Endophthalmitis due to infection with Enterococcus spp. progresses rapidly and often results in substantial and irreversible vision loss. Given that the frequency of this condition caused by vancomycin-resistant Enterococcus faecalis has been increasing, the development of novel therapeutics is urgently required.
KEYWORDS: Enterococcus, bacteriophage therapy, endophthalmitis, mouse, ophthalmology
ABSTRACT
Endophthalmitis due to infection with Enterococcus spp. progresses rapidly and often results in substantial and irreversible vision loss. Given that the frequency of this condition caused by vancomycin-resistant Enterococcus faecalis has been increasing, the development of novel therapeutics is urgently required. We have demonstrated the therapeutic potential of bacteriophage ΦEF24C-P2 in a mouse model of endophthalmitis caused by vancomycin-sensitive (EF24) or vancomycin-resistant (VRE2) strains of E. faecalis. Phage ΦEF24C-P2 induced rapid and pronounced bacterial lysis in turbidity reduction assays with EF24, VRE2, and clinical isolates derived from patients with E. faecalis-related postoperative endophthalmitis. Endophthalmitis was induced in mice by injection of EF24 or VRE2 (1 × 104 cells) into the vitreous. The number of viable bacteria in the eye increased to >1 × 107 CFU, and neutrophil infiltration into the eye was detected as an increase in myeloperoxidase activity at 24 h after infection. A clinical score based on loss of visibility of the fundus as well as the number of viable bacteria and the level of myeloperoxidase activity in the eye were all significantly decreased by intravitreous injection of ΦEF24C-P2 6 h after injection of EF24 or VRE2. Whereas histopathologic analysis revealed massive infiltration of inflammatory cells and retinal detachment in vehicle-treated eyes, the number of these cells was greatly reduced and retinal structural integrity was preserved in phage-treated eyes. Our results thus suggest that intravitreous phage therapy is a potential treatment for endophthalmitis caused by vancomycin-sensitive or -resistant strains of E. faecalis.
INTRODUCTION
Bacterial endophthalmitis can result from trauma or be a complication of ocular surgery or intravitreal injection, and it often leads to substantial loss of vision or even blindness. The frequency of postoperative endophthalmitis has increased recently in association with an increase in the performance of intraocular surgery and injection. Prompt diagnosis and appropriate treatment of endophthalmitis are necessary in order to prevent retinal damage and scarring. Most cases of postoperative endophthalmitis are caused by Gram-positive pathogens, such as Staphylococcus spp., Enterococcus spp., and Streptococcus spp. (1). Endophthalmitis caused by Enterococcus spp. has a worse prognosis in terms of final visual acuity than that caused by other bacteria, such as coagulase-negative Staphylococcus spp. and Staphylococcus aureus (1–3). Final visual acuity was found to be 20/400 or worse in 92.9% of individuals with postoperative endophthalmitis due to Enterococcus spp. (3).
Enterococcus spp. are resistant to a variety of commonly administered antibiotics, such as cephalosporins, aminoglycosides, and clindamycin. Enterococcus faecalis strains isolated from individuals with endophthalmitis from 2002 to 2012 showed higher MICs for various antibiotics than did those isolated from 1990 to 2001 (2, 4). Furthermore, cases of endophthalmitis due to vancomycin (VAN)-resistant Enterococcus spp. (VRE) have been described (5–12). Infection with E. faecalis was found to account for 16% of postoperative endophthalmitis cases in Japan, 25.4% in South Korea, and 31.1% in Sweden (13–15). The development of alternatives to antibiotics for the treatment of drug-resistant E. faecalis infection is therefore urgently needed.
Bacteriophages (phages) are present widely in environments such as soil, water, food, and the gastrointestinal tract. Bacteria infected with phages produce endolysin that disrupts the bacterial cell wall and results in cell death. The potential of phage-based therapy for bacterial infectious disease has thus been investigated, with three approaches to such therapy, administration of the phage itself, of phage-derived endolysin, or of phage-derived depolymerases (16, 17). Although the early attention paid to the potential of phage-based therapy waned after the introduction of antibiotics, there has been a resurgence of interest as a result of the growing problem of antibiotic resistance (18–20). We previously described ΦEF24C as a candidate therapeutic phage for E. faecalis infection. This phage is virulent and has a broad host range, being capable of inducing the lysis of many E. faecalis strains, including those resistant to VAN (21). We also showed that ΦEF24C-based therapy was effective in mouse models of sepsis induced by VRE (22). Moreover, we isolated a mutant strain of this phage, ΦEF24C-P2, that has a higher lytic activity against E. faecalis clinical strains than does the wild-type phage.
Although phages are able to lyse specific bacteria in vitro, the effects of phage therapy in vivo may depend on the type of agent, on the route, dose, and timing of administration, and on the tissue-specific microenvironment. With regard to the effects of phages on ocular bacterial infection, we previously showed that a single topical application of a phage in eyedrops was effective for the treatment of keratitis caused by Pseudomonas aeruginosa in mice (23). Intravitreal administration of a phage lytic enzyme was also shown to attenuate the development of S. aureus-induced endophthalmitis in mice (24). We have now examined the effects of intravitreal administration of the ΦEF24C-P2 phage on endophthalmitis caused by VAN-sensitive E. faecalis or VRE in mice.
RESULTS
Effects of phage ΦEF24C-P2 and VAN on the growth of E. faecalis strains in vitro.
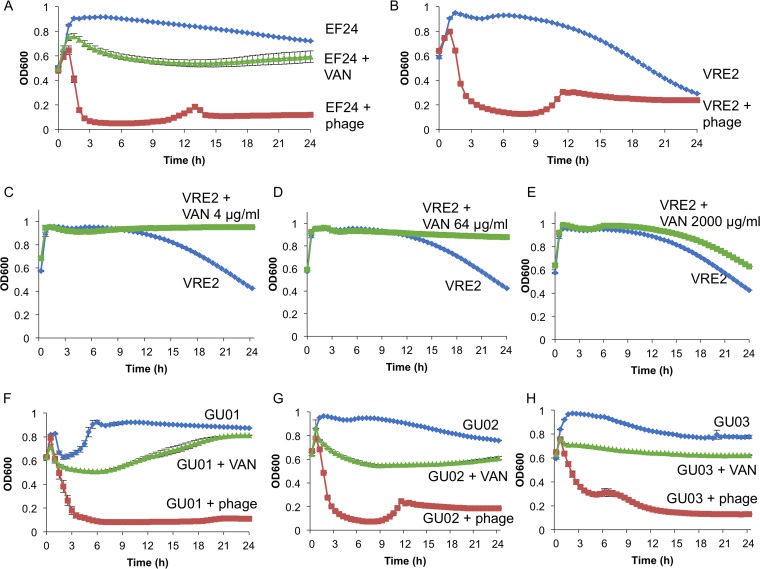
We first examined the effects of phage ΦEF24C-P2 and VAN in vitro on E. faecalis strains EF24 and VRE2, as well as on clinical isolates derived from patients with postoperative endophthalmitis due to E. faecalis. In the culture system adopted, all of the E. faecalis strains showed a spontaneous decrease in turbidity (transition to the decay phase), to a greater or lesser extent, after incubation for 8 h even in the absence of phage or VAN (Fig. 1). The addition of ΦEF24C-P2 at a multiplicity of infection of 100 induced marked bacteriolysis in both EF24 and VRE2 cultures within 3 h (Fig. 1A and B). Similarly, ΦEF24C-P2 induced rapid bacteriolysis in all three clinical isolates (Fig. 1F to H). On the other hand, although the presence of VAN at a concentration of 2,000 μg/ml resulted in a moderate decrease in turbidity for EF24 (Fig. 1A) and the three clinical isolates (Fig. 1F to H), the drug at concentrations of 4, 64, or 2,000 μg/ml had no such effect on the turbidity of VRE2 (Fig. 1C to E). In fact, VAN suppressed the transition of VRE2 to the decay phase at each concentration tested, although the reason for this effect is unclear. In all strains except GU01, the pronounced bacteriolysis induced by the phage was followed by a small increase in turbidity and a subsequent further decrease. It is not clear whether this pattern reflects an “evolutionary arms race” between the phage and host bacterium (25), but no substantial regrowth of any of the bacterial strains was apparent for up to 24 h after the onset of culture. Together, these results suggested that ΦEF24C-P2 might be effective for the treatment of E. faecalis infection regardless of the VAN sensitivity of the bacterium.
FIG 1.
Effects of phage ΦEF24C-P2 and vancomycin (VAN) on the growth of E. faecalis strains in vitro. (A) Effects of ΦEF24C-P2 and VAN (2,000 μg/ml) on the growth of E. faecalis strain EF24. Bacterial growth was monitored by measurement of optical density at 600 nm (OD600). (B) Effect of ΦEF24C-P2 on the growth of VRE2. (C to E) Effects of VAN at 4, 64, or 2,000 μg/ml, respectively, on the growth of VRE2. (F to H) Effects of ΦEF24C-P2 and VAN (2,000 μg/ml) on the growth of E. faecalis strains derived from patients with postoperative endophthalmitis (GU01, GU02, and GU03, respectively). All data are means ± SEM of triplicates from a representative experiment.
Effects of intravitreous administration of ΦEF24C-P2 on EF24-related endophthalmitis in mice.
We next examined the effects of ΦEF24C-P2 or the vehicle on noninfected eyes in mice. Macroscopic analysis and indirect ophthalmoscopy revealed that intravitreal administration of ΦEF24C-P2 or the vehicle alone did not induce a clinical inflammatory response, as evident from the lack of opacity in the anterior chamber and vitreous body at 18 and 66 h after injection (n = 6 eyes per condition). The clinical score based on loss of fundus visibility was thus 0 (on a scale of 0 to 4) under both conditions. Furthermore, there was no significant (P = 0.29, Student’s unpaired t test) difference in myeloperoxidase (MPO) activity (a marker of neutrophil infiltration) between eyes treated with ΦEF24C-P2 (0.096 ± 0.045 absorbance units) or the vehicle (0.045 ± 0.010 absorbance units) at 18 h after intravitreal administration (n = 6 eyes per condition). We also examined the scotopic electoretinogram (ERG) response at 66 h, 1 week, and 2 weeks after intravitreal administration of ΦEF24C-P2 (n = 6 eyes), with both the a-wave and b-wave (the responses generated from photoreceptors and the inner retina, respectively) showing no apparent difference compared with untreated eyes (data not shown), suggesting that retinal function was not affected by phage administration. Together, these results thus suggested that the administration of ΦEF24C-P2 did not induce an inflammatory response in the eye or manifest retinal toxicity.
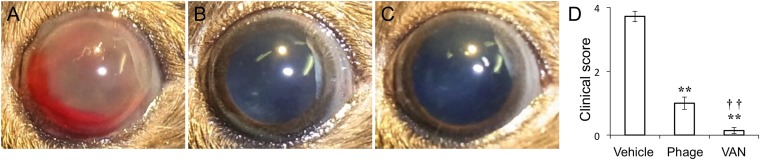
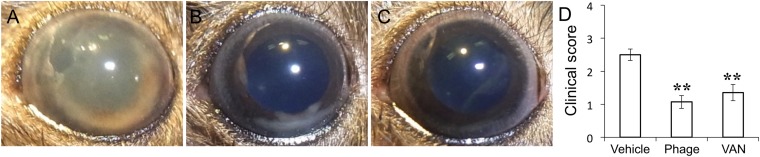
We then constructed a mouse model of E. faecalis endophthalmitis. Injection of 1 × 104 E. faecalis strain EF24 cells into the vitreous body resulted in the development of severe endophthalmitis at 24 h, with hemorrhage being apparent in the anterior chamber of most eyes (Fig. 2A). Macroscopic examination revealed that the ocular fundus was not visible as a result of hemorrhage and fibrin precipitation in the anterior chamber of vehicle-treated infected eyes. We examined the potential therapeutic effect of ΦEF24C-P2 compared with that of VAN. Intravitreous injection of ΦEF24C-P2 (1 × 108 plaque forming units [PFU]) or VAN (1 μg) at 6 h after EF24 injection resulted in a marked attenuation of clinical signs apparent at 24 h, with hemorrhage and fibrin not being detected in the anterior chamber and with the ocular fundus being visible (Fig. 2A to C). The clinical score of mice treated with ΦEF24C-P2 or VAN was thus significantly lower than that of control infected mice injected with the vehicle (Fig. 2D), with the clinical score of mice treated with VAN also being significantly lower than that of those treated with ΦEF24C-P2.
FIG 2.
Effects of intravitreous injection of phage ΦEF24C-P2 or VAN on the clinical signs of E. faecalis EF24-induced endophthalmitis in mice. (A to C) The right eye was injected intravitreously with 1 × 104 E. faecalis EF24 cells. Six hours after infection, vehicle (A), ΦEF24C-P2 (1 × 108 PFU) (B), or VAN (1 μg) (C) was administered intravitreously to the same eye. Representative photographs of the right eye at 24 h after EF24 injection are shown. (D) Clinical score related to loss of fundus visibility at 24 h after EF24 injection for the treated mice. Data are means ± SEM for 12 eyes in each group. **, P < 0.01 (Tukey-Kramer test) versus vehicle-treated eyes; ††, P < 0.01 (Tukey-Kramer test) versus phage-treated eyes.
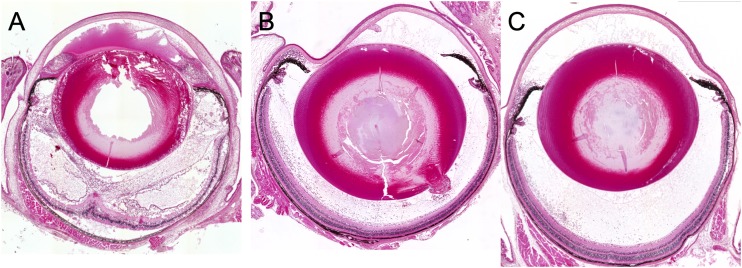
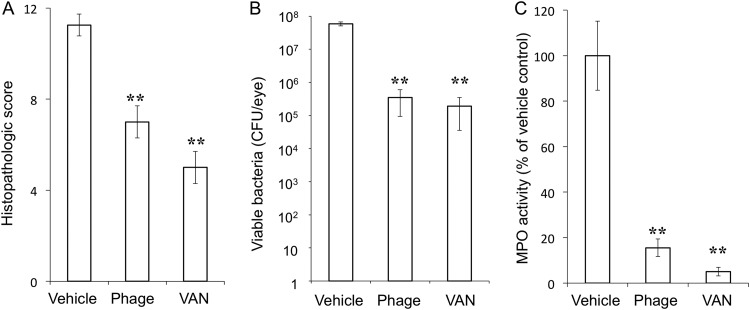
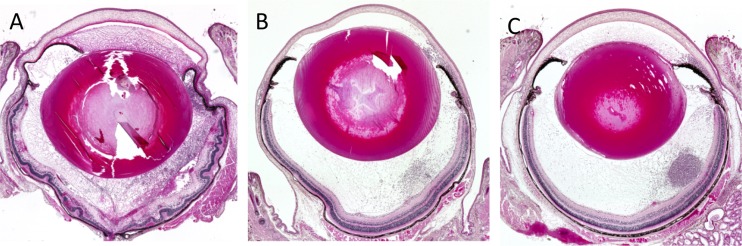
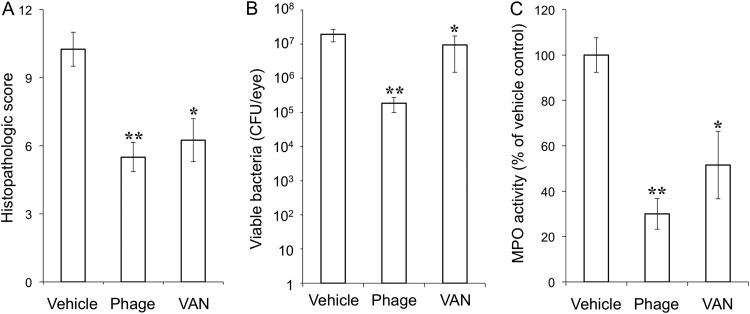
Eyeballs were subjected to histopathologic examination at 24 h after intravitreal injection of EF24. The eyes of vehicle-treated infected mice manifested corneal edema with cellular infiltrates, massive fibrinous exudate, and hemorrhage with inflammatory cells in the anterior chamber, as well as cellular infiltrates in the vitreous body and retina, retinal edema, and total retinal detachment (Fig. 3A). In contrast, inflammatory cell infiltration was attenuated in both ΦEF24C-P2-treated and VAN-treated eyes (Fig. 3B and C). The histopathologic score (scale of 0 to 12) was thus significantly decreased by injection of either ΦEF24C-P2 or VAN (Fig. 4A). Both the live bacterial load (Fig. 4B) and MPO activity (Fig. 4C) in eyes injected with ΦEF24C-P2 or VAN were also significantly reduced compared with those of eyes injected with the vehicle. The latter observation thus suggested that infiltration of neutrophils into the eye was suppressed by intravitreal injection of ΦEF24C-P2 or VAN. There was no significant difference in the histopathologic score, bacterial load, or MPO activity between the eyes treated with ΦEF24C-P2 and those treated with VAN.
FIG 3.
Histopathology of E. faecalis EF24-induced endophthalmitis in eyes treated with ΦEF24C-P2 or VAN. Eyes were excised at 24 h after infection with E. faecalis EF24 and subsequent intravitreal injection of vehicle (A), ΦEF24C-P2 (B), or VAN (C). The tissue was fixed, embedded in paraffin, sectioned at a thickness of 2 μm, and stained with hematoxylin-eosin.
FIG 4.
Histopathologic score, live bacterial load, and MPO activity for eyes with E. faecalis EF24-induced endophthalmitis treated with ΦEF24C-P2 or VAN. The histopathologic score for ocular inflammation (A), viable bacterial load (B), and MPO activity (C) were determined for eyes excised 24 h after infection with E. faecalis EF24 and subsequent intravitreal injection of vehicle, ΦEF24C-P2, or VAN. All data are means ± SEM for four eyes in each group. **, P < 0.01 (Tukey-Kramer test) versus vehicle-treated eyes.
We also examined the effects of phage therapy administered at a later time point (12 h) after infection. Intravitreous administration of ΦEF24C-P2 at this time also resulted in a significant improvement in the clinical score (3.30 ± 0.15 versus 3.80 ± 0.13; P < 0.05, Student’s unpaired t test) and reduced both the number of viable bacteria (1.3 × 106 versus 1.8 × 108, P < 0.05) and MPO activity (1.063 ± 0.058 versus 1.630 ± 0.195, P < 0.05) in the eye at 24 h after infection compared with administration of the vehicle (n = 6 eyes per condition).
Effects of intravitreous administration of ΦEF24C-P2 on VRE2-induced endophthalmitis in mice.
We next examined the effects of phage therapy on endophthalmitis induced by the VAN-resistant E. faecalis strain VRE2, which we confirmed was able to proliferate in the presence of vancomycin at 2,000 μg/ml in vitro (Fig. 1E). Injection of VRE2 into the vitreous induced endophthalmitis that completely obscured the fundus at 24 h as a result of fibrin precipitation in the anterior chamber or vitreous opacity (Fig. 5A). The disease severity appeared less than that evident with EF24-infected eyes, however. The clinical signs (Fig. 5A to C) and clinical score (Fig. 5D) for VRE2-infected eyes were attenuated markedly by intravitreal injection of ΦEF24C-P2 and to a lesser extent by injection of VAN at 6 h after infection compared with those apparent in vehicle-treated eyes. Histopathologic examination revealed inflammatory cell infiltration and retinal detachment in vehicle-treated eyes with VRE2-induced endophthalmitis, whereas cell infiltration was attenuated and retinal structure maintained in eyes treated with ΦEF24C-P2 or VAN (Fig. 6). The histopathologic score was thus significantly reduced by treatment with ΦEF24C-P2 and, to a lesser extent, by treatment with VAN (Fig. 7A). The number of viable bacteria (Fig. 7B) and MPO activity (Fig. 7C) in the eye were also significantly decreased by injection of ΦEF24C-P2 and, again, to a lesser extent by injection of VAN. There was no significant difference, however, in the histopathologic score, bacterial load, or MPO activity between the eyes treated with ΦEF24C-P2 and those treated with VAN.
FIG 5.
Effects of intravitreous injection of phage ΦEF24C-P2 or VAN on the clinical signs of VRE2-induced endophthalmitis in mice. (A to C) The right eye was injected intravitreously with 1 × 104 E. faecalis strain VRE2 cells. Six hours after infection, vehicle (A), ΦEF24C-P2 (1 × 108 PFU) (B), or VAN (1 μg) (C) was administered intravitreously to the same eye. Representative photographs of the right eye at 24 h after VRE2 injection are shown. (D) Clinical score at 24 h after VRE2 injection for the treated mice. Data are means ± SEM for 14 eyes in each group. *, P < 0.05; **, P < 0.01 (Tukey-Kramer test) versus vehicle-treated eyes.
FIG 6.
Histopathology of VRE2-induced endophthalmitis in eyes treated with ΦEF24C-P2 or VAN. Eyes were excised at 24 h after infection with E. faecalis strain VRE2 and subsequent intravitreal injection of vehicle (A), ΦEF24C-P2 (B), or VAN (C). The tissue was fixed, embedded in paraffin, sectioned at a thickness of 2 μm, and stained with hematoxylin-eosin.
FIG 7.
Histopathologic score, live bacterial load, and MPO activity in eyes with VRE2-induced endophthalmitis treated with ΦEF24C-P2 or VAN. The histopathologic score for ocular inflammation (A), viable bacterial load (B), and MPO activity (C) were determined for eyes excised 24 h after infection with E. faecalis strain VRE2 and subsequent intravitreal injection of vehicle, ΦEF24C-P2, or VAN. All data are means ± SEM for four eyes in each group. *, P < 0.05; **, P < 0.01 (Tukey-Kramer test) versus vehicle-treated eyes.
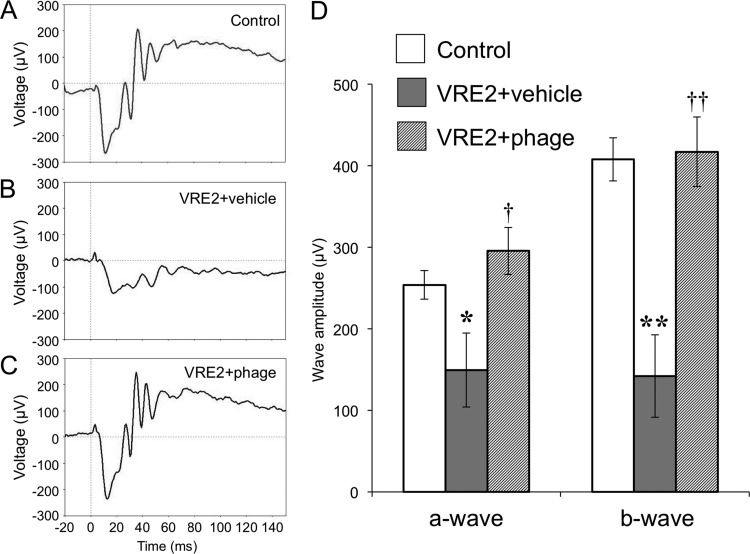
We also examined the scotopic ERG response in VRE2-infected eyes in order to evaluate retinal function. Eyes infected with VRE2 and treated with the vehicle showed a significant decrease in the amplitude of both the a-wave and b-wave at 24 h compared with uninfected control eyes (Fig. 8). In contrast, treatment with ΦEF24C-P2 at 6 h after VRE2 infection prevented the reduction in a-wave and b-wave amplitude, indicating that phage therapy preserved retinal function in VRE2-infected eyes.
FIG 8.
Retinal function in eyes with VRE2-induced endophthalmitis treated with ΦEF24C-P2. (A to C) Representative ERG responses for control (uninfected, untreated) eyes (A) and eyes at 24 h after infection with VRE2 and subsequent intravitreal injection of vehicle (B) or ΦEF24C-P2 (C). (D) Amplitude of the a-wave and b-wave determined from ERG recordings. Data are means ± SEM for 5 to 16 eyes in each group. *, P < 0.05; **, P < 0.01 (Tukey-Kramer test) versus control; †, P < 0.05; ††, P < 0.01 (Tukey-Kramer test) versus VRE2-infected eyes treated with vehicle.
Persistence of ΦEF24C-P2 in the eye.
We finally examined the number of viable phages in the eye at 18 and 66 h after intravitreal administration of ΦEF24C-P2 (1 × 108 PFU) with or without prior injection of E. faecalis EF24. The phage number did not differ significantly at either time point between eyes infected or not infected with bacteria (Fig. 9). Although the phage number declined in a time-dependent manner, between 1 × 105 and 1 × 106 PFU of ΦEF24C-P2 were still present in the eye at 66 h after phage administration.
FIG 9.
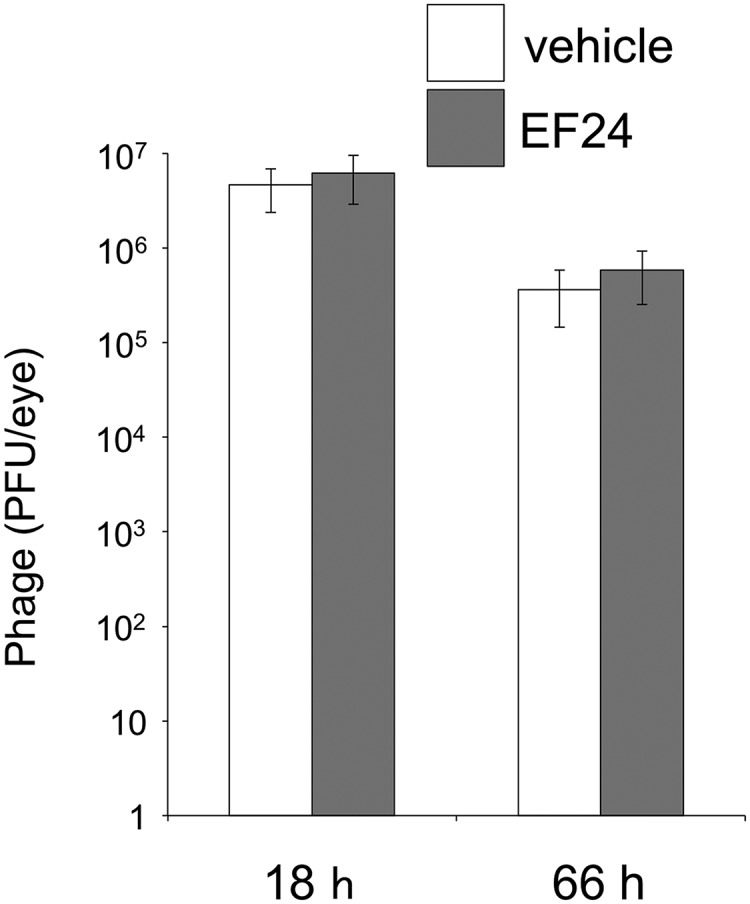
Persistence of ΦEF24C-P2 in the eyes of mice with or without endophthalmitis. Eyes were injected with ΦEF24C-P2 (1 × 108 PFU) at 6 h after injection of E. faecalis EF24 cells (1 × 104) or vehicle. The number of viable phages in the eye was determined at 18 and 66 h after intravitreal injection of ΦEF24C-P2. Data are means ± SEM for six eyes in each group.
DISCUSSION
We show here a therapeutic effect of intravitreous administration of a bacteriophage on E. faecalis-induced endophthalmitis in mice. Intravitreous injection of phage ΦEF24C-P2 thus resulted in a reduction in the load of bacteria, including the strains EF24 and VRE2, and improved disease outcome, with preservation of the structural integrity of the retina and suppression of neutrophil infiltration. Prompt elimination of bacteria before the induction of retinal damage and scarring is required to prevent blindness in endophthalmitis, and we also showed that this phage induced bacterial lysis in vitro faster than did VAN. Intravitreous administration is an efficient means of delivery of phages or other therapeutic agents to the site of infection in patients with endophthalmitis. Our results therefore suggest that intravitreous phage therapy is a potential option for the treatment of intraocular bacterial infection.
In the clinical setting, treatment of endophthalmitis with antibiotics is usually initiated before identification and susceptibility testing of the bacterial pathogen, given the rapid progression of the disease. With regard to the potential clinical application of phage therapy for endophthalmitis, phages would also need to be administered at the time of diagnosis in order to prevent irreversible retinal damage. Given that ΦEF24C-P2 is highly specific for E. faecalis, treatment with this phage may be effective only in cases of endophthalmitis caused by this bacterial species. Preparation of a phage cocktail to target relevant pathogens, including not only Enterococcus spp. but also Staphylococcus spp. and Streptococcus spp. (1), would thus be necessary for the clinical implementation of this therapeutic approach. This cocktail should also include phages such as ΦEF24C-P2 that are able to lyse antibiotic-resistant bacteria of each species. The intravitreal administration of such a phage cocktail may thus prove to be effective for the treatment of endophthalmitis even in cases refractory to antibiotic therapy.
In the present study, some bacteria remained in the eye after ΦEF24C-P2 treatment in mice, despite the rapid and extensive lysis of E. faecalis induced in vitro. In such in vitro experiments, phages are able to act directly on bacteria without impediment and thus show maximum lytic activity. However, endophthalmitis is established not only by bacteria directly but also as a result of complex interactions between the bacteria and endogenous components of the host, including the vitreous, infiltrated inflammatory cells, and products derived from inflammation, such as fibrin and tissue debris. It is thus possible that factors in the inflamed eye inhibit direct contact between phages and bacteria or interfere with phage lytic activity in vivo. The clinical application of phage therapy for endophthalmitis may require the development of agents or approaches that increase the bactericidal activity of the administered phages in vivo.
Active and passive phage therapies have been proposed, with the former being dependent on replication of the phage in the pathogen in vivo and the latter on just the initial phage dose (26). We previously developed an active phage therapy for lung-derived septicemia caused by S. aureus in mice (27). In the present study, the number of viable phages in the eye was similar for mice that had or had not received a prior injection of EF24, suggesting that ΦEF24C-P2 phage therapy for endophthalmitis is passive. Injection into the eyeball, which constitutes a closed space, delivers the phage directly and efficiently to the site of infection. Passive phage therapy is thought to have several advantages over the active mode (28). The high concentration of phage administered in passive phage therapy thus has the potential to suppress the emergence of drug-resistant bacteria (28) and possibly also that of phage-resistant bacteria. In addition, passive therapy is free of concerns regarding the production of progeny phage by the target bacteria in the patient (28).
The intravenous and percutaneous administration of personalized therapeutic phage cocktails was shown to rescue a patient from a life-threatening multidrug-resistant Acinetobacter baumannii infection (29). With regard to the application of phage therapy to ocular disease, a case of keratitis caused by VAN-resistant S. aureus was effectively treated by phage administration in eyedrops, a nasal spray, and intravenously (30). However, as far as we are aware, phage administration into the vitreous has not yet been described. We have now administered a phage intravitreally in mice and found it to have an antibacterial effect. We also found that the phage remained in the vitreous body for at least 66 h. Intracameral antibiotics were recently shown to reduce the risk of postoperative endophthalmitis (31), although hemorrhagic occlusive retinal vasculitis, a potentially blinding complication, has been associated with intracameral injection of VAN during cataract surgery (32–34). Given that phages may survive in the eye for several days, their administration into the eye at the end of surgery for prophylaxis of endophthalmitis might also be effective in the clinical setting. Again, however, such a prophylactic approach would likely require the development of a phage cocktail that targets the major relevant pathogens, including Enterococcus spp., Staphylococcus spp., and Streptococcus spp.
Endolysin is a peptidoglycan hydrolase produced by phages and has also been used for phage therapy. Intravitreous administration of endolysin in a mouse model of S. aureus-induced endophthalmitis was shown to be effective for sterilization and retinal protection (24). Although eyedrop administration can be performed frequently, intravitreal administration cannot. Endolysin is a protein and is susceptible to biological degradation, and it remains unknown how long endolysin is able to exert its effects in the eye.
Intravitreous administration of the antibiotics VAN and ceftazidime is a standard treatment for bacterial endophthalmitis and should be implemented promptly in patients diagnosed with or suspected of having this condition (35, 36). In the present study, there were no significant differences in the effects of ΦEF24C-P2 and VAN in vivo, with the exception of that on the clinical score in EF24-infected eyes, suggesting that the therapeutic efficacies of the phage and VAN are similar for experimental endophthalmitis. VAN treatment was associated with a reduction in the clinical and histopathologic scores and MPO activity in mice infected with VRE2, despite the lack of antibacterial activity in vitro and only a low level of such activity in vivo. The antimicrobial effect of VAN in vitro may depend on various experimental conditions, including the number of bacteria and the type of culture medium. In the present study, whereas VAN at a concentration of 2,000 μg/ml did not reduce the turbidity of VRE2 in a turbidity reduction assay, the MIC of VAN for VRE2 determined by antibiotic susceptibility testing according to the CLSI manual was 256 μg/ml. A high concentration of VAN in the vitreous might therefore have only a small antibacterial effect in vivo. In addition, some antibiotics have been found to inhibit not only bacterial viability but also toxin release as well as to have direct immunomodulatory properties. For example, VAN at therapeutic concentrations inhibits the production of tumor necrosis factor-α from human peripheral blood mononuclear cells stimulated by bacterial lipopolysaccharide (37, 38). It is thus possible that such an immunomodulatory action of VAN ameliorated the inflammatory response in VRE2-infected eyes.
Combinations of phages and antibiotics have recently been found to have synergistic effects (39). For example, combined therapy with phages and antibiotics was effective as a rescue treatment for severe septic peritonitis due to VRE in a mouse model (40). The growth of A. baumannii in vitro was also inhibited to a markedly greater extent by the combination of an antibiotic and a phage than by either agent alone (29). Such combinations also warrant investigation for their potential to treat ocular infectious disease.
Limitations of the present study include the lack of an examination of the effects of different phage doses and the lack of an extensive evaluation of the effects of the timing of phage administration. The development of phage therapy for clinical application will require further studies to determine the minimum effective concentration of phage for vitreous administration. Furthermore, the effectiveness of prophylactic administration of phage for the prevention of endophthalmitis also warrants investigation in future studies.
MATERIALS AND METHODS
Ethical treatment of animals.
This study was approved by the Committee for Care and Use of Laboratory Animals at Kochi University (permit number J-00061) and was performed in accordance both with the Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research and with institutional guidelines for animal research.
Bacterial strains, reagents, and culture media.
E. faecalis strains EF24 and VRE2 were as described previously (21, 22, 41). The E. faecalis strains GU01, GU02, and GU03 were isolated from three patients with postoperative endophthalmitis related to cataract surgery. EF24 was used as a host indicator strain for phage ΦEF24C-P2, and both EF24 and VRE2 were used for animal experiments. The MICs for VAN determined by antibiotic dilution susceptibility testing according to Clinical and Laboratory Standards Institute (CLSI) manual with E. faecalis EF24, GU01, GU02, and GU03 were 1.0, 1.0, 1.0, and 4.0 μg/ml, respectively. VRE2 manifested a high level of resistance to VAN, with a MIC of 256 μg/ml. All chemicals and reagents were obtained from Nacalai Tesque (Kyoto, Japan), Wako Chemicals (Osaka, Japan), or Sigma-Aldrich (St. Louis, MO), unless otherwise stated. Brain heart infusion (BHI) agar (Becton, Dickinson, and Company, Franklin Lakes, NJ) was used for bacterial and phage culture. EF agar base (Nissui Pharmaceutical, Tokyo, Japan) was used for counting CFUs of EF24 and VRE2. Double-layered agar based on BHI medium, with 1.5% and 0.5% agar as the lower and the upper layers, respectively, was used for evaluation of phage plaque formation.
Large-scale culture and purification of phage ΦEF24C-P2.
Phage ΦEF24C-P2 was described previously (41) and was amplified with E. faecalis strain EF24 in 200 to 250 ml of BHI broth. After complete bacterial lysis, the phage-containing fraction was collected by centrifugation, and polyethylene glycol 6000 and NaCl were added to final concentrations of 10% and 0.5 M, respectively. The suspension was stored at 4°C overnight, and a phage pellet was then obtained by centrifugation (10,000 × g, 20 min, 4°C), suspended in TM buffer (10 mM Tris-HCl [pH 7.2] and 5 mM MgCl2) containing DNase I (100 μg/ml) and RNase A (100 μg/ml), and incubated at 37°C for 30 min. The crude phage suspension was layered on top of a discontinuous gradient of 40%, 35%, and 30% iodixanol (Opti-Prep; Alere Technologies, Oslo, Norway) in physiological saline and was subjected to ultracentrifugation (50,000 × g, 2 h, 4°C). The phage band was collected and stored at 4°C until use. The phage concentration in PFU was measured with a plaque assay.
Turbidity reduction assay.
The lytic activity of ΦEF24C-P2 or VAN was assessed with a turbidity reduction assay performed as described previously (42). E. faecalis strains EF24, VRE2, GU01, GU02, and GU03 were grown in BHI broth at 37°C to an optical density at 600 nm (OD600) of 0.8. Each culture was centrifuged at 10,000 × g for 5 min at 4°C, and the cells were suspended in BHI broth to a final concentration of 2 × 109 cells/ml. The turbidity reduction assay was initiated by addition of an equal volume of ΦEF24C-P2 (final concentration of 2 × 1011 PFU/ml), VAN (final concentration of 2,000, 64, or 4 μg/ml), or the vehicle to the bacterial suspension in the wells of a 96-well plate, and the OD600 of each well was then monitored automatically every 30 min for 24 h with a microplate reader (HITS-S2; Scinics, Tokyo, Japan). All incubations were performed in triplicate.
Mouse model of endophthalmitis.
Specific pathogen-free female C57BL/6J mice at 7 weeks of age were obtained from Charles River Laboratories (Kanagawa, Japan) and housed under specific pathogen-free conditions at the animal facility of Kochi Medical School. E. faecalis strains EF24 and VRE2 were grown in 10 ml of BHI broth at 37°C to the logarithmic phase (∼100 Klett units, as measured with a Klett-Summerson colorimeter with filter no. 54) and were then isolated by centrifugation at 10,000 × g for 5 min at 4°C. The cell pellet was suspended in 10 ml of physiological saline, and the suspension was centrifuged again under the same conditions. The cells were then suspended in ∼1 ml of saline, and after appropriate dilution, turbidity (in Klett units) was measured to determine the bacterial cell number. One Klett unit was assumed to be equivalent to 7.9 × 106 E. faecalis cells/ml on the basis of standardization with bacterial cell numbers counted directly with a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA).
The mouse model of endophthalmitis was constructed as previously described (43). In brief, endophthalmitis of the right eye was induced by injection of 0.5 μl of physiological saline containing 1 × 104 E. faecalis EF24 or VRE2 cells into the vitreous with a 36-gauge needle. The left eye of each mouse was left untreated. At 6 h after bacterial injection, 1 × 108 PFU of phage ΦEF24C-P2 or 1 μg of VAN in 0.5 μl of physiological saline, or 0.5 μl of saline alone was administered into the vitreous of the right eye. We selected the concentration of VAN for in vivo administration on the basis of the dose administered in the clinical setting for the treatment of patients with endophthalmitis (36).
Macroscopic observation and indirect ophthalmoscopy.
At 24 h after bacterial injection, the infected eye was subjected to macroscopic analysis and to indirect ophthalmoscopy (Britescope BS-III LED; Neitz Instruments, Tokyo, Japan) for determination of a semiquantitative clinical score according to an established scale of 0 to 4 based on loss of fundus visibility (44), with slight modification, as follows: 0, a clear anterior chamber, clear vitreous, and clear view of the retina; 1, mild aqueous flare, mild vitreous haze, and slightly obscured view of the retina; 2, moderate aqueous flare, dense vitreal haze, poor pupil dilation, and moderately obscured view of the retina; 3, intense aqueous flare, opaque vitreous, and completely obscured view of the retina; and 4, bleeding in the anterior chamber.
Histopathologic analysis.
Mice were killed for eye isolation at 24 h after infection. The isolated eyes were fixed in Super Fix (Kurabo, Osaka, Japan), embedded in paraffin, sectioned at a thickness of 2 μm, and stained with hematoxylin-eosin. The sections were examined with a light microscope (Biorevo; Keyence, Osaka, Japan) and photographed. The severity of endophthalmitis was graded histopathologically on a scale of 0 to 12 as previously described (45).
Measurement of viable bacteria in the eye.
Eyes isolated at 24 h after infection were disrupted to release bacteria in 1.0 ml of ice-cold physiological saline with the use of a tissue homogenizer (mixer mill MM300; Qiagen, Venlo, the Netherlands) for 5 min at maximum speed. Portions of each homogenate were diluted with saline and plated on EF agar base for culture at 37°C for 48 h.
Assay of MPO activity.
The number of neutrophils in the eye was estimated by measurement of MPO activity as described previously (23), with slight modification. In brief, eyes isolated 24 h postinfection were homogenized in 1.0 ml of phosphate-buffered saline, the homogenate was centrifuged (10,000 × g, 15 min, 4°C), the resulting pellet was suspended in 0.03 ml of 50 mM potassium phosphate buffer (pH 6.0) containing 50 mM hexadecyltrimethylammonium bromide, and 0.07 ml of 50 mM potassium phosphate (pH 6.0) was then added to the suspension. Each sample was subjected to three freeze-thaw cycles and then centrifuged at 10,000 × g for 10 min at 4°C, after which 0.02 ml of the resulting supernatant was added to 0.05 ml of substrate reagent (R&D Systems, Minneapolis, MN), and the mixture was incubated for 20 min at room temperature. The reaction was terminated by the addition of 25 μl of 2 N H2SO4, and the absorbance at 450 nm was measured.
Analysis of retinal function.
The scotopic ERG response was determined as a measure of retinal function in mice with endophthalmitis. ERG readings were recorded with an acquisition system (PuREC; Mayo, Aichi, Japan) and light-emitting diode stimulator (LS-100; Mayo) at 24 h after infection as described previously (46), with slight modification. In brief, mice were dark-adapted overnight before ERG recording. Under dim red light, they were then anesthetized by intraperitoneal injection of a combination of medetomidine (Domitor; Nippon Zenyaku Kogyo, Tokyo, Japan) at 0.3 mg/kg, midazolam (Sandoz, Yamagata, Japan) at 4.0 mg/kg, and butorphanol (Vetorphale; Meiji Seika Pharma, Tokyo, Japan) at 5.0 mg/kg. Bilateral mydriasis was induced by topical application of 0.5% tropicamide and 0.5% phenylephrine (Santen Pharmaceutical, Osaka, Japan). A golden-ring electrode (Mayo) was placed on each cornea after topical anesthesia with 0.4% oxybuprocaine hydrochloride (Santen Pharmaceutical). A reference electrode (Mayo) was placed in the mouth, and a neutral electrode (Mayo) was attached to the tail. Scotopic a-wave and b-wave amplitude was recorded for both eyes with the use of flash ERG. The amplitude of the a-wave was measured from the baseline to the first negative peak, and that of the b-wave was measured from the a-wave peak to the first positive peak.
Measurement of viable phage in the eye.
At 6 h after injection of 0.5 μl of physiological saline with or without 1 × 104 E. faecalis EF24 into the vitreous, 1 × 108 PFU of phage ΦEF24C-P2 in 0.5 μl of physiological saline was administered into the vitreous. Eyes were isolated for determination of the concentration of phage at 18 or 66 h after intravitreal injection of ΦEF24C-P2.
Statistical analysis.
Quantitative data are presented as means ± standard error of the mean (SEM) and were analyzed with Student’s unpaired t test or the Tukey-Kramer test. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the Japan Society for the Promotion of Science KAKENHI grant JP 26462692 and by Novartis Pharma Research Grants 2017. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Norihito Morimoto for technical assistance.
REFERENCES
- 1.The Endophthalmitis Vitrectomy Study Group. 1996. Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol 122:830–846. doi: 10.1016/S0002-9394(14)70380-0. [DOI] [PubMed] [Google Scholar]
- 2.Kuriyan AE, Sridhar J, Flynn HW Jr., Smiddy WE, Albini TA, Berrocal AM, Forster RK, Belin PJ, Miller D. 2014. Endophthalmitis caused by Enterococcus faecalis: clinical features, antibiotic sensitivities, and outcomes. Am J Ophthalmol 158:1018–1023. doi: 10.1016/j.ajo.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todokoro D, Suzuki T, Kobayakawa S, Tomita H, Ohashi Y, Akiyama H. 2017. Postoperative Enterococcus faecalis endophthalmitis: virulence factors leading to poor visual outcome. Jpn J Ophthalmol 61:408–414. doi: 10.1007/s10384-017-0527-8. [DOI] [PubMed] [Google Scholar]
- 4.Scott IU, Loo RH, Flynn HW Jr., Miller D. 2003. Endophthalmitis caused by Enterococcus faecalis: antibiotic selection and treatment outcomes. Ophthalmology 110:1573–1577. doi: 10.1016/S0161-6420(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 5.Bains HS, Weinberg DV, Feder RS, Noskin GA. 2007. Postoperative vancomycin-resistant Enterococcus faecium endophthalmitis. Arch Ophthalmol 125:1292–1293. doi: 10.1001/archopht.125.9.1292. [DOI] [PubMed] [Google Scholar]
- 6.Esmaeli B, Holz ER, Ahmadi MA, Krathen RA, Raad II. 2003. Endogenous endophthalmitis secondary to vancomycin-resistant enterococci infection. Retina 23:118–119. doi: 10.1097/00006982-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Camarena JC, Bautista-de Lucio VM, Navas A, Ramirez-Miranda A, Graue-Hernandez EO. 2012. Delayed-onset post-keratoplasty endophthalmitis caused by vancomycin-resistant Enterococcus faecium. Case Rep Ophthalmol 3:370–374. doi: 10.1159/000344006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillier RJ, Arjmand P, Rebick G, Ostrowski M, Muni RH. 2013. Post-traumatic vancomycin-resistant enterococcal endophthalmitis. J Ophthalmic Inflamm Infect 3:42. doi: 10.1186/1869-5760-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera M, Pathengay A, Jindal A, Jalali S, Mathai A, Pappuru RR, Relhan N, Das T, Sharma S, Flynn HW. 2013. Vancomycin-resistant Gram-positive bacterial endophthalmitis: epidemiology, treatment options, and outcomes. J Ophthalmic Inflamm Infect 3:46. doi: 10.1186/1869-5760-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen J, Hartnett ME. 2017. Successful management of post-traumatic vancomycin-resistant Enterococcus endophthalmitis. Am J Ophthalmol Case Rep 5:117–118. doi: 10.1016/j.ajoc.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Desai RU, Pass AB, Saffra NA. 2010. Vancomycin-resistant enterococcal endophthalmitis. Arch Ophthalmol 128:794–795. doi: 10.1001/archophthalmol.2010.77. [DOI] [PubMed] [Google Scholar]
- 12.Tang CW, Cheng CK, Lee TS. 2007. Community-acquired bleb-related endophthalmitis caused by vancomycin-resistant enterococci. Can J Ophthalmol 42:477–478. doi: 10.3129/i07-057. [DOI] [PubMed] [Google Scholar]
- 13.Friling E, Lundstrom M, Stenevi U, Montan P. 2013. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg 39:15–21. doi: 10.1016/j.jcrs.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Kim HW, Kim SY, Chung IY, Lee JE, Lee JE, Park JM, Park JM, Han YS, Oum BS, Byon IS, Yun IH, Yoon HS, Park D, Jeong WJ, Yu BC, Park I, Bae T, Nam KY, Lee SJ. 2014. Emergence of Enterococcus species in the infectious microorganisms cultured from patients with endophthalmitis in South Korea. Infection 42:113–118. doi: 10.1007/s15010-013-0530-z. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Todokoro D, Kobayakawa S, Sotozono C, Eguchi S, Miyata K, Miyajima HB, Ike Y, Ohashi Y, Enterococci Endophthalmitis Working Group. 2014. Postcataract endophthalmitis caused by Enterococcus faecalis. Nippon Ganka Gakkai Zasshi 118:22–27. (In Japanese.) [PubMed] [Google Scholar]
- 16.Oliveira H, Costa AR, Ferreira A, Konstantinides N, Santos SB, Boon M, Noben JP, Lavigne R, Azeredo J. 2018. Functional analysis and antivirulence properties of a new depolymerase from a myovirus that infects Acinetobacter baumannii capsule K45. J Virol 93:e01163-18. doi: 10.1128/JVI.01163-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin H, Paff ML, Molineux IJ, Bull JJ. 2018. Antibiotic therapy using phage depolymerases: robustness across a range of conditions. Viruses 10:622. doi: 10.3390/v10110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone R. 2002. Bacteriophage therapy. Stalin’s forgotten cure. Science 298:728–731. doi: 10.1126/science.298.5594.728. [DOI] [PubMed] [Google Scholar]
- 19.Reardon S. 2014. Phage therapy gets revitalized. Nature 510:15–16. doi: 10.1038/510015a. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki S, Uchiyama J, Takemura-Uchiyama I, Daibata M. 2014. The age of the phage. Nature 509:S9. doi: 10.1038/509S9a. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama J, Rashel M, Maeda Y, Takemura I, Sugihara S, Akechi K, Muraoka A, Wakiguchi H, Matsuzaki S. 2008. Isolation and characterization of a novel Enterococcus faecalis bacteriophage phiEF24C as a therapeutic candidate. FEMS Microbiol Lett 278:200–206. doi: 10.1111/j.1574-6968.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 22.Uchiyama J, Rashel M, Takemura I, Wakiguchi H, Matsuzaki S. 2008. In silico and in vivo evaluation of bacteriophage phiEF24C, a candidate for treatment of Enterococcus faecalis infections. Appl Environ Microbiol 74:4149–4163. doi: 10.1128/AEM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda K, Ishida W, Uchiyama J, Rashel M, Kato S, Morita T, Muraoka A, Sumi T, Matsuzaki S, Daibata M, Fukushima A. 2012. Pseudomonas aeruginosa keratitis in mice: effects of topical bacteriophage KPP12 administration. PLoS One 7:e47742. doi: 10.1371/journal.pone.0047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh PK, Donovan DM, Kumar A. 2014. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother 58:4621–4629. doi: 10.1128/AAC.00126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jariah ROA, Hakim MS. 2019. Interaction of phages, bacteria, and the human immune system: evolutionary changes in phage therapy. Rev Med Virol: e2055. doi: 10.1002/rmv.2055. [DOI] [PubMed] [Google Scholar]
- 26.Brüssow H. 2005. Phage therapy: the Escherichia coli experience. Microbiology 151:2133–2140. doi: 10.1099/mic.0.27849-0. [DOI] [PubMed] [Google Scholar]
- 27.Takemura-Uchiyama I, Uchiyama J, Osanai M, Morimoto N, Asagiri T, Ujihara T, Daibata M, Sugiura T, Matsuzaki S. 2014. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes Infect 16:512–517. doi: 10.1016/j.micinf.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Cairns BJ, Payne RJ. 2008. Bacteriophage therapy and the mutant selection window. Antimicrob Agents Chemother 52:4344–4350. doi: 10.1128/AAC.00574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadlallah A, Chelala E, Legeais JM. 2015. Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol J 9:167–168. doi: 10.2174/1874364101509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsky L, Barrett G. 2018. Intracameral antibiotics for prophylaxis of postoperative endophthalmitis in Australia: a review. Clin Exp Ophthalmol 47:537–541. doi: 10.1111/ceo.13419. [DOI] [PubMed] [Google Scholar]
- 32.Todorich B, Faia LJ, Thanos A, Amin M, Folberg R, Wolfe JD, Todorich KM, Raphtis E, Ruby AJ, Williams GA, Hassan TS. 2018. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: a clinical-pathophysiological analysis. Am J Ophthalmol 188:131–140. doi: 10.1016/j.ajo.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Witkin AJ, Chang DF, Jumper JM, Charles S, Eliott D, Hoffman RS, Mamalis N, Miller KM, Wykoff CC. 2017. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology 124:583–595. doi: 10.1016/j.ophtha.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Witkin AJ, Shah AR, Engstrom RE, Kron-Gray MM, Baumal CR, Johnson MW, Witkin DI, Leung J, Albini TA, Moshfeghi AA, Batlle IR, Sobrin L, Eliott D. 2015. Postoperative hemorrhagic occlusive retinal vasculitis: expanding the clinical spectrum and possible association with vancomycin. Ophthalmology 122:1438–1451. doi: 10.1016/j.ophtha.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Fliney GD, Pecen PE, Cathcart JN, Palestine AG. 2018. Trends in treatment strategies for suspected bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol 256:833–838. doi: 10.1007/s00417-018-3910-3. [DOI] [PubMed] [Google Scholar]
- 36.Roth DB, Flynn HW Jr. 1997. Antibiotic selection in the treatment of endophthalmitis: the significance of drug combinations and synergy. Surv Ophthalmol 41:395–401. doi: 10.1016/S0039-6257(97)00005-2. [DOI] [PubMed] [Google Scholar]
- 37.Siedlar M, Szczepanik A, Wieckiewicz J, Pituch-Noworolska A, Zembala M. 1997. Vancomycin down-regulates lipopolysaccharide-induced tumour necrosis factor alpha (TNF alpha) production and TNF alpha-mRNA accumulation in human blood monocytes. Immunopharmacology 35:265–271. doi: 10.1016/S0162-3109(96)00156-7. [DOI] [PubMed] [Google Scholar]
- 38.Krehmeier U, Bardenheuer M, Voggenreiter G, Obertacke U, Schade FU, Majetschak M. 2002. Effects of antimicrobial agents on spontaneous and endotoxin-induced cytokine release of human peripheral blood mononuclear cells. J Infect Chemother 8:194–197. doi: 10.1007/s101560200036. [DOI] [PubMed] [Google Scholar]
- 39.Knezevic P, Curcin S, Aleksic V, Petrusic M, Vlaski L. 2013. Phage-antibiotic synergism: a possible approach to combatting Pseudomonas aeruginosa. Res Microbiol 164:55–60. doi: 10.1016/j.resmic.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Gelman D, Beyth S, Lerer V, Adler K, Poradosu-Cohen R, Coppenhagen-Glazer S, Hazan R. 2018. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res Microbiol 169:531–539. doi: 10.1016/j.resmic.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Uchiyama J, Takemura I, Satoh M, Kato S, Ujihara T, Akechi K, Matsuzaki S, Daibata M. 2011. Improved adsorption of an Enterococcus faecalis bacteriophage PhiEF24C with a spontaneous point mutation. PLoS One 6:e26648. doi: 10.1371/journal.pone.0026648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiki J, Nakamura T, Furusawa T, Ohno H, Takahashi H, Kitana J, Usui M, Higuchi H, Tanji Y, Tamura Y, Iwano H. 2018. Characterization of the lytic capability of a LysK-like endolysin, Lys-phiSA012, derived from a polyvalent Staphylococcus aureus bacteriophage. Pharmaceuticals (Basel) 11:25. doi: 10.3390/ph11010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, Campbell J, Swoboda JG, Walker S, Gilmore MS. 2011. Role of wall teichoic acids in Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci 52:3187–3192. doi: 10.1167/iovs.10-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiston EA, Sugi N, Kamradt MC, Sack C, Heimer SR, Engelbert M, Wawrousek EF, Gilmore MS, Ksander BR, Gregory MS. 2008. αB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun 76:1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozcimen M, Kurtoglu MG, Goktas S, Omeroglu E, Sakarya Y, Alpfidan I, Ozcimen S, Sakarya R, Yener HI, Demir LS, Saglam F. 2016. Daptomycin versus vancomycin in an Enterococcus faecalis endophthalmitis rabbit model. Curr Eye Res 41:232–239. doi: 10.3109/02713683.2015.1004722. [DOI] [PubMed] [Google Scholar]
- 46.Tomiyama Y, Fujita K, Nishiguchi KM, Tokashiki N, Daigaku R, Tabata K, Sugano E, Tomita H, Nakazawa T. 2016. Measurement of electroretinograms and visually evoked potentials in awake moving mice. PLoS One 11:e0156927. doi: 10.1371/journal.pone.0156927. [DOI] [PMC free article] [PubMed] [Google Scholar]