A plasmid-located fosfomycin resistance gene, fosA8, was identified from a CTX-M-15-producing Escherichia coli isolate recovered from urine. Identification of this gene was obtained by whole-genome sequencing. It encoded FosA8, which shares 79% and 78% amino acid identity with the most closely related FosA2 and FosA1 enzymes, respectively. The fosA8 gene was located on a transferable 50-kb plasmid of IncN type encoding high-level resistance to fosfomycin.
KEYWORDS: fosfomycin, FOS, Escherichia coli, fosA
ABSTRACT
A plasmid-located fosfomycin resistance gene, fosA8, was identified from a CTX-M-15-producing Escherichia coli isolate recovered from urine. Identification of this gene was obtained by whole-genome sequencing. It encoded FosA8, which shares 79% and 78% amino acid identity with the most closely related FosA2 and FosA1 enzymes, respectively. The fosA8 gene was located on a transferable 50-kb plasmid of IncN type encoding high-level resistance to fosfomycin. In silico analysis and cloning experiments identified fosA8 analogues (99% identity) in the genome of Leclercia decarboxylata, which is an enterobacterial species with natural resistance to fosfomycin. This finding adds L. decarboxylata to the list of enterobacterial species that are a reservoir of fosA-like genes which have been captured from the chromosome of a progenitor and are then acquired by E. coli.
INTRODUCTION
Fosfomycin is a phosphonic acid-derived antibiotic with a broad-spectrum bactericidal activity used as a first-line oral agent for uncomplicated urinary tract infections (1). It is receiving renewed interest worldwide as an antibiotic that contributes to the sparing of carbapenems in the treatment of infections due to extended-spectrum β-lactamase (ESBL)-producing isolates (2, 3). These ESBL producers are increasingly reported worldwide, particularly in Escherichia coli (4). Therefore, surveillance studies aiming to evaluate the spread and to identify the nature of the fosfomycin resistance determinants are becoming of growing importance for preserving the efficacy of that antibiotic.
Chromosomally encoded fosfomycin resistance mechanisms in E. coli include reduced drug uptake due to mutations in the genes encoding GlpT and UhpT transporters or mutations in the fosfomycin target which is the enzyme catalyzing the first step in peptidoglycan biosynthesis, namely, MurA. Reduced drug uptake remains the most frequent fosfomycin resistance mechanism (1, 5). Transferable and plasmid-encoded resistance mechanisms in E. coli are the FosA metalloenzymes, responsible for fosfomycin inactivation by catalyzing the conjugation of glutathione to fosfomycin (1, 5). FosA enzymes are Mn2+ and K+-dependent glutathione S-transferases that have been shown to inactivate fosfomycin in several Gram-negative bacterial species.
Among the seven known FosA variants, four (FosA3, FosA4, FosA5, and FosA6) have been identified as acquired resistance determinants in E. coli isolates (6–15). The FosA3 variant is the most widespread fosfomycin resistance determinant in E. coli, reported in human and animal isolates from Asia, Europe, and the United States (6, 7, 16–19). The natural reservoir of some plasmid-mediated fosA determinants has been identified. The fosA3, fosA5, fosATn2921, and fosA6 genes originate from Kluyvera georgiana, Klebsiella pneumoniae, Enterobacter cloacae, and Klebsiella pneumoniae, respectively (9, 10).
Recently, by performing a screening of fosfomycin resistance determinants among a collection of ESBL-producing E. coli isolates from Switzerland, we identified a single isolate harboring a plasmid-mediated fosfomycin resistance determinant that did not correspond to any known fosA gene (20). Therefore, the purpose of the study was to characterize this fosfomycin resistant determinant and to identify its origin.
RESULTS
Identification of the fosA8 gene.
E. coli isolate 376 was resistant to broad-spectrum cephalosporins, fluoroquinolones, kanamycin, tobramycin, and tetracycline (Table 1). It was also resistant to fosfomycin, according to the result of the rapid fosfomycin NP test (21), and had a fosfomycin MIC value of 1,024 μg/ml. This MIC value was reduced by 16-fold when phosphonoformate (PPF) was added, suggesting the presence of a FosA group glutathione-S-transferase activity (22). Using whole-cell DNA from E. coli 376 as the template, PCR amplifications were negative for the known fosA genes (fosA1 to fosA7 genes). Whole-genome sequencing (WGS) of E. coli 376 was then performed to possibly identify the mechanism that is responsible for fosfomycin resistance. A gene with a significant homology to fosA-like genes was identified. This novel fosA gene, which was named fosA8, encoded the FosA8 enzyme, which was 141 amino acids long and shared 66% to 79% amino acid identity with the FosA1 to FosA7 enzymes, the most closely related being FosA2. A BLAST search revealed that the same fosA8 gene was present in two E. coli strains isolated in the United States (GenBank accession no. CP041527.1 and CP019910.1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| J53 | Recipient strain for conjugation experiment; azide resistant | 34 |
| TOP10 | Recipient strain for cloning experiments | Invitrogen |
| 376 | Clinical isolate resistant to extended-spectrum cephalosporins, fluoroquinolones, kanamycin, tobramycin, tetracycline, and fosfomycin | This study |
| L. adecarboxylata DSMZ5077 | Natural fosfomycin-resistant enterobacterial species | This study |
| Plasmids | ||
| pACYC184 | Cloning plasmid; chloramphenicol and tetracycline resistant | New England Biolabs |
| p376 | Natural ca. 50-kb and IncN plasmid of E. coli 376 | This study |
| pFosA8 | Recombinant plasmid pACYC184 with a 789-bp PCR fragment (fosA8) | This study |
| pFosALA | Recombinant plasmid pACYC184 with a 789-bp PCR fragment (fosALA) | This study |
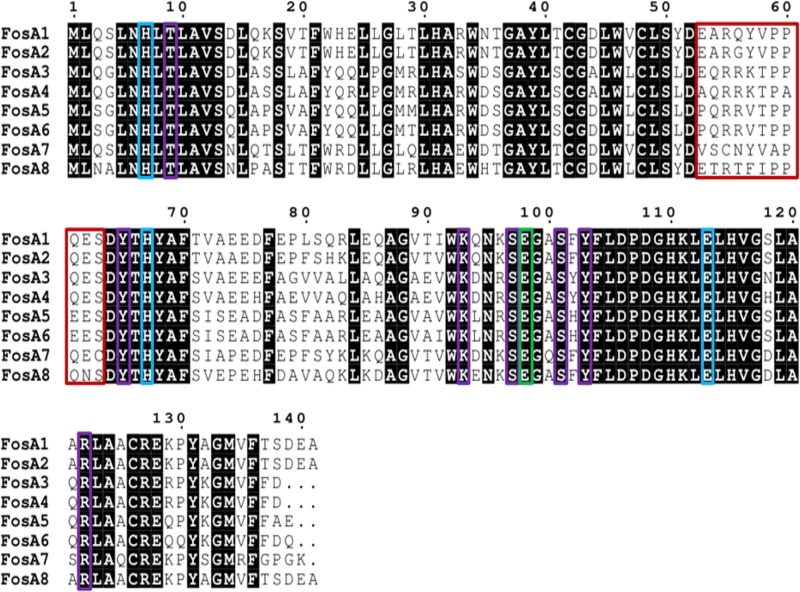
Phylogenetic analysis performed with FosA8 and the other FosA type enzymes revealed that FosA8 was distantly related to the FosA enzymes, representing a distinct cluster of FosA proteins (Fig. 1). FosA8 possessed conserved residues of FosA proteins that are implicated in the dimer formation of the FosA proteins, of Mn2+ and K+ binding, and to fosfomycin binding (1) (Fig. 2).
FIG 1.
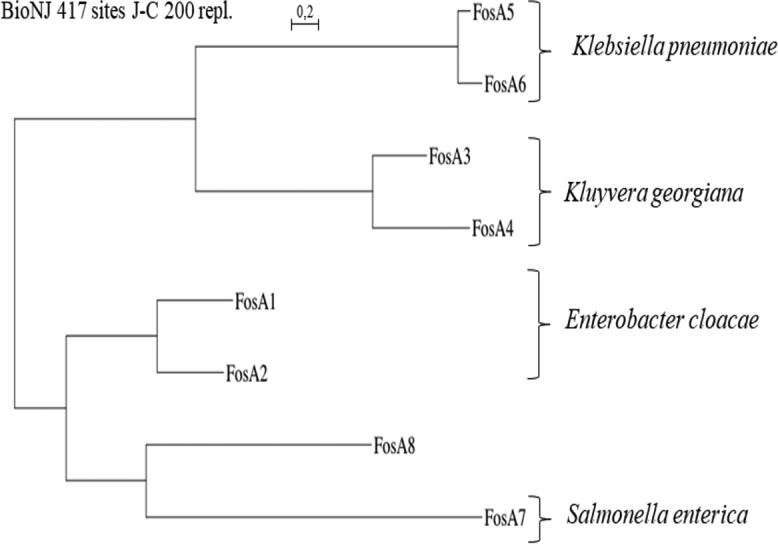
Phylogenetic tree obtained for the FosA proteins identified by distance method using the neighbor-joining algorithm (SeaView version 4 software [33]). Branch lengths are drawn to scale and are proportional to the number of amino acid substitutions with 500 bootstrap replications. The distance along the vertical axis has no significance. The enterobacterial species known as the natural reservoirs of these FosA proteins are shown on the right.
FIG 2.
Amino acid alignment of plasmid-mediated FosA proteins. Red, blue, green, and purple boxes bracket amino acids of the dimer interface loop and those implicated in Mn2+ coordination, in the K+ binding loop, and in fosfomycin binding, respectively (11).
Genetic context of the fosA8 gene.
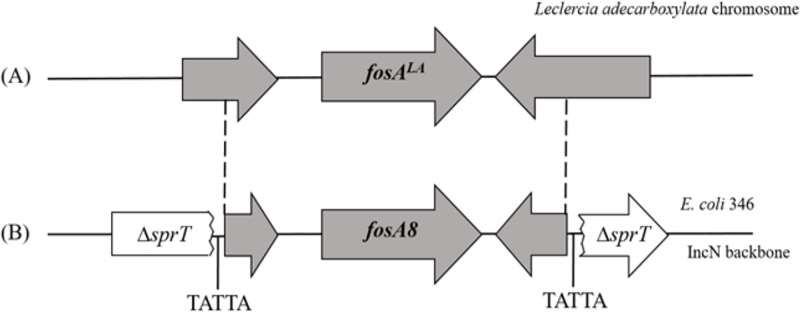
Analysis of the WGS of E. coli 376 showed that the fosA8 gene was located on a plasmid that also carried a kanamycin resistance marker. Mating-out assays showed that this plasmid was self-conjugative, at a frequency that was estimated to be ca. 10−5. This plasmid did not carry the blaCTX-M-15 ESBL gene that was identified in E. coli 376. PCR-based replicon typing and plasmid analysis following extraction by the Kieser method (23) performed with isolate 376 confirmed the WGS data and identified an IncN natural plasmid 50 kb in size. The fosA8 gene was inserted into the sprT gene and was bracketed by two direct repeat sequences (Fig. 3). The sprT gene encoded a hypothetical protein of unknown function (Fig. 3). The occurrence of identical direct repeats, TATTA, on each side of the DNA fragment encompassing the fosA8 gene strongly suggested that the insertion of the fosA8 gene onto its plasmid support resulted from a transposition event (Fig. 3).
FIG 3.
Genetic structures surrounding the fosALA gene on the chromosome of L. adecarboxylata (A) and on the fosA8 gene on plasmid p376 of E. coli 376 (B). The grey-shaded sequences indicate sequence identity between plasmid p376 and L. adecarboxylata DSMZ5077. TATTA direct repeats bracketing fosA8 on plasmid p376 are also indicated.
Origin of the fosA8 gene.
In silico analysis using the GenBank databases confirmed that fosA-like genes are present in the chromosomes of several enterobacterial species, such as those of Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, and Kluyvera ascorbata (9). FosA8 showed the highest percentage of amino acid identity (98%, differing in 3 amino acids) with a FosA protein, named FosALA, which is encoded by a gene identified in the chromosome of the enterobacterial species Leclercia adecarboxylata strain USDA-ARS-USMARC-60222 (GenBank accession no. CP013990.1). Accordingly, the close sequences located upstream and downstream of the fosA8 gene shared significant identity at the nucleotide level (99%) with those of L. adecarboxylata USDA-ARS-USMARC-60222 (Fig. 3).
Highly similar fosALA-like genes were identified in six chromosomes of Leclercia species available in the genome databases, including two L. adecarboxylata isolates (GenBank accession no. CP036199.1 and LR590464.1) and four Leclercia species isolates (GenBank accession no. CP026387.1, CP026167.1, CP031104.1 and CP031101.1), sharing from 85% to 97% nucleotide identity with fosALA.
The enterobacterial species L. adecarboxylata is rarely identified as a source of human infections and is naturally resistant to fosfomycin (24, 25).
In order to characterize the fosfomycin resistance conferred by FosLA, the L. adecarboxylata strain DSMZ5077 was analyzed. It carried a fosLA gene identical to that of L. adecarboxylata strain USDA-ARS-USMARC-60222. The fosfomycin resistance phenotype observed in L. adecarboxylata DSMZ5077 was antagonized by the addition of phosphonoformate (with the fosfomycin MIC value being decreased by 16-fold), indicating the role of FosALA for conferring fosfomycin resistance in that species. Both fosA8 and fosALA genes were cloned and expressed in E. coli TOP10. They conferred identical levels of resistance to fosfomycin (fosfomycin MIC, 1,024 mg/liter).
DISCUSSION
We report here the identification of a novel plasmid-mediated and transferable fosfomycin resistance determinant, FosA8, in E. coli. This enzyme conferred a high level of resistance to fosfomycin. Its <80% amino acid identity with known FosA proteins indicates the diversity of fosA genes that may circulate, remaining undetected when screening surveys based on molecular tools are performed. It is noteworthy that analysis of GenBank databases showed that this gene is present in two E. coli isolates recovered in the United States, indicating that it is already present on another continent.
The concomitant identification of the natural reservoir of the fosA8 gene, L. decarboxylata, adds to the growing list of enterobacterial species that naturally possess a fosA-like gene and from which those genes are translocated onto plasmids in E. coli (9, 10). In contrast to other enterobacterial species that possess these fosA-like genes, such as K. pneumoniae (9), fosA8-like genes are likely expressed at a high level in L. decarboxylata, since this species is naturally resistant to fosfomycin (25) and the fosfomycin resistance is inhibited by the addition of phosphonormate. The driving forces at the origin of transfer and the spread of fosA genes from their natural reservoir to a plasmid location in an enterobacterial species, E. coli, which does not possess a fosA gene, remain to be investigated.
This novel FosA determinant is reported from an E. coli isolate belonging to sequence type 40 (ST40) that is recognized as an emerging extraintestinal E. coli clone (26). Of note, the fosA8 and blaCTX-M-15 genes were not associated on the same plasmid. This is in contrast to the most commonly identified fosA gene, fosA3, which is always identified on plasmids carrying either ESBL genes, AmpC-type β-lactamase genes, or carbapenemase genes (16–19).
Nonetheless, identification of the FosA8 determinant from a CTX-M-producing isolate further highlights that ESBL producers are important reservoirs of transferable fosfomycin resistance determinants. This result may be a source of concern, since fosfomycin is becoming a first-line therapy for treating cystitis due to ESBL producers and the prevalence of ESBL producers is still rising worldwide.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli isolate 376 was isolated from urine in the context of screening 1,225 ESBL-producing E. coli isolates from a Swiss collection obtained from 2012 and 2013 (20). The list of strains and plasmids used in the study is shown in Table 1.
Fosfomycin resistance determination.
Two techniques were used to assess fosfomycin susceptibility. First, the agar dilution method using cation-adjusted Mueller-Hinton agar (MHA-CA, reference 64884; Bio-Rad, Marnes-la-Coquette, France) supplemented with 25 μg/ml glucose-6-phosphate was used to determine fosfomycin MICs, as recommended by Clinical and Laboratory Standards Institute (CLSI) guidelines (27). The breakpoints of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) were used as a reference (28). E. coli isolates with MICs of >32 μg/ml were categorized as resistant. Second, the rapid fosfomycin/E. coli NP test was performed as described by Nordmann et al. (21). The results of this test were read after 1 h 30 min of incubation at 35 ± 2°C.
FosA-like role in fosfomycin resistance.
The contribution of a potential FosA-like enzyme in fosfomycin resistance was assessed first by using the disk diffusion method as described by Nakamura et al. (22). Briefly, the fosfomycin-resistant E. coli 376 and the L. decarboxylata DBZ isolate at an ∼0.5 McFarland standard were inoculated onto Mueller-Hinton agar plates supplemented with 25 μg/ml of glucose-6-phosphate. Two disks containing 200 μg of fosfomycin, with and without 0.5 mg of the FosA inhibitor sodium phosphonoformate (PPF) (Sigma-Aldrich), were added to the plates, which were incubated at 35 ± 2°C overnight. Actually, PPF selectively inhibits the FosA-like proteins, which allows differentiation between plasmid-mediated FosA and other mechanisms of resistance to fosfomycin. An increase in the diameter of the growth inhibition zone by ≥5 mm in the presence of PPF is interpreted as FosA-related resistance.
Molecular analyses.
DNA was extracted from E. coli strain 376 with the QIAamp DNA minikit and the QIAcube workstation (Qiagen, Courtaboeuf, France), according to the manufacturer’s instructions. PCR amplification (Microsynth, Balgach, Switzerland) was performed to detect any known plasmid-mediated fosA genes (fosA1 to fosA7), as previously described (21). PCR conditions were 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, 52°C for 30 s and 72°C for 30 s, with a final extension of 72°C for 5 min. Sequences were analyzed with CloneManager Professional (Sci-Ed Software, Denver, CO, USA) (29). Whole-genome DNA of the fosA8-positive E. coli isolate and its transconjugant was extracted using a Sigma-Aldrich GenElute bacterial genomic DNA kit. Genomic libraries were assessed using a Nextera XT library preparation kit (Illumina Inc., San Diego, CA), and sequencing was performed using an Illumina MiniSeq system with 300-bp paired-end reads and a coverage of 50 times. The generated FastQ data were compiled and analyzed using the CLC Genomic Workbench (version 7.5.1; CLC Bio, Aarhus, Denmark). Reads were de novo assembled with automatic bubble and word size, and contigs with a minimum contig length of 800 nucleotides were generated using the mapping mode map reads back to contigs. The resulting contigs were uploaded into the Center for Genomic Epidemiology server (http://www.genomicepidemiology.org/). The plasmid replicon type and multilocus sequence type according to the Warwick typing scheme were determined using the PlasmidFinder (version 1.3) and MLST (version 1.8) programs, respectively (26, 30, 31). Sequence alignments was performed with ESPript 3.0 software, and construction of phylogenetic trees was performed with the SeaView alignment tool (version 4; Prabi, La Doua, Lyon, France) (32).
The fosA8 gene from E. coli 376 and the fosLA gene from L. adecarboxylata were cloned into the pACYC184 vector by using primers listed in Table 2 and expressed in E. coli TOP10.
TABLE 2.
Oligonucleotide sequences designed for this studya
| Primer | Sequence |
|---|---|
| fosA8 BamHI forward | 5′-GATGATGGATCCAGCGTGAAATGGAAGTGTGG-3′ |
| fosA8 SalI forward | 5′-GATGATGTCGACTAATACTGGTGATCCACGGC-3′ |
| fosA8 intern forward | 5′-AACCATCTGACCCTTGCTGT-3′ |
| fosA8 intern reverse | 5′-CGAGAAAATAGAACGACGCC-3′ |
Restriction sites are underlined; intern, internal to the fosA gene.
Plasmid analysis and conjugation.
Plasmid analysis was performed using the Kieser extraction method (23), followed by gel electrophoresis, in order to further confirm the size of the plasmid containing the fosA8 gene using E. coli strain 50192 harboring four plasmids of 154, 66, 48, and 7 kb as plasmid size markers (33). The determination of the plasmid incompatibility group was confirmed by PCR-based replicon typing (PBRT) (30). Conjugation experiments were performed using E. coli strain 376 and azide-resistant strain E. coli J53 as donor and recipient strains, respectively, as described previously (20). Both donor and recipient strains were cultured in exponential phase and then mixed on solid Luria Broth agar using filters at a 1:10 donor/recipient ratio. After 5 h of incubation, the filters were resuspended in 0.85% NaCl and the bacterial mixture was plated onto agar plates supplemented with fosfomycin (25 μg/ml) and sodium azide (100 μg/ml). The susceptibility of all E. coli transconjugants to fosfomycin was assessed by disk diffusion with azide- and fosfomycin-containing disks and by the PPF test (22).
Accession number(s).
The sequence data corresponding to the fosA8 gene reported in this paper was submitted to the GenBank nucleotide database under accession no. MN150127. In addition, the contigs corresponding to the whole genome of isolate 376 have been deposited as a BioProject under accession no. PRJNA558667.
ACKNOWLEDGMENTS
This work was funded by the University of Fribourg and by the Laboratoire Européen Associé INSERM Emerging Antibiotic Resistance in Gram-Negative Bacteria, University of Fribourg, Fribourg, Switzerland.
REFERENCES
- 1.Silver LL. 2017. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med 7:a025262. doi: 10.1101/cshperspect.a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 4.Pitout JDD, Laupland KB. 2008. Extended-spectrum β-lactamase producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 5.Cattoir V, Guérin F. 2018. How is fosfomycin resistance developed in Escherichia coli? Future Microbiol 13:1693–1696. doi: 10.2217/fmb-2018-0294. [DOI] [PubMed] [Google Scholar]
- 6.Alrowais H, McElheny CL, Spychala CN, Sastry S, Guo Q, Butt AA, Doi Y. 2015. Fosfomycin resistance in Escherichia coli, Pennsylvania, USA. Emerg Infect Dis 21:2045–2047. doi: 10.3201/eid2111.150750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benzerara Y, Gallah S, Hommeril B, Genel N, Decre D, Rottman M, Arlet G. 2017. Emergence of plasmid-mediated fosfomycin-resistance genes among Escherichia coli isolates, France. Emerg Infect Dis 23:1564–1567. doi: 10.3201/eid2309.170560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Q, Tomich AD, McElheny CL, Cooper VS, Stoesser N, Wang M, Sluis-Cremer N, Doi Y. 2016. Glutathione-S-transferase FosA6 of Klebsiella pneumoniae origin conferring fosfomycin resistance in ESBL-producing Escherichia coli. J Antimicrob Chemother 71:2460–2465. doi: 10.1093/jac/dkw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito R, Mustapha MM, Tomich AD, Callaghan JD, McElheny CL, Mettus RT, Shanks RMQ, Sluis-Cremer N, Doi Y. 2017. Widespread fosfomycin resistance in Gram-negative bacteria attributable to the chromosomal fosA gene. mBio 8:e00749-17. doi: 10.1128/mBio.00749-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito R, Pacey MP, Mettus RT, Sluis-Cremer N, Doi Y. 2018. Origin of the plasmid-mediated fosfomycin resistance gene fosA3. J Antimicrob Chemother 73:373–376. doi: 10.1093/jac/dkx389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klontz EH, Tomich AD, Günther S, Lemkul JA, Deredge D, Silverstein Z, Shaw JF, McElheny C, Doi Y, Wintrode PL, MacKerell AD Jr, Sluis-Cremer N, Sundberg EJ. 2017. Structure and dynamics of FosA-mediated fosfomycin resistance in Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 61:e01572-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Xu X, Guo Q, Wang P, Wang W, Wang M. 2015. Characterization of fosA5, a new plasmid-mediated fosfomycin resistance gene in Escherichia coli. Lett Appl Microbiol 60:259–264. doi: 10.1111/lam.12366. [DOI] [PubMed] [Google Scholar]
- 13.Rehman MA, Yin X, Persaud-Lachhman MG, Diarra MS. 2017. First detection of a fosfomycin resistance gene, fosA7, in Salmonella enterica serovar Heidelberg isolated from broiler chickens. Antimicrob Agents Chemother 61:e00410-17. doi: 10.1128/AAC.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Miao V, Kwong W, Xia R, Davies J. 2011. Identification of a novel fosfomycin resistance gene (fosA2) in Enterobacter cloacae from the Salmon River, Canada. Lett Appl Microbiol 52:427–429. doi: 10.1111/j.1472-765X.2011.03016.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang TY, Lu PL, Tseng SP. 2019. Update on fosfomycin-modified genes in Enterobacteriaceae. J Microbiol Immunol Infect 52:9–21. doi: 10.1016/j.jmii.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Lee SY, Park YJ, Yu JK, Jung S, Kim Y, Jeong SH, Arakawa Y. 2012. Prevalence of acquired fosfomycin resistance among extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother 67:2843–2847. doi: 10.1093/jac/dks319. [DOI] [PubMed] [Google Scholar]
- 17.Lupo A, Saras E, Madec JY, Haenni M. 2018. Emergence of blaCTX-M-55 associated with fosA, rmtB and mcr gene variants in Escherichia coli from various animal species in France. J Antimicrob Chemother 73:867–872. doi: 10.1093/jac/dkx489. [DOI] [PubMed] [Google Scholar]
- 18.Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. 2010. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother 54:3061–3064. doi: 10.1128/AAC.01834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie M, Lin D, Chen K, Wai Chi Chan E, Yao W, Wen S. 2016. Molecular characterization of Escherichia coli strains isolated from retail meat that harbor blaCTX-M and fosA3 genes. Antimicrob Agents Chemother 60:2450–2455. doi: 10.1128/AAC.03101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller L, Cimen C, Poirel L, Descombes MC, Nordmann P. 2019. Prevalence of fosfomycin resistance among ESBL-producing Escherichia coli isolates in the community, Switzerland. Eur J Clin Microbiol Infect Dis 38:945–949. doi: 10.1007/s10096-019-03531-0. [DOI] [PubMed] [Google Scholar]
- 21.Nordmann P, Poirel L, Mueller L. 2018. Rapid detection of fosfomycin resistance in Escherichia coli. J Clin Microbiol 57:e01531-18. doi: 10.1128/JCM.01531-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura G, Wachino J, Sato N, Kimura K, Yamada K, Jin W, Shibayama K, Yagi T, Kawamura K, Arakawa Y. 2014. Practical agar-based disk potentiation test for detection of fosfomycin-nonsusceptible Escherichia coli clinical isolates producing glutathione S-transferases. J Clin Microbiol 52:3175–3179. doi: 10.1128/JCM.01094-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. doi: 10.1016/0147-619X(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 24.Leclerc H. 1962. Étude biochimique d’Enterobacteriaceae pigmentées. Ann Inst Pasteur (Paris) 102:726–741. [PubMed] [Google Scholar]
- 25.Stock I, Burak S, Wiedemann B. 2004. Natural antimicrobial susceptibility patterns and biochemical profiles of Leclercia adecarboxylata strains. Clin Microbiol Infect 10:724–733. doi: 10.1111/j.1469-0691.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- 26.Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. 2015. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol 53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing; 28th informational supplement (M100–S28). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 29.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:320–324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 33.Vivian A. 1994. Plasmid expansion? Microbiology 140:213–214. doi: 10.1099/13500872-140-2-213-a. [DOI] [PubMed] [Google Scholar]
- 34.Jacoby GA, Han P. 1996. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol 34:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]