Abstract
Changes in whey protein (10%, w/v) induced by dry-heating (60 °C for 5 days at a relative humidity of 63%), wet-heating (85 °C for 30 min) or the two-combined heating in absence or presence of inulin (8%, w/v) were studied. Mixture of whey protein and inulin showed significantly higher absorbance at 290 nm than whey protein alone in all heating conditions while only dry-heated samples showed significantly increased absorbance value at 420 nm (p < 0.05). Whey protein after heating showed significantly lower zeta potential and inulin decreased the value of all heated samples further (p < 0.05) except for samples after dry-heating. Heating decreased the free sulfhydryl group content of whey protein samples while presence of inulin decreased further (p < 0.05). Dry-heating decreased while wet-heating increased the surface hydrophobicity of whey protein. Inulin had no effect on the surface hydrophobicity of heated whey protein under dry-heating but decreased under wet-heating.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00577-w) contains supplementary material, which is available to authorized users.
Keywords: Whey protein, Inulin, Dry heating, Wet heating
Introduction
Maillard reaction (MR) has been extensively documented for modifying the properties of whey protein significantly (Spotti et al., 2013). It can be divided into dry and wet heating processes which have sets of pros and cons respectively. In a dry heating reaction process, the reaction rate can be controlled due to the low diffusion of moisture because of a lower water content (Nasirpour et al., 2006). Dry heating requires a relative long reaction time and harsh conditions. It may form insoluble substances during this process. However, the slight change in color makes the products more suitable for food application. In addition to conjugating whey protein and polysaccharides by dry heating, attempts have also been achieved in forming complexes by wet heating method (Wang et al., 2013). In a wet heating reaction process, the reaction rate decreases due to dilution of the water-soluble reactant because of a higher water content (Nasirpour et al., 2006). Wet heating can control the reaction degree effectively, however, it may be involved with other interactions such as electrostatic, hydrogen binding and hydrophobic interactions and high temperature often causes protein aggregation (Perusko et al., 2015).
Whey proteins are among the most commonly employed functional ingredients in food formulations. They are a group of globular proteins which are sensitive to heating process. Whey protein molecules will denature and aggregate under heat treatment at a sufficiently high temperature via disulfide bond and/or hydrophobic interaction (Havea et al., 2004). Due to the compact structure and low molecular weight of whey proteins, application of native whey protein is limited to a relatively narrow field (Herceg et al., 2007). Heat induced modification may improve the functionality of whey proteins (Nicolai et al., 2011). However, the altered properties are ultimately determined by their molecular structure and interactions, as well as reaction conditions (Bryant and Mcclements, 1999).
Inulin is usually used as a food ingredient due to its improvement of the texture performance and functional benefits (Komatsu et al., 2013). It is a soluble fiber and composed of fructose units joined by β (2 → 1) glycoside bonds and terminates in glucose generally (Sołowiej et al., 2015). Since inulin has an active reducing-end residue in the molecule, it has been glycosylated to proteins to alter their properties. Recent research showed that Maillard products of ovomucoid and inulin had enhanced emulsion stability than ovomucoid alone significantly (Yap et al., 2007). For some food, such as bread, the quality can be improved when inulin was added which played a role through Maillard reaction (Poinot et al., 2010). In addition, in solution state, inulin may interact with protein via hydrophobic interaction (Ha et al., 2016).
The aims of this study were to analyze physicochemical properties of whey protein under dry-, wet- or combined state heating in absence or presence of inulin. All samples were measured and compared for absorbance, average particle size, zeta potential, free sulfhydryl group content and surface hydrophobicity.
Materials and methods
Materials
Whey protein isolate (WPI, 92% protein based on dry weight) was obtained from Fonterra Co-operative Group (Auckland, Netherlands). Composition of WPI is as follows (w/w): 0.36% fat, 1.6% ash, 0.7% lactose monohydrate and 0.07% calcium. Inulin was purchased from Hua-Cheng Bio-tech Co. Ltd. (Batch No. C228H3820191, Changchun, Jinlin, China). The product information as stated by manufacturer is: purity: 92% (w/w); degree of polymerization: 23–60; water content: 4.5%; minerals content: 0.2%; pH: 6.5 at concentration of 10% (w/v) in aqueous solution. 5,5′-Dithiobis-(2-nitrobenzoic acid) (DTNB) and 8-anilino-1-naphtalene sulfonic acid (ANS) were purchased from Sigma Aldrich (Sigma Aldrich Co., Ltd., St. Louis, MO, US). All other chemicals used were obtained from Beijing chemical works (Beijing, China).
Preparation of stock solution
WPI stock solution (20%, w/v) was prepared by dissolving accurately weighed whey protein powder slowly into deionized water with the help of magnetic stirring for 2 h at room temperature. The stock solution was stored at 4 °C overnight for further experiments.
Preparation of heating treated samples
Dry heating samples preparation
WPI stock solution was diluted to 10% (w/v) with deionized water, and then mixed with inulin powder to reach the final concentration of 8% (w/v) with the weight ratio of 5:4 by WPI to inulin under the condition of magnetic stirring. Sodium azide (0.02%, w/w) was added as a bactericide. The mixture solution was adjusted to pH 7.0 using sodium hydroxides (2 N) and then lyophilized for 24 h at 0.34 bar at − 54 °C using a freeze drier (Alpha 1–2, Mrtin Christ Corp., Germany). The obtained powder was incubated in an incubator (Spx-250B-Z, Shanghai Boxun Medical Biological Instrument Co., Ltd, China) at 60 °C for 5 days at a relative humidity of 63%. A low temperature of 60 °C (below the denaturation temperature of WPI) was selected to minimize the loss in protein quality (Nasirpour et al., 2006). The humidity (63%) which fell into the optimum water activity (Aw) range of 0.5–0.8 for Maillard reaction (Boekel, 2001) was obtained by using saturated potassium iodide solution at 60 °C, which was 63% (Yap et al., 2007). Dry-heated WPI (10%, w/v) without inulin was conducted in the same way. Dry heated samples were dissolved with deionized water to their original concentrations for further analysis.
Wet heating samples preparation
Diluted whey protein solution (10%, w/v) with or without inulin (8%, w/v) were adjusted to pH 7.0 and then heated at 85 °C for 30 min under the condition of magnetic stirring. The wet heating samples were obtained by cooling rapidly to room temperature.
Combined heating samples preparation
Dry-heated whey protein and mixture of whey protein and inulin were rehydrated to original volumes, and then heated at 85 °C for 30 min. After heating, all samples were cooled to room temperature quickly.
Determination of absorbance
Reconstituted suspensions of dry-heated samples and/or wet heated samples were determined for absorbance values at 290 and 420 nm in cuvettes (1 cm optical path length) by a UV–visible spectrophotometer (UV2550, Shimadzu Co., Ltd., Tokyo, Japan).
Particle size and zeta potential measurement
Particle size and zeta potential of all samples were measured according to our previous study (Zhang et al. 2019). Briefly, all samples were diluted to 1% (w/v) concentration with ultrapure water, and then measured for particle size and zeta potential by a Zetasizer model Nano-Z analyzer (Malvern Instruments Ltd, Worcestershire, UK). Particle size and zeta potential data analysis were performed according to Stokes–Einstein equation and Henry equation by the installed software of instrument, respectively.
Determination of free sulfhydryl group content
The free sulfhydryl group content of all samples was determined with Ellman’s reagent DTNB method by a UV–visible spectrophotometer (UV2550, Shimatzu Co., Ltd., Tokyo, Japan). The free sulfhydryl group content was calculated as the following equation:
where A412 = absorbance value, D = dilution factor, C = protein concentration (g/100 mL), 73.53 is obtained from 106/1.36 × 104, 1.36 × 104 was molar extinction coefficient.
Surface hydrophobicity measurement
All samples were diluted to the concentration of 0.005% (w/v). The surface hydrophobicity was determined by measuring the fluorescence intensity using ANS probe method by a Spectrofluorometer (RF-5301PC, Shimatzu Co., Ltd., Tokyo, Japan). ANS probe (20 μL, 8 mM) was added into 4 mL sample and then allowed to react in dark for 20 min before determination. Emission spectra were recorded from 400 to 700 nm at an excitation wavelength of 390 nm.
Data analysis
All data obtained from experiments were expressed as mean ± standard deviation (S.D.). The significant differences of data between samples and the control were calculated by using Version SPSS 20 (SPSS Inc., Chicago, IL, US). The significance level was set at p < 0.05. Data were checked for homogeneity by Leveneǐs test. When the data were homogeneous, one-way analysis of variance (ANOVA) was carried out and then a least squared differences (LSD) model was used. All the figures were drawn by origin 8.0 (Origin Lab Corp., Northampton, USA).
Results and discussion
Changes in absorbance of whey protein after heating in absence or presence of inulin
At the beginning of Maillard reaction, the hydrophilic reducing sugar pyrolyzed into ketone and aldehydes, which have the characteristic absorption peaks at 290 nm (Lertittikul et al., 2007). At the advanced stage, brown pigments called melanoidins can be produced and have characteristic absorption peaks at 420 nm (Rufián-Henares et al., 2006). Proteins containing aromatic amino acids also have a peak at about 280 nm (Zhang and Zhong, 2012). At lower turbidity and particle size, 400 nm is more sensitive for whey protein particles (Ryan et al., 2012). Therefore, absorbance at 290 and 400 nm of all the reconstituted suspension and solutions were determined and the results are shown in Fig. 1.
Fig. 1.
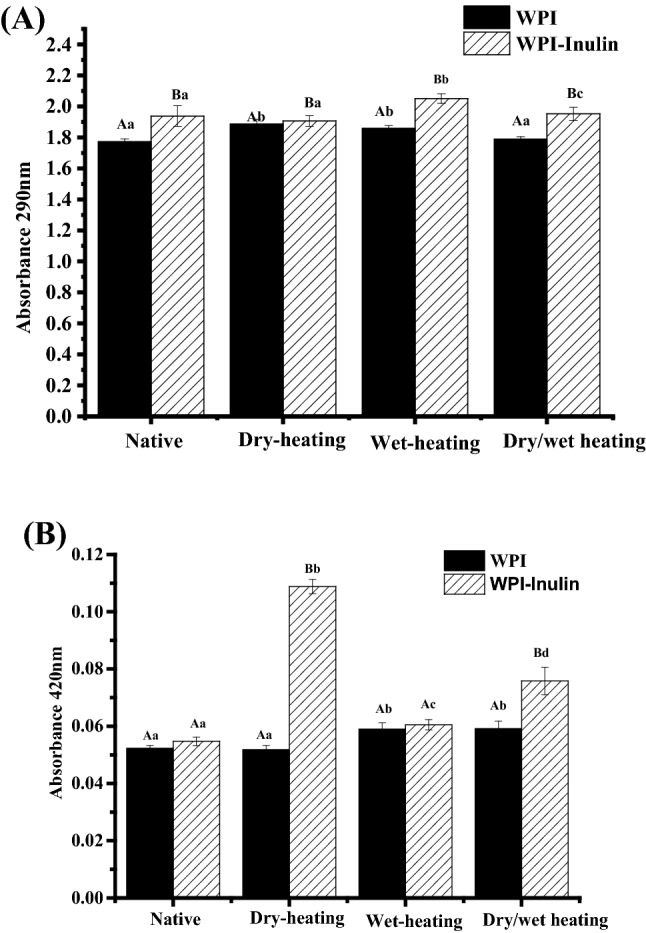
Changes in absorbance of whey protein after heating in absence or presence of inulin. Note: Different uppercase letters mean significant difference between WPI and WPI-Inulin samples. Different lower case letters denote significance between samples treated with different heating methods. The significant level is p < 0.05
Compared with whey protein at native state, dry-heating and combined dry/wet heating increased the absorbance of whey protein at 290 nm significantly (p < 0.05). According to manufacturer, the whey protein powder contains 0.7% (w/w) lactose monohydrate. Therefore, whey protein alone showed increased absorbance at 290 nm may due to the binding of residual lactose molecules induced by dry-heating. Similar results were reported by Yu et al. (2015) who found that the residual lactose in whey protein powder can be bonded to camel whey proteins during dry heating process. Schong and Famelart (2018) also reported the dry-heating induced MR of whey protein as a result of the presence of residual free lactose in the powder. In addition to the reason of MR, Qi et al. (2017) attributed the increased absorbance at about 280 nm to the denaturation and aggregation of whey proteins caused by heating. Whey protein after wet-heating treatment showed increased absorbance at 290 nm. Wet heating largely shortens the reaction time compared with dry heating, but high temperatures might cause protein aggregation and low degree of glycation (Qi et al., 2017). It was well known that whey protein at the 10% level underwent denaturation and aggregation when heated at 85 °C for 30 min (Sakandar et al., 2014), which may be responsible for the increased absorbance.
In native state, after mixing, WPI-Inulin showed significantly higher absorbance value at 290 nm may be due to the hydrophobic interaction of whey protein and inulin which increased the size of the mixture (Guo et al., 2018). As for different method, all WPI-Inulin mixtures showed significantly higher values than the respective WPI controls (p < 0.05), suggesting the interactions between the two substances induced by heating.
As shown in Fig. 1B, all samples showed significantly lower absorbance at 400 nm than at 290 nm (p < 0.05). For WPI only, dry-heating at 60 °C for 5 days did not improve the absorbance value at 400 nm while wet heating at 85 °C for 30 min improved the value significantly (p < 0.05). Influence of inulin on the absorbance of system are dependent of the heating type. For both native and wet-state heating conditions, presence of inulin did not affect the absorbance. However, under conditions of dry-heating, WPI-Inulin improved the absorbance value drastically (p < 0.05), indicating the development of advanced MR products. Similar results have been reported that obviously high absorbance value at 420 nm was observed for mixture of whey protein and dextran after dry-heating at 60 °C for 5 days (Laura et al., 2005) and 7 days (Sun et al., 2011) in comparison with whey protein alone.
Changes in particle size of whey protein after heating in absence or presence of inulin
Whey protein aggregates and glycosylated whey protein can be measured by the parameter of diameter. Dynamic Light Scattering (DLS) based on Brownian motion was used to measure this parameter and the results are shown in Fig. 2. Polydispersity Index (PDI) refers to the homogeneity of the system and the value can be considered as the applicability for determination. Sponton et al. (2015) pointed out that PDI values from 0.05 to 0.7 correspond to monodisperse system. Figure 2A indicated that all samples showed PDI values in the range of 0.42 ± 0.01 to 0.59 ± 0.01, which were monodisperse system with relatively narrow size distribution. Native samples showed low PDI values may due to the spherical shape and impact structure of whey proteins. Samples after wet heating treatment displayed significantly higher PDI values than those under other heating methods (p < 0.05). Wet-heating caused a less homogeneous system with increased PDI values may be due to the denaturation and generation of heat-induced strands at neutral pH in the system with broader size distribution (Li and Zhong, 2016; Mee et al., 2013). Presence of inulin increased the PDI values of system in the case of wet-heating and combined dry/wet heating. Similar results were reported by Ha et al. (2016) that increasing inulin level increased the PDI values of the mixture solution of whey protein and inulin after heating at 70 °C for 10 min.
Fig. 2.
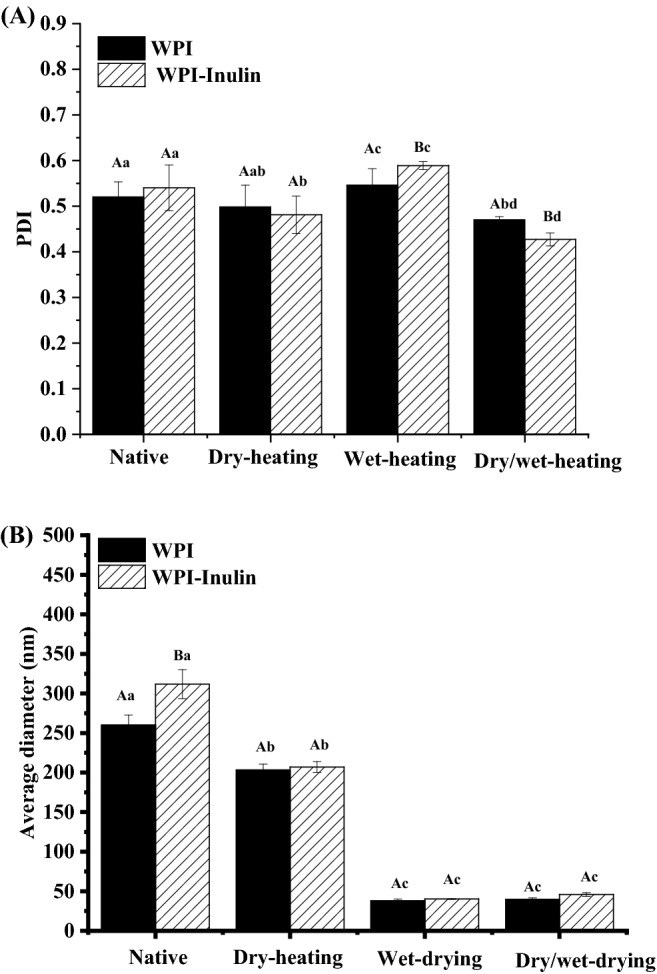
Changes in particle size of whey protein after heating in absence or presence of inulin. Note: Different uppercase letters mean significant difference between WPI and WPI-Inulin samples. Different lower case letters denote significance between samples treated with different heating methods. The significant level is p < 0.05
Figure 2B showed the average diameter of all reconstituted suspensions and solution samples (Also see Table S1). Compared with native samples, all treated samples showed significantly lower diameters (p < 0.05) may be due to the decreased hydro-layer of the molecules (Bech, 1981). Dry heated whey protein showed significantly different diameter in comparison with native sample, suggesting the MR and possible denaturation of whey protein during heating at 60 °C for 5 days. Protein dry heating is intensively used in food industry for viral and microbial decontamination of proteins and denaturation and aggregation of proteins may be noticed (Muhammad et al., 2012). Generally, samples after dry heating had significantly higher diameters than samples after wet heating treatment due to the thicker water layer around the protein molecules (p < 0.05). No significant difference was observed between samples after wet-heating and combined heating methods (p > 0.05), indicating that obvious whey protein aggregation may not occur when incubated at 60 °C for 5 days.
Native whey protein displayed mean diameter of 259.98 ± 12.75 nm and mixing with inulin resulted in a significantly higher diameter of 311.74 ± 18.43 nm (p < 0.05). The possible reason maybe that mixing whey protein and inulin formed a complex mainly via hydrogen bonding in solution (Ha et al., 2016). In other circumstances, presence of inulin increased the diameter of systems slightly compared with WPI alone. However, the difference was not significant. Our previous study also showed that inulin addition at the level of 5% did not affect the diameter of whey protein (8%) significantly after heating together at 85 °C for 30 min (Guo et al., 2018). The reason may be that inulin incorporating into whey protein peptide chain or absorbing on the surface of whey protein was not enough to generate significant changes.
Changes in zeta potential of whey protein after heating in absence or presence of inulin
The surface charge density of all reconstituted samples and solutions was determined and evaluated by zeta potential and the results are shown in Fig. 3 (Also see Table S1). According to general colloid chemistry principles, a dispersed system typically loses stability when the magnitude of the zeta potential is lower than approximate 30 mV (Bourbon et al., 2016). Native whey protein solution had a zeta potential of − 24.71 ± 1.83 mV and all the three heating treatments increased the absolute zeta potential values. Similar increased zeta potential for camel whey protein after dry-heating was found by Shima et al. (2018). Wet-drying method increased the absolute zeta potential of whey protein solution was also reported by Sun et al. (2018). Wet heating increasing the absolute zeta potential to a greater extent than dry-heating method, suggesting that wet-heating may cause exposure of more charged amino acid groups of whey proteins.
Fig. 3.
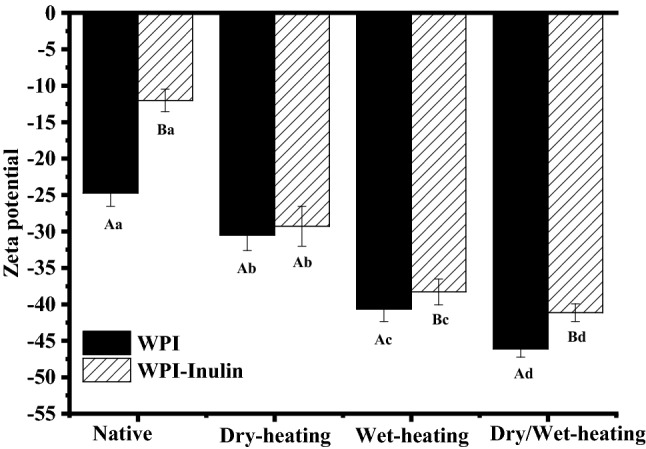
Changes in zeta potential of whey protein after heating in absence or presence of inulin. Note: Different uppercase letters mean significant difference between WPI and WPI-Inulin samples. Different lower case letters denote significance between samples treated with different heating methods. The significant level is p < 0.05
Mixture of WPI and inulin showed the similar trend related to heating method with WPI alone. Presence of inulin (− 12.02 ± 1.55 mV) reduced zeta potential magnitude of mixture may via shielding the surface charge of whey protein (Fig. 3). Similar results were reported by Tseng et al. (2008). Compared with native whey protein and inulin mixture solutions, dry heating and/or wet heating samples with or without inulin showed more stable properties revealed by the more negative zeta potentials. Samples undergone heat treatment at 85 °C for 30 min showed more negative values in the range of − 40 ~ 45 mV than samples after dry heating (p < 0.05), indicating a more stable state of the system.
Changes in free sulfhydryl group content of whey protein after heating in absence or presence of inulin
β-Lactoglobulin has one free sulfhydryl group, which is mainly responsible for whey proteins aggregation (Setiowati et al., 2017). Free sulfhydryl group content of all reconstituted suspension and solutions was determined and the results are shown in Fig. 4 (Also see Table S1). Compared with native samples, all heat-treated samples showed decreased free sulfhydryl group content due to the denaturation and aggregation of whey proteins (Visschers and de Jongh, 2005). For whey protein alone, wet-heated samples showed lower free sulfhydryl group content than dry-heated samples, suggesting the dominant denaturation and aggregation in the wet-state reaction. In presence of inulin, the free sulfhydryl group content was decreased for all heat-treated samples, suggesting that inulin may have enhanced the degree of whey protein aggregation under heating treatment.
Fig. 4.

Changes in free sulfhydryl group content of whey protein after heating in absence or presence of inulin. Note: Different uppercase letters mean significant difference between WPI and WPI-Inulin samples. Different lower case letters denote significance between samples treated with different heating methods. The significant level is p < 0.05
Changes in surface hydrophobicity of whey protein after heating in presence of inulin
Surface hydrophobicity is an indicator to the protein conformation change (Yang et al., 2018). Surface hydrophobicity of whey protein expressed as the ANS fluorescence intensity was determined and the results are shown in Fig. 5. Wet heating increased the surface hydrophobicity of whey protein, as shown in Fig. 5. As protein unfolds, more hydrophobic groups were exposed to the solvent, and the fluorescence intensity was increased. Dry-heating and combined heating decreased the fluorescence intensity. Decreased surface hydrophobicity for whey protein powder after spray drying was also reported by Nishanthi et al. (2017).
Fig. 5.
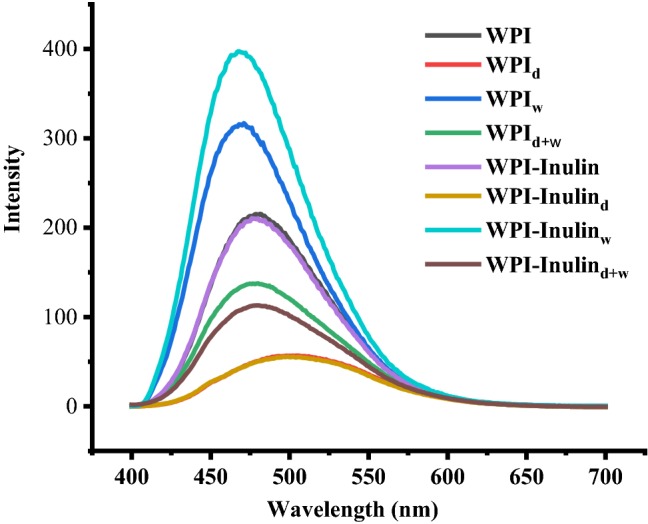
Changes in surface hydrophobicity of whey protein after heating in presence of inulin. Note: Samples with subscript “d” means samples dry-heated at 65 °C for 5 days; subscript “w” means samples heated at 85 °C for 30 min
In native and dry-heating state, the mixture of whey protein and inulin showed almost overlapped curves with that of controlled whey protein samples. Wet-heated whey protein in presence of inulin increased the surface hydrophobicity pronouncedly, indicating that inulin affected the conformation of whey protein more greatly. In combined heating condition, presence of inulin decreased the surface hydrophobicity. Obviously, mixture samples after heating at 85 °C for 30 min exhibited significantly higher hydrophobicity than samples incubated at 60 °C for 5 days (p < 0.05).
In conclusion, whey protein had physicochemical properties changes during heating process depending on the heating type and inulin presence. All the changes induced by heating may be result of whey protein denaturation and aggregate as well as Maillard reaction or hydrophobic interaction with inulin in systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The financial support for this project was provided by the Education Department of Jilin Province (JJKH20180170KJ).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Feng Gao, Email: g_f@jlu.edu.cn.
Xuefei Zhang, Email: zhangxf16@mails.jlu.edu.cn.
Hao Wang, Email: jlu_wh@126.com.
Xiaomeng Sun, Email: sunxm15@mails.jlu.edu.cn.
Jiaqi Wang, Email: jiaqi16@mails.jlu.edu.cn.
Cuina Wang, Phone: +86 431 87836362, Email: wangcuina@jlu.edu.cn.
References
- Bech AM. physical and chemical properties of whey proteins. Dairy Ind. Int. (1981)
- Boekel MA. Kinetic aspects of the Maillard reaction: a critical review. Die Nahr. 2001;45:150–159. doi: 10.1002/1521-3803(20010601)45:3<150::AID-FOOD150>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Bourbon AI, Pinheiro AC, Cerqueira MA, Vicente AA. Influence of chitosan coating on protein-based nanohydrogels properties and in vitro gastric digestibility. Food Hydrocolloid. 2016;60:109–118. doi: 10.1016/j.foodhyd.2016.03.002. [DOI] [Google Scholar]
- Bryant CM, Mcclements DJ. Ultrasonic spectrometry study of the influence of temperature on whey protein aggregation. Food Hydrocolloids. 1999;13:439–444. doi: 10.1016/S0268-005X(99)00018-1. [DOI] [Google Scholar]
- Guo MR, Wang H, Wang CN. Interactions between whey protein and inulin in a model system. J. Food Sci. Tech. 2018;55:4051–4058. doi: 10.1007/s13197-018-3331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HK, Jeon NE, Jin WK, Han KS, Yun SS, Lee MR. Physicochemical characterization and potential prebiotic effect of whey protein isolate/inulin nano complex. Korean J. Food Sci. Anim. Res. 2016;36:267–274. doi: 10.5851/kosfa.2016.36.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havea P, Carr AJ, Creamer LK. The roles of disulphide and non-covalent bonding in the functional properties of heat-induced whey protein gels. J. Dairy Res. 2004;71:328–330. doi: 10.1017/S002202990400024X. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Režek A, Lelas V, Krešić G, Franetović M. Effect of carbohydrates on the emulsifying, foaming and freezing properties of whey protein suspensions. J. Food Eng. 2007;79:279–286. doi: 10.1016/j.jfoodeng.2006.01.055. [DOI] [Google Scholar]
- Komatsu TR, Buriti FCA, Silva RCD, Lobo AR, Colli C, Gioielli LA, Saad SMI. Nutrition claims for functional guava mousses produced with milk fat substitution by inulin and/or whey protein concentrate based on heterogeneous food legislations. LWT - Food Sci. Technol. 2013;50:755–765. doi: 10.1016/j.lwt.2012.07.013. [DOI] [Google Scholar]
- Laura JC, Mar V, Pedro J, Martín-Álvarez, Agustín O, Rosina LF. Effect of the dry-heating conditions on the glycosylation of β-lactoglobulin with dextran through the Maillard reaction. Food Hydrocolloids. 2005;19:831–837. doi: 10.1016/j.foodhyd.2004.10.033. [DOI] [Google Scholar]
- Lertittikul W, Benjakul S, Tanaka M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein-glucose model system as influenced by pH. Food Chem. 2007;100:669–677. doi: 10.1016/j.foodchem.2005.09.085. [DOI] [Google Scholar]
- Li K, Zhong Q. Aggregation and gelation properties of preheated whey protein and pectin mixtures at pH 1.0–4.0. Food Hydrocolloids. 2016;60:11–20. doi: 10.1016/j.foodhyd.2016.03.009. [DOI] [Google Scholar]
- Mee RL, Ha NC, Ho KH, Won JL. Production and characterization of beta-lactoglobulin/alginate nanoemulsion containing coenzyme Q10: Impact of heat treatment and alginate concentrate. Korean J. Food Sci. Anim. Sci. 2013;33:67–74. doi: 10.5851/kosfa.2013.33.1.67. [DOI] [Google Scholar]
- Muhammad G, Valérie L, Saïd B, Thomas C. The physicochemical parameters during dry heating strongly influence the gelling properties of whey proteins. J. Food Eng. 2012;112:296–303. doi: 10.1016/j.jfoodeng.2012.05.006. [DOI] [Google Scholar]
- Nasirpour A, Scher J, Desobry S. Baby foods: formulations and interactions (a review) Crit. Rev. Food Sci. 2006;46:665–681. doi: 10.1080/10408390500511896. [DOI] [PubMed] [Google Scholar]
- Nicolai T, Britten M, Schmitt C. β-Lactoglobulin and WPI aggregates: formation, structure and applications. Food Hydrocolloid. 2011;25:1945–1962. doi: 10.1016/j.foodhyd.2011.02.006. [DOI] [Google Scholar]
- Nishanthi M, Chandrapala J, Vasiljevic T. Compositional and structural properties of whey proteins of sweet, acid and salty whey concentrates and their respective spray dried powders. Int. Dairy J. 2017;74:49–56. doi: 10.1016/j.idairyj.2017.01.002. [DOI] [Google Scholar]
- Perusko M, Al-Hanish A, Velickovic TC, Stanic-Vucinic D. Macromolecular crowding conditions enhance glycation and oxidation of whey proteins in ultrasound-induced Maillard reaction. Food chem. 2015;177:248–257. doi: 10.1016/j.foodchem.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Poinot P, Arvisenet G, Gruapriol J, Fillonneau C, Lebail A, Prost C. Influence of inulin on bread: kinetics and physico-chemical indicators of the formation of volatile compounds during baking. Food chem. 2010;119:1474–1484. doi: 10.1016/j.foodchem.2009.09.029. [DOI] [Google Scholar]
- Qi PX, Xiao Y, Wickham ED. Stabilization of whey protein isolate (WPI) through interactions with sugar beet pectin (SBP) induced by controlled dry-heating. Food Hydrocolloids. 2017;67:1–13. doi: 10.1016/j.foodhyd.2016.12.032. [DOI] [Google Scholar]
- Rufián-Henares JÁ, Guerra-Hernandez E, García-Villanova B. Colour measurement as indicator for controlling the manufacture and storage of enteral formulas. Food Control. 2006;17:489–493. doi: 10.1016/j.foodcont.2005.02.011. [DOI] [Google Scholar]
- Ryan KN, Vardhanabhuti B, Jaramillo DP, Zanten JHV, Coupland JN, Foegeding EA. Stability and mechanism of whey protein soluble aggregates thermally treated with salts. Food Hydrocolloids. 2012;27:411–420. doi: 10.1016/j.foodhyd.2011.11.006. [DOI] [Google Scholar]
- Sakandar HA, Imran M, Huma N, Ahmad S, Aslam HKW, Azam M, Muhammad S. Effects of polymerized whey proteins isolates on the quality of stirred yoghurt made from camel milk. J. Food Process. Technol. 2014;5:172–177. [Google Scholar]
- Setiowati AD, Saeedi S, Wijaya W, Meeren PVD. Improved heat stability of whey protein isolate stabilized emulsions via dry heat treatment of WPI and low methoxyl pectin: effect of pectin concentration, pH, and ionic strength. Food Hydrocolloids. 2017;63:716–726. doi: 10.1016/j.foodhyd.2016.10.025. [DOI] [Google Scholar]
- Schong E, Famelart MH. Dry heating of whey proteins leads to formation of microspheres with useful functional properties. Food Res. Int. 2018;113:210–220. doi: 10.1016/j.foodres.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Shima M, Maryam S, Farhadalavia, Zahra ED, Elnaz H, Nader S, Ali AMM. Effect of dry heating on physico-chemical, functional properties and digestibility of camel whey protein. Int. Dairy J. 2018;86:9–10. doi: 10.1016/j.idairyj.2018.06.015. [DOI] [Google Scholar]
- Sołowiej B, Glibowski P, Muszyński S, Wydrych J, Gawron A, Jeliński T. The effect of fat replacement by inulin on the physicochemical properties and microstructure of acid casein processed cheese analogues with added whey protein polymers. Food Hydrocolloids. 2015;44:1–11. doi: 10.1016/j.foodhyd.2014.08.022. [DOI] [Google Scholar]
- Spotti MJ, Perduca MJ, Piagentini A, Santiago LG, Rubiolo AC, Carrara CR. Gel mechanical properties of milk whey protein–dextran conjugates obtained by Maillard reaction. Food Hydrocolloids. 2013;31:26–32. doi: 10.1016/j.foodhyd.2012.08.009. [DOI] [Google Scholar]
- Sponton OE, Perez AA, Carrara CR, Santiago LG. Linoleic acid binding properties of ovalbumin nanoparticles. Colloids. Surf. B. 2015;128:219–226. doi: 10.1016/j.colsurfb.2015.01.037. [DOI] [PubMed] [Google Scholar]
- Sun WW, Yu SJ, Yang XQ, Wang JM, Zhang JB, Zhang Y. Study on the rheological properties of heat-induced whey protein isolate–dextran conjugate gel. Food Res. Int. 2011;44:3259–3263. doi: 10.1016/j.foodres.2011.09.019. [DOI] [Google Scholar]
- Sun XM, Wang CN, Guo MR. Interactions between whey protein or polymerized whey protein and soybean lecithin in model system. J. Dairy Sci. 2018;101:9680–9692. doi: 10.3168/jds.2018-14998. [DOI] [PubMed] [Google Scholar]
- Tseng YC, Xiong YL, Boatright WL. Effects of inulin/oligofructose on the thermal stability and acid-induced gelation of soy proteins. J. Food Sci. 2008;73:44–50. doi: 10.1111/j.1750-3841.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Visschers RW, de Jongh HH. Disulphide bond formation in food protein aggregation and gelation. Biotechnol. Adv. 2005;23:75–80. doi: 10.1016/j.biotechadv.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang WQ, Bao YH, Chen Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013;139:355–361. doi: 10.1016/j.foodchem.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Yang JQ, Liu GY, Zeng HB, Chen LY. Effects of high pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocolloids. 2018;83:275–286. doi: 10.1016/j.foodhyd.2018.05.020. [DOI] [Google Scholar]
- Yap M, Wong P, Kitts D. Comparison of physicochemical and antioxidant properties of monosaccharide and oligosaccharide modified egg white proteins, Chinese Institute of Food Science and Technology. 314–315 (2007)
- Yu SX, Mu TH, Zhang M, Ma MM, Zhao ZK. Effects of retrogradation and further acetylation on the digestibility and physicochemical properties of purple sweet potato flour and starch. Starch Stärke. 2015;67:892–902. doi: 10.1002/star.201500055. [DOI] [Google Scholar]
- Zhang XF, Sun XM, Gao F, Wang JQ, Wang CN. Systematical characterization of physicochemical and rheological properties of thermal induced polymerized whey protein: properties of thermal induced polymerized whey protein. J. Sci. Food Agric. 2019;99:923–932. doi: 10.1002/jsfa.9264. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhong QX. Binding between bixin and whey protein at pH 7.4 studied by spectroscopy and Isothermal Titration Calorimetry. J. Agric. Food Chem. 2012;60:1880–1886. doi: 10.1021/jf2050262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


