The mechanisms of terbinafine resistance in a set of clinical isolates of Trichophyton rubrum have been studied recently. Of these isolates, TIMM20092 also showed reduced sensitivity to azoles. The azole resistance of TIMM20092 could be inhibited by milbemycin oxime, prompting us to examine the potential of T. rubrum to develop resistance through multidrug efflux transporters.
Keywords: Trichophyton rubrum, dermatophytes, itraconazole, voriconazole, antifungal resistance, MFS transporters, ABC transporters
ABSTRACT
The mechanisms of terbinafine resistance in a set of clinical isolates of Trichophyton rubrum have been studied recently. Of these isolates, TIMM20092 also showed reduced sensitivity to azoles. The azole resistance of TIMM20092 could be inhibited by milbemycin oxime, prompting us to examine the potential of T. rubrum to develop resistance through multidrug efflux transporters. The introduction of a T. rubrum cDNA library into Saccharomyces cerevisiae allowed the isolation of one transporter of the major facilitator superfamily (MFS) conferring resistance to azoles (TruMFS1). To identify more azole efflux pumps among 39 ABC and 170 MFS transporters present within the T. rubrum genome, we performed a BLASTp analysis of Aspergillus fumigatus, Candida albicans, and Candida glabrata on transporters that were previously shown to confer azole resistance. The identified candidates were further tested by heterologous gene expression in S. cerevisiae. Four ABC transporters (TruMDR1, TruMDR2, TruMDR3, and TruMDR5) and a second MFS transporter (TruMFS2) proved to be able to operate as azole efflux pumps. Milbemycin oxime inhibited only TruMDR3. Expression analysis showed that both TruMDR3 and TruMDR2 were significantly upregulated in TIMM20092. TruMDR3 transports voriconazole (VRC) and itraconazole (ITC), while TruMDR2 transports only ITC. Disruption of TruMDR3 in TIMM20092 abolished its resistance to VRC and reduced its resistance to ITC. Our study highlights TruMDR3, a newly identified transporter of the ABC family in T. rubrum, which can confer azole resistance if overexpressed. Finally, inhibition of TruMDR3 by milbemycin suggests that milbemycin analogs could be interesting compounds to treat dermatophyte infections in cases of azole resistance.
INTRODUCTION
Tinea pedis and tinea unguium are mainly caused by two anthropophilic dermatophyte species, Trichophyton rubrum and Trichophyton interdigitale, with prevalences in Europe of 80% and 20%, respectively (1). Allylamines and azoles, targeting the ergosterol biosynthetic pathway, are the main oral and topical pharmacological options used to treat dermatophytosis. Allylamines, such as terbinafine, are inhibitors of the squalene epoxidase (SQLE), which is involved in the early steps of ergosterol biosynthesis (2). Its inhibition results in the accumulation of squalene, which is toxic to fungi (3, 4). Azoles, such as itraconazole (ITC) and voriconazole (VRC), act downstream of the SQLE reaction in the membrane during ergosterol synthesis. These antifungal drugs inhibit lanosterol 14-α-demethylase, resulting in the accumulation of sterol precursors (5). Terbinafine and azole compounds have been extensively used to treat dermatophyte infections.
Dermatophytes with reduced sensitivity to antifungal drugs have emerged in several countries (Switzerland, Denmark, and India) (6–10). Terbinafine resistance was imputed to single point mutations within the SQLE gene in 17 T. rubrum clinical isolates from Switzerland, leading to amino acid substitutions at one of four amino acid positions (Leu393, Phe397, Phe415, and His440) within the SQLE enzyme. T. rubrum isolates resistant to terbinafine had comparable ITC and VRC MICs, except for one isolate (TIMM20092) which showed reduced susceptibility to azole compounds. This strain was isolated from a patient with tinea pedis insensitive to standard systemic and topical terbinafine and ITC treatments. Of note, resistance of the growing fungus to azoles could be reversed by a subinhibitory concentration of milbemycin oxime, an inhibitor of ABC transporters. These findings led to the hypothesis that the overexpression of genes that encode transporters could be involved in azole resistance.
While several Aspergillus and Candida azole transporters belonging to the ATP-binding cassette (ABC) transporters or the major facilitator superfamily (MFS) transporters have been well characterized, much less is known about the involvement of transporters in the azole resistance in dermatophytes. Gene expression data have shown that azoles and other drugs induce the transcription of some ABC transporters in dermatophytes, including MDR1/PDR1, MDR2, and MDR4 (11–13). However, the direct involvement of these transporters in azole efflux has yet to be shown. The isolation of a strain resistant to azoles, in addition to terbinafine, from a patient insensitive to standard treatment incited us to examine the potential of T. rubrum to develop azole resistance through multidrug efflux transporters.
RESULTS
Reduced sensitivity of strain TIMM20092 to azoles.
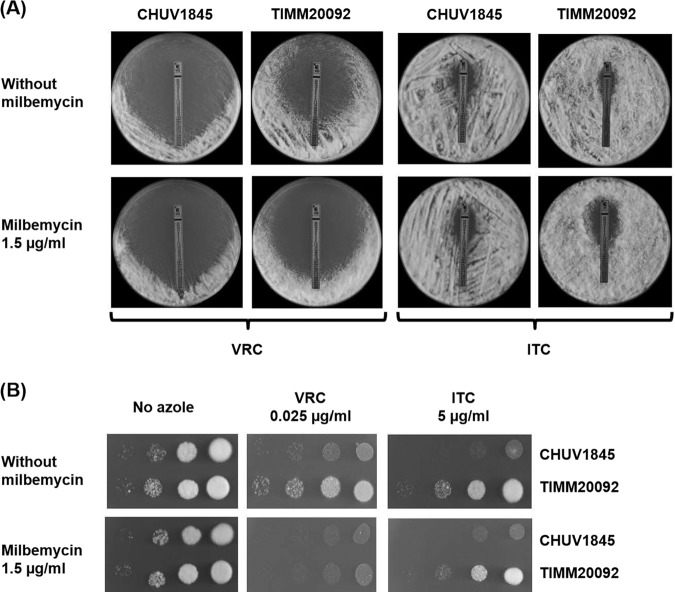
The MICs of VRC and ITC of TIMM20092 were measured at 0.063 μg/ml and 0.5 μg/ml, respectively, by the broth microdilution method. In the presence of 1.5 μg/ml milbemycin oxime, known to be an inhibitor of ABC transporters (14–16), the MIC of VRC decreased 75% to 0.015 μg/ml, and that of ITC decreased by 50% to 0.25 μg/ml. The values of 0.015 μg/ml (VRC) and 0.25 μg/ml (ITC) were close to those obtained for 10 clinical isolates with or without the presence of milbemycin oxime and are in line with values reported in the literature for T. rubrum (10, 17, 18). One of these clinical isolates, CHUV1845, was selected for comparison with TIMM20092 in further experiments.
A reduced sensitivity of TIMM20092 to ITC, similar to VRC, was clearly demonstrated by Etests and by spot tests on Sabouraud dextrose agar (SDA) containing the antifungal drugs (Fig. 1). The sensitivity of TIMM20092 to VRC was increased by adding 1.5 μg/ml milbemycin oxime to the agar medium, while resistance to ITC was only slightly affected. We found that at this concentration, milbemycin oxime did not affect fungal growth.
FIG 1.
Susceptibility and resistance of T. rubrum strains, CHUV1845 and TIMM20092, to voriconazole (VRC) and itraconazole (ITC) with and without 1.5 μg of milbemycin oxime. (A) Etest strips laced with an azole derivative were placed on fungal lawns (106 CFU) prepared on SDA plates. (B) Serial dilution drug susceptibility assays to VRC and ITC. T. rubrum spores were spotted at different dilutions on SDA plates, as described in Materials and Methods. The plates were incubated at 30°C for 7 days.
We sequenced the cyp51A gene encoding the azole target in TIMM20092 and CHUV1845. The sequence of cyp51A was identical to that of the sequences from the genomes of strains ATCC MYA-4607/CBS 118892 and CBS 288.86 submitted to UniProtKB (accession identifiers [IDs] F2SHH3 and A0A022VVX6, respectively). These results indicated that the resistance of TIMM20092 could not be attributed to a missense mutation of the drug target.
Identification of a new MFS antifungal transporter using a pool of plasmids from a previously constructed T. rubrum cDNA library.
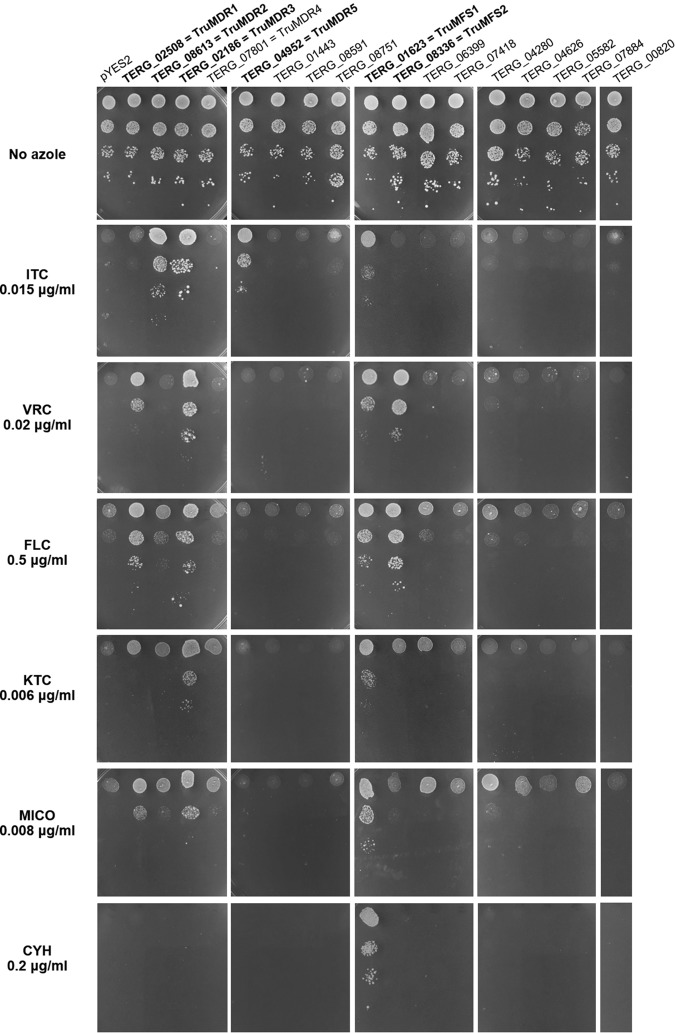
To explore the molecular basis of antifungal drug resistance in dermatophytes, we first transformed Saccharomyces cerevisiae Y02409 with a pool of plasmids from a previously constructed cDNA library, where T. rubrum cDNA sequences were cloned into the pYES2-DEST52 vector downstream of the GAL1 promoter for inducible, heterologous gene expression (19) (refer to Materials and Methods). Transformant cells were seeded on minimal medium with galactose (MMG) plates, which incorporated ITC and VRC at concentrations of 0.01 and 0.02 μg/ml, respectively. These concentrations were equivalent to about 5 times the MIC of S. cerevisiae Y02409 for both antifungal compounds. Five ITC-resistant and 12 VRC-resistant S. cerevisiae clones were obtained. The plasmid DNA of these clones was then isolated as previously described (20) and used for the transformation of Escherichia coli XL1-Blue. Sequence analysis revealed that all ampicillin-resistant E. coli clones obtained from each selected S. cerevisiae transformant contained a 2.1- to 2.3-kb cDNA insert with an identical 1.7-kb open reading frame (ORF). A BLAST search revealed that this ORF corresponded to TERG_01623, which encodes an MFS transporter. We designated this transporter TruMFS1. Unfortunately, no other transcripts containing a resistance gene were identified using this approach. TruMFS1 was able to transport all azoles tested, including miconazole (MICO), fluconazole (FLC), and ketoconazole (KTC). In addition, TruMFS1 was also able to transport cycloheximide (CYH) (Fig. 2).
FIG 2.
Susceptibility and resistance to itraconazole (ITC), voriconazole (VRC), fluconazole (FLC), ketoconazole (KTC), miconazole (MICO), and cycloheximide (CYH) of S. cerevisiae transformed with different plasmids encoding MFS and ABC transporters. The efflux pumps conferring drug resistance are highlighted in bold. Yeasts were spotted at different dilutions on MMG plates, as described in Materials and Methods. As a control, S. cerevisiae was spotted on plates without antifungal drugs. The plates were incubated at 30°C for 7 days. S. cerevisiae transformed with plasmid pYES2 was used as a control of susceptibility (top row).
Identification of an additional T. rubrum MFS transporter involved in azole resistance.
A search in the T. rubrum proteome for proteins with a domain signature for MFS transporters led to the identification of 170 potential proteins in the UniProtKB database (https://www.uniprot.org/) (see Table S1 in the supplemental material). To restrict this list to transporters potentially involved in azole resistance, we took advantage of studies performed with the closely related pathogenic fungus Aspergillus fumigatus, as well as with the well-studied pathogenic yeasts Candida albicans and Candida glabrata. AFUA_1G13800/mdrA was the only A. fumigatus MFS transporter for which involvement in azole resistance has been demonstrated (21), while additional MFS transporter genes of A. fumigatus were reported to be induced either by VRC (mfsB) (22) or by ITC (mdr3) (23). The BLAST analysis led to the identification of three transporters, TERG_08336, TERG_07418, and TERG_06399, whose products are the closest homologs of A. fumigatus mdrA, mfsB, and mdr3, respectively. The BLAST analysis also showed that the closest homolog of TruMFS1 that we identified earlier was aflT, a transporter whose gene is part of the aflatoxin cluster in the Aspergillus species (24). Seven MFS transporters from both C. albicans and C. glabrata led to the identification of five additional T. rubrum MFS candidates (TERG_00820, TERG_04280, TERG_04626, TERG_05582, and TERG_07884) (Table 1). There was no overlap between the sets of transporter genes identified from Aspergillus and Candida.
TABLE 1.
Identification of potential T. rubrum MFS transporters involved in azole resistance
| Locus name by organism | Transporter name(s)/synonyms | Resistance(s)or substrate informationa | PMID (reference) | T. rubrum homolog(s)c | Protein sequence identity, length of identical parts of proteins (aa)b |
|---|---|---|---|---|---|
| A. fumigatus | |||||
| AFUA_1G13800 | mdrA | VRC and FLC | 26933209 (21) | TERG_08336 (TruMFS2) | 64.4, 595 |
| AFUA_1G15490 | mfsB | VRC induced | 16622700 (22) | TERG_07418 | 65.1, 578 |
| AFUA_4G10000 | mdr3 | ITC induced | 15563516 (23) | TERG_06399 | 77.8, 803 |
| AFUA_1G12620 | aflT | Not aflatoxin | 17620135 (24) | TERG_01623 (TruMFS1) | 53.2, 494 |
| C. albicans | |||||
| CAALFM_CR04210CA | QDR1 | Not azoles | 24621232 (62) | TERG_04280 | 28.1, 509 |
| CAALFM_C305570WA | QDR2 | Not azoles | 24621232 (62) | TERG_04280 | 29.2, 504 |
| CAALFM_C601290CA | QDR3 | Not azoles | 24621232 (62) | TERG_00820 | 45.8, 697 |
| CAALFM_C701520WA | FLU1/TPO1 | Mycophenolic acid, FLC | 11065353 (63) | TERG_04626 | 49.5, 610 |
| CAALFM_C603170CA | MDR1/BMR1/BEN1 | Benomyl, CYH, methotrexate, FLC | 8031026 (64), 9210670 (65), 15273122 (66), 10844673 (67), 11568478 (68) | TERG_05582 | 35.2, 564 |
| CAALFM_C604610CA | NAG3/MDR97/TMP1 | CYH | 12076781 (69) | TERG_07884 | 56.4, 561 |
| CAALFM_C604620CA | NAG4 | CYH | 12076781 (69) | TERG_07884 | 57.1, 581 |
| C. glabrata | |||||
| CAGL0J09944g | AQR1 | Acetic acid, flucytosine, CLT | 23805133 (70) | TERG_04280 | 30.6, 492 |
| CAGL0G08624g | QDR2 | Quinidine, MICO, TIC, CLT, KTC | 23629708 (71) | TERG_04280 | 30.1, 583 |
| CAGL0G03927g | TPO1_1 | CLT | 26512119 (72) | TERG_04626 | 42.7, 518 |
| CAGL0E03674g | TPO1_2 | CLT | 26512119 (72) | TERG_04626 | 48.3, 478 |
| CAGL0H06017g | FLR1 | 5-Flucytosine | 28066366 (73) | TERG_05582 | 35.5, 512 |
| CAGL0H06039g | FLR2 | 5-Flucytosine | 28066366 (73) | TERG_05582 | 35.7, 566 |
| CAGL0I10384g | TPO3 | CLT, MICO, KTC, FLC | 24576949 (74) | TERG_07884 | 41.2, 450 |
Compounds that are found to be substrates or to induce gene expression and those that are not substrates are indicated. ITC, itraconazole; VRC, voriconazole; FLC, fluconazole; MICO, miconazole; TIC, tioconazole; CLT, clotrimazole; KTC, ketoconazole; CYH, cycloheximide.
aa, amino acids.
The efflux pumps conferring drug resistance are named between parentheses and highlighted in bold.
Full-length amino acid sequences of the identified transporters in T. rubrum were confirmed or deduced by alignment against a set of high-quality transporter amino acid sequences, namely, protein sequences of the model organisms S. cerevisiae, Aspergillus nidulans, A. fumigatus, C. albicans, and Trichophyton benhamiae (formerly Arthroderma benhamiae), which were reviewed by UniProtKB/Swiss-Prot. Subsequently, we cloned DNA sequences encoding the eight MFS transporters into pYES2 and transformed S. cerevisiae with the generated plasmids. Yeast transformants were then tested for resistance to ITC, VRC, FLC, KTC, and MICO, as described in Materials and Methods, to examine the capability of each transporter to operate as an efflux pump. Only the product of TERG_08336, designated TruMFS2, was able to provide VRC and FLC resistance but not ITC, KTC, or MICO resistance (Fig. 2). TruMFS2 was close to the A. fumigatus transporter mdrA (21). Surprisingly, none of the T. rubrum homologs of Candida MFS transporters used for the BLAST analysis appeared to be involved in resistance to any of the azoles tested (Fig. 2).
Identification of four T. rubrum ABC transporters involved in azole resistance.
A search in the proteome of T. rubrum in UniProtKB for proteins with a domain signature for ABC transporters led to the identification of 39 potential candidates (Table S2). Six of them were not actually transporters but derived from the ABC family since they have lost their transmembrane domains. Based on the literature, we screened 10 A. fumigatus, 4 C. albicans, and 3 C. glabrata transporters directly involved in azole resistance or induced by azoles (Table 2). The BLAST analysis identified 10 T. rubrum ABC transporters putatively involved in azole resistance. The only ABC transporter that did not have a homolog with >35% identity in T. rubrum was A. fumigatus atrF (AFUA_6G04360). In contrast, the products of TERG_02508 and TERG_02186, identified in T. rubrum, had homologs with >35% identity over a sequence of 1,000 amino acids in the three pathogenic fungi used for identification (Table 2). A. fumigatus mdr1/abcA (AFUA_5G06070) alone led to the identification of six different potential transporters of T. rubrum.
TABLE 2.
Identification of potential T. rubrum ABC transporters involved in azole resistance
| Locus name by organism | Transporter name(s)/synonyms | Resistance(s)or substrate informationa | PMID (reference) | T. rubrum homologsc | Protein sequence identity, length of identical parts of proteins (aa)b |
|---|---|---|---|---|---|
| A. fumigatus | |||||
| AFUA_3G07300 | atrI | ITC, VRC | 26933209 (21) | TERG_02508 (TruMDR1) | 58.6, 1,445 |
| AFUA_1G14330 | abcC/abcG1/cdr1B/atrE | ITC, VRC, POS | 16622700 (22), 23580559 (36), 22509997 (75) | TERG_02186 (TruMDR3), TERG_02508 (TruMDR1) | 61.7, 1,084; 50.8, 1,404 |
| AFUA_2G15130 | abcA_2 | FLC | 24123268 (76), 23796749 (77) | TERG_02186 (TruMDR3), TERG_02508 (TruMDR1) | 71.5, 1,085; 50.8, 1,404 |
| AFUA_5G06070 | mdr1/abcA | VRC induced | 16622700 (22) | TERG_08613 (TruMDR2), TERG_08693, TERG_00402, TERG_08751, TERG_04952 (TruMDR5), TERG_08591 | 68.5, 1,318; 50.1, 1,032; 49.1, 434; 41.7, 1,349; 40.7, 995; 38.7, 1,349 |
| AFUA_6G03470 | fmpD | Fumipyrrole | 25582336 (78) | TERG_08613 (TruMDR2) | 44.1, 1,279 |
| AFUA_1G17440 | abcA | Azoles (predicted) | 12172968 (79) | TERG_02186 (TruMDR3) | 57.0, 1,074 |
| AFUA_1G10390 | abcB | VRC induced | 16622700 (22) | TERG_01443 | 59.3, 933 |
| AFUA_1G12690 | mdr4 | VRC | 21321135 (25) | TERG_07801 (TruMDR4) | 60.0, 1,317 |
| AFUA_7G00480 | abcE | VRC induced | 16622700 (22) | TERG_08751, TERG_04952 (TruMDR5) | 53.8, 1,269; 39.2, 936 |
| AFUA_6G04360 | atrF | VRC | 26933209 (21) | —d | |
| C. albicans | |||||
| CAALFM_C603840CA | SNQ2 | Benomyl induced | 15273122 (66) | TERG_02508 (TruMDR1) | 42.8, 1,397 |
| CAALFM_C305220WA | CDR1 | FLC | 8585712 (34) | TERG_02186 (TruMDR3) | 45.3, 1,409 |
| CAALFM_C304890WA | CDR2 | Various azoles | 9043118 (35) | TERG_02186 (TruMDR3) | 44.7, 1,372 |
| CAALFM_C108070WA | CDR4 | Not FLC, CAS induced | 9767132 (80), 15917516 (81) | TERG_02186 (TruMDR3) | 45.3, 1,463 |
| C. glabrata | |||||
| CAGL0I04862g | SNQ2 | FLC | 18312269 (82) | TERG_02508 (TruMDR1) | 40.6, 1,495 |
| CAGL0M01760g | CDR1 | FLC | 11257032 (36) | TERG_02186 (TruMDR3) | 40,3, 1,506 |
| CAGL0M01760g | PDH1/CDR2 | FLC | 11257032 (36) | TERG_02186 (TruMDR3) | 45.3, 1,408 |
Compounds that are found to be substrates or to induce gene expression and those that are not substrates are indicated. ITC, itraconazole; VRC, voriconazole; FLC, fluconazole; POS, posaconazole; CAS, caspofungin. A. fumigatus abcA has been predicted to transport azoles by similarity but targeted disruption experiments did not confirm it.
aa, amino acids.
The efflux pumps conferring drug resistance are named between parentheses and highlighted in bold.
No homolog of A. fumigatus atrF has been found in T. rubrum.
As for MFS transporters, we checked the full-length amino acid sequences by comparing the T. rubrum protein sequences with those of the model organisms S. cerevisiae, A. nidulans, A. fumigatus, C. albicans and T. benhamiae. In particular, we made corrections in the intron-exon structure of the gene encoding TruMDR1 based on the alignment of the polypeptide sequence of this transporter with those of the closest transporters in the aforementioned organisms. The accession number of the updated sequence encoding TruMDR1 is GenBank accession no. MK787254. For two transporters encoded by TERG_08693 and TERG_00402, the full-length protein sequence could not be recovered, suggesting that they probably corresponded to products of pseudogenes, and they were not considered further in this study.
The eight remaining ABC transporters were tested in S. cerevisiae for their ability to provide resistance to azoles (Table 3). Four candidates, encoded by TERG_02508, TERG_08613, TERG_02186, and TERG_04952, were able to render the yeasts resistant to one or several azoles. TERG_02508 and TERG_08613 correspond to TruMDR1 and TruMDR2, respectively, which were previously reported (10, 11). However, for the first time, our study shows their direct involvement in the transport of azoles. The expression of TruMDR1 cDNA led to VRC, FLC, and MICO resistance, whereas the expression of TruMDR2 cDNA led to only ITC resistance (Fig. 2). The product of TERG_02186 was the only ABC transporter gene that rendered the yeasts resistant to all azoles tested and was designated TruMDR3 (Fig. 2). The product of TERG_04952, designated TruMDR5, gave clear resistance to ITC but not to other azoles (Fig. 2). The expression of TERG_07801, coding for TruMDR4, closely related to the A. fumigatus VRC-resistant transporter, mdr4 (25), surprisingly yielded no resistance, as was the case for TERG_01443, TERG_08591, and TERG_08751 (Fig. 2).
TABLE 3.
Characterization of potential T. rubrum transporters involved in azole resistance
| Locus name by type | Transportera name | Resistance for azoleb: |
Commentsc | |||||
|---|---|---|---|---|---|---|---|---|
| VRC | ITC | KTC | FLC | MIC | CYH | |||
| ABC transporters | ||||||||
| TERG_02508 | TruMDR1 | + | − | − | + | + | − | Close to A. fumigatus, C. albicans, and C. glabrata azole transporters |
| TERG_08613 | TruMDR2 | − | + | − | − | − | − | Close to A. fumigatus mdr1 |
| TERG_02186 | TruMDR3 | + | + | + | + | + | − | Close to A. fumigatus, C. albicans, and C. glabrata azole transporters |
| TERG_07801 | TruMDR4 | − | − | − | − | − | − | Close to A. fumigatus mdr4 |
| TERG_04952 | TruMDR5 | − | + | − | − | − | − | Close to A. fumigatus abcE |
| TERG_01443 | − | − | − | − | − | − | − | Close to A. fumigatus abcB |
| TERG_08591 | − | − | − | − | − | − | − | Close to A. fumigatus mdr1 |
| TERG_08751 | − | − | − | − | − | − | − | Close to A. fumigatus mdr1 and abcE |
| MFS transporters | ||||||||
| TERG_01623 | TruMFS1 | + | + | + | + | + | + | Close to A. fumigatus aflatoxin transporter aflT |
| TERG_08336 | TruMFS2 | + | − | − | + | − | − | Close to A. fumigatus mdrA |
| TERG_07418 | − | − | − | − | − | − | − | Close to A. fumigatus mfsB |
| TERG_06399 | − | − | − | − | − | − | − | Close to A. fumigatus mdr3 |
| TERG_04626 | − | − | − | − | − | − | − | Close to C. albicans FLU1 and C. glabrata TPO1_1 and TPO1_2 |
| TERG_07884 | − | − | − | − | − | − | − | Close to C. albicans MDR1 and C. glabrata FLR1 and FLR2 |
| TERG_05582 | − | − | − | − | − | − | − | Low similarities with C. albicans NAG3 and NAG4 and C. glabrata TPO3 |
| TERG_04280 | − | − | − | − | − | − | − | Low similarities with C. albicans QDR1 and QDR2 and C. glabrata AQR1 and QDR2 |
| TERG_00820 | − | − | − | − | − | − | − | Low similarities with C. albicans QDR3 |
Only transporters showing azole transporter activity have been named. The others are marked with a minus sign.
+, Constructs showing resistance in S. cerevisiae; −, no resistance.
Close homologs from A. fumigatus and/or Candida spp. are indicated.
Milbemycin oxime inhibits TruMDR3.
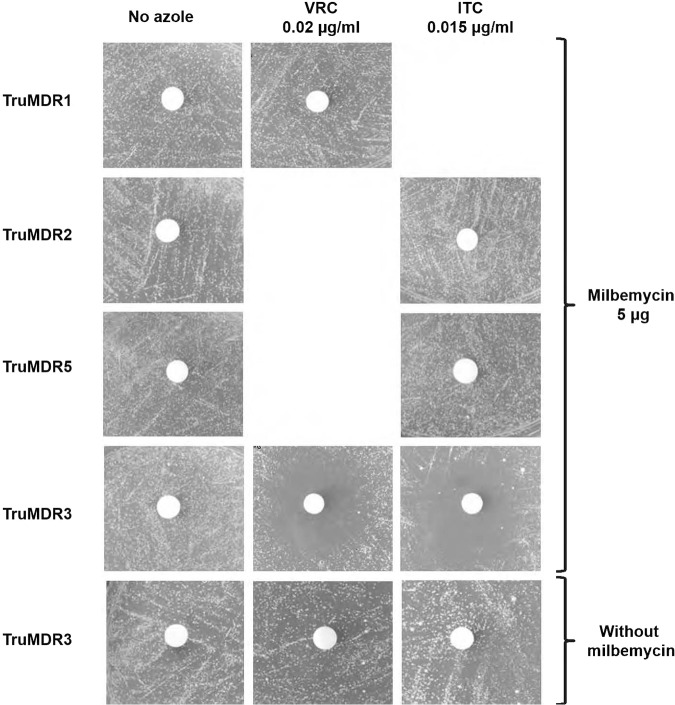
Using disk tests, we tested known inhibitors of ABC transporters, milbemycin oxime, ibuprofen, farnesol, haloperidol, promethazine hydrochloride, and curcumin (26–30), on the S. cerevisiae transformants, expressing individual transporters. S. cerevisiae cells were seeded to make a lawn on inducing MMG in the presence and absence of sub-MICs of VRC (0.02 μg/ml) or ITC (0.015 μg/ml). Cellulose disks were then placed on the surface of the medium and loaded with 5 μg of each inhibitor tested. Transformants expressing TruMDR3 were inhibited by milbemycin oxime (Fig. 3) but not by ibuprofen, farnesol, haloperidol, promethazine hydrochloride, or curcumin in the presence of either ITC or VRC (data not shown). Transformants expressing the genes encoding TruMDR1, TruMDR2, and TruMDR5 and the two MFS transporters TruMFS1 and TruMFS2 were not sensitive to milbemycin oxime and the other tested inhibitors. We concluded that milbemycin oxime was a specific inhibitor of TruMDR3.
FIG 3.
The effect of milbemycin oxime on TruMDR1, TruMDR2, TruMDR3, and TruMDR5. A total of 106 cells of S. cerevisiae transformants were seeded to make a lawn on MMG plates with a sub-MIC of ITC (0.5 μg/ml) or VRC (0.64 μg/ml) incorporated into the medium. Nonimpregnated cellulose disks, 6 mm in diameter, were then placed at the surface of the medium and individually saturated with 5 μg of milbemycin oxime. The plates were incubated at 30°C, and growth inhibition was observed after 5 days. Growth inhibition was recorded for TruMDR3. No effect was observed for TruMDR1, TruMDR2, and TruMDR5, or for TruMFS1 and TruMFS2 (not shown). Milbemycin oxime has only been tested when the transporter induced resistance to VRC or ITC.
TruMDR2 and TruMDR3 are overexpressed in the azole-resistant strain TIMM20092.
We examined the expression of transporter genes in the azole-resistant strain TIMM20092 and in CHUV1845 (azole-sensitive strain) using quantitative real-time reverse transcription-PCR (qRT-PCR). The basal expression of TruMDR2 and TruMDR3 increased 5- and 8-fold, respectively, in TIMM20092, compared to that in CHUV1845 (Fig. 4A). The other transporter genes (TruMDR1, TruMDR4, TruMDR5, TruMFS1, and TruMFS2) were expressed 2- to 4-fold more in TIMM20092 than in CHUV1845.
FIG 4.
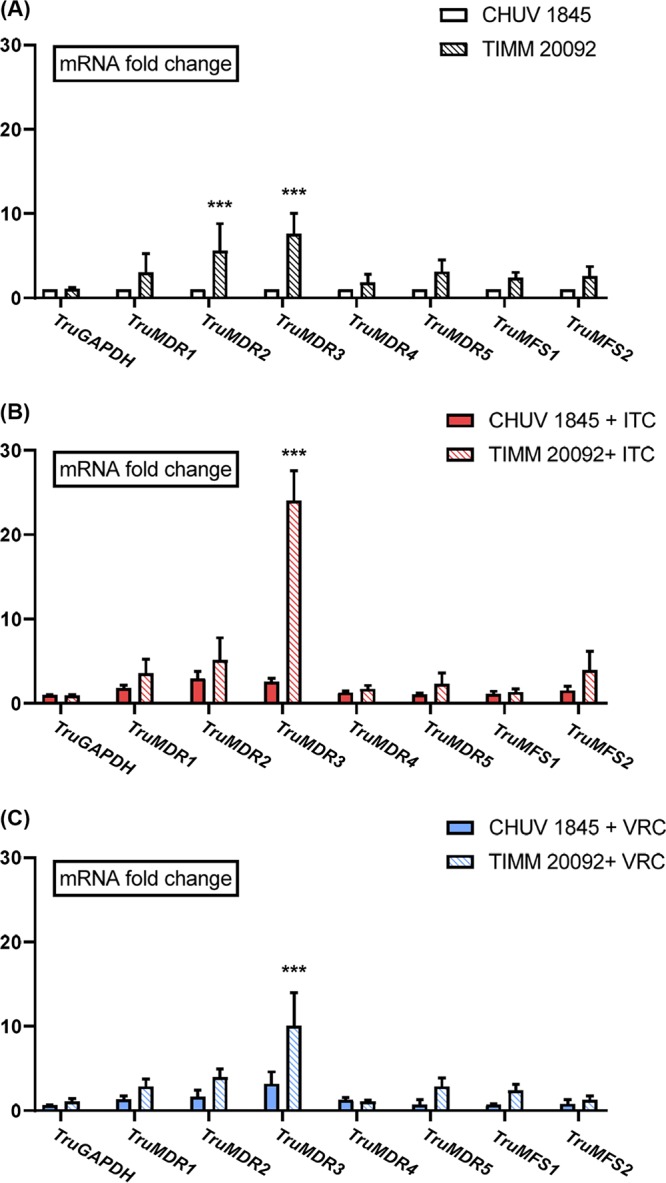
(A to C) Expression levels of genes encoding ABC and MFS transporters, as determined by qRT-PCR, in the absence of azole (A), upon exposure to itraconazole (ITC) (B), and upon exposure to voriconazole (VRC) (C). T. rubrum strains CHUV1845 and TIMM20092 were cultured for 7 days in SDB without an antifungal drug or SDB containing subinhibitory concentrations of ITC or VRC. The fold change represents the level of gene expression compared with that of T. rubrum CHUV1845 grown in SDB without antifungal drugs. The bars represent the standard deviation of the data obtained from three independent experiments. ***, P < 0.001.
ITC and VRC exposure increased significantly the transcription of TruMDR3 in both TIMM20092 and CHUV1845 (Fig. 4B and C). A slight increase in the expression of TruMDR1 and TruMDR2 was also observed upon ITC exposure in CHUV1845 but not in TIMM20092. There was no significant difference in the expression of TruMDR4, TruMDR5, TruMFS1, and TruMFS2 upon exposure to ITC or VRC in both strains.
Disruption of TruMDR3 in azole-resistant strain TIMM20092 leads to VRC sensitivity.
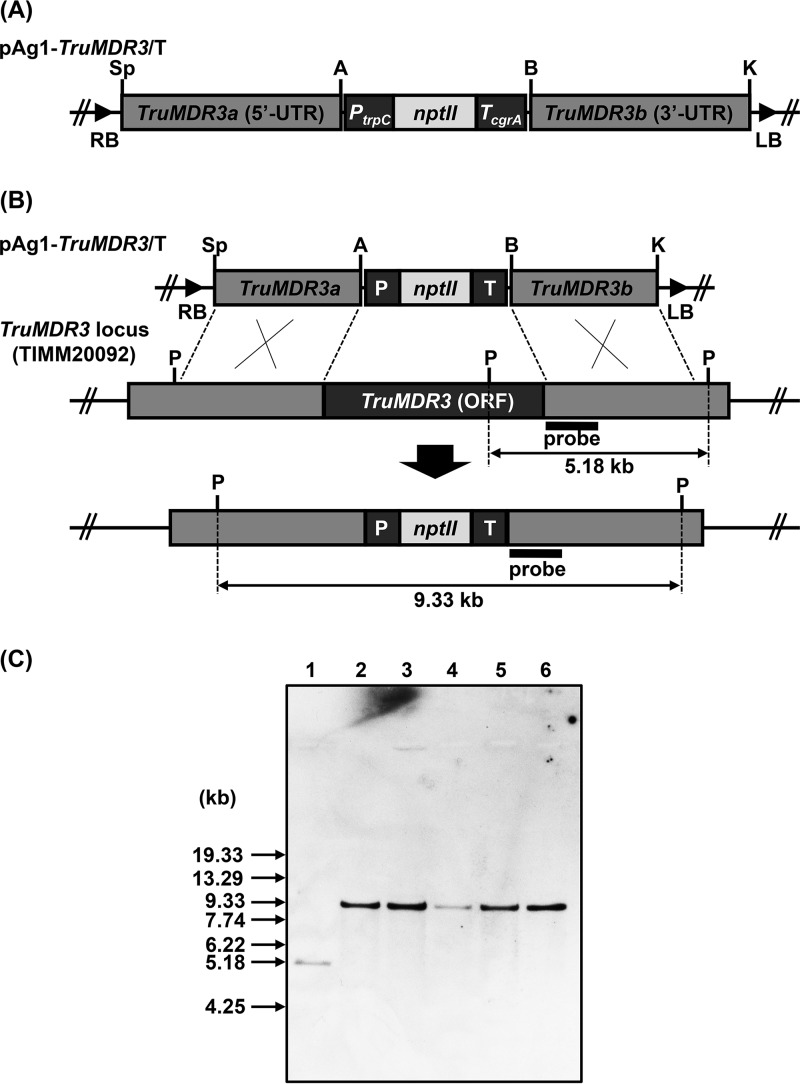
In order to examine the importance of TruMDR3 in azole resistance of TIMM20092, we carried out the disruption of the TruMDR3 locus by a gene replacement strategy (Fig. 5A and B). Since TIMM20092 is a wild-type clinical isolate, the targeting frequency of the TruMDR3 locus by homologous recombination in this strain was low. Of 150 G418-resistant transformants obtained by the A. tumefaciens-mediated transformation, 5 transformants have been found to be the desired TruMDR3 disruptants by PCR and Southern blotting (Fig. 5C).
FIG 5.
Disruption of the MDR3 (TruMDR3) gene of T. rubrum TIMM20092 by gene replacement strategy. (A) Schematic representation of the binary TruMDR3-targeting vector pAg1-TruMDR3/T. The nptII cassette is composed of Aspergillus nidulans trpC promoter (PtrpC), E. coli neomycin phosphotransferase gene (nptII), and the A. fumigatus cgrA terminator (TcgrA). LB and RB, left and right borders, respectively; A, ApaI; B, BamHI; K, KpnI; P, PstI; S, SpeI. (B) Schematic representation of the TruMDR3 locus before and after homologous recombination. (C) Southern blotting. Lane 1, TIMM20092 (parent strain); lanes 2 to 6, ΔTruMDR3-11, -22, -33, -39, and -45, respectively. A 509-bp fragment of the TruMDR3 locus was amplified by PCR with a pair of the primers P35 and P43 (Table S4) and used as a hybridization probe. DNA standard fragment sizes are shown on the left.
Spot tests on SDA medium and Etests show that the deletion of TruMDR3 in TIMM20092 mimics the addition of milbemycin and abolishes the resistance to VRC (Fig. 6A). This was confirmed by Etest (Fig. 6B). The deletion of TruMDR3 affected the resistance to ITC to a lesser extent, suggesting that another transporter could also be involved in ITC resistance of TIMM20092 (Fig. 6A and B).
FIG 6.
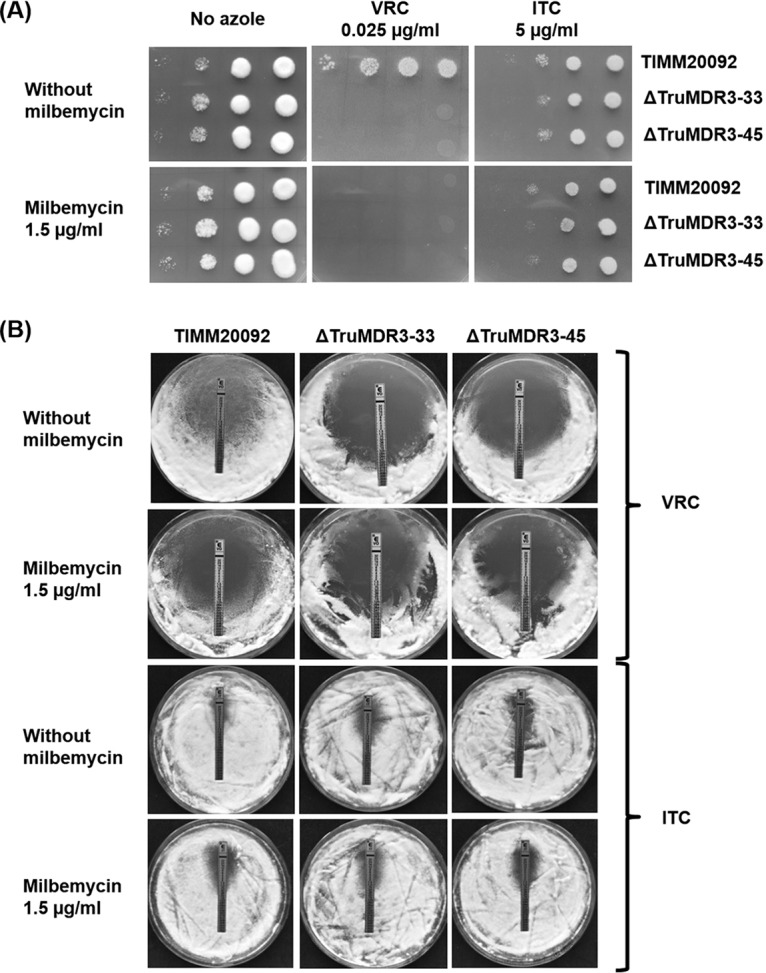
Susceptibility and resistance of T. rubrum strain TIMM20092, and two TruMDR3 mutants, ΔTruMDR3-33 and ΔTruMDR3-45, to voriconazole (VRC) and itraconazole (ITC). (A) Serial dilution drug susceptibility assays to VRC and ITC. T. rubrum spores were spotted at different dilutions on SDA plates, as described in Materials and Methods. The plates were incubated at 30°C for 7 days. (B) Etest strips laced with VRC and ITC were placed on fungal lawns (106 CFU) prepared on SDA plates.
DISCUSSION
For the first time, to our knowledge, we have described mechanisms of resistance to azole compounds in T. rubrum. Several mechanisms have been shown to be involved in nondermatophyte pathogenic fungi. These include the overexpression of the gene encoding lanosterol-14α-demethylase (erg11 in Candida, cyp51A in Aspergillus), or its variant (23, 31–33), and the overexpression of several multidrug efflux transporters (31, 33–38). We found in T. rubrum four ABC transporters, TruMDR1, TruMDR2, TruMDR3, and TruMDR5, and two MFS transporters, TruMFS1 and TruMFS2, able to act as efflux pumps for at least one of the azole compounds tested. TruMDR3 and TruMFS1 were able to transport all five azoles tested, showing them to have a broad specificity toward azoles. In addition, TruMFS1 has also been shown to be active toward CYH, suggesting that its substrate specificity is not restricted to azoles but is broader and could be considered a pleiotropic drug transporter. In contrast, TruMDR1 and TruMFS2 have been found to be efflux pumps for VRC and FLC (Fig. 2), which have similar molecular structures. The genes coding for two transporters, TruMDR2 and TruMDR3, were found to be overexpressed in a clinical isolate which showed reduced sensitivity to azoles, TIMM20092. Unlike terbinafine resistance in dermatophytes, the reduction in sensitivity of TIMM20092 to azoles was not mediated by a missense mutation in the antifungal drug target.
Antifungal susceptibility testing.
Spot tests, Etests, and microdilution assays were performed with adaptations from the Clinical and Laboratory Standards Institute (CLSI) guidelines (39) (use of SDA medium instead of RPMI 1640 and spectrophotometric measure of fungal growth) when working with T. rubrum, which was more experimentally demanding than other sporulating, fast-growing filamentous fungi (e.g., A. fumigatus) (40). The growth of T. rubrum isolates was faster, and mycelium was more abundant in Sabouraud dextrose broth (SDB) than in RPMI 1640 liquid or agar medium. Differences between MIC values in liquid and agar media were recorded for the same isolate. However, regardless of the method used, TIMM20092 was always more resistant than all other isolates for which the MIC appeared to be similar. The MIC of TIMM20092 always deviated from the standard MIC of the other strains.
Comparison of T. rubrum azole transporters with closest A. fumigatus homologs.
ITC is extensively used in therapy for dermatophyte infections (41). Recently, VRC, routinely used in clinical practice to treat fungal infections of the central nervous system (CNS) (42), was shown to be effective in treating dermatophytosis (43). Therefore, we first tested ITC and VRC on T. rubrum in our research on azole resistance involving transporters. Our first attempt to isolate dermatophyte azole transporters, using a pool of plasmids from a previously established cDNA library of T. rubrum, only led to the identification of an MFS type transporter, TERG_01623, whose closest homolog in Aspergillus is aflT. The gene encoding aflT is located within the aflatoxin gene cluster in Aspergillus species (24). The expression of the aflT gene in Aspergillus parasiticus is not controlled by the aflatoxin pathway-specific activator aflR, nor by the coactivator aflJ, and aflT does not have a significant role in aflatoxin secretion (44). It has not been demonstrated whether aflT plays a role in azole efflux. No other homologs of previously known Candida or Aspergillus azole transporters were isolated in the screen. This comes as no surprise since the cDNA bank used in this study was not produced under stress conditions with azoles that could induce the expression of genes encoding efflux pumps. In addition, the large size of transporters such as ABC transporters may have prevented them from being present in the library. To select additional azole transporter candidates in T. rubrum, we searched for homologs of azole transporters characterized in the well-studied fungal pathogens A. fumigatus, C. albicans, and C. glabrata and found five additional azole transporters among 16 candidates selected by the BLAST search.
Differences in substrate specificity were observed between azole transporters in A. fumigatus and their closest homologs in T. rubrum (Table 3). A. fumigatus mdr1/abcA has six 40 to 60% identical homologs in T. rubrum, but only two of them (TruMDR2 and TruMDR5) showed azole transport activity (Table 2). TruMDR2 is closest to A. fumigatus mdr1, while TruMDR5 shared a lower identity with A. fumigatus mdr1 than did TERG_08693, TERG_00402, and TERG_08751 products, which did not show any ITC or VRC transport activity. A difference in substrate specificity was also observed between TruMDR4 and its A. fumigatus homolog, mdr4. TruMDR4 showed no activity toward any of the five azoles tested (ITC, VRC, MICO, FLC, and KTC), while A. fumigatus mdr4 has been shown to be active toward ITC (25). Large gene families allow members to diverge rapidly throughout evolution with the consequence of a loss or a gain of functions, resulting in a great diversity of substrates and specificities in the case of transporters. TruMDR1 and TruMDR3 are close to all identified Candida ABC azole transporters (34, 35).
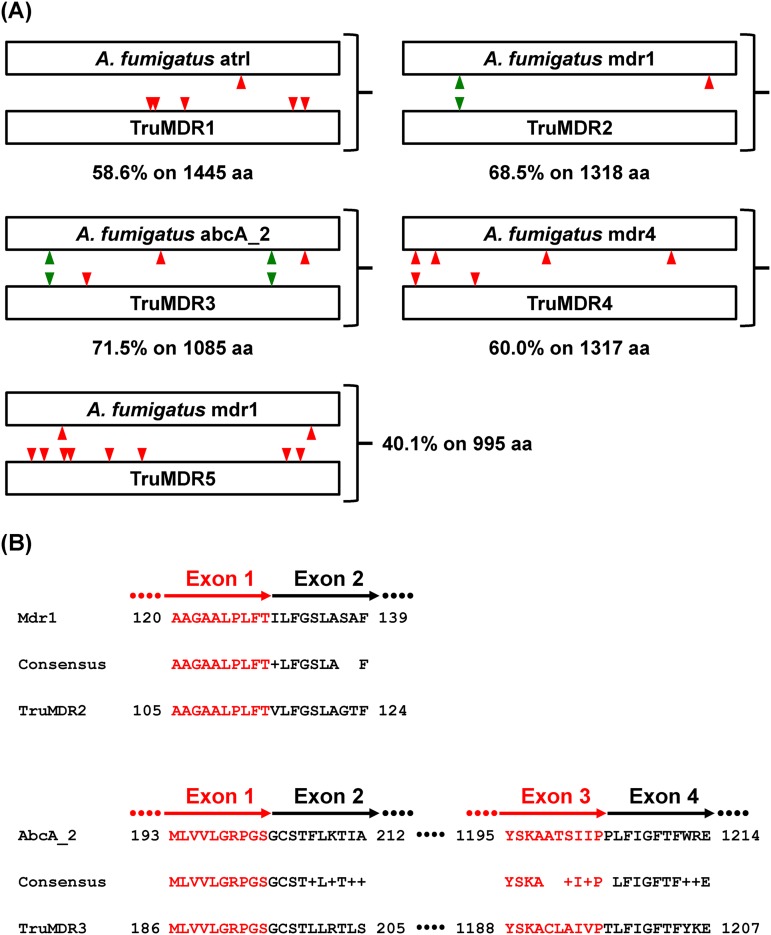
The intron-exon structures of genes encoding T. rubrum azole ABC transporters, and those of the closest homologs in A. fumigatus, were generally different (Fig. 7A and Table S3). The variability in the genetic models of ABC carriers cautions the use of the term orthologs between ABC transporter genes belonging to these fungal species. Only two pairs of genes could be considered orthologs, as follows: (i) TruMDR3 and the closest A. fumigatus homolog AFUA_2G15130/abcA_2, which had three and four introns, respectively, in which the positions of introns 1 and 3 were conserved (Fig. 7B); and (ii) TruMDR2 and A. fumigatus mdr1, which had one and two introns, respectively, in which intron 1 is conserved in the two genes (Fig. 7B). In contrast, TruMDR1 and the closest ABC transporter in A. fumigatus atrI (58% identity) cannot be considered orthologs. The unique intron in TruMDR1 and the five introns contained in A. fumigatus atrI are in different positions (Fig. 7A). Likewise, TruMDR4 (two introns) and TruMDR5 (eight introns) cannot be considered the orthologs of A. fumigatus mdr4 (four introns) and A. fumigatus mdr1 (one intron), respectively (Fig. 7A and Table S3).
FIG 7.
(A) Intron-exon structures of T. rubrum and A. fumigatus genes encoding ABC transporters. Green arrowheads indicate introns in ortholog positions. Red arrowheads indicate introns in other positions showing that the intron-exon structures of genes encoding T. rubrum azole ABC transporters and those of the closest homologs in A. fumigatus, are generally different. Refer to Table S3 for the amino acid positions in the transporter sequence, which correspond to the intron positions in the coding genes. (B) Comparison of the protein sequences encoded by the conserved intron region in TruMDR2 and A. fumigatus mdr1, and comparison of the protein sequences encoded by the two conserved intron regions in TruMDR1 and A. fumigatus abcA_2. The amino acid sequence coded by the 3′ extremity of the left exon is in red, and the amino acid sequence encoded by the 5′ extremity of the right exon is in black.
TruMDR3 plays a major role in azole resistance of strain TIMM20092.
The disruption of TruMDR3 in TIMM20092 abolished its resistance to VRC and reduced its resistance to ITC (Fig. 6). The reduced resistance to ITC is explained by the fact that the remaining TruMDR2, which is also significantly overexpressed in TIMM20092, is also an efflux pump for ITC, like TruMDR3 (Fig. 2). TruMDR3 was the only transporter to be inhibited by the multidrug resistance (MDR) inhibitor milbemycin oxime, and TIMM20092 lost its resistance to azoles after milbemycin oxime treatment. The sensitivity of TIMM20092 in the presence of milbemycin was comparable to that of the TIMM20092 ΔTruMDR3-33 and ΔTruMDR3-45 mutants (Fig. 6).
qRT-PCR analyses also showed that the gene encoding TruMDR3 was the most overexpressed in the azole-resistant strain TIMM20092, compared to the sensitive strain, CHUV1845 (Fig. 4A). Transcription of TruMDR3 was upregulated upon exposure to ITC and VRC in TIMM20092 and in CHUV1845 (Fig. 4B and C). Altogether, these results were consistent and suggested that overexpression of TruMDR3 was one of the major causes of the azole resistance in the TIMM20092 strain.
It should be noted that milbemycin inhibits both C. albicans CDR1 and CDR2 (14–16), whose amino acid sequences have approximately 50% identity with TruMDR3. TruMDR2 seems also to contribute to the azole resistance of TIMM20092, whereas TruMDR1 and TruMFS2 seem to play a weaker role in azole resistance of this clinical isolate. In contrast to findings by Martins et al. (13), TruMDR4 was not found to be overexpressed upon exposure to ITC. Its expression in S. cerevisiae did not result in S. cerevisiae resistance to ITC, and therefore, this transporter does not appear to contribute to azole resistance in T. rubrum.
The molecular mechanisms leading to the overexpression of azole transporters have been described in pathogenic fungi other than dermatophytes. For instance, point mutations in C. albicans TAC1, a transcription factor that regulates CDR1 and CDR2, were shown to mediate antifungal resistance through the overexpression of both genes encoding ABC transporters (45). A single base substitution in the hapE gene encoding the A. fumigatus transcription factor complex subunit led to azole resistance (46). Another A. fumigatus transcription factor, atrR, has been shown to play a pivotal role in a novel azole resistance mechanism by coregulating the drug target, Cyp51A, and putative drug efflux pump, abcC/cdr1B (47). The overexpression of genes coding for TruMDR2 and TruMDR3 leads to the assumption that there was a mutation gain of function in a transcription factor that remains to be identified in dermatophytes.
Conclusion and perspectives.
Our study highlights the role of the efflux pumps, specifically, the ability of the ABC transporter TruMDR3 to counteract the effect of azole treatments, in the case of azole-resistant dermatophytosis. Additional transporters, including TruMDR2, might also contribute to the natural azole resistance of strain TIMM20092. TruMDR2 and TruMDR3 are both overexpressed in TIMM20092, and both transport ITC. Further disruption studies including the double disruption of TruMDR2 and TruMDR3 would provide more clues about the contribution of each transporter to ITC resistance. However, targeted gene disruption is not straightforward in T. rubrum and challenges the creation of such mutants.
Unlike the other transporters tested in this study, TruMDR3 was specifically inhibited by milbemycin oxime. In contrast, previous studies showed that milbemycin A3 and A4, as well as their oxime derivatives, repressed drug efflux by inhibiting more than one transporter in C. glabrata and C. albicans (16, 48, 49). Azole resistance could also be reduced with milbemycin oxime in Indian Trichophyton mentagrophytes strains recently sent for examination (data not shown), suggesting overexpression of the MDR3 ortholog in these fungi. Dermatophyte isolates resistant to azole compounds or terbinafine have a high prevalence in India (9). The present study could be used as a basis for studying azole resistance in these isolates.
Milbemycin A3 and A4, as well as their oxime derivatives, are widely used in veterinary practice as antiparasitic drugs (50). A milbemycin derivative (moxidectin) was also used in humans to treat onchocerciasis (river blindness), a parasitic disease caused by the helminth Onchocerca volvulus (51). This study showed that such substances have low toxicity in humans. The drug resistance of fungal pathogens, including A. fumigatus, Candida species, and dermatophytes, poses a serious concern, given the limited number of antifungal drugs currently available (52–55). The increasing number of resistant isolates of pathogenic fungi in the world requires the development of new strategies to overcome resistance. Therefore, the combination of azoles with specific transporter inhibitors offers perspectives and potential solutions to treat dermatophyte infections in cases of azole resistance.
MATERIALS AND METHODS
Strains and plasmids.
T. rubrum TIMM20092 (6) and CHUV1845 were used in this study. The strains were stored as frozen stocks with 15% (vol/vol) glycerol at –80°C. S. cerevisiae strain Y02409 (MATa ura3Δ0 leu2Δ0 his3Δ1 met15Δ0 YOR153w::kanMX4; Euroscarf) and the expression vector pYES2 (Invitrogen-Life Technologies, Carlsbad, CA, USA) were used for heterologous expression of T. rubrum transporters. All plasmid subcloning experiments were performed in E. coli DH5α using the plasmid pUC57 (GeneCust, Ellange, Luxembourg). For TruMDR3 gene disruption in T. rubrum TIMM20092, Agrobacterium tumefaciens EHA105 was maintained as previously described (56).
Chemicals.
Itraconazole, milbemycin oxime, ibuprofen, farnesol, haloperidol, promethazine hydrochloride, and curcumin were purchased from Sigma-Aldrich (Buchs, Switzerland). Voriconazole (Vfend IV 200 mg) was acquired from Pfizer (Zurich, Switzerland). Milbemycin oxime and ibuprofen were dissolved in ethanol and distilled water (dH2O), respectively, at a concentration of 5 mg/ml. The other compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) to constitute stock solutions (5 mg/ml or 0.5 mg/ml for less soluble compounds). Stock solutions were stored at –20°C until use. Etest strips containing itraconazole (reference [Ref.] Etest, 0.002 to 32 μg/ml) and voriconazole (Ref. Etest, 0.002 to 32 μg/ml) were acquired from bioMérieux SA (Geneva, Switzerland) and stored at 4°C until use. Nonimpregnated cellulose disks (bioMérieux) were used for the diffusion assays.
Growth media.
T. rubrum strains were grown on Sabouraud dextrose agar (SDA) and liquid medium (SDB) (Bio-Rad, Hercules, CA, USA). Liquid cultures were performed without shaking for 7 days at 30°C. Complete medium for S. cerevisiae consisted of 1% (wt/vol) Bacto yeast extract (Difco Laboratories, Detroit, MI, USA), 2% (wt/vol) Bacto peptone (Difco Laboratories), and 2% (wt/vol) dextrose (YPD). S. cerevisiae synthetic minimal medium (MMD) supplemented with histidine, leucine, methionine, and tryptophan (20 μg/ml) was prepared according to Sherman (57), with 2% (wt/vol) dextrose as the carbon source. Minimal medium with galactose (MMG) was prepared as MMD, but 2% (wt/vol) galactose was added as the carbon source instead of dextrose. MMG was used for the expression of genes cloned in the pYES2 expression vector under the control of the GAL1 promoter. MMD and MMG plates were made with 2% (wt/vol) agar.
Drug susceptibility assays for T. rubrum.
T. rubrum cultures were grown on 1/10 SDA (0.1% [wt/vol] peptone, 0.2% [wt/vol] dextrose, 2% [wt/vol] agar) for 14 days at 30°C. Sporulation was monitored by microscopy. T. rubrum spores were collected using sterile swabs and suspended in 3 ml of sterile dH2O. To obtain standardized conidial stock suspensions, absorbance values at a wavelength of 600 nm were determined (GeneQuant 1300 spectrophotometer; GE Healthcare Life Sciences, Marlborough, MA, USA) and diluted to a value of 1.0. The viable spore count was determined by inoculating serial decimal dilutions of the conidial stock suspensions on SDA plates. When considering the stock suspensions, an optical density (OD) value of 1.0 was found to correspond to 2.2 × 107 to 2.3 × 107 CFU/ml.
MICs were determined according to guidelines for the broth the microdilution method of the Clinical and Laboratory Standards Institute (CLSI) (38) but using SDB instead of RPMI medium (40). After incubation, the plates were read using a microtitration plate spectrophotometer (Multiskan Ascent OD595 nm; Thermo Fisher, Switzerland) set at a wavelength of 595 nm. The MIC90 was defined as the lowest concentration of the drug present in the wells showing growth inhibition of 90% or higher, in comparison to absorbance values obtained without antifungal drugs. For Etest assays, strips laced with an azole derivative were placed on fungal lawns (106 CFU) prepared on SDA plates. For susceptibility assays on agar plates, 10 μl of serial dilutions of the stock suspensions (100 to 103, which corresponded to 105 to 102 spores, respectively) was spotted on SDA plates containing the desired concentration of antifungal drugs. The dishes were incubated at 30°C for 7 days.
T. rubrum cyp51A sequencing.
T. rubrum genomic DNA was isolated from freshly growing mycelia using a DNeasy plant minikit (Qiagen, Hilden, Germany). A DNA fragment encoding T. rubrum Cyp51A was amplified by PCR with a standard protocol using homologous sense and antisense primers P1 and P2, respectively, (Table S4) and 200 ng of T. rubrum genomic DNA. DNA sequencing was performed by Microsynth (Balgach, Switzerland).
T. rubrum cDNA library for heterologous expression in S. cerevisiae.
A cDNA bank of T. rubrum had already been prepared in the plasmid pSPORT6 (58). An RNA sample was prepared from T. rubrum, which was grown for 10 days in liquid soy protein medium (2 g soy protein [Supro 1711; Protein Technologies International] per liter), to favor the expression of genes encoding proteases (58–60). The cDNA fragments, which were cloned in pSPORT6, were then transferred to the pYES2-DEST52 plasmid using the Invitrogen Life Technologies Gateway technology for gene expression in S. cerevisiae.
Bioinformatic identification of T. rubrum putative multidrug efflux pumps.
We performed a BLASTp analysis with A. fumigatus, C. albicans, and C. glabrata on T. rubrum recorded ABC and MFS transporters, which were known to confer azole and terbinafine resistance (Tables 1 and 2). Transporters in T. rubrum, with over 35% and 25% identity with any tested transporters of A. fumigatus and the Candida species, respectively, were retained for further investigations.
Expression of T. rubrum ABC and MFS transporters in S. cerevisiae.
cDNA fragments encoding the MFS transporters TERG_04280, TERG_06399, TERG_07418, and TERG_08336 and all selected ABC transporters except for TERG_08612 were obtained by gene synthesis (GeneCust) and cloned into pUC57 between the BamHI and NotI sites of the multiple-cloning site. cDNA fragments encoding TERG_04626, TERG_05582, and TERG_07884 transporters were obtained by PCR using a standard protocol (56, 57), with the pairs of homologous primers P3-P4, P5-P6, and P7-P8, respectively (Table 1), and 200 ng of DNA prepared from 106 clones of the cDNA library as a target.
Expression plasmids were constructed by cloning cDNA fragments encoding transporters into the vector pYES2 for gene expression in S. cerevisiae. Each pUC57 construct hosting a cDNA fragment was digested by the restriction enzymes BamHI and NotI, and the cDNA fragment was inserted end to end into the expression vector of S. cerevisiae, pYES2, and digested by the same enzymes. Likewise, PCR fragments for which BamHI and NotI sites were previously designed at the 5′ end of the primers were inserted into pYES2, digested by the same enzymes.
A DNA fragment encoding the TERG_08613 ORF was previously constructed as follows: the P9-P10, P11-P12, and P13-P14 sense and antisense primer pairs (Table S4) were used to amplify three contiguous fragments of T. rubrum genomic DNA. Subsequently, the three PCR products were digested with BamHI/NgoMVI, NgoMVI/XhoI, and XhoI/NotI, respectively, and inserted end to end into pYES2 digested with BamHI and NotI.
S. cerevisiae transformations were performed with 1.0 μg of pYES2 constructs, using a transformation kit (Invitrogen-Life Technologies) according to the manufacturer’s recommendations. The selection of URA3 transformants was performed using MMD plates without uracil. Transformants were seeded onto MMG plates without uracil for heterologous expression of T. rubrum transporters under the control of the GAL1 promoter.
Drug susceptibility assays for S. cerevisiae transformants.
S. cerevisiae transformants were tested for antifungal resistance using serial dilution drug susceptibility assays and Etests. Yeasts were grown to mid-log phase (optical density at 600 nm [OD600], 1.0) at 30°C in liquid MMG, which contained the required amino acids. Each culture was diluted to an OD600 of 1.0. An OD value of 1.0 was found to correspond to about 107 CFU/ml in the yeast suspensions. Subsequently, for the serial dilution drug susceptibility assays, 10 μl of serial dilutions (10° to 104) was spotted onto MMG plates containing the desired concentration of antifungal drug.
For testing inhibitors on transporters, 106 S. cerevisiae transformant cells were seeded to make a lawn on MMG agar-solidified medium plates, which incorporated a sub-MIC of ITC (0.015 and 0.0075 μg/ml) or VRC (0.02 μg/ml). Nonimpregnated cellulose disks, 6 mm in diameter, were then placed at the surface of the medium and individually saturated with 5 μg of each tested inhibitor. The plates were incubated at 30°C, and growth inhibition was observed after 5 days.
Total RNA extraction and quantitative real-time reverse transcription-PCR.
T. rubrum CHUV1845 and TIMM20092 were grown in 50 ml of SDB, in 500-ml tissue culture flasks, without an antifungal drug and in the presence of 0.05 μg/ml of ITC or 0.02 μg/ml of VRC. Plugs from fresh fungal cultures were used as inoculates. Liquid cultures were conducted at 30°C, without shaking. After 7 days, the growing mycelia from each strain were collected, frozen, and ground under liquid nitrogen. Total RNA was extracted using the RNeasy plant minikit (Qiagen) and treated with DNase I (Qiagen). First-strand cDNA was synthesized using a high-capacity RNA-to-cDNA kit (Applied Biosystems, Carlsbad, CA, USA). The qRT-PCR analysis was performed using Power SYBR green PCR master mix on a StepOne real-time PCR system (Applied Biosystems) under standard conditions, according to the manufacturer’s recommendations.
We used primers designed by Martins et al. (13) to amplify TruACT (TERG_06637), TruGAPDH (TERG_04402), TruMDR1 (TERG_02508), TruMDR2 (TERG_08613), and TruMDR4 (TERG_07801). The primers used to amplify TruMDR3 (TERG_02186), TruMDR5 (TERG_04952), TruMFS1 (TERG_01623), and TruMFS2 (TERG_08336) were designed in this work and are listed in Table S4. Dissociation curves of the qPCR-amplified products were plotted to confirm the absence of nonspecific products or primer dimers. The expression levels of the genes encoding transporters were examined as relative fold changes compared to their levels in the CHUV1845 strain. Gene expression was normalized to the actin gene (TruACT) using two primers, P15 and P16 (Table S4), and the relative quantification of gene expression was calculated according to the 2ΔΔCT method (where CT is the threshold cycle). The statistical significance of SQLE gene expression levels among strains was evaluated using Student's t test.
Construction of a transformation vector for targeted-gene disruption.
A TruMDR3-targeting vector, pAg1-TruMDR3/T (Fig. 5A and Table S5), was constructed as follows. Approximately 2.4 to 2.6 kb of the upstream and downstream fragments of TruMDR3 (TERG_02186) was amplified from TIMM20092 total DNA by PCR with the P33-P34 and P35-36 primer pairs (Table S4), respectively. The nptII cassette, which is composed of the promoter sequence of Aspergillus nidulans trpC gene (PtrpC), nptII, and the terminator of A. fumigatus cgrA gene (TcgrA), was amplified from the plasmid vector pSP72-PcFLP (Table S5) by PCR with the P44-45 primer pair (Table S4). The three amplified fragments were digested with SpeI/ApaI, BamHI/KpnI, or ApaI/BamHI and cloned into SpeI/KpnI doubly digested pAg1 (61) to generate pAg1-TruMDR3/T.
The PCRs were performed using PrimeSTAR high-sensitivity (HS) DNA polymerase (TaKaRa Bio). All of the internal ApaI, BamHI, KpnI, and SpeI sites contained in the amplified fragments were inactivated by overlap extension PCR with the P33-P37, P38-P39, P40-P34, P35-P41, and P42-P36 primer pairs (Table S4). If necessary, the amplified fragments were gel purified with a QIAEX II gel extraction kit (Qiagen), subcloned into HincII-digested pUC118, and sequenced.
Fungal genetic transformation.
T. rubrum TIMM20092 was transformed by the A. tumefaciens-mediated transformation (ATMT) method, as described previously (56), with several minor modifications. After cocultivation, nylon membranes were transferred onto Sabouraud dextrose agar (SDA) medium containing 250 μg/ml G418 (Sigma-Aldrich) and overlaid with 10 ml of SDA supplemented with the same concentration of G418. The plates were further overlaid after 24 h with 10 ml of SDA containing 300 μg/ml G418 and then incubated for 6 to 7 days. The colonies regenerating on the selective medium were considered putative G418-resistant clones and transferred onto potato dextrose agar (PDA) medium supplemented with 100 μg/ml G418, 500 μg/ml cycloheximide, 50 μg/ml chloramphenicol, and 200 μg/ml cefotaxime sodium (Sanofi-Aventis), if necessary.
Screening of the desired transformants.
The desired transformants were finally screened by PCR and Southern blotting. Total DNA was extracted according to a method described previously (56). Aliquots of 50 to 100 ng of the total DNA were used as the templates in the PCRs. For Southern blotting, aliquots of approximately 10 μg of the total DNA were digested with an appropriate restriction enzyme, separated by electrophoresis on 0.8% (wt/vol) agarose gels, and transferred onto Hybond-N+ membranes (GE Healthcare Ltd.). Southern hybridization was performed using an ECL Direct nucleic acid labeling and detection system (GE Healthcare Ltd.), according to the manufacturer’s instructions. Of the 5 T. rubrum transformants lacking the TruMDR3 gene that were obtained, the ΔTruMDR3-33 and ΔTruMDR3-45 mutants were deposited as TIMM40001 and TIMM40002, respectively, in the culture collection of the Teikyo University Institute of Medical Mycology (TIMM).
Data availability.
The updated sequences were submitted to GenBank with the following identification numbers: MK787243 (TERG_02186/TruMDR3), MK787253 (TERG_01443), MK787254 (TERG_02508/TruMDR1), MK787255 (TERG_04280), MK787256 (TERG_04952/TruMDR5), MK787257 (TERG_06399), MK787258 (TERG_07418), MK787259 (TERG_08336/TruMFS2), MK787260 (TERG_08591), MK787261 (TERG_08751), MK787262 (TERG_08613/TruMDR2), and MK787263 (TERG_07801/TruMDR4).
Supplementary Material
ACKNOWLEDGMENTS
We thank Gionata Marazza, Bellinzona (TI, Switzerland) for collecting samples, from which the TIMM20092 strain was isolated, and Christine Pich (CHUV and University of Lausanne) for the statistical analyses performed.
The in silico analysis performed by the Swiss-Prot group of the SIB Swiss Institute of Bioinformatics was supported by the Swiss Federal Government through the State Secretariat for Education, Research and Innovation (SERI).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00863-19.
REFERENCES
- 1.Baran R, Hay R, Haneke E, Tosti A. 2006. Onychomycosis, the current approach to diagnosis and therapy, 2nd ed Taylor and Francis, Abington, United Kingdom, p 1–145. [Google Scholar]
- 2.Petranyi G, Ryder NS, Stütz A. 1984. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science 224:1239–1941. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- 3.Ryder NS. 1992. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol 126(Suppl 39):2–7. doi: 10.1111/j.1365-2133.1992.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Valachovic M, Garaiova M, Holic R, Hapala I. 2016. Squalene is lipotoxic to yeast cells defective in lipid droplet biogenesis. Biochem Biophys Res Commun 469:1123–1128. doi: 10.1016/j.bbrc.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 5.Van den Bossche H, Marichal P, Gorrens J, Coene MC, Willemsens G, Bellens D, Roels I, Moereels H, Janssen PA. 1989. Biochemical approaches to selective antifungal activity. Focus on azole antifungals. Mycoses 32(Suppl 1):35–52. doi: 10.1111/j.1439-0507.1989.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, Salamin K, Fratti M, Monod M. 2017. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother 61:e00115-17. doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Digby SS, Hald M, Arendrup MC, Hjort SV, Kofoed K. 2017. Darier disease complicated by terbinafine-resistant Trichophyton rubrum: a case report. Acta Derm Venerol 97:139–140. doi: 10.2340/00015555-2455. [DOI] [PubMed] [Google Scholar]
- 8.Schøsler L, Andersen LK, Arendrup MC, Sommerlund M. 2018. Recurrent terbinafine resistant Trichophyton rubrum infection in a child with congenital ichthyosis. Pediatr Dermatol 35:259–260. doi: 10.1111/pde.13411. [DOI] [PubMed] [Google Scholar]
- 9.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, Meis JF, Chowdhary A. 2018. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 61:477–484. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 10.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, Narang T, Chakrabarti A. 2018. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother 62:e02522-17. doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervelatti EP, Fachin AL, Ferreira-Nozawa MS, Martinez-Rossi NM. 2006. Molecular cloning and characterization of a novel ABC transporter gene in the human pathogen Trichophyton rubrum. Med Mycol 44:141–147. doi: 10.1080/13693780500220449. [DOI] [PubMed] [Google Scholar]
- 12.Fachin AL, Ferreira-Nozawa MS, Maccheroni W Jr, Martinez-Rossi NM. 2006. Role of the ABC transporter TruMDR2 in terbinafine, 4-nitroquinoline N-oxide and ethidium bromide susceptibility in Trichophyton rubrum. J Med Microbiol 55:1093–1099. doi: 10.1099/jmm.0.46522-0. [DOI] [PubMed] [Google Scholar]
- 13.Martins MP, Franceschini ACC, Jacob TR, Rossi A, Martinez-Rossi NM. 2016. Compensatory expression of multidrug-resistance genes encoding ABC transporters in dermatophytes. J Med Microbiol 65:605–610. doi: 10.1099/jmm.0.000268. [DOI] [PubMed] [Google Scholar]
- 14.Holmes AR, Lin YH, Niimi K, Lamping E, Keniya M, Niimi M, Tanabe K, Monk BC, Cannon RD. 2008. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob Agents Chemother 52:3851–3862. doi: 10.1128/AAC.00463-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niimi K, Harding DR, Holmes AR, Lamping E, Niimi M, Tyndall JD, Cannon RD, Monk BC. 2012. Specific interactions between the Candida albicans ABC transporter Cdr1p ectodomain and a d-octapeptide derivative inhibitor. Mol Microbiol 85:747–767. doi: 10.1111/j.1365-2958.2012.08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva LV, Sanguinetti M, Vandeputte P, Torelli R, Rochat B, Sanglard D. 2013. Milbemycins: more than efflux inhibitors for fungal pathogens. Antimicrob Agents Chemother 57:873–886. doi: 10.1128/AAC.02040-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessup CJ, Warner J, Isham N, Hasan I, Ghannoum MA. 2000. Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J Clin Microbiol 38:341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura T, Asahara M, Yamamoto M, Yamaura M, Matsumura M, Goto K, Rezaei-Matehkolaei A, Mirhendi H, Makimura M, Makimura K. 2014. In vitro susceptibility of dermatomycoses agents to six antifungal drugs and evaluation by fractional inhibitory concentration index of combined effects of amorolfine and itraconazole in dermatophytes. Microbiol Immunol 58:1–8. doi: 10.1111/1348-0421.12109. [DOI] [PubMed] [Google Scholar]
- 19.Moir DT, Davidow LS. 1991. Production of proteins by secretion from yeast. Methods Enzymol 194:491–507. doi: 10.1016/0076-6879(91)94037-d. [DOI] [PubMed] [Google Scholar]
- 20.Togni G, Sanglard D, Quadroni M, Foundling SI, Monod M. 1996. Acid proteinase secreted by Candida tropicalis: functional analysis of preproregion cleavages in C. tropicalis and Saccharomyces cerevisiae. Microbiology 142:493–503. doi: 10.1099/13500872-142-3-493. [DOI] [PubMed] [Google Scholar]
- 21.Meneau I, Coste AT, Sanglard D. 2016. Identification of Aspergillus fumigatus multidrug transporter genes and their potential involvement in antifungal resistance. Med Mycol 54:616–627. doi: 10.1093/mmy/myw005. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Ferreira ME, Malavazi I, Savoldi L, Brakhage AA, Goldman NH, Kim HS, Nierman WC, Goldman GH. 2006. Transcriptome analysis of Aspergillus fumigatus exposed to voriconazole. Curr Genet 50:32–44. doi: 10.1007/s00294-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Li H, Li R, Bu D, Wan Z. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother 55:31–37. doi: 10.1093/jac/dkh507. [DOI] [PubMed] [Google Scholar]
- 24.Carbone I, Ramirez-Prado JH, Jakobek JL, Horn BW. 2007. Gene duplication, modularity and adaptation in the evolution of the aflatoxin gene cluster. BMC Evol Biol 7:111. doi: 10.1186/1471-2148-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. 2011. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother 55:2092–2097. doi: 10.1128/AAC.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelini A, Iezzi M, Di Febbo C, Di Ilio C, Cuccurullo F, Porreca E. 2008. Reversal of P-glycoprotein-mediated multidrug resistance in human sarcoma MES-SA/Dx-5 cells by nonsteroidal anti-inflammatory drugs. Oncol Rep 20:731–735. [PubMed] [Google Scholar]
- 27.Sharma M, Prasad R. 2011. The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans. Antimicrob Agents Chemother 55:4834–4843. doi: 10.1128/AAC.00344-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Černáková L, Dižová S, Gášková D, Jančíková I, Bujdáková H. 2019. Impact of farnesol as a modulator of efflux pumps in a fluconazole-resistant strain of Candida albicans. Microb Drug Resist 25:805–812. doi: 10.1089/mdr.2017.0332. [DOI] [PubMed] [Google Scholar]
- 29.Iwaki K, Sakaeda T, Kakumoto M, Nakamura T, Komoto C, Okamura N, Nishiguchi K, Shiraki T, Horinouchi M, Okumura K. 2006. Haloperidol is an inhibitor but not substrate for MDR1/P-glycoprotein. J Pharm Pharmacol 58:1617–1622. doi: 10.1211/jpp.58.12.0008. [DOI] [PubMed] [Google Scholar]
- 30.Molnár J, Szabo D, Mándi Y, Mucsi I, Fischer J, Varga A, König S, Motohashi N. 1998. Multidrug resistance reversal in mouse lymphoma cells by heterocyclic compounds. Anticancer Res 18:3033–3038. [PubMed] [Google Scholar]
- 31.Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martínez M, Calabrese D, Sanglard D, Patterson TF. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abastabar M, Hosseini T, Valadan R, Lagzian M, Haghani I, Aslani N, Badali H, Nouripour-Sisakht S, Nazeri M, Gholami S, Vakili M, Bowyer P, Shokohi T, Hedayati MT. 2019. Novel point mutations in cyp51A and cyp51B genes associated with itraconazole and posaconazole resistance in Aspergillus clavatus isolates. Microb Drug Resist 25:652–662. doi: 10.1089/mdr.2018.0300. [DOI] [PubMed] [Google Scholar]
- 33.Morschhäuser J. 2002. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta 1587:240–248. doi: 10.1016/s0925-4439(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 34.Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother 39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanglard D, Ischer F, Monod M, Bille J. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 36.Sanglard D, Ischer F, Bille J. 2001. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob Agents Chemother 45:1174–1183. doi: 10.1128/AAC.45.4.1174-1183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morschhäuser J, Barker KS, Liu TT, BlaB-Warmuth J, Homayouni R, Rogers PD. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog 3:e164. doi: 10.1371/journal.ppat.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, Ramage G, Denning DW, Bowyer P. 2013. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother 68:1486–1496. doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 40.Laurent A, Monod M. 2017. Production of Trichophyton rubrum microspores in large quantities and its application to evaluate amorolfine/azole compound interactions in vitro. Mycoses 60:581–586. doi: 10.1111/myc.12632. [DOI] [PubMed] [Google Scholar]
- 41.Poirier JM, Cheymol G. 1998. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 35:461–473. doi: 10.2165/00003088-199835060-00004. [DOI] [PubMed] [Google Scholar]
- 42.Wiederhold NP, Pennick GJ, Dorsey SA, Furmaga W, Lewis JS Jr, Patterson TF, Sutton DA, Fothergill AW. 2014. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother 58:424–431. doi: 10.1128/AAC.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filho AMS, Ventura CG, Criado PR, Del Negro GB, Freitas RS, Luiz OC, Giudice MC, Neto ED, Benard G. 2017. Hemodialysis and kidney transplantation as predisposing conditions to onychomycosis. Nephron 137:38–46. doi: 10.1159/000475674. [DOI] [PubMed] [Google Scholar]
- 44.Chang PK, Yu J, Yu JH. 2004. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet Biol 41:911–920. doi: 10.1016/j.fgb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Coste A, Turner V, Ischer F, Morschhäuser J, Forche A, Selmecki A, Berman J, Bille J, Sanglard D. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camps SM, Dutilh BE, Arendrup MC, Rijs AJ, Snelders E, Huynen MA, Verweij PE, Melchers WJ. 2012. Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7:e50034. doi: 10.1371/journal.pone.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagiwara D, Miura D, Shimizu K, Paul S, Ohba A, Gonoi T, Watanabe A, Kamei K, Shintani T, Moye-Rowley WS, Kawamoto S, Gomi K. 2017. A novel Zn2-Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co-regulating cyp51A and cdr1B expressions. PLoS Pathog 13:e1006096. doi: 10.1371/journal.ppat.1006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. 2009. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev 22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker B, Izumikawa K, Tsai HF, Bennett JE. 2014. Milbemycin A4 oxime as a probe of azole transport in Candida glabrata. FEMS Yeast Res 14:755–761. doi: 10.1111/1567-1364.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dryden M, Payne P. 2005. Preventing parasites in cats. Vet Ther 6:260–267. [PubMed] [Google Scholar]
- 51.Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, Rock D, Chen C-Y, Schwertschlag US. 2003. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol 43:1108–1115. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- 52.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger S, El Chazli Y, Babu AF, Coste AT. 2017. Azole resistance in Aspergillus fumigatus: a consequence of antifungal use in agriculture? Front Microbiol 8:1024. doi: 10.3389/fmicb.2017.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. 2016. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol 7:2173. doi: 10.3389/fmicb.2016.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz IR, Patterson TF. 2018. The emerging threat of antifungal resistance in transplant infectious diseases. Curr Infect Dis Rep 20:2. doi: 10.1007/s11908-018-0608-y. [DOI] [PubMed] [Google Scholar]
- 56.Yamada T, Makimura K, Satoh K, Umeda Y, Ishihara Y, Abe S. 2009. Agrobacterium tumefaciens-mediated transformation of the dermatophyte, Trichophyton mentagrophytes: an efficient tool for gene transfer. Med Mycol 47:485–494. doi: 10.1080/13693780802322240. [DOI] [PubMed] [Google Scholar]
- 57.Sherman F, Wakem P. 1991. Mapping yeast genes. Methods Enzymol 194:38–57. doi: 10.1016/0076-6879(91)94006-x. [DOI] [PubMed] [Google Scholar]
- 58.Jousson O, Léchenne B, Bontems O, Capoccia S, Mignon B, Barblan J, Quadroni M, Monod M. 2004. Multiplication of an ancestral gene encoding secreted fungalysin preceded species differentiation in the dermatophytes Trichophyton and Microsporum. Microbiology 150:301–310. doi: 10.1099/mic.0.26690-0. [DOI] [PubMed] [Google Scholar]
- 59.Jousson O, Léchenne B, Bontems O, Mignon B, Reichard U, Barblan J, Quadroni M, Monod M. 2004. Secreted subtilisin gene family in Trichophyton rubrum. Gene 339:79–88. doi: 10.1016/j.gene.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Monod M, Léchenne B, Jousson O, Grand D, Zaugg C, Stöcklin R, Grouzmann E. 2005. Aminopeptidases and dipeptidyl-peptidases secreted by the dermatophyte Trichophyton rubrum. Microbiology 151:145–155. doi: 10.1099/mic.0.27484-0. [DOI] [PubMed] [Google Scholar]
- 61.Zhang A, Lu P, Dahl-Roshak AM, Paress PS, Kennedy S, Tkacz JS, An Z. 2003. Efficient disruption of a polyketide synthase gene (pks1) required for melanin synthesis through Agrobacterium-mediated transformation of Glarea lozoyensis. Mol Genet Genomics 268:645–655. [DOI] [PubMed] [Google Scholar]
- 62.Shah AH, Singh A, Dhamgaye S, Chauhan N, Vandeputte P, Suneetha KJ, Kaur R, Mukherjee PK, Chandra J, Ghannoum MA, Sanglard D, Goswami SK, Prasad R. 2014. Novel role of a family of major facilitator transporters in biofilm development and virulence of Candida albicans. Biochem J 460:223–235. doi: 10.1042/BJ20140010. [DOI] [PubMed] [Google Scholar]
- 63.Calabrese D, Bille J, Sanglard D. 2000. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146:2743–2754. doi: 10.1099/00221287-146-11-2743. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Yaacov R, Knoller S, Caldwell GA, Becker JM, Koltin Y. 1994. Candida albicans gene encoding resistance to benomyl and methotrexate is a multidrug resistance gene. Antimicrob Agents Chemother 38:648–652. doi: 10.1128/aac.38.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White TC. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 41:1482–1487. doi: 10.1128/AAC.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karababa M, Coste AT, Rognon B, Bille J, Sanglard D. 2004. Comparison of gene expression profiles of Candida albicans azole-resistant clinical isolates and laboratory strains exposed to drugs inducing multidrug transporters. Antimicrob Agents Chemother 48:3064–3079. doi: 10.1128/AAC.48.8.3064-3079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wirsching S, Michel S, Morschhäuser J. 2000. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol Microbiol 36:856–865. doi: 10.1046/j.1365-2958.2000.01899.x. [DOI] [PubMed] [Google Scholar]
- 68.Kohli A, Gupta V, Krishnamurthy S, Hasnain SE, Prasad R. 2001. Specificity of drug transport mediated by CaMDR1: a major facilitator of Candida albicans. J Biosci 26:333–339. doi: 10.1007/BF02703742. [DOI] [PubMed] [Google Scholar]
- 69.Yamada-Okabe T, Yamada-Okabe H. 2002. Characterization of the CaNAG3, CaNAG4, and CaNAG6 genes of the pathogenic fungus Candida albicans: possible involvement of these genes in the susceptibilities of cytotoxic agents. FEMS Microbiol Lett 212:15–21. doi: 10.1111/j.1574-6968.2002.tb11238.x. [DOI] [PubMed] [Google Scholar]
- 70.Costa C, Henriques A, Pires C, Nunes J, Ohno M, Chibana H, Sá-Correia I, Teixeira MC. 2013. The dual role of Candida glabrata drug:H+ antiporter CgAqr1 (ORF CAGL0J09944g) in antifungal drug and acetic acid resistance. Front Microbiol 4:170. doi: 10.3389/fmicb.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costa C, Pires C, Cabrito TR, Renaudin A, Ohno M, Chibana H, Sá-Correia I, Teixeira MC. 2013. Candida glabrata drug:H+ antiporter CgQdr2 confers imidazole drug resistance, being activated by transcription factor CgPdr1. Antimicrob Agents Chemother 57:3159–3167. doi: 10.1128/AAC.00811-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pais P, Costa C, Pires C, Shimizu K, Chibana H, Teixeira MC. 2016. Membrane proteome-wide response to the antifungal drug clotrimazole in Candida glabrata: role of the transcription factor CgPdr1 and the drug:H+ antiporters CgTpo1_1 and CgTpo1_2. Mol Cell Proteomics 15:57–72. doi: 10.1074/mcp.M114.045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pais P, Pires C, Costa C, Okamoto M, Chibana H, Teixeira MC. 2016. Membrane proteomics analysis of the Candida glabrata response to 5-flucytosine: unveiling the role and regulation of the drug efflux transporters CgFlr1 and CgFlr2. Front Microbiol 7:2045. doi: 10.3389/fmicb.2016.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa C, Nunes J, Henriques A, Mira NP, Nakayama H, Chibana H, Teixeira MC. 2014. Candida glabrata drug:H+ antiporter CgTpo3 (ORF CAGL0I10384g): role in azole drug resistance and polyamine homeostasis. J Antimicrob Chemother 69:1767–1776. doi: 10.1093/jac/dku044. [DOI] [PubMed] [Google Scholar]
- 75.Bowyer P, Mosquera J, Anderson M, Birch M, Bromley M, Denning DW. 2012. Identification of novel genes conferring altered azole susceptibility in Aspergillus fumigatus. FEMS Microbiol Lett 332:10–19. doi: 10.1111/j.1574-6968.2012.02575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paul S, Diekema D, Moye-Rowley WS. 2013. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot Cell 12:1619–1628. doi: 10.1128/EC.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paul S, Moye-Rowley WS. 2013. Functional analysis of an ATP-binding cassette transporter protein from Aspergillus fumigatus by heterologous expression in Saccharomyces cerevisiae. Fungal Genet Biol 57:85–91. doi: 10.1016/j.fgb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macheleidt J, Scherlach K, Neuwirth T, Schmidt-Heck W, Straßburger M, Spraker J, Baccile JA, Schroeder FC, Keller NP, Hertweck C, Heinekamp T, Brakhage AA. 2015. Transcriptome analysis of cyclic AMP-dependent protein kinase A-regulated genes reveals the production of the novel natural compound fumipyrrole by Aspergillus fumigatus. Mol Microbiol 96:148–162. doi: 10.1111/mmi.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langfelder K, Gattung S, Brakhage AA. 2002. A novel method used to delete a new Aspergillus fumigatus ABC transporter-encoding gene. Curr Genet 41:268–274. doi: 10.1007/s00294-002-0313-z. [DOI] [PubMed] [Google Scholar]
- 80.Franz R, Michel S, Morschhäuser J. 1998. A fourth gene from the Candida albicans CDR family of ABC transporters. Gene 220:91–98. doi: 10.1016/s0378-1119(98)00412-0. [DOI] [PubMed] [Google Scholar]
- 81.Liu TT, Lee RE, Barker KS, Lee RE, Wei L, Homayouni R, Rogers PD. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob Agents Chemother 49:2226–2236. doi: 10.1128/AAC.49.6.2226-2236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torelli R, Posteraro B, Ferrari S, La Sorda M, Fadda G, Sanglard D, Sanguinetti M. 2008. The ATP-binding cassette transporter-encoding gene CgSNQ2 is contributing to the CgPDR1-dependent azole resistance of Candida glabrata. Mol Microbiol 68:186–201. doi: 10.1111/j.1365-2958.2008.06143.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The updated sequences were submitted to GenBank with the following identification numbers: MK787243 (TERG_02186/TruMDR3), MK787253 (TERG_01443), MK787254 (TERG_02508/TruMDR1), MK787255 (TERG_04280), MK787256 (TERG_04952/TruMDR5), MK787257 (TERG_06399), MK787258 (TERG_07418), MK787259 (TERG_08336/TruMFS2), MK787260 (TERG_08591), MK787261 (TERG_08751), MK787262 (TERG_08613/TruMDR2), and MK787263 (TERG_07801/TruMDR4).