REPLY
We appreciate the comments by Kalil et al. regarding the results of the study by Geriak et al. (1). We are aware of the methodological limitations mentioned, as they are discussed in our paper. As a result, we acknowledge that our study falls short of the robust expectations of what would be considered a “practice-changing” clinical trial. We applaud a critical commentary on these limitations given the potential implications on practice.
The letter by Kalil et al. implies that the standard of care monotherapy patient group had a higher mortality than the combination therapy patients because the deck was stacked against them with respect to comorbidities. They highlight the “disadvantages” of the monotherapy patients without mentioning the “disadvantages” of the combination group, all of which are numerical rather than statistical differences. While the higher procalcitonin and C-reactive protein concentrations suggest “a more inflammatory state” in the monotherapy group, it is important to note that white blood cell count, interleukin-10 concentrations, and number of endocarditis patients were higher in the combination patient group. In previous studies, only age, endovascular source, and renal insufficiency are consistent predictors of mortality in Staphylococcus aureus bacteremia (2). The 26% in-hospital/30-day mortality seen with standard of care patient population in the Geriak study is well within expectations of mortality for methicillin-resistant S. aureus (MRSA) bacteremia (2). The difference in this study was the 0% mortality in the combination group, despite having Charlson comorbidity and Pitt bacteremia scores that were similar to those of the monotherapy group.
Kalil et al. state that the randomization in this trial was “flawed.” We point out that a matter of chance brought more patients in the monotherapy arm (23 patients) than the combination arm (17 patients). The percentage of patients in each group (57.5%/42.5%) was similar to the allocation of patients in a recent small randomized trial of fecal microbiota transplant (55%/45%) reported by Juul et al. (3). This can occur during early enrollment due to chance but will often even out over time with continued randomization, which could not occur in our study due to early termination.
Kalil et al. are correct that one patient either way would sway results away from statistical significance due to the small number of patients, but as the study was not carried out in a blinded manner, continuation of this study posed an ethical dilemma that could not allow continuation for the sake of statistical perfection. A data safety monitoring board, while ideal, was not funded for, and an nonblinded study such as this where investigators were concerned of the adverse outcomes befalling monotherapy patients, would likely have not changed the results of the study or its early discontinuation. Thus, only a larger blinded study would be able to overcome these limitations, but at what cost?
We believe the results of this study achieved after 40 patients accurately reflect a survival benefit of early daptomycin plus ceftaroline versus vancomycin in MRSA bacteremia, albeit in a suboptimal fashion. The results are consistent with our previous unpublished drug utilization evaluation (DUE) of daptomycin plus ceftaroline versus vancomycin for MRSA bacteremia from two hospitals initiated by antimicrobial stewardship personnel prior to this study. The DUE involved a retrospective analysis of 11 pretrial patients (the first such treated patients at our institution) treated with daptomycin plus ceftaroline matched (age within 10 years, renal function, and infection source) to 22 vancomycin-treated patients and showed a strong (but not statistically significant) survival benefit in daptomycin plus ceftaroline patients (Fig. 1). Interestingly, mortality with the combination treatment was 0%.
FIG 1.
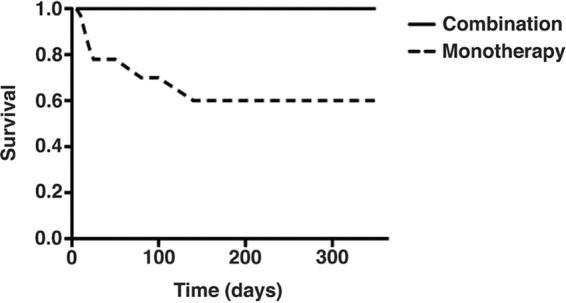
Retrospective survival analysis of the first 11 patients receiving combination therapy with daptomycin plus ceftaroline (n = 11) at our hospital as part of a drug utilization evaluation compared to matched patients (1:2 ratio based on source of bacteremia, age, and renal function) receiving vancomycin monotherapy (n = 22). These were all pretrial patients. The P values for the difference in mortality at different times follow: after 30 days, P = 0.2; after 60 days, P = 0.14; after 1 year P = 0.06.
The main conclusion of our study should be that there are potentially better treatment options for use in MRSA bacteremia. However, our study fails to provide the answer as to the optimal treatment that collectively integrates efficacy, tolerability, and cost of therapy. Therefore, the results of our study should direct scientific leadership toward a next step to compare other treatments to vancomycin in MRSA bacteremia, such as daptomycin plus an antistaphylococcal beta lactam or vancomycin plus ceftaroline, or perhaps even ceftaroline alone. The favorable daptomycin plus fosfomycin data for MRSA bacteremia from Spanish investigators is of interest (4). The results of the recent CAMERA-2 trial examining vancomycin plus flucloxacillin versus vancomycin alone for MRSA bacteremia reiterated the nephrotoxicity risk that has been seen with patients treated with vancomycin plus piperacillin-tazobactam (5, 6). This adverse effect overshadowed any positive outcomes, such as more rapid MRSA bacteremia clearance with that combination, and cautions against the use of vancomycin plus a penicillin as combination therapy in MRSA bacteremia. Some preliminary data suggest possible favorable outcomes in MRSA bacteremia with vancomycin plus cefazolin, without nephrotoxicity, but final results are awaited (7).
MRSA bacteremia mortality has not improved in decades, perhaps because a perfect “practice-changing trial” is still awaited. As Djulbebovic and Hozo point out, the rigor of acceptance of published research findings as true should vary based as a function of “acceptable regret,” that is, our ability to tolerate the consequences of making a wrong decision in accepting the research hypothesis (here, mean drug acquisition costs of $8,000 to $10,000 per patient) balanced against the expected benefits of the findings (here, reduced mortality) (8). We observed improvements in survivability of our patients, an additional observation to a growing list of observations that combination therapies may well be the next mainstream management of MRSA bacteremia. The results of our study are directing us toward improving patient outcomes in treating MRSA bacteremia by steering us away from vancomycin monotherapy. At the end of the day, we completely agree with Kalil that our trial is not a definitive daptomycin plus ceftaroline versus standard monotherapy for MRSA bacteremia trial, but after reading and analyzing it, would you be willing to put your patients at stake in a definitive trial?
Footnotes
This is a response to a letter by Kalil et al. https://doi.org/10.1128/AAC.00900-19.
REFERENCES
- 1.Geriak M, Haddad F, Rizvi K, Rose W, Kullar R, LaPlante K, Yu M, Vasina L, Ouellette K, Zervos M, Nizet V, Sakoulas G. 2019. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 63:e02483-18. doi: 10.1128/AAC.02483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juul FE, Garborg K, Bretthauer M, Skudal H, Øines MN, Wiig H, Rose Ø, Seip B, Lamont JT, Midtvedt T, Valeur J, Kalager M, Holme Ø, Helsingen L, Løberg M, Adami HO. 2018. Fecal microbiota transplantation for primary Clostridium difficile infection. N Engl J Med 378:2535–2536. doi: 10.1056/NEJMc1803103. [DOI] [PubMed] [Google Scholar]
- 4.Pujol M, Miro JM, Shaw E, et al. 2018. Daptomycin plus fosfomycin versus daptomycin monotherapy for methicillin-resistant Staphylococcus aureus bacteremia. A multicenter, randomized clinical trial, abstr LB3. IDWeek 2018. https://idsa.confex.com/idsa/2018/webprogram/Paper74232.html#. [DOI] [PMC free article] [PubMed]
- 5.Hall P. 2019. Highlights from ECCMID 2019. Lancet Infect Dis 19:582. doi: 10.1016/S1473-3099(19)30223-3. [DOI] [PubMed] [Google Scholar]
- 6.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. 2018. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults. A systematic review and meta-analysis. Crit Care Med 46:12–20. doi: 10.1097/CCM.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 7.Trinh TD, Zasowski EJ, Lagnf AM, Shtia S, Dhar S, Mynatt R, Pogue JM, Rybak MJ. 2017. Combination vancomycin/cefazolin (VAN/CFZ) for methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections (BSI). Open Forum Infect Dis 4(Suppl 1):S281. doi: 10.1093/ofid/ofx163.631. [DOI] [Google Scholar]
- 8.Djulbegovic B, Hozo I. 2007. When should potentially false research findings be considered acceptable? PLoS Med 4:e26. doi: 10.1371/journal.pmed.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]


