Abstract
Coriandrum sativum is an important spice plant known for its unique fragrance. Coriander oil is also one of the major essential oils in world global market. The oil yield varies with different coriander varieties; and the content and quality of oil is governed by several factors. In recent times, a variety of technologies have been exploited to improve phyto-compounds including essential oils. In this present study, Methyl jasmonate (MeJA) was amended in medium and the yield of essential oil was measured and compared in different cultivating tissues. The cultured tissues were nonembryogenic callus and embryogenic tissues (induction, proliferation and maturation stages of embryos). MeJA acts as a signaling molecule in accumulating secondary metabolites. Four different MeJA treatments i.e. T1 = 50, T2 = 100, T3 = 150 and T4 = 200 μM, along with a control (T0) were used and the yield of coriander essential oil was estimated in different in vitro cultivating tissues by using Gas chromatography–mass spectrometry (GC–MS). The addition of MEJA enriched essential oil yield, maximum oil being in maturation stage of embryos at T3 (150 μM). Other added treatments also had varied stimulatory role. The addition of MeJA induced stress as the stress marker enzymes like superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) content were high compared to non treated tissue (T0). In T4, the CAT activity was maximum i.e. 5.83 and 6.28 mg−1 protein min−1 in Co-1 and RS respectively in matured somatic embryos. The SOD activity was also high at maturation stage of embryos at T4 (5.3 mg−1 protein min−1 in RS). The APX activity on the other, was high (3.32 mg−1 protein min−1) in induction stage of embryogenesis at T3. The comparative biochemical (sugar, protein and proline) analyses of tissues were performed and presented that had high and low essential oil. MeJA induced stress may help in accumulating essential oils in C. sativum.
Keywords: Callus, Embryogenic callus, Elicitor, Essential oil, Methyl jasmonate
Introduction
Coriandrum sativum L. is an annual herb belonging to the family Apiaceae (Singh et al. 2012). The genus Coriandrum has two species, C. tordylium—a wild species and C. sativum, a cultivated one. It is a diploid cross pollinated spice crop, native to southern Europe and western Mediterranean region; and is presently cultivated worldwide (Innocent et al. 2011). India produces coriander significantly in which states like Gujarat, Karnataka, Maharashtra and Rajasthan are the major centres of production (Anonymous 2001). Based on seed size, C. sativum has been divided into large—(vulgare or macrocarpum) and small seeded varieties (microcarpum), former grown widely in tropical and subtropical climates and later one cultivated in temperate climates such as Russia, eastern and central European countries (Small 1997). Coriander oil is among the 20 major essential oils in world market (Lawrence 1992), and its commercial importance depends on fragrance, chemical and physical properties of oil (Smallfield et al. 2001). The dried coriander seeds contain essential oil (0.03% to 2.6%) with linalool as main component (Coskuner and Karababa 2007; Eikani et al. 2007). The oil yield varies with different coriander varieties with yield percentage from 0.10 to 1.8 (Fuente et al. 2003). However, the essential oil yield and quality is governed by several intrinsic factors (Fuente et al. 2003). The linalool content of essential oil in ripened coriander fruits is noted to be more than that of mature fruits (Diederichsen 1996).
The plants are the biochemical factories for the production of primary metabolites like sugars, amino acids and fatty acids; similarly it also produces diverse secondary metabolites such as alkaloids, flavonoids, volatile oils, glycosides etc. with pharmaceutical significance (Namdeo 2007). These can be classified into three main groups: the terpenes, phenolics and nitrogen-containing compounds (Loc et al. 2014) and are found in low concentrations under in vivo conditions that makes extraction from natural vegetation difficult and expensive (Zabala et al. 2010). The essential volatile oils are complex mixtures of low molecular weight (usually less than 500 daltons) compounds produced by plants. The constituents of essential oils like terpenes, terpenoids and phenolics exhibit lipophilic nature and are produced in cytoplasm and plastids of plant cells via malonic acid, mevalonic acid and methyl-d-erythritol-4-phosphate pathways (Voon et al. 2012). Monoterpenes, sesquiterpenes and their oxygenated derivatives are the major group of chemical entities in essential oils (Carson et al. 2006) and their effectiveness is decided mostly by the main components (Bakkali et al. 2008). The lipophilic nature of essential oils appears to play a crucial role for their antimicrobial activity by disrupting membrane permeability and osmotic balance of the cell. Terpenes are hydrocarbon with several isoprene (C5H8) units while the terpenoids are biochemical modification of terpenes via enzymes that add oxygen molecules or remove methyl group (Caballero et al. 2003). In general, terpenoids are more antimicrobial than the terpenes (Burt 2004). Out of 3000 essential oils produced by about 2000 plant species, 300 are important from commercial point of view with annual production of 40,000–60,000 tonnes with estimated market value of 700 million US$, which indicates growing essential oils consumption level worldwide (Djilani and Dicko 2012). The essential oils producing plants belong to various genera distributed to around 60 families, some families such as Alliaceae, Apiaceae, Asteraceae, Lamiaceae, Myrtaceae, Poaceae and Rutaceae are well known for their ability to produce essential oils of medicinal and industrial value (Vigan 2010; Hammer and Carson 2011). Plants of these families are exploited for essential oil production at commercial level, for example coriander, anise, dill and fennel oils are extracted from Coriandrum sativum, Pimpinella anisum, Anethum graveolens and Foeniculum vulgare plants respectively belonging to the family Apiaceae and are known for their antifungal, antibacterial, anticancer and antiviral activities (Raut and Karuppayil 2014). Essential oils or some related components are used in perfumes and make-up products, sanitary products, dentistry, agriculture, as food preservatives, additives and as natural remedies (Bakkali et al. 2008). Essential oils are also used in food and pharmaceutical industries owing to various medicinal (therapeutic, antimicrobial and antioxidant and other) activities (Teixeira et al. 2013; Veeresham 2012; Purnobasuki and Utami 2015). These compounds are also used as herbicides, pesticides and anticancer compounds (Burfield and Reekie 2005). The essential oils have a role in plant defense and pollinator attraction and play in plants’ fitness under environmental variation, results in quantitative and qualitative product variation. The essential oil yield and composition depends on various factors including plant genetics, developmental stage, application of fertilizers, geographic location, surrounding climate, stress during growth or maturity, the post harvest drying, storage and the method of extraction (Alvarez-Castellanos and Pascual-Villalobos 2003). In recent years, due to renewed interest in natural products, plant essential oils receive more focus on phytomedicine (Zu et al. 2010). The essential oils may play a very key role in integrated pest management programmes in future and may provide immense opportunity to agriculture sectors in developing plant based compounds against pest and other related issues. There are several reports available on essential oil from in vivo grown plants but only a few studies have been conducted for the extraction of essential oil and oil composition variations in regenerated C. sativum varieties (Khodadadi et al. 2013).
In vitro cell cultures offer an alternative technique for the production of these metabolites which can further be enhanced by the application of elicitors (Zhao et al. 2005; Roat and Ramawat 2009; Mehpara and Mujib 2017). Elicitors are either biotic or abiotic in nature. Biotic elicitors are of biological origin such as MeJA, polysaccharides derived from cell walls from plant cells (pectin or cellulose) or micro-organisms (chitin or glucans), glycoproteins or intracellular proteins which activates or inactivates a number of enzymes or ion channels through receptor binding mechanism (Veersham 2004). Abiotic elicitors on the other, are substances of non-biological origin such as inorganic salts like Cu and Cd ions, Ca2+ and physical factors like temperature, high pH etc. An elicitor is added to culture medium as external stimuli, changes cell metabolism, causes a stress in culture and activates secondary metabolite synthesis (Radman et al. 2003; Zhao et al. 2005; Vasconsuelo and Boland 2007). The effects of various elicitors (yeast extract, MeJA, salicylic acid and chitin) on enhancement of secondary compounds production in plant cell cultures has been studied in different plant groups like Solenostemon scutellarioides (Sahu et al. 2013), callus cultures of Rosa hybrida (Ram et al. 2013), hairy root cultures of soybean (Theboral et al. 2014), cell suspension cultures of Catharanthus roseus (Saiman et al. 2015) and adventitious root cultures of Eleutherococcus koreanum (Lee et al. 2015). Among various elicitors, MeJA has been extensively studied in enhancing secondary compounds in a number of plants like A. annua for artemisinin (Baldi and Dixit 2008), in Melastoma malabathricum for anthocyanin (See et al. 2011), in Glycyrrhiza inflata hairy roots cultures for glycyrrhizin (Wongwicha et al. 2011), diterpenoid in Salvia sclarea (Kuzma et al. 2009). The cell cultures of Solanum hainanense showed increased solasodine production at 50 μM MeJA (Loc et al. 2014). The treatment with MJ influences increased the yield of phytosterols in whole plant cultures of L. paucicostata (Suh et al. 2013). It is considered to be a universal elicitor for enhancing secondary metabolite synthesis through signal transduction in several plant cells and tissues (Chehab et al. 2008). Signalling elements appear to be engaged in signal transduction, initiated by elicitor addition involving ion channels, protein kinases, G-proteins, ROS, cyclic GMP, fatty acids and their derivatives. MeJA application also reported to induce systemic synthesis of PR-proteins (Ding et al. 2002). Due to low molecular weight, MeJA is transported readily in plants and activate genes (Oliveira et al. 2015). In this present article, we therefore, analysed the impact of MEJA on essential oil yield of various in vitro cultivated tissues in C. sativum. The biochemical alteration in response to MeJA-generated stress linked high oil yield was also investigated in tissues.
Materials and methods
Two C. sativum, (Co-1 and Rajendra swathi, RS) varieties’ seeds were procured from National Research Centre for Seed Spices (NRCSS) Ajmer, Rajasthan, India. The seeds were cleaned with running tap water by using saturated solution of cetrimide, a detergent. The seeds were surface sterilized with 0.1% (w/v) HgCl2 for 2 min, washed four times with autoclaved double-distilled water. Finally, the seeds were placed on 1/2 MS medium (Murashige and Skoog 1962) devoid of plant growth regulators (PGRs). The seeds were germinated within 7–10 days and the various parts of germinating seedlings were used for callus induction purposes.
Induction of callus
Hypocotyl, root and cotyledon of 1 week old in vitro germinated seedlings were cultured on MS medium supplemented with different concentrations (0.5–2.0 mg/l) of 2, 4-dichlorophenoxy acetic acid, 2, 4-D and α-Naphthalene acetic acid, NAA (0.5–1.0 mg/l). Callus was induced at variable intensity, and was maintained for proliferation.
Somatic embryo formation
Granular embryogenic callus was formed from 2,4-D induced callus within 4–5 weeks of incubation. Different stages of embryos i.e. globular, heart-, torpedo- and cotyledonary somatic embryos were differentiated at variable numbers on different PGR amended concentrations primarily with NAA (0.5–2.0 mg/l) and BA (0.5 mg/l). The entire process of embryogeny was divided into induction, proliferation, maturation and germination stages of embryos. Each stages of embryogenesis has specific requirement of PGRs.
Elicitor treatment
The methyl jasmonate, MeJA was procured from Sigma Aldrich (USA) and the solution was prepared by dissolving with 90% ethanol and the required volume was obtained by using DDW. Four different concentrations (T1, T2, T3 and T4) along with a control (T0) were prepared and added to the medium using 0.45 μm filters (Axiva Sichem Biotech, India):
Essential oil study
Extraction
Five grams of crushed dried sample was subject to hydro distillation in a glass Clevenger type apparatus for 2 h. The obtained oil was separated and the yield was measured as volume of essential oil (ml) per gram of sample (Ramezani et al. 2009). The liquid–liquid extraction step was performed using hexane. The oil was stored in a freezer at − 4 °C until analyzed by GC–MS.
Analysis by Gas chromatography–mass spectrometry (GC–MS)
GC–MS analysis was performed using an Agilent gas chromatography model 6890 N coupled to a 5793 inert gas mass selective detector. The compounds were separated on a cross-linked fused silica capillary column HP5-MS (30m × 250 μm × 0.25 μm). The head pressure of the carrier gas helium (high purity) was 50 kPa. The temperature programmed was set at an initial 60 °C, followed by an increase by 10 °C min−1–200 °C and held for 15 min. The MS detector was operated in the full scan mode with 70 eV electrons ionization, by scanning a mass range of m/z 35–450 in 0.45 s. The system was computer-controlled using Agilent GC–MSD ChemStation. The compounds were identified by matching their mass spectra with the flavour spectral library with a resemblance percentage above 90%.
Assessment of enzyme activities
Fresh tissues such as non-embryogenic callus, somatic embryos at different stages i.e. induction, proliferation and maturation stages were homogenized in 2.0 ml 0.1 M extraction buffer (0.1 M K-phosphate, 0.5 mM EDTA, 1.0 mM ascorbic acid, pH 7.5). After centrifugation at 104 rpm for 20 min, the supernatant was used for enzyme analysis.
Catalase (CAT) activity
Following Aebi’s (1984) method, the CAT activity was estimated by measuring a decrease in absorbance at 240 nm of reaction mixture containing 1.0 ml of 0.5 M reaction phosphate buffer (Na-phosphates, pH 7.5), 0.1 ml EDTA, 0.2 ml enzyme extract and 0.1 ml H2O2. The reaction was run for 3 min. One unit of enzyme determines the amount necessary to decompose 1.0 μM of H2O2 per min. The CAT activity was calculated by using co-efficient of absorbance 0.036 mM−1 cm−1.
Ascorbate peroxidase (APX) activity
The method developed by Nakano and Asada (1981) was used for APX activity. To the mixture of 1.0 ml sodium buffer (0.1 M, pH 7.2), 0.1 ml of EDTA and 0.1 ml of tissue extract, 1.0 ml of 0.5 mM ascorbate was added and the reaction was run for 3 min at 25 °C. The APX activity was assessed by observing the change in absorbance due to ascorbate degradation by APX and calculated by using the coefficient of absorbance 2.81 mM−1 cm−1. The activity of APX was expressed in EU mg−1 protein min−1.
Superoxide dismutase (SOD) activity
The SOD was estimated following Dhindsa et al. (1981) method. The callus tissue (0.1 g) was homogenised in 2.0 ml of extraction mixture (0.5 M phosphate buffer (pH 7.3), 3.0 mM EDTA, 1.0% (w/v) Polyvinylpyrollidone (PVP), 1.0% (v/v) Triton X100) and centrifuged at 104 for 10 min. The SOD activity in the supernatant was assayed by adding 0.1 ml tissue extract with 1.5 ml reaction buffer, 0.2 ml methionine, 0.1 ml each of 1.0 M NaCO3, 2.25 mM Nitro Blue Tetrazolium (NBT) solution, 3.0 mM EDTA, riboflavin; and 1.0 ml of Millipore H2O was taken in test tubes and was incubated under light for 10 min at 25 °C. A 50% reduction in colour is 1.0 unit and the enzyme activity was expressed in EU mg−1 protein min−1.
Biochemical analysis
Estimation of sugar
For sugar estimation, Dey’s (1990) method was followed. Callus tissue of 0.1 g was extracted twice with 90% alcohol at 60 °C. Final volume of extract was made up to 10 ml by adding DDW. After mixing 0.5 ml of aliquot with 0.5 ml of 5% phenol, 1.0 ml concentrated sulphuric acid was added and cooled in air. The optical density was measured at 485 nm.
Estimation of proline
Proline estimation was made according to Bates et al. (1973). About 0.05 g of callus was homogenized in 2.0 ml of 3.0% aqueous sulphosalicylic acid under cold conditions; the homogenate was filtered through Whatman filter paper (No. 1). Filtrate (1.0 ml) was added along with 1.0 ml ninhydrin and 1.0 ml glacial acetic acid; and the reaction mixture was incubated for 1 h at 100 °C. The reaction was terminated in ice bath and 2.0 ml toluene was added to reaction mixture. Proline content was measured by spectrophotometric assay at 520 nm.
Estimation of protein
Protein estimation was made as described by Bradford (1976). The homogenate of 0.25 g callus in 1.5 ml phosphate buffer (0.1 M, pH 7.0) under pre-cold conditions was centrifuged at 104 rpm for 10 min and 1.0 ml supernatant was added with 0.5 ml Trichloroacetic acid (10%). After centrifugation, the pellets were washed with acetone and dissolved in 1.0 ml of 0.1 N NaOH. To 1.0 ml of aliquot, 1.0 ml of Bradford reagent was added and the optical density was measured at 595 nm.
Statistical analysis
All the experiments were performed two to three times by using varied explants (hypocotyl, root, cotyledon and somatic embryos). The values are means of three replicates from two experiments with six to nine embryos in each replicate. Means were compared by analysis of variance (ANOVA) with Duncans Multiple Range Test (DMRT) (Duncan 1955) at p ≤ 0.05. The analysis was made with SPSS 15.0 for Windows.
Results
Callus induction and somatic embryogenesis
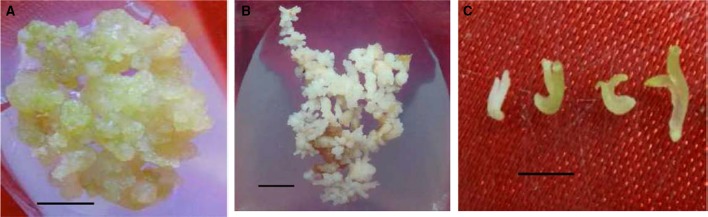
The seed germinated hypocotyl, root and cotyledon were used for the establishment of callus. The callus induction medium was amended with various concentrations and combinations of plant growth regulators, PGRs. The response of callus induction ability varied with coriander variety, use of explants, PGRs and the concentrations and combinations of added PGRs. The Co-1 coriander variety was more responsive in producing callus compared to the RS variety. Of the three various explants tested, hypocotyl was noted to be more responsive compared to the other two explants i.e. root and cotyledon. The callus induction percentage was quite high at low 2, 4-D concentrations; NAA was again very efficient in inducing callus at lower levels. The induced callus (Fig. 1a) was sub cultured at regular interval of 4 weeks and on 2, 4-D amended medium, the callus transformed into embryogenic tissue characterized by their granular appearance. The tissue started to differentiate embryos (Fig. 1b) on callus surfaces, and the supplementation of 2,4-D was found essential for inducing somatic embryos on which heterogeneous mixture of embryos were formed. The number of globular and heart shaped embryos were more and later torpedo and cotyledonary embryos (Fig. 1c) were noticed. Beside 2, 4-D, successful embryo differentiation was also noticed in NAA and BA added medium. The ‘hypocotyl-callus’ showed better embryo differentiation ability (over 70% or more) compared to ‘cotyledon–and root’ callus (data not shown). The embryos proliferated well in the same medium but embryo maturation was high on GA3 added medium in which embryos were distinct, green and elongated with two clear polar ends. The embryos germinated into plantlets in the same maturation medium amended with BAP. The entire regeneration process from ‘callus to plantlet’ was mediated through somatic embryogenesis by passing through non- embryogenic and embryogenic (induction, embryo proliferation, maturation/germination) stages.
Fig. 1.
a Non embryogenic callus, b embryogenic callus with embryos and c isolated embryos of C. sativum (a, b bar = 0.5 cm; c bar = 1 cm)
Effect of MeJA on essential oil accumulation
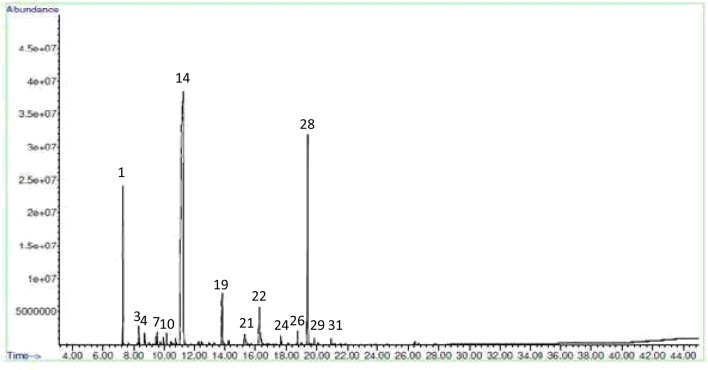
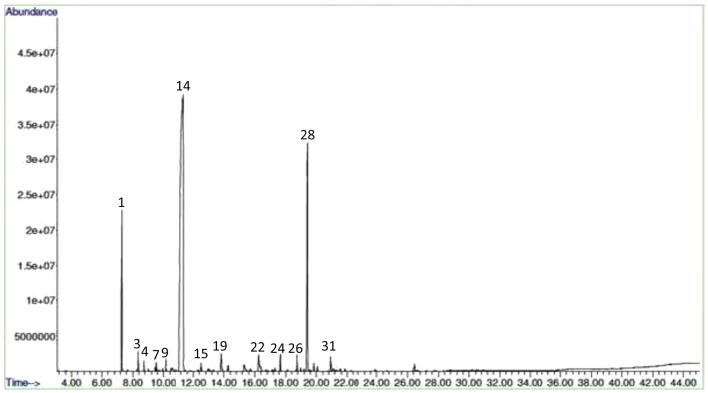
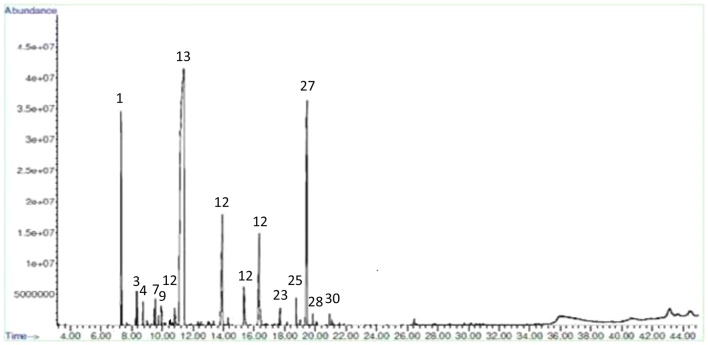
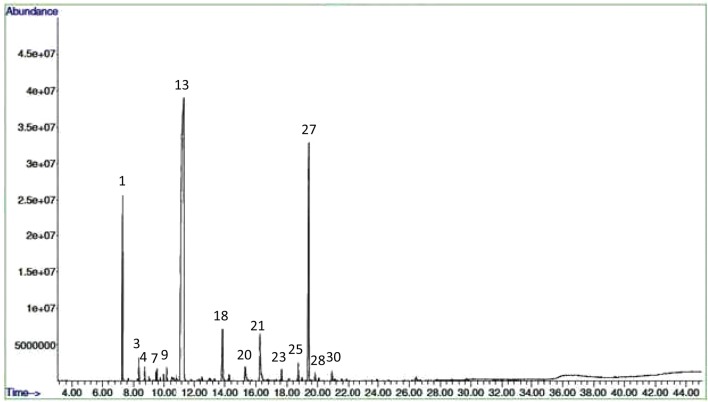
The essential oil was present in embryogenic induction-, proliferation- and maturation stages of somatic embryos while it was absent in non-embryogenic callus. In Co-1, the mature stages of somatic embryos had high essential oil content compared to control (Figs. 2 and 3) and other stages of embryos. The amendment of MeJA as an elicitor improved essential oil yield in all different stages of somatic embryos and with increasing MeJA concentrations, the essential oil accumulation was found more in both the two varieties. Figures 4 and 5 show the GC-MS spectra of essential oil of matured somatic embryos in RS with and without MeJA treatment. It also indicated that the essential oil accumulation in maturing somatic embryos was 0.30% in Co-1 while 0.50% accumulation was noted in RS. The maximum accumulation of 0.43% was noted in Co-1 at T2 and 0.75% in RS at T3 after 3 weeks of elicitation (Table 1). The proliferating embryos also had higher oil level (0.38%) at T2 after 3 weeks of elicitation in Co-1 and the increase was about 0.67% at T3 in RS. The chemical composition of essential oil in control and in MeJA elicited treatment (300 μm) especially at mature somatic embryos coriander varieties is presented in Tables 2 and 3. The composition of essential oil in elicited and non-elicited cultures was nearly the same; in maturing somatic embryos, linalool accumulation was quite high compared to other constituents such as α-Pinene and Geraniol, which accumulated poorly in treated cultures (Table 4).
Fig. 2.
GC-MS spectra of essential oil of mature somatic embryos (T0) of Co-1 variety of C. sativum
Fig. 3.
GC-MS spectra of essential oil of mature somatic embryos (T2) of Co-1 variety of C. sativum
Fig. 4.
GC-MS spectra of essential oil of mature somatic embryos (T0) of RS variety of C. sativum
Fig. 5.
GC-MS spectra of essential oil of mature somatic embryos (T3) of RS variety of C. sativum
Table 1.
The effects of MeJA (300 μM) with different concentrations of PGRs on embryo induction, proliferation and maturation frequency of CO-1 and RS varieties of Coriandrum sativum
| PGR (mg/l) | CO-1 | RS | ||||||
|---|---|---|---|---|---|---|---|---|
| 2,4-D | NAA | BA | No. of embryo’s induced per culture (50 g) | Embryo proliferation (%) | Embryo maturation (%) | No. of embryo’s induced per culture (50 g) | Embryo proliferation (%) | Embryo maturation (%) |
| 0.5 | 0.0 | 0.0 | 54.3 ± 2.5b | 00.00 | 00.00 | 63.0 ± 3.5a | 00.00 | 00.00 |
| 1.0 | 0.0 | 0.0 | 57.5 ± 2.0a | 00.00 | 00.00 | 59.3 ± 2.0b | 00.00 | 00.00 |
| 0.0 | 0.5 | 0.25 | 00.00 | 76.7 ± 3.4a | 84.3 ± 2.0b | 00.00 | 80.0 ± 2.8a | 88.3 ± 3.0b |
| 0.0 | 1.0 | 0.25 | 00.00 | 57 ± 2.6b | 79.5 ± 2.2a | 00.00 | 64.5 ± 2.3b | 83.5 ± 4.2a |
Mean values within a column followed by different letters are significantly different (at p = 0.05) according Duncan’s multiple range test (DMRT)
Table 2.
Essential oil percentage (w/v) in methyl jasmonate treated in vitro cultures/tissues after 3 weeks of treatment
| Treatment | Essential oil percentage (w/v) | |||||
|---|---|---|---|---|---|---|
| Co-1 | RS | |||||
| Embryogenic Induction stage | Proliferation stage of embryo | Maturation stage of embryo | Embryogenic Induction stage | Proliferation stage of embryo | Maturation stage of embryo | |
| T0 | 0.25 ± 0.017 c | 0.28 ± 0.015 d | 0.30 ± 0.02 d | 0.34 ± 0.017 d | 0.42 ± 0.026 e | 0.50 ± 0.02d |
| T1 | 0.29 ± 0.02 b | 0.32 ± 0.02 c | 0.34 ± 0.023 c | 0.41 ± 0.027 c | 0.53 ± 0.025 d | 0.63 ± 0.02 c |
| T2 | 0.31 ± 0.023 a | 0.38 ± 0.017 a | 0.43 ± 0.025 a | 0.46 ± 0.026 a | 0.58 ± 0.023 c | 0.69 ± 0.026 b |
| T3 | 0.28 ± 0.021 b | 0.35 ± 0.025 b | 0.40 ± 0.027 b | 0.44 ± 0.02 b | 0.67 ± 0.021 a | 0.75 ± 0.03 a |
| T4 | 0.25 ± 0.02 c | 0.33 ± 0.023 c | 0.36 ± 0.023 c | 0.40 ± 0.021 c | 0.62 ± 0.023 b | 0.70 ± 0.027 b |
Mean values within a column followed by different letters are significantly different (at p = 0.05) according to DMRT
Table 3.
Chemical composition (% of compounds) of essential oil in control and MeJA elicited (300 µm) mature somatic embryos of coriander Co-1 variety
| S. no | Compound | RT | Percentage | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |||
| 1 | α-Pinene | 7.28 | 7.39 | 7.32 | 7.20 | 6.50 | 7.0 |
| 2 | Camphene | 7.65 | 0.07 | 0.08 | 0.07 | 0.06 | 0.07 |
| 3 | β-Pinene | 8.34 | 0.81 | 0.78 | 0.8 | 0.76 | 0.77 |
| 4 | β-Myrcene | 8.71 | 0.44 | 0.40 | 0.42 | 0.37 | 0.41 |
| 5 | Octanal | 8.99 | 0.13 | 0.14 | 0.13 | 0.10 | 0.11 |
| 6 | P-cymene | 9.44 | 0.27 | 0.25 | 0.21 | 0.14 | 0.18 |
| 7 | Limonene | 9.51 | 0.39 | 0.38 | 0.32 | 0.34 | 0.30 |
| 8 | Cis-Ocimene | 9.73 | 0.09 | 0.09 | 0.07 | 0.04 | 0.05 |
| 9 | β-Ocimene | 9.93 | 0.21 | 0.23 | 0.21 | 0.09 | 0.15 |
| 10 | ϒ-Terpinene | 10.14 | 0.42 | 0.40 | 0.43 | 0.39 | 0.43 |
| 11 | Trans-Linalool oxide | 10.47 | 0.20 | 0.19 | 0.18 | 0.18 | 0.17 |
| 12 | n-octanol | 10.57 | 0.31 | 0.29 | 0.30 | 0.32 | 0.29 |
| 13 | α-Terpinolene | 10.79 | 0.38 | 0.38 | 0.30 | 0.24 | 0.26 |
| 14 | Linalool | 11.32 | 60.20 | 60.23 | 60.47 | 66.66 | 60.49 |
| 15 | Camphor | 12.30 | 0.10 | 0.10 | 0.09 | 0.11 | 0.12 |
| 16 | Citronella | 12.50 | 0.22 | 0.26 | 0.21 | 0.42 | 0.39 |
| 17 | Borneol | 12.99 | 0.26 | 0.29 | 0.25 | 0.28 | 0.29 |
| 18 | 4-terpineol | 13.32 | 0.15 | 0.17 | 0.13 | 0.11 | 0.10 |
| 19 | α-Terpineol | 13.83 | 3.63 | 3.40 | 2.54 | 1.18 | 2.26 |
| 20 | Decanal | 14.26 | 0.38 | 0.35 | 0.37 | 0.38 | 0.37 |
| 21 | Nerol | 15.30 | 1.36 | 1.43 | 1.10 | 1.19 | 1.30 |
| 22 | Geraniol | 16.26 | 3.28 | 3.21 | 3.32 | 3.35 | 3.16 |
| 23 | Furan/n-octylfuran | 17.31 | 0.08 | 0.07 | 0.10 | 0.17 | 0.19 |
| 24 | Undecanal | 17.66 | 0.49 | 0.45 | 0.62 | 0.73 | 0.69 |
| 25 | Myrtenyl acetate | 18.13 | 0.14 | 0.11 | 0.15 | 0.12 | 0.14 |
| 26 | Citronellol acetate | 18.74 | 0.65 | 0.67 | 0.64 | 0.63 | 0.66 |
| 27 | Neryl acetate | 18.99 | 0.16 | 0.15 | 0.14 | 0.14 | 0.13 |
| 28 | Geranyl acetate | 19.42 | 13.99 | 13.90 | 13.79 | 13.45 | 13.38 |
| 29 | Dodecanal | 19.83 | 0.30 | 0.35 | 0.31 | 0.30 | 0.28 |
| 30 | Caryophyllene | 20.10 | 0.11 | 0.10 | 0.16 | 0.19 | 0.19 |
| 31 | 2-Dodecenal | 20.92 | 0.38 | 0.44 | 0.52 | 0.65 | 0.59 |
| 32 | Tridecanal | 21.10 | 0.15 | 0.11 | 0.19 | 0.25 | 0.22 |
| 33 | 2-Octylfuran | 21.55 | 0.07 | 0.09 | 0.08 | 0.12 | 0.11 |
| 34 | Tetradecanal | 21.86 | 0.07 | 0.06 | 0.09 | 0.10 | 0.09 |
Table 4.
Chemical composition (% of compounds) of essential oil in control and MeJA elicited (300 µm) mature somatic embryos of coriander RS variety
| S. no | Compound | RT | Percentage | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |||
| 1 | α-Pinene | 7.28 | 7.38 | 7.29 | 7.12 | 6.55 | 6.68 |
| 2 | Camphene | 7.65 | 0.08 | 0.08 | 0.09 | 0.14 | 0.016 |
| 3 | β-Pinene | 8.33 | 0.70 | 0.68 | 0.77 | 0.73 | 0.71 |
| 4 | β-Myrcene | 8.71 | 0.45 | 0.49 | 0.44 | 0.42 | 0.46 |
| 5 | Octanal | 8.99 | 0.11 | 0.13 | 0.09 | 0.08 | 0.12 |
| 6 | P-cymene | 9.44 | 0.28 | 0.25 | 0.26 | 0.28 | 0.29 |
| 7 | Limonene | 9.52 | 0.47 | 0.52 | 0.49 | 0.59 | 0.56 |
| 8 | β-Ocimene | 9.93 | 0.24 | 0.21 | 0.18 | 0.08 | 0.13 |
| 9 | §-Terpinene | 10.15 | 0.42 | 0.45 | 0.43 | 0.40 | 0.43 |
| 10 | Trans-Linalool oxide | 10.47 | 0.18 | 0.14 | 0.17 | 0.18 | 0.21 |
| 11 | n-octanol | 10.56 | 0.25 | 0.27 | 0.31 | 0.28 | 0.30 |
| 12 | α-Terpinolene | 10.79 | 0.44 | 0.37 | 0.34 | 0.28 | 0.27 |
| 13 | Linalool | 11.29 | 60.58 | 62.73 | 61.89 | 66.98 | 65.45 |
| 14 | Camphor | 12.29 | 0.19 | 0.22 | 0.18 | 0.15 | 0.12 |
| 15 | Citronella | 12.49 | 0.20 | 0.18 | 0.26 | 0.31 | 0.33 |
| 16 | Borneol | 12.97 | 0.23 | 0.26 | 0.25 | 0.30 | 0.28 |
| 17 | 3-cyclohexen-1-ol | 13.31 | 0.12 | 0.10 | 0.13 | 0.08 | 0.10 |
| 18 | Terpineol | 13.82 | 4.06 | 3.95 | 4.10 | 3.94 | 4.03 |
| 19 | Decanal | 14.25 | 0.34 | 0.31 | 0.33 | 0.31 | 0.28 |
| 20 | Nerol | 15.29 | 1.33 | 1.21 | 1.19 | 0.90 | 1.05 |
| 21 | Geraniol | 16.25 | 3.63 | 3.22 | 3.35 | 2.10 | 2.47 |
| 22 | Furan | 17.31 | 0.07 | 0.07 | 0.06 | 0.08 | 0.09 |
| 23 | Undecanal | 17.66 | 0.47 | 0.41 | 0.44 | 0.51 | 0.49 |
| 24 | Myrtenyl acetate | 18.13 | 0.13 | 0.10 | 0.12 | 0.15 | 0.16 |
| 25 | Citronellol acetate | 18.74 | 0.60 | 0.58 | 0.62 | 0.64 | 0.61 |
| 26 | Neryl acetate | 18.99 | 0.14 | 0.17 | 0.13 | 0.12 | 0.13 |
| 27 | Geranyl acetate | 19.41 | 13.23 | 13.20 | 13.28 | 13.35 | 13.31 |
| 28 | Dodecanal | 19.83 | 0.24 | 0.21 | 0.20 | 0.23 | 0.24 |
| 29 | Caryophyllene | 20.10 | 0.10 | 0.12 | 0.14 | 0.13 | 0.13 |
| 30 | 2-Dodecenal | 20.92 | 0.33 | 0.29 | 0.32 | 0.39 | 0.38 |
| 31 | 2-Octylfuran | 21.55 | 0.06 | 0.06 | 0.09 | 0.11 | 0.10 |
| 32 | Tetradecanal | 21.86 | 0.07 | 0.08 | 0.06 | 0.07 | 0.06 |
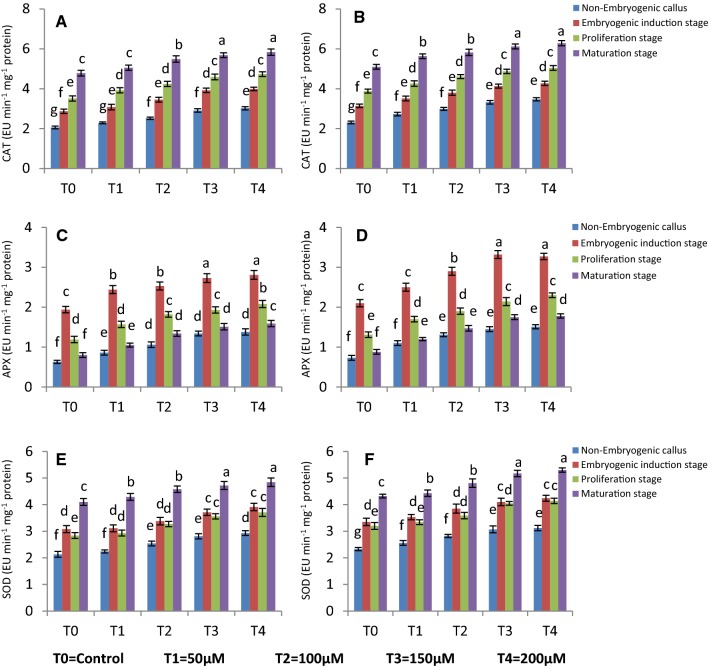
CAT, APX and SOD activity
The amendment of elicitor induces stress in cultured tissues, which also produces oil more compared to non treated culture. It is therefore necessary to study the stress level of different tissues exposed to MeJA. Various antioxidant enzymes are investigated for this purpose. The CAT activity was observed more in maturation stages of embryos followed by proliferation-, induction and non embryogenic callus tissues. In T4, the maximum CAT activity i.e. 5.83 and 6.28 mg−1 protein min−1 was noted in Co-1 and RS respectively in matured somatic embryos. In proliferation stage of embryos, equally high level of CAT activity i.e. 4.73 mg−1 protein min−1 and 5.04 mg−1 protein min−1 was noted in Co-1 and RS after 3 weeks of elicitation. The CAT activity increased linearly with increased elicitor concentrations and duration of exposure in both the two coriander varieties. The APX activity was however, high in induction stage of embryogenesis, maximum APX activity being 3.32 mg−1 protein min−1 at T3 in RS, followed by proliferation stages of embryos in 2.14 and 2.3 mg−1 protein min−1 in Co-1 and RS respectively. Non embryogenic callus had least APX activity (1.38 mg−1 protein min−1 in Co−1 and 1.51 mg−1 protein min−1 in RS).The SOD activity was highest at maturation stage of embryos, followed by embryogenic induction-, proliferation and non embryogenic stages (Fig. 6). With increase in MeJA concentration, the SOD activity also increased and maximum SOD activity was at T4 after 3 weeks of elicitation (4.84 and 5.3 mg−1 protein min−1 in Co-1 and RS respectively). In non embryogenic callus, the SOD activity was comparatively low in Co-1 (2.93 mg−1 protein min−1) and RS (3.12 mg−1 protein min−1).
Fig. 6.
Effect of different concentrations of MeJA on a, b catalase activity; c, d APX activity; e, f SOD activity of different stages in CO-1 and RS respectively after 3weeks. Values are mean ± standard deviation of three experiments. Mean values in the bars followed by different letters are significantly different (at p = 0.05) according to DMRT
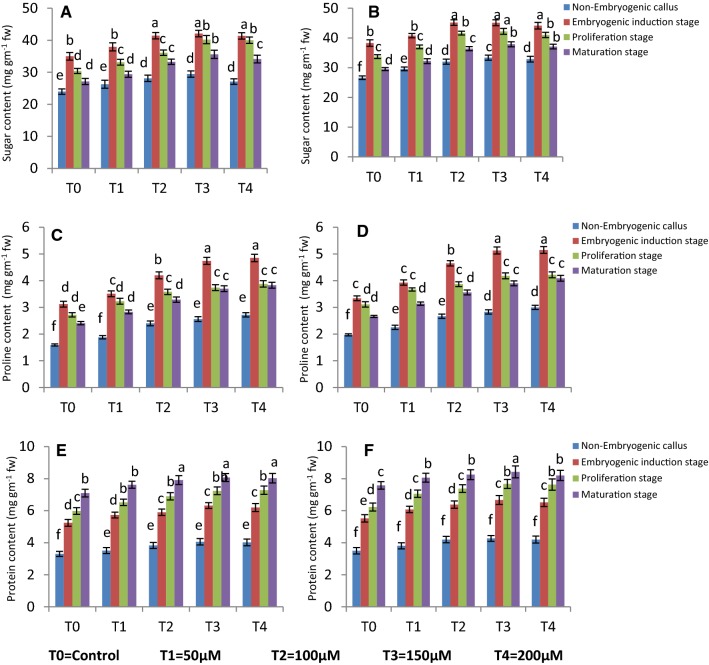
Biochemical (sugar, proline and protein) attributes
The comparative biochemical analyses of tissues were conducted that had high and low essential oil yield in C. sativum. The influence of MeJA in altering biochemical profiles was also investigated as it produced stress in culture. The present analysis showed differences in profiles between embryogenic and non-embryogenic callus in which RS is biochemically rich compared to Co-1 coriander variety. Sugar content was more at induction stage of somatic embryos compared to non-embryogenic callus stages and with MeJA amendment sugar levels increased further. Maximum sugar content was 39.93 mg gm−1 fw in Co-1 after 2 weeks in T3 and while the sugar level was 44.27 mg gm−1 fw sugar in RS after 3 weeks of treatment. The advanced stages of somatic embryos had higher levels of sugar especially at T3 (35.6 and 37.87 mg gm−1 fw in Co-1 and RS respectively). Similarly, proline level was higher at induction stage of somatic embryos as compared to other tissues/stages while the level was least in non-embryogenic callus. The proline content was very high at embryo induction stage, treated with MeJA. The maximum content was in T4 after 3 weeks of elicitation (4.85 and 5.15 mg gm−1 fw in Co-1 and RS respectively). In maturation stage of embryos, proline level was 3.83 and 4.09 mg gm−1 fw at T4 in Co-1 and RS coriander variety respectively. The protein content of various in vitro cultivated tissues was also measured, producing high and low essential oil in C. sativum. Protein content was maximum in maturation stage of embryo, followed by proliferation stage of embryos. In maturation embryo stage and in MeJA T4 treatment, higher levels of protein i.e. 8.43 mg gm−1 fw was noted in RS (Fig. 7). The proliferation stage of embryos also had increased levels of protein in RS (7.66 mg gm−1 fw protein) in T3. The protein level was comparatively low i.e. 4.06 mg gm−1 fw and 4.27 mg gm−1 fw in non-embryogenic callus tissue of Co-1 and RS in T3 treatment after 3 weeks of elicitation.
Fig. 7.
Effect of different concentrations of MeJA on a, b sugar content; c, d proline content; e, f protein content of different stages in CO-1 and RS respectively after 3 weeks. Values are mean ± standard deviation of three experiments. Mean values in the bars followed by different letters are significantly different (at p = 0.05) according to DMRT
Discussion
In this present study, the influence of MeJA was investigated on essential oil yield in in vitro grown tissues in C. sativum and we noted a positive influence on essential oil synthesis. The effect of MeJA concentrations and exposure time was quite remarkable on oil yield in both the two varieties of coriander. The yield of oil was improved significantly with MeJA addition particularly at T3. There are many similar reports on MeJA induced synthesis of terpenoids/other secondary metabolites from in vitro cultured tissues (Ramakrishna and Ravishankar 2011; Ribkahwati et al. 2015). In this C. sativum study, the essential oil was absent in callus tissue and was started to accumulate with the differentiation of tissues; and the yield was maximum in maturation stages of somatic embryo. The essential oil was however, reported in callus in other studied plant materials like Foeniculum vulgare (Khodadadi et al. 2013), cotton (Rodriguez-Saona et al. 2001), sweet basil (Deschamps and Simon 2006), lima bean and gerbera (Liang et al. 2006) and it also improved synthesis of alkaloids (Szopa et al. 2012; Danaee et al. 2015). In our study, the higher level of MeJA (300 μM) was noted to be very efficient for stimulating essential oil compared to lower levels (100 μM and 200 μM) while MeJA concentration at 100 μM was observed to be more active in other studied materials (Kim et al. 2013; Mangas et al. 2006; Scholz et al. 2009). The mechanism of elicitation is however, diverse in different plant groups and the elicitation mechanism includes activities at various levels viz. cell wall, cytosolic messenger molecules and biosynthetic pathway. Radman et al. (2003) suggested the occurrence of ‘elicitor-receptor interaction’ is the basis for rapid array of biochemical responses. Our results in C. sativum suggest that MeJA acts as an inducer in enhancing essential oil yield, but how it improved yield and altered composition still not known clearly. In other studies, elicitors are found to bind receptor protein, present in plasma membrane and activated specific genes through signal transduction pathways (as a part of defense response) and promoted secondary metabolites biosynthesis (Mishra et al. 2011). During stress-induced secondary metabolite enrichment, Jasmonates acts as a regulatory signal to coordinate the activation of multiple biosynthetic genes (Pauwels et al. 2009; Howe 2010) as jasmonates are plant hormones, having the ability to regulate gene expression in plant defense responses and are being used as potent elicitor in improving secondary product accumulation in a number of studied materials (Santino et al. 2013). JA and its methyl ester MeJA both are considered important signalling compounds in elicitation process, help accumulating various secondary metabolites (Shimizu et al. 2010). In the process of bioproduct accumulation, MeJA treatment up-regulated genes participate in JA biosynthesis, and other associated genes encoding stress protective proteins (Wang et al. 2015). Other lines of research however, indicated that MeJA acts as a secondary messenger in a wide range of signalling pathways (Zhao et al. 2005) and the transcript levels vary with MeJA concentrations in different species, such as in Angelica gigas, AgPAL and AgC4H expression levels reached highest at 300 mM MeJA, which showed over-production of secondary metabolites (Park et al. 2011). In contrast, transcript levels of phenyl-propanoid biosynthetic genes ArPAL, ArC4H and Ar4CL were high and reached maximum at low 50 mM MeJA level (Kim et al. 2013).
As the callus transforms into embryogenic callus, biochemical profiles also alter simultaneously. In this coriander study, we noted differences in biochemical and enzyme activities in embryogenic and non-embryogenic callus. The alteration in biochemical attributes in embryogenic callus with non-embryogenic tissue or with differentiated embryo was previously reported in several investigated plants (Singh et al. 2011; Fatima et al. 2011). Kumar and Kumari (2010) observed alteration of biochemical profile and enzyme activities during cellular differentiation process, this and similar other findings helped to understand the metabolic changes occur in developmental route (Singh et al. 2009; Santos et al. 2005). Here, the enzyme activities were less in non-embryogenic callus compared to embryogenic one, but it was high at specific embryo stages. In Hevea brasiliensis similar increased peroxidase activity was noted during embryo organization time (Silva et al. 2014). The medium was amended with MeJA in order to improve alkaloid yield but this also causes stress in culture. Cells/plants are however, equipped with antioxidant and scavenging systems to mitigate the adverse cellular situation. The antioxidant enzymes such as CAT, SOD and APX are usually used to evaluate biochemical and physiological responses of plants exposed to biotic/abiotic stresses (Gill and Tuteja 2010). In our study, CAT activity was increased after elicitor treatment and this increased enzyme activity was noted to be due to H2O2 synthesis, which promotes ROS accumulation in cultivated tissues (Gao et al. 2011). The enzyme activity is considered to be more at the onset of stress and with increasing stress the enzyme activity accumulates further and decreases later/at the end of stress (Ying et al. 2015). The induction of oxidative stress in response to MeJA has been reported earlier, and with an increased enzyme activities the possible damages are prevented (Chong et al. 2005). SOD is also believed to provide first line of defense against toxic impacts of reactive oxygen species by converting H2O2 to H2O and O2 and conversion of O2-radicals to H2O2 and O2. In our observations, the activities of SOD and APX enhanced with increasing stress duration whereas CAT activities decreased as compared to the control. An increase in SOD and APX and decrease in CAT activity was reported in previous studies during drought stress (Pan et al. 2006). The reduced SOD, CAT activity and increased POD activity was also reported in response to drought conditions (Simova-Stoilova et al. 2007). In this studied material, the CAT activity was inversely proportional to the increasing MeJA levels. Broetto et al. (2002) noted decreased CAT activity due to adverse effect of salts, causing alteration in protein/enzyme structure. The increased SOD activity may be partly due to increased metabolic activity or an increased rate of SOD biosynthesis under stress influence (Pham et al. 2015). This enhanced activity may also be seen as an adaptive reaction of cells to changes in oxidative stress process (Chakrabarti and Patra 2013).
The addition of MeJA influences biochemical attribute accumulation like sugar, proline and protein as was observed in our present investigation. Both the two varieties showed increased sugar accumulation under higher MeJA levels. More accumulation of sugar facilitates osmotic adjustment (Mahajan and Tuteja 2005), functions as metabolic signals (Aghaleh et al. 2009) and has a critical role in osmoprotection, carbon preservation, membrane stability and radical scavenging (Parvaiz and Satyawati 2008). There are several other previous reports indicating increased soluble carbohydrates levels in tolerant varieties, exposed to salt (Ansari et al. 2013). In this present study, we noted increased protein and decreased soluble sugar levels in maturation stage of somatic embryo, which is in confirmatory with the findings of Kumar and Kumari (2010). Soluble carbohydrates and fructans are considered as sensitive markers in the selection of tolerant genotypes under salt stress (Parvaiz and Satyawati 2008). The accumulation of higher levels of carbohydrates under salt prevents plants from oxidative damage and it also helps in maintaining protein structure (Hajihashemi et al. 2006). Soluble sugars scavenge ROS as well by improving synthesis of nicotinamide adenine dinucleotide phosphate (NADPH) at high glucose levels, while free sugar reduction may be due to MeJA induced oxidative stress (Couee et al. 2006). Proline seems to be widely distributed osmolyte in plants under stress conditions (Bhaskara et al. 2015). In this study, proline levels increased with increasing MeJA concentrations in both the two varieties and this increased proline accumulation may be due to protein breakdown. In our study proline levels were found more at induction stage of somatic embryogenesis as compared to other embryogenic stages, which are in conformity with the previous findings of Fatima et al. (2015). These observations show that the accumulation of proline in response to stress help in adaptive response to plants. Beside osmoprotectant, proline also acts as a sink for energy to regulate redox potentials (Sharma and Dietz 2006), it protects macromolecules against denaturation and helps in reducing acidity in cell. The proline works in osmotic adjustment in promoting higher resistance to plants under adverse conditions, minimizes various negative effects and plays an essential role in defense mechanism by providing carbon, nitrogen and energy following stress (Aktas et al. 2007). Proline is a special osmolyte and a compatible solute in plant cells, which assists in preserving cell turgor under low water levels (Bidabadi et al. 2012). With increasing MeJA concentration the total soluble protein content increased at different developmental stages of embryos and the findings are very similar to Gao et al. (2011) observation in which total soluble protein content increased in response to biotic elicitor application in Euphorbia pekinensis. Similar enhancement in protein content has been reported in several other tolerant plant species under salt stress conditions (Najaphy et al. 2010). Low levels of protein, also found in cases may be due to degradation of biomolecules like enzymes (Nunes et al. 2008), which failed to maintain cell turgor pressure under stress situation (Ashraf and Harris 2004). Proteins are one of the several biochemical compounds, which accumulated during stress as a strategy to adapt various stress conditions and in this present study, protein levels increased with increasing MeJA concentrations. Similar observation was reported in other plants, dealt with various stress conditions (Lakra et al. 2016). Thus the amendment of MeJA increased stress level in medium (as was observed from increasing antioxidant enzymes’ levels) may stimulate in synthesizing higher levels of essential oils in in vitro cultivated coriander tissues. To our best knowledge this is the first ever study of essential oil after MeJA elicitation in coriander in vitro.
Acknowledgements
The first author is thankful to University Grant Commission for awarding Junior Research Fellowship. The authors are also grateful to the Department of Botany, Jamia Hamdard for providing necessary facility and to Central Instrumentation facilities for providing other help.
Author contributions
MA performed all the experimental works; Other scientists involved in this study helped in designing experiments, preparing tables, figures and photoplates; and AM supervised and edited manuscript for final submission.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no conflict of interest.
Ethical approval
This article does not require any experiment or study with human participants or animals.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Aghaleh M, Niknam V, Ebrahimzadeh H, Razavi K. Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicornia persica and S. europaea. Biol Plantarum. 2009;53:243–248. [Google Scholar]
- Aktas LY, Akca BTH, Parlak S. Role of abscisic acid and proline treatment on induction of antioxidant enzyme activities and drought tolerance responses of Laurus nobilis L. seedlings. Fen Bilimleri Dergisi. 2007;28:14–27. [Google Scholar]
- Alvarez-Castellanos PP, Pascual-Villalobos MJ. Effect of fertilizer on yield and composition of flowerhead essential oil of Chrysanthemum coronarium (Asteraceae) cultivated in Spain. Ind Crops Prod. 2003;17:77–81. [Google Scholar]
- Anonymous . The Wealth of India, a dictionary of Indian raw materials and industrial products. New Delhi: National Institute of Science Communication, CSIR; 2001. pp. 203–206. [Google Scholar]
- Ansari MI, Yadav A, Lal R. An-overview on invertase in sugarcane. Bioinformation. 2013;9(9):464–465. doi: 10.6026/97320630009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Baldi A, Dixit VK. Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour Tech. 2008;99(11):4609–4614. doi: 10.1016/j.biortech.2007.06.061. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bhaskara GB, Yang TH, Verslues PE. Dynamic proline metabolism: importance and regulation in water limited environments. Front Plant Sci. 2015;6:484. doi: 10.3389/fpls.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidabadi SS, Meon S, Wahab Z, Subramaniam S, Mahmood M. In vitro selection and characterization of water stress tolerant lines among ethyl methanesulphonate (EMS) induced variants of banana (Musa spp., with AAA genome) Aust J Crop Sci. 2012;6:567–575. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Ann Biochem. 1976;72:248–253. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broetto F, Luttge U, Ratajczak R. Influence of light intensity and salt treatment on mode of photosynthesis and enzymes of the antioxidative response system of Mesembryanthemum crystallinum. Funct Plant Biol. 2002;29:13–23. doi: 10.1071/PP00135. [DOI] [PubMed] [Google Scholar]
- Burfield T, Reekie SL. Mosquitoes, malaria and essential oils. Int J Aromather. 2005;15:30–41. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods1a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Caballero B, Trugo LC, Finglas PM. Encyclopedia of food sciences and nutrition. 2. Amsterdam: Academic Press; 2003. [Google Scholar]
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Patra PK. Effect of fluoride on superoxide dismutase activity in four common crop plants. Fluoride. 2013;46(2):59–62. [Google Scholar]
- Chehab EW, Kaspi R, Savchenko T, Rowe H, Negre-Zakharov F, Kliebenstein D, Dehesh K. Distinct roles of jasmonates and aldehydes in plant-defence responses. PLoS One. 2008;3(4):1–10. doi: 10.1371/journal.pone.0001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TM, Abdullah MA, Fadzillah NM, Lai OM, Lajis NH. Jasmonic acid elicitation of anthraquinones with some associated. Enzyme Microb Technol. 2005;36:469–477. [Google Scholar]
- Coskuner Y, Karababa E. Physical properties of coriander seed (Coriandrum sativum L.) J Food Eng. 2007;80(2):408–416. [Google Scholar]
- Couee I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot. 2006;57:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- Danaee M, Farzinebrahimi R, Kadir MA, Sinniah UR, Mohamad R, Taha RM. Effects of MeJA and SA elicitation on secondary metabolic activity, antioxidant content and callogenesis in Phyllanthus pulcher. Braz J Bot. 2015;38(2):265–272. [Google Scholar]
- Deschamps C, Simon JE. Terpenoid essential oil metabolism in basil (Ocimum basilicum L.) following elicitation. J Essent Oil Res. 2006;18:618–621. [Google Scholar]
- Dey PM. Methods in plant biochemistry. In: Dey PM, editor. Carbohydrates. 2. London: Academic Press; 1990. [Google Scholar]
- Dhindsa RH, Plumb-Dhindsa P, Thorpe TA. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot. 1981;32:93–101. [Google Scholar]
- Diederichsen A (1996) Results of a characterization of a germplasm collection of coriander (Coriandrum sativum L.) in the Gatersleben genebank. In: Inter. Symp. Breeding Res. Med. Aromatic Plants, June 30-July 4, Quedlinburg, Germany, pp 45-48
- Ding CK, Wang CY, Gross KC, Smith DL. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- Djilani A, Dicko A. The therapeutic benefits of essential oils. In: Bouayed J, Bohn T, editors. Nutrition, Well-being and Health. Croatia: In Tech; 2012. pp. 155–178. [Google Scholar]
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11(1):1. [Google Scholar]
- Eikani MH, Golmohammad F, Rowshanzamir S. Supercritical water extraction of essential oils from coriander seeds (Corinadrum sativum L) J Food Eng. 2007;80(2):735–740. [Google Scholar]
- Fatima S, Mujib A, Samaj J. Anti-oxidant enzyme responses during in vitro embryogenesis in Catharanthus roseus. J Hort Sci Biotechnol. 2011;86(6):569–574. [Google Scholar]
- Fatima S, Mujib A, Tonk D. NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tiss Organ Cult. 2015;121(2):445–458. [Google Scholar]
- Fuente EB, Gil A, Lenardis AE, Pereira ML, Suarez SA, Ghersa CM, Grass MY. Response of winter crops differing in grain yield and essential oil production to some agronomic practices and environmental gradient in the Rolling Pampa, Argentina. Agric Ecosys Environ. 2003;99:159–169. [Google Scholar]
- Gao F, Yong Y, Dai C. Effects of endophytic fungal elicitor on two kinds of terpenoids production and physiological indexes in Euphorbia pekinensis suspension cells. J Med Plants Res. 2011;5(18):4418–4425. [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Hajihashemi S, Kiarostami K, Enteshari S, Saboora A. The effects of salt stress and paclobutrazol on some physiological parameters of two salt-tolerant and salt-sensitive cultivars of wheat. Pak J Biol Sci. 2006;9(7):1370–1374. [Google Scholar]
- Hammer KA, Carson CF. Antibacterial and antifungal activities of essential oils. In: Thormar H, editor. Lipids and essential oils as antimicrobial agents. Chichester: John Wiley and Sons Ltd; 2011. pp. 255–306. [Google Scholar]
- Howe GA. The roles of hormones in defence against insects and disease. Jasmonates. In: Davies PJ, editor. Plant hormones. Biosynthesis, signal transduction, action! Dordrecht: Kluwer Academic Publishers; 2010. pp. 646–680. [Google Scholar]
- Innocent BX, Fathima MSA, Dhanalakshmi Studies on the immouostimulant activity of Coriandrum sativum and resistance to Aeromonas hydrophila in Catla catla. J Appl Pharm Sci. 2011;1(7):132–135. [Google Scholar]
- Khodadadi E, Aharizad S, Mohammadi SA, Khodadadi E, Kosarinasab M, Sabzi M. Chemical composition of essential oil compounds from the callus of fennel (Foeniculum vulgare Miller.) Int J Agron Agricul Res. 2013;3(11):1–6. [Google Scholar]
- Kim YB, Kim JK, Romij-Uddin M, Xu H, Park WT, Tuan P, Li X, Chung E, Lee JH, Park SU. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS One. 2013;8(5):e64199. doi: 10.1371/journal.pone.0064199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SP, Kumari BDR. Effect of primary and secondary somatic embryogenesis in Safflower (Carthamus tinctorius L) at morphological and biochemical levels. Am-Eurasian J Agric Environ Sci. 2010;8(6):784–792. [Google Scholar]
- Kuzma L, Bruchajzer E, Wysokinska H. Methyl jasmonate effect on diterpenoid accumulation in Salvia sclarea hairy root culture in shake flasks and sprinkle bioreactor. Enz Microb Technol. 2009;44:406–410. [Google Scholar]
- Lakra N, Tomar PC, Mishra SN. Growth response modulation by putrescine in Indian mustard Brassica Juncea L. under multiple stress. Ind J Exp Biol. 2016;54:262–270. [PubMed] [Google Scholar]
- Lawrence BM (1992) A planning scheme to evaluate new aromatic plants for the flavor and fragrance industries, In: Janick J, Simon JE (eds), Proceedings of the second national symposium. New Crops: exploration, research, and commercialization. John Wiley and Sons, Inc., New York, pp 620–627
- Lee EJ, Park SY, Paek KP. Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant Cell Tiss Organ Cult. 2015;120:1–10. [Google Scholar]
- Liang Y-S, Kim HK, Lefeber AWM, Erkelens C, Choi YH, Verpoorte R. Identification of phenylpropanoids in methyl jasmonate treated Brassica rapa leaves using two-dimensional nuclear magnetic resonance spectroscopy. J Chromatogr A. 2006;1112(1–2):148–155. doi: 10.1016/j.chroma.2005.11.114. [DOI] [PubMed] [Google Scholar]
- Loc NH, Anh NHT, Khuyen LTM, An TNT. Effects of yeast extract and methyl jasmonate on the enhancement of solasodine biosynthesis in cell cultures of Solanum hainanense Hance. J BioSci Biotech. 2014;3(1):1–6. [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arc Biochem Biophy. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusido RM, Piol TM, Palazn J. The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry. 2006;67:2041–2049. doi: 10.1016/j.phytochem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Mehpara M, Mujib A. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Braz J Pharm. 2017;27:549–556. [Google Scholar]
- Mishra J, Singh M, Palni LMS, Nandi SK. Assessment of genetic fidelity of encapsulated microshoots of Picrorhiza kurroa. Plant Cell Tissue Organ Cult. 2011;104:181–186. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. [Google Scholar]
- Najaphy A, Khamssi NN, Mostafaie A, Mirzaee H. Effect of progressive water deficit stress on proline accumulation and protein profiles of leaves in chickpea. Afri J Biotech. 2010;9:7033–7036. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Namdeo AG. Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev. 2007;1(1):69–79. [Google Scholar]
- Nunes C, de Sousa AS, da Silva JM, Fevereiro MPS, da Silva AB. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environ Exp Bot. 2008;63:289–296. [Google Scholar]
- Oliveira MB, Juniorb ML, Grossi-de-Sac MF, Petrofeza S. Exogenous application of methyl jasmonate induces a defense response and resistance against Sclerotinia sclerotiorum in dry bean plants. J Plant Physiol. 2015;182(15):13–22. doi: 10.1016/j.jplph.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wu LJ, Yu ZL. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch) Plant Growth Regul. 2006;49:157–165. [Google Scholar]
- Park SY, Cho HM, Moon HK, Kim YW, Paek KY. Genotypic variation and aging effects on the embryogenic capability of Kalopanax septemlobus. Plant Cell Tissue Organ Cult. 2011;105:265–270. [Google Scholar]
- Parvaiz A, Satyawati S. Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ. 2008;54(3):89–99. [Google Scholar]
- Pauwels L, Inze D, Goossens A. Jasmonate-inducible gene: what does it mean? Trends Plant Sci. 2009;14:87–91. doi: 10.1016/j.tplants.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Pham NT, Kim JG, Jung S. Differential antioxidant responses and perturbed porphyrin biosynthesis after exposure to oxyfluorfen and methyl viologen in Oryza sativa. Int J Mol Sci. 2015;16:16529–16544. doi: 10.3390/ijms160716529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnobasuki RH, Purnobasuki H, Isnaeni, Utami ESW. Quantity essential oil from rose callus leaf (Rosa hybrid L. variety Hybride tea purple): results of light elicitation. J Chem Pharma Res. 2015;7(4):496–499. [Google Scholar]
- Radman R, Saez T, Bucke C, Keshavarz T. Elicitation of plant and microbial systems. Biotechnol App Biochem. 2003;37:91–102. doi: 10.1042/ba20020118. [DOI] [PubMed] [Google Scholar]
- Ram M, Prasad KV, Singh SK, Hada BS, Kumar S. Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tissue Organ Cult. 2013;113(3):459–467. [Google Scholar]
- Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani S, Rahmanian M, Jahanbin R, Mohajeri F, Rezai MR, Solaimani B. Diurnal changes in essential oil content of coriander (Coriandrum sativum L.) aerial parts from Iran. Res J Biol Sci. 2009;4(3):277–281. [Google Scholar]
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Indus Crops Prod. 2014;62:250–264. [Google Scholar]
- Ribkahwati, Purnobasuki H, Isnaeni, Utami ESW. Quantity essential oil from rose callus leaf (Rosa hybrid L. variety Hybride tea purple): results of light elicitation. J Chem Pharma Res. 2015;7(4):496–499. [Google Scholar]
- Roat C, Ramawat KG. Elicitor induced accumulation of stilbenes in cell suspension cultures of Cayratia trifoliata (L.) Domin. Plant Biotechnol Rep. 2009;3:135–138. [Google Scholar]
- Rodriguez-Saona C, Crafts-Brandner SJ, Pare PW, Henneberry TJ. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J Chem Ecol. 2001;27(4):679–695. doi: 10.1023/a:1010393700918. [DOI] [PubMed] [Google Scholar]
- Sahu R, Gangopadhyay M, Dewanjee S. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Solenostemon scutellarioides. Acta Physiol Plant. 2013;35(5):1473–1481. [Google Scholar]
- Saiman MZ, Mustafa NR, Choi YH, Verpoorte R, Schulte AE. Metabolic alterations and distribution of five-carbon precursors in jasmonic acid-elicited Catharanthus roseus cell suspension cultures. Plant Cell Tissue Organ Cult. 2015;122(2):351–362. [Google Scholar]
- Santino A, Taurino M, Domenico SD, Bonsegna S, Poltronieri P, Pastor V, Flors V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013;32:1085–1098. doi: 10.1007/s00299-013-1441-2. [DOI] [PubMed] [Google Scholar]
- Santos MO, Romano E, Yotoko KSC, Tinoco MLP, Dias BBA, Aragao FJL. Characterization of the cacao somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogeesis. Plant Sci. 2005;168:723–729. [Google Scholar]
- Scholz M, Lipinski M, Leupold M, Luftmann H, Harig L, Ofir R, Fischer R, Prüfer D, Muller KJ. Methyl jasmonate induced accumulation of kalopanaxsaponin I in Nigella sativa. Phytochemistry. 2009;70(4):517–522. doi: 10.1016/j.phytochem.2009.01.018. [DOI] [PubMed] [Google Scholar]
- See KS, Bhatt A, Keng CL. Effect of sucrose and methyl jasmonate on biomass and anthocyanin production in cell suspension culture of Melastoma malabathricum (Melastomaceae) Rev Biol Trop. 2011;59(2):597–606. [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ. The significance of amino acids and amino-acid derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Maeda K, Kato M, Shimomura K. Methyl jasmonate induces anthocyanin accumulation in Gynura bicolor cultured roots. In Vitro Cell Dev. Biol Plant. 2010;46:460–465. [Google Scholar]
- Silva MMR, Ulisses C, Medeiros MJLM, Granja MMC, Willadino L, Camara T. Antioxidant enzymes activity in embryogenic and non-embryogenic tissues in sugarcane. Acta Biol Colom. 2014;19(2):203–210. [Google Scholar]
- Simova-Stoilova L, Demirevska K, Petrova T, Tsenov N, Feller U. Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (Triticum aestivum L.) varieties to long-term field drought. Plant Growth Regul. 2007;58:107–117. [Google Scholar]
- Singh SR, Singh R, Dhawan AK. Biochemical changes related to shoot differentiation in callus cultures of Tylophora indica Wight and Arn. J Indian Bot Soci. 2009;88(3,4):49–53. [Google Scholar]
- Singh N, Yadav K, Kumari S, Renu Metabolic changes during differentiation in callus cultures of Stevia rebaudiana (bertoni) J Phytol. 2011;3(3):63–67. [Google Scholar]
- Singh SK, Kakan RK, Meena RS, Pancholy A, Pathak R, Raturi A. Studies on genetic divergence among Indian varieties of a spice herb, Coriandrum sativum. J Environ Biol. 2012;33:781–789. [PubMed] [Google Scholar]
- Small E. Culinary herbs. Ottawa: NRC Research Press; 1997. pp. 219–225. [Google Scholar]
- Smallfield BM, van Klink JW, Perry NB, Dodds G. Coriander spice oil: effects of fruit crushing and distillation time on yield and composition. J Agric Food Chem. 2001;49:118–123. doi: 10.1021/jf001024s. [DOI] [PubMed] [Google Scholar]
- Suh HW, Hyun SH, Kim SH, Lee SY, Choi HK. Metabolic profiling and enhanced production of phytosterols by elicitation with methyl jasmonate and silver nitrate in whole plant cultures of Lemna paucicostata. Process Biochem. 2013;48:1581–1586. [Google Scholar]
- Szopa A, Ekiert H, Szewczyk A, Fugas E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. Divaricata (Tenore) Gams. under different light conditions. Plant Cell Tissue Org Cult. 2012;110:329–336. [Google Scholar]
- Theboral J, Sivanandhan G, Subramanyam K, Arun M, Selvaraj N, Manickavasagam M, Ganapathi A. Enhanced production of isoflavones by elicitation in hairy root cultures of Soybean. Plant Cell Tissue Organ Cult (PCTOC) 2014;117(3):477–481. [Google Scholar]
- Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JM, Saraiva JA, Nunes ML. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crops Prod. 2013;43:587–595. [Google Scholar]
- Vasconsuelo A, Boland R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007;172:861–877. [Google Scholar]
- Veeresham C. Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res. 2012;3(4):200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigan M. Essential oils: renewal of interest and toxicity. Eur J Dermatol. 2010;20:685–692. doi: 10.1684/ejd.2010.1066. [DOI] [PubMed] [Google Scholar]
- Voon CH, Bhat R, Rusul G. Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Compr Rev Food Sci Food Saf. 2012;11:34–55. [Google Scholar]
- Wang J, Qian J, Yao L, Lu Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Biores Biopros. 2015 doi: 10.1186/s40643-014-0033-5. [DOI] [Google Scholar]
- Wongwicha W, Tanaka H, Shoyama Y, Putalun W. Methyl jasmonate elicitation enhances glycyrrhizin production in Glycyrrhiza inflata hairy roots cultures. Z Naturforsch. 2011;66:423–428. doi: 10.1515/znc-2011-7-815. [DOI] [PubMed] [Google Scholar]
- Ying YQ, Song LL, Jacobs DF, Mei L, Liu P, Jin SH, Jia S, Wu JS. Physiological response to drought stress in Camptotheca acuminate seedlings from two provenances. Front Plant Sci. 2015;6:361. doi: 10.3389/fpls.2015.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala MA, Angarita M, Restrepo JM, Caicedo LA, Perea M. Elicitation with methyl-jasmonate stimulates peruvoside production in cell suspension cultures of Thevetia peruviana. Vitro Cell Dev Biol Plant. 2010;46:233–238. [Google Scholar]
- Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–294. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Zu YG, Yu HM, Liang L, Fu YJ, Efferth T, Liu X, Wu N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules. 2010;15:3200–3210. doi: 10.3390/molecules15053200. [DOI] [PMC free article] [PubMed] [Google Scholar]