VL-2397 is an antifungal drug with a novel mechanism of action, rapid fungicidal in vitro activity, and potent in vivo activity against Aspergillus fumigatus, including azole-resistant strains. VL2397-101, a phase 1 first-in-human, randomized, double-blind, placebo-controlled dose-escalation study, was conducted in healthy adults to determine the safety, tolerability, and pharmacokinetics (PK) of single and multiple ascending intravenous (i.v.) doses of VL-2397.
KEYWORDS: Aspergillus fumigatus, VL-2397, antifungal agents, clinical trials, pharmacokinetics
ABSTRACT
VL-2397 is an antifungal drug with a novel mechanism of action, rapid fungicidal in vitro activity, and potent in vivo activity against Aspergillus fumigatus, including azole-resistant strains. VL2397-101, a phase 1 first-in-human, randomized, double-blind, placebo-controlled dose-escalation study, was conducted in healthy adults to determine the safety, tolerability, and pharmacokinetics (PK) of single and multiple ascending intravenous (i.v.) doses of VL-2397. All dosing cohorts were fully enrolled; all subjects completed the safety follow-up. A safety committee reviewed the safety data for each dosing cohort prior to recommending the initiation of each subsequent cohort. No serious adverse events (SAEs) occurred; the majority of treatment-emergent adverse events (TEAEs) were mild and self-limited. The most common drug-related TEAEs were infusion site reactions. No clinically concerning trends were noted in vital signs, electrocardiograms, physical examinations, or safety laboratory results. Following single infusions of VL-2397, the overall and maximum exposures rose less than proportionally with increasing doses from 3 mg to 1,200 mg as indicated by area under the concentration-time curve over 24 h (AUC24) and maximum concentration (Cmax). No signs of VL-2397 accumulation were observed following i.v. infusions of 300, 600, and 1,200 mg every 24 h (q24h) for 7 days. Renal elimination played a major role in total body clearance, with up to 47% of unmetabolized drug in urine 24 h after administration at single doses of >30 mg. Overall, VL-2397 dosing in the study appeared to be safe and well tolerated in the healthy subjects. The safety profile, consistent PK, and lack of drug accumulation support further development of VL-2397 in patients with invasive aspergillosis.
INTRODUCTION
Development of new drugs to treat invasive fungal infections (IFIs) in immunocompromised patients, including acute leukemia patients and allogeneic hematopoietic stem cell transplant recipients, remains urgent. A major cause of morbidity and mortality in these patients is acute invasive aspergillosis (IA), most commonly caused by Aspergillus fumigatus (1). Although azoles and polyenes are available as primary treatments for IA, the 6-week all-cause-mortality rate remains high (∼20%) for both drug classes (2–4). Major limitations of current antifungal drugs include toxicity, drug-drug interactions, and emerging drug resistance (5). Despite the medical needs, only one new antifungal drug has been recently approved (isavuconazole in 2015) as a primary treatment for IA; no new class of antifungal agents has been made commercially available for IFIs in the past decade, and only a few antifungal agents are currently in development (5).
VL-2397 (previously known as ASP2397) is an investigational antifungal drug candidate derived from a natural product that was discovered in a leaf litter fungus, Acremonium persicinum, collected from a Malaysian national park (6). VL-2397 was found to protect against lethal outcomes following Aspergillus fumigatus infection in both a silkworm larva model and a murine model for invasive pulmonary aspergillosis (7). VL-2397 has a novel mechanism of antifungal action that differentiates it from existing classes of antifungal drugs which target fungal cell wall or plasma membrane components. VL-2397 is a cyclic hexapeptide siderophore containing aluminum in lieu of iron and is actively transported through a membrane-bound transporter called siderophore iron transporter 1 (Sit1) (6, 8) to enter fungal cells. Siderophore iron transporters are used by various fungi to transport iron-bound siderophores from the environment into fungal cells; iron is a critical factor for growth and survival of Aspergillus fumigatus and other fungal pathogens (9–11). Mammalian cells do not possess Sit1 (12); therefore, VL-2397 will not enter human cells using this mechanism, potentially allowing for a favorable safety profile due to its selective uptake by fungal cells. Recent studies indicate that Sit1-mediated uptake is essential for VL-2397 susceptibility and that antifungal activity is independent of aluminum importation by fungal cells (13).
VL-2397 has demonstrated in vitro activity against Aspergillus species, including those that are resistant to azoles, and in addition has shown activity against some of the other filamentous fungi, such as Fusarium species, that are extremely difficult to treat (8). VL-2397 has demonstrated rapid fungicidal activity in vitro and a rapid inhibition of hyphal elongation against Aspergillus fumigatus (8). In mouse models of IA, including challenge studies with azole-resistant Aspergillus isolates, treatment with VL-2397 provided high survival rates and reduced fungal colony counts in the lungs of infected mice (14, 15). Lastly, VL-2397 appears to have a low propensity for cytochrome P450-mediated drug-drug interactions as well as a low potential for off-target activity with a variety of cellular proteins tested (16). Collectively, these attributes support VL-2397 as a future frontline treatment for IA.
A first-in-human phase 1 study was conducted to examine the safety, tolerability, and pharmacokinetic (PK) profiles of VL-2397 in healthy human subjects who were randomized to one of seven single-ascending-dose (SAD) cohorts or one of four multiple-ascending-dose (MAD) cohorts.
RESULTS
Subject disposition and analysis populations.
A total of 96 subjects ranging from 19 to 55 years of age were enrolled into one of seven SAD or four MAD cohorts (Table 1). Infusions in the initial cohort 7 subjects (cohort 7X) were discontinued early due to infusion occlusion alarms resulting from cumulative filtration of drug product. Consequently, the 8 subjects were replaced and dosed (cohort 7) following introduction of an exchangeable filtered extension set. All 16 subjects were included in the safety population, but only the 8 replacement subjects (cohort 7) were included in the PK analysis.
TABLE 1.
Dosing regimens
| Cohort | VL-2397 dose (mg) per infusion | Dose frequency | VL-2397 concn (mg/ml) | Infusion vol (ml) | Calculated infusion time (min) |
|---|---|---|---|---|---|
| 1 | 3 | 1 | 0.12 | 25 | 6 |
| 2 | 10 | 1 | 0.12 | 83 | 20 |
| 3 | 30 | 1 | 0.12 | 250 | 60 |
| 4 | 100 | 1 | 1.2 | 83 | 20 |
| 5 | 300 | 1 | 1.2 | 250 | 60 |
| 6 | 600 | 1 | 1.2 | 500 | 120 |
| 7 | 1,200 | 1 | 1.2 | 1,000 | 240 |
| 8 | 300 | 7 | 1.2 | 250 | 60 |
| 9 | 600 | 7 | 1.2 | 500 | 120 |
| 10 | 1,200 | 7 | 1.2 | 1,000 | 240 |
| 11 | 300 | Every 8 h for days 1–7 | 1.2 | 250 | 60 |
| 600 | Every 24 h for days 8–21 | 1.2 | 500 | 120 |
Subject demographics and baseline characteristics.
Table 2 shows that the baseline characteristics of the subjects in the VL-2397 groups and the placebo groups in SAD and MAD cohorts were similar with respect to age, sex, ethnicity, race, and body mass index. Overall, subjects among all cohorts were of a mean age ranging from 34 to 41 years, predominantly male (≥67%), of Hispanic or Latino ethnicity (≥50%), and white (≥79%) and had a mean body mass index ranging from 22 to 28 kg/m2.
TABLE 2.
Demographic and baseline characteristics (safety population)
| Characteristic | SAD |
MAD cohorts 8–10 |
MAD cohort 11 |
|||
|---|---|---|---|---|---|---|
| Placebo (n = 16) | VL-2397 (n = 48) | Placebo (n = 6) | VL-2397 (n = 18) | Placebo (n = 2) | VL-2397 (n = 6) | |
| Age, yrs | ||||||
| Mean (SD) | 38.5 (9.2) | 41.0 (9.3) | 40.0 (9.6) | 40.8 (9.5) | 34.0 (8.5) | 36.2 (8.9) |
| Median (range) | 41 (19–55) | 41.0 (22–55) | 41.5 (26–52) | 43.5 (21–52) | 34.0 (28–40) | 35.0 (23–48) |
| Sex, no. (%) | ||||||
| Male | 13 (81) | 32 (67) | 6 (100) | 12 (67) | 2 (100) | 6 (100) |
| Female | 3 (19) | 16 (33) | 0 | 6 (33) | 0 | 0 |
| Ethnicity, no. (%) | ||||||
| Hispanic | 9 (56) | 29 (60) | 5 (83) | 13 (72) | 1 (50) | 5 (83) |
| Non-Hispanic | 7 (44) | 19 (40) | 1 (17) | 5 (28) | 1 (50) | 1 (17) |
| Race, no. (%) | ||||||
| White | 16 (100) | 38 (79) | 5 (83) | 15 (83) | 2 (100) | 5 (83) |
| Black | 0 | 9 (19) | 1 (17) | 1 (6) | 0 | 0 |
| Other | 0 | 1 (2) | 0 | 2 (11) | 0 | 1 (17) |
| BMI, kg/m2 | ||||||
| Mean (SD) | 26.3 (2.8) | 25.9 (2.7) | 28.0 (1.4) | 26.5 (2.7) | 22.0 (2.3) | 27.4 (1.4) |
| Median (range) | 27.0 (21–30) | 26.4 (20–29.8) | 28.4 (25.9–29.5) | 26.6 (21.7–30.0) | 22.0 (20.4–23.7) | 27.5 (25.3–29.0) |
Safety evaluation.
(i) SAD cohorts 1 to 7. Among the SAD cohorts, 25 of the 64 subjects (39%) reported a total of 47 treatment-emergent adverse events (TEAEs), including 26 related to the study drug (active drug or placebo). Forty of these TEAEs were mild in severity, 5 were moderate, and 2 were potentially life-threatening. The potentially life-threatening TEAEs were elevations in creatine phosphokinase (CK) reported for 2 subjects (both in the 100-mg VL-2397 cohort) that were unrelated to study drug and were attributed by the principal investigator (PI) to subjects’ engaging in strenuous activity in the Arizona heat. No serious adverse events (SAEs) or subject discontinuations due to TEAEs occurred. The total number of TEAEs reported in each of the SAD cohorts among VL-2397 recipients did not appear to correlate with increasing doses of VL-2397. The incidence of TEAEs reported by subject in SAD cohorts 1 to 7 is summarized in Table 3.
TABLE 3.
Subjects with treatment-emergent adverse events by system organ class and preferred term (safety population) for SAD cohorts
| Subject group or adverse eventa | No. (%) of subjects receiving indicated treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | VL-2397, SAD |
|||||||||
| 3 mg (cohort 1) | 10 mg (cohort 2) | 30 mg (cohort 3) | 100 mg (cohort 4) | 300 mg (cohort 5) | 600 mg (cohort 6) | 1,200 mg (cohort 7) | Unknown (cohort 7X) | Total | ||
| Total dosed | 16 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 48 (100) |
| Subjects with TEAEs | 7 (44) | 4 (67) | 3 (50) | 2 (33) | 4 (67) | 2 (33) | 1 (17) | 2 (33) | 0 | 18 (38) |
| Subjects without TEAEs | 9 (56) | 2 (33) | 3 (50) | 4 (67) | 2 (33) | 4 (67) | 5 (83) | 4 (67) | 6 (100) | 30 (63) |
| Cardiac disorders | 2 (13) | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 1 (2) |
| Bradycardia | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tachycardia | 1 (6) | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 1 (2) |
| Gastrointestinal disorders | 1 (6) | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 2 (4) |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (2) |
| Nausea | 1 (6) | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| General disorders and administration site conditions | 3 (19) | 2 (33) | 1 (17) | 2 (33) | 0 | 0 | 1 (17) | 1 (17) | 0 | 7 (15) |
| Feeling cold | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Influenza-like illness | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infusion site erythema | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infusion site extravasation | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Infusion site hemorrhage | 1 (6) | 1 (17) | 1 (17) | 0 | 0 | 0 | 1 (17) | 0 | 0 | 3 (6) |
| Infusion site pain | 2 (13) | 1 (17) | 0 | 1 (17) | 0 | 0 | 0 | 1 (17) | 0 | 3 (6) |
| Infusion site swelling | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Edema, peripheral | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Vessel puncture site hemorrhage | 1 (6) | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Vessel puncture site pain | 1 (6) | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Infections and infestations | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Viral infection | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injury, poisoning, and procedural complications | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Excoriation | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Investigations | 2 (13) | 2 (33) | 1 (17) | 0 | 2 (33) | 0 | 0 | 0 | 0 | 5 (10) |
| Blood creatine phosphokinase increase | 1 (6) | 0 | 0 | 0 | 2 (33) | 0 | 0 | 0 | 0 | 2 (4) |
| Blood creatinine increase | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood pressure increase | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Lipase increase | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Neutrophil count decrease | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Metabolism and nutrition disorders | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertriglyceridemia | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 1 (2) |
| Back pain | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 1 (2) |
| Nervous system disorders | 2 (13) | 1 (17) | 0 | 0 | 1 (17) | 1 (17) | 0 | 0 | 0 | 3 (6) |
| Dizziness | 2 (13) | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Dizziness, postural | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 | 1 (17) | 1 (17) | 0 | 0 | 0 | 2 (4) |
| Respiratory, thoracic, and mediastinal disorders | 1 (6) | 0 | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 1 (2) |
| Epistaxis | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 1 (2) |
| Skin and subcutaneous tissue disorders | 1 (6) | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Dry skin | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) |
| Pruritus, generalized | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Adverse events are classified according to MedDRA, version 19.0.
The most common TEAEs reported were pain and hemorrhage at the infusion site. Pain at the infusion site was reported for three subjects receiving VL-2397 (3 mg, 30 mg, and 1,200 mg, respectively) and two subjects receiving placebo; hemorrhage at the infusion site was reported for three subjects receiving VL-2397 (3 mg, 10 mg, and 600 mg, respectively) and one subject receiving placebo. These infusion site TEAEs started 2 h to 3 days after the start of the infusion and lasted 3 to 14 days. Six of these 9 TEAEs were related to the study drug.
Several subjects in the SAD cohorts who received VL-2397 experienced laboratory- or vital sign-related TEAEs that were related to the study drug. One subject in the 3-mg cohort experienced a decreased absolute neutrophil count of 400/μl on day 2, while another subject in the 3-mg cohort experienced a transient elevation in blood pressure to 182/113 mm Hg on day 1, 12 min after dosing; one subject in the 10-mg cohort experienced an elevated lipase of 92 IU/liter on day 3; and one subject in the 100-mg cohort experienced tachycardia on day 1 approximately 6 h after dosing. All of these TEAEs resolved while on study. No electrocardiographic evidence of QT interval abnormalities was observed. The only electrocardiogram (ECG)-related TEAE was mild bradycardia on day 1 in one subject who received placebo.
(ii) MAD cohorts 8 to 10. Among MAD cohorts 8 to 10, all 24 (100%) subjects reported a total of 121 TEAEs (109 mild, 10 moderate, 1 severe, and 1 potentially life-threatening CK elevation which was not related to the study drug), of which 100 were related to the study drug. The total number of TEAEs reported in cohort 10 (1,200 mg of VL-2397) was higher than in the lower-dose MAD cohorts, suggesting a possible relationship of TEAEs to dose of VL-2397. No SAEs were reported. The incidence of TEAEs reported by subject in MAD cohorts 8 to 10 is summarized in Table 4.
TABLE 4.
Subjects with treatment-emergent adverse events by system organ class and preferred term (safety population) for MAD cohorts
| Subject group or adverse eventa | No. (%) of subjects receiving indicated treatment |
||||||
|---|---|---|---|---|---|---|---|
| Placebo, 7 days | VL-2397, MAD/7 days |
Placebo, 28 days | VL-2397, 28 days, 900/600 mgb | ||||
| 300 mg (cohort 8) | 600 mg (cohort 9) | 1,200 mg (cohort 10) | Total | ||||
| Total dosed | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 18 (100) | 2 (100) | 6 (100) |
| Subjects with TEAEs | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 18 (100) | 1 (50) | 5 (83) |
| Subjects without TEAEs | 0 | 0 | 0 | 0 | 0 | 1 (50) | 1 (17) |
| Blood and lymphatic system disorders | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Lymphadenopathy | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Cardiac disorders | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 |
| Tachycardia | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye disorders | 0 | 1 (17) | 0 | 1 (17) | 2 (11) | 0 | 0 |
| Eye pruritus | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Lid sulcus deepened | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Ocular hyperemia | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Gastrointestinal disorders | 2 (33) | 1 (17) | 1 (17) | 3 (50) | 5 (28) | 0 | 0 |
| Constipation | 1 (17) | 1 (17) | 0 | 2 (33) | 3 (17) | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 2 (33) | 2 (11) | 0 | 0 |
| Dry mouth | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Flatulence | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| General disorders and administration site conditions | 6 (100) | 6 (100) | 5 (83) | 5 (83) | 16 (89) | 0 | 5 (83) |
| Catheter site pain | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Chest pain | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Chills | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Feeling hot | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Hunger | 2 (33) | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Infusion site discomfort | 1 (17) | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Infusion site erythema | 2 (33) | 3 (50) | 1 (17) | 2 (33) | 6 (33) | 0 | 0 |
| Infusion site extravasation | 1 (17) | 0 | 1 (17) | 2 (33) | 3 (17) | 0 | 0 |
| Infusion site hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Infusion site induration | 3 (50) | 3 (50) | 0 | 2 (33) | 5 (28) | 0 | 0 |
| Infusion site edema | 0 | 1 (17) | 1 (17) | 1 (17) | 3 (17) | 0 | 0 |
| Infusion site pain | 5 (83) | 4 (67) | 4 (67) | 5 (83) | 13 (72) | 0 | 0 |
| Infusion site pruritus | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Peripheral swelling | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Thirst | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Vessel puncture site hemorrhage | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 2 (33) |
| Vessel puncture site pain | 0 | 2 (33) | 0 | 0 | 2 (11) | 0 | 1 (17) |
| Infections and infestations | 1 (17) | 0 | 0 | 1 (17) | 1 (6) | 1 (50) | 0 |
| Carbuncle | 0 | 0 | 0 | 0 | 0 | 1 (50) | 0 |
| Pyelonephritis, acute | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Urinary tract infection | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Viral infection | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 |
| Injury, poisoning, and procedural complications | 1 (17) | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Arthropod bite | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Laceration | 1 (17) | 0 | 0 | 0 | 0 | 0 | 0 |
| Investigations | 0 | 1 (17) | 1 (17) | 1 (17) | 3 (17) | 0 | 1 (17) |
| Aspartate aminotransferase increase | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Blood creatine phosphokinase increase | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Blood creatinine increase | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Blood pressure diastolic increase | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Blood triglyceride increase | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Urine output increase | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 1 (17) | 1 (17) | 0 | 2 (11) | 0 | 1 (17) |
| Back pain | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Flank pain | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Musculoskeletal discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Myalgia | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 0 |
| Pain in extremity | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Nervous system disorders | 0 | 1 (17) | 3 (50) | 3 (50) | 7 (39) | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Dysgeusia | 0 | 1 (17) | 2 (33) | 0 | 3 (17) | 0 | 0 |
| Headache | 0 | 0 | 1 (17) | 3 (50) | 4 (22) | 0 | 0 |
| Psychiatric disorders | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Anxiety | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Renal and urinary disorders | 0 | 0 | 2 (33) | 0 | 2 (11) | 0 | 0 |
| Micturition urgency | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Pollakiuria | 0 | 0 | 1 (17) | 0 | 1 (6) | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Skin and subcutaneous tissue disorders | 0 | 1 (17) | 0 | 3 (50) | 4 (22) | 0 | 4 (67) |
| Acne | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Dermatitis, contact | 0 | 0 | 0 | 0 | 0 | 0 | 4 (67) |
| Ecchymosis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) |
| Erythema | 0 | 1 (17) | 0 | 0 | 1 (6) | 0 | 1 (17) |
| Papule | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Pruritus | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Pruritus, generalized | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Rash papular | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Vascular disorders | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
| Flushing | 0 | 0 | 0 | 1 (17) | 1 (6) | 0 | 0 |
Adverse events are classified according to MedDRA, version 19.0.
300 mg q8h on days 1 to 7 and 600 mg q24h on days 8 to 28.
The most common TEAEs reported in MAD cohorts 8 to 10 were pain, induration, erythema, and extravasation at the infusion site, the majority related to the study drug. Pain at the infusion site was reported for 14 subjects receiving VL-2397 (4 receiving 300 mg, 5 receiving 600 mg, and 5 receiving 1,200 mg) and 5 subjects receiving placebo, induration at the infusion site was reported for 6 subjects receiving VL-2397 (3 receiving 300 mg, 1 receiving 600 mg, and 2 receiving 1,200 mg) and 3 subjects receiving placebo, erythema at the infusion site was reported for 6 subjects receiving VL-2397 (3 receiving 300 mg, 1 receiving 600 mg, and 2 receiving 1,200 mg) and 2 subjects receiving placebo, and extravasation at the infusion site was reported for 3 subjects receiving VL-2397 (1 receiving 600 mg and 2 receiving 1200 mg) and 1 subject receiving placebo. These infusion site TEAEs were observed up to 1 day after the start of infusion and lasted from 1 h to 18 days.
Constipation was reported for three subjects receiving VL-2397 (1 receiving 300 mg and 2 receiving 1,200 mg) and one subject receiving placebo. Headache was reported for four subjects receiving VL-2397 (1 receiving 600 mg and 3 receiving 1,200 mg). These TEAEs started 2 to 24 h after infusion and lasted 15 min to 26 days. All of these TEAEs were related to the study drug.
Two subjects (receiving 1,200 mg of VL-2397) were discontinued from dosing by the PI due to TEAEs related to the study drug as follows.
(i) A 47-year-old male developed an elevation in serum creatinine from normal (1.17 mg/dl) at baseline to 1.48 mg/dl which was detected after receipt of the second dose of VL-2397, resulting in discontinuation of the study drug. The serum creatinine remained elevated for 10 days, with values ranging from 1.29 to 1.52 mg/dl. Urinalysis revealed only microscopic hematuria; creatinine clearance remained normal throughout. The serum creatinine returned to normal by day 17 without intervention. It was subsequently established that the subject had had elevated serum creatinine levels as high as 1.32 mg/dl during participation in previous studies at the clinical research site.
(ii) A 43-year-old female developed a severe generalized rash approximately 10 h after receiving the second dose of VL-2397. The subject also reported moderate pruritus, mild flushing, and anxiety. Physical examination revealed a fine, erythematous papular rash on the anterior and posterior aspects of the torso as well as on the right forearm, left arm, and right upper thigh. The subject was afebrile; blood pressure was elevated (159/104 mm Hg). Oral diphenhydramine was administered for the pruritus. The study drug was discontinued after her dose on day 2 but the rash continued to spread to the bilateral upper and lower legs, bilateral forearms, and buttocks. The subject subsequently developed chills, nausea, constipation, and headache and was treated with acetaminophen and topical hydrocortisone. On day 9, the subject was examined by a dermatologist, who diagnosed the rash as either a drug rash or contact dermatitis and prescribed topical flurandrenolide (Cordran) lotion for the persistent rash. The rash and pruritus resolved by day 27.
No additional subjects in MAD cohorts 8 to 10 who received VL-2397 experienced any laboratory- or vital sign-related TEAE related to the study drug.
(iii) MAD cohort 11. Among cohort 11 subjects, 6 (75%) of the 8 subjects reported a total of 23 AEs, including 3 TEAEs related to the study drug. Twenty-one of these TEAEs were mild and two of these TEAEs were moderate in severity. No SAEs or subject discontinuations due to TEAEs were observed in this cohort. The incidence of TEAEs by subject for MAD cohort 11 is summarized in Table 4.
The most common TEAEs in MAD cohort 11 were contact dermatitis and hemorrhage at the venipuncture site, neither related to the study drug. Contact dermatitis was reported for four subjects receiving VL-2397, but this TEAE was not related to the study drug. Two subjects receiving VL-2397 reported a mild hemorrhage at the venipuncture site, starting approximately 7 h after the start of infusion, which lasted from 2 to 26 days.
No subject in MAD cohort 11 who received VL-2397 experienced a laboratory- or vital sign-related TEAE related to the study drug.
(iv) Overall safety assessment. Daily doses of up to 1,200 mg of VL-2397 (cohorts 1 to 10) for up to 7 days administered by peripheral i.v. infusion, and 600 to 900 mg (300 mg thrice) daily for 28 days administered by peripherally inserted central catheter infusion (cohort 11), appeared to be safe and well tolerated in healthy male and female subjects in this study. No SAEs occurred; the majority of TEAEs were mild and self-limited.
The most common TEAEs were infusion site reactions. No treatment- or dose-related safety trends were observed from vital sign, electrocardiogram, or physical examination results in any of the 11 cohorts. Likewise, no clinically notable laboratory shifts were observed postdose from baseline with respect to serum chemistry, hematology, or urinalysis parameters. As noted above, only the 1,200-mg dose led to study drug discontinuations, both due to TEAEs: one of mild increase in serum creatinine and the other of severe rash, the latter being the only severe TEAE observed in the trial. Both subjects completed the study; their TEAEs resolved during follow-up.
Pharmacokinetic evaluation.
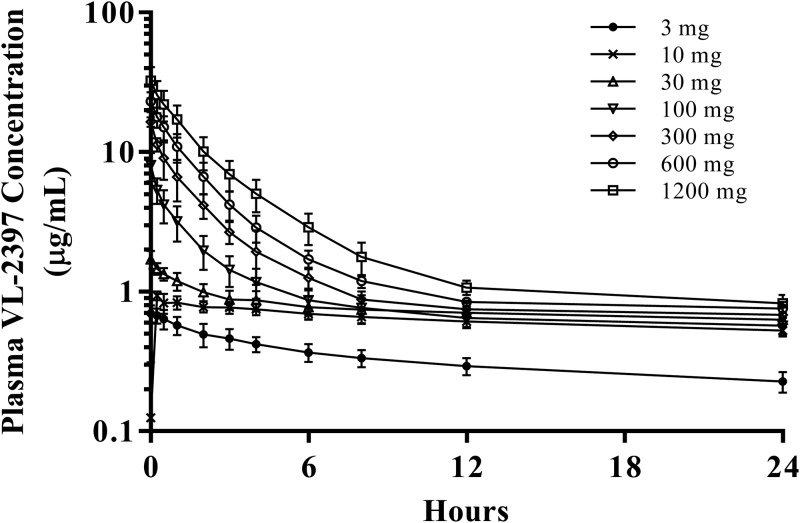
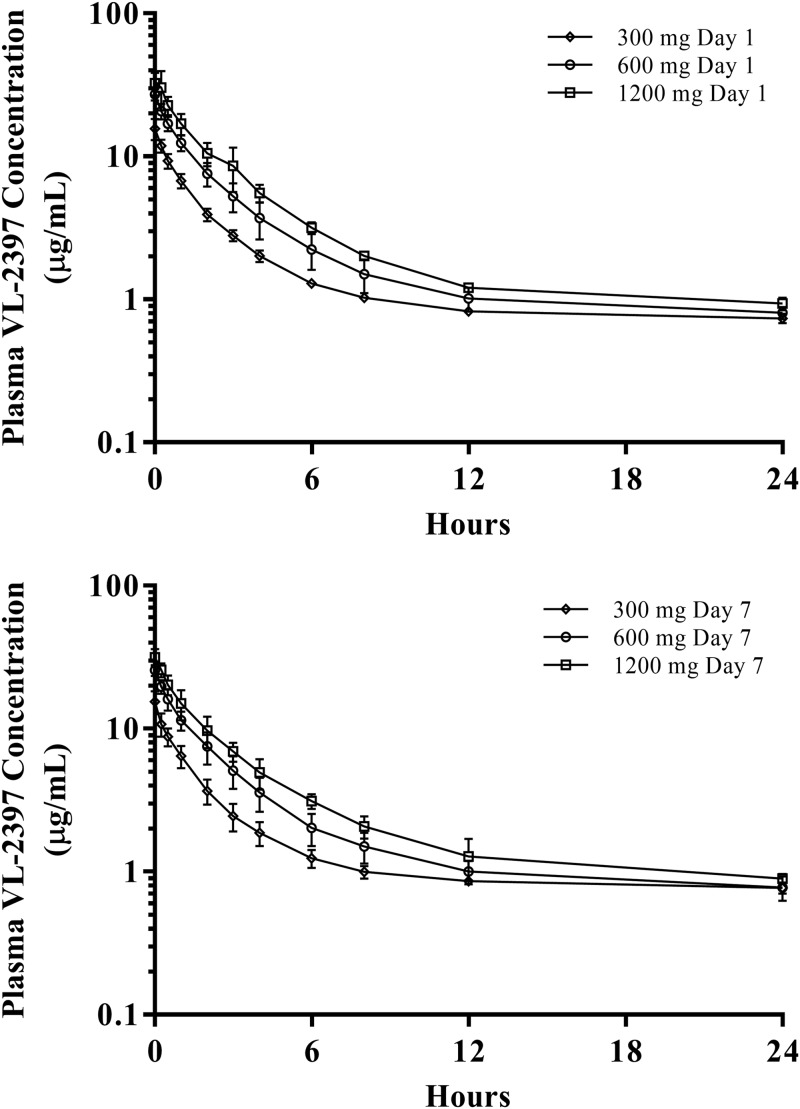
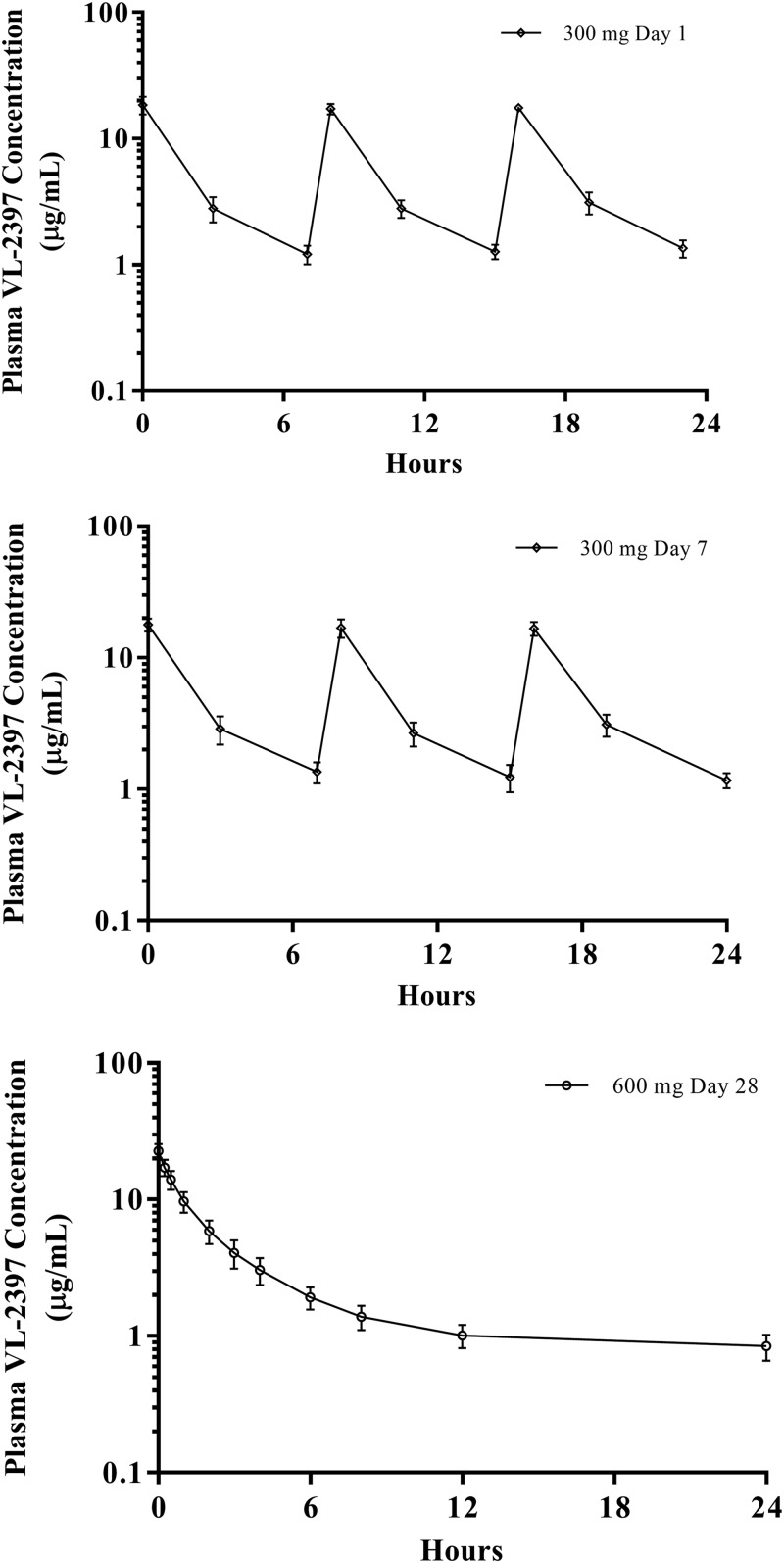
All subjects provided blood (plasma) and urine samples for PK analysis. Table 5 gives the PK parameters for the SAD cohorts and MAD cohorts. The arithmetic mean plasma VL-2397 concentrations versus time are shown in Fig. 1 to 4.
TABLE 5.
Pharmacokinetic parameters from SAD and MAD cohorts
| Cohort | Value for indicated pharmacokinetic parametera
|
||||||
|---|---|---|---|---|---|---|---|
| Geometric mean (CV [%]) |
Arithmetic mean ± SD |
||||||
| AUC24 (ng·h/ml) | Cmax (ng/ml) | kel (1/h) | t1/2 (h) | CL (liters/h) | Vz (liters) | CumFe (%) | |
| SAD cohorts | |||||||
| 3 mg (cohort 1) | 7.8857 (14.2) | 0.7358 (10.3) | 0.008133 ± 0.0013878 | 87.53 ± 16.353 | 0.09135 ± 0.022816 | 11.20 ± 1.5866 | 7.067 ± 3.47 |
| 10 mg (cohort 2) | 15.4443 (8.8) | 1.234 (14.3) | 0.009279 ± 0.0010692 | 75.52 ± 8.5285 | 0.1414 ± 0.021891 | 15.23 ± 1.4101 | 22.070 ± 8.02 |
| 30 mg (cohort 3) | 18.5793 (17.3) | 1.711 (13.4) | 0.008700 ± 0.0018200 | 82.29 ± 15.197 | 0.3571 ± 0.16464 | 39.62 ± 8.9732 | 33.162 ± 12.97 |
| 100 mg (cohort 4) | 24.9795 (20) | 7.88 (21.4) | 0.009849 ± 0.0010563 | 71.00 ± 7.0047 | 1.258 ± 0.29296 | 126.7 ± 18.233 | 46.578 ± 8.99 |
| 300 mg (cohort 5) | 47.0997 (11) | 16.44 (7.7) | 0.009737 ± 0.0012295 | 72.26 ± 10.192 | 2.632 ± 0.36779 | 271.4 ± 27.767 | 45.577 ± 15.10 |
| 600 mg (cohort 6) | 81.4473 (15) | 22.82 (16.2) | 0.009216 ± 0.0013854 | 76.66 ± 11.629 | 3.677 ± 0.47223 | 401.5 ± 36.147 | 47.621 ± 6.21 |
| 1,200 mg (cohort 7) | 161.0445 (19.9) | 31.62 (23.2) | 0.009799 ± 0.00053708 | 70.91 ± 3.7825 | 4.932 ± 0.83043 | 501.7 ± 69.940 | 38.493 ± 4.54 |
| MAD cohorts, day 1 | |||||||
| 300 mg (cohort 8) | 48.4377 (6.7) | 15.4 (18.1) | ND | ND | ND | ND | 61.448 ± 5.05 |
| 600 mg (cohort 9) | 98.8501 (10.2) | 27.03 (8.9) | ND | ND | ND | ND | 43.819 ± 8.34 |
| 1,200 mg (cohort 10) | 169.8596 (11.5) | 35.11 (22.8) | ND | ND | ND | ND | 49.272 ± 5.74 |
| 900/600 mg (cohort 11) | NP | 18.3 (15.2) | ND | ND | ND | ND | 51.052 ± 2.93 |
| MAD cohorts, day 7 | |||||||
| 300 mg (cohort 8) | 47.2314 (9.9) | 15.27 (16.4) | 0.009465 ± 0.00092852 | 73.82 ± 7.2095 | 6.376 ± 0.58297 | ND | 45.427 ±12.57 |
| 600 mg (cohort 9) | 94.0712 (12.7) | 25.49 (8.2) | 0.007971 ± 0.0017315 | 91.09 ± 23.081 | 6.422 ± 0.84004 | ND | 43.662 ± 18.12 |
| 1,200 mg (cohort 10) | 162.5545 (16) | 31.33 (13.6) | 0.006951 ± 0.00071033 | 100.5 ± 10.351 | 7.451 ± 1.1520 | ND | 51.077 ± 15.76 |
| 900/600 mg (cohort 11) | NP | 17.7 (11.4) | NP | NP | NP | ND | 44.451 ± 11.47 |
| MAD cohort 11, day 28 | |||||||
| 900/600 mg (cohort 11) | 83.9347 (14.4) | 22.64 (11.5) | 0.008760 ± 0.00054125 | 79.37 ± 4.8150 | 7.208 ± 0.99768 | ND | 43.368 ± 10.23 |
kel, elimination rate constant; Vz, volume of distribution; ND, parameter not determined; NP, parameter not presented.
FIG 1.
Arithmetic mean plasma VL-2397 concentrations versus time following single i.v. infusions of VL-2397 (SAD), cohorts 1 to 7. Shown are mean values (±SDs) for each time point for each of the seven SAD cohorts. The mean concentrations detected at 240 h after dosing ranged from 0.04 to 0.1 μg/ml.
FIG 2.
Arithmetic mean plasma VL-2397 concentrations versus time following multiple i.v. infusions of VL-2397 (MAD/7 days), cohorts 8 to 10. Shown are mean values (±SDs) for each time point for each of the three MAD cohorts that received 7 daily doses, measured 24 h after the first dose (upper graph) or 24 h after the 7th dose (lower graph). The mean concentrations detected at 240 h after the 7th day of dosing ranged from 0.1 to 0.2 μg/ml.
FIG 3.
Arithmetic mean plasma VL-2397 concentrations versus time following multiple i.v. infusions of VL-2397 (MAD/28 days). Shown are mean values (±SDs) for each time point for MAD cohort 11, measured 24 h after dose 1 (upper graph), dose 7 (middle graph), or dose 28 (lower graph). The mean concentration detected at 240 h after the last dosing was 0.14 μg/ml.
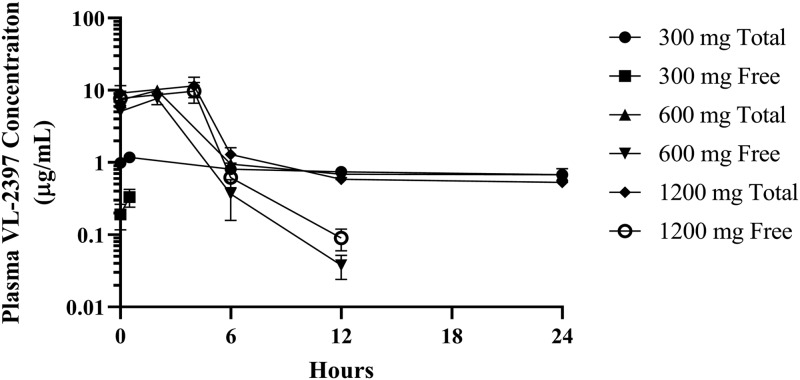
FIG 4.
Arithmetic mean plasma VL-2397 concentrations versus time for free drug and total drug, SAD cohorts 3, 6, and 7. Shown are mean values (±SDs) for each time point for SAD cohort 3, 6, and 7, measured over a 24-h period after each dose. The plasma concentrations for free drug 1 h after administration of the 30-mg dose and 24 h after the 600-mg and 1,200-mg doses were below the detection limit of the assay.
As shown in Table 5 and Fig. 1, following administration of single i.v. infusions of VL-2397 (SAD cohorts 1 to 7), the overall and maximum VL-2397 exposures based on area under the concentration-time curves (AUCs) and maximum concentration (Cmax), respectively, rose with increasing VL-2397 doses from 3 to 1,200 mg (Table 5). The AUC and Cmax values rose less than proportionally with increasing doses from 3 to 1,200 mg. Arithmetic mean VL-2397 circulating half-life (t1/2) values were comparable across all dose levels and ranged from approximately 71 to 88 h. These t1/2 values represent only the terminal phase and do not reflect the disposition of VL-2397 during the dosing interval. Arithmetic mean clearance (CL) values rose with increasing VL-2397 doses from 3 to 1,200 mg, with mean values ranging from approximately 0.0913 to 4.93 liters/h for CL.
Nonclinical plasma protein binding studies showed that zinc-α2-glycoprotein (ZAG) bound the drug at a very high affinity and binding was saturated above the 30-mg dose (unpublished data). Saturation of plasma binding components is illustrated in Fig. 4, showing clearance of free drug compared to total drug. At 30 mg, the Cmax for free drug was less than that for total drug and was below the detectable limit 1 h after initiation of dose administration. In contrast, the Cmax for free drug for the 600-mg and 1,200-mg doses was comparable to the Cmax for total drug. The concentration of free drugs was below the detectable limit 24 h after initiation of dose administration.
As shown in Table 5 and Fig. 2, following administration of the first dose (day 1) of multiple i.v. infusions of VL-2397 once daily (q24h) for 7 days (MAD/7 days, cohorts 8 to 10), the overall and maximum VL-2397 exposures (based on AUC and Cmax, respectively) rose with increasing VL-2397 doses from 300 to 1,200 mg. Geometric mean AUC over 24 h (AUC24) and Cmax values for MAD/7 days dose levels were comparable with the corresponding dose level in SAD cohorts 5, 6, and 7 (Table 5).
As shown in Table 5, following administration of the last dose (day 7) of multiple i.v. infusions of VL-2397 (MAD/7 days, cohorts 8 to 10), the geometric mean overall and maximum VL-2397 exposures based on AUC and maximum steady-state drug concentration (Cmax,ss), respectively, rose with increasing VL-2397 doses from 300 to 1,200 mg. For MAD cohorts 8 to 10, the VL-2397 AUC rose in a relatively dose-proportional manner with increasing doses from 300 mg to 1,200 mg, while the increase in Cmax (based on Cmax on day 1 and Cmax,ss on day 7) was less than dose proportional. Arithmetic mean t1/2 values tended to increase with increasing VL-2397 doses and ranged from approximately 74 to 101 h following 300- to 1,200-mg dose levels. Mean VL-2397 CLss values were comparable across all dose levels following 300, 600, and 1,200 mg of VL-2397 q24h for 7 days (MAD/7 days, cohorts 8, 9, and 10) and were also comparable with the administration of 600 mg q24h for 21 days (MAD/28 days, cohort 11) with mean values ranging from approximately 6.38 to 7.45 liters/h.
No signs of VL-2397 accumulation were observed following multiple i.v. infusions of 300, 600, and 1,200 mg of VL-2397 Q24H for 7 days. The mean accumulation ratios (last dose divided by first dose) for AUC and Cmax were <1.0 for all doses. No signs of VL-2397 accumulation were observed following thrice-daily infusions of 300 mg for 7 days followed by daily infusions of 600 mg for 21 days.
Overall, the median times after administration when VL-2397 reached maximum concentration (Tmax) were observed at the end of VL-2397 infusion.
Renal elimination plays a major role in total body clearance of VL-2397 at dose levels higher than 10 mg. Following a single i.v. infusion of VL-2397, the cumulative fraction of VL-2397 excreted in urine (CumFe) rose with increasing VL-2397 doses of 3 to 100 mg and ranged from approximately 7 to 47%, respectively; however, CumFe did not increase further at doses higher than 100 mg, with comparable CumFe values following 100 to 1,200 mg (CumFe ranged from approximately 38 to 48%). The same trend was observed following multiple i.v. infusions of 300, 600, and 1,200 mg of VL-2397 for 7 days, with mean CumFe ranging from approximately 43 to 61%, and approximately 43% following 600 mg of VL-2397 q24h for 21 days (CumFe on days 7 and 21 might include additional minimal cumulative amounts, approximately <4%, from the previous dose levels).
DISCUSSION
This randomized, double-blind, placebo-controlled, ascending-dose study evaluated the safety and PK of VL-2397 in healthy adults receiving (i) a single dose of up to 1,200 mg, (ii) multiple doses (7 days) of 300 mg, 600 mg, and 1,200 mg, and (iii) multiple doses (28 days) of 300 mg thrice daily for 7 days followed by 600 mg daily for 21 days. All enrolled subjects completed their study visits.
VL-2397 was deemed safe and well tolerated at all tested dose levels by the Safety Review Committee (SRC).
No SAEs were observed. Most TEAEs were mild in severity, the most common TEAEs being infusion site reactions. Incidence of TEAEs did not appear to increase with rising VL-2397 dose level. Potentially life-threatening elevations in CK were observed in 2 subjects (both in the 100-mg VL-2397 cohort); these were deemed unrelated to the study drug, attributable to strenuous activity in extreme heat, and not accompanied by renal dysfunction. Two subjects in the highest-dosing cohort (1,200 mg daily for 7 days) experienced TEAEs related to VL-2397 which resulted in discontinuation of dosing following 2 doses. One subject had a mild elevation in serum creatinine that returned to normal by day 17. The other subject developed a severe generalized erythematous papular rash that resolved approximately 1 month after onset; this was the only severe TEAE in the entire study. The severities of related TEAEs in VL-2397 recipients were mostly mild.
The plasma PK of VL-2397 was generally well characterized in the SAD and MAD cohorts. In both SAD and MAD cohorts, AUC and Cmax rose in a manner nonproportional to dose. Pharmacokinetic results for the SAD cohorts were very similar to the PK parameters for the MAD cohorts. Following multiple i.v. infusions of VL-2397 q24h for 7 days, the mean VL-2397 accumulation ratios based on the total extent and peak exposure were comparable across all 3 dose levels (300, 600, and 1,200 mg), with mean values of <1.0. An accumulation ratio of <1.0 was also observed following administration of 300 mg of VL-2397 q8h for 7 days. Accordingly, based on these results, no evidence of VL-2397 accumulation in plasma was seen following 7 days of dosing. Similarly, no signs of VL-2397 accumulation were observed following daily infusions of 600 mg for 21 days.
Renal elimination was found to play a major role in total body clearance of VL-2397 at dose levels higher than 10 mg. Following a single i.v. infusion of VL-2397, the CumFe for unmetabolized drug rose with increasing VL-2397 doses of 3 to 100 mg and ranged from approximately 7 to 47% of the injected dose, respectively. However, CumFe did not increase further at doses higher than 100 mg, indicating that plasma protein binding of VL-2397 was saturated between the 30-mg and 100-mg doses. The same trend was observed following multiple i.v. infusions of 300, 600, and 1,200 mg of VL-2397 for 7 days, with mean CumFe values ranging from approximately 44 to 51%; CumFe of approximately 43% was observed following treatment with 600 mg of VL-2397 q24h for 21 days. These results are similar to the nonclinical results of an absorption/distribution/metabolism/excretion study conducted with rats, in which the cumulative urinary excretion was 64% up to 168 h after intravenous administration of an 8-mg/kg (of body weight) dose of 14C-labeled VL-2397 (31.4% of the dose was in the feces [unpublished data]). The plateau of CumFe values at the 100-mg dose is indicative of saturation of protein binding in blood. Protein saturation binding is further supported by the plasma concentration of free drug versus total drug shown in Fig. 4, where the Cmax values for the free drug and total drug were comparable for the 600-mg and 1,200-mg doses, whereas the Cmax for the free drug was below the Cmax for total drug at the 30-mg dose. Figure 4 further shows that the majority of drug is in the free fraction and that it is cleared 24 h after initiation of administration.
Pharmacokinetic-pharmacodynamic (PK-PD) target attainment analysis was conducted to determine appropriate dosing for a planned phase 2 study in patients with invasive aspergillosis. The PK data from the VL2397-101 phase 1 study in conjunction with data from a mouse invasive pulmonary aspergillosis study were used to select a phase 2 dosing regimen. The PK-PD driver of efficacy for VL-2397 was determined to be free area under the plasma concentration-versus-time curve from time zero to 24 h postdose/MIC (AUC0–24:MIC), and a value of 8.40 was associated with a 2-log reduction in Aspergillus fumigatus lung burden in the mouse model (17). The PK-PD target attainment analysis indicated that a VL-2397 dosage of 600 mg once daily for up to 4 weeks was predicted to provide adequate VL-2397 target attainment up to an MIC of 4 μg/ml against Aspergillus fumigatus (17), which is a higher MIC than observed for a panel of 49 A. fumigatus isolates (range, 0.06 to 0.5 μg/ml) (8).
A recently published population PK modeling paper describing the use of 1,908 VL-2397 concentrations from the subjects in the phase 1 trial proposed nonlinear, saturable binding kinetics of VL-2397 (18). The major serum binding protein for VL-2397 is zinc-α2-glycoprotein (ZAG), which was proposed by the authors as the likely primary source of nonlinearity. Further studies will be important to better understand the mechanisms underlying the observed nonlinearity.
VL-2397 is a siderophore that exerts its antifungal effect by a novel iron transporter-dependent mechanism of action distinct from all existing antifungal drug classes that are used to treat IA, including azoles, amphotericin B, and echinocandins. Siderophores produced by Aspergillus fumigatus such as triacetylfusarinine C and ferrocrocin comprise a major iron acquisition pathway (9, 10), and iron and siderophore synthesis are well-recognized virulence factors (11) for this filamentous fungus. Studies are in progress to elucidate intracellular components in Aspergillus fumigatus downstream of Sit-1-mediated uptake that are crucial for the mechanism of antifungal action. Recent studies indicate that Sit1-mediated uptake is essential for VL-2397 susceptibility and that antifungal activity is independent of aluminum importation by fungal cells (13). A previous publication indicated that the apo-form (metal-free form) of VL-2397 does not have antifungal activity in vivo (7). Further elucidation of VL-2397’s antifungal mechanism may provide insights into developing derivative molecules with a broader antifungal spectrum.
The most recent Infectious Diseases Society of America guidelines recommend voriconazole as primary treatment for IA and isavuconazole or lipid formulations of amphotericin B as alternative treatment options (19). The emergence of azole-resistant fungal strains during the past several decades is believed to be attributable to long-term azole therapy in addition to environmental azole use as fungicidal agents in agriculture (20, 21). Worldwide, 3.2% of >3,700 Aspergillus fumigatus isolates sampled from 19 countries were azole resistant (22). In some countries, such as the Netherlands, >25% of clinical Aspergillus isolates carry azole resistance alleles; the mortality rate in patients with azole-resistant isolates approaches 100% (23). VL-2397 has shown antifungal activity in mice challenged with azole-resistant Aspergillus fumigatus (14), supporting its azole-independent mechanism of action and potential utility for clinical treatment of humans infected with azole-resistant isolates.
In addition to rising azole-resistant fungal strains, existing antifungal drugs indicated for treatment of IA also have appreciable toxicity/tolerance concerns and potential for drug-drug interactions that require intensive therapeutic drug monitoring (1, 5). High-risk patients with suspected IA are also receiving a variety of treatments for their underlying cancer or transplant, with a number of coadministered drugs exhibiting known drug-drug interactions with azoles (24). Use of alternative antifungal drug candidates such as VL-2397, which do not appear to be metabolized by cytochrome P450 isozymes, in contrast to azoles (16), may provide an attractive treatment option for this very sick population. The safety data to date for healthy volunteers suggest that VL-2397 may also offer an advantage of lower toxicity than those of existing antifungal drugs.
Conclusions.
VL-2397 appears to be safe and well tolerated, with favorable plasma PK profiles in healthy subjects in this phase 1 study, supporting its advancement to a phase 2 study for the treatment of patients with invasive aspergillosis.
MATERIALS AND METHODS
All pertinent study documents, including protocol and informed consent, were reviewed and approved by an institutional review board prior to study initiation. The studies were designed and monitored in compliance with good clinical practice and in accordance with the ethical principles set forth in the Declaration of Helsinki. Vical Incorporated sponsored and funded this single-center study conducted at Celerion (Tempe, AZ).
VL-2397 preparation and administration.
VL-2397 drug product was formulated in 20% ethanol–80% propylene glycol at a concentration of 150 mg/ml. For the 3-mg, 10-mg, and 30-mg doses, 0.2 ml of drug product was injected into an infusion bag containing 250 ml of 5% dextrose in water (D5W), yielding a final drug concentration of 0.12 mg/ml. For the 100-mg to 1,200-mg doses, 2 ml of VL-2397 drug product was injected into an infusion bag containing 250 ml of D5W. The infusion rate was held constant at 250 ml/h, and the dose was controlled by the infusion time. Table 1 lists the dose, drug concentration in D5W, and the infusion time for all 11 cohorts.
Study design and treatment.
VL2397-101 was a first-in-human, randomized, double-blind, placebo-controlled SAD and MAD phase 1 study of VL-2397 in healthy adults 18 to 55 years of age.
This study included seven SAD cohorts followed by three 7-day MAD cohorts, and culminating in a 28-day MAD cohort. Eight male or female adult subjects 18 to 55 years of age were randomized 3:1 within each cohort to receive either VL-2397 or placebo using a peripheral i.v. catheter in all but the final cohort, in which a peripherally inserted central catheter was used to avoid the discomfort associated with frequent peripheral i.v. catheter changes that would have been required during a 28-day period. Investigational products comprised active VL-2397 in D5W and placebo (D5W alone) administered via i.v. infusion. The pharmacy ensured that the 3:1 randomization schedule was followed when preparing each dose. Treatment assignments were blinded to the sponsor, subjects, investigators, and all site staff except for pharmacy and infusion personnel.
Subjects resided in a clinical research unit in Arizona for observation and prespecified assessments until 2 days after the last dose of the study drug (days 3, 9, and 30 for the SAD cohorts, MAD cohorts 8 to 10, and MAD cohort 11, respectively).
Inclusion criteria.
Randomized subjects were healthy male or female subjects 18 to 55 years of age at the time of consent. Female subjects could be enrolled only if either surgically sterile or postmenopausal. Screening hematology, clinical chemistries, coagulation, and urinalysis results were to be consistent with overall good health as determined by the PI and renal function criteria met, including creatinine and creatinine clearance within normal limits. Body mass index values were to be inclusive between 18 and 30 kg/m2. Subjects were to be willing and able to provide written informed consent and to comply with the requirements of the study, including inpatient stay and completion of all study-related procedures.
Exclusion criteria.
Subjects were excluded from the study if any of the following exclusion criteria were met: any major chronic systemic disease; any acute illness within 14 days of study screening; participation in an investigational drug study within 30 days, use of prescription medications within 14 days, or use of over-the-counter medications within 7 days prior to the first planned drug administration; history of tobacco or alcohol use within 6 months or 12 months, respectively, prior to the first planned drug administration; history of significant allergic drug reactions or known allergy to dextrose, corn, or corn products; blood donation within the last 3 months; positive laboratory tests for pregnancy, hepatitis B virus surface antigen, hepatitis C virus antibody, and human immunodeficiency virus antibody; women who were pregnant, breastfeeding, or of childbearing potential.
Study populations.
Analysis populations were the safety population (all subjects who received at least one dose of active drug or placebo) and PK population (all subjects who received active drug and provided at least one quantifiable plasma and/or urine concentration of VL-2397).
Safety assessments.
All subjects were monitored for TEAEs and SAEs from the start of the inpatient stay until end of study visits on days 11 (SAD cohorts), 17 (MAD cohorts 8 to 10), and 38 (MAD cohort 11). Vital signs, 12-lead ECGs, hematology and serum chemistry evaluations, and urinalysis were performed at screening (days −28 to −2), at baseline (day −1), and at specified time points during the study depending upon cohort and assessment. The PI assessed the severity and causality for each TEAE.
Individual subjects were to be discontinued from receiving further study drug based on developing any of the following stopping rules as determined by the investigator: (i) serum creatinine increase of ≥0.3 mg/dl or an increase of ≥50% above baseline regardless of relationship to study drug; (ii) any grade 3 or 4 AE, including laboratory abnormality per Common Terminology Criteria for Adverse Events, version 4.0, that was considered related to study participation; and (iii) an SAE that was considered related to study participation.
An SRC, which included the sponsor medical monitor, PI, and an independent safety consultant, reviewed the safety data in each cohort after the last dose to provide recommendations regarding dose progression to the next cohort. Any single daily dose administered in the MAD cohorts did not exceed the highest dose delivered in the prior SAD cohorts.
Pharmacokinetic assessments.
For the SAD cohorts, blood samples were collected for plasma PK profile analysis predose (within approximately 30 min to 4.5 h prior to start of infusion), midway into the infusion, at the end of the infusion, at 15 and 30 min (0.25 and 0.5 h, respectively), and at 1, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h relative to the end of the day 1 infusion. Follow-up blood samples were also drawn on days 5 and 11 as part of the PK profile. For the MAD cohorts, blood samples were collected predose (within approximately 30 min to 4.5 h prior to the start of infusion), midway into the infusion, at the end of the infusion, at 15 and 30 min (0.25 and 0.5 h, respectively), and at 1, 2, 3, 4, 6, 8, 12, and 24 h (prior to dosing on day 2) relative to the end of the day 1 infusion. During the 7-day infusion period, additional blood samples were drawn within approximately 60 min prior to dosing on days 3, 4, 5, and 6. On day 7, blood samples were collected predose (within approximately 60 min to 4.5 h prior to the start of infusion), midway into the infusion, at the end of the infusion, at 15 and 30 min, and at 1, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h relative to the end of the day 7 infusion. On days 11 and 17, follow-up blood samples were collected for plasma PK assessments. For MAD cohort 11, administration of drug q8h for 7 days, samples for a 24-h PK profile (predose within 60 min prior to the start of infusion, midway into the infusion, at the end of the infusion, and at 3, 7, 8, 11, 15, 16, and 19 h relative to the end of the first infusion on day 1) were collected. Samples from the 7- and 8-h time points were collected immediately before and after the administration of the second dose on day 1. Samples from the 15- and 16-h time points were collected immediately before and after the administration of the third dose on day 1. Additional blood was drawn just prior to the first infusion on days 2 through 6.
The noncompartmental PK parameters were calculated from the plasma VL-2397 concentration-time data using Phoenix WinNonlin, version 6.3. Extensive PK sampling from plasma and urine was performed for all subjects following study drug administration.
For total VL-2397 concentration determination, human plasma samples containing VL-2397 standardized with a VL-2397 internal reference standard (VL-2397-IS) and sodium heparin as the anticoagulant were precipitated using acetonitrile. The supernatant was collected and evaporated, and the residue was reconstituted. For unbound VL-2397, human plasma samples containing VL-2397 and sodium heparin as the anticoagulant were dialyzed against phosphate-buffered saline. An aliquot of the dialysate was internally standardized with VL-2397-IS and extracted using solid-phase extraction (SPE). For determination of VL-2397 concentration following urine excretion, human urine samples containing VL-2397 and internally standardized with VL-2397-IS were diluted and isolated by SPE. An aliquot from the reconstituted residue of total drug, or the eluent from the SPE for unbound drug or urine, was analyzed by reversed-phase high-performance liquid chromatography (HPLC) using a Restek Allure Biphenyl column. The mobile phase was nebulized using heated nitrogen in a Z-spray source/interface set to electrospray in the positive ionization mode. Ionized compounds were detected by tandem mass spectrometry. All sample preparations and HPLC assays were validated with respect to accuracy (<15% standard deviation [SD]), precision (<15% coefficient of variation [CV]), linearity, sensitivity, and specificity at MicroConstants, Inc. (San Diego, CA). The assay method for measuring VL-2397 in human plasma has a calibration range from 2.00 to 1,000 ng/ml. The method is reproducible and rugged, with no significant matrix interference in the lots of human plasma tested.
Statistical methods.
The study was not powered for inferential statistical analyses. The sample size for this study was selected without statistical considerations. Descriptive statistics, including the numbers and percentages for categorical variables and the numbers, means, and SDs and the medians, minimums, and maximums for continuous variables, were summarized by cohort (i.e., dose) and study drug (VL-2397 or placebo). A statistical analysis plan was prepared and finalized before database lock and analysis of data were performed. All statistical analyses were performed using SAS, version 9.3.
Safety was assessed in the safety analysis population. Safety was evaluated by presenting summaries of TEAEs, ECGs, clinical laboratory evaluations (hematology, chemistry panel, and urinalysis), and vital signs. All TEAEs that occurred during this study were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 19.1.
A TEAE was defined as an adverse event that was starting or worsening during the time of or after study drug administration. The incidence of TEAEs was summarized by system organ class and preferred term, by relationship to study drug, and by severity. In addition, the incidence of serious TEAEs and the incidence of TEAEs leading to discontinuation of study drug were summarized by system organ class and preferred term. Descriptive statistics for clinical laboratory test results and vital signs, and for changes from baseline, were summarized by time point. Incidences of potentially clinically significant clinical laboratory results and vital signs were also summarized by time point. The numbers and percentages of abnormal ECGs were summarized by time point.
Plasma and urine concentrations and PK parameter values were imported into SAS and all descriptive statistics were calculated using SAS, version 9.3. Pharmacokinetics was assessed in the PK analysis population and included Cmax, AUC, clearance, elimination rate constant, and terminal half-life. For each plasma concentration-time curve, PK parameters were determined directly from inspection of the data or calculated using noncompartmental methods with validated PK software (Phoenix WinNonlin, version 6.3). Urine concentration data were used to determine renal excretion and drug recovery rates.
ACKNOWLEDGMENTS
Haran T. Schlamm (HTS Pharma Consulting, Inc.) provided medical writing assistance. Chris Rubino (Institute of Clinical Pharmacodynamics) provided assistance with PK/PD analyses. We are grateful to Erin Castelloe, who served as an independent safety consultant on the SRC.
During conduct of the study, all authors except for Danielle Armas were employees of, and held stock options in, Vical Incorporated and were involved in the study design, study conduct, data analysis, and/or writing of internal study reports. All coauthors were involved in the preparation and approval of the manuscript.
REFERENCES
- 1.Patterson TF. 2010. Aspergillus species, p 3241–3255. In Mandell GL, Bennett JE, Dolin R (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 7th ed, vol 2 Churchill Livingstone Elsevier Press, Philadelphia, PA. [Google Scholar]
- 2.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomized-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 3.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. 2015. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 4.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, Heussel CP, Lortholary O, Rieger C, Boehme A, Aoun M, Horst HA, Thiebaut A, Ruhnke M, Reichert D, Vianelli N, Krause SW, Olavarria E, Herbrecht R. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad Trial). Clin Infect Dis 44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 5.Perfect JR. 2017. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16:603–616. doi: 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura I, Yoshimura S, Masaki T, Takase S, Ohsumi K, Hashimoto M, Furukawa S, Fujie A. 2017. ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J Antibiot (Tokyo) 70:45–51. doi: 10.1038/ja.2016.107. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura I, Kanasaki R, Yoshikawa K, Furukawa S, Fujie A, Hamamoto H, Sekimizu K. 2017. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J Antibiot 70:41–44. doi: 10.1038/ja.2016.106. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura I, Ohsumi K, Yoshikawa K, Kanasaki R, Masaki T, Takase S, Hashimoto M, Fujie A, Nakai T, Matsumoto S, Takeda S, Akamatsu S, Uchida S, Maki K. 2014. ASP2397: a novel natural product with potent fungicidal activity against Aspergillus spp—a new mode of action and in vitro activity, abstr F-1590. Abstr Intersci Conf Antimicrob Agents Chemother.
- 9.Haas H. 2012. Iron—a key nexus in the virulence of Aspergillus fumigatus. Front Microbiol 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas H. 2014. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat Prod Rep 31:1266–1276. doi: 10.1039/c4np00071d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, Haynes K, Haas H. 2004. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med 200:1213–1219. doi: 10.1084/jem.20041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiang T, Baillie DL. 2005. Comparison of the yeast proteome to other fungal genomes to find core fungal genes. J Mol Evol 60:475–483. doi: 10.1007/s00239-004-0218-1. [DOI] [PubMed] [Google Scholar]
- 13.Dietl A-M, Misslinger M, Aguiar MM, Ivashov V, Teis D, Pfister J, Decristoforo C, Hermann M, Sullivan SM, Smith LR, Haas H. 2019. The siderophore transporter Sit1 determines susceptibility to the antifungal VL-2397. Antimicrob Agents Chemother 63:e00807-19. doi: 10.1128/AAC.00807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura I, Nakai T, Matsumoto S, Takeda S, Akamatsu S, Uchida S, Koide Y, Mitori H, Noto T, Maki K. 2014. ASP2397: a novel product with potent fungicidal activity against Aspergillus species—in vivo activity against A. fumigatus, abstr F-1591. Abstr Intersci Conf Antimicrob Agents Chemother. [DOI] [PMC free article] [PubMed]
- 15.Nakamura I, Ochbuchi M, Akabane T, Akamatsu S, Matsumoto S, Takeda S, Smith L, Sullivan S. 2015. Pharmacokinetic and pharmacodynamics characterization of ASP2397, a novel potent antifungal agent with activity against invasive pulmonary aspergillosis, abstr F-746. Abstr Intersci Conf Antimicrob Agents Chemother.
- 16.Nakamura I, Ohbuchi M, Matsumoto S, Smith L, Sullivan S. 2017. Characterization of potential drug interactions and off-target activities of VL-2397, a novel antifungal agent against invasive aspergillosis, abstr 235. Abstr ASM Microbe.
- 17.Rubino CM, Smith LR, Mammen M, Hopkins AM, Lakota EA, Sullivan SM. 2017. Pharmacokinetics-pharmacodynamic target attainment analysis to support VL-2397 dose selection for a phase 2 trial in patients with invasive aspergillosis, abstr 1515. Abstr IDWeek.
- 18.Kovanda LL, Sullivan SM, Smith LR, Desai AV, Bonate PL, Hope WW. 2019. Population pharmacokinetic modeling of VL-2397, a novel antifungal agent: analysis of a single- and multiple-ascending-dose study in healthy subjects. Antimicrob Agents Chemother 63:e00163-19. doi: 10.1128/AAC.00163-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MN, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:433–442. doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verweij PE, Chowdhary A, Melchers WJG, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhary A, Sharma C, Meis JF. 2017. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S436–S444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 22.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekotter A, Lass-Florl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJG, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. 2018. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 24.Nett JE, Andes DR. 2016. Antifungal agents spectrum of activity, pharmacology and clinical indications. Infect Dis Clin North Am 30:51–83. doi: 10.1016/j.idc.2015.10.012. [DOI] [PubMed] [Google Scholar]