Abstract
Background
Family history has been shown to be associated with increased risk of atrial fibrillation (AF). However, the specific AF characteristics that travel with a family history have not yet been elucidated. The purpose of this study was to determine whether a family history of AF is associated with specific patient characteristics in a worldwide, remote cohort.
Methods
From the Health eHeart Study, an internet-based prospective cohort, we performed a cross-sectional analysis of AF participants who reported their family history and completed questionnaires regarding their medical conditions and AF symptoms. We assessed demographics, cardiovascular comorbidities, and AF symptom characteristics in AF participants with and without a family history of AF.
Results
In multivariable analysis of 5,884 participants with AF (mean age 59.9 ± 14.5, 59% male, 92% white), female sex (odds ratio [OR]=1.35, 95% CI, 1.17-1.54, p<0.0001) and birth in the U.S. (OR=2.54, 95% CI, 2.12-3.05, p<0.0001) were independently associated with having a family history of AF. Having a family history of AF was also more commonly associated with symptoms of shortness of breath (OR=1.40, 95% CI, 1.07-1.82, p=0.014), chest pain, pressure, or discomfort (OR=1.95, 95% CI, 1.22-3.13, p=0.0052), and feeling generally “off” about oneself (OR=1.84, 95% CI, 1.27-2.67, p=0.0013).
Conclusions
Patients with a family history of AF are more likely to be female, be US-born, and experience symptoms of AF, suggesting underlying mechanistic differences between those with and without family history of AF.
Keywords: Atrial fibrillation; Family history; Genetics, Heritability; Phenotype
Introduction
Atrial fibrillation (AF) is the most common arrhythmia, affecting millions of Americans and rapidly increasing in both incidence and prevalence [1-4]. AF doubles mortality and is a common cause of stroke [1,2]. Though the mechanisms underlying AF remain largely unknown, established risk factors, such as age, male sex, white race, hypertension, and other comorbidities, have been identified [5,6]. A family history of AF has similarly emerged as a well-established risk factor for the disease [5-9]. Several common genetic variants have been associated with an increased susceptibility to AF [8,10,11], but the mechanisms underlying those associations remain unclear. One previous registry-based study in the US suggested that patients with a family history of AF develop the disease at a younger age, have less comorbidities, and are more symptomatic [12], but no additional studies have examined these relationships. We therefore sought to compare the characteristics of AF patients with and without a family history of the disease in a worldwide, remote cohort.
Methods
Study design
We utilized data collected between March 8, 2013 and October 25, 2017 from the Health eHeart Study (www.health-eheartstudy.org), an online-based prospective, longitudinal cohort study. English-speaking adults each with an active email were recruited through academic institutions, lay press, social media and promotional events. Upon enrollment, all participants provided informed consent electronically and were asked to complete a series of online questionnaires regarding demographics, personal and family medical history, habits, symptoms, and quality of life [Supplementary Table 1]. The Health eHeart Study was approved by the UCSF Institutional Review Board.
Supplementary Table 1. Online questionnairesfrom the Health eHeart Study.
| Basic demographics | |
|---|---|
| 1. What is your biological sex? | o Male o Female |
| 2. Where were you born (country)? | o U.S.A. o Mexico o China o India o Philippines o Other country |
| 3. What is your racial background? Check all that apply. | o Black or African American o White o Asian (including South Asian and Asian Indian) o Native Hawaiian or Pacific Islander o American Indian or Alaska Native o Some other race o Don’t know |
| 4. Are you of Hispanic, Latino or Spanish origin or ancestry? | o No o Yes, Mexican, Mexican American or Chicano o Yes, Puerto Rican o Yes, Cuban o Yes, Other or Mixed Hispanic, Latino or Spanish origin o Don’t know |
| Medical history | |
| 1. Hypertension | o Yes o No o Don’t know |
| 2. Diabetes? Do not include pre-diabetes. | o Yes o No o Don’t know |
| 3. Coronary artery disease (blockages in your heart vessels) or angina (chest pain)? | o Yes o No o Don’t know |
| 4. A heart attack? | o Yes o No o Don’t know |
| 5. Congestive Heart Failure (CHF, Heart Failure)? | o Yes o No o Don’t know |
| 6. Stroke or TIA (Transient Ischemic Attack or Mini-Stroke)? | o Yes o No o Don’t know |
| 7. Do you or have you ever had a congenital heart disease (a heart birth defect)? | o Yes o No o Don’t know |
| 8. Sleep apnea (obstructive sleep apnea, OSA)? | o Yes o No o Don’t know |
| 9.COPD (emphysema, chronic bronchitis, obstructive pulmonary disease)? | o Yes o No o Don’t know |
| 10. Asthma, to the point that you use inhalers daily or have been to the hospital for your asthma | o Yes o No o Don’t know |
| 11. A cardiac arrest? | o Yes o No o Don’t know |
| 12. Do you have an implanted device for your heart? If you have one, you were given a card which has this information on it. | o No o Pacemaker (not an ICD) o ICD (Implantable Cardioverter-Defibrillator) o Implanted Loop Recorder or rhythm monitor (e.g., Reveal, Confirm) o Other |
| Smoking history | |
| 1. Have you ever smoked cigarettes regularly (at least 1 cigarette per day and a total of 100 cigarettes in your lifetime)? | o Yes o No |
| 2. Do you smoke now? | o Daily o Some days o No |
| Alcohol history | |
| 1. Did you drink any alcoholic beverages in the past year? | o No o Yes o Don’t know o I refuse to answer |
| 2. Did you drink alcohol more than once or twice in the past? | o No o Yes o Don’t know o I refuse to answer |
| 3. How many drinks of wine do you usually have per week? A drink is a 5-ounce glass. Round down. | _____ drinks per week |
| 4. How many drinks of beer do you usually have per week? One beer is a 12-ounce glass, can, or bottle. Round down. | _____ drinks per week |
| 5. How many drinks per week do you usually have of hard liquor? Count each shot, which is 1 ½ ounces, as one drink. Round down | _____ drinks per week |
| 6. During the past 24 hours, how many drinks have you had? | _____ drinks per week |
| 7. Approximately how many years ago did you stop drinking? Round do the nearest year except round ½ down; e.g., record 1 ½ as 1). | _____ years |
| 8. What was the usual number of drinks you consumed per week before you stopped? Write in 00 if less than one drink per week. | _____ drinks per week |
| Atrial fibrillation history | |
| 1. Did you have any symptoms (such as palpitations, dizziness, shortness of breath, chest discomfort, difficulty exercising, or generalized ‘feeling bad’) when you were first diagnosed (or prior to)? | o Yes o No o Don’t know |
| 2. Are you in atrial fibrillation all the time? | o Yes o No. It comes and goes on its own o No. It has stopped because of a shock to your heart or because of a medication o Don’t know |
| 3. Have you ever had a shock to your check or cardioversion? | o Yes o No o Don’t know |
| 4. Have you ever had an ablation for your atrial fibrillation? | o Yes o No |
| 5. What symptoms do you have when you have atrial fibrillation? It’s OK if you only experience these symptoms sometimes. Check all that apply. | o I never have symptoms o Palpitations or irregular or “funny” heartbeats o Shortness of breath of difficulty breathing o Difficulty exercising or exerting o Chest pain, pressure, or discomfort o Dizziness o Feeling generally tired o Feeling generally “off” your normal self o Other o Don’t know |
Assessment of atrial fibrillation and family history
AF was determined by responses to the question, “Have you ever been told by a doctor or nurse that you have, or have been treated for, atrial fibrillation (in the past or currently)?” with response options “Yes”, “No” and “Don’t know.” We included only participants who responded “yes” and treated those who responded as “Don’t know” as missing. This approach was previously validated using medical record data among 42 patients [13]. To identify those with any family history of AF, we included participants who reported any family member (either immediate or extended) with AF. If participants were unsure, the answer was considered negative. Participants were considered to have a first-degree family history of AF if they self-reported at least one biological sister, brother, father, or mother with AF.
Covariate ascertainment
Self-identified race was categorized as white, black, Asian, Native Hawaiian/Pacific Islander, American Indian, or other. Hispanic ethnicity was also assessed. Smoking status was ascertained as never, past, or current smoker, with regular use defined as at least 1 cigarette per day or a total of 100 cigarettes in one’s lifetime. Alcohol use was assessed through self-report of consumption over the past year and number of drinks a week. Medical history was determined by participant report that they had specifically received a diagnosis of one of the following from a healthcare professional [Supplementary Table 1]: hypertension, diabetes, coronary artery disease, heart attack, congestive heart failure, cerebrovascular accident (stroke or transient ischemia attack), congenital heart disease, and obstructive sleep apnea. Participants with AF were also asked specific questions regarding their AF history and associated symptoms.
Statistical analysis
Normally distributed continuous variables are presented as means ± SD and were compared using unpaired t-tests. Non-normally distributed continuous variables are presented as medians and interquartile ranges and were compared using Wilcoxon rank-sum tests. Categorical variables were compared using χ2 tests. Multivariable analysis was performed with logistic regression analysis, including only co-variates that exhibited p values < 0.05 in unadjusted analyses. We first performed an analysis to assess relationships between demographics, medical comorbidities, habits and a family history of AF; we then analyzed relationships between a family history and characteristics of the participant’s AF itself (such as AF type and associated symptoms) after adjusting for relevant demographics, medical conditions and habits. All analyses were performed using SAS Version 9.4. Two-tailed p values < 0.05 were considered statistically significant.
Results
Any family history of atrial fibrillation
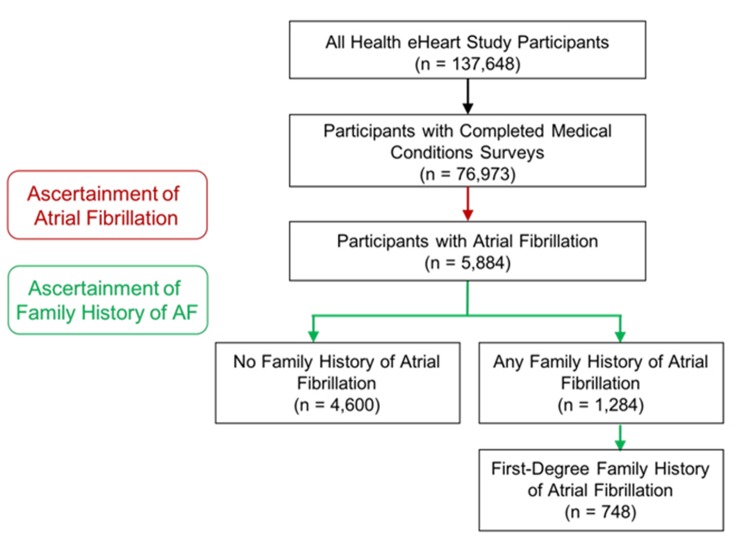
At the time of study analysis, 76,973 of 137,648 Health eHeart participants (49.4%) had completed the survey for medical conditions. Of those, 5,884 (7.6%) reported a diagnosis of AF. Of those with AF, 1,284 (21.8%) had a family history of AF [Figure 1] and [Supplementary Figure 1]. [Table 1] shows the baseline characteristics among those with and without a family history of AF. Those with a family history of AF tended to be older, female, more often from the US, and less often with a history of coronary artery disease or a history of a cerebrovascular accident [Table 1]. In addition, those with a family history were more likely to experience symptomatic AF when they were first diagnosed and continued to manifest more symptoms of AF than AF patients without a family history.
Figure 1. Geographical distribution of Health eHeart participants with atrial fibrillation.
Each dot represents at least one participant in a given zipcode. Blue dots indicate those with a family history of atrial fibrillation, while orange dots indicate those without a family history of atrial fibrillation.
Supplementary Figure 1. Health eHeart Study enrollment of atrial fibrillation participants with and without family history of atrial fibrillation.
Table 1. Baseline characteristics of atrial fibrillation participants with and without any family history of the disease.
| No Family History of AF (n = 4600) | Family History of AF (n = 1284) | p-value | |
|---|---|---|---|
| Basic demographics | |||
| Age, mean ± SD, years | 56.9 ± 15.4 | 60.4 ± 11.2 | <0.0001 |
| Sex | <0.0001 | ||
| Male | 2009 (61%) | 647 (52%) | |
| Female | 1269 (39%) | 607 (48%) | |
| Country of birth | <0.0001 | ||
| USA | 2336 (71%) | 1085 (87%) | |
| Other | 939 (29%) | 169 (13%) | |
| Race/Ethnicity, n (%) | 0.17 | ||
| Black | 50 (1%) | 20 (2%) | |
| White | 2998 (92%) | 1144 (91%) | |
| Asian | 89 (3%) | 29 (2%) | |
| Native Hawaiian | 4 (0.1%) | 0 (0%) | |
| American Indian | 8 (0.2%) | 9 (0.7%) | |
| Other | 51 (2%) | 15 (1%) | |
| Don't know | 3 (0.09%) | 1 (0.08%) | |
| Hispanic (ethnicity) | 170 (5%) | 50 (4%) | 0.09 |
| Medical history | |||
| Hypertension | 2357 (51%) | 676 (53%) | 0.38 |
| Diabetes | 589 (13%) | 155 (12%) | 0.48 |
| Coronary artery disease | 1029 (22%) | 245 (19%) | 0.011 |
| Heart attack | 583 (13%) | 151 (12%) | 0.38 |
| Congestive heart failure | 734 (16%) | 187 (15%) | 0.22 |
| Stroke or TIA | 533 (12%) | 120 (9%) | 0.023 |
| Congenital heart disease | 433 (9%) | 102 (8%) | 0.10 |
| Obstructive sleep apnea | 1238 (27%) | 346 (27%) | 0.85 |
| COPD | 354 (8%) | 104 (8%) | 0.70 |
| Asthma | 544 (12%) | 155 (12%) | 0.91 |
| Cardiac arrest | 316 (7%) | 81 (6%) | 0.43 |
| Implantable device | 3802 (84%) | 1088 (85%) | 0.33 |
| Smoking history | |||
| History of smoking regularly | 910 (53%) | 707 (56%) | 0.05 |
| Current smoker | 63 (4%) | 46 (4%) | 0.99 |
| Alcohol Use | |||
| Did you drink alcoholic beverages in the past year? | 1334 (77%) | 971 (77%) | 0.93 |
| Did you drink alcohol more than once or twice in the past? | 248 (63%) | 186 (66%) | 0.54 |
| Drinks of wine/week | 4.2 ± 28.1 | 3.4 ± 5.9 | 0.38 |
| Drinks of beer/week | 1.9 ± 9.5 | 1.3 ± 3.4 | 0.061 |
| Drinks of hard liquor/week | 1.4 ± 4.2 | 1.4 ± 4.4 | 0.098 |
| Drinks in the past 24 hours | 0.9 ± 2.6 | 0.9 ± 1.7 | 0.071 |
| Approximately how many years ago did you stop drinking? | 56.3 ± 289.4 | 46.2 ± 243.5 | 0.70 |
| What is the usual number of drinks you consumed per week before you stopped? | 13.3 ± 35.9 | 10.2 ± 19.6 | 0.29 |
| Atrial fibrillation history | |||
| Symptoms when first diagnosed? | 3092 (76%) | 1005 (80%) | 0.006 |
| Paroxysmal AF | 1953 (48%) | 627 (50%) | 0.29 |
| Hx of cardioversion | 1286 (32%) | 394 (31%) | 0.80 |
| Hx of AF ablation | 976 (24%) | 330 (26%) | 0.12 |
| Atrial fibrillation symptoms (check all that apply) | |||
| Never have symptoms | 540 (12%) | 138 (11%) | 0.33 |
| Palpitations | 2675 (58%) | 807 (63%) | 0.0025 |
| SOB | 364 (8%) | 152 (12%) | <0.0001 |
| Difficulty exercising | 94 (2%) | 25 (2%) | 0.83 |
| Chest pain/pressure/discomfort | 78 (2%) | 36 (3%) | 0.01 |
| Dizziness | 85 (2%) | 16 (1%) | 0.14 |
| Feeling generally tired | 69 (2%) | 21 (2%) | 0.73 |
| Feeling generally "off" your normal self | 117 (3%) | 57 (4%) | 0.0004 |
| Other | 45 (1%) | 12 (1%) | 0.89 |
| Don't know | 540 (12%) | 139 (11%) | 0.14 |
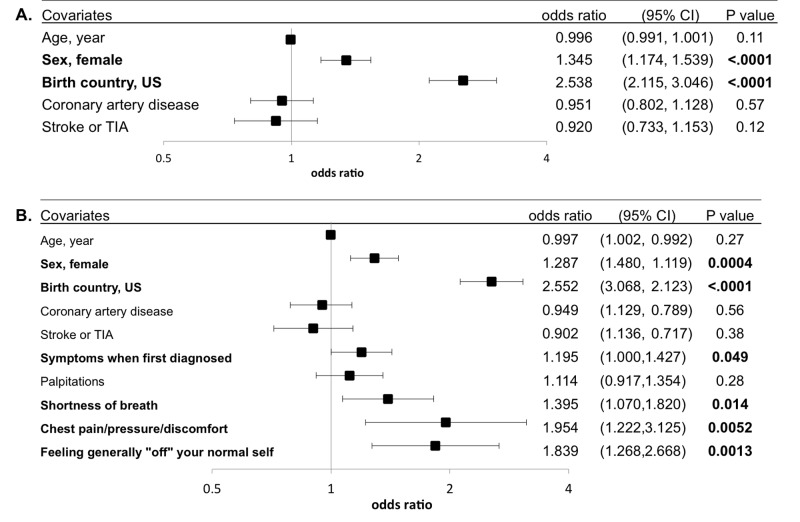
In a multivariable adjusted analysis including relevant demographics, past medical history and habits, those with a family history of AF had a statistically significant 35% greater odds of being female and also had more than 2-fold greater odds of being born in the US [Figure 2]. After including AF-related history and symptoms that met criteria for inclusion in the multivariate model, being female and being born in the US remained significantly associated with a family history of AF [Figure 2]. In addition, AF patients with a family history of AF were more likely to report AF-related shortness of breath, chest pain, pressure, or discomfort, or feeling “off” about one’s normal self after adjusting for baseline characteristics [Figure 2].
Figure 2. Multivariable adjusted relationships between participant characteristics and any family history of AF.
(A) Adjusted for relevant demographics, medical history and habits. (B) Additionally adjusted for relevant AF- and symptom-related history. Models were adjusted for all covariates listed. Statistically significant relationships are in bold. Y error bars denote 95% confidence intervals.
First-degree family history of atrial fibrillation
Of those with AF, 768 (13.7%) had at least one first degree family member with AF. Baseline characteristics of those with and without first-degree family history are reported in [Table 2]. Those with a first-degree family history of AF were more likely to be older, female, and from the US, but less likely to be of Hispanic ethnicity and have diabetes, coronary artery disease, and congenital heart disease [Table 2]. Though there was no significant differences in having paroxysmal AF or history of cardioversion, those with a first-degree family history of AF were more likely to have had an AF ablation. As with those with any family history of AF, those with a first degree family history were more likely to experience a variety of symptoms during their AF episodes [Table 2].
Table 2. Baseline characteristics of atrial fibrillation participants with and without a first-degree family history of the disease.
| No First-Degree Family History of AF (n = 5136) | First-Degree Family History of AF (n = 748) | p-value | |
|---|---|---|---|
| Basic demographics | |||
| Age, mean ± SD, years | 57.0 ± 15.5 | 58.7 ± 12.8 | 0.0003 |
| Sex | 0.004 | ||
| Male | 2266 (60%) | 390 (54%) | |
| Female | 1540 (40%) | 336 (46%) | |
| Country of birth | <0.0001 | ||
| USA | 2801 (74%) | 620 (85%) | |
| Other | 1002 (26%) | 106 (15%) | |
| Race/Ethnicity, n (%) | 0.076 | ||
| Black | 64 (2%) | 6 (0.83%) | |
| White | 3458 (91%) | 684 (94.34%) | |
| Asian | 103 (3%) | 15 (2.07%) | |
| Native Hawaiian | 4 (0.1%) | 0 (0.00%) | |
| American Indian | 17 (0.5%) | 0 (0.00%) | |
| Other Don't know | 61 (2%) 3 (0.08%) | 5 (0.69%) 1 (0.1%) | |
| Hispanic (ethnicity) | 198 (5%) | 22 (3%) | 0.012 |
| Medical history | |||
| Hypertension | 2657 (52%) | 376 (50%) | 0.45 |
| Diabetes | 667 (13%) | 77 (10%) | 0.038 |
| Coronary artery disease | 1148 (22%) | 126 (17%) | 0.0006 |
| Heart attack | 662 (13%) | 72 (10%) | 0.011 |
| Congestive heart failure | 818 (16%) | 103 (14%) | 0.13 |
| Stroke or TIA | 583 (11%) | 70 (9%) | 0.10 |
| Congenital heart disease | 488 (10%) | 47 (6%) | 0.0041 |
| Obstructive sleep apnea | 1376 (27%) | 208 (28%) | 0.68 |
| COPD | 398 (8%) | 60 (8%) | 0.85 |
| Asthma | 617 (12%) | 82 (11%) | 0.36 |
| Cardiac arrest | 357 (7%) | 40 (5%) | 0.090 |
| Implantable device | 832 (16%) | 105 (14%) | 0.10 |
| Smoking history | |||
| History of smoking regularly | 1047 (46%) | 324 (44%) | 0.35 |
| Current smoker | 86 (4%) | 23 (3%) | 0.41 |
| Alcohol Use | |||
| Did you drink alcoholic beverages in the past year? | 1732 (77%) | 573 (79%) | 0.29 |
| Did you drink alcohol more than once or twice in the past? | 336 (65%) | 98 (64%) | 0.82 |
| Drinks of wine/week | 4.0 ± 24.9 | 3.6 ± 5.3 | 0.76 |
| Drinks of beer/week | 1.7 ± 8.1 | 1.5 ± 5.6 | 0.76 |
| Drinks of hard liquor/week | 1.4 ± 4.0 | 1.4 ± 5.0 | 0.77 |
| During past 24 hours, how many drinks? | 0.8 ± 2.4 | 1.0 ± 1.5 | 0.25 |
| Approximately how many years ago did you stop drinking? | 57.7 ± 291.4 | 32.5 ± 181.1 | 0.42 |
| What is the usual number of drinks you consumed per week before you stopped? | 12.4 ± 32.5 | 10.4 ± 19.2 | 0.55 |
| Atrial fibrillation history | |||
| Symptoms when first diagnosed? | 3517 (75%) | 580 (78%) | 0.20 |
| Paroxysmal AF | 2215 (48%) | 365 (49%) | 0.53 |
| Hx of cardioversion | 1448 (31%) | 232 (31%) | 0.96 |
| Hx of AF ablation | 1098 (24%) | 208 (28%) | 0.012 |
| Atrial fibrillation symptoms (check all that apply) | |||
| Never have symptoms | 610 (12%) | 68 (0.09%) | 0.026 |
| Palpitations | 3008 (59%) | 474 (63%) | 0.012 |
| SOB | 432 (8%) | 84 (11%) | 0.011 |
| Difficulty exercising | 103 (2%) | 16 (2%) | 0.81 |
| Chest pain/pressure/discomfort | 89 (2%) | 25 (3%) | 0.0029 |
| Dizziness | 93 (2%) | 8 (1%) | 0.15 |
| Feeling generally tired | 79 (2%) | 11 (1%) | 0.89 |
| Feeling generally "off" your normal self | 133 (3%) | 41 (5%) | <0.0001 |
| Other | 45 (1%) | 12 (2%) | 0.58 |
| Don't know | 610 (12%) | 68 (9%) | 0.026 |
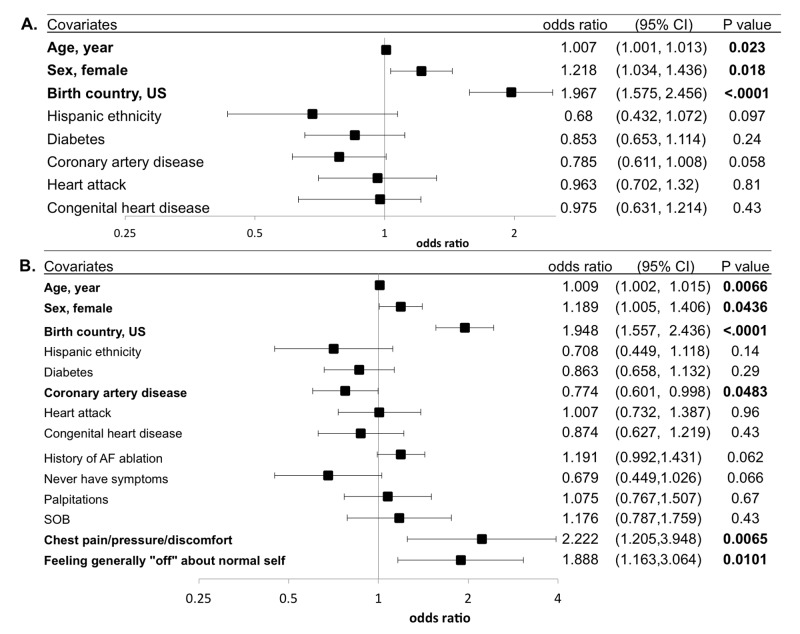
In a multivariable adjusted analysis including demographics, medical history and habits, older age, female sex, and being born in the US were each significantly associated with having a first-degree family history of AF [Figure 3]. When AF characteristics (including AF type, AF-related history, and AF-related symptoms) were also added to the multivariable model, having a first-degree family history of AF was significantly associated with reporting symptoms of chest pain, pressure, or discomfort and feeling generally “off” about oneself during AF episodes [Figure 3].
Figure 3. Multivariable adjusted relationships between participant characteristics and a first-degree family history of AF.
(A) Adjusted for relevant demographics, medical history and habits. (B) Additionally adjusted for relevant AF- and symptom-related history. Models were adjusted for all covariates listed. Statistically significant relationships are in bold. Y error bars denote 95% confidence intervals.
Discussion
Among a large, remote cohort of AF patients, a family history of AF was more commonly observed in women and those born in the US. Those with a family history of AF exhibited more symptomatic AF. Our study validates the results of a previous registry-based study that females and those with more symptoms during AF are more likely to report a family history of the disease [12], extending those findings to a worldwide cohort.
The reasons for the consistent relationship between female sex and a family history of AF are unclear. This would appear to run contrary to the consistent observation that women are at a lower risk for AF than men [7,16,17]. Of note, the mechanisms underlying that difference have not been fully elucidated, may be multifactorial, and may be related to differences in body (and left atrial) size and or hormonal influences [18-20]. It is important to acknowledge that women may simply be more likely to report a family history of AF (even in the absence of an actual greater prevalence of a family history) because they are more attune to their family members’ health history [21]. This itself may yet be clinically relevant information when considering the reliability of the family history from men versus women. Assuming there is truly a relationship between female sex and a family history of AF, these findings may point to some sex-related mechanisms that affect the penetrance of AF-related genes. In light of the overall greater prevalence of AF among men, such a finding would also suggest that the sex-specific differences influencing AF risk would be potent enough to otherwise suppress the emergence of AF in the general population of women.
In our international cohort, we were able to demonstrate that US-born participants were more likely to report an AF family history. Again, it is difficult to know whether this has more to do with the awareness of health problems and AF among American families versus a “true” phenomenon. It is possible that there are some genetic differences that render certain populations more prone to AF among those more likely to migrate to the US. There may also be some gene-environment interactions that are disproportionally influenced by some particular exposure in the US.
It is well known that AF patients can experience a variety of sensations during their episodes, ranging from completely asymptomatic to suffering debilitating symptoms [22]. While some of this variability is likely related to ventricular rates and differences in AV nodal conduction properties, the reasons some individuals are more or less symptomatic remain largely unknown. In addition to hemodynamic effects, there are likely neurologic and psychological components related to sensitivity to changes in heart rate and rhythm and reactions to stress [23]. The relationship between having a family history of AF and having more symptomatic AF was very consistent in our cohort, both before and after adjustment for potential confounders and mediators. Those with a family history more commonly described shortness of breath, chest pain, pressure, or discomfort, and feeling “off” during their AF episodes. A possible explanation is that those who tend to be more symptomatic will seek out more family members with AF. Interestingly, it is also possible that having symptomatic AF itself is an inherited characteristic, which would certainly lend itself to becoming more apparent among family members. Inherited AF tends to be more dominant in otherwise healthier and younger individuals with the disease [9,12,24], who are more likely to have robust AV nodal conduction and thus more likely experience symptoms from rapid ventricular rates. While we demonstrated that older age was associated with having a first-degree family history, we were not able to determine the age of diagnosis with our database. Previous studies have reported that earlier diagnosis of AF in patients and their first-degree relatives is associated with higher risk of AF [5-7]. Finally, previous studies have suggested that women tend to experience more AF-related symptoms and worse quality-of-life than men [25-27]. As the relationship between female sex and a family history of AF as well as between symptoms and a family history of AF remained statistically significant after each was adjusted for the other, those previous studies may reveal a heritable AF subtype relevant to both relationships.
Our study has several potential limitations. As eluded to above, these data were based on self-report. However, as also mentioned, even if this explains the results observed, there may be clinically relevant lessons that can be gleaned from the data. We previously validated the accuracy of an AF diagnosis in the Health eHeart Study and found it to be very accurate among a small number of patients with available medical records.[15] In addition, for any misclassification of AF to be important, there would need to be a differential effect by predictor (such as family history of AF) for results to be affected. Although the mean age of our study cohort was 60 and more than 10% were of some race/ethnicity other than non-Hispanic white, Health eHeart Study participants are not completely representative of the general population (particularly as they require some ability to interact on the internet). However, this should limit generalizability of our findings rather than internal validity. We acknowledge that “any family history” is both broad and potentially vague, but our analyses restricted to just a first degree family history did not yield meaningfully different results. Finally, it is possible that we were not aware of or did not include other covariates that may have been important.
Conclusion
Among individuals with AF, a family history of the disease is more common in women, those born in the US, and those with symptomatic AF. These differences may help in understanding mechanisms underlying AF when a family history of the disease is present and may suggest that symptomatic AF reflects a particular biological subtype.
References
- 1.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur. Heart J. 2006 Aug;27 (16):1979–2030. doi: 10.1093/eurheartj/ehl176. [DOI] [PubMed] [Google Scholar]
- 2.Chugh S S, Blackshear J L, Shen W K, Hammill S C, Gersh B J. Epidemiology and natural history of atrial fibrillation: clinical implications. J. Am. Coll. Cardiol. 2001 Feb;37 (2):371–8. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 3.Zulkifly Hanis, Lip Gregory Y H, Lane Deirdre A. Epidemiology of atrial fibrillation. Int. J. Clin. Pract. 2018 Mar;72 (3) doi: 10.1111/ijcp.13070. [DOI] [PubMed] [Google Scholar]
- 4.Naccarelli Gerald V, Varker Helen, Lin Jay, Schulman Kathy L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 2009 Dec 01;104 (11):1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin E J, Levy D, Vaziri S M, D'Agostino R B, Belanger A J, Wolf P A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994 Mar 16;271 (11):840–4. [PubMed] [Google Scholar]
- 6.Benjamin Emelia J, Chen Peng-Sheng, Bild Diane E, Mascette Alice M, Albert Christine M, Alonso Alvaro, Calkins Hugh, Connolly Stuart J, Curtis Anne B, Darbar Dawood, Ellinor Patrick T, Go Alan S, Goldschlager Nora F, Heckbert Susan R, Jalife José, Kerr Charles R, Levy Daniel, Lloyd-Jones Donald M, Massie Barry M, Nattel Stanley, Olgin Jeffrey E, Packer Douglas L, Po Sunny S, Tsang Teresa S M, Van Wagoner David R, Waldo Albert L, Wyse D George. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009 Feb 03;119 (4):606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox Caroline S, Parise Helen, D'Agostino Ralph B, Lloyd-Jones Donald M, Vasan Ramachandran S, Wang Thomas J, Levy Daniel, Wolf Philip A, Benjamin Emelia J. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004 Jun 16;291 (23):2851–5. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 8.Lubitz Steven A, Yin Xiaoyan, Fontes João D, Magnani Jared W, Rienstra Michiel, Pai Manju, Villalon Mark L, Vasan Ramachandran S, Pencina Michael J, Levy Daniel, Larson Martin G, Ellinor Patrick T, Benjamin Emelia J. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010 Nov 24;304 (20):2263–9. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyen Nina, Ranthe Mattis F, Carstensen Lisbeth, Boyd Heather A, Olesen Morten S, Olesen Søren-Peter, Wohlfahrt Jan, Melbye Mads. Familial aggregation of lone atrial fibrillation in young persons. J. Am. Coll. Cardiol. 2012 Sep 04;60 (10):917–21. doi: 10.1016/j.jacc.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Ellinor Patrick T, Shin Jordan T, Moore Rachel K, Yoerger Danita M, MacRae Calum A. Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation. 2003 Jun 17;107 (23):2880–3. doi: 10.1161/01.CIR.0000077910.80718.49. [DOI] [PubMed] [Google Scholar]
- 11.Christophersen Ingrid Elisabeth, Ravn Lasse Steen, Budtz-Joergensen Esben, Skytthe Axel, Haunsoe Stig, Svendsen Jesper Hastrup, Christensen Kaare. Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrhythm Electrophysiol. 2009 Aug;2 (4):378–83. doi: 10.1161/CIRCEP.108.786665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudbjartsson Daniel F, Arnar David O, Helgadottir Anna, Gretarsdottir Solveig, Holm Hilma, Sigurdsson Asgeir, Jonasdottir Adalbjorg, Baker Adam, Thorleifsson Gudmar, Kristjansson Kristleifur, Palsson Arnar, Blondal Thorarinn, Sulem Patrick, Backman Valgerdur M, Hardarson Gudmundur A, Palsdottir Ebba, Helgason Agnar, Sigurjonsdottir Runa, Sverrisson Jon T, Kostulas Konstantinos, Ng Maggie C Y, Baum Larry, So Wing Yee, Wong Ka Sing, Chan Juliana C N, Furie Karen L, Greenberg Steven M, Sale Michelle, Kelly Peter, MacRae Calum A, Smith Eric E, Rosand Jonathan, Hillert Jan, Ma Ronald C W, Ellinor Patrick T, Thorgeirsson Gudmundur, Gulcher Jeffrey R, Kong Augustine, Thorsteinsdottir Unnur, Stefansson Kari. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007 Jul 19;448 (7151):353–7. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 13.Firouzi Mehran, Ramanna Hemanth, Kok Bart, Jongsma Habo J, Koeleman Bobby P C, Doevendans Pieter A, Groenewegen W Antoinette, Hauer Richard N W. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ. Res. 2004 Aug 20;95 (4):e29–33. doi: 10.1161/01.RES.0000141134.64811.0a. [DOI] [PubMed] [Google Scholar]
- 14.Gundlund Anna, Fosbøl Emil Loldrup, Kim Sunghee, Fonarow Gregg C, Gersh Bernard J, Kowey Peter R, Hylek Elaine, Mahaffey Kenneth W, Thomas Laine, Piccini Jonathan P, Peterson Eric D. Family history of atrial fibrillation is associated with earlier-onset and more symptomatic atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am. Heart J. 2016 May;175 ():28–35. doi: 10.1016/j.ahj.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Dixit Shalini, Pletcher Mark J, Vittinghoff Eric, Imburgia Kourtney, Maguire Carol, Whitman Isaac R, Glantz Stanton A, Olgin Jeffrey E, Marcus Gregory M. Secondhand smoke and atrial fibrillation: Data from the Health eHeart Study. Heart Rhythm. 2016 Jan;13 (1):3–9. doi: 10.1016/j.hrthm.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd-Jones Donald M, Wang Thomas J, Leip Eric P, Larson Martin G, Levy Daniel, Vasan Ramachandran S, D'Agostino Ralph B, Massaro Joseph M, Beiser Alexa, Wolf Philip A, Benjamin Emelia J. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004 Aug 31;110 (9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 17.Heeringa Jan, van der Kuip Deirdre A M, Hofman Albert, Kors Jan A, van Herpen Gerard, Stricker Bruno H Ch, Stijnen Theo, Lip Gregory Y H, Witteman Jacqueline C M. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur. Heart J. 2006 Apr;27 (8):949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 18.McManus David D, Yin Xiaoyan, Gladstone Rachel, Vittinghoff Eric, Vasan Ramachandran S, Larson Martin G, Benjamin Emelia J, Marcus Gregory M. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc. 2016 Sep 14;5 (9) doi: 10.1161/JAHA.116.004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus Gregory M, Yang Yanfei, Varosy Paul D, Ordovas Karen, Tseng Zian H, Badhwar Nitish, Lee Byron K, Lee Randall J, Scheinman Melvin M, Olgin Jeffrey E. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm. 2007 Feb;4 (2):138–44. doi: 10.1016/j.hrthm.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seko Yuta, Kato Takao, Haruna Tetsuya, Izumi Toshiaki, Miyamoto Shoichi, Nakane Eisaku, Inoko Moriaki. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci Rep. 2018 Apr 23;8 (1) doi: 10.1038/s41598-018-24875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma Nidhi, Chakrabarti Subho, Grover Sandeep. Gender differences in caregiving among family - caregivers of people with mental illnesses. World J Psychiatry. 2016 Mar 22;6 (1):7–17. doi: 10.5498/wjp.v6.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rienstra Michiel, Lubitz Steven A, Mahida Saagar, Magnani Jared W, Fontes João D, Sinner Moritz F, Van Gelder Isabelle C, Ellinor Patrick T, Benjamin Emelia J. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation. 2012 Jun 12;125 (23):2933–43. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sears Samuel F, Serber Eva R, Alvarez Luis G, Schwartzman David S, Hoyt Robert H, Ujhelyi Michael R. Understanding atrial symptom reports: objective versus subjective predictors. Pacing Clin Electrophysiol. 2005 Aug;28 (8):801–7. doi: 10.1111/j.1540-8159.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 24.Kääb Stefan, Darbar Dawood, van Noord Charlotte, Dupuis Josée, Pfeufer Arne, Newton-Cheh Christopher, Schnabel Renate, Makino Seiko, Sinner Moritz F, Kannankeril Prince J, Beckmann Britt M, Choudry Subbarao, Donahue Brian S, Heeringa Jan, Perz Siegfried, Lunetta Kathryn L, Larson Martin G, Levy Daniel, MacRae Calum A, Ruskin Jeremy N, Wacker Annette, Schömig Albert, Wichmann H-Erich, Steinbeck Gerhard, Meitinger Thomas, Uitterlinden André G, Witteman Jacqueline C M, Roden Dan M, Benjamin Emelia J, Ellinor Patrick T. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur. Heart J. 2009 Apr;30 (7):813–9. doi: 10.1093/eurheartj/ehn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zöller Bengt, Ohlsson Henrik, Sundquist Jan, Sundquist Kristina. High familial risk of atrial fibrillation/atrial flutter in multiplex families: a nationwide family study in Sweden. J Am Heart Assoc. 2012 Dec 31;2 (1) doi: 10.1161/JAHA.112.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball Jocasta, Carrington Melinda J, Wood Kathryn A, Stewart Simon. Women versus men with chronic atrial fibrillation: insights from the Standard versus Atrial Fibrillation spEcific managemenT studY (SAFETY). PLoS ONE. 2013;8 (5) doi: 10.1371/journal.pone.0065795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheuermeyer Frank Xavier, Mackay Martha, Christenson Jim, Grafstein Eric, Pourvali Reza, Heslop Claire, MacPhee Jan, Ward John, Heilbron Brett, McGrath Lorraine, Humphries Karin. There Are Sex Differences in the Demographics and Risk Profiles of Emergency Department (ED) Patients With Atrial Fibrillation and Flutter, but no Apparent Differences in ED Management or Outcomes. Acad Emerg Med. 2015 Sep;22 (9):1067–75. doi: 10.1111/acem.12750. [DOI] [PubMed] [Google Scholar]