Abstract
Lactobacillus brevis KU15153 was isolated from kimchi and probiotic characterization was performed including analysis of its antimicrobial and antioxidant effects. Lactobacillus rhamnosus GG (LGG) was used as a probiotic control. L. brevis KU15153 survived under artificial gastric conditions and was non-hemolytic, showed antibiotic susceptibility, and did not produce carcinogenic β-glucuronidase. L. brevis KU15153 adhered strongly to HT-29 cells in the direct adherent assay and showed high cell surface hydrophobicity. Particularly, L. brevis KU15153 showed antimicrobial activity against the food-borne pathogens Escherichia coli ATCC 25922, Listeria monocytogenes ATCC 15313, Salmonella Typhimurium P99, and Staphylococcus aureus KCCM 11335. Antioxidant activity was assessed using the DPPH radical scavenging assay and β-carotene and linoleic acid inhibition assay. L. brevis KU15153 showed higher antioxidant activity than LGG. These results suggest that L. brevis KU15153 has potential for use as a probiotic organism.
Keywords: Probiotic, Kimchi, Lactobacillus brevis, Antimicrobial effect, Antioxidant effect
Introduction
Kimchi, a traditional fermented food and representative ethnic food in Korea, has been reported as a healthy food (Bae et al., 2018; Park et al., 2018). Kimchi is prepared from Chinese cabbage or radish with various seasonings such as ground red pepper, garlic, ginger, fish sauce, shrimp sauce, and other ingredients (Lee et al., 2014). Kimchi is commonly stored at a low temperature for several months to multiple years. During fermentation, diverse lactic acid bacteria (LAB) are present, such as Lactobacillus sp., Leuconostoc sp., Lactococcus sp., Weissella sp., and Pediococcus sp. (Park et al., 2018). Particularly, Lactobacillus sp. become the predominant species during incipient fermentation; these bacteria produce lactic acid and decrease the pH (Lee et al., 2016). LAB strains were investigated to determine their probiotic properties and diverse effects including anti-obesity, antioxidant, and antidiabetic activities.
Probiotic are defined by the World Health Organization as live microorganisms having a health benefit to the host when administered at adequate levels (FAO/WHO, 2002; Kimoto-Nira et al., 2015; Vitali et al., 2012). Probiotics have diverse functional effects such as antioxidant, antidiabetic, antiallergy, blood lipids reducing, anti-inflammatory, and anticancer effects to improve host health (Jeon et al., 2016; Lee et al., 2015). Probiotics should be investigated to determine their characteristics including resistance to gastric and bile with adhesion effects in the human intestinal tract to evaluate probiotic potential (Son et al., 2018).
LAB are generally recognized as “generally recognized as safe” and used as food supplements and food preservation because of their diverse probiotic properties (Aarti et al., 2017; Son et al., 2018). Lactobacillus acidophilus, L. rhamnosus, L. helveticus, L. bulgaricus, L. plantarum, L. salivarius, L. reuteri, L. casei, L. brevis, Bifidobacterium infantis, B. lactis, B. bifidum, Pediococcus acidophilus, and Streptococcus thermophilus are representative LAB probiotic strains. Among these strains, L. brevis is commonly isolated from fermented foods, plants, and the human intestinal tract and have been investigated for their probiotic potential (Lee et al., 2014; Sharma et al., 2017). Lactobacillus brevis KB290 was reported to reduce the incidence of influenza (Sharma et al., 2017; Waki et al., 2014). L. brevis CD2 showed anti-inflammatory effects and L. brevis B23 was found to have antimicrobial effect (Riccia et al., 2007; Rushdy and Gomaa, 2013).
The aim of the present study was to assess the probiotic potential of L. brevis KU15153 isolated from kimchi such as its stability to gastric conditions, safety, antimicrobial activity, and antioxidant effects.
Materials and methods
Microorganisms and culture conditions
Lactobacillus brevis KU15153 was isolated from kimchi, and was grown in lactobacilli MRS broth (BD BBL, Franklin Lakes, NJ, USA). Lactobacillus rhamnosus GG (LGG, KCTC 5033) was used as a commercial probiotic strain and was obtained from the Korean Collection for Type Cultures (Jeolla-do, Korea). Escherichia coli ATCC 25922, Listeria monocytogenes ATCC 15313, Salmonella Typhimurium P99, and Staphylococcus aureus KCCM 11335 were incubated in tryptic soy broth (TSB; BD BBL, Sparks, MD, USA) at 37 °C for 24 h.
To obtain pellets of the LAB strains, the strains were centrifuged (14,240 × g, 5 min, 4 °C) and washed twice with peptone water. The washed LAB cells were resuspended in peptone water.
Tolerance to artificial gastric juice and bile acid
The resistance of LAB strains to artificial gastric juice and bile salts was determined as described by Lee et al. (2015) with some modifications. LAB strains were grown in MRS broth at 37 °C for 24 h and then resuspended in the MRS broth (pH 2.5) containing 0.3% (w/v) pepsin (Sigma-Aldrich, St. Louis, MO, USA) for 3 h at 37 °C. To assess the tolerance of the strains to bile salt, an overnight culture of the LAB strains was resuspended in MRS broth containing 0.3% (w/v) oxgall and incubated for 24 h at 37 °C. Survival rates were confirmed by counting the viable cells on MRS plates.
Hemolytic activity
The LAB strains were streaked onto Columbia agar containing 5% (w/v) sheep blood and incubated for 24 h at 37 °C for hemolysis (Jeon et al., 2017; Taheur et al., 2016). α-Hemolysis, β-hemolysis, and γ-hemolysis appeared as a green-hued zone, clear zone, and no clear zone the around the colonies, respectively. E. coli ATCC 25922, S. Typhimurium P99, and S. aureus KCCM 11335 and were used as positive controls for α- and for β-hemolysis, respectively.
Antibiotic susceptibility
The antibiotic susceptibilities of the LAB strains were measured according to Clinical and Laboratory Standards Institute guidelines (CLSI, 2014). The disc diffusion method was used to evaluate the susceptibility to clinically important antibiotics, such as ampicillin, gentamicin, kanamycin, streptomycin, tetracycline, ciprofloxacin, chloramphenicol, and doxycycline. The LAB strains (1 × 106 CFU/mL) were spread onto MRS agar, and antibiotic discs were placed on the MRS agar surface. After incubation for 24 h at 37 °C, the diameters (mm) of the clear zones were measured.
Production of enzymes
Enzyme production of was analyzed using the API ZYM kit (BioMerieux, Marcy-lÈtoile, France). The LAB strains were incubated in MRS broth at 37 °C for 24 h. The overnight cultures of LAB strains were centrifuged (14,240 × g, 5 min, 4 °C). The cell pellet was resuspended in PBS (phosphate-buffered saline, Hyclone, Logan, UT, USA). Next, 65 μL of the LAB strains (106 CFU/mL) was inoculated into each cupule. After incubation at 37 °C for 4 h, ZYM A and ZYM B reagents were sequentially dropped into each cupule. Enzyme production was measured by substrate hydrolysis measured as the color change of the sample.
Adhesion ability to HT-29 cells
HT-29 cells were obtained from the Korean Cell Line Bank (KCLB 30038, Seoul, Korea). The culture conditions were RPMI 1640 medium (Hyclone) with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin, and incubated at 37 °C with 5% CO2.
For the adhesion ability, HT-29 cells were seeded into 24-well polystyrene plates at 1 × 105 cells/well. And the plates incubated at 37 °C in a 5% CO2 incubator. After incubation for 24 h, 2 × 107 CFU/well of the LAB strains were added to pre-seeded HT-29 cells and incubated for 2 h and then washed thrice with PBS. Next, 1 mL of 1% (v/v) Triton X-100 (Sigma-Aldrich) solution was added to detach the bacteria during incubation for 10 min. After diluting the detached LAB strains, the strains were spread onto MRS agar and incubated for 24 h at 37 °C.
Cell surface hydrophobicity test
The hydrophobicity of LAB strains was measured as described previously with some modifications (Saini and Tumar, 2017; Taheur et al., 2016). Three solvents were used to characterize the cell surface: chloroform (monopolar and Lewis-acid solvent), ethyl acetate (monopolar and Lewis-base solvent), and xylene (apolar solvent). The LAB were incubated in MRS broth at 37 °C for 24 h. These cultures were centrifuged at 14,240 × g for 5 min. The culture supernatants were washed twice and resuspended in PBS. The resuspended cells were adjusted to an OD600 of 0.5 (ODInitial). Next, 3 mL of the resuspended cells was mixed with 1 mL of solvents (chloroform, ethyl acetate, and xylene) and pre-incubated for 10 min at 37 °C. The mixture was mixed for 1 min and incubated at 37 °C for 20 min. Incubated mixture was separated into two phases (water and solvent). One milliliter of the aqueous phase was collected and the absorbance was measured at 600 nm (ODTime). The cell surface hydrophobicity was expressed as follows:
Antimicrobial activity to pathogens
The antimicrobial activity of the LAB strains was determined using a modified deferred method described by Son et al. (2017). For this assay, indicator pathogenic strains, such as L. monocytogenes ATCC 15313, S. Typhimurium, E. coli ATCC 25922, and S. aureus KCCM 11335 were used. To assess the antimicrobial activity, 3 μL of LAB strains (approximately 109 CFU/mL), was spotted onto MRS agar and incubated for 24 h at 37 °C. Next, 100 μL of the indicator pathogens strain (approximately 106 CFU/mL) was inoculated into 4 mL of TSA soft agar, and the soft agar was overlaid. The plate was incubated for 24 h at 37 °C. The clear zones were measured and represented as diameters (mm).
Inhibition of adherence of pathogens to HT-29 cells
The inhibition of pathogen adherence was evaluated by using modified method described by Jeon et al. (2017). HT-29 cells (1 × 105 cells/well) were seeded into 24-well plates and incubated at 37 °C in a 5% CO2 incubator for 24 h. Approximately 106 CFU/well of pathogens with or without L. brevis KU15153 were adjusted and incubated at 37 °C for 2 h. After incubation, non-adherent cells were washed twice with PBS. And then the cells were added to 1 mL of 1% (v/v) Triton X-100 solution to detach the HT-29 cells. The mixture was spread onto Oxford, XLD, EMB, and MSA agar for E. coli, L. monocytogenes, S. Typhimurium, and S. aureus, respectively, to count the number of viable cells.
Auto-aggregation and co-aggregation assay
Auto-aggregation was measured as the auto-aggregation percentage (Lee et al., 2015). Bacterial cells were centrifuged at 14,240 × g for 10 min and washed twice with PBS. The bacterial cells were adjusted to an OD600 of 0.3 ± 0.05. To evaluate auto-aggregation, each bacteria suspension (4 mL) was incubated at 37 °C for 4 and 24 h. The absorbance was read at 600 nm at 0, 4, and 24 h after incubation. Auto-aggregation was expressed as follows:
where ODTime and ODInitial represented the absorbance at a specific incubation time and initial time, respectively.
To examine co-aggregation, each LAB bacterial suspension (2 mL) was mixed with each pathogen (2 mL) and incubated at 37 °C for 4 and 24 h. The absorbance was measured at 600 nm. Co-aggregation was expressed as follows:
where ODP, ODL, and ODMix represented absorbance of pathogen, LAB, and mixed strains, respectively.
Antioxidant activity
Lactobacillus brevis KU15153 and LGG were cultured at 37 °C for 24 h. The LAB strains were centrifuged (14,240 × g, 5 min, 4 °C) and washed twice with PBS. The washed bacterial cells were resuspended in PBS to 107 CFU/mL.
DPPH radical scavenging assay was measured as described by Lee et al. (2015). First, 120 μL of resuspended LAB strains (107 CFU/mL) was added to 120 μL of 400 μM DPPH solution (Das and Goyal, 2015) in similar proportions. The mixture was shaken and reacted at 37 °C for 30 min in the dark space. The absorbance was measured at 517 nm. The percentage scavenging of DPPH radicals was expressed as follows:
The β-carotene-linoleic inhibition assay was conducted as described by Jang et al. (2018). A β-carotene solution was prepared by adding 2 mg of β-carotene, 44 μL of linoleic acid, and 0.2 mL of Tween 80 in 10 mL of chloroform. Next, the chloroform in mixtures were removed using a rotary evaporator at 40 °C and then the mixture was immediately mixed with 100 mL of distilled water. Part of this mixture (4.5 mL) was added to 0.5 mL of LAB strains (107 CFU/mL). The mixture was incubated at 50 °C water bath for 2 h. The absorbance was measured at 470 nm, and the remaining β-carotene in the mixture was expressed as follows:
Statistical analysis
The results for each treatment were obtained in triplicate, and a one-way analysis of variance (SPSS software version 19; IBM, Armonk, NY, USA) was performed to determine the significance of differences among the mean values.
Results and discussion
Tolerance to artificial gastric juice and bile acid
Probiotic organisms must survive in low pH environments (pH 2.5–3.5) and under the 0.3% bile salt conditions of the gastrointestinal tract (Lee et al., 2015). The tolerance of L. brevis KU15153 and LGG to gastric conditions is presented in Table 1. The cell number of L. brevis KU15153 decreased by 0.15 log CFU/mL under artificial gastric conditions (70.79% survival rate) and increased by 0.65 log CFU/mL under bile salt conditions (104.47% survival rate). However, the cell number of LGG showed survival rates of 52.48% and 101.91% under artificial gastric conditions and bile salt conditions, respectively. L. rhamonsus KCTC 12202BP and L. brevis G1 showed 81.28% and 87.09% survival rates at pH 2.5 in the presence of 0.3% pepsin, respectively (Son et al., 2017). L. rhamonsus KCTC 12202BP and L. brevis G1 survival increased in 0.3% oxgall for 24 h. These results indicate that compared to LGG, the survival of L. brevis KU15153 was higher survival in artificial gastric and bile salts.
Table 1.
Tolerance to artificial gastric acid and bile salts and adherence to intestinal cells of LGG and L. brevis KU15153
| Treatment | Cell no. (Log CFU/mL) | |
|---|---|---|
| LGG | L. brevis KU15153 | |
| Tolerance to artificial gastric acid and bile salts | ||
| Initial cell no. | 8.76 ± 0.06c | 8.30 ± 0.02d |
| pH 2.5, 0.3% (w/v) pepsin, 3 h | 8.48 ± 0.03b | 8.15 ± 0.10c |
| 0.3% (w/v) oxgall, 24 h | 9.04 ± 0.03d | 8.95 ± 0.02e |
| Adhesion to HT-29 cell | ||
| Initial cell no. | 8.77 ± 0.04c | 7.94 ± 0.01b |
| Adhesion cell no. | 7.51 ± 0.14a | 6.89 ± 0.06a |
LGG, L. rhamnosus GG
Values are expressed as mean ± standard deviation
a−eThe superscript letters in the same row indicate statistical differences (p < 0.05)
Hemolytic activity
Lactobacillus brevis KU15153 and LGG showed no hemolysis (γ-hemolysis) when incubated on sheep blood plates at 37 °C for 24 h under anaerobic conditions (data not shown). However, S. aureus KCCM 11335 showed α-hemolysis. Additionally, S. Typhimurium P99 and E. coli ATCC 25922 showed β-hemolysis. Therefore, the selected L. brevis KU15153 is non-pathogenic and considered as safe organism for human health.
Antibiotic susceptibility of the LAB strains
Antibiotic susceptibility tests of probiotics are necessary to determine the safety of these organisms in humans or animals. L. brevis KU15153 was resistant to streptomycin and ciprofloxacin and susceptible to ampicillin, gentamycin, kanamycin, tetracycline, chloramphenicol, and doxycycline (data not shown). In a previous study, L. brevis KU15006 was resistant to gentamycin, kanamycin, streptomycin, and ciprofloxacin, while ampicillin, tetracycline, chloramphenicol, and doxycycline susceptible to the other antibiotics (Son et al., 2017).
Enzyme production determined using API ZYM kit
Enzyme production is an important criterion among probiotic properties (Cole et al., 1989). Increases in β-glucuronidase in the feces are involved in gastric cancer and inflammatory bowel disease (Mroczynska et al., 2013). Thus, probiotic strains should not produce carcinogenic enzymes such as β-glucuronidase. L. brevis KU15153 produced various non-hazardous enzymes including acid phosphatase, leucine arylamidase, naphthol-AS-BI-phosphohydrolase, and valine arylamidase, and did not produce the hazardous β-glucuronidase. LGG did not produce β-glucuronidase (data not shown).
Ability to adhere to HT-29 cells
The adherence ability of HT-29 cells is essential for identifying potential probiotic bacteria. The capacity of LAB strains to adhere to HT-29 cells was assessed, and the results are shown in Table 1. The initial inoculum of L. brevis KU15153 was 7.94 log CFU/well. After 2 h, the number of adherent bacteria to HT-29 cells was 6.89 log CFU/well for LGG (5.62% adhesion rate). LGG showed a lower adhesion rate than L. brevis KU15153 (8.91% adhesion rate). Leuconostoc mesenteroides H40, L. plantarum (FI10604, Lb41, and Ln1), L. brevis FI10700, and Lactobacillus perolens FI10842) showed variable adhesion rates (2.86–12.37%) (Jang et al., 2018; Jeon et al., 2016; Son et al., 2018). As a result, the intestinal adhesion ability of L. brevis KU15153 was higher than that of LGG and showed values acceptable for use as a probiotic.
Cell surface hydrophobicity test
The cell surface hydrophobicity of a probiotic is associated with its adhesion ability. The cell surface hydrophobicity results are shown in Fig. 1. L. brevis KU15153 showed higher affinity in chloroform (69.25%) than in ethyl acetate (19.88%) and xylene (44.34%). Lee et al. (2015) reported that the hydrophobicity values of L. lactis KC24 were 54.41, 18.44, and 3.66% in chloroform, xylene, and ethyl acetate, respectively. Therefore, L. brevis KU15153 was characterized as s Lewis acid; these characteristics in solvents may greatly influence adhesion ability (p < 0.05) (Taheur et al., 2016).
Fig. 1.
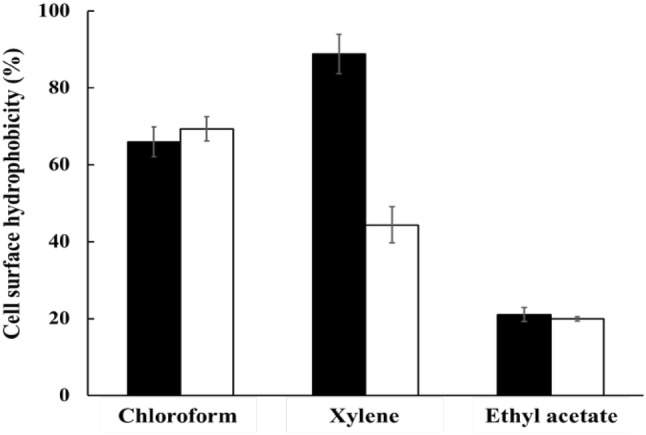
Cell surface hydrophobicity of LGG and L. brevis KU15153 to solvents. Filled square, LGG (L. rhamnosus GG); Open square, L. brevis KU15153. Each value represents the mean ± standard deviation, and different letters on each bar represent significant difference between values (p < 0.05)
Antimicrobial activity using modified deferred method
The antimicrobial activity of L. brevis KU15153 was assessed using a modified deferred method (Table 2). L. brevis KU15153 showed distinct antibacterial activity against E. coli ATCC 25922, L. monocytogenes ATCC 15313, S. Typhimurium P99, and S. aureus KCCM 11335. Among the pathogens, S. aureus KCCM 11335 showed the largest inhibition zone. These antimicrobial activities originated from the production of metabolites such organic acids (lactic acid, acetic acid, etc.), hydrogen peroxide, diacetyl, and bacteriocin, among other molecules (Oliveira et al., 2017; Šušković et al., 2010).
Table 2.
Antagonistic effects against foodborne pathogens of LGG and L. brevis KU15153 by deferred method
| Pathogens | Inhibitory diameter (mm) | |
|---|---|---|
| LGG | L. brevis KU15153 | |
| Escherichia coli ATCC 25922 | 18.67 ± 4.73b | 13.00 ± 2.00a |
| Listeria monocytogenes ATCC 15313 | 22.33 ± 0.58b | 14.67 ± 1.15ab |
| Salmonella Typhimurium P99 | 3.00 ± 0.00a | 16.67 ± 2.89ab |
| Staphylococcus aureus KCCM 11335 | 22.33 ± 8.69b | 18.00 ± 3.00b |
LGG, L. rhamnosus GG
All values are mean ± standard deviation
a–bThe superscript letters in the same row indicate statistical differences (p < 0.05)
Inhibition of adherence of pathogens to HT-29 cells
Adhesion to HT-29 cells was assessed by counting the number of bacteria adhered to HT-29 cells (Table 3). E. coli ATCC 25922, L. monocytogenes ATCC 15313, S. Typhimurium P99, and S. aureus KCCM 11335 numbers were reduced to 0.59, 0.12, 0.95, and 0.49 CFU/mL, respectively. Particularly, S. Typhimurium P99 with L. brevis KU15153 to HT-29 cell was decreased remarkably. In a previous study, adhesion of L. monocytogenes and S. aureus to Caco-2 cells was reduced by L. lactis KC24 (Lee et al., 2015). These results indicate that L. brevis KU15153 can modify the intestinal microflora by inhibiting adhesion of pathogens to the intestines.
Table 3.
Inhibition activity of L. brevis KU15153 against adherence of foodborne pathogens to HT-29 cells
| Pathogens | Adherent cell no. (Log CFU/mL) | |
|---|---|---|
| Pathogen | Pathogens with L. brevis KU15153 | |
| Escherichia coli ATCC 25922 | 7.49 ± 0.84b | 6.90 ± 0.09d |
| Listeria monocytogenes ATCC 15313 | 5.17 ± 0.17a | 5.05 ± 0.14b |
| Salmonella Typhimurium P99 | 5.70 ± 0.11a | 4.75 ± 0.26a |
| Staphylococcus aureus KCCM 11335 | 6.99 ± 0.02b | 6.50 ± 0.01c |
All values are mean ± standard deviation
a–dThe superscript letters in the same row indicate statistical differences (p < 0.05)
Auto-aggregation and co-aggregation
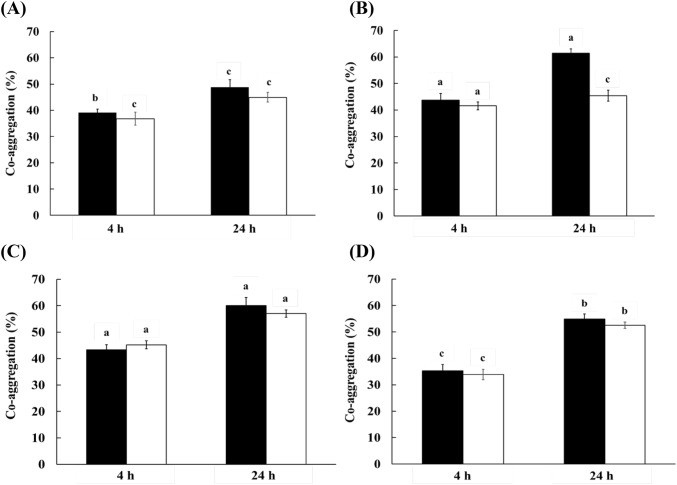
Auto-aggregation and co-aggregation were evaluated to assess colonization of bacteria on intestinal cells (Fig. 2). At 4 h incubation, L. brevis KU15153 and LGG showed 21.44% and 22.68% auto-aggregation abilities, respectively. After 24 h incubation, auto-aggregation of L. brevis KU15153 (52.55%) was higher than that of LGG (44.70%). The co-aggregation activity of L. brevis KU15153 with pathogens was 40-55% after 24 h of incubation. Co-aggregation of L. brevis KU15153 with pathogens showed a lower coaggregation ability than LGG. In another study, co-aggregation of L. lactis KC24 with pathogens (L. monocytogenes and S. aureus) was the 29.28–74.11%. These auto-aggregation and co-aggregation abilities of L. brevis KU15153 suggest that this strain helps to prevent colonization or modification under the conditions of the intestine (Lee et al., 2015).
Fig. 2.
Co-aggregation activities of LGG and L. brevis KU15153 with pathogens. (A) LAB with Listeria monocytogenes ATCC 15313, (B) LAB with Salmonella Typhimurium P99, (C) LAB with Escherichia coli ATCC 25922, and (D) LAB with Staphylococcus aureus KCCM 11335. Filled square, LGG (L. rhamnosus GG); open square, L. brevis KU15153. Each value represents the mean ± standard deviation and different letters on each bar represent significant difference between values (p < 0.05)
Antioxidant activity
The antioxidant activity of LAB strains plays an important role in the protection from free radicals (Ren et al., 2014). The antioxidant activity of L. brevis KU15153 was measured by DPPH radical scavenging and β-carotene bleaching assays (Table 4). The DPPH radical scavenging and β-carotene bleaching inhibitory activities of L. brevis KU15153 were higher than those of LGG. L. brevis KU15153 and LGG showed DPPH radical scavenging activities of 44.14% and 19.21% at 107 CFU/mL, respectively. The β-carotene bleaching activities of L. brevis KU15153 and LGG were 71.62% and 64.25%, respectively. Aarti et al. (2017) reported that the DPPH radical scavenging activity of L. brevis LAP2 increased in a concentration-dependent manner (18.8–68.35% at 108–109 CFU/mL). Some L. brevis strains (O-9 and LSe) showed low DPPH radical scavenging activities with values of 0% and 9.3% (Shakibaie et al., 2017; Uugantsetseg and Batjargal, 2014). These antioxidant effects were reported to be strain-specific based on the cell wall composition, enzyme, metabolite production, etc. (Oh et al., 2018).
Table 4.
Antioxidant activities of LGG and L. brevis KU15153 with different mechanisms
| Microorganisms | Antioxidant activity (%) | |
|---|---|---|
| DPPH radical scavenging activity | β-Carotene bleaching inhibitory activity | |
| LGG | 19.21 ± 2.92a | 64.25 ± 6.27a |
| L. brevis KU15153 | 44.14 ± 0.23b | 71.62 ± 6.87a |
LGG, L. rhamnosus GG
All values are mean ± standard deviation
a–bThe superscript letters in the same row indicate statistical differences (p < 0.05)
In conclusion, L. brevis KU15153 isolated from kimchi showed potential probiotic properties such as stability under gastric conditions and adherence to intestinal cells. Additionally, L. brevis KU15153 showed antibiotic susceptibility and hemolytic activity, and thus appears to be safe. L. brevis KU15153 may have modified the intestinal conditions by inhibiting pathogenic strains and adhering pathogens to intestinal cell. Its antioxidant activity was higher than that of LGG in DPPH radical scavenging and β-carotene bleaching inhibitory activities. Therefore, L. brevis KU15153 may be useful as a probiotic organism and can be applied in the functional food industry as a safe food additive.
Acknowledgements
This research was supported by the Agri-food R&D Performance Follow-up Support Program (2018100713) of the Korean Ministry of Agriculture, Food and Rural Affairs.
Compliance with ethical standards
Conflict of interest
Jang, Lee, and Paik declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hye Ji Jang, Email: gpwljjj@naver.com.
Na-Kyoung Lee, Email: nakyoung_lee@nate.com.
Hyun-Dong Paik, Email: hdpaik@konkuk.ac.kr.
References
- Aarti C, Khusro A, Varghese R, Varghese R, Arasu MV, Agastian P, Al-Dhabi NA, Ilavenil S, Choi KC. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT-Food Sci. Technol. 2017;86:438–446. doi: 10.1016/j.lwt.2017.07.055. [DOI] [Google Scholar]
- Bae G, Kim J, Kim H, Seok JH, Lee DB, Kim KH, Chung MS. Inactivation of norovirus surrogates by kimchi fermentation in the presence of black raspberry. Food Control. 2018;91:390–396. doi: 10.1016/j.foodcont.2018.04.025. [DOI] [Google Scholar]
- CLSI. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. M100–S24. Clinical and Laboratory Standards Institute, Wayne, PA, USA (2014)
- Cole CB, Fuller R, Carter SM. Effect of probiotic supplements of Lactobacillus acidophilus and Bifidobacterium adolescentis 2204 on β-glucosidase and β-glucuronidase activity in the lower gut of rats associated with a human faecal flora. Microb. Ecol. Health Dis. 1989;2:223–225. doi: 10.3109/08910608909140223. [DOI] [Google Scholar]
- Das D, Goyal A. Antioxidant activity and γ-aminobutyl acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT Food Sci. Technol. 2015;61:263–268. doi: 10.1016/j.lwt.2014.11.013. [DOI] [Google Scholar]
- FAO/WHO. Joint FAO/WHO (Food and Agriculture Organization/World Health Organization) Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. Ontario: London (2002)
- Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from Kimchi. J. Food Sci. Technol. 2018;55:3174–3180. doi: 10.1007/s13197-018-3245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon EB, Son SH, Jeewanthi RKC, Lee NK, Paik HD. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Sci. Biotechnol. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HL, Lee NK, Yang SJ, Kim WS, Paik HD. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017;26:1641–1648. doi: 10.1007/s10068-017-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto-Nira H, Suzuki S, Suganuma H, Moriya N, Suzuki C. Growth characteristics of Lactobacillus brevis KB290 in the presence of bile. Anaerobe. 2015;35:96–101. doi: 10.1016/j.anaerobe.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Lee KW, Shim JM, Park SK, Heo HJ, Kim HJ, Ham KS, Kim JH. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT-Food Sci. Technol. 2016;71:130–137. doi: 10.1016/j.lwt.2016.03.029. [DOI] [Google Scholar]
- Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Sci. Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- Lee NK, Kim SY, Han KJ, Eom SJ, Paik HD. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sci. Technol. 2014;58:130–134. doi: 10.1016/j.lwt.2014.02.028. [DOI] [Google Scholar]
- Mroczynska M, Gałecka M, Szachta P, Kamoda D, Libudzisz Z, Roszak D. β-Glucuronidase and β-glucosidase activity in stool specimens of children with inflammatory bowel disease. Pol. J. Microbiol. 2013;62:319–325. [PubMed] [Google Scholar]
- Oh NS, Joung JY, Lee JY, Kim Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLos One. 2018;13(2):e0192021. doi: 10.1371/journal.pone.0192021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LC, Silveira AMM, Monteiro AS, Santos VL, Nicoli JR, Azevedo VAC, Soares SC, Dias-Souza MV, Nardi RMD (2017). In silico prediction, in vitro antibacterial spectrum, and physicochemical properties of a putative bacteriocin produced by Lactobacillus rhamnosus strain L156.4. Font. Microbiol. 8: 876 (2017) [DOI] [PMC free article] [PubMed]
- Park SE, Seo SH, Kim EJ, Na CS, Son HS. Effects of different fermentation temperatures on metabolites of Kimchi. Food Biosci. 2018;23:100–106. doi: 10.1016/j.fbio.2018.03.009. [DOI] [Google Scholar]
- Ren D, Li C, Qin Y, Yin R, Du S, Ye F, Liu C, Liu H, Wang M, Li Y, Sun Y, Li X, Tian M, Jin N. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe. 2014;30:1–10. doi: 10.1016/j.anaerobe.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Riccia DN, Bizzini F, Perilli MG, Polimeni A, Trichieri V, Amicosante G, Cifone MG. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007;13:367–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- Rushdy AA, Gomaa EZ. Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Ann. Microbiol. 2013;63:81–90. doi: 10.1007/s13213-012-0447-2. [DOI] [Google Scholar]
- Saini K, Tumar SK. In vitro evaluation of probiotic potential of Lactobacillus cultures of human orgin capable of selenium bioaccumulation. LWT-Food Sci. Technol. 2017;84:497–504. doi: 10.1016/j.lwt.2017.05.034. [DOI] [Google Scholar]
- Shakibaie M, Mohammadi-Khorsand T, Adeli-Sardou M, Jafari M, Amirpour-Rostami S, Ameri A, Forootanfar H. Probiotic and antioxidant properties of selenium-enriched Lactobacillus brevis Lse isolated from an Iranian traditional dairy product. J. Trace Elem Med Biol. 2017;40:1–9. doi: 10.1016/j.jtemb.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kaur J, Lee S, Park YS. Molecular discrimination of Lactobacillus brevis strains isolated from food products in South Korea using multilocus sequence typing. LWT-Food Sci. Technol. 2017;86:337–343. doi: 10.1016/j.lwt.2017.08.016. [DOI] [Google Scholar]
- Son SH, Jeon HL, Jeon EB, Lee NK, Park YS, Kang DK, Paik HD. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci. Technol. 2017;85:181–186. doi: 10.1016/j.lwt.2017.07.018. [DOI] [Google Scholar]
- Son SH, Jeon HL, Yang SJ, Sim MH, Kim YJ, Lee NK, Paik HD. Probiotic lactic acid bacteria isolated from traditional Korean fermented foods based on β-glucosidase activity. Food Sci. Biotechnol. 2018;27:123–129. doi: 10.1007/s10068-017-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šušković J, Kos B, Beganović J, Pavunc AL, Habjanić K, Matošić S. Antimicrobial activity – the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010;48:296–307. [Google Scholar]
- Taheur FB, Kouidhi B, Fdhila K, Elabed H, Slama RB, Mahdouani K, Bakhrouf A, Chaieb K. Anti-bacterial and anti-biofilm activity of probiotic bacteria against oral pathogens. Microb. Pathog. 2016;97:213–220. doi: 10.1016/j.micpath.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Uugantsetseg E, Batjargal B. Antioxidant activity of probiotic lactic acid bacteria isolated from Mongolian airag. Mong. J. Chem. 2014;15:73–78. doi: 10.5564/mjc.v15i0.327. [DOI] [Google Scholar]
- Vitali B, Minervini G, Rizzello CG, Spisni E, Maccaferri S, Brigidi P, Gobbetti M, Cagno RD. Novel probiotic candidates for humans isolated from raw fruits and vegetables. Food Microbiol. 2012;31:116–125. doi: 10.1016/j.fm.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Waki N, Matsumoto M, Fukui Y, Suganuma H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among school children: An open-label pilot study. Lett. Appl. Microbiol. 2014;59:565–571. doi: 10.1111/lam.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]