cumulative egg production of caged A. stephensi populations per reproductive cycle. Egg production during successive reproductive cycles in TG and NTG mosquitoes. A-C) Total number of eggs per reproductive cycle for TG and NTG mosquitoes. D-E) Cumulative egg production after each reproductive cycle for caged TG and NTG mosquito populations. This experiment was replicated three times using different mosquito cohorts.
Supplemental Figure 3: Cumulative egg production of A. stephensi per individual mosquito and relative to population survival. Cumulative egg production for individual mosquitoes was calculated by dividing the number of eggs produced in each cycle by the number of surviving TG or NTG mosquitoes. A-C) Cumulative numbers of eggs per individual mosquito per reproductive cycle for TG and NTG mosquitoes. D) Egg production was compared relative to percent population survival in the cage populations to assess whether a difference in egg production due to the transgene was observed. TG lines are denoted by solid symbols and NTG by open symbols. Each symbol represents successive reproductive cycle and represents the amount of eggs produced during that clutch relative to the percentage of mosquitoes still alive in the cage population. Linear regression slopes between TG and NTG mosquitoes in a replicate were found to not be significantly different in two of the replicates (Rep. 2 p=0.806; Rep. 3 p=0.148) and only modestly significant in the third (Rep. 3 p=0.0473) indicating that egg production per mosquito was not significantly impacted by transgene expression.
Supplemental Figure 4. Relative expression of ILP transcripts in TG and NTG A. stephensi. Graph represents means (ΔCt) transcript expression of five ILP genes in RNA samples prepared from pooled mosquito tissues at different time points: ILP expression in mosquito heads (A-E), abdominal body walls (F-J), and midguts (K-O) are shown. ΔCt values great than 20 indicate minimal expression of the ILP in that tissue. Data were analyzed by Student’s paired t-test; significant p-values are shown, n = 5.
Supplemental Figure 5. Alignment of A. stephensi ILP2/3 and dilp2/3. Amino acid sequences were aligned using Clustal Omega. The more highly conserved A-chain of A. stephensi ILP2 is more similar to dilp2 and, likewise, for A. stephensi ILP3 and dilp3. Unique amino acid conservation between A. stephensi ILP2/dilp2 are highlighted in green and A. stephensi ILP3/dilp3 in yellow. In contrast, the B-chain shows slightly more conservation between A. stephensi ILP2/dilp3 (green) and A. stephensi ILP3/dilp2 (yellow).
Supplemental Table 1: List of primer sequences used for qPCR in this study.