Abstract
The anti-skin inflammatory activities of rose petal extracts have been described in our previous study. Because skin inflammation is closely linked to skin aging, our study investigated the effects of Rosa gallica petals on skin aging-related activities such as skin whitening and anti-wrinkle properties. Each sample was prepared via extraction using different ethanol ratios with the objective of evaluationg optimal extraction conditions for industrial application. Aqueous 50% (v/v) EtOH extract of R. gallica petal significantly suppressed tyrosinase activity, melanin production, and solar UV-induced matrix metalloproteinase-1, a hall mark of wrinkle formation. In addition, the aqueous 50% (v/v) EtOH extract showed the highest antioxidative effect and had highest flavonoid contents, consistent with the reported anti-aging effects. Overall, our findings suggest that R. gallica petals extracts exhibit anti-aging effects. Furthermore, 50% EtOH extraction, in particular, was optimal for the highest anti-aging, and anti-oxidative effects as well as to obtain the highest flavonoid content.
Keywords: Rosa gallica, Skin aging, Flavonoid, Anti-oxidative effect
Introduction
Skin plays a vital role as a protective barrier against harmful factors associated with heredity and genetics, environmental issues, hormonal changes, and metabolic processes (Mukherjee et al., 2006). Among these, environmental factors, such as exposure to solar ultraviolet (UV) radiation, act as key mediators that contribute to premature aging (Laga and Murphy, 2009). Main symptoms of skin-aging, which occurs as a result of photo-aging, included deep wrinkles, abnormal pigmentation and elasticity (Farage et al., 2013; Wlaschek et al., 2001).
Pigmentation is a symptom of aging, which is caused by an abnormal production of melanin, resulting in a variety of skin disorders including freckles, melasma, age spots, and other hyperpigmentation syndromes (D’Mello et al., 2016; Seo et al., 2003). In the melanogenesis pathway, tyrosinase is important as the rate-limiting enzymes that converts l-tyrosine to l-DOPA and oxidizes l-DOPA to form DOPA-quinone (Akhtar et al., 2015). Therefore, inhibition of tyrosinase is strongly associated with melanin synthesis. Moreover, wrinkle formation, caused by the loss of collagen fibrils and elastase, is another characteristic of photoaging. Matrix metalloproteinase-1 (MMP-1), secreted by human skin fibroblasts, is mainly responsible for collagen degradation during the photo-aging process (Pandel et al., 2013). Downregulation of MMP-1 expression, which inhibits collagen degradation, may enhance wrinkle-improving functions.
For the above stated reasons, the use of tyrosinase inhibitors or MMP inhibitors is considered a promising strategy for the alleviation of skin photo-aging. Previous studies have indicated that retinol, garlic extract (caffeine acid, and S-ally cysteine) and phytoceramide, kojic acid, arbutin, and vitamin C may act as skin aging inhibitors (Couteau and Coiffard, 2016; Kim et al., 2013). However, the use of these substance involves certain limitations such as various side effects including cytotoxicity, odor, and coloration (Yamakoshi et al., 2003). Therefore, current studies are focused on the development of safer, naturally-derived components which confer effective skin photo-protection.
Rosa species, grown worldwide, are considered as a good source of dietary supplements. Rose petal extract (RPE), which contains elements such as phenolic acid, flavonols and anthocyanins, have been reported to exhibit many beneficial effects, such as anti-skin inflammatory activities, in addition to other biological roles (Bitis et al., 2017; Lee et al., 2018; Masek et al., 2017; Navarro-Gonzalez et al., 2015). Because these properities of RPE are known to be related to skin aging, it has been proposed as a potential candidate for skin protection. However, little is known about the effect of RPE on skin aging. Additionally, water and ethanol are considered to be the best extraction solvents because of polarity differences and safety of use (Abarca-Vargas et al., 2016). Thus, samples with different ratios of water and ethanol (0, 10, 30, 50, 70, 90, and 100% ethanol RPE) were prepared via extraction.
The purpose of this study was to investigate the effect of RPE on skin whitening and wrinkle improvement. RPE extracts with different extraction solvent ratios (0, 10, 30, 50, 70, 90 and 100% ethanol/water solvents) were tested to determine the optimal solvent ratio for extraction.
Materials and methods
Reagent
Rosa gallica petals were imported from Turkey through GN Bio (Gyeonggi, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), Penicillin–streptomycin–neomycin, and 0.5% trypsin–EDTA were purchased from GIBCO® Invitrogen (Auckland, NZ, USA). Specific antibodies against tyrosinase and β-actin were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). Primary antibody of MMP-1 was obtained from R&D systems™. All other chemicals, including alpha-melanocyte stimulating hormone (α-MSH), mushroom tyrosinase, and l-DOPA (l-3,4-dihydroxyphenylalanine) were purchased from Sigma-Aldrich Co., LLC (St. Louis, MO, USA).
Sample preparation
Rose petals were mixed with 100 mL of 0, 10, 30, 50, 70, 90, and 100% (v/v) EtOH (absolute ethanol). Soluble components were then extracted in 80 °C water using a reflux condenser. The extract was filtered through filter paper number 2 (Whatman, Maidstone, England), vacuum-concentrated and subsequently dissolved in distilled water and freeze-dried, to be used as samples for the functional analysis test.
Cell culture
B16F10 melanoma cells were purchased from the Korean Cell Line Bank (Seoul, Korea). Human dermal fibroblast (HDF) cells were obtained from Dr. Jin Ho Chung (College of Medicine, Seoul National University, Seoul, Korea). Both cells were cultured in DMEM supplemented with 10% fetal bovine serum (v/v) and 1% (v/v) penicillin under 37 °C, and 5% CO2 conditions.
Cell viability
Cell viability was measured via MTS [3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]assay. Cells were seeded on 96-well plates and grown until confluent. Then, cells were starved with serum-free DMEM overnight and treated with the samples at indicated concentrations for 24 h. After exposure to the samples, 20 μL of MTS solution was allowed to react with the cells for 1 h. Absorbance was measured using a microplate reader (Sunrise-Basic Tecan, Tecan Austria GmbH Grödig, Austria) at 570 nm.
In vitro mushroom tyrosinase activity
The in vitro tyrosinase assay was conducted according to methods described previously (Kim et al., 2015). In brief, 40 µL of each sample was added to the assay buffer (20 µL of 0.1 M potassium phosphate), followed by incubation for 30 min with 20 µL of mushroom tyrosinase (0.02 mg/mL). Then, 40 µL of substrate (l-DOPA) was added to each mixture. The reaction was allowed to proceed at room temperature for 15 min before the formation of dopachrome was analyzed by measuring absorbance at 475 nm using a microplate reader (Infinite®2000 PRO, Tecan, Switzerland).
Evaluation of melanin production
The melanin production assay was performed according to previously described-protocol (Friedmann and Gilchrest, 1987; Gordon et al., 1989). B16F10 cells (8 × 103 cells) were seeded on 6-well plates with 2 mL culture media. After 24 h, samples were pretreated with the cells for 1 h, following which α-MSH (100 nM) was exposed to the cells. The cells were collected after 72 h, and melanin production was measured using a microplate reader (Infinite®2000 PRO, Tecan, Switzerland) at 495 nm.
Western blot
Protein samples were obtained from cells, using 1× Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA). Protein concentration was estimated using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, San José, CA, USA). Protein samples were loaded on to a 10% SDS–polyacrylamide gel (Bio-Rad Laboratories, Hercules, California, USA) for electrophoresis and then transferred to an Immobilon P membrane (Millipore, Billerica, MA, USA). The PVDF membrane was blocked with 5% fat-free milk for 1 h and the membrane was treated with specific primary antibody overnight at 4 °C. Protein bands were detected using a chemiluminescence detection kit (GE Healthcare, NJ, USA) after hybridization with an HRP-conjugated secondary antibody (Cell Signaling).
DPPH radical scavenging assay
DPPH radical scavenging activity was measured as follows; 0.2 mL each of extract was added to 3 mL of -ethanol, to which 0.8 mL of 400 μM DPPH dissolved in ethanol was added. This mixture was vortexed for 10 s and maintained at room temperature for 10 min, and absorbance was measured at 517 nm (Wang et al., 1999). DPPH radical scavenging activity was expressed as a percentage of the absorbance of the group to which no DPPH was added. All experiments were replicated a minimum of 3 times.
Total flavonoid content
Total flavonoid content in the extracts was measured using the aluminum chloride method (Jia et al., 1999) which was modificated using catechin. To 100 μL of the extract, 500 μL of distilled water and 30 μL NaNO2 were added, following which 60 μL AlCl3 was added 6 min later. After 5 min, 200 μL of 1 M NaOH was added and the brought up to 1 mL with distilled water. The solution was mixed well and centrifuged at 15,928g, at 4 °C, for 5 min. After centrifugation, 200 μL of the supernatant was obtained and absorbance was measured using a microplate reader (Infinite®2000 PRO; Tecan, Switzerland) at 510 nm.
Statistical analysis
Experiments were conducted in triplicate, and data are expressed as the mean ± standard deviation (SD). Student’s t tests were used for single statistical comparisons. Statistical significance was set at (#) = p < 0.05 and (##) = p < 0.001 for comparison with the untreated control; (*) = p < 0.05 and (**) = p < 0.001 for comparison with the α-MSH-treated group.
Results and discussion
Extraction of the petals of Rosa gallica according to different solvent ratio
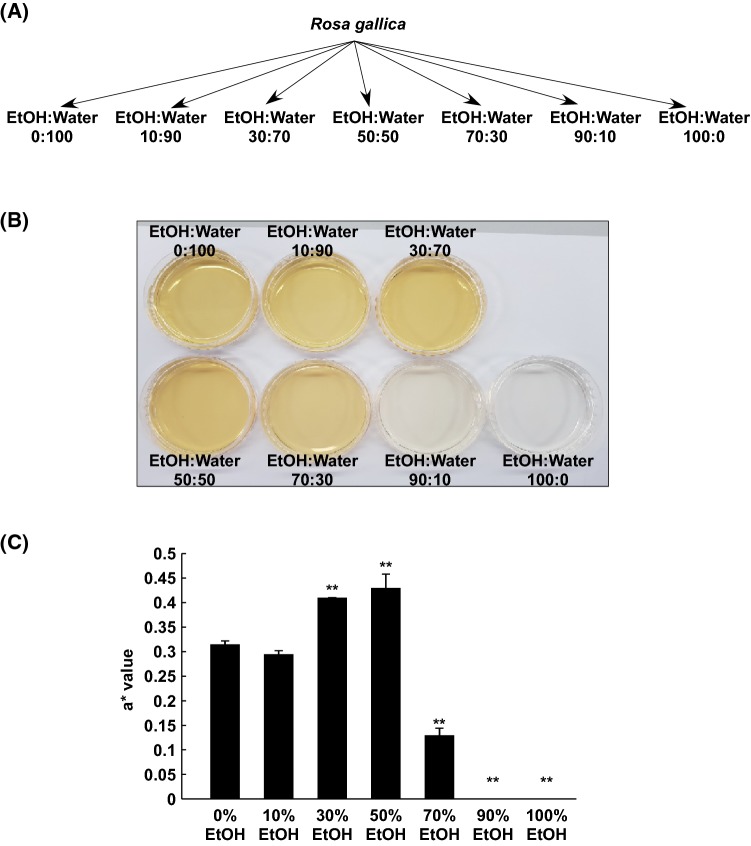
Previously RPE was produced via aqueous 70% (v/v) EtOH extraction (Lee et al., 2018). However, if RPE is intended for purposes of industrial application, extraction conditions must be optimal. In order to investigate optimal extraction conditions of RPE for anti-skin aging activity, various solvents were used for RPE (Fig. 1A). Interestingly, the color of each extract varied, based on visual observation (Fig. 1B). The color of EtOH extracts, 90% or higher, was clear, whereas 50% (v/v) EtOH extract appeared to be the most reddish. The result of colorimeter revealed an a* value for redness for 50% EtOH, which showed the highest level among the extracts (Fig. 1C).
Fig. 1.
Extraction of the petals of Rosa gallica. Sample preparation of EtOH extracts from Rosa gallica petals (A). Images of various EtOH extracts from Rosa gallica petals (B). The a* value measurement of color-difference meter (C). Data represent 3 independent experiments, which gave similar results. The (**) sign indicates a significant (p < 0.001) decrease
Effect of the extracts of rose petal on skin whitening activity
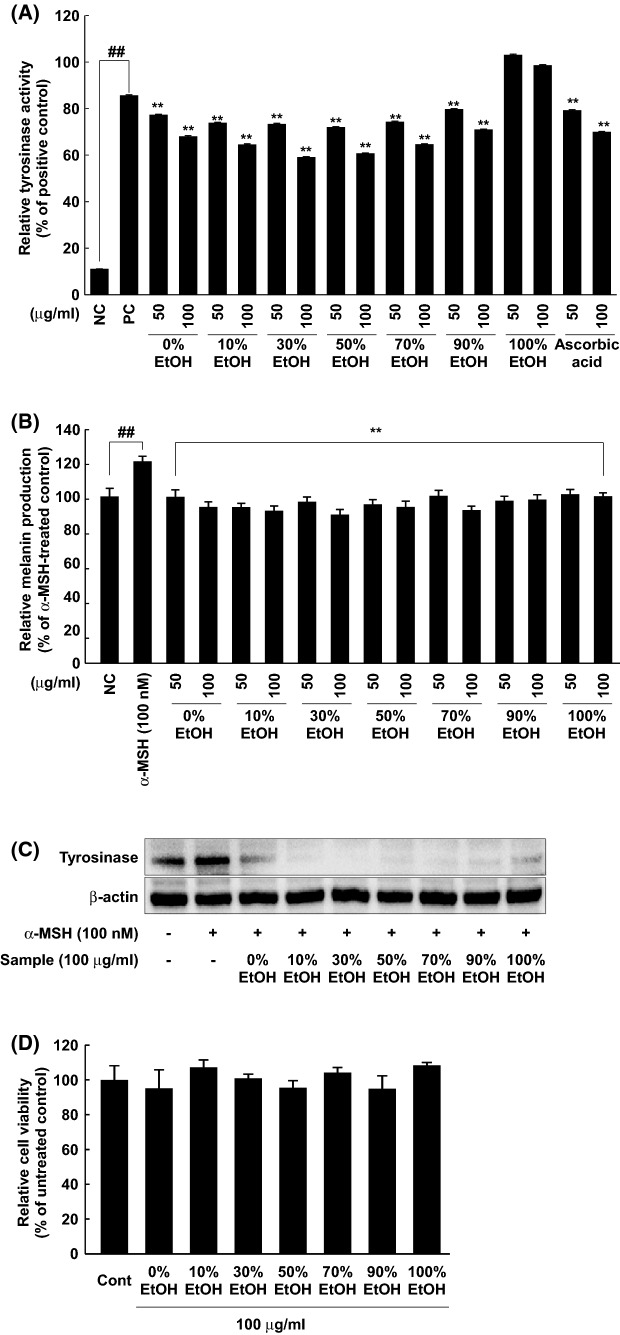
Since tyrosinase is a key enzyme involved in hyperpigmentation via melanin production (Chang, 2009), we analyzed the inhibitory effect of the extracts on tyrosinase activity in vitro. Most extracts, except 100% EtOH, revealed dose-dependent regression on in vitro tyrosinase activity (Fig. 2A). Furthermore, 30% and 50% EtOH extracts showed stronger inhibitory effects than ascorbic acid. Ascorbic acid was described as a skin whitening agents in previous literature (Chang, 2009). To evaluate skin whitening activity of the extracts in a cellular model, melanin production was assessed in B16F10 melanoma cells following treatment with each extract. A 100 nM portion of α-MSH increased melanin production and every extract of rose petal inhibited melanin production (Fig. 2B). Furthermore, 30% and 50% EtOH extracts showed the strongest inhibitory effet on tyrosinase activity and melanin production in vitro, respectively. Under similar conditions, the effect of each of the extracts on tyrosinase expression was examined. Also, α-MSH increased tyrosinase expression and 100 μg/mL each of the extracts attenuated tyrosinase expression (Fig. 2C). Treatment with extracts at 100 μg/mL did not cause cell cytotoxicity in B16F10 melanoma cells (Fig. 2D). Thus, inhibition of melanin by RPE is attributed to the dual fuction of tyrosinase activity and expression the absence of cytotoxicity.
Fig. 2.
Anti-melanogenic activity of EtOH extracts of Rosa gallica petals via suppression of tyrosinase expression/activity. The effect of the extracts on tyrosinase activity was evaluated using mushroom tyrosinase enzyme (NC, negative control; PC, positive control) (A). The effect of the extracts on melanin production was measured. Detailed procedure was described in Materials and methods (B). Tyrosinase expression was measured following treatment of the extracts. Western blot analysis was used to reveal the effect of the extracts on the tyrosinase expression (C). Cytotoxicity of the extracts in B16F10 melanoma cells was estimated. MTS assay was performed to verify non-cytotoxic concentration of the extracts as described in Materials and methods (Cont, untreated control) (D). Data represent 3 independent experiments, which gave similar results. The (##) sign indicates a significant (p < 0.001) increase, and (**) indicates a significant (p < 0.001) decrease
Effect of the RPE on solar UV (sUV)-induced MMP-1 expression in human dermal fibroblasts
Inhibitors of MMP-1 may be regarded as anti-wrinkle agents (Park et al., 2018; Yang et al., 2018). This is because MMP-1 is a key enzyme, responsible for wrinkle formation during the skin aging process (Pittayapruek et al., 2016). To determine anti-wrinkle effects of RPE, MMP-1 expression was evaluated in human dermal fibroblasts using Western blot analysis. MMP-1 expression was dramatically increased by sUV irradiation (30 kJ/cm2), and all extracts inhibited sUV-induced MMP-1 expression (Fig. 3A). Whereas 100% EtOH extracts exhibited mild effect, 50% EtOH extracts displayed the strongest suppressing activity on sUV-induced MMP-1 expression. Next, we investigated MMP-1 production levels in cultured media, because MMP-1 is a secreted protein for collagen degradation. Similar to that shown in Fig. 3A, MMP-1 level in cultured media was suppressed by each extract (Fig. 3B). In particular, 50% EtOH extract showed the highest suppressive activity on sUV-induced MMP-1 expression, and 100% EtOH extract exhibited a mild effect compared to that by other extracts.
Fig. 3.
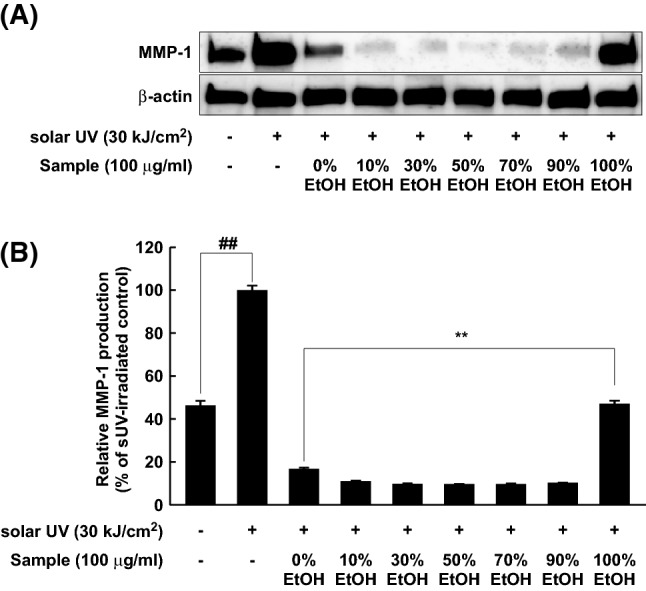
Inhibitory effect of EtOH extracts of Rosa gallica petals on sUV-induced MMP-1 expression. Each extract was allowed to react with the cells for 1 h and sUV (solar UV) was irradiated (NC, negative control). After 48 h of sUV exposure, the media and cell lysate were collected. Specific primary antibody against MMP-1 was used for MMP-1 protein detection in cell lysate (A). Detailed procedure was represented in Materials and methods. The MMP-1 production level in cultured media was evaluated by using ELISA kit for detection active MMP-1 (B). Data represent 3 independent experiments, which gave similar results. The (##) sign indicates a significant (p < 0.001) increase, and (**) indicates a significant (p < 0.001) decrease
Anti-oxidative activity and total flavonoid contents of the rose petal extracts
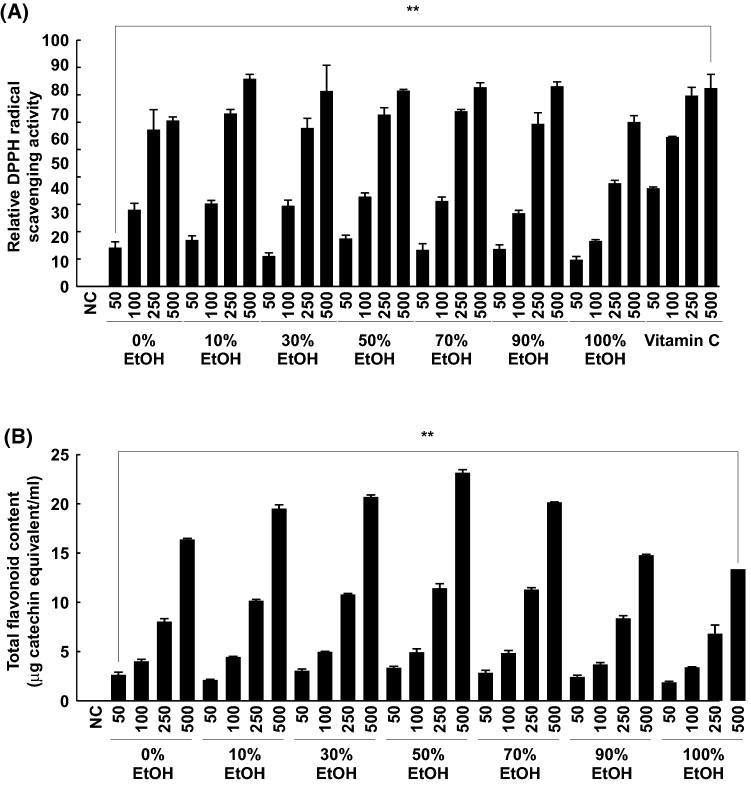
Antioxidants that inhibit ROS production are known to reduce hyperpigmentation or prevent UV-induced melanin production (Yamakoshi et al., 2003). DPPH radical scavenging activity was determined to compare the antioxidant activity of the extracts. Interestingly, every extract showed drastic, dose-dependent, radical scavenging activity. The 500 μg/mL samples showed anti-oxidative activity similar to vitamin C. Among the extracts, the 50% EtOH extract showed the strongest anti-oxidative effect at the lowest concentration (50 μg/mL) (Fig. 4A).
Fig. 4.
Anti-oxidative activity and total flavonoid content in the extracts from the petals of Rosa gallica. The anti-oxidative activity of the extracts was verified by DPPH radical scavenging assay (NC, negative control) (A). Vitamin C was used as the positive control. Total flavonoid content in the extracts was measured as described in “Materials and methods” (B). Data are representative of three independent experiments. (**) denote statistical significance (p < 0.001) as compared with controls
There are many different compounds such as terpenes, flavonoids, and anthocyanins in rose petals (Knapp et al., 1998; Kumar et al., 2008; Oka et al., 1998; Schieber et al., 2005). These chemicals represent many biological activities (Kumar et al., 2009). In particular, flavonoids have been described as the main group of phenolic compounds responsible for biological properties of antioxidant and anti-inflammatory effects (Du et al., 2016; Jung et al., 2015). Next, the total flavonoid content of each extract was measured. Large amounts of flavonoids were present in each extract (Fig. 4B). The results indicated that the 50% EtOH extract contained the highest flavonoid content among the samples. This trend was similar to that observed in the anti-oxidative assay result.
The dark red color of the 50% EtOH solvent of rose petals is assumed to be related to anthocyanin, a flavonoid (Fig. 1). This result substantiates the findings of a previous study (Oancea et al., 2012). According to the previous study, anthocyanins in blueberry (Vaccinium orymbosum L.) were best-extracted in 50% EtOH solvent than in any other solvent, including 60%, 70% and 80% EtOH (Oancea et al., 2012). Naturally, anthocyanins occur as glycosides. Thus we surmised that the polarity of the anthocyanins may have been accorded by the 50% EtOH solvent. The strong anti-oxidative activity of anthocyanins is well demonstrated in various model systems (Kalt et al., 2003; Rice-Evans et al., 1995). Because 50% EtOH rose petal extract had the highest anthocyanin content, the anti-oxidative activity of 50% EtOH extract was the strongest among the 50 μg/mL extracts.
In summary, our study demonstrated the potential of RPE as anti-skin aging ingredient. For purpose of industrial application, optimization of extraction conditions is essential in order to reduce production costs. The current study investigated solvents most suitable for Rose petal extraction, with reference to anti-skin aging activity. The flavonoids in rose petals were extracted best by the 50% EtOH solvent. This extract showed the strongest anti-oxidative activity as well as anti-skin aging activity such as skin whitening and wrinkle suppression activity. Clinical studies and stability analyses may be required to test its suitability for industrial application.
Acknowledgements
This research was supported by Main Research Program (E0183112-02) of the Korea Research Food Institute (KFRI) funded by the Ministry of Science, ICT & Future Planning and by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (118060-03-2-HD020).
Compliance with ethical standards
Conflict of interest
There is no conflict of interest among the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eun Ju Shin, Email: Shin.Eun-ju@kfri.re.kr.
Ah-ram Han, Email: lambo3@kfri.re.kr.
Myung-hee Lee, Email: mhlee83@kfri.re.kr.
Young-Ran Song, Email: Song.Young-ran@kfri.re.kr.
Kwang Min Lee, Email: kmlee@kfri.re.kr.
Tae-Gyu Nam, Email: ntg97@kfri.re.kr.
Pomjoo Lee, Email: kuempire@gmail.com.
Sung-Young Lee, Email: mbiotec@hotmail.com.
Tae-Gyu Lim, Phone: +82-63-219-9423, Email: tglim82@kfri.re.kr, Email: tglim83@kfri.re.kr.
References
- Abarca-Vargas R, Malacara CFP, Petricevich VL. Characterization of chemical compounds with antioxidant and cytotoxic activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) extracts. Antioxidants 5: 45 (2016) [DOI] [PMC free article] [PubMed]
- Akhtar MN, Sakeh NM, Zareen S, Gul S, Lo KM, Ul-Haq Z, Shah SAA, Ahmad S. Design and synthesis of chalcone derivatives as potent tyrosinase inhibitors and their structural activity relationship. J. Mol. Struct. 2015;1085:97–103. doi: 10.1016/j.molstruc.2014.12.073. [DOI] [Google Scholar]
- Bitis L, Sen A, Ozsoy N, Birteksoz-Tan S, Kultur S, Melikoglu G. Flavonoids and biological activities of various extracts from Rosa sempervirens leaves. Biotechnol. Biotechnol. Equi. 2017;31:299–303. doi: 10.1080/13102818.2016.1277956. [DOI] [Google Scholar]
- Chang TS. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteau C, Coiffard L. Overview of skin whitening agents: drugs and cosmetic products. Cosmetics. 2016;3:27. doi: 10.3390/cosmetics3030027. [DOI] [Google Scholar]
- D’Mello SA, Finlay GJ, Baguley BC, Askarian-Amiri ME. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016;17:7. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Sun G, Zhang X, Liu Y. Compararisons and correlations of phenolic profiles and anti-oxidant activities of seventeen varieties of pineapple. Food Sci. Biotechnol. 2016;25:445–451. doi: 10.1007/s10068-016-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv. Wound Care. 2013;2:5–10. doi: 10.1089/wound.2011.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J. Cell Physiol. 1987;133:88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- Gordon PR, Mansur CP, Gilchrest BA. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J. Invest. Dermatol. 1989;92:565–572. doi: 10.1111/1523-1747.ep12709595. [DOI] [PubMed] [Google Scholar]
- Jia Z, Tang MC, Wu JM. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Jung H, Lee HJ, Cho H, Lee K, Kwak HK, Hwang KT. Antocyanins in Rubus fruits and antioxidant and anti-inflamatory activities in RAW 264.7 cells. Food Sci. Biotechnol. 24: 187–1886 (2015)
- Kalt W, Lawand C, Ryan DAJ, McDonald JE, Donner H, Forney CF. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage. J. Am. Soc. Hortic. Sci. 128: 917–923 (2003)
- Kim SR, Jung YR, An HJ, Kim DH, Jang EJ, Choi YJ, Moon KM, Park MH, Park CH, Chung KW, Bae HR, Choi YW, Kim ND, Chung HY. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS One. 2013;8:e73877. doi: 10.1371/journal.pone.0073877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Baek EJ, Lee EJ, Yeom MH, Park JS, Lee KW, Kang NJ. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp. Dermatol. 2015;24:150–152. doi: 10.1111/exd.12586. [DOI] [PubMed] [Google Scholar]
- Knapp H, Straubinger M, Fornari S, Oka N, Watanabe N, Winterhalter P. (S)-3,7-dimethyl-5-octene-1,7-diol and related oxygenated monoterpenoids from petals of Rosa damascena Mill. J. Agri. Food Chem. 1998;46:1966–1970. doi: 10.1021/jf970987x. [DOI] [Google Scholar]
- Kumar N, Bhandari P, Singh B, Gupta AP, Kaul VK. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J. Sep. Sci. 2008;31:262–267. doi: 10.1002/jssc.200700372. [DOI] [PubMed] [Google Scholar]
- Kumar N, Bhandari P, Singh B, Bari SS. Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009;47:361–367. doi: 10.1016/j.fct.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Laga AC, Murphy GF. The translational basis of human cutaneous photoaging: on models, methods, and meaning. Am. J. Pathol. 2009;174:357–360. doi: 10.2353/ajpath.2009.081029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Nam TG, Lee I, Shin EJ, Han AR, Lee P, Lee SY, Lim TG. Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food Sci. Nutr. 2018;6:2560–2567. doi: 10.1002/fsn3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek A, Latos M, Chrzescijanska E, Zaborski M. Antioxidant properties of rose extract (Rosa villosa L.) measured using electrochemical and UV/Vis spectrophotometric methods. Int. J. Electrochem. Sci. 2017;12:10994–11005. doi: 10.20964/2017.11.72. [DOI] [Google Scholar]
- Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin. Interv. Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Gonzalez I, Gonzalez-Barrio R, Garcia-Valverde V, Bautista-Ortin AB, Periago MJ. Nutritional composition and antioxidant capacity in edible flowers: characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2015;16:805–822. doi: 10.3390/ijms16010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea S, Stoia M, Coman D. Effects of extraction conditions on bioactive anthocyanin content of Vaccinium corymbosum in the perspective of food applications. Procedia Eng. 2012;42:489–495. doi: 10.1016/j.proeng.2012.07.440. [DOI] [Google Scholar]
- Oka N, Ikegami A, Ohki M, Sakata K, Yagi A, Watanabe N. Citronellyl disaccharide glycoside as an aroma precursor from rose flowers. Phytochemistry. 1998;47:1527–1529. doi: 10.1016/S0031-9422(97)00526-8. [DOI] [Google Scholar]
- Pandel R, Poljsak B, Godic A, Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:7. doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EK, Lee HJ, Lee H, Kim JH, Hwang J, Koo JI, Kim SH. The Anti-wrinkle mechanism of melatonin in UVB treated HaCaT keratinocytes and hairless mice via inhibition of ROS and sonic hedgehog mediated inflammatory proteins. Int. J. Mol. Sci. 2018;19:6. doi: 10.3390/ijms19071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 17 (2016) [DOI] [PMC free article] [PubMed]
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- Schieber A, Mihalev K, Berardini N, Mollov P, Carle R. Flavonol glycosides from distilled petals of Rosa damascena Mill. Z. Naturforsch. C. 2005;60:379–384. doi: 10.1515/znc-2005-5-602. [DOI] [PubMed] [Google Scholar]
- Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: recent prospects. J. Agric. Food Chem. 2003;51:2837–2853. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- Wang MF, Jin Y, Ho CT. Evaluation of resveratrol derivatives as potential antioxidants and identification of a reaction product of resveratrol and 2,2-diphenyl-1-picryhydrazyl radical. J. Agric. Food Chem. 1999;47:3974–3977. doi: 10.1021/jf990382w. [DOI] [PubMed] [Google Scholar]
- Wlaschek M, Tantcheva-Poor I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, Schuller J, Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B. 2001;63:41–51. doi: 10.1016/S1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003;16:629–638. doi: 10.1046/j.1600-0749.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- Yang JE, Ngo HTT, Hwang E, Seo SA, Park SW, Yi TH. Dietary enzyme-treated Hibiscus syriacus L. protects skin against chronic UVB-induced photoaging via enhancement of skin hydration and collagen synthesis. Arch. Biochem. Biophys. 662: 190–200 (2018) [DOI] [PubMed]