Abstract
Background
Several randomized trials have evaluated the efficacy of prophylactic magnesium (Mg) supplementation in prevention of post-operative atrial fibrillation (POAF) in patients undergoing cardiac artery bypass grafting (CABG). We aimed to determine the role of prophylactic Mg in 3 different settings (intraoperative, postoperative, intraoperative plus postoperative) in prevention of POAF.
Methods
A systemic literature search was performed (until January 19, 2019) using PubMed, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials to identify trials evaluating Mg supplementation post CABG. Primary outcome of our study was reduction in POAF post CABG.
Results
We included a total of 2,430 participants (1,196 in the Mg group and 1,234 in the placebo group) enrolled in 20 randomized controlled trials. Pooled analysis demonstrated no reduction in POAF between the two groups (RR 0.90; 95% CI, 0.79-1.03; p=0.13; I2=42.9%). In subgroup analysis, significant reduction in POAF was observed with postoperative Mg supplementation (RR 0.76; 95% CI, 0.58-0.99; p=0.04; I2=17.6%) but not with intraoperative or intraoperative plus postoperative Mg supplementation (RR 0.77; 95% CI, 0.49-1.22; p = 0.27; I2=49% and RR 0.92; 95% CI, 0.68-1.24; p = 0.58; I2=51.8%, respectively).
Conclusions
Magnesium supplementation, especially in the postoperative period, is an effective strategy in reducing POAF following CABG.
Keywords: Magnesium, Atrial Fibrillation, Coronary artery bypass grafting, CABG
Introduction
Coronary artery bypass grafting (CABG) is the mainstay for the treatment of coronary artery disease in select patient population unless contraindicated [1]. During the cardiopulmonary bypass (CBP), cardioplegic perfusion is intermittently discontinued (15 minutes to up to 30 minutes, depending upon institutional practice) for distal anastomoses construction during which the myocardium is predisposed to ischemic injury [2-5], thereby resulting in ischemic-reperfusion injury [6] and/or reperfusion-induced atrial/ventricular arrhythmias [7,8]. New onset atrial fibrillation is the most common arrhythmia observed postoperatively with incidence ranging from 25% to 40%; typically peaking on post-operative day 2 [9-12]. Development of post-operative atrial fibrillation (POAF) also increases the risk of heart failure, stroke and deterioration in patient's hemodynamic status resulting in increased in-hospital mortality [13,14].
Multiple randomized clinical trials have evaluated the role of prophylactic magnesium (Mg) supplementation for prevention of POAF, with conflicting results [15-34]. With increasing evidence (and addition of new trials) we aimed to assess the role of prophylactic Mg supplementation in reduction of POAF. In addition, we also evaluated the role of prophylactic Mg in three different settings (intraoperative, postoperative, or in combination) in prevention of POAF.
Methods
Search Strategy and Study Selection
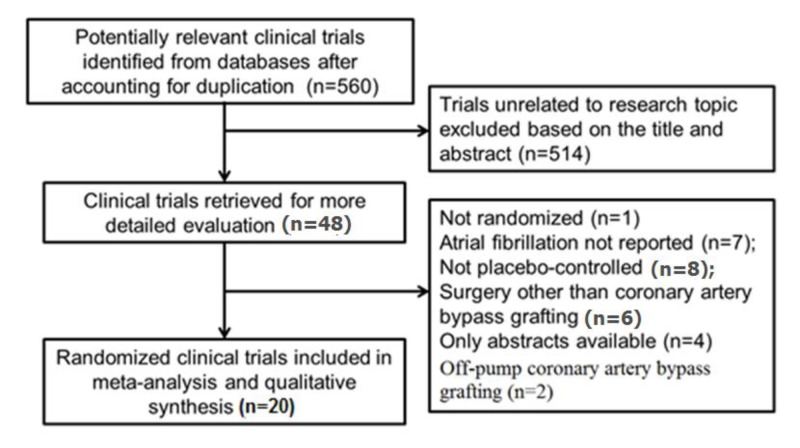
We searched PubMed, The Cochrane Library, EMBASE, EBSCO, Web of Science and CINAHL databases from inception through January 19, 2019 to identify trials evaluating Mg supplementation in patients undergoing CABG surgery using the key words: magnesium, coronary artery bypass grafting, CABG and atrial fibrillation. The eligibility criteria for our systematic review and meta-analysis included: (1) randomized controlled study design; (2) human subjects undergoing CABG surgery only; (3) received Mg supplementation intraoperatively, postoperatively or in combination; (4) reported periprocedural incidence of atrial fibrillation; and (5) literature published in English. All studies without a comparator arm, undergoing concomitant valve repair, studies that did not report clinical outcomes, off-pump CABG surgery and observational studies/case reports were excluded from the analysis [Figure 1]. We used the longest available follow-up data from the individual studies for our analysis.
Figure 1. Process of study selection (PRISMA statement).
Data extraction and Quality appraisal
Clinical, interventional, and outcome data were extracted from individual studies by 2 independent abstractors (RC and JG) and entered into a data extraction form. This included information about study design, patient characteristics (age, gender, Mg supplementation, POAF, length of stay, aortic cross clamp time and follow up period). Jadad score was independently calculated by 2 investigators (RC and JG) [Table 1] [35]. Any disparities between the two investigators were discussed with a third investigator (MT) until consensus was reached. Final results were reviewed by senior investigators.
Table 1. Characteristics of participating studies (data presented as control group/study group).
| Study name | No. of patients | Mean age (years) | Men (%) | Mean LVEF (%) | Previous MI (%) | Blinding | Infusion | Total amount (mmol) | Duration of aortic clamping (mean) | POAF (n) | Follow-up duration (hrs) | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intraoperative Magnesium supplementation | ||||||||||||
| Shakerinia et al 1996 | 25/25 | 65/67 | 68/64 | 65/67 | 72/80 | NS | MgSO4 | NA | NA | 8/5 | 24 | 1 |
| Yeatman et al 2002 | 200/200 | 63/64 | 78/83 | NA | NA | DB | MgSO4 | 20 | 47/49 | 45/58 | NA | 3 |
| Begogul et al 2003 | 50/50 | 61/64 | 88/86 | 40/40 | 14/18 | DB | MgSO4 | 16 | 44/40 | 3/2 | 24 | 2 |
| Hayashi et al 2004 | 35/35 | NA | 66/74 | 52/50 | NA | NS | MgSO4 | NA | 62/47 | 11/3 | NA | 1 |
| Ji et al 2006 | 20/20 | 56/59 | 60/70 | 47/49 | 12/11 | NS | MgSO4 | NA | 59/61 | 8/2 | NA | 3 |
| Casalino et al 2008 | 49/48 | 66/68 | 74/75 | 54/56 | 40/43 | NS | MgSO4 | 32 | 38/37 | 5/4 | 120 | 2 |
| Svagzdiene et al 2009 | 106/52 | 65/65 | NA | 44/46 | NA | NS | MgSO4 | NA | 47/52 | 28/15 | 72 | 1 |
| Postoperative Magnesium supplementation | ||||||||||||
| Fanning et al 1991 | 50/49 | 62/59 | 78/71 | 49/50 | 42/35 | DB | MgSO4 | 84 | 66/66 | 14/7 | 96 | 4 |
| Colquhoun et al 1993 | 64/66 | 59/57 | 80/83 | NA | 53/45 | DB | MgCl | 50 | 52/51 | 15/11 | 96 | 4 |
| Parikka et al 1993 | 71/69 | 54/57 | 82/84 | 59/61 | NA | NS | MgSO4 | 70 | NA | 18/20 | 48 | 2 |
| Nurozler et al 1996 | 25/25 | 54/56 | 92/9% | 66/67 | 28/32 | DB | MgSO4 | 100 | 52/46 | 5/1 | 120 | 2 |
| Jensen et al 1997 | 28/29 | 61/61 | 100/100 | NA | NA | DB | MgSO4 | 110 | NA | 10/10 | 72 | 4 |
| Treggiari-Venzi et al 2000 | 51/47 | 65/65 | 84/89 | 57/62 | 45/3% | DB | MgSO4 | 48 | 103/91 | 14/11 | 72 | 5 |
| Behmanesh et al 2006 | 50/50 | 63/66 | 93/81 | NA | 50/36 | NS | MgSO4 | NA | 44/44 | 21/10 | 168 | 3 |
| Intra- + Postoperative magnesium supplementation | ||||||||||||
| Caspi et al 1995 | 48/50 | 62/60 | 83/89 | 49/48 | NA | NS | MgSO4 | 48 | 45/50 | 18/22 | 36 | 4 |
| Solomon et al 2000 | 82/85 | 61/62 | 73/80 | 54/53 | NA | NS | MgSO4 | 150 | 63/60 | 16/19 | 24 | 4 |
| Bert et al 2001 | 60/63 | 64/63 | 83/8% | 49/48 | NA | NS | MgSO4 | 49 | 60/55 | 23/24 | 96 | 4 |
| Forlani et al 2002 | 50/54 | 64/64 | 88/85 | 55/52 | 66/65 | NS | MgSO4 | 37 | 47/48 | 19/8 | 720 | 4 |
| Geertman et al 2004 | 73/74 | 62/64 | 79/79 | NA | NA | DB | MgSO4 | 50 | 48/50 | 19/25 | 36 | 4 |
| Hazelrigg et al 2004 | 97/105 | 64/62 | 68/74 | 51/53 | NA | DB | MgSO4 | NA | 55/61 | 41/32 | 120 | 4 |
Outcome Variables
The primary outcome of our study was reduction in POAF burden. In order to assess possible differences in the timing of Mg administration, we further divided trials into three subdivisions (secondary outcomes): intraoperative, postoperative and a combination of intra-and postoperative Mg administration.
Statistical Analysis
We conducted a meta-analysis of summary statistics from the individual trials because detailed, patient-level data were not available for all trials. Summary estimates and 95% confidence intervals (CI) were reported for continuous variables as difference in means. Mantel-Haenszel risk ratio (RR) fixed effects model was used to summarize data across treatment arms. We evaluated heterogeneity of effects using the Higgins I-squared (I2) statistic [36]. In cases with heterogeneity (defined as I2 >25%), random effects models of DerSimonian and Laird [37] were used. Publication bias was estimated visually by funnel plots [38,39]. If any bias was observed, further bias quantification was measured using the Begg-Mazumdar test [40], and Egger test [38]. All analyses were conducted using Comprehensive Meta-Analysis 2.0 software (Biostat, Inc., Englewood, NJ)
Results
We included 20 randomized controlled trials [15-34] with a total of 2,430 patients - 1,196 patients in Mg supplementation group, while 1,234 patients in the placebo group. [Table 1] describes the baseline characteristics of included studies including patient demographics, Mg regimens, and incidence of POAF. [Table 2] describes the differences in baseline characteristics between Mg supplementation and placebo groups of included studies.
Table 2. Baseline demographics of study population.
RR=Relative Risk; SMD=Standardized Mean Difference
| Baseline Characteristic | Mg supplementation | Placebo | N | Studies (n) | RR or SMD (95% CI) | Heterogeneity P value | Heterogeneity I2 (%) | P for overall effect |
|---|---|---|---|---|---|---|---|---|
| Age, yrs | 62.3 | 61.6 | 2,008 | 15 | 0.21 (0.03 to 0.40) | 0.02 | 75.58 | <0.0001 |
| Males, % | 79.6 | 78.4 | 1,986 | 16 | 1.02 (0.98 to 1.06) | 0.97 | 0 | 0.37 |
| Hypertension, % | 49.1 | 48.0 | 669 | 7 | 0.96 (0.86 to 1.09) | 0.73 | 0 | 0.55 |
| Diabetes mellitus, % | 21.0 | 18.0 | 1,169 | 9 | 1.11 (0.73 to 1.67) | 0.02 | 53.99 | 0.63 |
| History of myocardial infarction, % | 47.3 | 48.6 | 966 | 11 | 0.97 (0.86 to 1.10) | 0.88 | 0 | 0.62 |
| Preoperative use of beta-blockers, % | 63.0 | 67.8 | 1,723 | 15 | 0.95 (0.87 to 1.03) | 0.06 | 39.26 | 0.21 |
| Need for vasopressors post-surgery, % | 29.5 | 31.9 | 958 | 7 | 0.83 (0.62 to 1.12) | 0.11 | 42.09 | 0.22 |
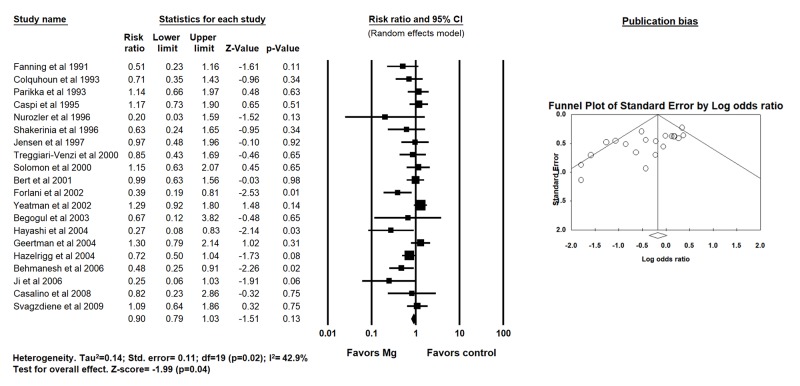
Four hundred and thirty patients received Mg intraoperatively, 335 patients received Mg postoperatively while 431 patients received Mg both intra- and post-operatively. By using random-effects model, pooled analysis for the primary outcome demonstrated no difference in POAF between the two groups (22% versus [vs.] 29% for Mg and placebo groups respectively, RR 0.90; 95% CI, 0.79-1.03; p = 0.13; I2=42.9%) [Figure 2].
Figure 2. Forest plot demonstrating the effects of magnesium supplementation compared to placebo on post operative atrial fibrillation after CABG surgery (random effects model).
No significant difference was observed between the two groups for length of stay (6.75 days vs 6.77 days for Mg and placebo arm respectively, SMD 0; 95%CI -0.13 - 0.13, p=1.00; I2=0%), perioperative myocardial infarction (MI) (2.7% vs. 2.2% for Mg and placebo groups respectively, RR 1.26, 95% CI, 0.67 - 2.38, p=0.47; I2=0%), perioperative mortality (0.6% vs 0.6% for Mg and placebo groups respectively, RR 1.06, 95% CI, 0.43 - 2.62, p=0.90; I2=0%), aortic cross-clamping time (53 minutes vs. 55 minutes for Mg and placebo groups respectively, SMD -0.12, 95% CI -0.55 - 0.32, p=0.60;I2=95%) and duration of CPB (89 minutes vs. 88 minutes for Mg and placebo groups respectively, SMD 0.30, 95% CI -0.05 - 0.66, p=0.09; I2=91%).
Intraoperative Magnesium supplementation subgroup
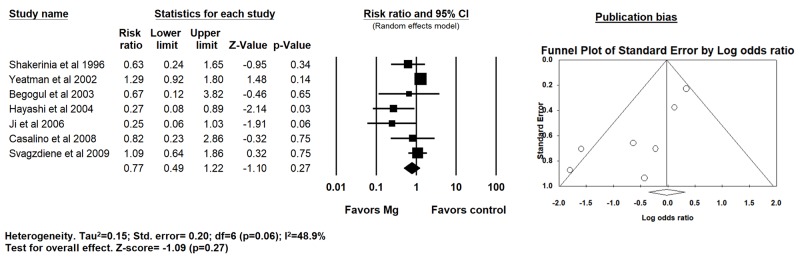
In 7 trials that evaluated prophylactic intraoperative Mg supplementation, 16% patients had POAF in the intraoperative Mg arm vs. 24% in the placebo arm with no reduction in POAF (RR 0.77; 95% CI: 0.49 - 1.22; p=0.27; I2=48.9%) [Figure 3].
Figure 3. Forest plot demonstrating the effects of intraoperative magnesium supplementation compared to placebo on post operative atrial fibrillation after CABG surgery (random effects model).
There were no significant differences observed between the two groups for perioperative MI (2.1% for Mg and placebo groups respectively, RR 1.00; 95% CI 0.29 - 3.40, p=1.00, I2=0%), perioperative mortality (0.3% vs. 0.5% for Mg and placebo groups respectively, RR 1.44, 95% CI 0.23 - 9.04, p=0.70; I2=18.16%), aortic cross-clamping time (SMD -0.10, 95% CI -1.46 - 1.28, p=0.89; I2=98.33%) and duration of CPB (SMD 0.77, 95% CI -0.14 - 1.67, p=0.09; I2=96.15%).
Postoperative Magnesium supplementation subgroup
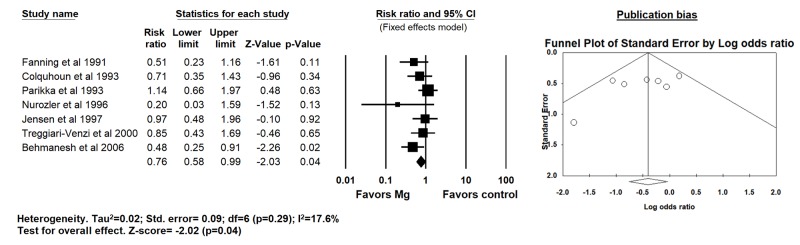
Seven trials that evaluated postoperative Mg supplementation, there was a significant reduction in the incidence of POAF (20% vs 29% for Mg and placebo groups respectively, RR 0.76; 95% CI 0.58 - 0.99; p=0.04; I2=17.6%) [Figure 4].
Figure 4. Forest plot demonstrating the effects of postoperative magnesium supplementation compared to placebo on post operative atrial fibrillation after CABG surgery (fixed effects model since I2<25%).
There were no significant differences observed between the two groups for perioperative MI (1.9% vs. 2.0% for Mg and placebo groups respectively, RR 0.98; 95% CI 0.25 - 3.77, p=0.97; I2=0%), perioperative mortality (0.5% vs. 0.9% for Mg and placebo groups respectively, RR 0.79, 95% CI 0.17 - 3.66, p=0.77; I2=0%), aortic cross-clamping time (SMD -0.32, 95% CI -0.74 - 0.10, p=0.14; I2=75.28%) and duration of CPB (SMD -0.08, 95% CI -0.38 - 0.21, p=0.57; I2=51%).
Intraoperative plus Postoperative Magnesium supplementation subgroup
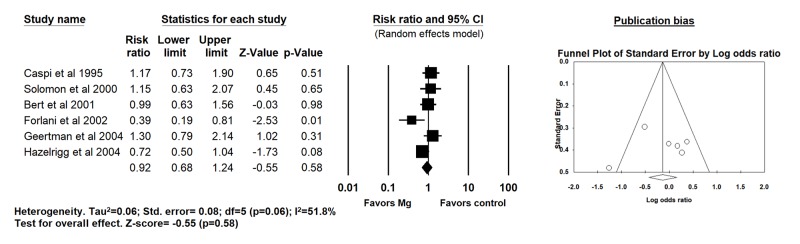
In six trials evaluating a combined intra and postoperative magnesium supplementation strategy, no reduction in POAF (31% vs 34% for Mg and placebo groups respectively, RR 0.92; 95% CI 0.68 - 1.24; p=0.58; I2=51.8%) [Figure 5], perioperative MI (RR 1.60; 95% CI 0.66 - 3.90, p=0.30; I2=0%), perioperative death (RR 1.14; 95% CI 0.28 - 4.65, p=0.86; I2=0%) and aortic cross-clamp time (SMD 0.03, 95% CI -0.15 - 0.22, p=0.73; I2=40%) was observed. However, CPB time was significantly more in Mg group compared to placebo (90 minutes vs. 85 minutes, respectively, SMD 0.19, 95% CI 0.003 - 0.37, p=0.04; I2=0%).
Figure 5. Forest plot demonstrating the effects of intraoperative+postoperative magnesium supplementation compared to placebo on post operative atrial fibrillation after CABG surgery (random effects model).
Publication bias and Quality appraisal
A significant publication bias was identified overall for POAF [Table 3]. Upon further stratification based on timing of Mg administration, publication bias was significant for intra-operative strategy only. No publication bias was observed for perioperative MI, mortality, aortic cross-clamping time and duration of CPB. The publication bias observed did not change even after adjustments using Duval and Tweedie’s trim and fill and addition of imputed studies.
Table 3. Summary of Egger’s and Begg’s test for publication bias.
p-value of <0.05 indicates publication bias
| Outcomes | Egger’s test p-value | Begg’s test p-value |
|---|---|---|
| Overall POAF | 0.002 | 0.008 |
| POAF (Intraoperative Mg) | 0.01 | 0.13 |
| POAF (Postoperative Mg) | 0.18 | 0.54 |
| POAF (Intra- + Postoperative Mg) | 0.84 | 1.00 |
Discussion
The current meta-analysis analyzed 2,430 patients and demonstrated a significant reduction in POAF among patients undergoing on-pump CABG surgery who received prophylactic Mg supplementation in the postoperative period only. No significant differences were observed in perioperative MI, mortality, aortic cross-clamp time or duration of CPB between the two groups. To the best of our knowledge, this is the first meta-analysis demonstrating the role of prophylactic Mg supplementation (and different administration strategies) in patients undergoing on-pump CABG surgery in preventing POAF [41-46].
The precise mechanism by which prophylactic Mg supplementation reduces POAF remains unclear. Hypomagnesaemia has been shown to be proarrhythmic with studies demonstrating an increased risk of atrial and ventricular arrhythmias [47,48]. In addition, studies have shown that serum Mg levels do not correlate with myocardial tissue magnesium levels [49], with low extracellular Mg associated with abnormalities of depolarization, repolarization and automaticity [50]. Mg supplementation therefore significantly increases atrial refractoriness by prolonging the action potential duration and atrial effective refractory period [51-53]. A possible explanation to the efficacy of postoperative Mg supplementation in reducing POAF, as observed in our study, likely stems from the myocardial Mg depletion in immediate postoperative period (circulating volume dilution from extracorporeal support, use of diuretics which promotes Mg excretion and/or norepinephrine induced redistribution of Mg from intracellular to extracellular compartment). Myocardial Mg depletion would not be reflected on serum Mg levels; and therefore could be responsible for provoking atrial arrhythmias despite normal serum Mg levels. Magnesium supplementation in the postoperative period possibly offsets this process. In addition, POAF predominantly occurs between postoperative day 1 and day 4 with the peak incidence at day 2, which is often associated with hypomagnesemia. This time course also correlates with increased sympathetic activation (from surgical stress and exaggerated by β-blockers withdrawal) and has been associated with POAF. Therefore, prophylactic Mg supplementation postoperatively may attenuate adrenergic medicated automaticity and reduce the incidence of POAF as observed in this study. Interestingly, there was no reduction in POAF in patients with intraoperative or intraoperative plus post-operative magnesium supplementation. The exact explanation remains uncertain. Theoretically, the duration of aortic cross-clamp time and CPB might be responsible for POAF reduction, nonetheless no significant differences were observed between the two groups.
Development of POAF after CABG adds a potentially preventable significant burden to healthcare and is associated with increased length of hospital stay. In a study by Aranki et al, length of stay increased from 9.3 ± 19.6 days to 15.3 ± 28.6 days (p=0.001), which was estimated to an additional charge of $10,055 for in-patient hospital charges per patient [54]. Multiple agents have been explored to reduce the incidence of POAF after CABG including beta-blockers, anti-arrhythmic agents (sotalol and amiodarone) and Mg supplementation. Amongst these agents, Mg is the least likelihood of drug interactions and side effects, is readily available, well tolerated by patients and inexpensive [55].
Due to multiple randomized clinical trials exploring the role and utility of Mg prophylaxis, several meta-analyses have been conducted in the past. The results of our study contrasts from the previously reported meta-analyses by Gu et al and De Oliveira et al, both of which demonstrated an overall reduction in POAF with magnesium supplementation (RR=0.64; 0.50-0.83 and OR=0.69; CI 0.53-0.90, respectively) [43,44]. In a sub-analysis by De Oliveira et al comparing POAF between high-quality and low-quality studies, no reduction of POAF was found with magnesium supplementation in higher quality studies but a significant reduction was seen with low-quality studies. However, no such differences were found in our sub-analysis without any significant reduction in POAF when stratified by high or low-quality studies (data not shown).
There are several limitations in this study. First, the studies included in this study span a time of 25 years during which there has been tremendous evolution in the surgical techniques. Second, majority of trials included in our analysis did not specify concomitant use of beta-blockers, which might have overestimated the effectiveness of Mg in the postoperative sub-group. Finally, a publication bias was observed in the overall results of the study and the included trials had diverse dosing regimens, mode of supplementation and follow-up time period. However, no significant heterogeneity was observed for POAF reduction in the postoperative Mg supplementation group.
Acknowledgement
None
Conclusions
Magnesium supplementation, especially in the postoperative period, is an effective strategy in reducing POAF following on-pump CABG surgery. Further large randomized controlled trials are needed to validate our results and whether this reduced incidence of POAF would translate into reducing length of stay and healthcare cost.
References
- 1.Hillis L David, Smith Peter K, Anderson Jeffrey L, Bittl John A, Bridges Charles R, Byrne John G, Cigarroa Joaquin E, DiSesa Verdi J, Hiratzka Loren F, Hutter Adolph M, Jessen Michael E, Keeley Ellen C, Lahey Stephen J, Lange Richard A, London Martin J, Mack Michael J, Patel Manesh R, Puskas John D, Sabik Joseph F, Selnes Ola, Shahian David M, Trost Jeffrey C, Winniford Michael D, Jacobs Alice K, Anderson Jeffrey L, Albert Nancy, Creager Mark A, Ettinger Steven M, Guyton Robert A, Halperin Jonathan L, Hochman Judith S, Kushner Frederick G, Ohman E Magnus, Stevenson William, Yancy Clyde W. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2012 Jan;143 (1):4–34. doi: 10.1016/j.jtcvs.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Calafiore A M, Teodori G, Mezzetti A, Bosco G, Verna A M, Di Giammarco G, Lapenna D. Intermittent antegrade warm blood cardioplegia. Ann. Thorac. Surg. 1995 Feb;59 (2):398–402. doi: 10.1016/0003-4975(94)00843-v. [DOI] [PubMed] [Google Scholar]
- 3.Ali I M. Interrupted warm blood cardioplegia. Eur J Cardiothorac Surg. 1996;10 (6) doi: 10.1016/s1010-7940(96)80123-0. [DOI] [PubMed] [Google Scholar]
- 4.Minatoya K, Okabayashi H, Shimada I, Tanabe A, Nishina T, Nandate K, Kunihiro M. Intermittent antegrade warm blood cardioplegia for CABG: extended interval of cardioplegia. Ann. Thorac. Surg. 2000 Jan;69 (1):74–6. doi: 10.1016/s0003-4975(99)01384-3. [DOI] [PubMed] [Google Scholar]
- 5.Randomised trial of normothermic versus hypothermic coronary bypass surgery. The Warm Heart Investigators. Lancet. 1994 Mar 05;343 (8897):559–63. [PubMed] [Google Scholar]
- 6.Tünerir B, Colak O, Alataş O, Beşoğul Y, Kural T, Aslan R. Measurement of troponin T to detect cardioprotective effect of trimetazidine during coronary artery bypass grafting. Ann. Thorac. Surg. 1999 Dec;68 (6):2173–6. doi: 10.1016/s0003-4975(99)01126-1. [DOI] [PubMed] [Google Scholar]
- 7.Komori S, Li B, Matsumura K, Takusagawa M, Sano S, Kohno I, Osada M, Sawanobori T, Ishihara T, Umetani K, Ijiri H, Tamura K. Antiarrhythmic effect of magnesium sulfate against occlusion-induced arrhythmias and reperfusion-induced arrhythmias in anesthetized rats. Mol. Cell. Biochem. 1999 Sep;199 (1-2):201–8. doi: 10.1023/a:1006938010925. [DOI] [PubMed] [Google Scholar]
- 8.Caputo M, Bryan A J, Calafiore A M, Suleiman M S, Angelini G D. Intermittent antegrade hyperkalaemic warm blood cardioplegia supplemented with magnesium prevents myocardial substrate derangement in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 1998 Dec;14 (6):596–601. doi: 10.1016/s1010-7940(98)00247-4. [DOI] [PubMed] [Google Scholar]
- 9.Lauer M S, Eagle K A, Buckley M J, DeSanctis R W. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989 Mar 1;31 (5):367–78. doi: 10.1016/0033-0620(89)90031-5. [DOI] [PubMed] [Google Scholar]
- 10.Cox J L. A perspective of postoperative atrial fibrillation in cardiac operations. Ann. Thorac. Surg. 1993 Sep;56 (3):405–9. doi: 10.1016/0003-4975(93)90871-e. [DOI] [PubMed] [Google Scholar]
- 11.Vecht R J, Nicolaides E P, Ikweuke J K, Liassides C, Cleary J, Cooper W B. Incidence and prevention of supraventricular tachyarrhythmias after coronary bypass surgery. Int. J. Cardiol. 1986 Nov;13 (2):125–34. doi: 10.1016/0167-5273(86)90137-3. [DOI] [PubMed] [Google Scholar]
- 12.Gerges Christian, Gerges Mario, Skoro-Sajer Nika, Zhou Yi, Zhang Lixia, Sadushi-Kolici Roela, Jakowitsch Johannes, Lang Marie B, Lang Irene M. Hemodynamic Thresholds for Precapillary Pulmonary Hypertension. Chest. 2016 Apr;149 (4):1061–73. doi: 10.1378/chest.15-0928. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhof Paulus, Benussi Stefano, Kotecha Dipak, Ahlsson Anders, Atar Dan, Casadei Barbara, Castella Manuel, Diener Hans-Christoph, Heidbuchel Hein, Hendriks Jeroen, Hindricks Gerhard, Manolis Antonis S, Oldgren Jonas, Popescu Bogdan Alexandru, Schotten Ulrich, Van Putte Bart, Vardas Panagiotis. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016 Oct 07;37 (38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 14.Mostafa Ashraf, El-Haddad Mohamed A, Shenoy Maithili, Tuliani Tushar. Atrial fibrillation post cardiac bypass surgery. Avicenna J Med. 2012 Jul;2 (3):65–70. doi: 10.4103/2231-0770.102280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanning W J, Thomas C S, Roach A, Tomichek R, Alford W C, Stoney W S. Prophylaxis of atrial fibrillation with magnesium sulfate after coronary artery bypass grafting. Ann. Thorac. Surg. 1991 Sep;52 (3):529–33. doi: 10.1016/0003-4975(91)90918-g. [DOI] [PubMed] [Google Scholar]
- 16.Colquhoun I W, Berg G A, el-Fiky M, Hurle A, Fell G S, Wheatley D J. Arrhythmia prophylaxis after coronary artery surgery. A randomised controlled trial of intravenous magnesium chloride. Eur J Cardiothorac Surg. 1993;7 (10):520–3. doi: 10.1016/1010-7940(93)90049-h. [DOI] [PubMed] [Google Scholar]
- 17.Parikka H, Toivonen L, Pellinen T, Verkkala K, Järvinen A, Nieminen M S. The influence of intravenous magnesium sulphate on the occurrence of atrial fibrillation after coronary artery by-pass operation. Eur. Heart J. 1993 Feb;14 (2):251–8. doi: 10.1093/eurheartj/14.2.251. [DOI] [PubMed] [Google Scholar]
- 18.Caspi J, Rudis E, Bar I, Safadi T, Saute M. Effects of magnesium on myocardial function after coronary artery bypass grafting. Ann. Thorac. Surg. 1995 Apr;59 (4):942–7. doi: 10.1016/0003-4975(95)00050-u. [DOI] [PubMed] [Google Scholar]
- 19.Nurözler F, Tokgözoglu L, Pasaoglu I, Böke E, Ersoy U, Bozer A Y. Atrial fibrillation after coronary artery bypass surgery: predictors and the role of MgSO4 replacement. J Card Surg. 1996 Nov 1;11 (6):421–7. doi: 10.1111/j.1540-8191.1996.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 20.Shakerinia T, Ali I M, Sullivan J A. Magnesium in cardioplegia: is it necessary? Can J Surg. 1996 Oct;39 (5):397–400. [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen B M, Alstrup P, Klitgård N A. Magnesium substitution and postoperative arrhythmias in patients undergoing coronary artery bypass grafting. Scand. Cardiovasc. J. 1997;31 (5):265–9. doi: 10.3109/14017439709069546. [DOI] [PubMed] [Google Scholar]
- 22.Treggiari-Venzi M M, Waeber J L, Perneger T V, Suter P M, Adamec R, Romand J A. Intravenous amiodarone or magnesium sulphate is not cost-beneficial prophylaxis for atrial fibrillation after coronary artery bypass surgery. Br J Anaesth. 2000 Nov;85 (5):690–5. doi: 10.1093/bja/85.5.690. [DOI] [PubMed] [Google Scholar]
- 23.Solomon A J, Berger A K, Trivedi K K, Hannan R L, Katz N M. The combination of propranolol and magnesium does not prevent postoperative atrial fibrillation. Ann. Thorac. Surg. 2000 Jan;69 (1):126–9. doi: 10.1016/s0003-4975(99)01187-x. [DOI] [PubMed] [Google Scholar]
- 24.Bert A A, Reinert S E, Singh A K. A beta-blocker, not magnesium, is effective prophylaxis for atrial tachyarrhythmias after coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2001 Apr;15 (2):204–9. doi: 10.1053/jcan.2001.21959. [DOI] [PubMed] [Google Scholar]
- 25.Forlani Stefano, De Paulis Ruggero, de Notaris Stefano, Nardi Paolo, Tomai Fabrizio, Proietti Igino, Ghini Anna S, Chiariello Luigi. Combination of sotalol and magnesium prevents atrial fibrillation after coronary artery bypass grafting. Ann. Thorac. Surg. 2002 Sep;74 (3):720–5. doi: 10.1016/s0003-4975(02)03773-6. [DOI] [PubMed] [Google Scholar]
- 26.Yeatman Mark, Caputo Massimo, Narayan Pradeep, Lotto Attilio A, Ascione Raimondo, Bryan Alan J, Angelini Gianni D. Magnesium-supplemented warm blood cardioplegia in patients undergoing coronary artery revascularization. Ann. Thorac. Surg. 2002 Jan;73 (1):112–8. doi: 10.1016/s0003-4975(01)03270-2. [DOI] [PubMed] [Google Scholar]
- 27.Beşoğul Y, Tünerir B, Ozdemir C, Aslan R. Magnesium-flush infusion into the aortic root just before reperfusion reduces the requirement for internal defibrillation and early post-perfusion arrhythmias. J. Int. Med. Res. 2003 Jul 23;31 (3):202–9. doi: 10.1177/147323000303100306. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi Yoshitaka, Ohtani Masakatsu, Sawa Yoshiki, Hiraishi Taizo, Akedo Hiroshi, Kobayashi Yasuhiko, Matsuda Hikaru. Minimally-diluted blood cardioplegia supplemented with potassium and magnesium for combination of 'initial, continuous and intermittent bolus' administration. Circ. J. 2004 May;68 (5):467–72. doi: 10.1253/circj.68.467. [DOI] [PubMed] [Google Scholar]
- 29.Geertman Hans, van der Starre Peter J A, Sie Hauw T, Beukema Willem P, van Rooyen-Butijn Michelle. Magnesium in addition to sotalol does not influence the incidence of postoperative atrial tachyarrhythmias after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2004 Jun;18 (3):309–12. doi: 10.1053/j.jvca.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Hazelrigg Stephen R, Boley Theresa M, Cetindag Ibrahim B, Moulton Kreigh P, Trammell Gary L, Polancic Joan E, Shawgo Tilitha S, Quin Jacquelyn A, Verhulst Stephen. The efficacy of supplemental magnesium in reducing atrial fibrillation after coronary artery bypass grafting. Ann. Thorac. Surg. 2004 Mar;77 (3):824–30. doi: 10.1016/j.athoracsur.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Behmanesh Saeid, Tossios Paschalis, Homedan Hossam, Hekmat Khosro, Hellmich Martin, Müller-Ehmsen Jochen, Schwinger Robert H G, Mehlhorn Uwe. Effect of prophylactic bisoprolol plus magnesium on the incidence of atrial fibrillation after coronary bypass surgery: results of a randomized controlled trial. Curr Med Res Opin. 2006 Aug;22 (8):1443–50. doi: 10.1185/030079906X115649. [DOI] [PubMed] [Google Scholar]
- 32.Ji B, Liu J, Liu M, Feng Z, Wang G, Lu F, Long C. Effect of cold blood cardioplegia enriched with potassium-magnesium aspartate during coronary artery bypass grafting. J Cardiovasc Surg (Torino) 2006 Dec;47 (6):671–5. [PubMed] [Google Scholar]
- 33.Casalino Stefano, Tesler Ugo F, Novelli Eugenio, Stelian Edmond, Renzi Luca, Alessi Claudio, Lanzillo Guido, Cerin Gheorghe, Diena Marco. The efficacy and safety of extending the ischemic time with a modified cardioplegic technique for coronary artery surgery. J Card Surg. 2008 Oct 22;23 (5):444–9. doi: 10.1111/j.1540-8191.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 34.Svagzdiene Milda, Sirvinskas Edmundas, Benetis Rimantas, Raliene Laima, Simatoniene Violeta. Atrial fibrillation and changes in serum and urinary electrolyte levels after coronary artery bypass grafting surgery. Medicina (Kaunas) 2009;45 (12):960–70. [PubMed] [Google Scholar]
- 35.Jadad A R, Moore R A, Carroll D, Jenkinson C, Reynolds D J, Gavaghan D J, McQuay H J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17 (1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 36.Cipriani Andrea, Furukawa Toshi A, Salanti Georgia, Chaimani Anna, Atkinson Lauren Z, Ogawa Yusuke, Leucht Stefan, Ruhe Henricus G, Turner Erick H, Higgins Julian P T, Egger Matthias, Takeshima Nozomi, Hayasaka Yu, Imai Hissei, Shinohara Kiyomi, Tajika Aran, Ioannidis John P A, Geddes John R. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018 Apr 07;391 (10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7 (3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315 (7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterne Jonathan A C, Sutton Alex J, Ioannidis John P A, Terrin Norma, Jones David R, Lau Joseph, Carpenter James, Rücker Gerta, Harbord Roger M, Schmid Christopher H, Tetzlaff Jennifer, Deeks Jonathan J, Peters Jaime, Macaskill Petra, Schwarzer Guido, Duval Sue, Altman Douglas G, Moher David, Higgins Julian P T. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011 Jul 22;343 () doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 40.Begg C B, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994 Dec;50 (4):1088–101. [PubMed] [Google Scholar]
- 41.Shiga Toshiya, Wajima Zen'ichiro, Inoue Tetsuo, Ogawa Ryo. Magnesium prophylaxis for arrhythmias after cardiac surgery: a meta-analysis of randomized controlled trials. Am. J. Med. 2004 Sep 01;117 (5):325–33. doi: 10.1016/j.amjmed.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly S J. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart. 2005 May;91 (5):618–23. doi: 10.1136/hrt.2004.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu Wan-Jie, Wu Zhen-Jie, Wang Peng-Fei, Aung Lynn Htet Htet, Yin Rui-Xing. Intravenous magnesium prevents atrial fibrillation after coronary artery bypass grafting: a meta-analysis of 7 double-blind, placebo-controlled, randomized clinical trials. Trials. 2012 Apr 20;13 () doi: 10.1186/1745-6215-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Oliveira Gildasio S, Knautz Jennifer S, Sherwani Saadia, McCarthy Robert J. Systemic magnesium to reduce postoperative arrhythmias after coronary artery bypass graft surgery: a meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2012 Aug;26 (4):643–50. doi: 10.1053/j.jvca.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Burgess David C, Kilborn Michael J, Keech Anthony C. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur. Heart J. 2006 Dec;27 (23):2846–57. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- 46.Alghamdi Abdullah A, Al-Radi Osman O, Latter David A. Intravenous magnesium for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and meta-analysis. J Card Surg. 2005 Apr 28;20 (3):293–9. doi: 10.1111/j.1540-8191.2005.200447.x. [DOI] [PubMed] [Google Scholar]
- 47.Klevay Leslie M, Milne David B. Low dietary magnesium increases supraventricular ectopy. Am. J. Clin. Nutr. 2002 Mar;75 (3):550–4. doi: 10.1093/ajcn/75.3.550. [DOI] [PubMed] [Google Scholar]
- 48.Guideri G, Lehr D, Horowitz S. Enhanced incidence of isoproterenol-induced ventricular fibrillation in the magnesium-deficient rat. J Am Coll Nutr. 1985;4 (2):139–55. [PubMed] [Google Scholar]
- 49.Millane T A, Jennison S H, Mann J M, Holt D W, McKenna W J, Camm A J. Myocardial magnesium depletion associated with prolonged hypomagnesemia: a longitudinal study in heart transplant recipients. J. Am. Coll. Cardiol. 1992 Oct;20 (4):806–12. doi: 10.1016/0735-1097(92)90177-o. [DOI] [PubMed] [Google Scholar]
- 50.Roden D M, Iansmith D H. Effects of low potassium or magnesium concentrations on isolated cardiac tissue. Am. J. Med. 1987 Mar 20;82 (3A):18–23. doi: 10.1016/0002-9343(87)90128-8. [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen H S, Thomsen P E. The electrophysiological effects of intravenous magnesium on human sinus node, atrioventricular node, atrium, and ventricle. Clin Cardiol. 1989 Feb;12 (2):85–90. doi: 10.1002/clc.4960120204. [DOI] [PubMed] [Google Scholar]
- 52.Christiansen E H, Frost L, Andreasen F, Mortensen P, Thomsen P E, Pedersen A K. Dose-related cardiac electrophysiological effects of intravenous magnesium. A double-blind placebo-controlled dose-response study in patients with paroxysmal supraventricular tachycardia. Europace. 2000 Oct;2 (4):320–6. doi: 10.1053/eupc.2000.0123. [DOI] [PubMed] [Google Scholar]
- 53.Redwood S R, Taggart P I, Sutton P M, Bygrave A, Bashir Y, Purkayastha D D, Camm A J, Treasure T. Effect of magnesium on the monophasic action potential during early ischemia in the in vivo human heart. J. Am. Coll. Cardiol. 1996 Dec;28 (7):1765–9. doi: 10.1016/S0735-1097(96)00373-7. [DOI] [PubMed] [Google Scholar]
- 54.Aranki S F, Shaw D P, Adams D H, Rizzo R J, Couper G S, VanderVliet M, Collins J J, Cohn L H, Burstin H R. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996 Aug 01;94 (3):390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 55.Trappe Hans-Joachim, Brandts Bodo, Weismueller Peter. Arrhythmias in the intensive care patient. Curr Opin Crit Care. 2003 Oct;9 (5):345–55. doi: 10.1097/00075198-200310000-00003. [DOI] [PubMed] [Google Scholar]