LETTER
Mycobacterium abscessus, a rapidly growing nontuberculous mycobacterium (NTM), is increasingly recognized as an important pathogen causing soft tissue and lung infections as well as severe disseminated infections in immunocompromised patients (1, 2). Due to the intrinsic resistance to most of the classic antituberculous drugs and many other antibiotics, infections with M. abscessus are extremely difficult to manage (3, 4). The spread of strains which acquired additional resistance-causing mutations and the requirement for extended treatment periods, often leading to severe adverse events, stress the need for identification of new compounds active against this pathogen (2). Recent drug screening efforts have focused on testing of preselected compounds and compound libraries containing substances with known antimycobacterial or antibacterial activity which led to identification of some compounds inhibiting growth of M. abscessus (5–9). Given the high attrition rate of hit and lead compounds during preclinical and clinical development, additional efforts may be needed to identify promising clinical candidates against M. abscessus. Phenotypic whole-cell screening of large, diverse synthetic small-molecule libraries led to the identification of potent and selective antituberculous drugs, some of which have been tested successfully in clinical trials (8, 10, 11). While the expected hit rate for these classic M. tuberculosis whole-cell screens is between 0.3 and 1%, this important information is missing for M. abscessus strains (12).
Here, we report on a head-to-head comparison of M. abscessus and Mycobacterium tuberculosis in a whole-cell phenotypic screen using a diverse chemical library of 10,000 synthetic small molecules (World Diversity Set III, SPECS, Netherlands) (13). We established a resazurin microtiter assay (REMA) for M. abscessus in analogy to the M. tuberculosis REMA which was previously described (14, 15). Log phase M. abscessus ATCC 19977 with an optical density at 600 nm (OD600) of 0.0001 was incubated in 96-well plates at 37°C in Middlebrook 7H9 broth supplemented with 10% ADC (albumin, dextrose, catalase), 0.5% glycerol, and 0.05% Tween 80. After 72 hours, resazurin was added (0.025% wt/vol), and fluorescence was measured after 2 hours of incubation (excitation at 560 nm and emission at 590 nm). When using dimethyl sulfoxide (DMSO) and clarithromycin (20 μM) as controls, the assay had a Z-factor of 0.78 in a 96-well microplate format. Molecules were tested in parallel REMA against M. abscessus or M. tuberculosis Erdman in duplicates at concentrations of 20 μM and 10 μM, respectively.
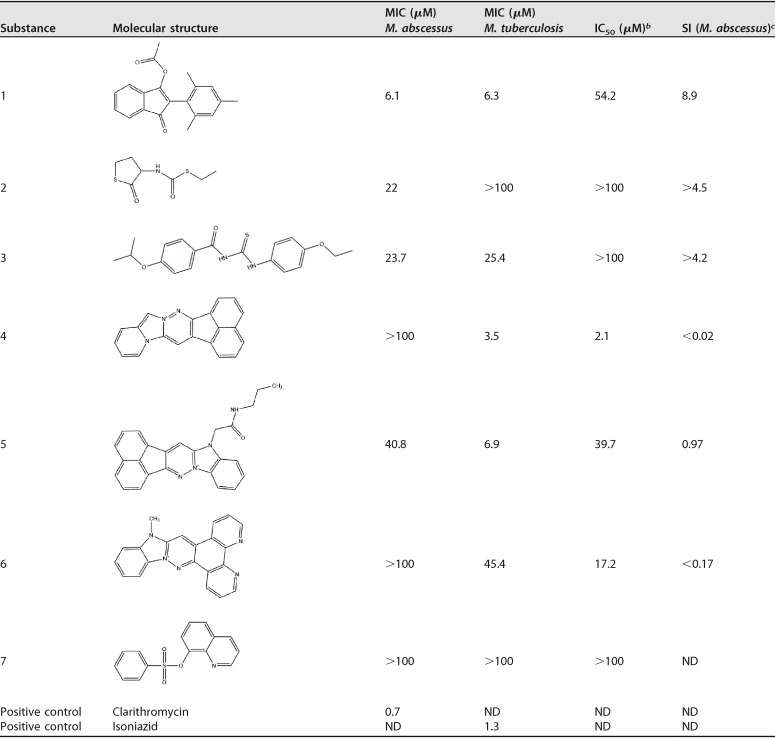
In the M. tuberculosis primary screen, we observed the expected hit rate of 0.7% (72 hits). In contrast to that, the M. abscessus screen revealed only 7 substances causing growth inhibition of 50% or higher (hit rate of 0.07%) (Table 1). M. abscessus hits were retested, followed by MIC determination in M. abscessus and M. tuberculosis using 2-fold serial dilutions and a starting concentration of 100 μM. To estimate the cellular toxicity (50% inhibitory concentration [IC50]) of the seven hit compounds, we used standard human hepatoma HepG2 cell cytotoxicity assays and calculated the corresponding selectivity indices (SI) (IC50/MIC). Growth inhibition of M. abscessus was confirmed for all 7 hit compounds. However, only compound 1 had a MIC below 20 μM (6.1 μM). The selectivity index for this substance was 8.9 (Table 1). Hit compound 2, a carbamothioate, and hit compound 3, a thiourea, had MICs of 22 μM and 23.7 μM, respectively. IC50 cytotoxicity values were >100 μM. A database search revealed that both substances display known antituberculous activity (Collaborative Drug Discovery Vault) (16). This also holds true for the imidazo-pyridazine compounds 4 to 6, all which displayed an SI of <1 due to pronounced cytotoxicity. Hit compound 7, a quinoline, did not reach a MIC of ≤100 μM.
TABLE 1.
Properties of M. abscessus REMA hit compoundsa
ND, not determined.
IC50, half maximal inhibitory concentration, measured in human hepatoma HepG2 cells.
SI, selectivity index (MIC against M. abscessus/IC50).
In conclusion, we show that high-throughput screening of randomly selected molecules yields extremely low hit rates for compounds targeting M. abscessus. Despite using a two-times-higher compound concentration in the M. abscessus assay, the hit rate was 10 times lower than results obtained for M. tuberculosis. From a total of seven hits, only one showed a MIC below 10 μM and moderate cytotoxicity. Five of the seven M. abscessus hits were either positive in our M. tuberculosis REMA or had been described previously as hits in other M. tuberculosis screening campaigns (16). Upon testing of M. abscessus hits against M. tuberculosis, we identified only one substance (number 2) which seems to possess selective activity against M. abscessus (Table 1). The disappointing results observed in this M. abscessus small-molecule screening study can be explained by the high intrinsic drug resistance of NTM that might reflect an evolutional adaptation to hostile environments (soil and water) (4, 17). NTM have a highly flexible system of gene regulation altering growth rate, metabolism, and inducible expression of genes directly facilitating drug resistance (e.g., genes encoding efflux pumps). One example is the transcriptional regulator WhiB7, which is involved in various mechanisms of inducible drug resistance in mycobacteria (18–22). Recently, it was shown that WhiB7 confers species-specific patterns of gene induction that might explain differences in drug susceptibilities among different mycobacterial species. For M. abscessus, it was shown that resistance to amikacin is induced by the WhiB7-regulated gene eis2, which seems to be unique for this species (23).
Based on our findings, we suggest retesting and repurposing substances that have already been tested positive in screens targeting M. tuberculosis and Gram-positive bacteria, rather than performing large-scale phenotypic screening against M. abscessus (5–7, 24, 25). In addition, known antimycobacterial drugs may exhibit synergistic effects as shown for combinations of various drug classes acting against M. abscessus (26–28). Changing assay conditions and using different media may also increase hit rates, as recently shown for experiments performed using Mueller-Hinton broth (6). Whether this will translate into compounds with good in vivo activity against M. abscessus requires further investigation.
ACKNOWLEDGMENTS
J.R. receives funding from the Thematic Translational Unit Tuberculosis (TTU TB, grant numbers TTU 02.806 and 02.905) of the German Center of Infection Research (DZIF). Financial support was also received from the German Research Foundation (DFG RY 159) and the Center for Molecular Medicine Cologne (ZMMK—CAP8). J.J.M. receives funding from DZIF (stipend TI 07.001_Malin_00 [TTU TB]).
REFERENCES
- 1.Petrini B. 2006. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 114:319–328. doi: 10.1111/j.1600-0463.2006.apm_390.x. [DOI] [PubMed] [Google Scholar]
- 2.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Daniels T, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Humphrey H, Isalska B, Jensen-Fangel S, Jönsson B, Jones AM, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luthra S, Rominski A, Sander P. 2018. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol 9:2179. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 5.Low JL, Wu ML, Aziz DB, Laleu B, Dick T. 2017. Screening of TB actives for activity against nontuberculous mycobacteria delivers high hit rates. Front Microbiol 8:1539. doi: 10.3389/fmicb.2017.01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter A, Strauch A, Chao J, Ko M, Av-Gay Y. 2018. Screening of preselected libraries targeting Mycobacterium abscessus for drug discovery. Antimicrob Agents Chemother 62:e00828-18. doi: 10.1128/AAC.00828-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz DB, Low JL, Wu ML, Gengenbacher M, Teo JWP, Dartois V, Dick T. 2017. Rifabutin is active against Mycobacterium abscessus complex. Antimicrob Agents Chemother 61:e00155-17. doi: 10.1128/AAC.00155-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu ML, Aziz DB, Dartois V, Dick T. 2018. NTM drug discovery: status, gaps and the way forward. Drug Discov Today 23:1502–1519. doi: 10.1016/j.drudis.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaushik A, Ammerman NC, Martins O, Parrish NM, Nuermberger EL. 2019. In vitro activity of new tetracycline analogs omadacycline and eravacycline against drug-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 63. doi: 10.1128/AAC.00470-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechartier B, Rybniker J, Zumla A, Cole ST. 2014. Tuberculosis drug discovery in the post-post-genomic era. EMBO Mol Med 6:158–168. doi: 10.1002/emmm.201201772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann J, Rybniker J, Muller R. 2017. Novel and revisited approaches in antituberculosis drug discovery. Curr Opin Biotechnol 48:94–101. doi: 10.1016/j.copbio.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Manjunatha UH, Smith PW. 2015. Perspective: challenges and opportunities in TB drug discovery from phenotypic screening. Bioorg Med Chem 23:5087–5097. doi: 10.1016/j.bmc.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 13.SPECS. 2019. World Diversity Set 3 factsheet. https://www.specs.net/transfer.php?code=SPECSpH9TJQygpSymZUO1Lv9TLJA0p2uyMKEsI0EGZl5jMTM8H3OyL3AiESyeBJWuJGW4. Accessed 3 July 2019.
- 14.Rybniker J, Chen JM, Sala C, Hartkoorn RC, Vocat A, Benjak A, Boy-Röttger S, Zhang M, Székely R, Greff Z, Orfi L, Szabadkai I, Pató J, Kéri G, Cole ST. 2014. Anticytolytic screen identifies inhibitors of mycobacterial virulence protein secretion. Cell Host Microbe 16:538–548. doi: 10.1016/j.chom.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Rybniker J, Vocat A, Sala C, Busso P, Pojer F, Benjak A, Cole ST. 2015. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun 6:7659. doi: 10.1038/ncomms8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekins S, Clark AM, Dole K, Gregory K, McNutt AM, Spektor AC, Weatherall C, Litterman NK, Bunin BA. 2018. Data mining and computational modeling of high-throughput screening datasets. Methods Mol Biol 1755:197–221. doi: 10.1007/978-1-4939-7724-6_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 15:149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Burian J, Ramon-Garcia S, Howes CG, Thompson CJ. 2012. WhiB7, a transcriptional activator that coordinates physiology with intrinsic drug resistance in Mycobacterium tuberculosis. Expert Rev Anti Infect Ther 10:1037–1047. doi: 10.1586/eri.12.90. [DOI] [PubMed] [Google Scholar]
- 19.Ramon-Garcia S, Ng C, Jensen PR, Dosanjh M, Burian J, Morris RP, Folcher M, Eltis LD, Grzesiek S, Nguyen L, Thompson CJ. 2013. WhiB7, an Fe-S-dependent transcription factor that activates species-specific repertoires of drug resistance determinants in actinobacteria. J Biol Chem 288:34514–34528. doi: 10.1074/jbc.M113.516385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiman DE, Raghunand TR, Agarwal N, Bishai WR. 2006. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob Agents Chemother 50:2836–2841. doi: 10.1128/AAC.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burian J, Yim G, Hsing M, Axerio-Cilies P, Cherkasov A, Spiegelman GB, Thompson CJ. 2013. The mycobacterial antibiotic resistance determinant WhiB7 acts as a transcriptional activator by binding the primary sigma factor SigA (RpoV). Nucleic Acids Res 41:10062–10076. doi: 10.1093/nar/gkt751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris RP, Nguyen L, Gatfield J, Visconti K, Nguyen K, Schnappinger D, Ehrt S, Liu Y, Heifets L, Pieters J, Schoolnik G, Thompson CJ. 2005. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 102:12200–12205. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst-Hess K, Rudra P, Ghosh P. 2017. Mycobacterium abscessus WhiB7 regulates a species-specific repertoire of genes to confer extreme antibiotic resistance. Antimicrob Agents Chemother 61:e01347-17. doi: 10.1128/AAC.01347-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Run E, Arthur M, Mainardi JL. 2018. In vitro and intracellular activity of imipenem combined with rifabutin and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 62:e00623-18. doi: 10.1128/AAC.00623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, Addrizzo-Harris DJ, O’Donnell AE, Marras TK, Flume PA, Loebinger MR, Morgan L, Codecasa LR, Hill AT, Ruoss SJ, Yim JJ, Ringshausen FC, Field SK, Philley JV, Wallace RJ Jr, van Ingen J, Coulter C, Nezamis J, Winthrop KL, CONVERT Study Group. 2018. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT): a prospective, open-label, randomized study. Am J Respir Crit Care Med 198:1559. doi: 10.1164/rccm.201807-1318OC. [DOI] [PubMed] [Google Scholar]
- 26.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Select beta-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrob Agents Chemother 63:e02613-18. doi: 10.1128/AAC.02613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pryjma M, Burian J, Thompson CJ. 2018. Rifabutin acts in synergy and is bactericidal with frontline Mycobacterium abscessus antibiotics clarithromycin and tigecycline, suggesting a potent treatment combination. Antimicrob Agents Chemother 62:e00283-18. doi: 10.1128/AAC.00283-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. 2012. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 56:6324–6327. doi: 10.1128/AAC.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]