Abstract
Background Endoscopic mucosal resection (EMR) with snare is the recommended technique to resect non-invasive colorectal neoplastic lesions between 10 and 30 mm in diameter. The objective of EMR is to resect completely the neoplastic tissue en bloc and preferably with free margins (R0), avoiding recurrences. Anchoring the tip of the snare in the submucosa is a technical trick that allows snare sliding to be reduced and larger pieces to be caught. The aim of the present study was to evaluate the effectiveness and safety of anchoring-EMR (A-EMR).
Methods This was a retrospective analysis of A-EMR procedures for lesions of diameter between 10 and 30 mm (endoscopic evaluation) performed consecutively in four French centers between May 2017 and January 2018. A-EMR was routinely performed for all EMR using Olympus conventional snares (10 or 25 mm). The primary outcome was evaluation of the proportion of R0 resections.
Results A total of 141 A-EMR procedures were performed by 10 operators. Mean lesion size was 19.8 mm. Anchoring was feasible in 96.5 % of cases. There were 81.6 % en bloc resections and 70.2 % R0 resections, with the percentage of procedures decreasing with increasing lesion size (82.8 % < 20 mm, 55.3 % 21 – 30 mm, and 50.0 % > 30 mm, P = 0.002). Complete perforations closed endoscopically occurred in 3/141 cases (2.1 %); none occurred in lesions < 20 mm in size (0 /87).
Conclusion The A-EMR technique appears to be promising with a high proportion of R0 for lesions of 10 – 20 mm in size without any perforations. It could also offer an alternative to endoscopic submucosal dissection (ESD), or to hybrid techniques to reach R0 for lesions between 20 and 30 mm in size.
Introduction
The quality of endoscopic resections has become a focal point in endoscopy research where the target is techniques with higher rates of complete resection for small polyps (cold snare for lesions < 10 mm in diameter) or large lesions (endoscopic submucosal dissection (ESD), for large lesions > 30 mm in size) 1 2 . Endoscopic mucosal resection (EMR) with snare is the technique recommended to resect non-invasive colorectal neoplastic lesions (10 – 30 mm) effectively and with low morbidity 1 . Nevertheless, such resections are only curative when the lesion is resected in a single specimen (en bloc) with free margins (R0 resection). In the case of piecemeal resection (more than one piece) or in the case of incomplete en bloc resection (undetermined – Rx, or invaded margins – R1), the risk of local recurrence increases and a second colonoscopy is recommended within 6 months to detect and resect potential local recurrence. Thus, improving the quality of EMR to increase the rate of R0 resection and to reduce the need for follow-up procedures should become a target of research. It has been reported that conventional EMR is associated with 62 – 65 % en bloc resection for lesions < 20 mm in diameter but R0 resection has never been evaluated precisely 1 3 4 .
We previously described the anchoring EMR (A-EMR) technique 5 which consists of creating a small hole in the submucosa with the tip of a conventional EMR snare to anchor the snare tip in the surrounding margin to reduce sliding and to enlarge the snare opening. This technique is currently used in four centers in France and in this study, we retrospectively assess the current effectiveness and safety of A-EMR.
Methods
Design
This was a retrospective multicenter pilot study in four French endoscopy tertiary care centers with experience in EMR and ESD, and where A-EMR was routinely practiced. In total, 10 operators participated in the study. They had each performed >200 EMR procedures before beginning A-EMR.
Inclusion criteria
The study included all consecutive A-EMR procedures attempted between May 2017 and December 2017 with en bloc intent for sessile or flat lesions between 10 and 30 mm in size. Evaluation of lesion size was done endoscopically; however, size is known to be imprecisely measured this way and may be influenced by the operator’s experience. The classic landmark used to help in size evaluation is the comparison with an open biopsy forceps (measuring about 7 mm in length). Once the specimen was resected, it was systematically stretched on cork using needles and accurately measured.
Exclusion criteria
The study excluded lesions with pedunculated shape (Ip of Paris classification) or with invasive shape (ulcerated type III of Paris classification), recurrent or residual lesions after previous resection as well as other causes of severe fibrosis (ulcerative colitis), and lesions with a high risk of superficial adenocarcinoma requiring ESD.
Anchoring-EMR procedure
Video 1 Snare shape depending on pressure and an example of the procedure.
In all cases, 10 or 25 mm conventional snares from Olympus were used (SD-210U, Olympus, Tokyo, Japan) according to lesion size. Snare size was chosen by the operator during the procedure, and use of a distal cap fitted to the colonoscope tip was left to the operator’s discretion. After injection of colored fluid (indigo carmine blue, Carmine, SERB, Paris, France) into the submucosal layer, anchoring of the snare tip was performed by creating a small hole in the mucosa a small distance from the lesion edges using electric cutting current (Endocut Q, Erbe, Tübingen, Germany). This incision aimed to reach the submucosa and securely anchor the snare tip there ( Fig. 1 , Video 1 ).
Fig. 1.
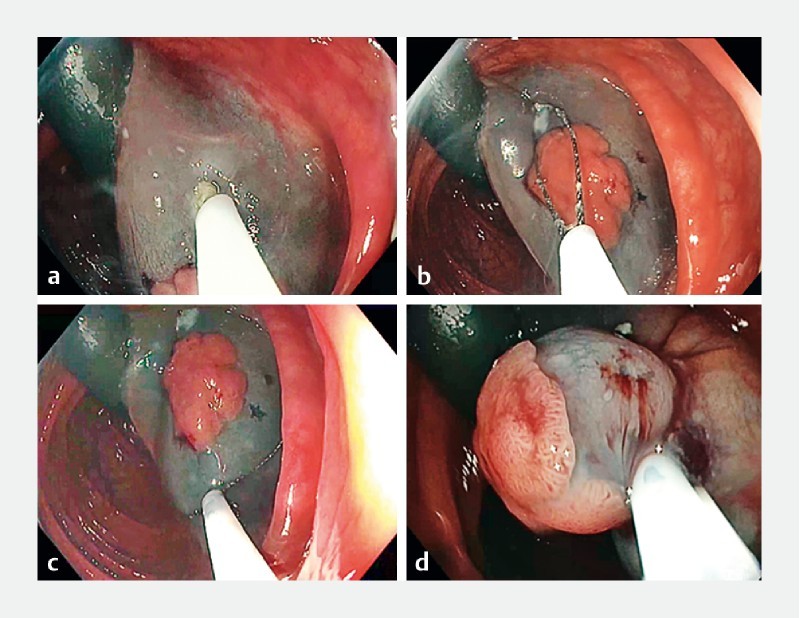
Anchoring procedure. First an incision is made into the mucosa using the tip of the snare ( a ) followed by anchoring of the tip and snare opening (oval shape of snare) ( b ). Then, pressure on the snare leads to a larger round shape ( c ) and then resection is performed after snare closure ( d ).
Then the snare was deployed progressively and adjusted around the lesion trying to respect free margins between lesion edges and snare closure. Anchoring allowed the snare to be enlarged after application of pressure ( Fig. 1 and Fig. 2 ). Once closed, resection was performed as usual with the Endocut Q. Immediately after resection, the resected area was assessed to detect both muscular damage according to Sydney’s classification (partial damage with target sign or complete transmural perforation) 6 and residual neoplastic tissue. In the case of perforation, endoscopic closure was attempted. If a single snare EMR resected the whole lesion without residual tissue, resection was considered to be en bloc endoscopically. In the case of residual tissue detected using white light, virtual chromoendoscopy and magnification if needed, additional snare resection(s) (with or without anchoring) was performed leading to a piecemeal resection automatically considered to be R1. After resection, the specimen was stretched on cork with needles and fixed in buffered formalin for pathological assessment. Lesions were then sliced into 2 mm sections followed by analysis of resection margins to assess R0 status. Pathologists used their conventional technique to analyze the margins and were not aware of the future retrospective evaluation.
Fig. 2.

Snare shape depending on amount of pressure on the anchoring point. The snare is oval without pressure on the tip ( a ) and becomes round when a pressure is applied to the tip ( b ).
Outcomes
Primary outcome was the proportion of R0 resections defined histologically by the presence of lateral and deep free margins around the lesion after A-EMR.
Secondary outcomes assessed after A-EMR were: the characteristics of R0 resection (size, operator, distal cap use); the proportion of successful A-EMR defined by the ability to anchor the snare in the submucosa without slipping of the snare during closure (tip of the snare fixed in the submucosa throughout snare closure); the proportion of en bloc resection defined endoscopically; the proportion of immediate and delayed adverse events (within the first month) including perforation (complete or partial, i. e. with target sign) and bleeding, as well as the proportion of adverse events leading to further surgery.
Data collection
All data were retrieved from the endoscopy, pathology, and hospitalization reports and were collected, anonymized, and collated in a spreadsheet (Excel; Microsoft, Redmond, WA, United States). Data were verified by an independent research assistant (MMG).
Statistical analysis
Baseline characteristics and outcome variables were described by the mean, standard deviation (SD) and range for continuous variables, and by frequencies and percentages for categorical variables. Comparisons were performed using Fisher’s exact test. Multivariate analysis was performed by penalized logistic regression, including all factors significant in univariate analysis. A P value < 0.05 was considered to be statistically significant. All analyses were performed using R software version 3.4.2. (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ ).
Ethics
This study was in compliance with the Declaration of Helsinki and received approval from the ethics committee of the Hospices Civils de Lyon (March 7, 2018). All of the patients in the study consented to participate.
Declaration
This study was declared on the database of the United States National Library of Medicine (clinicaltrials.gov) under the number NCT03467451.
Results
From May 2017 to December 2017, 141 consecutive lesions in 125 patients (mean age: 65.4 years; range 30 – 89 years) were resected by 10 operators in four French centers using the A-EMR technique. Among them, 112 patients had 1 lesion (89.6 %), 10 had 2 (8.0 %), and 3 patients had 3 lesions (2.4 %). The size of 6 lesions was not determined pathologically as these were piecemeal resections; the mean (pathologically determined) size of the 135 lesions was 19.8 mm (range 8 – 40 mm; SD: 7.1). Lesions were adenomas or intramucosal adenocarcinomas in 89/141 cases (63.1 %), sessile serrated lesions in 51 cases (36.2 %), and invasive submucosal adenocarcinoma in one case (0.7 %). All lesions included in the study were endoscopically determined to be between ≥ 10 and ≤ 30 mm; according to pathological assessment, there were 87 lesions between 8 and 20 mm, 38 lesions between 21 and 30 mm, and 10 lesions > 30 mm; the characteristics of the resected lesions are presented in Table 1 .
Table 1. Characteristics of lesions resected by A-EMR.
| Characteristics | Lesions, n = 141 |
| Location | |
|
24 (17.0) |
|
53 (37.6) |
|
9 (6.4) |
|
16 (11.3) |
|
13 (9.2) |
|
15 (10.6) |
|
11 (7.8) |
|
0 |
| Pathologically-determined size | |
|
87 (64.4) |
|
38 (28.1) |
|
10 (7.4) |
|
6 |
| Paris classification | |
|
52 (36.9) |
|
2 (1.4) |
|
67 (47.5) |
|
5 (3.5) |
|
1 (0.7) |
|
3 (2.1) |
|
11 (7.8) |
|
0 |
| Histology subtype | |
|
89 (63.1) |
|
51 (36.2) |
|
1 (0.7) |
|
0 |
Technical results
The injection before EMR was performed with saline serum in 113 cases (80.1 %), with hyaluronic acid (0.4 % solution; Sigmavisc, Life Partners Europe, Paris, France) in 24 (17.0 %), and with a glycerol mixture in 4 cases (2.8 %). Snare anchoring through the mucosa was feasible in 136 cases (96.5 %). In five cases, anchoring was not feasible since the snare slipped away from the anchoring point before complete snare closure. A cap was fitted to the tip of the colonoscope in 54 cases (38.3 %), no cap was used in 54 cases (38.3 %), and data were missing in 33 cases (23.4 %). A 25 mm snare was used in 127 cases (90.1 %) and a 10 mm snare in 14 cases (9.9 %). Endoscopically, en bloc resection was performed in 115/141 cases (81.6 %).
Histology results
R0 resections were obtained in 99/141 cases (70.2 %). The proportion of R0 resections obtained was significantly different according to lesion large diameter: 82.8 % (72/87) for lesions <20 mm in size, 55.3 % (21/38) for lesions between 20 and 30mm in size, and 50.0 % (5/10) for those > 30 mm in size ( P = 0.002). When anchoring was attempted but not achieved (5 cases), the proportion of R0 resections was 0 % although the proportion obtained when anchoring was feasible was 72.8 % and the difference was statistically significant ( P = 0.002). There was no statistically significant difference in the proportion of R0 resections according to the other factors investigated, including operator or type of solution injected into the submucosa ( Table 2 ). There were three operators with a proportion of R0 below the overall value (70.2 %) and two who attained 100 % R0. In multivariate analysis adjusted for the size of the lesion, the effect of the success of anchoring was still significant ( P value = 0.025).
Table 2. En bloc and R0 resection according to lesion and procedure characteristics.
| Characteristics | n | En bloc resection (%) | R0 resection (%) | P value |
| Success of anchoring | 0.002 | |||
|
136 | 115 (84.6) | 99 (72.8) | |
|
5 | 0 (0.0) | 0 (0.0) | |
|
0 | 0 | 0 | |
| Pathology-determined size | 0.002 | |||
|
87 | 80 (92.0) | 72 (82.8) | |
|
38 | 26 (68.4) | 21 (55.3) | |
|
10 | 8 (80.0) | 5 (50.0) | |
|
6 | 6 | 6 | |
| Center | 0.737 | |||
|
41 | 33 (80.5) | 27 (65.9) | |
|
55 | 47 (85.5) | 38 (69.1) | |
|
21 | 16 (76.2) | 15 (71.4) | |
|
24 | 19 (79.2) | 19 (79.2) | |
|
0 | 0 | 0 | |
| Operator | 0.399 | |||
|
5 | 5 (100) | 5 (100) | |
|
7 | 5 (71.4) | 5 (71.4) | |
|
13 | 9 (69.2) | 6 (46.2) | |
|
12 | 10 (83.3) | 6 (50.0) | |
|
12 | 10 (83.3) | 9 (75.0) | |
|
21 | 16 (76.2) | 15 (71.4) | |
|
21 | 19 (90.5) | 16 (76.2) | |
|
25 | 21 (84.0) | 17 (68.0) | |
|
24 | 19 (79.2) | 19 (79.2) | |
|
1 | 1 (100) | 1 (100.0) | |
|
0 | 0 | 0 | |
| Histology subtype | 0.339 | |||
|
90 | 70 (77.8) | 66 (73.3) | |
|
51 | 45 (88.2) | 33 (64.7) | |
|
0 | 0 | 0 | |
| Cap assisted | 0.283 | |||
|
54 | 45 (83.3) | 42 (77.8) | |
|
54 | 44 (81.5) | 36 (66.7) | |
|
33 | 33 | 33 | |
| Injection medium | 0.671 | |||
|
113 | 93 (82.3) | 77 (68.1) | |
|
4 | 3 (75.0) | 3 (75.0) | |
|
24 | 19 (79.2) | 19 (79.2) | |
|
0 | 0 | 0 | |
Adverse events
Perforations occurred in 5/141 resections (3.5 %) in 125 patients (5/125, 4.0 %) including complete transmural perforation in three cases (2.1 %) and partial perforation with target sign in two cases (1.4 %). Complete perforations never occurred for lesions < 20 mm in size (0/87, 0 %), in one case for lesions between 20 and 30 mm in size (1/38, 2.6 %), and in two cases >30 mm in size (2/10, 20 %). All were treated conservatively with endoscopic closure using hemoclips, and none led to salvage surgery. One delayed bleeding (melena) occurred after 24 hours (0.7 %) and which stopped spontaneously without a new colonoscopy; blood transfusion was not necessary.
Discussion
Anchoring-EMR is effective in achieving en bloc and R0 resections of colorectal neoplasia lesions, in particular, those <20 mm in size. The results presented herein for the A-EMR technique compare well with the literature as it is reported that only 62 – 65 % of resections are en bloc with conventional EMR 1 3 4 7 ; interestingly, it is difficult to compare R0 resection rates as they do not seem to be reported in studies evaluating EMR.
Current quality indicators for endoscopic resections require en bloc resection for small polyps (< 10 mm), and on this basis, cold snaring of these is strongly recommended 1 . For larger lesions (> 30 mm), EMR is mostly piecemeal in intent, whereas ESD has a high R0 resection rate 8 9 10 11 . Nevertheless, ESD is time consuming and requires expensive devices and thorough training. Alternative hybrid strategies have been developed to push the boundaries of ESD indications, but the resulting R0 resection rate was far from perfect and, although marginally quicker, these use the same devices 12 which does not meaningfully change the cost.
The A-EMR technique associates a simple technical trick with conventional EMR snares and with a good overall proportion of en bloc and R0 resections in different colon segments, including more than 60 % of resections in the ascending colon, known to be a technically challenging location. In this study, when considering only lesions < 20 mm in size, which are the most suitable for en bloc EMR according to ESGE guidelines 1 , A-EMR achieved a high quality level as the proportion of R0 was comparable to that reported for ESD in Japanese studies 8 13 14 , but without any perforations. This is of interest because, as in the case of R0 resections for non-invasive neoplasia, the risk of local recurrence is theoretically null and therefore there is probably no need for follow-up colonoscopy to detect local recurrence 15 despite the recent ESGE guidelines 16 .
For larger lesions, although there was no comparator group, the proportion of R0 may be better than for conventional EMR, but does not reach that obtained with ESD, and it is of note that perforations occurred in such cases. Among these, the proportion of perforations was 6.2 %, which is considerably higher than the 1.2 % reported after conventional EMR in comparably sized lesions 17 . Anchoring could lead to a deeper catching and, as a result, to an increased risk of perforation, but comparative data are needed to evaluate the morbidity of this technique compared to conventional EMR. The latter point highlights the main limitation of this study.
A general point to consider is that, in light of the relatively high perforation rate in this series, operators using A-EMR should be careful if en bloc resection is attempted for a lesion > 2 cm in size. Furthermore, the number of procedures for each operator was low, which precluded analysis of a potential operator effect on the R0 result. This was further compounded by the non-standardized solution used for the submucosal cushion and the non-systematic use of a distal cap. Although not significant in this sample, a larger difference between the frequency of en bloc (88.2 %) and R0 resections (64.7 %) appeared for sessile serrated lesions than for adenomas (respectively, 77.8 and 73.3 %). This larger difference underlines the difficult delineation of sessile serrated lesions endoscopically and then the technical challenge to remove those lesions with free margins as previously reported 18 ; this could affect the results in a future comparative study. Another point to consider is that the expertise of the physicians involved is also a limitation since most of them were also expert in ESD and were used to accessing the submucosa, which may affect the generalizability of the results.
Our opinion is that the main limitation of conventional EMR is the lack of snare fixation at the distal point with a risk of snare sliding to the lesion edge leading to incomplete (R1), uncertain (Rx) or piecemeal resections. This is a postulation that led us to create and develop this method of A-EMR.
To summarize, the A-EMR technique appears to be promising with a high proportion of R0 for lesions of 10 to 20 mm diameter without any perforation. It could also offer an alternative to ESD or to hybrid techniques to reach R0 for lesions between 20 and 30 mm saving time and money by using conventional devices. Randomized comparative studies are required using standardized procedures to conclude as to the potential benefits of this technique.
Acknowledgments
We sincerely thank Pr Jean-François Bretagne for reviewing this article.
Footnotes
Competing interests None
References
- 1.Ferlitsch M, Moss A, Hassan C et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270–297. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Kashida H, Saito Y et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–434. doi: 10.1111/den.12456. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida N, Naito Y, Inada Y et al. Multicenter study of endoscopic mucosal resection using 0.13% hyaluronic acid solution of colorectal polyps less than 20 mm in size. Int J Colorectal Dis. 2013;28:985–991. doi: 10.1007/s00384-012-1631-3. [DOI] [PubMed] [Google Scholar]
- 4.Woodward T, Crook J E, Raimondo M et al. Improving complete EMR of colorectal neoplasia: a randomized trial comparing snares and injectate in the resection of large sessile colon polyps. Gastrointest Endosc. 2015;81:673–681. doi: 10.1016/j.gie.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Jacques J, Legros R, Charissoux A et al. Anchoring the snare tip by means of a small incision facilitates en bloc endoscopic mucosal resection and increases the specimen size. Endoscopy. 2017;49:E39–E41. doi: 10.1055/s-0042-121009. [DOI] [PubMed] [Google Scholar]
- 6.Burgess N G, Bassan M S, McLeod D et al. Deep mural injury and perforation after colonic endoscopic mucosal resection: a new classification and analysis of risk factors. Gut. 2017;66:1779–1789. doi: 10.1136/gutjnl-2015-309848. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida N, Naito Y, Inada Y et al. Endoscopic mucosal resection with 0.13% hyaluronic acid solution for colorectal polyps less than 20 mm: a randomized controlled trial. J Gastroenterol Hepatol. 2012;27:1377–1383. doi: 10.1111/j.1440-1746.2012.07166.x. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Fukuzawa M, Matsuda T et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343–352. doi: 10.1007/s00464-009-0562-8. [DOI] [PubMed] [Google Scholar]
- 9.Fujiya M, Tanaka K, Dokoshi T et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583–595. doi: 10.1016/j.gie.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Zhang X-H, Ge J et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J Gastroenterol. 2014;20:8282–8287. doi: 10.3748/wjg.v20.i25.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi N, Yoshitake N, Hirahara Y et al. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728–733. doi: 10.1111/j.1440-1746.2011.06942.x. [DOI] [PubMed] [Google Scholar]
- 12.Toyonaga T, Man-i M, Chinzei R et al. Endoscopic treatment for early stage colorectal tumors: the comparison between EMR with small incision, simplified ESD, and ESD using the standard flush knife and the ball tipped flush knife. Acta Chir Iugosl. 2010;57:41–46. doi: 10.2298/aci1003041t. [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Uraoka T, Yamaguchi Y et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Itabashi M, Shimada Y et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pioche M, Walter T. Endoscopic removal of colorectal T1 cancers: Why is a 1-year follow-up recommended by ESGE when resection is R0 and curative? Endosc Int Open. 2019;7:E816–E817. doi: 10.1055/a-0900-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan C, Wysocki P T, Fuccio L et al. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guideline. Endoscopy. 2019;51:266–277. doi: 10.1055/a-0831-2522. [DOI] [PubMed] [Google Scholar]
- 17.Bronsgeest K, Huisman J F, Langers A et al. Safety of endoscopic mucosal resection (EMR) of large non-pedunculated colorectal adenomas in the elderly. Int J Colorectal Dis. 2017;32:1711–1717. doi: 10.1007/s00384-017-2892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohl H, Srivastava A, Bensen S P et al. Incomplete polyp resection during colonoscopy – results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–800. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]


