Ibrexafungerp is a first-in-class glucan synthase inhibitor. In vitro activity was determined for 89 Candida glabrata isolates with molecularly identified FKS1 or FKS2 mutations conferring resistance to the echinocandins. All isolates were resistant to at least one echinocandin (i.e., anidulafungin, caspofungin, or micafungin) by broth microdilution. Results for ibrexafungerp were compared with those for each echinocandin.
KEYWORDS: Candida glabrata, SCY-078, ibrexafungerp, FKS, echinocandins, resistance, antifungal
ABSTRACT
Ibrexafungerp is a first-in-class glucan synthase inhibitor. In vitro activity was determined for 89 Candida glabrata isolates with molecularly identified FKS1 or FKS2 mutations conferring resistance to the echinocandins. All isolates were resistant to at least one echinocandin (i.e., anidulafungin, caspofungin, or micafungin) by broth microdilution. Results for ibrexafungerp were compared with those for each echinocandin. Ibrexafungerp had good activity against all echinocandin-resistant C. glabrata isolates.
TEXT
Candida glabrata infection is one of the primary causes of candidemia in the United States and accounts for more than one-third of all candidemia isolates (1, 2). Compared to other common candidemia species, such as Candida albicans, Candida tropicalis, and Candida parapsilosis, C. glabrata infection exhibits higher rates of azole antifungal resistance (1). Hence, the echinocandins have been the first line of therapy for C. glabrata infections (3). Echinocandin resistance in C. glabrata isolates is generally ∼3% to 4% but can be as high as 20% to 30% in individual institutions (4). Even more alarming, recent data show that ∼9% of C. glabrata isolates that are resistant to fluconazole are also resistant to the echinocandins (3). This elevated resistance to the most commonly used antifungals highlights the need for additional treatment options.
The major mechanisms of resistance to the echinocandins in C. glabrata isolates are known. Mutations occur within a subunit of glucan synthase, the target of the echinocandins. The majority of resistant C. glabrata isolates contain mutations in one of two hot spot (HS) regions in Fks1 or Fks2. Isolates with FKS mutations have shown various degrees of susceptibility to echinocandins depending on the mutation (3).
Ibrexafungerp (IBX) is a novel glucan synthase inhibitor. IBX is a triterpenoid that offers oral bioavailability (5–7). It shows in vitro and in vivo activity against multiple Candida species and has activity against some echinocandin-resistant isolates (5, 8–10). With few options remaining for treatment, evaluation of novel antifungal agents is imperative.
The study collection consisted of 89 C. glabrata bloodstream isolates known to have elevated MICs against the echinocandins. Sequencing to confirm the presence of an FKS mutation was performed as previously described (3). All isolates were tested according to Clinical and Laboratory Standards Institute reference methodology M27-A4 (11). C. parapsilosis ATCC 22019 and Candida krusei ATCC 5268 isolates were included as quality controls.
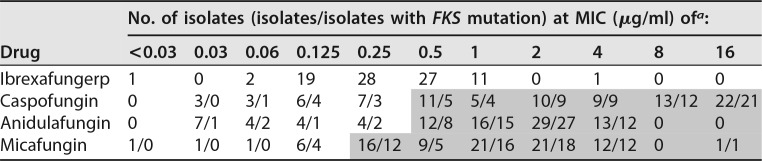
Twenty-three of the isolates had mutations in FKS1 HS1 encoding six unique mutations (Table 1). These included 13 isolates with an S629P mutation, 1 isolate with an S629T mutation, 6 isolates with an R631G mutation, 1 isolate with a D632V mutation, 1 isolate with an I634V mutation, and 1 isolate with a D632Y mutation. No mutations were detected in the FKS1 HS2 region.
TABLE 1.
Distribution of ibrexafungerp by echinocandin MIC value
Resistant MIC values are shaded.
Fifty-three of the isolates had mutations in FKS2 HS1encoding six unique mutations. These included 38 isolates with an S663P mutation, 4 isolates with a D666V mutation, 5 isolates with an F659Y mutation, 1 isolate with a P667H mutation, 2 isolates with an S663F mutation, and 3 isolates with a deletion at 658F. No mutations were detected in the FKS2 HS2 region.
Seven isolates had mutations in FKS1 HS1 and FKS2 HS1 simultaneously. These included, respectively, 1 isolate with S629P and S663P mutations, 3 isolates with S629P and D666V mutations, 1 isolate with S629T and F659Y mutations, 1 isolate with R631G and S663P mutations, and 1 isolate with R631G and D666V mutations.
Interestingly, 20 isolates were resistant to at least one echinocandin but no FKS mutation, including 13 isolates with resistance to a single echinocandin, 3 isolates with resistance to two echinocandins, and 4 isolates with resistance to all three echinocandins. These isolates may represent an unknown mechanism of resistance or the inherent variability in antifungal susceptibility testing, which shows that when tested multiple times, a single isolate can have MIC values that differ by as much as two dilutions. Not all of the isolates with resistance to only a single echinocandin were resistant to caspofungin, although problems with in vitro testing with caspofungin have been noted, and this may be reflected in isolates 34, 43, and 77, shown in Table 1 (12).
The MIC values of IBX ranged from <0.03 to 4 μg/ml (Table 1). The mode was 0.25 μg/ml, and the MIC50 and MIC90 were 0.25 and 1.0 μg/ml, respectively. The MIC values for the echinocandins among the same collection ranged from 0.03 to 4 μg/ml for anidulafungin, 0.03 to >16 μg/ml for caspofungin, and 0.008 to >16 μg/ml for micafungin (Table 2).
TABLE 2.
MIC values for ibrexafungerp and three echinocandins, with FKS sequencing results for each isolate
| Isolate ID | MIC (μg/ml) of: |
Gene and hot spot region(s)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| Anidulafungin | Caspofungin | Micafungin | Ibrexafungerp | FKS1 HS1 | FKS1 HS2 | FKS2 HS1 | FKS2 HS2 | |
| 1 | 1 | 0.5 | 1 | 1 | S629P | WT | WT | WT |
| 24 | 2 | 8 | 1 | 0.25 | S629P | WT | WT | WT |
| 28 | 0.5 | 0.5 | 0.125 | 0.5 | S629P | WT | WT | WT |
| 31 | 0.5 | 0.125 | 0.125 | 0.5 | S629P | WT | WT | WT |
| 32 | 4 | >16 | 2 | 0.5 | S629P | WT | WT | WT |
| 68 | 2 | 2 | 0.5 | 1 | S629P | WT | WT | WT |
| 72 | 2 | 16 | 4 | 0.5 | S629P | WT | WT | WT |
| 84 | 4 | 8 | 2 | 0.125 | S629P | WT | WT | WT |
| 85 | 2 | >16 | 2 | 0.125 | S629P | WT | WT | WT |
| 80 | 1 | 8 | 1 | 0.125 | S629P | WT | S663P | WT |
| 38 | 2 | 4 | 1 | 0.5 | S629P | WT | D666V | WT |
| 48 | 0.125 | 0.125 | 0.5 | 0.25 | S629P | WT | D666V | WT |
| 63 | 1 | 2 | 1 | 0.5 | S629P | WT | D666V | WT |
| 50 | 2 | 4 | 0.125 | 0.25 | S629T | WT | F659Y | WT |
| 2 | 2 | 4 | 2 | 0.25 | WT | WT | S663P | WT |
| 3 | 4 | >16 | 4 | 0.5 | WT | WT | S663P | WT |
| 4 | 2 | 8 | 4 | 1 | WT | WT | S663P | WT |
| 5 | 0.5 | 16 | 4 | 0.5 | WT | WT | S663P | WT |
| 7 | 2 | 16 | 4 | 1 | WT | WT | S663P | WT |
| 8 | 1 | 16 | 4 | 1 | WT | WT | S663P | WT |
| 9 | 4 | >16 | 4 | 0.5 | WT | WT | S663P | WT |
| 12 | 2 | 2 | 0.5 | 0.5 | WT | WT | S663P | WT |
| 14 | 0.5 | 1 | 2 | 0.25 | WT | WT | S663P | WT |
| 15 | 4 | >16 | 4 | 0.5 | WT | WT | S663P | WT |
| 18 | 4 | 8 | 2 | 0.25 | WT | WT | S663P | WT |
| 19 | 2 | 1 | 2 | 0.5 | WT | WT | S663P | WT |
| 22 | 2 | >16 | 2 | 0.5 | WT | WT | S663P | WT |
| 23 | 2 | >16 | 2 | 0.25 | WT | WT | S663P | WT |
| 30 | 4 | >16 | 2 | 0.5 | WT | WT | S663P | WT |
| 37 | 4 | 16 | 2 | 0.5 | WT | WT | S663P | WT |
| 40 | 1 | 2 | 1 | 0.25 | WT | WT | S663P | WT |
| 41 | 4 | 16 | 4 | 0.5 | WT | WT | S663P | WT |
| 45 | 1 | 16 | 2 | 0.25 | WT | WT | S663P | WT |
| 46 | 1 | 4 | 1 | 0.5 | WT | WT | S663P | WT |
| 53 | 4 | >16 | 2 | 0.5 | WT | WT | S663P | WT |
| 55 | 2 | 8 | 2 | 0.5 | WT | WT | S663P | WT |
| 62 | 2 | 8 | 1 | 0.25 | WT | WT | S663P | WT |
| 67 | 2 | 8 | 2 | 0.25 | WT | WT | S663P | WT |
| 69 | 2 | 8 | 1 | 0.25 | WT | WT | S663P | WT |
| 70 | 2 | 8 | 1 | 0.25 | WT | WT | S663P | WT |
| 71 | 2 | >16 | 4 | 1 | WT | WT | S663P | WT |
| 73 | 1 | 8 | 1 | 0.5 | WT | WT | S663P | WT |
| 74 | 1 | 8 | 1 | 0.5 | WT | WT | S663P | WT |
| 75 | 4 | >16 | 4 | 0.125 | WT | WT | S663P | WT |
| 76 | 2 | 4 | 2 | 0.25 | WT | WT | S663P | WT |
| 82 | 2 | 4 | 2 | 0.25 | WT | WT | S663P | WT |
| 87 | 1 | 4 | 1 | 0.125 | WT | WT | S663P | WT |
| 88 | 2 | >16 | 1 | 1 | WT | WT | S663P | WT |
| 89 | 2 | 4 | 2 | 1 | WT | WT | S663P | WT |
| 90 | 2 | 4 | 1 | 0.125 | WT | WT | S663P | WT |
| 36 | 2 | 16 | 4 | 0.5 | R631G | WT | S663P | WT |
| 6 | 2 | 2 | 0.25 | 0.25 | WT | WT | P667H | WT |
| 10 | 1 | 0.5 | 0.25 | 0.5 | WT | WT | F659Y | WT |
| 11 | 1 | 2 | 0.25 | 0.25 | WT | WT | F659Y | WT |
| 57 | 1 | 2 | 0.25 | 0.25 | WT | WT | F659Y | WT |
| 59 | 0.5 | 1 | 0.25 | 0.5 | WT | WT | F659Y | WT |
| 13 | 0.5 | 0.25 | 0.125 | 0.25 | WT | WT | S663F | WT |
| 25 | 2 | 2 | 0.25 | 0.125 | WT | WT | S663F | WT |
| 17 | 0.25 | 0.25 | 0.5 | 0.0625 | R631G | WT | WT | WT |
| 20 | 0.06 | 0.125 | 0.25 | 0.25 | R631G | WT | WT | WT |
| 21 | 0.25 | 0.5 | 1 | 0.0625 | R631G | WT | WT | WT |
| 29 | 0.06 | 0.125 | 0.25 | <0.03 | R631G | WT | WT | WT |
| 16 | 0.5 | 0.25 | 0.5 | 0.125 | R631G | WT | D666V | WT |
| 33 | 0.5 | 0.5 | 0.25 | 0.125 | WT | WT | del658F | WT |
| 39 | 4 | 16 | >16 | 4 | WT | WT | del658F | WT |
| 86 | 2 | >16 | 2 | 0.25 | WT | WT | del658F | WT |
| 42 | 1 | 1 | 0.25 | 0.5 | D632V | WT | WT | WT |
| 54 | 0.03 | 0.06 | 0.25 | 0.25 | I634V | WT | WT | WT |
| 61 | 1 | 2 | 0.25 | 0.125 | D632Y | WT | WT | WT |
| 26 | 0.5 | 0.5 | 1 | 0.125 | WT | WT | WT | WT |
| 34 | 0.125 | 0.5 | 0.06 | 0.125 | WT | WT | WT | WT |
| 35 | 0.125 | 0.5 | 0.125 | 0.25 | WT | WT | WT | WT |
| 43 | 0.03 | 0.5 | 0.008 | 1 | WT | WT | WT | WT |
| 44 | 0.03 | 0.06 | 0.5 | 0.125 | WT | WT | WT | WT |
| 47 | 2 | 2 | 1 | 1 | WT | WT | WT | WT |
| 49 | 0.03 | 0.03 | 0.5 | 0.125 | WT | WT | WT | WT |
| 51 | 0.06 | 0.125 | 0.25 | 0.125 | WT | WT | WT | WT |
| 52 | 0.06 | 0.06 | 0.25 | 0.25 | WT | WT | WT | WT |
| 56 | 0.03 | 0.03 | 0.25 | 0.25 | WT | WT | WT | WT |
| 58 | 0.5 | 0.5 | 0.125 | 0.125 | WT | WT | WT | WT |
| 60 | 0.03 | 0.125 | 0.25 | 0.5 | WT | WT | WT | WT |
| 64 | 0.5 | 0.25 | 0.5 | 0.125 | WT | WT | WT | WT |
| 65 | 0.25 | 0.25 | 1 | 0.125 | WT | WT | WT | WT |
| 66 | 0.5 | 0.25 | 1 | 0.25 | WT | WT | WT | WT |
| 77 | 0.125 | 0.5 | 0.03 | 1 | WT | WT | WT | WT |
| 78 | 0.03 | 0.03 | 2 | 0.25 | WT | WT | WT | WT |
| 79 | 1 | 1 | 1 | 0.125 | WT | WT | WT | WT |
| 81 | 0.25 | 0.25 | 0.5 | 0.25 | WT | WT | WT | WT |
| 83 | 4 | 8 | 2 | 0.5 | WT | WT | WT | WT |
WT, wild type.
IBX showed consistent activity against all of the FKS mutations in C. glabrata. One isolate with a deletion at 658F in the FKS2 HS1 region displayed a high MIC of 4 μg/ml, although the other two isolates with the same mutation had MICs of 0.125 and 0.25 μg/ml.
This report describes in vitro susceptibilities of echinocandin-resistant C. glabrata with the novel glucan synthase inhibitor IBX. There currently are no breakpoints for IBX and C. glabrata. Although FKS mutations confer resistance to the echinocandins, the same mutations do not confer resistance to IBX. Although these results are promising, they need to be further evaluated in vivo. In conclusion, IBX is a promising candidate to combat echinocandin-resistant C. glabrata infections and is a welcome addition to the limited antifungal treatment options.
ACKNOWLEDGMENTS
We are grateful to the members of the Mycotic Diseases Branch for their support.
The findings and conclusions in this work are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto E, Boyken L, Tendolkar S, McDanel J, Castanheira M, Pfaller M, Diekema D. 2014. Candidemia surveillance in Iowa: emergence of echinocandin resistance. Diagn Microbiol Infect Dis 79:205–208. doi: 10.1016/j.diagmicrobio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. 2014. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother 58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkow EL, Angulo D, Lockhart SR. 2017. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against clinical isolates of Candida auris. Antimicrob Agents Chemother 61:e00435-17. doi: 10.1128/AAC.00435-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepak AJ, Marchillo K, Andes DR. 2015. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother 59:1265–1272. doi: 10.1128/AAC.04445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin EL, Long L, Isham N, Borroto-Esoda K, Barat S, Angulo D, Wring S, Ghannoum M. 2019. A novel 1,3-β-d-glucan inhibitor, ibrexafungerp (formerly SCY-078), shows potent activity in the lower pH environment of vulvovaginitis. Antimicrob Agents Chemother 63:e02611-18. doi: 10.1128/AAC.02611-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. 2014. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez-Ortigosa C, Perez WB, Angulo D, Borroto-Esoda K, Perlin DS. 2017. De novo acquisition of resistance to SCY-078 in Candida glabrata involves FKS mutations that both overlap and are distinct from those conferring echinocandin resistance. Antimicrob Agents Chemother 61:e00833-17. doi: 10.1128/AAC.00833-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. 2017. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother 61:e00161-17. doi: 10.1128/AAC.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts—4th ed. CLSI document M27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]