Abstract
The perceived aroma of oolong tea is primarily and directly affected by its infusion matrix, and the release persistence of headspace volatiles can better illustrate the persistent aroma. Headspace solid phase microextraction coupled with gas chromatography–mass spectrometry was performed to analyze the headspace constituents of oolong tea. The presence of the infusion matrix seemed to prevent the headspace release of certain odorants. The release of indole, nerolidol, and α-farnesene was also remarkably enhanced or depressed (2.70, 1.56, and 0.69-fold in tea infusion versus in dry leaves). Moreover, the amount of volatile species gradually decreased with increased water ratio. Eight odorants were determined to be stable and persistent during continuous infusion, whereas six were determined to be less persistent (gradually decreased or stopped releasing). The volatile dilution test further confirmed the persistent release of nerolidol.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00587-8) contains supplementary material, which is available to authorized users.
Keywords: Volatiles, Oolong tea, Infusion matrix, Aroma persistence, Headspace solid phase microextraction (HS-SPME)
Introduction
Oolong tea is a partially fermented tea, with an annual world production of over 250,000 tons (Mei and Wang, 2017). The most important attributes of oolong tea are its pleasant and persistent aroma, which can be fruity, floral, roasted or honey-like. Consisting of hundreds of volatile compounds (Ho et al., 2015), oolong tea aroma is mainly formed during the manufacturing process (e.g., withering, tossing, and baking). Endogenous enzymatic hydrolysis of glycosidic precursors can release plenty of floral/fruity odorants (such as linalool, geraniol, benzyl alcohol, and methyl salicylate) (Ogawa et al., 1995), and the derived volatiles from carotenoids photooxidation, lipids oxidation, and Maillard reaction are also responsible for aroma formation in oolong tea (Chen et al., 2013; Ho et al., 2015). Various factors, including tea variety (Lin et al., 2013), raw materials (Zeng et al., 2017b), manufacturing process (Zeng et al., 2016), and storage conditions (Chen et al., 2013), have been reported to influence the volatiles contained in oolong tea. In addition, a number of odor-active components have been determined in green tea (Katsuno et al., 2014; Kumazawa and Masuda, 2002), black tea (Schuh and Schieberle, 2006), and jasmine tea (Ito et al., 2002), and are suspected to be key contributors to the perceived aroma.
Nevertheless, the sensory aroma of oolong tea is perceived and evaluated during daily infusions. Accordingly, the perceived aroma is primarily and directly affected by the aroma sources (e.g., dry leaves, tea infusion, infused leaves), water ratio, infusion frequency, and the infusion matrix. Further hydrolysis of volatile precursors in tea infusion has been reported, with geraniol significantly increased in black tea infusion, compared to its concentration in the leaves (Schuh and Schieberle, 2006). Aroma release affected by the infusion matrix of Longjing tea has also been presented (Cheng et al., 2008). The effect on volatile solubility and headspace amount by the interactions with nonvolatile components (e.g., catechins and caffeine) in wine and other foods has been well documented in the literature (Dufour and Bayonove, 1999; Robinson et al., 2009). Therefore, it is interesting to systematically study the headspace release of oolong tea volatiles during the infusion process, particularly under the different situations mentioned above.
For this aim, the volatile profiles of dry leaves and tea infusions, and the impact of water ratio and infusion frequency on headspace release were comparatively studied. Headspace odorants most affected by the infusion matrix were explored, since the influences from tea liquid are crucial but are normally ignored when exploring the key odorants contributing to perceived aroma. And a model tea infusion matrix was prepared to investigate the effects of the concentration of individual odorants within the infusion on headspace release. The release persistence of headspace volatiles after continuous infusion was also examined, especially after the 3rd infusion, and this might tell us the odorants contribute to the persistent aroma of oolong tea. The headspace solid phase microextraction and gas chromatography (HS-SPME–GC–MS) technique was performed in the present study to analyze headspace constituents, due to its advantages of convenient and solvent-free (Caprioli et al., 2012; Romeo et al., 2007). The headspace analytes were trapped on the fiber, in which case interference from the infusion matrix can be drastically reduced (Elmore et al., 1997; Jelen et al., 1998; Riu-Aumatell et al., 2004).
Materials and methods
Materials and chemicals
Oolong tea samples (Tieguanyin, produced in autumn) were collected from local producers of Anxi County of China in late November. Benzyl alcohol (purity ≥ 99.5%), 2-phenylethanol (purity ≥ 99.5%), indole (purity ≥ 99.5%), nerolidol (purity of 97%), and glucose (purity ≥ 99.5%) were purchased from Shanghai Aladdin Reagent Company (Shanghai, China). Tea polyphenols (> 98% HPLC grade) were obtained from Shaanxi Sciphar Natural Products Co., Ltd, (Xian, China). Caffeine (purity ≥ 98.5%) was purchased from Sigma-Aldrich China, Inc. (Shanghai, China).
Oolong tea brewing
Ten gram of tea sample was infused with 100 mL boiling tap water, and the tea liquid was poured out after 6 min of brewing. The freshly prepared tea infusion and 10 g of dry tea sample were used for subsequent brewing to compare their headspace volatiles. Three oolong tea samples were used for comparison.
For tea infusions brewed by tea/water ratios of 1:3, 1:10, 1:20, and 1:50 (w/v), a 10-g tea sample infused by 30 mL boiling water, a 10-g tea sample infused by 100 mL boiling water, a 5-g tea sample infused by 100 mL boiling water, and a 2-g tea sample infused by 100 mL boiling water were prepared separately. The tea liquid was poured out after 6 min of brewing and used for volatile analysis.
For each infusion frequency test, a 10-g tea sample was infused with 100 mL boiling water, and the tea liquid was poured out after 2 min of brewing. Then, a volume of boiling water equivalent to that of the poured tea liquid was added to continue the next infusion. Fifty milliliters of tea liquid from the 1st, 2nd, 3rd, 5th, and 7th infusion was used for volatile analysis.
Model infusion and volatile dilution test
A simple model infusion of oolong tea consisting of 1.5 g/L tea polyphenols, 1.5 g/L glucose, and 2.0 g/L caffeine was prepared with boiling tap water. Separately, 5 μL benzyl alcohol (or its diluted solution), 5 μL 2-phenylethanol (or its diluted solution), 1 μL nerolidol (or its diluted solution), and 0.01 g indole (or its diluted solution) diluted in 100 mL freshly prepared model infusion were prepared as the solutions for analysis. The volatile solution was maintained for 5 min in a 50 °C water bath to facilitate volatilization. Three milliliters of headspace was directly injected into the injector set of GC/MS for analysis.
HS-SPME/GC–MS procedure
For analysis of the volatiles during the infusion process, the analyzed sample was transferred to a 100 mL glass septum flask. For analysis of the volatiles during the infusion process, the analyzed sample was transferred to a 100 mL glass septum flask. SPME device (Supelco, Bellefonte, PA, USA) was rapidly inserted into the headspace for volatile extraction. The SPME fibre was coated with 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB). Before each extraction, the SPME fibre was preconditioned for 5 min in the injection port of the GC at 220 °C. The headspace extraction was kept in a water bath at 50 °C, and lasted for 40 min. Agilent 6890 gas chromatograph coupled to an Agilent 5973N mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) was used to perform volatile detection and identification. The GC oven was equipped with an HP-INNOWax capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies). The SPME fiber was desorbed at 220 °C in the GC injection port in splitless mode for 3.5 min. Helium (purity of 99.999%) was used as the carrier gas at a constant flow rate of 1 mL/min. The temperature program was set as following: 50 °C for 5 min, 3 °C/min to 220 °C, and held for 5 min, and finally 10 °C/min to 240 °C and held for 5 min. The ion source temperature was set at 200 °C, and MS was scanned at 70 eV between 35 and 600 amu. Peak area was calculated by ChemStation software (Agilent Technologies). Peak identification was made by searching the MS data library (NIST98) and further comparing the retention index (RI) with published data. Kovats’ retention indices for each peak were calculated by using an n-alkane mixture (C8–C20; Sigma-Aldrich, St Louis, MO, USA) under the same GC conditions. Volatile detection was carried out in triplicate for each sample.
Statistical and multivariate analysis
The relative percentages (relative content) of the aligned peaks were obtained by peak-area normalization and imported to form a data set for multivariate analysis. Principal component analysis (PCA) and loading S-plot were performed by SIMCA-P+ (version 11.0, Umetrics, Umeå, Sweden). S-plot was declared with weight (w) and correlation (pcorr) based on orthogonal partial least squares discriminant analysis (OPLS-DA) in order to screen the compounds most responsible for group differences. This visualization tool is used for the identification of possible interesting compounds for orthogonal variation (Wiklund et al., 2008). The raw data set was mean-centered and Pareto-scaled before performing S-plot.
Results and discussion
Headspace volatiles influenced by the infusion matrix
The differences in headspace volatiles between dry leaves and tea infusion (with leaves) might tell us the influence of the infusion matrix, particularly for the volatiles that are most affected. Forty-eight common volatiles were clearly detected in the dry leaves of three oolong tea samples, whereas only 36 common volatiles were detected in samples of tea infusion (Table S1 in the Supplementary Materials). At least 12 volatiles could not be detected in the headspace of the tea liquid. Consistent with the previous study of Christian and Schieberle (Schuh and Schieberle, 2006), it was also discovered that more volatiles were identified in the dry leaves of Darjeeling black tea compared to the hot water infusion.
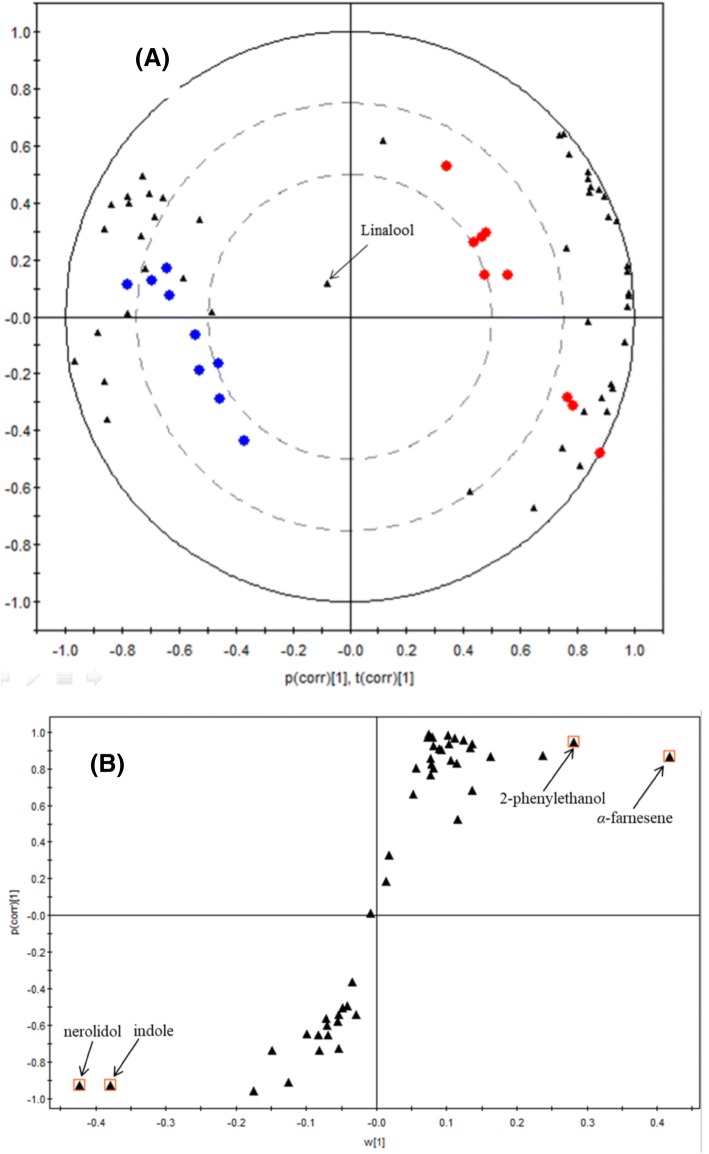
To have a better visualization of the headspace volatiles most affected by tea matrix, PCA using volatiles data was performed between the two groups. Bi-plot containing object scores and variable loadings were drawn, as showed in Fig. 1A. A clear separation of dry leaves (red balls) and tea infusion (blue balls) could be observed, indicating that the volatile profiles were quite different between the two groups. Linalool seemed to be the most stable volatile either between the two groups or among different analyses. An S-plot was further generated for the sake of the most differential volatiles between the two groups (Fig. 1B). The X-axis represents the weights of the correlation between X-matrix and group scores, while the Y-axis represents the correlation coefficient (Wiklund et al., 2008). The upper right region of the S-plot reveals that α-farnesene and 2-phenylethyl alcohol are the two most differential volatiles in the dry leaves (18.24 and 2.93% in dry leaves versus 9.62% and undetectable in tea infusion). In contrast, the lower left region reveals that nerolidol and indole are the two most differential volatiles in tea infusion (13.86 and 13.81% in tea infusion versus 8.86 and 5.11% in dry leaves).
Fig. 1.
Multivariate analysis of headspace volatiles for dry leaves and tea infusions. (A) PC1 versus PC2 bi-plot, combined with observation scores and variable loadings. ‘Red circle’: dry leaves; ‘blue circle’: tea infusion; ‘filled triangle’: volatiles. (B) S-plot with interesting volatiles most responsible for orthogonal variation highlighted by the red squares. ‘filled triangle’: volatiles. (Color figure online)
When exploring the key odorants contributing to perceived aroma, the influences from tea liquid are crucial but are normally ignored. The headspace release of nerolidol and indole were significantly enhanced with the existence of the infusion matrix. Nerolidol appeared to have rose-like and apple-like odors (Zeng et al., 2017a), and indole appeared to have animal-like (Kumazawa and Masuda, 2002) and floral (Katsuno et al., 2014) odors; both were characterized as the key odorants of tea (Kumazawa and Masuda, 2002; Ma et al., 2014). However, benzyl alcohol, 2-phenylethanol, and linalool oxides, documented as potent key odorants of tea (Ito et al., 2002; Yang et al., 2013), could barely volatilize into the headspace; therefore, they unlikely contributed to the perceived aroma of oolong tea directly.
Alteration of headspace volatiles with the increase of water ratio
Since particular differences in tea volatiles could occur while brewing in hot water, it was also interesting to study the influences of water ratio on volatile release. Tea liquids (without leaves) brewed by tea/water ratios of 1:3, 1:10, 1:20, and 1:50 (w/v) were prepared and analyzed. It is worth noting that the tea/water ratio of approximately 1:50 (w/v) is most frequently used for daily consumption and sensory evaluation of tea. As shown in Table 1, the amount of volatile species in the headspace of tea liquids gradually decreased with the increase of water ratio. In total, 30 volatiles were clearly detected while brewing with a tea/water ratio of 1:3, whereas only 20 volatiles were detected at the ratio of 1:50. Moreover, much fewer volatiles could be detected in tea liquid without leaves compared to dry leaves (48 volatiles) or tea infusion with leaves (36 volatiles).
Table 1.
Relative contents of headspace volatiles at different tea/water ratios
| Compounds | RI | Relative content (%) (mean ± SD)b | |||
|---|---|---|---|---|---|
| 1:3 | 1:10 | 1:20 | 1:50 | ||
| Dodecane | 1200 | 0.24 ± 0.07 | ND | ND | ND |
| (E)-β-Ocimene | 1257 | 4.24 ± 0.76 | 3.11 ± 0.37 | 1.81 ± 0.35 | 0.98 ± 0.14 |
| Tridecane | 1300 | 0.70 ± 0.04 | ND | ND | ND |
| 2-Ethenyl-1,1-dimethyl-3-methylidene-cyclohexane | 1316 | 5.31 ± 0.89 | 3.42 ± 0.20 | 1.44 ± 0.30 | ND |
| (E)-β-Farnesene | 1673 | 2.08 ± 0.36 | 2.48 ± 0.54 | 3.46 ± 0.53 | 3.48 ± 0.02 |
| β-Bisabolene | 1724 | 0.47 ± 0.16 | 0.53 ± 0.09 | ND | ND |
| α-Bergamotene | 1729 | 0.94 ± 0.12 | 0.86 ± 0.10 | 0.93 ± 0.10 | 0.84 ± 0.02 |
| Naphthalene | 1735 | 1.73 ± 0.61 | 2.52 ± 0.50 | 2.69 ± 0.50 | 4.13 ± 0.62 |
| α-Farnesene | 1754 | 10.16 ± 0.58 | 7.87 ± 0.25 | 3.97 ± 0.68 | 1.70 ± 0.06 |
| 1-Methylnaphthalene | 1776 | 0.14 ± 0.01 | 0.14 ± 0.03 | 0.20 ± 0.00 | ND |
| Hydrocarbon | 26.01 ± 1.56 | 20.93 ± 0.53 | 14.49 ± 1.89 | 11.14 ± 0.68 | |
| Linalool | 1562 | 1.59 ± 0.10 | 1.76 ± 0.31 | 1.50 ± 0.07 | 1.62 ± 0.02 |
| Benzyl alcohol | 1837 | 0.39 ± 0.00 | 0.26 ± 0.00 | ND | ND |
| 2-Phenylethyl alcohol | 1897 | 6.52 ± 1.23 | 3.20 ± 0.66 | 1.89 ± 0.17 | ND |
| Nerolidol | 12.66 ± 0.54 | 19.81 ± 1.91 | 23.59 ± 1.13 | 23.59 ± 5.74 | |
| Alcohols | 21.16 ± 1.33 | 25.03 ± 2.53 | 26.98 ± 0.75 | 25.21 ± 2.88 | |
| Isopentyl hexanoate | 1471 | 0.78 ± 0.05 | 0.69 ± 0.12 | ND | ND |
| (Z)-3-Hexenyl butanoate | 1475 | 0.55 ± 0.05 | 0.75 ± 0.06 | ND | ND |
| (Z)-3-Hexenyl hexanoate | 1664 | 1.76 ± 0.07 | 2.27 ± 0.07 | 1.50 ± 0.09 | 1.14 ± 0.26 |
| Methyl salicylate | 1769 | 0.49 ± 0.08 | 0.65 ± 0.18 | 0.68 ± 0.04 | 0.54 ± 0.14 |
| Phenethyl isobutyrate | 1843 | 0.92 ± 0.11 | 1.02 ± 0.14 | 1.05 ± 0.12 | 1.11 ± 0.30 |
| 2-Phenylethyl butanoic acid, 2-methyl-, 2-phenylethyl ester | 1968 | 2.41 ± 0.13 | 3.44 ± 0.51 | 3.58 ± 0.33 | 5.01 ± 1.33 |
| Esters | 6.91 ± 0.09 | 8.82 ± 0.51 | 6.80 ± 0.40 | 7.79 ± 1.02 | |
| Nonanal | 1406 | 0.84 ± 0.20 | ND | ND | ND |
| Benzaldehyde | 1531 | 1.58 ± 0.10 | 1.49 ± 0.37 | 2.40 ± 0.28 | 3.87 ± 0.77 |
| Benzeneacetaldehyde | 1648 | 3.34 ± 0.33 | 3.59 ± 0.45 | 4.32 ± 0.42 | 4.93 ± 0.58 |
| Aldehydes | 5.75 ± 0.62 | 5.08 ± 0.73 | 6.72 ± 0.22 | 8.80 ± 0.20 | |
| 6-Methyl-5-hepten-2-one | 1348 | 1.55 ± 0.33 | 1.51 ± 0.13 | 1.69 ± 0.24 | 2.21 ± 0.36 |
| β-Ionone | 1905 | ND | 0.25 ± 0.03 | 0.15 ± 0.04 | 0.18 ± 0.00 |
| (Z)-Jasmone | 1912 | 0.49 ± 0.09 | 0.57 ± 0.05 | 0.43 ± 0.04 | 0.28 ± 0.04 |
| Ketones | 2.04 ± 0.29 | 2.33 ± 0.18 | 2.27 ± 0.13 | 2.67 ± 0.18 | |
| Benzyl nitrile | 1916 | 5.81 ± 0.35 | 4.77 ± 0.21 | 4.56 ± 0.26 | 3.87 ± 0.18 |
| Unknown-1a | 1955 | 0.65 ± 0.04 | 0.91 ± 0.17 | 1.01 ± 0.05 | 1.12 ± 0.22 |
| Unknown-2a | 2.48 ± 0.14 | 2.66 ± 0.21 | 2.17 ± 0.15 | 2.20 ± 0.18 | |
| Indole | 17.80 ± 1.54 | 18.19 ± 0.93 | 20.24 ± 1.95 | 17.63 ± 1.38 | |
| Total | 88.60 ± 1.09 | 88.73 ± 1.96 | 85.24 ± 2.97 | 80.44 ± 3.73 | |
| (Number of volatiles) | (29) | (27) | (23) | (20) | |
RI retention indices
aMass spectral ions (relative abundance in %): unknown-1: m/z = 104 (100), 207 (50); unknown-2: m/z = 104 (100), 105 (38), 77 (25), 103 (20). retention time: unknown-1: 38.92 min; unknown-2: 43.75 min
bRelative content, percent normalized peak areas (n = 3)
It seemed that the high ratio of hot water might prevent headspace release of some volatiles. It is presumed that these ‘suppressed’ volatiles might be barely soluble in hot water (such as the hydrocarbons) or, on the contrary, easily dissolved in tea infusion (such as benzyl alcohol and 2-phenethyl alcohol), thus prevented in their release into the headspace. The high water ratio would also influence matrix-volatile interaction, which might change headspace partition coefficients of the volatiles. Dodecane, tridecane, and especially nonanal were not detected at the tea/water ratio of 1:10, while headspace release of β-bisabolene, benzyl alcohol, isoamyl hexanoate, and (Z)-3-hexenyl butyrate seemed to be completely suppressed at the tea/water ratio of 1:20. No volatilizations of 1-methylnaphthalene and 2-phenethyl alcohol could be detected at the tea/water ratio of 1:50. In addition, relative contents of (E)-β-ocimene, α-farnesene, and benzyl cyanide also significantly decreased with the increase of water ratio. Interestingly, the relative contents of (E)-β-farnesene, naphthalene, nerolidol, benzaldehyde, phenylacetaldehyde, 2-phenylethyl 2-methylbutyrate, and 6-methyl-5-heptene-2-one in the headspace increased with the water ratio.
Nerolidol, indole, phenylacetaldehyde, and benzyl nitrile were the four principal headspace volatiles determined for oolong tea liquid (without leaves), jointly represented approximately half of the headspace extracts (Table 1). Nerolidol, indole, and phenylacetaldehyde were determined to have rose/apple (Zeng et al., 2017a), animal-like/floral (Katsuno et al., 2014; Qin et al., 2013), and honey-like (Kumazawa and Masuda, 2002) odors, respectively. Considering their high amount and FD factors (Kumazawa and Masuda, 2002; Ma et al., 2014), these three compounds were possible likely the key odorants of oolong tea infusion. Benzyl nitrile has also been reported to be highly relevant to the roasted aroma of tea (Togari et al., 1995).
Release persistence of volatiles during continuous infusing
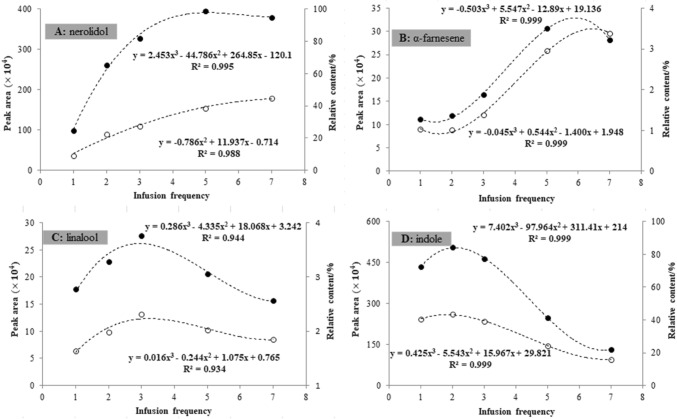
In general, oolong tea can be infused multiple times (normally 3–7 times for daily consumption), while the persistence and richness of the perceived aroma also varies. Therefore, it appeared interesting to examine the volatility persistence of individual odorants after continuous infusion. For this purpose, tea liquids from the 1st, 2nd, 3rd, 5th, and 7th infusion of oolong tea were analyzed, and the headspace intensities (peak areas and relative contents) of the volatiles were compared (Table S2 in the Supplementary Materials). The impact of infusion frequency on headspace release was quite evident, and different dynamic changes could be observed, represented by individual odorants of nerolidol, α-farnesene, linalool, and indole (Fig. 2).
Fig. 2.
Dynamic changes of the headspace release of individual odorants with respect to infusion frequency. ‘Filled circle’: peak area; ‘open circle’: relative content
Nerolidol is a persistent and rich odorant, and its peak area increased from 98.75 × 104 (the 1st infusion) to 394.75 × 104 (the 5th infusion) and remained at 379.89 × 104 at the 7th infusion (Fig. 2A). In addition, the relative content of nerolidol continued to rise from the 1st infusion (9.13%) to the 7th infusion (44.70%), indicating its possible dominant contribution to the persistent aroma of oolong tea. Alpha-farnesene was another persistent odorant, since its headspace intensity was still increasing after infusing five times, as presented in Fig. 2B. During continuous infusion, the dynamic release of linalool showed an upward parabolic curve (Fig. 2C). Moreover, persistent release could also be observed for linalool since the headspace concentration remained at 56.7 (peak area) and 80.1% (relative content) at the 7th infusion compared to the 3rd infusion. Relatively, indole seemed to release much faster but less persistent. A significant decrease in headspace release could be observed after the initial stage of continuous infusion, and the peak area and relative contents remained at only 26.2 and 36.5%, respectively, at the 7th infusion compared to the 2nd infusion (Fig. 2D).
Volatile persistence of dilution test in model infusion
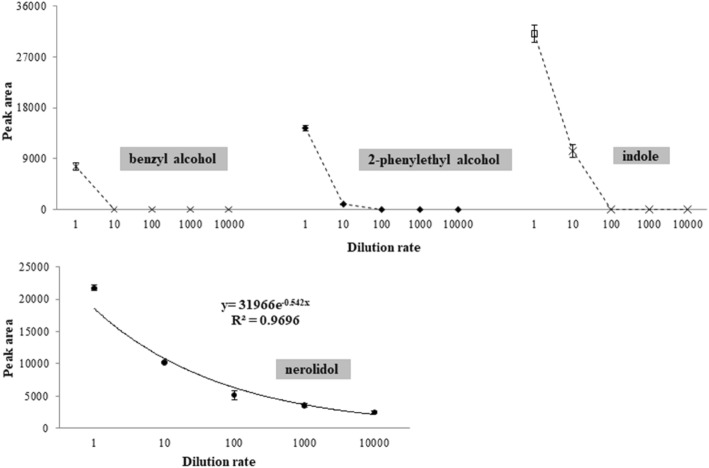
The influence of the tea matrix on headspace release was investigated in a model infusion of oolong tea, with individual odorants diluted in that system. The model infusion was prepared in the presence of tea polyphenols, caffeine, and soluble sugar, which have been reported as the major nonvolatile components in oolong tea infusions (Kausar et al., 2013; Wang et al., 2010). Nerolidol (a dominant and persistent odorant), indole (a dominant but less persistent odorant), benzyl alcohol (not abundant in dry leaves and is not released into the headspace of infusions), and 2-phenylethanol (abundant in dry leaves but not released into the headspace of infusions) were selected for the dilution test.
The headspace amounts (peak area) of all four odorants decreased when diluted in the model infusion, as shown in Fig. 3. Benzyl alcohol (initial concentration of 50 ppm, v/v) showed no release at the dilution ratio of 1:10. The headspace amount of 2-phenylethanol (initial concentration of 50 ppm, v/v) remained at only 6.7% at the same dilution ratio, and there was no release at the dilution ratio of 1:100. A dramatic decrease in headspace release could also be observed for indole (initial concentration of 0.1 g/L), which disappeared at the dilution ratio of 1:100. However, the peak area for indole remained at 33.3% through 10 dilutions, indicating a more persistent release in the headspace of the model infusion compared to benzyl alcohol and 2-phenylethanol. Interestingly, the headspace amount of nerolidol (initial concentration of 10 ppm, v/v) was shown to decrease exponentially (y = 31966e−0.542x, R2 = 0.9696) with dilution ratio. In addition, 11.3% amount still remained even after being diluted 10,000 times, indicating extremely persistent volatilization of this dominant volatile. Together with the data obtained in the tea/water ratio test, the infusion matrix was likely to have a particularly enhanced effect on the headspace release of nerolidol.
Fig. 3.
Dynamic changes of the headspace amount of individual odorants with respect to dilution ratio
Essential oils only account for approximately 0.01% of the dry matter of tea (Cheng et al., 2008; Fanaro et al., 2012; Shimoda et al., 1995). Accordingly, benzyl alcohol and 2-phenylethanol content would be far less than the 5 ppm (v/v) contained in oolong tea infusion (tea/water ratio of 1:20). Combined with the data on water solubility (4.105 × 104 mg/L and 2.199 × 104 mg/L, respectively, in water at 25 °C; the ChemSpider database, http://www.chemspider.com), benzyl alcohol and 2-phenylethanol levels are probably not sufficient to be released into the headspace of oolong tea. As presented in our previous work (Lin et al., 2013), indole accounted for 4.49–12.03% (relative content) of the total volatiles in oolong tea (dry leaves), much more than in the other teas (Baldermann et al., 2014). The high amount of indole might ensure its headspace release, though high solubility in tea infusion was probable (1.529 × 103 mg/L in water at 25 °C; the ChemSpider database, http://www.chemspider.com). Further studies on the specific solubilities of tea odorants in the infusion matrix would help us to better understand their performance in the headspace.
Interesting volatiles during oolong tea infusion
It is proposed in our work that the headspace performance of individual odorants should primarily determine their availability and contribution to the sensory system. Nineteen odorants divided into three groups according to the release performance we had investigated are summarized in Table 2. The five volatiles (i.e., benzyl alcohol, 2-phenylethanol, nonanal, isoamyl hexanoate, and β-bisabolene) that were not released into the infusion headspace were grouped as ‘no contribution to perceived aroma’. They might have a considerable amount in the tea leaves but could hardly volatilize into the headspace of the tea infusion, presumably due to their high solubility and/or interaction with the infusion matrix. Eight volatiles, including nerolidol, α-farnesene, (E)-β-farnesene, (Z)-jasmone, methyl salicylate, naphthalene, (E)-β-ocimene, and linalool, exhibited a ‘stable and persistent’ headspace release with variations in aroma source, water ratio, and infusion frequency. Combined with data concerning odor type and sensory threshold, these eight volatiles possibly are likely the key odorants of oolong tea, especially after the 3rd infusion. Similarly, six volatiles (i.e., indole, phenethyl isobutyrate, benzyl nitrile, 6-methyl-5-hepten-2-one, benzaldehyde, and benzeneacetaldehyde) were determined as ‘less persistent’ odorants that might contribute to the initial aroma perceived during tea brewing, however their roles as odorants were dramatically weakened along with continuous infusing.
Table 2.
Descriptions of some interesting volatiles contained in oolong tea infusion
| Compounds | RI | Relative contenta,b (%) | Aroma performance | Odor description |
|---|---|---|---|---|
| Nonanal | ND | No contribution | Orange-like (Kumazawa and Masuda, 2002) | |
| Isopentyl hexanoate | ND | No contribution | Fruity (Rodríguez et al., 2015) | |
| β-Bisabolene | ND | No contribution | Orange (Macleod and Pieris, 1984) | |
| Benzyl alcohol | ND | No contribution | Fruity (Ito et al., 2002) | |
| 2-Phenylethyl alcohol | ND | No contribution | Rose (Qin et al., 2013) | |
| (E)-β-Ocimene | 1257 | 0.98 ± 0.14 | Stable and persistent | Herbal (Zeng et al., 2017a) |
| Linalool | 1562 | 1.62 ± 0.02 | Stable and persistent | Floral (Katsuno et al., 2014; Qin et al., 2013) |
| (E)-β-Farnesene | 1673 | 3.48 ± 0.02 | Stable and persistent | Citric, sweet, wood (Rodríguez et al., 2015) |
| Naphthalene | 1735 | 4.13 ± 0.62 | Stable and persistent | Naphthalene (Yang et al., 2008) |
| α-Farnesene | 1754 | 1.70 ± 0.06 | Stable and persistent | Fruity, herbal (Zeng et al., 2017a) |
| Methyl salicylate | 1769 | 0.54 ± 0.14 | Stable and persistent | Sweet (Qin et al., 2013) |
| (Z)-Jasmone | 1912 | 0.28 ± 0.04 | Stable and persistent | Floral, green (Katsuno et al., 2014; Qin et al., 2013) |
| Nerolidol | 23.59 ± 5.74 | Stable and persistent | Floral, fruity (Qin et al., 2013) | |
| 6-Methyl-5-hepten-2-one | 1348 | 2.21 ± 0.36 | Less persistent | Sweet, fruity (Qin et al., 2013) |
| Benzaldehyde | 1531 | 3.87 ± 0.77 | Less persistent | Fragrant, sweet (Qin et al., 2013) |
| Benzeneacetaldehyde | 1648 | 4.93 ± 0.58 | Less persistent | Sweet, almond (Qin et al., 2013; Zhu et al., 2017) |
| Benzyl nitrile | 1916 | 3.87 ± 0.18 | Less persistent | Stale, hay (Macleod and Panchasara, 1983) |
| Phenethyl isobutyrate | 1.11 ± 0.30 | Less persistent | Fruity, Rose (Rodríguez et al., 2015) | |
| Indole | 17.63 ± 1.38 | Less persistent | Floral, amimalic (Katsuno et al., 2014; Qin et al., 2013) |
RI retention indices
aData of tea liquid (without leaves) analyzed at the tea/water ratio of 1:50 (g/mL)
bND: not detected in the infusion headspace
In summary, the influences of infusion matrix on headspace release of volatiles could be clearly observed during oolong tea brewing. The presence of infusion matrix might prevent the headspace release of certain odorants (especially benzyl alcohol, 2-phenylethyl alcohol, linalool oxide, and nonanal). During continuous infusion, eight odorants were determined to be stable and persistent, whereas six were determined to be less persistent. Volatile dilution test in a model tea infusion further confirmed the persistent release of nerolidol, while depressed release or no release was observed for indole, benzyl alcohol, and 2-phenylethanol. The headspace performance of the volatiles during brewing would primarily and directly affect the perceived aroma and could better identify the key odorants responsible for daily tea aroma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by National Natural Science Foundation of China under Grant No. 31800582, and Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ14C160003.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jie Lin, Email: linjie@zafu.edu.cn.
Yuanxu Shi, Email: 346150774@qq.com.
Chunwang Dong, Email: dongchunwang@163.com.
Xiaochang Wang, Phone: + 86 571 88982380, Email: xcwang@zju.edu.cn.
References
- Baldermann S, Yang Z, Katsuno T, Tu VA, Mase N, Nakamura Y, Watanabe N. Discrimination of green, oolong, and black teas by GC–MS analysis of characteristic volatile flavor compounds. Am. J. Anal. Chem. 2014;05:620–632. doi: 10.4236/ajac.2014.59070. [DOI] [Google Scholar]
- Caprioli G, Cortese M, Cristalli G, Maggi F, Odello L, Ricciutelli M, Sagratini G, Sirocchi V, Tomassoni G, Vittori S. Optimization of espresso machine parameters through the analysis of coffee odorants by HS-SPME–GC/MS. Food Chem. 2012;135:1127–1133. doi: 10.1016/j.foodchem.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Kuo PC, Yang ML, Li FY, Tzen JTC. Effects of baking and aging on the changes of phenolic and volatile compounds in the preparation of old Tieguanyin oolong teas. Food Res. Int. 2013;53:732–743. doi: 10.1016/j.foodres.2012.07.007. [DOI] [Google Scholar]
- Cheng Y, Huynh-Ba T, Blank I, Robert F. Temporal changes in aroma release of Longjing tea infusion: interaction of volatile and nonvolatile tea components and formation of 2-butyl-2-octenal upon aging. J. Agric. Food Chem. 2008;56:2160–2169. doi: 10.1021/jf073132l. [DOI] [PubMed] [Google Scholar]
- Dufour C, Bayonove CL. Interactions between wine polyphenols and aroma substances. An insight at the molecular level. J. Agric. Food Chem. 1999;47:678–684. doi: 10.1021/jf980314u. [DOI] [PubMed] [Google Scholar]
- Elmore JS, Erbahadir A, Mottram DS. Comparison of dynamic headspace concentration on Tenax with solid phase microextraction for the analysis of aroma volatiles. J. Agric. Food Chem. 1997;45:2638–2641. doi: 10.1021/jf960835m. [DOI] [Google Scholar]
- Fanaro GB, Duarte RC, Santillo AG, Pinto e Silva MEM, Purgatto E, Villavicencio ALCH. Evaluation of γ-radiation on oolong tea odor volatiles. Radiat. Phys. Chem. 2012;81:1152–1156. doi: 10.1016/j.radphyschem.2011.11.061. [DOI] [Google Scholar]
- Ho CT, Zheng X, Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4:9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Ito Y, Sugimoto A, Kakuda T, Kubota K. Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac. J. Agric. Food Chem. 2002;50:4878–4884. doi: 10.1021/jf020282h. [DOI] [PubMed] [Google Scholar]
- Jelen HH, Wlazly K, Wasowicz E, Kaminski E. Solid-phase microextraction for the analysis of some alcohols and esters in beer: comparison with static headspace method. J. Agric. Food Chem. 1998;46:1469–1473. doi: 10.1021/jf9707290. [DOI] [Google Scholar]
- Katsuno T, Kasuga H, Kusano Y, Yaguchi Y, Tomomura M, Cui J, Yang Z, Baldermann S, Nakamura Y, Ohnishi T, Mase N, Watanabe N. Characterisation of odorant compounds and their biochemical formation in green tea with a low temperature storage process. Food Chem. 2014;148:388–395. doi: 10.1016/j.foodchem.2013.10.069. [DOI] [PubMed] [Google Scholar]
- Kausar T, Akram K, Kwon JH. Comparative effects of irradiation, fumigation, and storage on the free amino acids and sugar contents of green, black and oolong teas. Radiat. Phys. Chem. 2013;86:96–101. doi: 10.1016/j.radphyschem.2012.12.011. [DOI] [Google Scholar]
- Kumazawa K, Masuda H. Identification of potent odorants in different green tea varieties using flavor dilution technique. J. Agric. Food Chem. 2002;50:5660–5663. doi: 10.1021/jf020498j. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang P, Pan Z, Xu H, Luo Y, Wang X. Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS-SPME/GC–MS. Food Chem. 2013;141:259–265. doi: 10.1016/j.foodchem.2013.02.128. [DOI] [PubMed] [Google Scholar]
- Ma C, Qu Y, Zhang Y, Qiu B, Wang Y, Chen X. Determination of nerolidol in teas using headspace solid phase microextraction–gas chromatography. Food Chem. 2014;152:285–290. doi: 10.1016/j.foodchem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Macleod AJ, Panchasara SD. Volatile aroma components, particularly glucosinolate products, of cooked edible mushroom (Agaricus bisporus) and cooked dried mushroom. Phytochemistry. 1983;22:705–709. doi: 10.1016/S0031-9422(00)86966-6. [DOI] [Google Scholar]
- Macleod AJ, Pieris NM. Volatile aroma constituents of Sri Lankan ginger. Phytochemistry. 1984;23:353–359. doi: 10.1016/S0031-9422(00)80332-5. [DOI] [Google Scholar]
- Mei Y, Wang Z. Investigation report on national production and marketing of Oolong Tea in 2016. Guangdong Tea Industry 1–8 (2017) (in Chinese)
- Ogawa K, Moon JH, Guo W, Yagi A, Watanabe N, Sakata K. A study on tea aroma formation mechanism: alcoholic aroma precursor amounts and glycosidase activity in parts of the tea plant. Z. Naturforsch. C Biosci. 1995;50:493–498. doi: 10.1515/znc-1995-7-805. [DOI] [PubMed] [Google Scholar]
- Qin Z, Pang X, Chen D, Cheng H, Hu X, Wu J. Evaluation of Chinese tea by the electronic nose and gas chromatography–mass spectrometry: correlation with sensory properties and classification according to grade level. Food Res. Int. 2013;53:864–874. doi: 10.1016/j.foodres.2013.02.005. [DOI] [Google Scholar]
- Riu-Aumatell M, Castellari M, Lopez-Tamames E, Galassi S, Buxaderas S. Characterisation of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS. Food Chem. 2004;87:627–637. doi: 10.1016/j.foodchem.2003.12.033. [DOI] [Google Scholar]
- Robinson AL, Ebeler SE, Heymann H, Boss PK, Solomon PS, Trengove RD. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J. Agric. Food Chem. 2009;57:10313–10322. doi: 10.1021/jf902586n. [DOI] [PubMed] [Google Scholar]
- Rodríguez RM, Pando RB, Suárez BV. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT Food Sci. Technol. 2015;64:1342–1353. doi: 10.1016/j.lwt.2015.07.056. [DOI] [Google Scholar]
- Romeo V, Ziino M, Giuffrida D, Condurso C, Verzera A. Flavour profile of capers (Capparis spinosa L.) from the Eolian Archipelago by HS-SPME/GC–MS. Food Chem. 2007;101:1272–1278. doi: 10.1016/j.foodchem.2005.12.029. [DOI] [Google Scholar]
- Schuh C, Schieberle P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006;54:916–924. doi: 10.1021/jf052495n. [DOI] [PubMed] [Google Scholar]
- Shimoda M, Shigematsu H, Shiratsuchi H, Osajima Y. Comparison of the odor concentrates by SDE and adsorptive column method from green tea infusion. J. Agric. Food Chem. 1995;43:1616–1620. doi: 10.1021/jf00054a037. [DOI] [Google Scholar]
- Togari N, Kobayashi A, Aishima T. Relating sensory properties of tea aroma to gas chromatographic data by chemometric calibration methods. Food Res. Int. 1995;28:485–493. doi: 10.1016/0963-9969(95)00028-3. [DOI] [Google Scholar]
- Wang K, Liu F, Liu Z, Huang J, Xu Z, Li Y, Chen J, Gong Y, Yang X. Analysis of chemical components in oolong tea in relation to perceived quality. Int. J. Food Sci. Technol. 2010;45:913–920. doi: 10.1111/j.1365-2621.2010.02224.x. [DOI] [Google Scholar]
- Wiklund S, Johansson E, Sjöström L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008;80:115–122. doi: 10.1021/ac0713510. [DOI] [PubMed] [Google Scholar]
- Yang Z, Baldermann S, Watanabe N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013;53:585–599. doi: 10.1016/j.foodres.2013.02.011. [DOI] [Google Scholar]
- Yang DS, Lee KS, Jeong OY, Kim KJ, Kays SJ. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2008;56:235–240. doi: 10.1021/jf072360c. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhou Y, Gui J, Fu X, Mei X, Zhen Y, Ye T, Du B, Dong F, Watanabe N, Yang Z. Formation of volatile tea constituent indole during the oolong tea manufacturing process. J. Agric. Food Chem. 2016;64:5011–5019. doi: 10.1021/acs.jafc.6b01742. [DOI] [PubMed] [Google Scholar]
- Zeng L, Liao Y, Li J, Zhou Y, Tang J, Dong F, Yang Z. α-Farnesene and ocimene induce metabolite changes by volatile signaling in neighboring tea (Camellia sinensis) plants. Plant Sci. 2017;264:29–36. doi: 10.1016/j.plantsci.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhou Y, Fu X, Mei X, Cheng S, Gui J, Dong F, Tang J, Ma S, Yang Z. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017;237:488–498. doi: 10.1016/j.foodchem.2017.05.137. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen F, Wang L, Niu Y, Xiao Z. Evaluation of the synergism among volatile compounds in Oolong tea infusion by odour threshold with sensory analysis and E-nose. Food Chem. 2017;221:1484–1490. doi: 10.1016/j.foodchem.2016.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.