Rezafungin (CD101) is a novel echinocandin under development for once-weekly intravenous (i.v.) dosing. We evaluated the pharmacodynamics (PD) of rezafungin against 4 Candida tropicalis and 4 Candida dubliniensis strains, using the neutropenic mouse invasive candidiasis model. The area under the concentration-time curve (AUC)/MIC was a robust predictor of efficacy (R2 = 0.93 and 0.72, respectively).
KEYWORDS: Candida tropicalis, Candida dubliniensis, pharmacodynamics, rezafungin, echinocandin
ABSTRACT
Rezafungin (CD101) is a novel echinocandin under development for once-weekly intravenous (i.v.) dosing. We evaluated the pharmacodynamics (PD) of rezafungin against 4 Candida tropicalis and 4 Candida dubliniensis strains, using the neutropenic mouse invasive candidiasis model. The area under the concentration-time curve (AUC)/MIC was a robust predictor of efficacy (R2 = 0.93 and 0.72, respectively). The stasis free-drug 24-h AUC/MIC target exposure for the group ranged from 3 to 25, whereas the 1-log-kill free-drug 24-h AUC/MIC target exposure ranged from 4.3 to 62. These values are similar to those found in previous rezafungin PD studies with other Candida spp. Based on recent surveillance susceptibility data, AUC/MIC targets are likely to be exceeded for >99% of C. tropicalis and C. dubliniensis isolates with the previously studied human dose of 400 mg i.v. once weekly.
TEXT
Rezafungin is a novel echinocandin with broad activity against fungal pathogens, including all Candida and Aspergillus species (1–5). Similar to other echinocandin drugs, rezafungin targets 1,3-β-d-glucan synthesis. Unlike other echinocandins, which are administered once daily, rezafungin has the pharmacokinetic (PK) advantage of a prolonged terminal half-life of ∼133 h in humans, which allows for extended-interval dosing (6). Once-weekly dosing regimens have been studied in phase 1 and 2 trials demonstrating dose-proportional PK with relatively low interindividual variability and very favorable safety profiles (6).
Invasive candidiasis is the most common invasive fungal infection of hospitalized patients and the fourth most common cause of nosocomial bloodstream infections leading to high morbidity and mortality (7–10). Historically, Candida albicans has been the predominant causative species; however, in recent years, epidemiological shifts in prevalence have occurred (11–17). Indeed, in many parts of the world, non-albicans Candida species outnumber C. albicans strains. For example, a recent large epidemiological study of Candida bloodstream isolates from the Asia-Pacific region demonstrated that C. tropicalis isolates were almost equal in prevalence to C. albicans isolates, and, in sum, non-albicans Candida species accounted for almost 65% of all bloodstream isolates over the 2-year study (18). As previous studies have demonstrated species-specific pharmacodynamic (PD) targets for the echinocandin group against different Candida species, we sought to examine the PD efficacy of rezafungin against C. tropicalis and C. dubliniensis in a neutropenic mouse infection model. This included determining the target area under the concentration-time curve (AUC)/MIC exposures to assist in an optimal dosing-regimen design for infections with these species, estimation of the probability of clinical target attainment, and proposal of preliminary susceptibility breakpoints.
Four strains each of C. tropicalis and C. dubliniensis were selected for in vivo studies. Unless noted by ATCC number, each of the strains was a clinical isolate and was chosen based on similar growth in untreated animals (Table 1). Antimicrobial susceptibility testing was performed according to CLSI broth microdilution guidelines (19). The MIC values for rezafungin ranged from 0.016 to 0.06 mg/liter against the strain panels selected for both species (Table 1). The neutropenic mouse disseminated candidiasis model was used for all experiments. Neutropenia was induced by subcutaneous cyclophosphamide injection on days −4 (150 mg/kg), −1, +3, and +6 (100 g/kg) to ensure neutropenia throughout the 7-day experiment. Three mice were included in each treatment and control group. Mice were inoculated with one of the eight strains at 5.98 ± 0.06 log10 CFU/ml via the lateral tail vein. Antifungal treatment with rezafungin began 2 h after inoculation. Groups of animals were subjected to five rezafungin dosing regimens that varied from 0.25 to 64 mg/kg. Drug was administered intraperitoneally. We recently characterized the PK in this mouse model across the same dose range (20), and those data were utilized to calculate PK exposures for the present study. Given the prolonged half-life in mice (28 to 41 h), rezafungin doses were administered on days 0, 3, and 6. After 7 days, mice were euthanized for determination of fungal burden using quantitative CFU cultures from kidney homogenates. Organism burden in each treatment group was compared with fungal burden at the start of therapy and analyzed using the sigmoid maximum effect (Emax) model (Hill equation). Free-drug concentration was determined using a protein binding value of 99.2% in mice (20). The PK/PD parameter AUC/MIC was used for analysis because it is the demonstrated parameter linked to efficacy for echinocandins and rezafungin in previous PK/PD studies (20–22).
TABLE 1.
Susceptibility testing results, fitness, and PD target exposures for net stasis and 1-log kill for rezafungin in the neutropenic mouse disseminated candidiasis model
| Species | Strain | MIC (mg/liter) | Growth in untreated animals (CFU/kidneys) | Net stasis |
1-Log kill |
||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg every 72 h) | Free-drug AUC0–168/MIC | Free-drug avg AUC0–24/MIC | Dose (mg/kg every 72 h) | Free-drug AUC0–168/MIC | Free-drug avg AUC0–24/MIC | ||||
| C. tropicalis | 98-234 | 0.03 | 4.46 | 2.28 | 108.6 | 15.51 | 4.06 | 180.7 | 25.82 |
| ATCC 750 | 0.016 | 4.74 | 1.02 | 107.6 | 15.37 | 2.42 | 214.5 | 30.64 | |
| CAY2597 | 0.06 | 3.30 | 1.81 | 44.7 | 6.39 | 3.23 | 73.5 | 10.50 | |
| 1751 | 0.03 | 4.46 | 1.22 | 65.4 | 9.35 | 2.49 | 117.0 | 16.71 | |
| C. dubliniensis | 90 | 0.06 | 4.29 | 1.65 | 41.6 | 5.94 | 2.18 | 52.2 | 7.46 |
| 991460 | 0.06 | 4.52 | 1.02 | 28.6 | 4.09 | 23.51 | 431.5 | 61.64 | |
| 1031899 | 0.03 | 3.77 | 3.92 | 175.3 | 25.05 | 10.34 | 404.4 | 57.77 | |
| 1032127 | 0.06 | 3.86 | 0.66 | 21.5 | 3.07 | 1.09 | 30.1 | 4.30 | |
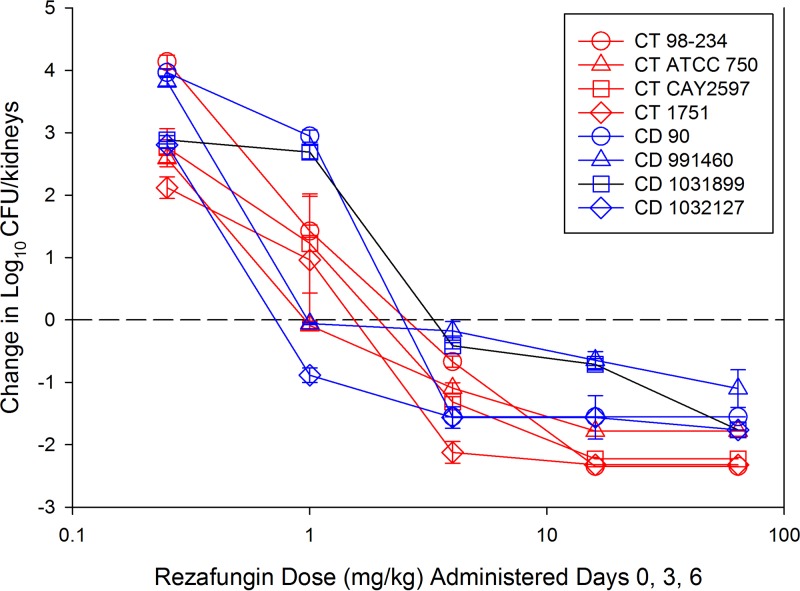
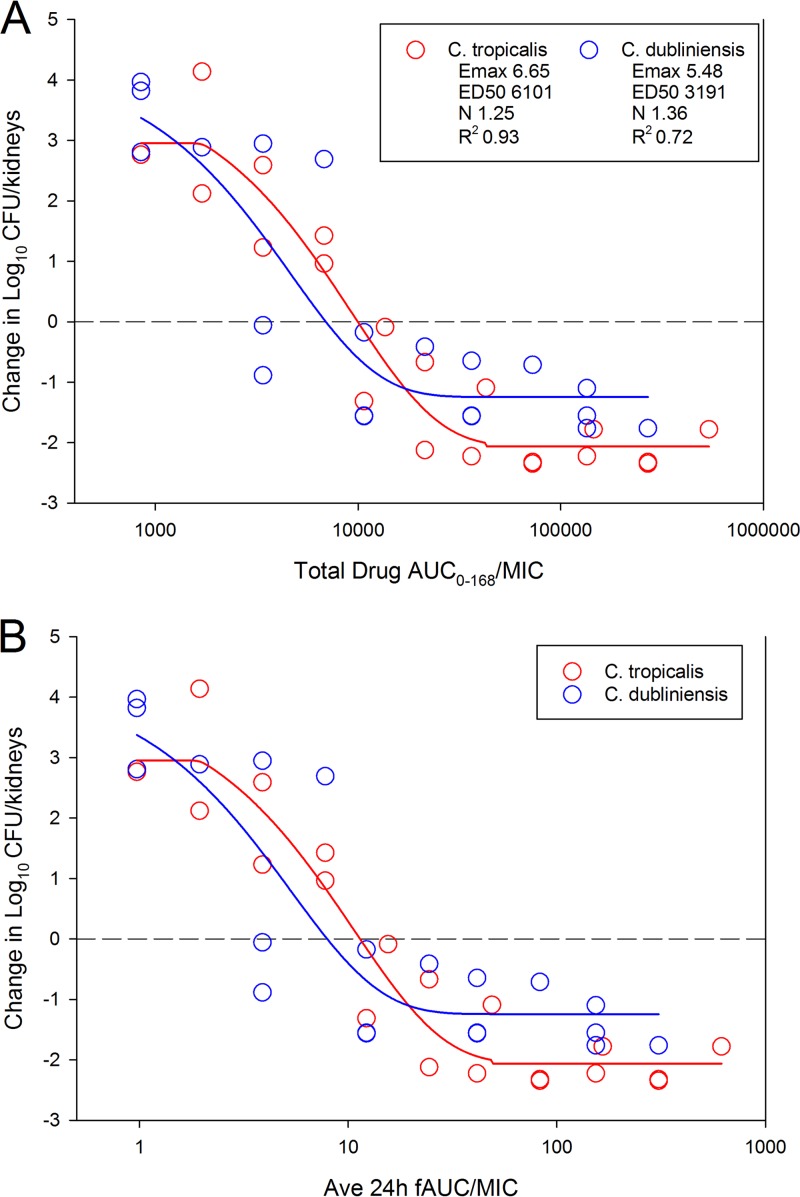
The results of the dose-ranging studies against C. tropicalis and C. dubliniensis are shown in Fig. 1. Dose-dependent activity was noted against all strains, with a >1-log10 kill, compared to fungal burden at the start of therapy, achieved for all strains. Compared with vehicle-treated controls, rezafungin-treated animals achieved an almost 6-log10 reduction in fungal burden. When the treatment data were modeled according to the AUC/MIC index, there was a robust relationship, with R2 values of 0.93 for C. tropicalis and 0.72 for C. dubliniensis (Fig. 2) strains. The doses necessary to achieve net stasis and a 1-log reduction compared with the start of therapy were calculated for each organism and are shown in Table 1. The respective 7-day (0 to 168 h) AUC/MIC target exposures for each endpoint (stasis and 1-log kill) were calculated, as was the average 24-h AUC/MIC. The latter was included to allow for comparison with other echinocandins in this model, which have traditionally been reported using 24-h AUC/MIC exposures. Free-drug 24-h average AUC/MIC targets ranged from 3 to 25 and 1-log kill from 4.3 to 62 for all organisms studied (Table 1).
FIG 1.
In vivo rezafungin dose-response curves for 4 C. tropicalis (CT) and 4 C. dubliniensis (CD) strains. Each symbol represents the mean and standard deviation (error bars) of the change in fungal burden in kidneys from 3 mice. Dashed horizontal line, burden at the start of therapy (0 h). Points above line, net increase in fungal burden. Points below line, net decrease in fungal burden over the 7-day experiment.
FIG 2.
Relationship between rezafungin treatment effect and the PK/PD AUC/MIC index for C. tropicalis and C. dubliniensis isolates. Each symbol represents the mean change in fungal burden in kidneys from 3 mice. Dashed horizontal line, burden at the start of therapy (0 h). Points above line, net increase in fungal burden. Points below line, net decrease in fungal burden over the 7-day experiment. Rezafungin PK/PD exposure-response relationship using total-drug AUC over the full treatment duration (total-drug AUC0–168/MIC) (A) and using the average free-drug 24-h AUC over the 7-day experiment (average free-drug AUC0–24/MIC) (B).
Previous neutropenic mouse invasive candidiasis PK/PD studies with rezafungin against C. albicans, Candida glabrata, Candida parapsilosis, and Candida auris species similarly demonstrated potent activity (20, 22). The 24-h free-drug average AUC/MIC target demonstrated in the previous studies was 0.5 to 3 for net stasis, which interestingly was numerically lower than that for other echinocandin (e.g., caspofungin, micafungin, and anidulafungin) PK/PD studies in this model. In the current study, we expanded the understanding of PD activity of rezafungin to C. tropicalis and C. dubliniensis strains, which are important pathogens in many areas worldwide (23). We demonstrated that the in vivo exposure-response activity of rezafungin against C. tropicalis and C. dubliniensis isolates was comparable to that in previous animal model studies with other Candida spp. These data also align with previous in vitro evaluations of rezafungin activity, which demonstrated relatively equipotent activity against C. tropicalis and C. dubliniensis compared with other Candida spp. (2, 3). The PK of rezafungin in humans has been well characterized (6), including population PK modeling and target attainment analysis for C. albicans and C. glabrata isolates (24, 25). According to the highest PK/PD target for each species identified in this study (24-h free-drug AUC/MIC, 15.5 for C. tropicalis and 25 for C. dubliniensis), the target exposure was met or exceeded for C. tropicalis organisms with a rezafungin MIC of ≤0.5 mg/liter and for C. dubliniensis organisms with a rezafungin MIC of ≤0.25 mg/liter. To put this in context, previous in vitro susceptibility studies for rezafungin have generated MIC90 values of 0.06 mg/liter for C. tropicalis (n = 274) and 0.12 mg/liter for C. dubliniensis (n = 102) isolates (2, 3, 26–28). Thus, the current rezafungin dosing regimen studied for clinical use would be expected to be efficacious for these two organism groups. Further clinical studies of rezafungin that examine patient outcomes in the context of PK exposure and MIC distribution are necessary to confirm these in vivo results.
In summary, rezafungin demonstrated potent in vivo efficacy against C. tropicalis and C. dubliniensis isolates in the neutropenic mouse disseminated candidiasis model. To our knowledge, this is the first in vivo PK/PD study in this model to use C. dubliniensis isolates. The PK/PD targets identified for net stasis and 1-log kill were similar to those in previous studies of rezafungin against other Candida spp. Integrating these PK/PD targets in the context of expected human drug exposure based on PK studies and known MIC distributions suggests that rezafungin is likely to be highly efficacious for these two important pathogen groups.
ACKNOWLEDGMENT
This study was supported by funding from Cidara Therapeutics, Inc.
REFERENCES
- 1.Wiederhold NP, Locke JB, Daruwala P, Bartizal K. 2018. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J Antimicrob Chemother 73:3063–3067. doi: 10.1093/jac/dky280. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. 2017. Activity of a long-acting echinocandin (CD101) and seven comparator antifungal agents tested against a global collection of contemporary invasive fungal isolates in the SENTRY 2014 Antifungal Surveillance Program. Antimicrob Agents Chemother 61:e02045-16. doi: 10.1128/AAC.02045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. 2017. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents 50:352–358. doi: 10.1016/j.ijantimicag.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Hall D, Bonifas R, Stapert L, Thwaites M, Shinabarger DL, Pillar CM. 2017. In vitro potency and fungicidal activity of CD101, a novel echinocandin, against recent clinical isolates of Candida spp. Diagn Microbiol Infect Dis 89:205–211. doi: 10.1016/j.diagmicrobio.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Berkow EL, Lockhart SR. 2018. Activity of CD101, a long-acting echinocandin, against clinical isolates of Candida auris. Diagn Microbiol Infect Dis 90:196–197. doi: 10.1016/j.diagmicrobio.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Sandison T, Ong V, Lee J, Thye D. 2017. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 61:e01627-16. doi: 10.1128/AAC.01627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE, NIAID Mycosis Study Group. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37:634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 8.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 9.Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, Callegari A, Righi E. 2015. Clinical and therapeutic aspects of candidemia: a five year single centre study. PLoS One 10:e0127534. doi: 10.1371/journal.pone.0127534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassetti M, Giacobbe DR, Vena A, Trucchi C, Ansaldi F, Antonelli M, Adamkova V, Alicino C, Almyroudi MP, Atchade E, Azzini AM, Carannante N, Carnelutti A, Corcione S, Cortegiani A, Dimopoulos G, Dubler S, Garcia-Garmendia JL, Girardis M, Cornely OA, Ianniruberto S, Kullberg BJ, Lagrou K, Le Bihan C, Luzzati R, Malbrain M, Merelli M, Marques AJ, Martin-Loeches I, Mesini A, Paiva JA, Peghin M, Raineri SM, Rautemaa-Richardson R, Schouten J, Brugnaro P, Spapen H, Tasioudis P, Timsit JF, Tisa V, Tumbarello M, van den Berg C, Veber B, Venditti M, Voiriot G, Wauters J, Montravers P. 2019. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care 23:219. doi: 10.1186/s13054-019-2497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol 49:396–399. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother 55:561–566. doi: 10.1128/AAC.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. 2011. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in intensive care unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int J Antimicrob Agents 38:65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. 2012. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004–2008. Diagn Microbiol Infect Dis 74:323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73:45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Guinea J. 2014. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 18.Tan TY, Hsu LY, Alejandria MM, Chaiwarith R, Chinniah T, Chayakulkeeree M, Choudhury S, Chen YH, Shin JH, Kiratisin P, Mendoza M, Prabhu K, Supparatpinyo K, Tan AL, Phan XT, Tran TT, Nguyen GB, Doan MP, Huynh VA, Nguyen SM, Tran TB, Van Pham H. 2016. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol 54:471–477. doi: 10.1093/mmy/myv114. [DOI] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard —3rd ed CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. 2018. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob Agents Chemother 62:e02154-17. doi: 10.1128/AAC.02154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepak AJ, Andes DR. 2014. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5:a019653. doi: 10.1101/cshperspect.a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepak AJ, Zhao M, Andes DR. 2018. Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob Agents Chemother 62:e01572-18. doi: 10.1128/AAC.01572-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Xu YC, Hsueh PR. 2016. Epidemiology of candidemia and antifungal susceptibility in invasive Candida species in the Asia-Pacific region. Future Microbiol 11:1461–1477. doi: 10.2217/fmb-2016-0099. [DOI] [PubMed] [Google Scholar]
- 24.Lakota EA, Ong V, Flanagan S, Rubino CM. 2018. Population pharmacokinetic analyses for rezafungin (CD101) efficacy using phase 1 data. Antimicrob Agents Chemother 62:e02603-17. doi: 10.1128/AAC.02603-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bader JC, Lakota EA, Flanagan S, Ong V, Sandison T, Rubino CM, Bhavnani SM, Ambrose PG. 2018. Overcoming the resistance hurdle: pharmacokinetic-pharmacodynamic target attainment analyses for rezafungin (CD101) against Candida albicans and Candida glabrata. Antimicrob Agents Chemother 62:e02614-17. doi: 10.1128/AAC.02614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castanheira M, Messer SA, Rhomberg PR, Dietrich RR, Pfaller MA. 2018. Activity of a long-acting echinocandin rezafungin (CD101) and comparator antifungal agents tested against contemporary invasive fungal isolates: SENTRY 2016, abstr Friday-485. Abstr ASM Microbe, Atlanta, GA. American Society of Microbiology, Washington, DC. [Google Scholar]
- 27.Pfaller MA, Messer SA, Rhomberg PR, Schaefer BA, Castanheira M. 2018. Activity of a long-acting echinocandin rezafungin tested against invasive fungal isolates collected worldwide, abstr 2400. Abstr ID Week 2018, San Francisco, CA. Infectious Diseases Society of America, Arlington, VA. [Google Scholar]
- 28.Toth Z, L F, Locke JB, Kardos G, Nagy F, Kovacs R, Szekely A, Borman AM, Majoros L. 2019. Activity of rezafungin against common and rare Candida species in vitro, abstr P2161. Abstr European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, Netherlands. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]