Piperacillin-tazobactam (TZP) is frequently used to treat severe hospital-acquired infections in children. We performed a single-center, pharmacokinetic (PK) trial of TZP in children ranging in age from 2 months to 6 years from various clinical subpopulations.
KEYWORDS: children, pediatrics, pharmacokinetics, piperacillin-tazobactam, population pharmacokinetics, sepsis
ABSTRACT
Piperacillin-tazobactam (TZP) is frequently used to treat severe hospital-acquired infections in children. We performed a single-center, pharmacokinetic (PK) trial of TZP in children ranging in age from 2 months to 6 years from various clinical subpopulations. Children who were on TZP per the standard of care were prospectively included and assigned to receive a dose of 80 mg/kg of body weight every 6 h infused over 2 h (ages 2 to 5 months) or a dose of 90 mg/kg every 8 h infused over 4 h (ages 6 months to 6 years). Separate population PK models were developed for piperacillin and tazobactam using nonlinear mixed-effects modeling. Optimal dosing was judged based on the ability to maintain free piperacillin concentrations above the piperacillin MIC for enterobacteria and Pseudomonas aeruginosa for ≥50% of the dosing interval. Any untoward event occurring during treatment was collected as an adverse event. A total of 79 children contributed 174 PK samples. The median (range) age and weight were 1.7 years (2 months to 6 years) and 11.4 kg (3.8 to 27.6 kg), respectively. A 2-compartment model with first-order elimination best described the piperacillin and tazobactam data. Both final population PK models included weight and concomitant furosemide administration on clearance and weight on the volume of distribution of the central compartment. The optimal dosing regimens in children with normal renal function, based on the piperacillin component, were 75 mg/kg/dose every 4 h infused over 0.5 h in infants ages 2 to ≤6 months and 130 mg/kg/dose every 8 h infused over 4 h in children ages >6 months to 6 years against bacteria with MICs up to 16 mg/liter. A total of 44 children (49%) had ≥1 adverse event, with 3 of these (site infiltrations) considered definitely associated with the extended infusions.
TEXT
Piperacillin-tazobactam (TZP) is a combination of ureidopenicillin and a beta-lactamase inhibitor frequently used to treat severe hospital-acquired infections in infants and children. Similar to other beta-lactams, the piperacillin component exerts bactericidal activity in a time-dependent manner. Consequently, the percentage of time that the free piperacillin concentration remains above the MIC (fT>MIC) is the surrogate pharmacodynamic (PD) parameter that best correlates with efficacy (1). Optimal results are observed when the fT>MIC is ≥50% (2, 3). In critically ill adults, a higher threshold of an fT>MIC of 100% was correlated with greater efficacy (4, 5).
The treatment of sepsis is increasingly challenged by the emergence of bacterial resistance. As bacteria become resistant, evidenced by higher MICs, it is more difficult to achieve appropriate exposure and meet the surrogate PD target for efficacy (fT>MIC ≥ 50%) with a traditional short infusion (0.5 h). Extended TZP infusions are associated with better PD target attainment and decreased mortality (6, 7), improvement in clinical cure (8–10), and cost reductions in adults (11, 12). Data on extended infusions in children are scarce, and this alternative dosing strategy may compromise the administration of other therapies by occupying intravenous access for prolonged periods. This is particularly important in children with limited vascular access. Therefore, the efficacy, safety, and pharmacokinetics (PK) of extended TZP infusions need to be better established.
Piperacillin and tazobactam are both eliminated by the kidneys through a combination of glomerular filtration and tubular secretion (13). These elimination pathways undergo maturation during childhood, suggesting differences in TZP drug disposition in infants and young children relative to adults. In children, published results suggest benefits from using extended TZP infusions, but studies are mostly limited to simulation analyses in small specific populations (14–20), with only one PK study prospectively evaluating dosing regimens with extended infusions (21). In neonates, short TZP infusions were sufficient to achieve the PD target, suggesting no additional advantages of extended infusions in this population (22). The optimal dosing regimens with extended infusions and the age threshold at which those are beneficial are unknown. Our study aimed to better characterize the PK and safety of TZP when administered with extended infusions in children ≥2 months to 6 years old and to provide dosing recommendations in this age group.
RESULTS
Study population.
A total of 89 children received extended TZP infusions. Their median (range) age and body weight (WT) were 1.5 years (2 months to 6 years) and 11.4 kg (3.8 to 27.6 kg), respectively (Table 1). The distribution of children according to hospitalization units was as follows: 42 (47%) were from general pediatrics/surgery units, 19 (21%) were from hematology-oncology units, and 28 (31%) were from the pediatric intensive care unit (PICU). The median (range) duration of treatment with extended TZP infusions was 3.4 days (0 to 15 days). Microbiology cultures were sent for 80 (90%) children, and 32 (36%) children had at least one positive culture. The main foci of infection were intra-abdominal (27%), respiratory (22%), and urinary tract (22%), and the most commonly isolated pathogens were Staphylococcus aureus (28%), Enterobacter cloacae (25%), and Klebsiella species (25%).
TABLE 1.
Clinical characteristicsa
| Characteristic | Value |
|
|---|---|---|
| All children (n = 89) | Children with PK samplesb (n = 79) | |
| No. (%) of male patients | 51 (57) | 43 (54) |
| No. (%) of Caucasian patients | 78 (88) | 69 (87) |
| Median (range) age (yr) | 1.5 (0.2–6.3) | 1.7 (0.2–6.3) |
| No. (%) of children in the following age group: | ||
| ≥2–5 mo | 15 (17) | 13 (16) |
| ≥6 mo–6 yr | 74 (83) | 66 (84) |
| Median (range) wt (kg) | 11.4 (3.8–27.6) | 11.4 (3.8–27.6) |
| No. (%) of children in the following hospitalization unit: | ||
| General pediatrics/surgery | 42 (47) | 35 (44) |
| Hematology-oncology | 19 (21) | 18 (23) |
| PICU | 28 (31) | 26 (33) |
| Median (range) PICU scorec | ||
| PIM2 | 2.6 (0.2–60) | 2.6 (0.2–60) |
| PELOD | 1.5 (0–41) | 2 (0–41) |
| Median (range) duration of TZP extended-infusion treatment (days) | 3.4 (0–14.7) | 3.7 (0–14.7) |
| No. (%) of children receiving the following comedication: | ||
| Furosemide | 22 (25) | 20 (25) |
| Vancomycin | 17 (19) | 13 (16) |
| Tobramycin | 25 (28) | 23 (29) |
| Median (range) peak serum creatinine value (μmol/liter) | 38 (21–69) | 38 (21–69) |
PELOD, pediatric logistic organ dysfunction; PICU, pediatric intensive care unit; PIM2, pediatric index of mortality 2; PK, pharmacokinetics; TZP, piperacillin-tazobactam.
Subjects with at least one PK sample collected.
Data are for 28 of all children and 26 children with PK samples.
PK specimens.
Of the 89 children enrolled, 79 (89%) had PK samples, with a median (range) of 2 (1 to 4) PK samples per subject. TZP was discontinued before PK samples were collected in 10 (11%) children. A total of 174 PK samples were collected, with 9 (5%) and 11 (6%) samples having values below the quantification limit (BQL) for piperacillin and tazobactam, respectively. Samples with values BQL were excluded from analysis.
Piperacillin population PK (PopPK) model.
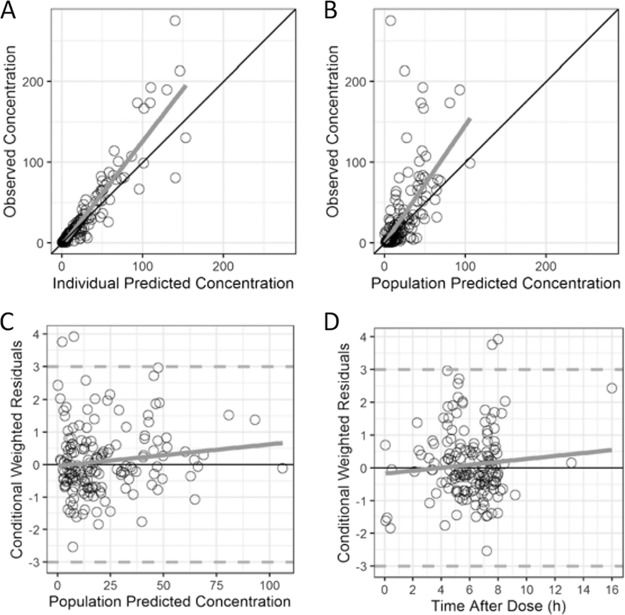
A two-compartment PK model with first-order elimination fitted the data well (Fig. 1). Saturable elimination was evaluated using a Michaelis-Menten equation and resulted in an objective function value (OFV) similar to that achieved with first-order elimination. Estimated allometric exponents resulted in a better fit to characterize body WT in relationship to PK parameters and were estimated to be 1.40 for clearance (CL) and intercompartmental clearance (Q) and 1.26 for the volume of distribution of the central compartment (Vc) and the volume of distribution of the peripheral compartment (Vp) (Table 2). After accounting for WT, the concomitant administration of furosemide (as a dichotomous variable) on CL resulted in a significant reduction in the OFV (see Table S4 in the supplemental material). No other covariates reached statistical significance. Thus, the final model included WT and concomitant furosemide administration for CL and WT only for Vc, Vp, and Q (Table 2 and Table S4). Between-subject variability (BSV) was 46% for CL and was fixed to 0 for Vc, Vp, and Q, as it could not be precisely estimated (failed to converge, unsuccessful covariance step, or high shrinkage). A proportional residual error model characterized the unexplained residual variability and was estimated to be 46%. Diagnostic plots, bootstraps, and a visual predictive check (VPC) showed a good fit for the final model, with underprediction for piperacillin concentrations of >100 mg/liter (Table 2, Fig. 2, and Fig. S1).
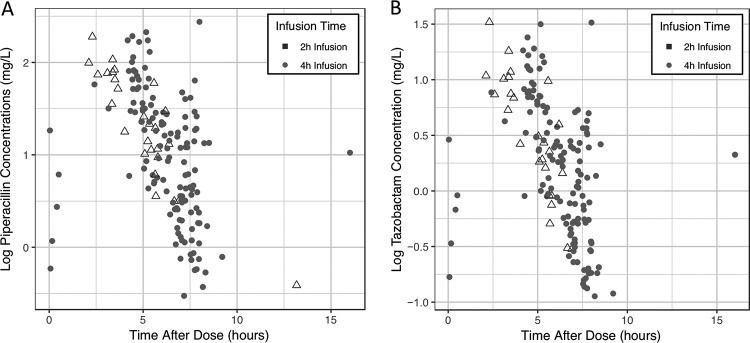
FIG 1.
Piperacillin (A) and tazobactam (B) concentrations versus time.
TABLE 2.
Final piperacillin model and bootstrap analysisa
| Parameter | Final model |
Bootstrap analysis (n = 1,000) |
|||||
|---|---|---|---|---|---|---|---|
| Point estimate | RSE (%) | 95% CI | Shrinkage (%) | 2.5th percentile | Median | 97.5th percentile | |
| Typical value | |||||||
| CL (liters/h) | 3.92 | 17.4 | 2.58 to 5.26 | 2.74 | 3.72 | 5.35 | |
| Vc (liters) | 4.87 | 27.2 | 2.28 to 7.46 | 2.47 | 4.35 | 8.24 | |
| Q (liters/h) | 0.25 | 86.2 | −0.17 to 0.68 | 0.05 | 0.21 | 1.24 | |
| Vp (liters) | 0.49 | 47.9 | 0.03 to 0.95 | 0.19 | 0.66 | 8.46 | |
| Covariate effect | |||||||
| WT on CL and Q (exponent) | 1.40 | 13.2 | 1.03 to 1.76 | 0.95 | 1.36 | 1.78 | |
| Furosemide on CL | −0.28 | 97.7 | −0.83 to 0.26 | −0.64 | −0.29 | −0.06 | |
| WT on Vc and Vp (exponent) | 1.26 | 23.4 | 0.68 to 1.84 | 0.32 | 1.16 | 1.95 | |
| Variance model | |||||||
| IIV (CL) | 0.44 | 17.6 | 0.29 to 0.59 | 4.8 | 0.27 | 0.42 | 0.57 |
| IIV (Vc) | |||||||
| IIV (Q) | |||||||
| IIV (Vp) | |||||||
| Proportional error | 0.46 | 10.5 | 0.37 to 0.56 | 15 | 0.36 | 0.45 | 0.53 |
CI, confidence interval; CL, clearance; IIV, interindividual variability; Q, intercompartmental clearance; RSE, residual standard error; Vc, volume of distribution of the central compartment; Vp, volume of distribution of the peripheral compartment; WT, body weight.
FIG 2.
Goodness-of-fit plots for the final piperacillin model. The final piperacillin diagnostic plots are of the observed versus individual predicted concentrations (A) or population predicted concentrations (B) and the conditional weighted residuals versus the population predicted concentration (C) or the time after the dose (D).
Tazobactam population PK model.
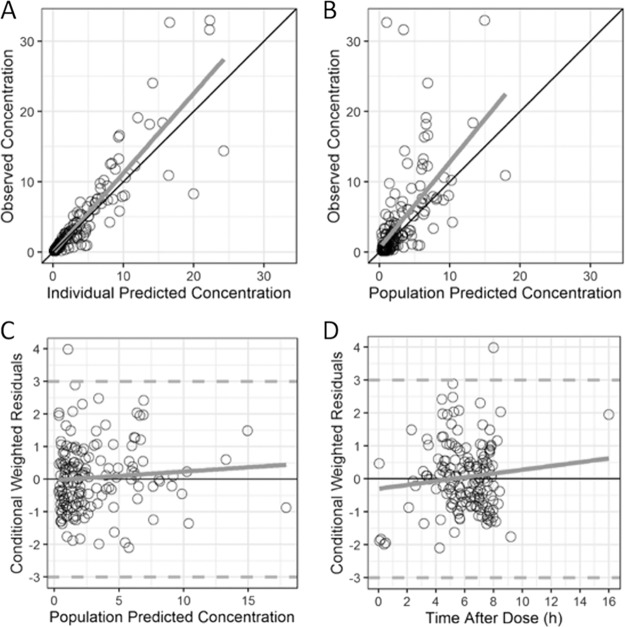
A two-compartment PK model with first-order elimination best described the data (Fig. 1). After accounting for WT using an estimated exponent (1.24 on CL and Q and 1.06 on Vc and Vp) allometric relationship, concomitant administration of furosemide as a dichotomous variable on CL resulted in a significant reduction in the OFV. No other covariates reached statistical significance. Thus, the final model included WT and concomitant furosemide administration for CL and WT only for Vc (Table 3 and Table S5). BSV was 39% for CL and was fixed to 0 for Vc, Vp, and Q. A proportional residual error model characterized the unexplained residual variability and was estimated to be 43%. Diagnostic plots, bootstrap analyses, and VPC showed a good fit for the final model (Table 3, Fig. 3, and Fig. S2).
TABLE 3.
Final tazobactam model and bootstrapa
| Parameter | Final model |
Bootstrap analysis (n = 1,000) |
|||||
|---|---|---|---|---|---|---|---|
| Point estimate | RSE (%) | 95% CI | Shrinkage (%) | 2.5th percentile | Median | 97.5th percentile | |
| Typical value | |||||||
| CL (liters/h) | 3.15 | 12.7 | 2.37 to 3.93 | 2.34 | 3.10 | 4.15 | |
| Vc (liters) | 3.79 | 19.7 | 2.33 to 5.25 | 2.25 | 3.69 | 5.97 | |
| Q (liters/h) | 0.20 | 45.0 | 0.02 to 0.38 | 0.06 | 0.20 | 0.48 | |
| Vp (liters) | 3.65 | 46.6 | 0.32 to 6.98 | 0.30 | 3.19 | 7.85 | |
| Covariate effect | |||||||
| WT on CL and Q (exponent) | 1.24 | 16.1 | 0.85 to 1.62 | 0.77 | 1.24 | 1.69 | |
| Furosemide on CL | −0.29 | 66.3 | −0.66 to 0.09 | −0.55 | −0.30 | −0.04 | |
| WT on Vc and Vp (exponent) | 1.06 | 31.4 | 0.41 to 1.71 | 0.30 | 1.09 | 1.85 | |
| Variance model | |||||||
| IIV (CL) | 0.37 | 19.8 | 0.23 to 0.52 | 6 | 0.23 | 0.37 | 0.51 |
| IIV (Vc) | |||||||
| IIV (Vp) | |||||||
| IIV (Q) | |||||||
| Proportional error | 0.43 | 10.1 | 0.34 to 0.52 | 16 | 0.11 | 0.17 | 0.24 |
CI, confidence interval; CL, clearance; IIV, interindividual variability; Q, intercompartmental clearance; RSE, residual standard error; Vc, volume of distribution of the central compartment; Vp, volume of distribution of the peripheral compartment; WT, body weight.
FIG 3.
Goodness-of-fit plots for the final tazobactam model. The final tazobactam diagnostic plots are of the observed versus individual predicted concentrations (A) or population predicted concentrations (B) and the conditional weighted residuals versus the population predicted concentration (C) or the time after the dose (D).
Assessment of dose-exposure relationship.
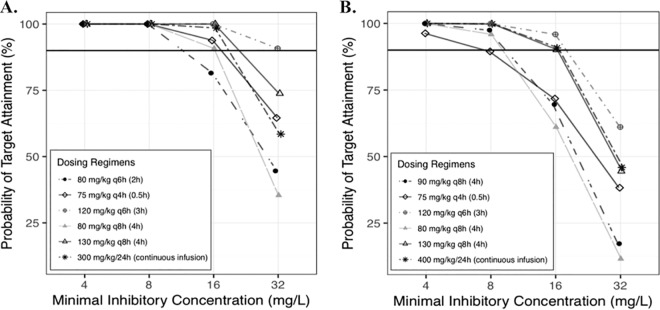
Using the final piperacillin PK model derived in the current study, simulations were conducted by assuming the absence of concomitant furosemide therapy. At a MIC of 16 mg/liter, the dosing regimens used in our study reached probabilities of target attainment (PTAs) of 82% in infants ≥2 to 5 months of age and 70% in infants and children from ≥6 months to 6 years of age for our primary PD target of an fT>MIC of ≥50%. The optimal dosing regimens for a MIC of 16 mg/liter were 75 mg/kg of body weight/dose every 4 h infused over 0.5 h in infants ages 2 to ≤6 months (total daily dose, 450 mg/kg/day; total infusion time, 3 h) and 130 mg/kg/dose every 8 h infused over 4 h in children ages >6 months to 6 years (total daily dose, 390 mg/kg/day; total infusion time, 12 h) (Fig. 4). Alternatively, 80 mg/kg/dose every 8 h infused over 4 h may be considered in infants 2 to ≤6 months of age because of its lower total daily dose (240 mg/kg/day), although it implies a longer daily infusion time (12 h). At a MIC of 32 mg/liter, 120 mg/kg/dose every 6 h given over 3 h was the optimal dosing regimen in infants 2 to ≤6 months of age (total daily dose, 480 mg/kg/day; total infusion time, 12 h), while none of the tested dosing regimens were optimal in children ages >6 months to 6 years. Continuous infusions were necessary to reach our secondary PD target of an fT>MIC of 100% at a MIC of 16 mg/liter, i.e., a dose of 300 mg/kg/24 h in infants ages 2 to ≤6 months and a dose of 400 mg/kg/24 h in children ages >6 months to 6 years.
FIG 4.
Target attainment rates by MIC of free piperacillin concentrations for 50% of the dosing interval in 1,000 simulated children ages 2 to 6 months (A) and 6 months to 6 years (B). The solid horizontal black lines represent the 90% target attainment rate. q4h, q6h, and q8h, every 4, 6, and 8 h, respectively.
Using the final tazobactam PK model derived in the current study, simulations were conducted in our population to assess free tazobactam exposure using our recommended dosing regimens. The median (range) percentage of the time that the free tazobactam concentrations were greater than 4 mg/liter was 100% (80 to 100%).
Safety.
A total of 44 children (49%) experienced at least 1 adverse event (AE), with 10 children (11%) experiencing at least 1 severe AE. Thirty-two clinical AEs occurred in 28 children, and 42 laboratory AEs occurred in 32 children (Tables S2 and S3). Three of the observed AEs were considered to be definitely associated with TZP (infusion site infiltration). Isolated eosinophilia was observed in 1 (1%) child and was the only laboratory AE considered to be probably associated with TZP. The clinical picture was not compatible with a drug reaction with eosinophilia and systemic symptoms (DRESS), and eosinophilia resolved while continuing TZP treatment. In 2 children, TZP treatment was stopped prematurely because of a possible association with an AE (cellulitis and severe rash). Fifty (72%) AEs resolved without clinical consequence, and 19 (28%) were ongoing at discharge from hospital or 30 days following the last extended-infusion dose if the child was still hospitalized.
DISCUSSION
This is the largest population PK study performed using extended TZP infusions in infants and children and also the first to include children from different clinical subpopulations. A two-compartment model best described our data, as previously reported in adults and children (14, 15, 17, 23, 24). A two-compartment model was preferred, as it resulted in a lower OFV and more plausible PK parameter estimates compared with those achieved with a one-compartment model relative to those previously published in the literature. Although of debatable significance, expressing CL estimates per kilogram of body weight was our only way to compare our results with those previously published in the literature. Given this limitation, we found that our population estimate for piperacillin CL (0.34 liters/h/kg) was in the upper range of the values previously reported in children (0.20 to 0.33 liters/h/kg) (14–17, 21, 25). A potential explanation for this difference includes a younger median age in our study population. Previous studies included older children and cohorts with a wider age range (up to 18 years versus up to 6 years in our study population) (15, 21). Renal tubular secretion and glomerular filtration mature rapidly following birth. Renal tubular secretion and glomerular filtration reach maturation at 1 and 2 years of age, respectively (26). Therefore, when expressed per kilogram of body weight, CL appears to be higher in young children than in older children and adults (27), although the actual CL (expressed in liters per hour) remains lower. Finally, our observed higher CL-per-kilogram estimate may also be due to the absence of significant organ dysfunctions in our population, as shown by a low median pediatric logistic organ dysfunction (PELOD) score, despite enrollment in the PICU. Our population estimates for piperacillin Vc and Vp were in the upper range of the values previously reported in children (14–16, 21, 25).
Our population estimate for tazobactam CL (0.28 liters/h/kg) was consistent with previously reported values in older children (0.19 to 0.33 liter/h/kg) (17, 21, 25). Our population estimates for Vc and Vp were higher than those previously described by De Cock et al. (17) using a two-compartment model (0.33 and 0.32 liter/kg, respectively, in our study versus 0.13 and 0.11 liter/kg, respectively, in the study by De Cock et al. [17]). Because tazobactam is highly hydrophilic, a higher volume of distribution per kilogram of body weight is expected in children less than 1 year of age, where the relative proportion of body water to fat is higher than that in older children (28). Our studied population’s young age may therefore explain our higher observed tazobactam total volume of distribution.
Age, either part of a maturation function or not, was previously found to be a significant covariate for piperacillin CL in neonates (22) and children (age range, 2 months to 15 years) (17). Although age was not a significant covariate in our model, we believe that fitted allometric exponents captured the influence of body size as well as that of renal maturation. When exploring different PK models with fixed allometric exponents, we found that age as part of a sigmoid maturation model was a significant covariate on CL. However, estimated allometric exponents resulted in a better fit and a more parsimonious model than fixed allometric exponents with maturation as a covariate. This is consistent with the available literature, supporting the inaccuracy of fixed allometric exponents in young infants and children undergoing organ maturation (29, 30). Our estimated allometric exponents for WT on CL were greater than 1 (1.40 and 1.24 for piperacillin and tazobactam, respectively), suggesting that CL increased more rapidly than WT in this young population. These results are consistent with renal maturation occurring at a higher rate relative to body WT in young children.
Our results suggest that both piperacillin and tazobactam CL decrease when furosemide is administered concomitantly. Furosemide was associated with a 25% reduction in the piperacillin and tazobactam CL. To our knowledge, this association has not been previously described. Despite normal serum creatinine (SCR) values, furosemide may be a surrogate for renal failure, leading to decreased piperacillin and tazobactam excretion, as the SCR value is an unreliable marker of acute kidney injury in children (31). Indeed, furosemide is often used in children with acute kidney injury, as it typically leads to reduced urine output, prompting clinicians to administer diuretics in an attempt to force diuresis. Therefore, the observed association between furosemide and decreased CL may in fact represent an association between acute kidney injury and decreased CL. The decreased CL in children on concomitant furosemide therapy may also be explained by drug-drug interactions at the organic anion transporter (OAT) level. Piperacillin, tazobactam, and furosemide are partly eliminated via active tubular secretion via OAT1/3 (32, 33). It is possible that furosemide has a higher affinity for the OAT1/3 transporter and creates a competitive inhibition, limiting the excretion of piperacillin and tazobactam. Given the wide TZP therapeutic index, we do not believe that concomitant furosemide administration requires routine TZP dose adjustments. However, it may contribute to the achievement of higher TZP concentrations.
Our piperacillin dose-exposure simulations indicated that the standard maximum dose of 300 to 400 mg/kg/24 h with 0.5-h infusions does not reach the surrogate PD target for efficacy in infants and children without acute kidney injury and in the absence of concomitant furosemide therapy. In infants 2 to 6 months of age, daily doses of 450 mg/kg divided every 4 h with 0.5-h infusions were required against bacteria with MICs of 16 mg/liter. Of note, no safety data are available on such higher doses in this age group, and the alternative lowest total daily doses of 240 mg/kg divided every 8 h with 4-h infusions should be considered if venous access allows it. Daily doses of up to 600 mg/kg were found to be safe for the treatment of pulmonary exacerbations in children with cystic fibrosis, although they were potentially associated with a reversible serum sickness-like syndrome (34, 35). The safety of high piperacillin doses has not been evaluated in children <2 years of age. The AEs most commonly associated with TZP include gastrointestinal disturbances and cutaneous reactions (36). Severe AEs, such as seizures and significant transaminitis, were also described using standard dosing regimens but were not reported with high piperacillin doses in children (34, 35). More safety data on high piperacillin dosing regimens are needed.
Regarding MICs beyond the susceptibility breakpoint (16 mg/liter) for Enterobacteriaceae and Pseudomonas aeruginosa, we found that higher doses (up to 480 mg/kg/day) achieved the target for a MIC of 32 mg/liter (intermediate susceptibility) in infants 2 to ≤6 months of age. However, none of the tested regimens were optimal in children >6 months of age at MICs of >16 mg/liter. These results support Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobials Susceptibility Testing (EUCAST) guidelines fixing the susceptibility breakpoint at ≤16 mg/liter (37, 38). Continuous infusions were necessary to reach our more stringent PD target of a fT>MIC of 100% and could be considered to treat infections in critically ill children caused by bacteria with MICs up to 16 mg/liter. Moreover, significant variability in PK parameters remained in our model, and therapeutic drug monitoring may be considered to optimize TZP exposure in critically ill children with severe infections.
PK/PD target values are based on antimicrobial efficacy, which relies on the piperacillin component for TZP. Therefore, simulations were performed using the final piperacillin PK model and PK/PD targets referred to piperacillin concentrations (39). Tazobactam has little antimicrobial activity itself, but through irreversible beta-lactamase inhibition, it allows piperacillin activity by preventing its breakdown (40). The PK/PD index associated with tazobactam efficacy is the fraction of time that the free concentration is above a threshold. This threshold depends on the amount of beta-lactamases produced but has been shown to be less than 4 mg/liter (40). In our population, simulations showed that free tazobactam concentrations were above 4 mg/liter for the whole dosing interval, supporting the efficacy of our recommended dosing regimens with regard to the tazobactam component.
Nearly half of our population had an AE (44 children; 49%). However, most of these events were considered mild (83%) and unrelated or unlikely to have been related to TZP (82%). Infusion site infiltration was the only AE definitely associated with extended-infusion TZP. Site infiltration is a well-known complication of peripheral catheter use, and the incidence (3%) that we observed using extended infusions was comparable to that previously described by investigators using standard dosing regimens (36, 41). Gastrointestinal discomfort (diarrhea, abdominal pain) was the most commonly observed clinical AE (13%) and was considered probably associated with TZP, but its incidence was similar to the previously described incidence with standard dosing regimens (36).
Our study has limitations. Our final models include significant between-subject and residual variabilities, possibly because of our sparse sampling approach and opportunistic design. Our final piperacillin model also shows underprediction for piperacillin concentrations of >100 mg/liter, most likely due to the limited number of samples with very high concentrations. However, this should not impact our dosing recommendations, as those are not based on maximal concentrations (Cmax) but are based on the percentage of the time that concentrations remain above the MIC (fT>MIC). Although we included children from different hospitalization units, our PICU population was not extremely ill and had low PELOD and pediatric index of mortality 2 (PIM2) scores. This may have prevented us from characterizing specific changes in PK parameters associated with the early phase of severe sepsis (i.e., renal hyperfiltration with increased CL) and with significant organ dysfunction (i.e., decreased CL). Similarly, the exclusion of children with stage 2 acute kidney injury or greater prevents us from applying our results to children with impaired renal function. Lastly, our safety assessment is limited by the short duration of treatment with extended TZP infusions (median, 3.4 days). However, our study also has considerable strengths. It is the largest study evaluating the PK and safety of extended TZP infusions in infants and children. Extended TZP infusions were feasible and well tolerated. It also is the first study to describe a potential drug-drug interaction between furosemide and TZP. Both are very commonly administered medications in children, especially in intensive care units (ICUs), and our findings warrant further studies.
Conclusion.
Based on PD target achievement, the optimal dosing regimens in children with normal renal function were 75 mg/kg/dose every 4 h infused over 0.5 h in infants 2 to ≤6 months of age (total daily dose, 450 mg/kg/day; total infusion time, 3 h) and 130 mg/kg/dose every 8 h infused over 4 h in children >6 months to 6 years of age (total daily dose, 390 mg/kg/day; total infusion time, 12 h) against bacteria with MICs of up to 16 mg/liter. Infants and children over 6 months of age need extended TZP infusions for optimal PD target achievement. Further data on the safety of high-dose piperacillin regimens are needed.
MATERIALS AND METHODS
Design and study population.
We conducted a single-center, open-label opportunistic PK and safety study of TZP administered per standard of care to infants and children ≥2 months to 6 years of age with suspected or confirmed infection. Enrollment occurred from January 2016 to December 2017. Based on available PK parameters in the literature (25) and on a previous simulation study conducted by our group (20), enrollment was stratified by (i) age group (cohort 1, ≥2 months to 5 months of age; cohort 2, ≥6 months to 6 years of age) and (ii) clinical subpopulations (general pediatrics/surgery units, hematology-oncology units, and pediatric intensive care unit [PICU]). Children were excluded if they had insufficient venous access to allow extended infusion, a history of anaphylaxis to beta-lactams, stage 2 acute kidney injury or greater (defined as a doubling of the baseline serum creatinine [SCR] concentration, when available, or doubling of the normal value for age [42]), chronic renal insufficiency, or cystic fibrosis or if they were supported either by extracorporeal membrane oxygenation (ECMO) or by renal replacement therapy. In addition to baseline demographics, we collected results from laboratory tests if they were done per the standard of care (e.g., complete blood count and blood urea nitrogen [BUN], SCR, alanine aminotransferase [ALT], aspartate aminotransferase [AST], and albumin concentrations), concomitant medication of interest (the renally excreted and/or nephrotoxic compounds vancomycin, tacrolimus, amphotericin B, gentamicin, tobramycin, furosemide, and acyclovir), and microbiological laboratory assessments. The pediatric logistic organ dysfunction (PELOD) (43) and pediatric index of mortality 2 (PIM2) (44) scores were also collected for children in the PICU. PELOD was measured on day 1 of extended TZP infusion, using the most severe values over the 24 h. PIM2 was measured at ICU admission, using the first value of each variable measured within the period of time from first contact by the ICU team (in either the ICU, emergency department, or hospital ward or outside the hospital) to ICU admission. The CHU Sainte-Justine Institutional Review Board approved our protocol (approval number NCT32987). Written informed consent was obtained from the parents or legal guardian of each child, and oral assent was obtained from the child when the child was ≥5 years old and able to understand.
Drug dosing.
Once the participant was enrolled, the TZP dosing regimen was adjusted to meet the study protocol: 80 mg/kg/dose every 6 h infused over 2 h in infants from cohort 1 (age, ≥2 to 5 months) and 90 mg/kg/dose every 8 h infused over 4 h in infants and children from cohort 2 (age, ≥6 months to 6 years). Study dosing regimens were determined following previous PK simulations using nonlinear mixed-effect modeling (Phoenix NLME and Trial Simulator software) and piperacillin clearance (CL) and volume of distribution (V) parameters from the available literature (25). These dosing regimens aimed to optimize PD target achievement (fT>MIC ≥ 50%) for bacteria with MICs up to 16 mg/liter, which represents the piperacillin MIC for enterobacteria and P. aeruginosa (37, 38).
Sample collection, analytical method, and PK analysis.
A maximum of 4 PK samples per patient was collected using an opportunistic approach, where timing was dependent on standard-of-care laboratory assessments. Blood was collected (200 μl of whole blood/sample) in EDTA-containing microcontainers and placed on ice for a maximum of 1 h before processing. Plasma was then separated via centrifugation (3,000 rpm for 10 min at 4°C) and transferred to polypropylene tubes. Plasma was frozen in a −80°C freezer for a maximum of 15 months before analysis.
Piperacillin and tazobactam concentrations were quantified using validated liquid chromatography-tandem mass spectrometry assays. The chromatography system and mass spectrometer used were an Agilent 1200 series high-performance liquid chromatography system and an Agilent 6400 series triple-quadrupole mass spectrometer, respectively. The linear concentration ranges of piperacillin and tazobactam were 0.3 to 275 mg/liter and 0.1 to 32.9 mg/liter, respectively. The lower limit of quantification (LLOQ) was 0.1 mg/liter for both piperacillin and tazobactam. The intra-assay accuracies of the quality control samples (0.1, 0.3, 4, 160, and 400 mg/liter) ranged from 96.0% to 102.0% for piperacillin and from 92.5% to 99.2% for tazobactam.
Piperacillin and tazobactam PK data were analyzed separately with nonlinear mixed-effect modeling using the software NONMEM (version 7.3). One- and two-compartment models were evaluated. The potential effects of covariates were evaluated if a relationship was first suspected based on physiological plausibility. A lognormal distribution was assumed for modeling the interindividual variabilities of the PK parameters. The relationship between the interindividual variability of the PK parameters and covariates was assessed using visual inspection of scatter and box plots (continuous and categorical variables, respectively). Body weight (WT) was assumed to be a significant covariate and was included in the model as a starting point using an exponent allometric relationship normalized on our population median WT. Both estimated and fixed (0.75 for CL and 1 for V) allometric exponents were evaluated. The following covariates were evaluated: age, sex, concomitant medications (vancomycin, tacrolimus, amphotericin B, gentamicin, tobramycin, furosemide, and acyclovir), hospitalization units, maturation (Hill equation) for renal glomerular filtration (45) and active tubular secretion (46), and SCR, BUN, and albumin concentrations. The Hill equation was also evaluated using both estimated and fixed parameters (Hill coefficient and maturation half-life [TM50]) based on the literature (17, 47). Inclusion of covariates in the model was performed using a stepwise forward additive approach using a P value of 0.05 (change in OFV [ΔOFV] = 3.84 for 1 degree of freedom) and a backward elimination approach using a P value of 0.01 (ΔOFV = 6.63 for 1 degree of freedom). Missing values were imputed to be the last value carried forward. All models were run with the first-order conditional estimation with interaction (FOCEI). The model fit was evaluated using successful minimization, goodness-of-fit plots, plausibility, the precision of parameter estimates, and a visual predictive check (VPC).
Assessment of dose-exposure relationship.
The final piperacillin PK model was used to assess the dose-exposure relationship, as TZP dosing is based on the piperacillin component (48). A virtual population of 1,000 children with baseline characteristics similar to those of our study population was used to simulate a variety of dosing regimens and measure the proportion of simulated children who achieved the PD target. The free piperacillin concentration was calculated as 70% of the total predicted concentration (13). Simulated dosing regimens ranged from 50 to 150 mg/kg/dose given every 4 to 8 h infused over 0.5 to 4 h, with the maximum total daily dose being 600 mg/kg/day (34, 35) (see Table S1 in the supplemental material). Continuous infusions from 300 mg/kg/day to 600 mg/kg/day were also simulated. The bactericidal property of TZP is attributed to its piperacillin component, and therefore, target values are based on free piperacillin concentrations and MICs refer to those of piperacillin (39). An fT>MIC of ≥50% was defined as the primary PD target for efficacy. Additionally, probabilities of target attainment (PTAs) were calculated for a more stringent PD target of an fT>MIC of 100%, as this target has been proposed in the literature and is increasingly used in critically ill patients (4). PTAs were calculated across MICs of 4 to 32 mg/liter. Optimal dosing regimens were stratified by age group and selected according to their capacity to reach a ≥90% PTA for a MIC of 16 mg/liter while using minimal total daily doses and infusion times. These optimal dosing regimens were then simulated in our population to assess tazobactam exposure, based on our final tazobactam model. The free tazobactam concentration was calculated to be 70% of the total predicted concentration (13).
Safety.
An adverse event (AE) was defined as any untoward event, regardless of the relationship to the study drug, which occurred more than 24 h following the first extended TZP infusion dose up to 3 days following the last extended TZP infusion dose. Clinical and laboratory events were defined a priori, based on the available literature (Tables S2 and S3) (49). Each AE was followed until resolution or, if ongoing, until discharge from the hospital or 30 days following the last extended TZP infusion dose, whichever came first.
Supplementary Material
ACKNOWLEDGMENTS
The assay measuring piperacillin and tazobactam concentrations was performed at OpAns, LLC, Durham, NC, by Alan Cunningham (analyst), Amy Delinsky (analyst), Christine Grosse (responsible scientist), and Kenneth C. Lewis (chief executive officer). We thank the staff and research team at CHU Sainte-Justine for their support of this study.
This work was funded by the SickKids Foundation and a CIHR-Institute of Human Development, Child and Youth Health (IHSCYH) new investigator research grant. Julie Autmizguine receives salary support from the Fonds de Recherche Santé Québec (FRQS) and does consulting for Astellas Pharma Inc. Jean Lavigne and Nastya Kassir are paid consultants at Certara. The remaining authors have no relevant conflicts to disclose.
Céline Thibault and Julie Autmizguine wrote the manuscript; Julie Autmizguine designed the research; Céline Thibault (primary author), Julie Autmizguine (study principal investigator), Catherine Litalien (collaborator), and Yves Théorêt (collaborator) performed the research; and Jean Lavigne, Nastya Kassir, and Céline Thibault analyzed the data.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01260-19.
REFERENCES
- 1.Turnidge JD. 1998. The pharmacodynamics of beta-lactams. Clin Infect Dis 27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 2.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig WA. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis 158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 3.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 4.Wong G, Brinkman A, Benefield RJ, Carlier M, De Waele JJ, El Helali N, Frey O, Harbarth S, Huttner A, McWhinney B, Misset B, Pea F, Preisenberger J, Roberts MS, Robertson TA, Roehr A, Sime FB, Taccone FS, Ungerer JP, Lipman J, Roberts JA. 2014. An international, multicentre survey of beta-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 69:1416–1423. doi: 10.1093/jac/dkt523. [DOI] [PubMed] [Google Scholar]
- 5.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, Bellomo R, Lipman J. 2016. Continuous versus intermittent beta-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 194:681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Zhang C, Zhou Q, Wang Y, Chen L. 2015. Clinical outcomes with alternative dosing strategies for piperacillin/tazobactam: a systematic review and meta-analysis. PLoS One 10:e0116769. doi: 10.1371/journal.pone.0116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Lipman J. 2013. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis 56:236–244. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, Rai V, Wong KK, Hasan MS, Abd Rahman AN, Jamal JA, Wallis SC, Lipman J, Staatz CE, Roberts JA. 2016. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 42:1535–1545. doi: 10.1007/s00134-015-4188-0. [DOI] [PubMed] [Google Scholar]
- 10.Ram R, Halavy Y, Amit O, Paran Y, Katchman E, Yachini B, Kor S, Avivi I, Ben-Ami R. 2018. Extended versus bolus infusion of broad-spectrum beta-lactams for febrile neutropenia: an unblinded randomized trial. Clin Infect Dis 67:1153–1160. doi: 10.1093/cid/ciy258. [DOI] [PubMed] [Google Scholar]
- 11.Bao H, Lv Y, Wang D, Xue J, Yan Z. 2017. Clinical outcomes of extended versus intermittent administration of piperacillin/tazobactam for the treatment of hospital-acquired pneumonia: a randomized controlled trial. Eur J Clin Microbiol Infect Dis 36:459–466. doi: 10.1007/s10096-016-2819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunetti L, Poustchi S, Cunningham D, Toscani M, Nguyen J, Lim J, Ding Y, Nahass RG. 2015. Clinical and economic impact of empirical extended-infusion piperacillin-tazobactam in a community medical center. Ann Pharmacother 49:754–760. doi: 10.1177/1060028015579427. [DOI] [PubMed] [Google Scholar]
- 13.Daniel KP, Krop LC. 1996. Piperacillin-tazobactam: a new beta-lactam-beta-lactamase inhibitor combination. Pharmacotherapy 16:149–162. [PubMed] [Google Scholar]
- 14.Cies JJ, Shankar V, Schlichting C, Kuti JL. 2014. Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J 33:168–173. doi: 10.1097/INF.0b013e3182a743c7. [DOI] [PubMed] [Google Scholar]
- 15.Cies JJ, Jain J, Kuti JL. 2015. Population pharmacokinetics of the piperacillin component of piperacillin/tazobactam in pediatric oncology patients with fever and neutropenia. Pediatr Blood Cancer 62:477–482. doi: 10.1002/pbc.25287. [DOI] [PubMed] [Google Scholar]
- 16.Beranger A, Benaboud S, Urien S, Moulin F, Bille E, Lesage F, Zheng Y, Genuini M, Gana I, Renolleau S, Hirt D, Treluyer JM, Oualha M. 2019. Piperacillin population pharmacokinetics and dosing regimen optimization in critically ill children with normal and augmented renal clearance. Clin Pharmacokinet 58:223–233. doi: 10.1007/s40262-018-0682-1. [DOI] [PubMed] [Google Scholar]
- 17.De Cock P, van Dijkman SC, de Jaeger A, Willems J, Carlier M, Verstraete AG, Delanghe JR, Robays H, Vande Walle J, Della Pasqua OE, De Paepe P. 2017. Dose optimization of piperacillin/tazobactam in critically ill children. J Antimicrob Chemother 72:2002–2011. doi: 10.1093/jac/dkx093. [DOI] [PubMed] [Google Scholar]
- 18.Delvallee M, Mazingue F, Abouchahla W, Delebarre M, Wallet F, Courcol R, Kipnis E, Dessein R. 2013. Optimization of continuous infusion of piperacillin-tazobactam in children with fever and neutropenia. Pediatr Infect Dis J 32:962–964. doi: 10.1097/INF.0b013e318298dfb8. [DOI] [PubMed] [Google Scholar]
- 19.Courter JD, Kuti JL, Girotto JE, Nicolau DP. 2009. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr Blood Cancer 53:379–385. doi: 10.1002/pbc.22051. [DOI] [PubMed] [Google Scholar]
- 20.Thibault C, Kassir N, Theoret Y, Varin F, Litalien C, Autmizguine J. 2017. Dose-exposure simulation for piperacillin-tazobactam dosing strategies in infants and young children. J Popul Ther Clin Pharmacol 24:e33-344. [DOI] [PubMed] [Google Scholar]
- 21.Nichols K, Chung EK, Knoderer CA, Buenger LE, Healy DP, Dees J, Crumby AS, Kays MB. 2016. Population pharmacokinetics and pharmacodynamics of extended-infusion piperacillin and tazobactam in critically ill children. Antimicrob Agents Chemother 60:522–531. doi: 10.1128/AAC.02089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen-Wolkowiez M, Watt KM, Zhou C, Bloom BT, Poindexter B, Castro L, Gao J, Capparelli EV, Benjamin DK Jr, Smith PB. 2014. Developmental pharmacokinetics of piperacillin and tazobactam using plasma and dried blood spots from infants. Antimicrob Agents Chemother 58:2856–2865. doi: 10.1128/AAC.02139-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felton TW, Hope WW, Lomaestro BM, Butterfield JM, Kwa AL, Drusano GL, Lodise TP. 2012. Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob Agents Chemother 56:4087–4094. doi: 10.1128/AAC.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterfield JM, Lodise TP, Beegle S, Rosen J, Farkas J, Pai MP. 2014. Pharmacokinetics and pharmacodynamics of extended-infusion piperacillin/tazobactam in adult patients with cystic fibrosis-related acute pulmonary exacerbations. J Antimicrob Chemother 69:176–179. doi: 10.1093/jac/dkt300. [DOI] [PubMed] [Google Scholar]
- 25.Reed MD, Goldfarb J, Yamashita TS, Lemon E, Blumer JL. 1994. Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother 38:2817–2826. doi: 10.1128/aac.38.12.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. 2006. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet 45:1077–1097. doi: 10.2165/00003088-200645110-00003. [DOI] [PubMed] [Google Scholar]
- 27.Chen N, Aleksa K, Woodland C, Rieder M, Koren G. 2006. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol 21:160–168. doi: 10.1007/s00467-005-2105-4. [DOI] [PubMed] [Google Scholar]
- 28.Friis-Hansen B. 1971. Body composition during growth. In vivo measurements and biochemical data correlated to differential anatomical growth. Pediatrics 47(Suppl 2):264. [PubMed] [Google Scholar]
- 29.Calvier EA, Krekels EH, Valitalo PA, Rostami-Hodjegan A, Tibboel D, Danhof M, Knibbe CA. 2017. Allometric scaling of clearance in paediatric patients: when does the magic of 0.75 fade? Clin Pharmacokinet 56:273–285. doi: 10.1007/s40262-016-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmood I. 2010. Theoretical versus empirical allometry: facts behind theories and application to pharmacokinetics. J Pharm Sci 99:2927–2933. doi: 10.1002/jps.22073. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg JH, Parikh CR. 2017. Biomarkers for diagnosis and prognosis of AKI in children: one size does not fit all. Clin J Am Soc Nephrol 12:1551–1557. doi: 10.2215/CJN.12851216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebner T, Ishiguro N, Taub ME. 2015. The use of transporter probe drug cocktails for the assessment of transporter-based drug-drug interactions in a clinical setting-proposal of a four component transporter cocktail. J Pharm Sci 104:3220–3228. doi: 10.1002/jps.24489. [DOI] [PubMed] [Google Scholar]
- 33.Wen S, Wang C, Duan Y, Huo X, Meng Q, Liu Z, Yang S, Zhu Y, Sun H, Ma X, Yang S, Liu K. 2018. OAT1 and OAT3 also mediate the drug-drug interaction between piperacillin and tazobactam. Int J Pharm 537:172–182. doi: 10.1016/j.ijpharm.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 34.McCarty JM, Tilden SJ, Black P, Craft JC, Blumer J, Waring W, Halsey NA. 1988. Comparison of piperacillin alone versus piperacillin plus tobramycin for treatment of respiratory infections in children with cystic fibrosis. Pediatr Pulmonol 4:201–204. doi: 10.1002/ppul.1950040403. [DOI] [PubMed] [Google Scholar]
- 35.Reed MD, Stern RC, Myers CM, Klinger JD, Yamashita TS, Blumer JL. 1987. Therapeutic evaluation of piperacillin for acute pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 3:101–109. doi: 10.1002/ppul.1950030212. [DOI] [PubMed] [Google Scholar]
- 36.Pfizer Canada Inc. 2014. Tazocin®, piperacillin and tazobactam powder for injection. Product monograph Wyeth Holdings Corporation, Pfizer Canada Inc, Kirkland, Quebec, Canada. [Google Scholar]
- 37.Clinical Laboratory Standards Institute. 2019. Performance standard for antimicrobial susceptibility testing, 29th ed. CLSI supplement M100 Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 38.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. http://www.eucast.org. Accessed 12 June 2019.
- 39.Schoonover LL, Occhipinti DJ, Rodvold KA, Danziger LH. 1995. Piperacillin/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. Ann Pharmacother 29:501–514. doi: 10.1177/106002809502900510. [DOI] [PubMed] [Google Scholar]
- 40.Nicasio AM, VanScoy BD, Mendes RE, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2016. Pharmacokinetics-pharmacodynamics of tazobactam in combination with piperacillin in an in vitro infection model. Antimicrob Agents Chemother 60:2075–2080. doi: 10.1128/AAC.02747-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Paula DHG, Tura BR, Lamas CDC. 2012. Adverse events related to intravenous antibiotic therapy: a prospective observational study in the treatment of infective endocarditis. BMJ Open 2:e001189. doi: 10.1136/bmjopen-2012-001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acute Kidney Injury Working Group. 2012. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 43.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. 2003. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 44.Slater A, Shann F, Pearson G. 2003. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 45.Anderson BJ, Holford NH. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 46.Hayton WL. 2000. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci 2:E3. doi: 10.1208/ps020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holford N, Heo YA, Anderson B. 2013. A pharmacokinetic standard for babies and adults. J Pharm Sci 102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 48.Taketomo CHJ, Kraus D. 2016. Pediatric & neonatal dosage handbook, 23rd ed. Lexi-Comp, Inc, Hudson, OH. [Google Scholar]
- 49.England A, Wade K, Smith PB, Berezny K, Laughon M. 2016. Optimizing operational efficiencies in early phase trials: The Pediatric Trials Network experience. Contemp Clin Trials 47:376–382. doi: 10.1016/j.cct.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.