Abstract
Hepatocellular carcinoma (HCC) is one of the top causes of cancer mortality worldwide. Although HCC has been researched extensively, there is still a need for novel and effective therapeutic interventions. There is substantial evidence that initiation of carcinogenesis in liver cirrhosis, a leading cause of HCC, is mediated by cancer stem cells (CSCs). CSCs were also shown to be responsible for relapse and chemoresistance in several cancers, including HCC. MicroRNAs (miRNAs) constitute important epigenetic markers that regulate carcinogenesis by acting post-transcriptionally on mRNAs, contributing to the progression of HCC. We have previously shown that co-culture of cancer cells with mesenchymal stem cells (MSCs) could induce the reprogramming of MSCs into CSC-like cells. In this review, we evaluate the available data concerning the epigenetic regulation of miRNAs through methylation and the possible role of this regulation in stem cell and somatic reprogramming in HCC.
Keywords: miRNA, cancer stem cell, methylation, mesencymal stem cells, hepatocellular carcinoma
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most frequent primary malignancy of the liver. It is the third leading cause of mortality associated with cancer worldwide (Yang and Roberts, 2010; Dhanasekaran et al., 2012). HCC is a multifactorial disease that is influenced by several risk factors. It typically develops as a result of underlying liver disease and is commonly associated with cirrhosis (Huang et al., 2013). The major HCC-risk factors include viral infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), which leads to liver cirrhosis and accounts for 75% of HCC cases (El-Serag, 2002). Other factors attributed to HCC include alcohol abuse, intake of food contaminated with aflatoxin and toxic chemical exposure, including dimethylformamide, dimethylacetamide, trichloroethylene, tetrachloroethylene, carbon tetrachloride and chloroform (Malaguarnera et al., 2012). In addition, Obesity is one of the highly recent factors that plays a significant role in developing non-alcoholic fatty liver disease (NAFLD) (Cholankeril et al., 2017). It can progress in many stages starting with lipid deposition in hepatocytes' cytoplasm and can lead to non-alcoholic steatohepatitis (NASH) (Marrero et al., 2002; Guzman et al., 2008; Reddy et al., 2012; White et al., 2012). NASH is the severe stage of NAFLD indicated by hepatocyte injury, uncontrolled inflammation, hepatocyte ballooning, cell death, infiltration of inflammatory cells, and collagen deposition (Guzman et al., 2008; Reddy et al., 2012). NASH has been determined to be one of the important events in promoting hepatic carcinogenesis (Ip and Wang, 2014).
Tissue damage and fibrosis result from chronic inflammation and oxidative stress, leading to cirrhosis and eventually tumor initiation, progression, and even metastasis (Lau and Lai, 2008; Shariff et al., 2009; Cabrera and Nelson, 2010). Although only ~10–20% of HCC patients are eligible for surgical interference at the time of diagnosis, liver transplantation remains the first choice for treatment (Ji et al., 2009a). Furthermore, patients suffer a high frequency of relapse, and in patients who experience curative resection, the 5-year survival rate is 30–40% (Budhu et al., 2008). The low detection and high recurrence rates for the curable stages of HCC have increased interest in investigations of the molecular mechanisms underlying this disease (He et al., 2015).
Cancer Stem Cells (CSCs)
The failure of conventional treatments to completely eliminate invasive tumor cells is thought to be due to the presence of a small subset of cancer cells, termed CSCs, which are accountable for cancer progression, metastasis, recurrence, and drug resistance. CSCs have been classified as immortal tumor-initiating cells which have pluripotent and self-renewal capacity (Chen et al., 2013). CSCs have been identified in numerous solid tumors, such as breast cancer, colon cancer, and HCC (Szotek et al., 2006; O'Brien et al., 2007; Kawai et al., 2015). CSCs were found to have a main contribution in tumor heterogeneity and to contribute to drug resistance (Beck and Blanpain, 2013; Bedard et al., 2013; Klein, 2013; Meacham and Morrison, 2013). While the origin of CSCs remains unclear, the proposed mechanisms for their generation include cell fusion, genetic mutations in stem cells, and regulatory factors within the tumor microenvironment (TME) (Bu and Cao, 2012). In addition, signaling pathways and genes that regulate stem cell differentiation, as Wnt/β-catenin, transforming growth factor β (TGF-β), and microRNAs (miRNAs), could contribute to the control and maintenance of CSC differentiation (Bedard et al., 2013; Meacham and Morrison, 2013). The Wnt/β-catenin signaling pathway seems to play major roles in the development of CSCs and in self-renewal, tumorigenesis, and cancer chemoresistance (Espada et al., 2009; Eaves and Humphries, 2010; Mohammed et al., 2016).
Characteristics of miRNAs
MiRNAs are small non-coding RNA molecules consisting of 21−23 nucleotides. They control gene expression by base pairing with the messenger RNAs (mRNAs) (Lu et al., 2005; Griffiths-Jones et al., 2006). Transcripts are regulated through either degradation or translational repression (Bartel, 2004). Full complementarity between a miRNA and an mRNA results in full degradation of the target mRNA. However, defects in perfect complementarity leads to less translation of the target gene without affecting the level of mRNA (Lewis et al., 2005; Cummins and Velculescu, 2006). MiRNAs target up to 90% of human genes (Miranda et al., 2006) and can be found in exons or introns of coding or non-coding genes, with their transcription dependent on genomic localization (Baskerville and Bartel, 2005; Lin et al., 2006). Although miRNAs have their own promoters and are self-sufficiently expressed some miRNAs that share the same transcriptional regulation are ordered in clusters. Hundreds of miRNAs have been known by molecular cloning and bioinformatics approaches in plants and animals (Bushati and Cohen, 2007; Liu et al., 2014). Interestingly, a subgroup of miRNAs, namely, epi-miRNAs, control the expression of epigenetic marks, such as DNA methyltransferases (DNMTs), histone deacetylases (HDACs), and polycomb genes, either directly or indirectly. DNA methylation has a key role in gene expression regulation via maintaining the stability of gene silencing. In mammals, DNA methylation takes place by covalent modification of cytosine residues through the addition of a methyl group to the fifth position of a cytosine ring, particularly in the CpG dinucleotides. This process is mediated by members of the DNMTs enzymes family (Chuang and Jones, 2007). Therefore, miRNAs function as both genetic and epigenetic regulators (Valeri et al., 2009).
miRNAs control many cellular processes in eukaryotes, such as rate of growth, development, differentiation potential, cell cycle progression, and apoptosis, and their abnormal expression affects many human diseases (Valeri et al., 2009; Krol et al., 2010; Wahid et al., 2010; Pritchard et al., 2012). In addition to serving as essential players in tumor development, miRNAs have a role as possible biomarkers for cancer (Calin and Croce, 2006). Indeed, miRNA profiles reflect the stages of tumors and their developmental lineages (George and Mittal, 2010). MiRNAs have been found to modulate CSCs and metastasis. They can also act as oncogenes and tumor suppressors. Due to their functions as oncogenes and tumor suppressor genes, these miRNAs have been referred to as oncomirs (George and Mittal, 2010).
miRNAs and HCC
Recent studies on liver miRNAs investigated the overexpression of specific miRNAs or the inhibition of other miRNAs both in vitro and in vivo. These studies showed the crucial biological roles of miRNAs for proper liver function (Takata et al., 2013). In HCC, Murakami et al. initially reported dysregulated miRNA expression, with four miRNAs, namely, miR-18, miR-92, miR-20, and precursor miR-18 being inversely associated with the extent of HCC development (Murakami et al., 2006). Later, several studies confirmed that miRNAs play an essential regulatory role in hepatic carcinogenesis progression and malignant transformation. Some miRNAs showed abnormal expression during the progression of liver cancer (Zhao et al., 2009). The expression of some miRNAs was shown to influence HCC development via dysregulation of a number of cancer-associated molecular pathways, including TGF-β, p53, WNT/β-catenin, P13K/AKT/mTOR, RAS/MAPS, MET, and MYC (Negrini et al., 2011). Many oncogenic miRNAs have shown aberrant expression in HCC, including miR-1275 (Shaalan et al., 2018), miR-17-5p (Habashy et al., 2016), miR-96-5p, miR-182-5p (Assal et al., 2015), miR-155 (El Tayebi et al., 2015), and miR-181a (Lashine et al., 2011). Other tumor suppressor miRNAs involved in HCC include miR-34a (Yacoub et al., 2016), miR-486-5p (Youness et al., 2016), miR-615-5p (El Tayebi et al., 2012), and miR-Let7i (Fawzy et al., 2016). Genome-wide approaches have identified hundreds of miRNAs in HCC tumor tissues that were to be dysregulated compared to non-tumor tissues (Borel et al., 2012). MiR-122 is among many unique and well-studied dysregulated miRNAs that are highly expressed specifically in human liver. In HCC patients, a shorter recurrence time were attributed to lower levels of miR-122. While elevated expression of cyclin G1, a target of miR-122, was associated with a lower survival rate. Moreover, miR-122 acts as a tumor suppressor in HCC, and was subsequently reported to be downregulated in around 70% of cases (Callegari et al., 2013). MiR-221 is another critical oncogenic miRNA that is upregulated in 70–80% of HCC cases. Its overexpression leads to enhanced proliferation potential, migration, invasion, rate of growth, and decreased the rate of apoptosis in HCC patients (Fornari et al., 2008). Additionally, miR-221 modulates several gene targets involved in cancer-related pathways, including PTEN (P13K/AKT/mTOR), CDKN1B/p27, and CDKN1C/p57 (Fornari et al., 2008; Garofalo et al., 2009).
Due to their non-invasive detection, good specificity, and sensitivity, miRNAs are considered effective biomarkers for HCC (Shen et al., 2016). MiR-155-5p, miR-206, miR-21-5p, and miR-212-3p. MiR-155-5p and miR-21-5p which are reported as biomarkers for the prognosis of HCC in tissues, were found to have upregulated expression levels. On the other hand others were down-regulated (Han et al., 2013; Wang et al., 2014; Yunqiao et al., 2014; Tu et al., 2015). Circulating miR-122-5p and miR-16-5p could be used as presumed biomarkers for HCC. MiR-122-5p and miR-16-5p belong to this group which were particularly detected to be up and down-regulated, respectively (Cho et al., 2015; El-Abd et al., 2015).
Most often, elevated expression of miR-18b-5p, miR-200a-3p, miR-200b-3p, miR-21-5p, miR-224-5p, and miR-29-5p in tissue were mostly reported to be HCC. In addition, miR-139-5p was down-regulated. Therefore, they were beneficial for diagnosis of HCC (Zhu et al., 2012; Murakami et al., 2013; Dhayat et al., 2014; Han et al., 2014; Li T. et al., 2014; Amr et al., 2016).
Circulating miRNAs were proposed as prognostic biomarkers and reported to be linked to tissue invasion and metastasis. Those biomarkers included miR-122-5p, miR-17-5p, miR-182-5p, miR-21-5p, miR-24-3p, and miR-331-3p, all were up-regulated in the group reported to have a low-survival rate (Zheng et al., 2013; Meng et al., 2014; Chen et al., 2015; Wang L.-J. et al., 2015; Xu Y. et al., 2015). Meanwhile, the serum miR-150-5p was highly expressed in HCC patients after surgical operation, however following tumor relapse its expression levels were reversed (Yu F. et al., 2015).
In tissues, high expression of miR-150-5p and miR-29a-5p in combination of low expression of miR-101-3p, miR-126-3p, miR-127-3p, miR-139-5p, and miR-214-3p have tumor-suppressor roles and consequently have potential use as diagnostic biomarkers for HCC (Zhu et al., 2012; Han et al., 2014; Li T. et al., 2014; Peveling-Oberhag et al., 2014; Xie et al., 2014; Zhou et al., 2014; Wang S. et al., 2015). The association between the circulating miR-101-3p, miR-122-5p, miR-125b-5p, miR-139-5p, miR-150-5p, miR-16-5p, miR-181a-5p, miR-199a-3p, miR-199a-5p, miR-203a-3p, miR-21-5p, miR-22-3p, miR-29b-3p, miR-375, let-7b-5p, and tumor suppressor render them potential biomarkers for differentiating HCC from healthy controls (Zhou J. et al., 2011; Luo et al., 2013; Li T. et al., 2014; Tan et al., 2014; Xie et al., 2014; Chen et al., 2015; Jiang et al., 2015; Wang S. et al., 2015; Yin et al., 2015; Yu F. et al., 2015; Hung et al., 2016). Contrarily, miR-101-3p, miR-122-5p, miR-125b-5p, miR-130a-3p, miR-146a-5p, miR-214-3p, and miR-99a-5p were known as tumor suppressors in HCC and played the role of prognostic indicators for HCC (Zhang et al., 2012; Wang et al., 2013; Li B. et al., 2014; Rong et al., 2014; Tsang et al., 2014; Xie et al., 2014; Xu Q. et al., 2015). Serum miR-1-3p, miR-101-3p, miR-122-5p, miR-150-5p, miR-203a-3p, and miR-30c-5p were linked to tumor suppression, and new independent parameters of overall survival in HCC (Köberle et al., 2013; Xie et al., 2014; Cho et al., 2015; Liu D. et al., 2015; Xu Y. et al., 2015; Yu F. et al., 2015).
As miRNAs expression levels can be used as biomarkers for HCC diagnosis and prognosis, miRNA specific methylation patterns are of importance for therapeutic applications as well. Acting as a tumor suppressor miRNA, decreased expression of miR-10a due to hypermethylation can be used as a biomarker for early HCC diagnosis and risk assessment (Shen et al., 2012). Furthermore, some miRNAs methylation patterns can be HCC cell-specific and therefore used as diagnostic biomarkers. Such miRNAs cell-specific diagnostic methylation patterns include the hypermethylation of miR-129-2, miR-34a, and miR-148a (Anwar et al., 2013; Lu et al., 2013). Also, hypermethylation of mir-9-1 has been shown to be a biomarker for poor diagnosis and aggressiveness (Anwar et al., 2013). In addition to their implications in diagnosis and prognosis, miRNA specific aberrant methylation patterns can be used for therapeutic applications. For example, administration of miR-124, which is hypermethylated in HCC, stopped HCC progression in animal models and was considered safe. Moreover, sorafenib (anti-cancer drug) increased the expression of miR-1274, which is hypermethylated in HCC, leading to an increased response to therapy (Zhou C. et al., 2011).
miRNAs and CSCs in HCC
miRNAs play essential roles in regulating CSCs (Garg, 2015), and in regulating apoptosis in CSCs by acting on mRNAs of apoptosis proteins or regulating mRNAs that are downstream targets in specific apoptotic pathways. These control mechanisms aid in the regulation of metastasis, drug resistance, tumor invasion, pluripotency, and self-renewal potential.
The tumorigenicity of liver CSCs was found to be significantly suppressed by inhibition of miR-181. This miRNA regulates the differentiation potential of liver CSCs through activating transcription factors, including caudal homeobox gene 2 (CDX2) and GATA6, and negatively regulating the Wnt/β-catenin pathway via nemo-like kinase (NLK) (Ji et al., 2009b; Leal and Lleonart, 2013; Bessède et al., 2014). MiR-Let-7 and miR-Lin28 have been reported to be related to the rate of growth and metastasis of HCC. Lin28 is highly expressed in normal embryonic stem cells (ESCs). It maintains the self-renewal of liver CSCs by inhibiting the interaction of Let-7 with the mature miRNA. Let-7 degradation, which is caused by excessively active Lin28 and c-MYC, dis-equilibrates liver CSCs, leading to accelerated growth and metastasis of HCC (Heo et al., 2008). MiRNAs positively regulate liver CSCs via high expression of EpCAM, which is a prominent marker of liver CSCs. This upregulation is mediated by inhibition of TGF-β by downstream transcription factors of miR-18, such as CDX2, GATA6, and NLK. The EpCAM intracellular domain (EpICD) enters the nucleus and induces overexpression of cyclin D1, c-MYC, and miR-181 after binding to LIM domain protein 2 (FHL2), β-catenin and lymphoid enhancer factor 1 (Lef-1) (Ji et al., 2009b). Another group showed that TGF-β1 downregulate TP53INP1 by targeting miR-155 and promote epithelial-mesenchymal transition (EMT) and liver CSC phenotypes (Liu F. et al., 2015). The Wnt/β-catenin signaling pathway that regulates tumor heterogeneity is mainly related to miRNA, but the mechanism by which this balance between liver CSCs and cancer cells is maintained has not been elucidated.
Based on previous studies, some miRNAs expression was reported to be dysregulated in both HCC and CSCs. In Table 1, we compiled the mutually dysregulated miRNAs to establish the links between these miRNAs and the initiation and progression of HCC.
Table 1.
miRNAs whose expression was reported to be dysregulated in HCC and CSCs.
| miRNA | Expression and biological function in HCC | Expression and biological function in CSCs |
|---|---|---|
| miR-Let-7a | Downregulated (Connolly et al., 2008) MiR-Let-7 family act as tumor suppressors via regulating expression of oncogenes including RAS, COL1A2, and AT-hook 2 high mobility group (Johnson et al., 2005; Shi et al., 2017) |
Downregulated (Yata et al., 2015) The let-7 miRNA family has been reported to maintain the state of differentiation and self-renewal inhibition of CSCs by targeting Lin28, H-RAS, and HMGA2 (Ali Hosseini Rad et al., 2013; Sun et al., 2016) |
| miR-Let-7b miR-Let-7c miR-Let-7d miR-Let-7e miR-Let-7f miR-Let-7g |
Downregulated (Gramantieri et al., 2007) Act as tumor suppressors by targeting mRNA of RAS, COL1A2, and AT-hook 2 group |
Downregulated (Peter, 2009) miR-Let-7 family is involved in the differentiation of CSCs and maintenance of stemness by targeting Lin28 which have a negative effect on Let-7, and inhibits the expression of H-RAS and HMGA2 (Ali Hosseini Rad et al., 2013) |
| miR-9 | Upregulated (Wang et al., 2008) MiR-9 increases growth and metastasis (Chen et al., 2015). It targets directly the 3′UTR of PPAR alpha and regulates its expression levels in liver cancer cells. MiR-9 is highly over expressed in liver cancer patients. It acts as tumor suppressor, and could have a therapeutic effect (Chen et al., 2015) |
Upregulated (Schraivogel et al., 2011) miR-9 is responsible for maintaining stemness and promotion of CD 133+ cell proliferation by targeting CAMTA 1 (Takahashi et al., 2014; Wang L.-J. et al., 2015) |
| miR-16 | Upregulated (Huang et al., 2009) MiR-16 targets and regulates Bcl-2 and suppresses Bcl-2 protein expression by binding to the 3′UTR of Bcl-2 mRNA. Bcl-2 is one of the well-known anti apoptotic protein members in Bcl-2 family that control cancer cells response to drugs (Wang et al., 2018). RHepG2 cells, the multidrug resistant subgroup of human HCC HepG2 cells were reported to have higher levels of miR-16 and P-glycoprotein which is associated with multidrug resistance and lower level of Bcl-2 (Fregni et al., 2018) |
Upregulated (Caruso et al., 2012) miR-16 regulates proliferation and self-renewal by targeting bmi1 (B lymphoma Mo-MLV insertion region 1 homolog) (Shimono et al., 2015) |
| miR-17 | Upregulated (Huang et al., 2009) The miR-17-92 cluster is the first oncogenic miRNAs identified in human (El-Badawy et al., 2017). This cluster targets thrombospondin-1 (TSP1), proangiogenic targets, and connective tissue growth factor (CTGF) to increase the potential of tumor angiogenesis (Sung et al., 2013) |
Upregulated (Schraivogel et al., 2011) Promotion of CD 133+ cell proliferation by targeting CAMTA 1 (Takahashi et al., 2014) |
| miR-20a | Upregulated (Connolly et al., 2008) Involved in proliferation and recurrence of HCC (Zheng et al., 2013). MiR-20 a reduced the endogenous level of myeloid cell leukemia sequence 3′UTR Mcl-1 protein in HCC (Liu et al., 2012) |
Upregulated (Caruso et al., 2012) Regulating and enhancing stemness properties (Yu F. et al., 2015) |
| miR-21 | Upregulated (Connolly et al., 2008) Involved in progression and metastasis (Xie et al., 2014). miR-21 inhibited KLF5 gene by targeting its 3′-UTR. KLF5 gene play a role in cancer as a tumor inhibitor (Wang et al., 2012) |
Upregulated (Caruso et al., 2012) enhance stemness properties of CSCs by targeting TGFβR2 (Ali Hosseini Rad et al., 2013) |
| miR-24 | Upregulated (Huang et al., 2009) Has a role in metastasis and invasion (Zhou et al., 2014) miRNA-24 binds to the 3′-UTR of p53 mRNA and down regulates its expression |
Upregulated (Roscigno et al., 2017) Involved in maintaining stemness markers It has been postulated that miR-24 survived stem cells from apoptosis in the hypoxia conditions through an FIH1–HIFα pathway as it have HIF binding site (Peveling-Oberhag et al., 2014) |
| miR-27a | Upregulated (Connolly et al., 2008) MiRNA-2a regulates PPAR-γ expression through promoting HCC cell proliferation |
Upregulated (Caruso et al., 2012) Promotes angiogenesis and metastasis through targeting ENPP1 (Shimono et al., 2015) |
| miR-29c | Downregulated (Su et al., 2009) miR-29c targets SIRT1 oncogene thus acting as a tumor suppressor (Hung et al., 2016) |
Upregulated (Caruso et al., 2012) |
| miR-96 | Upregulated (Wang et al., 2008) MiR-96 over expression targets SOX6 that regulates proliferation potential, invasion and migration (Liu Y. et al., 2011) |
Downregulated (Shimono et al., 2015) |
| miR-34a | Downregulated (Sun et al., 2017; Xia et al., 2017; Ren et al., 2018) Involved in inhibition of invasion and migration potential Through c-Met signaling pathway (Rong et al., 2014) miR-34a targeted c-Met resulting in decreasing the mRNA and protein levels; thus, decrease phosphorylation of extracellular signal-regulated kinases 1 and 2 (Li N. et al., 2009) |
Downregulated (Wheeler et al., 2009; Stadler et al., 2010) Involved in inhibiting the self-renewal and metastasis of CSCs (Tsang et al., 2014) and suppression of asymmetric cell division in CSCs by targeting NOTCH 1 (Wang et al., 2013; Takahashi et al., 2014) |
| miR-93 | Upregulated (Thurnherr et al., 2016) MiR-93 over expression in-vitro enhanced HCC cell migration and invasion by targeting Programmed cell death 4 (PDCD4) gene (Zhang et al., 2012) |
Downregulated (Caruso et al., 2012) |
| miR-495 | Upregulated (Yang et al., 2013) miR-495 targets IGFIR and regulates ERK and AKT pathways, therefore inhibiting invasion and proliferation potential of HCC cells (Kim et al., 2012) |
Upregulated (Hwang-Verslues et al., 2011) Enhance stemness properties of CSCs by targeting REDD1 (Ali Hosseini Rad et al., 2013) |
| miR-1246 | Downregulated (El-Halawany et al., 2015) miR-1246 enhances the cell migration by targeting 3′UTR of the cell adhesion molecule 1 and downregulating its expression (Ma et al., 2010) |
Upregulated (Eshelman and Yochum, 2016; Zhang et al., 2016) Maintains properties of CSCs by targeting Wnt/beta-catenin pathway (Lou et al., 2018) |
| miR-210 | Upregulated (Yang et al., 2016) miR-210 targets SMAD4 and STAT6 to promote tumor angiogenesis (Huang et al., 2008) |
Upregulated (Bao et al., 2012) Promotes metastasis, proliferation, and self-renewal of CSCs by targeting E-cadherin (Tang et al., 2018) |
| miR-18 | Upregulated (Liu et al., 2017) The inhibition of miR-18 enhances the migration of HCC by targeting Smad2 (Li L. et al., 2015) |
Upregulated (Turchi et al., 2013) Modulates tumorigenesis (Mens and Ghanbari, 2018) |
| miR-191 | Upregulated (He et al., 2011) miR-191 promotes proliferation, tumor growth of HCC cells, and apoptosis by targeting TIMP3, TMC7, SOX4, and IL1A (Chang et al., 2012) |
Upregulated (Xu W. et al., 2015) |
| miR-7 | Downregulated (Yu et al., 2016) miR-7 targets oncogenes such as Cdr 1 and acts tumor suppressor (Lu et al., 2013) |
Downregulated (Zhang et al., 2014) Inhibits metastasis by targeting SETDB1 (Shimono et al., 2015) |
| miR-150 | Downregulated (Thurnherr et al., 2016) miR-150 acts as tumor suppressor by inhibiting GAB1 expression and downregulating ERK activation |
Upregulated (Liu D. Z. et al., 2015) increases proliferation by targeting Wnt signaling pathway (Shimono et al., 2015) |
| miR-145 | Downregulated (Thurnherr et al., 2016) Downregulated (Thurnherr et al., 2016) miR-145 regulates growth rate and proliferation and the re-expression of miR-145 resulting in cell apoptosis. It has been reported that insulin-like growth factor (IGF) is an important oncogenic pathway in HCC. Meanwhile, miR-145 targets insulin receptor substrate (IRS1)-1, IRS2, and insulin-like growth factor 1 receptor (Law et al., 2012) | Downregulated (Yu Y. et al., 2015; Zhou et al., 2017) Targets ROCK1, Oct-4, Sox2, and Klf 4 genes and inhibits tumorigenesis, invasion, and stemness (Shi et al., 2017) |
| miR-101 | Downregulated (Su et al., 2009) Involved in promoting apoptosis and suppressing tumorigenicity (Jiang et al., 2015) via targets RAB5A, STMN1, and ATG4D (Xu Y. et al., 2013) |
Upregulated (Caruso et al., 2012) |
| miR-141 | Downregulated (Gramantieri et al., 2007) MiR-141 targets Tiam1 genes and inhibits HCC cells migration, proliferation and invasion potential in-vitro (Schoolmeesters et al., 2009) |
Downregulated (Gregory et al., 2008) inhibits the proliferation of CSCs by suppressing Wnt signaling pathway (Shimono et al., 2015) |
| miR-142 | Downregulated (Gramantieri et al., 2007) | Upregulated (Caruso et al., 2012; Chapnik et al., 2014) Inhibits the proliferation of CSCs by suppressing Wnt signaling pathway (Shimono et al., 2015) |
| miR-155 | Upregulated (Wang et al., 2008) Enhances proliferation, growth, and tumorigenesis and decreases apoptosis (Gramantieri et al., 2007; Peter, 2009) |
Upregulated (Caruso et al., 2012) |
| miR-183 | Upregulated (Wang et al., 2008) Regulates carcinogenesis (Takahashi et al., 2014). MiR-183 suppress apoptosis in HCC cells through targeting Programmed cell death 4 (PDCD4). PDCD4 mediates its proapototic function in human HCC by being involved in the TGF-β1-induced apoptotic pathway (Li et al., 2010) |
Downregulated (Dambal et al., 2015; Leung et al., 2015) Maintains EMT and self-renewal of CSCs by targeting SNAI2, SMAD4, and bmi1 (Shimono et al., 2015) |
| miR-185 | Downregulated (Huang et al., 2009) In HCC: miR-185 induces HCC proliferation by targeting the DNTM1 3′UTR luciferase (Chang et al., 2014) |
Upregulated (Caruso et al., 2012) |
| miR-194 | Downregulated (Huang et al., 2009) miR-194 induces apoptosis by targeting MAP4K4 (Peuget et al., 2014) |
Upregulated (Caruso et al., 2012) maintains self-renewal by targeting bmi1 (Shimono et al., 2015) |
| miR-200a | Downregulated (Murakami et al., 2006) Involved in enhancing proliferation and carcinogenesis (Caruso et al., 2012). Meanwhile, it regulates the invasion and migration of HCC cells by targeting Foxa2 (Chen et al., 2017) |
Downregulated (Pode-Shakked et al., 2013) Involved in inhibiting EMT, BMI1, Sox2, Klf4, and Notch signaling, and reducing the stemness properties and mammosphere formation of CSCs by targeting ZEB1 and ZEB 2, SIP1, BMI-1, and Klf4 (Ali Hosseini Rad et al., 2013; Shimono et al., 2015) |
| miR-200b | Downregulated (Huang et al., 2009) Involved in enhancing proliferation and carcinogenesis (Caruso et al., 2012) miR-200b suppressed the expression of BMI1 and ZEB1, moreover ZEB1 promotes CD13, CD24, and EPCAM resulting in the upregulation of CD13 and CD24 so, the miR-200-ZEB1 circuit regulates stemness in HCC and differentiates between HCC contains CD13+/CD24+ CSCs from EpCAM + CSCs (Tsai et al., 2017) |
Downregulated (Pode-Shakked et al., 2013) Involved in inhibiting EMT, BMI1, Sox2, Klf4, and Notch signaling, and reducing the stemness properties of CSCs by targeting ZEB1, SIP1, Bmi-1, and Klf4 (Ali Hosseini Rad et al., 2013; Shimono et al., 2015) |
| miR-214 | Downregulated (Wang et al., 2008) MiR-214 acts as a suppressor to HCC by downregulating β-catenin signaling pathway (Nie et al., 2011) |
Upregulated (Zhang et al., 2006) Maintains properties of CSCS by targeting CTNNB1 (Lou et al., 2018) |
| miR-215 | Downregulated (Su et al., 2009) miR-215 showed significant upregulation in HCC serum, and thus functions as a biomarker for early diagnosis in HCC patients miR-215 is significantly correlated with the important genes in Wnt/β-catenin pathway including. β-catenin, APC, and c-myc (Ashmawy et al., 2017) |
Upregulated (Caruso et al., 2012) Acts as tumor suppressor (Ullmann et al., 2019) Mechanistically, miR-215 targets by a cell cycle-regulated nuclear and centrosome protein, which suppress the miRNA resulted in the induction of P53, G2 arrest, and P21 and decrease the proliferation (Song et al., 2010) |
| miR-221 | Upregulated (Huang et al., 2009) Involved in enhancing proliferation and tumorigenicity (Sun et al., 2017; Ren et al., 2018) |
Upregulated (Shimono et al., 2009) Promotes progression of CSCs by targeting PTEN (Li et al., 2017) |
| miR-222 | Upregulated (Huang et al., 2009) Involved in enhancing tumorigenesis (Ren et al., 2018) and increase the growth rate by targeting the cyclin-dependent kinase inhibitor p27 (Song et al., 2017) |
Upregulated (Shimono et al., 2009) Promotes progression of CSCs by targeting PTEN (Li et al., 2017) |
| miR-424 | Downregulated (Su et al., 2009) MiR-424 is involved in tumorigenesis of HCC by suppression of c-Myb. It directly targets the 3UTR of c-Myb and induces inhibition of proliferation and invasion in HCC cells (Zhang et al., 2011) |
Downregulated (Yata et al., 2015) |
| miR-34a | Downregulated (Peveling-Oberhag et al., 2014; Xie et al., 2014; Yu F. et al., 2015) MiR-34a induces apoptosis of HCC and decreases its proliferation by targeting the expression of HDAC1. It acts as a 3′UTR to regulate its expression on HCC cells (Peveling-Oberhag et al., 2014) |
Downregulated (Zhou et al., 2014; Wang S. et al., 2015) Inhibits self-renewal properties of CSCs by targeting CD44 and Notch1 (Ali Hosseini Rad et al., 2013) |
| miR-378 | Downregulated (Xu Y. et al., 2015) Rs1076064 (pri-miR-378) variant genotypes contributed to the expression of the miR-378 in decreasing the risk of HCC (Mei et al., 2013) |
No data |
| miR-24 | Upregulated (Li T. et al., 2014) In HCC: miR-24 regulates oncogenes by binding to 3′-UTR of P53 (Hassan et al., 2010) |
Upregulated (Wang L.-J. et al., 2015) Promotes apoptosis resistance through regulating BimL (Roscigno et al., 2017) |
| miR-29c | Downregulated (Xu Y. et al., 2015) In HCC: miR-29c targets oncogenic by binding to 3′UTR in SIRT1 (Sun et al., 2009) |
Upregulated (Murakami et al., 2013) |
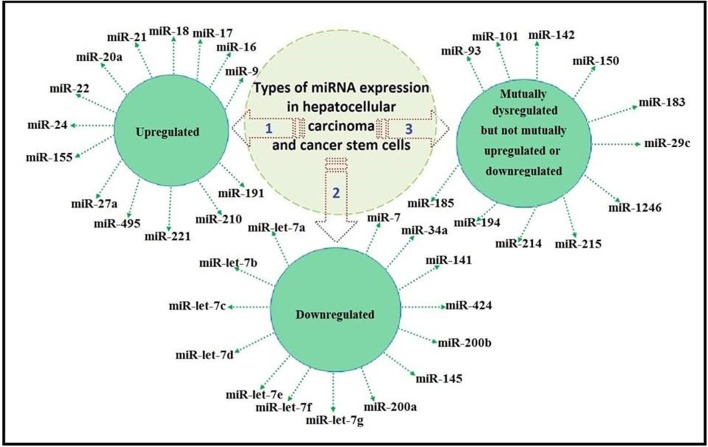
We classified the previously reported miRNAs as mutually upregulated, mutually downregulated, and mutually dysregulated but not mutually upregulated or mutually downregulated, as illustrated in Figure 1.
Figure 1.
Classification of miRNA expression as mutually upregulated, mutually downregulated, and mutually dysregulated but not mutually upregulated or downregulated.
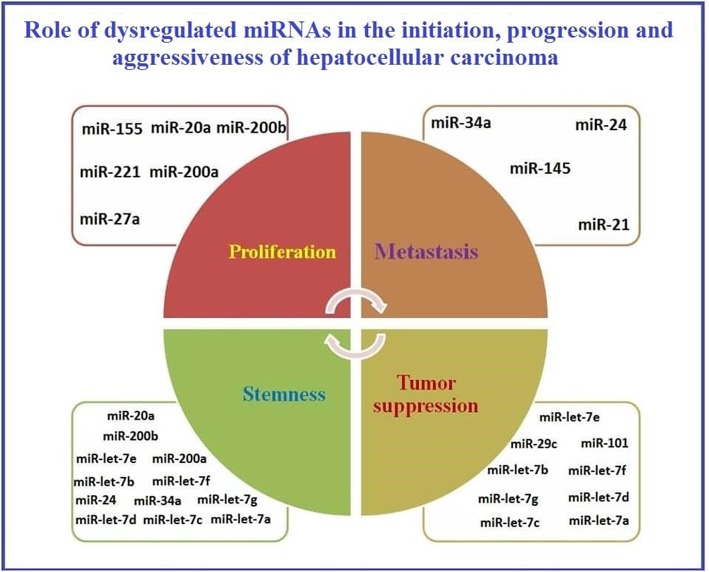
A schematic presentation of the role of dysregulated miRNAs in HCC initiation, progression, and aggressiveness is presented in Figure 2.
Figure 2.
Representation of the role of dysregulated miRNAs in initiation, progression, and aggressiveness of Hepatocellular carcinoma.
As reported previously, MSCs aid in cancer development by enhancing the metastatic capability of tumor cells (Hill et al., 2017). This has been also reasoned by the fact that MSCs can home to tumor microenvironment mainly due to the action of stromal cell-derived factor 1 (SDF-1) (Gao et al., 2009). After homing, MSCs start to trans-differentiate into cancer associated fibroblasts mainly due to the action of TGF-beta1 (Ghaderi and Abtahi, 2018). Afterwards, cancer associated fibroblasts (CAFs) start to induce metastasis in neighboring tumor cells by inducing EMT (Wang et al., 2018). Some of the significant genes involved in such pro-metastatic signature of the tumor MSCs have been identified in lung cancer and they include GREM1, LOXL2, ADAMTS12, and ITGA11 (Fregni et al., 2018). Also, as investigated by our research group, soluble factors secreted from cancer cells when cocultured with MSCs have shown to induce cancer stem cell-like characteristics in the cocultured MSCs (El-Badawy et al., 2017). Relating the previous information, we are trying to highlight the mutual dysregulated miRNA in HCC, CSCs, and MSCs to investigate whether miRNAs play a vital role in the acquirement of MSCs to pro-metastatic characteristics or development into Cancer stem cells- like cells or even CAFs. So, in Table 2, we summarize the roles of miRNAs that are mutually dysregulated in HCC, CSC, and in MSCs. The functions of these miRNAs may provide insight into their regulatory roles in the development of cancer (Schraivogel et al., 2011). Based on these proposed functions (Table 2), we classified these miRNAs according to their roles in MSC differentiation.
Table 2.
The roles of the mutually dysregulated miRNAs in HCC and CSC, in MSC differentiation.
| miRNA | Role in MSCs |
|---|---|
| miR-let-7 family | Inhibits adipogenesis and migration of cells (Sung et al., 2013) |
| miR-16 | Enhances myogenesis and G1 arrest (Liu et al., 2012; Wang et al., 2012) |
| miR-17 | Enhances osteogenesis (Liu Y. et al., 2011) |
| miR-20a | Enhances osteogenesis (Zhang et al., 2011) |
| miR-21 | Enhances both osteogenesis and adipogenesis but inhibits proliferation and aids in survival under hypoxic conditions (Nie et al., 2011; Kim et al., 2012; Mei et al., 2013) |
| miR-24 | Enhances adipogenesis and inhibits osteogenesis (Sun et al., 2009; Hassan et al., 2010) |
| miR-27a | Inhibits osteogenesis (Schoolmeesters et al., 2009; Hassan et al., 2010) |
| miR-141 | Inhibits osteogenesis (Itoh et al., 2009) |
| miR-145 | Inhibits chondrogenesis (Tong et al., 2011; Martinez-Sanchez et al., 2012) |
| miR-155 | Inhibits adipogenesis and immune regulation (Skårn et al., 2012; Xu C. et al., 2013) |
| miR-194 | Inhibits chondrogenesis (Xu et al., 2012) |
| miR-200a | Inhibits osteogenesis (Thurnherr et al., 2016) |
| miR-221 | Inhibits adipogenesis (El-Halawany et al., 2015) |
| miR-222 | Inhibits adipogenesis (El-Halawany et al., 2015) |
Figure 3 shows the potential pathways that may be involved in the reprogramming of MSCs and their acquisition of CSC-like characteristics after co-culture with cancer cells.
Figure 3.
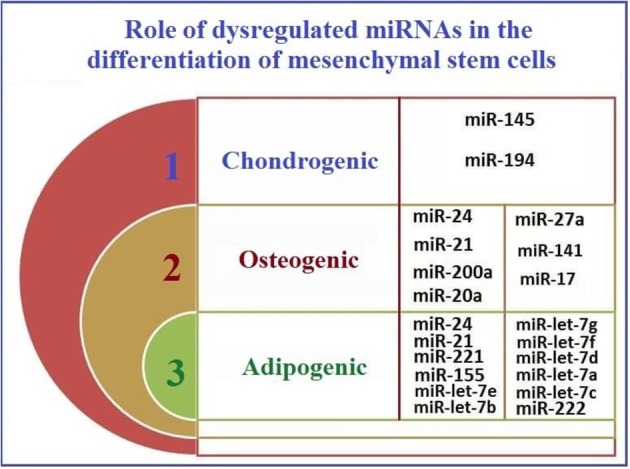
The roles of mutually dysregulated miRNAs in HCC and CSC, in MSC differentiation.
The relationship between the expression of miRNAs expression, their target genes' expression, and the fate of HCC is detailed in Table 3.
Table 3.
The relationship among miRNAs' expression, target genes' expression, and the fate of HCC.
| miRNA | Expression | Target genes | Expression of target genes | Fate of HCC |
|---|---|---|---|---|
| miR-Let-7a | ||||
| miR-Let-7b | ||||
| miR-Let-7c | ||||
| miR-Let-7d | RAS oncogenes | Suppression | ||
| miR-Let-7e | ||||
| miR-Let-7f | ||||
| miR-Let-7g | ||||
| miR-200 a | Foxa2 | Enhanced proliferation and carcinogenesis | ||
| miR-200 b | Downregulation | BMI1 | Upregulation | |
| miR-145 | ROCK 1 | Increased tumorigenesis and invasion | ||
| miR-34a | HDAC1 | Inhibition of invasion and migration | ||
| miR-141 | TIAM1 | Inhibition of proliferation, invasion, and migration | ||
| miR-7 | CDR1 | Suppression | ||
| miR-424 | C-myb | Inhibition of invasion and proliferation | ||
| miR-9 | PPAR alpha | Increase growth and metastasis | ||
| miR-16 | Bcl-2 | Inhibition of proliferation, invasion, and metastasis | ||
| miR-17 | TSP 1 | Increased tumorigenesis | ||
| miR-18 | SMAD2 | Inhibition of migration | ||
| miR-20a | MCl-1 | Increased proliferation and recurrence | ||
| miR-21 | KLFS | Increased progression and metastasis | ||
| miR-24 | P53 | Increased Metastasis and invasion | ||
| miR-27a | PPAR-y | Increased proliferation | ||
| miR-495 | IGFlR | Inhibition of proliferation and invasion | ||
| miR-191 | Upregulation | TIMP3 | Downregulation | Increased proliferation and tumorigenesis |
| miR-155 | Oncogenesis and casein kinase 1-α (CK1-α) | Increased proliferation and tumorigenesis and decreased apoptosis | ||
| miR-221 | NFkB and downstream genes such as (bcl-2/MMP-9 and VEGF) | Increased proliferation and tumorigenesis | ||
| miR-22 | 3UTR of CD147 | – | ||
| miR-210 | SMAD4-STAT6 | Increased angiogenesis | ||
| miR-1246 | Downregulated | CADM1 | Upregulated | Increased migration |
| miR-29c | Downregulated | SIRT 1 | Upregulated | Suppression |
| miR-214 | Downregulated | CTNNB 1 | Upregulated | Suppression |
| miR-215 | Downregulated | Upregulated | Suppression | |
| miR-142 | Downregulated | THBS4–TGF-β | Upregulated | Increased migration, invasion, and metastasis |
| miR-150 | Downregulated | GAB1 | Upregulated | Suppression |
| miR-93 | Upregulated | PDCD 4 | Downregulated | Increased migration and invasion |
| miR-183 | Upregulated | ETS2 and EGR1 | Downregulated | Carcinogenesis |
| miR-185 | Downregulated | DNTM1 | Upregulated | Increased proliferation |
| miR-194 | Downregulated | MAP4K4 | Upregulated | Increased proliferation |
| miR-101 | Downregulated | 3′UTR of WT-PTEN | Upregulated | Promotion of apoptosis and suppression |
Dysregulated miRNA Methylation in HCC
Genome-wide abnormal DNA methylation of miRNA host genes in HCC was recently reported. One study analyzed tumor and neighboring normal non-tumor tissues in 62 HCC patients. This analysis was performed using Infinium Human Methylation Analysis Bead Chips. One hundred ten miRNAs from 64 different host genes were covered in this analysis through assessing the methylation of 254 CpG sites. Methylation levels were found to be significantly different at 54 CpG sites from 27 host genes between tumor and neighboring normal non-tumor tissues (Shen et al., 2012). In addition, the expression of three identified miRNAs were measured. MiR-10a was downregulated in tumor tissues and therefore its action on its oncogenic target genes as a tumor suppressor miRNA diminished. This decline appeared to be related to hypermethylation of the host genes. Accordingly, aberrant methylation and expression of miRNAs were considered valuable molecular biomarkers for HCC early diagnosis (Shen et al., 2012). In another study, miRNA genes, from HCC cells and normal liver hepatocytes, showed significantly different profiles of global DNA methylation. In the same study, in HCC cells, miRNAs CpG-poor regions were more commonly hypomethylated rather than being hypermethylation (He et al., 2015). Investigations using miRNA expression microarray data identified 10 dysregulated miRNAs in HCC that are regulated by DNA methylation. Of the 10 studied miRNAs, miR-23a/27a and miR-25/93/106b constituted two miRNA clusters in which five miRNAs were upregulated. On the other hand, the other five miRNAs including miR-375, miR-195, miR-497, miR-378, and miR-148a were downregulated (He et al., 2015). The cluster containing miR-25/93/106b, with upregulated expression, was required for cell proliferation including the anchorage-independent growth. It was also shown to target the E2F1 transcription factor in HCC, which inhibits apoptosis (Li Y. et al., 2009). Additionally, miR-331-3p was shown to target PHLPP, a protein that plays a central role in inducing apoptosis and reducing metastasis (Ma et al., 2010; Liu J. et al., 2011; Chang et al., 2014; Peuget et al., 2014). These data provide further evidence for the potential role of miR-331-3p in HCC metastasis. The miR-23a/27a cluster, with upregulated expression, enhanced anti-apoptotic pathways in addition to promoting cells proliferation in HCC (Huang et al., 2008). In another study, miR-429 functioned by manipulating liver tumor-initiating cells to target the RBBP4/E2F1/OCT4 axis and was upregulated in HCC due to four aberrant hypomethylated upstream sites (Li L. et al., 2015).
AEG-1 and ATG7 were found to be targets for miR-375, one of the previously mentioned epigenetically downregulated miRNAs, which makes miR-375 a tumor suppressor miRNA in HCC (Chang et al., 2012; He et al., 2012). When overexpressed, miR-375 inhibited both migration and invasion in HCC (He et al., 2012). Cell growth was also inhibited by the action of the miR-195/497, which targeted vital cell cycle regulators, leading to G1 arrest in HCC (Furuta et al., 2013). As reported using a bioinformatics approach, CDK4, which is involved in chemotherapy-mediated tumor cell apoptosis, was predicted to be a potential miR-195 target (Yang et al., 2011). MiR-378 suppressed HCC tumor growth, which was originally caused by HBV infection. MiR-378 was found to directly target the insulin-like growth factor 1 receptor (IGF1R) (Li et al., 2013). IGF2BP1, highly involved in liver cancer progression by promoting metastasis, was reported as an expected miR-378 target (Gutschner et al., 2014). Acting as a tumor suppressor miRNA by targeting c-Met which is an oncogene, miR-148 has also been shown to target DNMT1 in hepatocytes leading to the induction of liver-specific phenotype (Gailhouste et al., 2013). MiR-148a is among the five epigenetically downregulated miRNA by methylation and since DNMT1 and DNMT3B are considered to be two of its targets and responsible for its methylation, miR-148a together with DNMT1 and DNMT3B constitute a positive feedback mechanism which in the case of HCC leads to miR-148a downregulation (Duursma et al., 2008; Pan et al., 2010).
Due to several reports of miRNA hypomethylation in HCC, DNA hypomethylation was shown to play a significant role in miRNA regulation. Moreover, DNA methylation might be responsible for the abnormal expression of these miRNAs. Accordingly, further studies on these dysregulated miRNAs are needed (He et al., 2015).
In HCC, miRNAs have been shown to be regulated through epigenetic markers, specifically by DNA methylation. In Table 4, we review reported methylation-regulated miRNAs in HCC. One of the few studies in this area reported that methylation of miR-203 in CSCs plays a role in EMT and increases cancer progression (Taube et al., 2013). A summary of the available data concerning the epigenetic control of miRNA expression in HCC via methylation is provided in Table 4. Also, Table 4 shows the role the methylation of these miRNAs plays in the progression of HCC.
Table 4.
miRNAs regulated by methylation in HCC.
| miRNA | Function/target | Impact of methylation | Status | Expression upon methylation |
|---|---|---|---|---|
| miR-148a | Acts as a tumor suppressor (Pan et al., 2014) | Enhanced tumorigenesis and HCC progression | Hypermethylation (He et al., 2015) | Downregulation |
| miR-375 | Acts as a tumor suppressor by inhibiting metastasis (Xie D. et al., 2017) 34 | Increased metastasis and HCC progression | Hypermethylation (He et al., 2015) | Downregulation |
| miR-195 | It acts as a tumor suppressor through metastasis inhibition (Wang M. et al., 2015) | Enhanced tumorigenesis and HCC progression | Hypermethylation (He et al., 2015) | Downregulation |
| miR-497 | It acts as a tumor suppressor by inhibiting metastasis angiogenesis (Yan et al., 2015) | Enhanced angiogenesis and metastasis | Hypermethylation (He et al., 2015) | Downregulation |
| miR-378 | Acts as a tumor suppressor (Li et al., 2013) | Enhanced proliferation | Hypermethylation (He et al., 2015) | Downregulation |
| miR-106b | Targets DAB2 (Sun et al., 2018) | Proliferation and migration | Hypomethylation (He et al., 2015) | Upregulation |
| miR-25 | Inhibits RhoGDI1 (Wang C. et al., 2015) | Promotion of both migration and invasion | Hypomethylation (He et al., 2015) | Upregulation |
| miR-93 | Targets PDCD4 (Ji et al., 2017) | Enhanced metastasis and invasion | Hypomethylation (He et al., 2015) | Upregulation |
| miR-23a | Acts as an oncomiR (Bao et al., 2014) | Onset of HCC | Hypomethylation (He et al., 2015) | Upregulation |
| miR-27a | Targets the peroxisome proliferator-activated receptor γ gene (Li S. et al., 2015) | Increased proliferation capacity | Hypomethylation (He et al., 2015) | Upregulation |
| miR-10a | Is reported to have several functions. It can promote migration and invasion, while inhibiting angiogenesis capacity in HCC and reduce metastasis capability by targeting Â1-integrin and MMP-2 (Tang, 2013) | After hypermethylation, metastasis and angiogenesis are expected to be reduced. On the other hand, invasion, and migration capabilities would be reduced | Hypermethylation (Shen et al., 2012) | Downregulation |
| miR-10b | Acts by targeting CSMD1, RhoC, uPAR, and MMPs (Liao et al., 2014; Zhu et al., 2016) | Hypermethylation of this miRNA is expected to reduce levels of migration, proliferation, and invasion potential | Hypermethylation (Shen et al., 2012) | Downregulation |
| miR-196b | Targets FOXP2 (Yu et al., 2018) | Decreased metastasis, proliferation, and migration potential | Hypermethylation (Shen et al., 2012) | Downregulation |
| miR-1 | It acts as an oncomiR (Hu et al., 2015) | Decreased proliferation and migration potential | Hypermethylation (Datta et al., 2008) | Silenced |
| miR-124 | Acts as a tumor suppressor by targeting Baculoviral IAP repeat containing 3 (BIRC3) gene (Cao et al., 2018) | Promotion of proliferation and migration potential | Hypermethylation (Furuta et al., 2010) | Silenced |
| miR-125b | Acts as a tumor suppressor by targeting (TAZ) transcriptional co-activator (Li J. et al., 2015) | Increased migration and invasion | Hypermethylation (Alpini et al., 2011) | Silenced |
| miR-203 | Acts as a tumor suppressor by targeting survivin (Wei W. et al., 2013) | Increased proliferation potential | Hypermethylation (Furuta et al., 2010) | Silenced |
| miR-1247 | Acts as a tumor suppressor with Wnt3 being its target (Chu et al., 2017) | Increased proliferation and invasion potential | Hypermethylation (Anwar et al., 2013) | Downregulation |
| miR-132 | Acts as a tumor suppressor by targeting PIK3R3 (Hu et al., 2015) | Increased proliferation, invasion, and migration potential | Hypermethylation (Wei X. et al., 2013) | Downregulation |
| miR-320 | Acts as a tumor suppressor where c-Myc is its target (Xie F. et al., 2017) | Increased proliferation and invasion | Hypermethylation (Shen et al., 2012) | Downregulation |
| miR-596 | No data available | No data available | Hypermethylation (Anwar et al., 2013) | Downregulation |
| miR-663 | Inhibits proliferation by targeting the HMGA2 gene (Huang et al., 2016) | Increased proliferation and invasion | Hypermethylation (Potapova et al., 2011) | Downregulation |
| miR-9 | Different data are reported: It may act as a tumor suppressor role by targeting TAZ (WWTR1) (Higashi et al., 2015). Moreover, it targets KLF17 and increases the migration and invasion properties (Sun et al., 2013) | Data reported that hypermethylation could lead to HCC progression (Higashi et al., 2015), while other reports suggested that hypermethylation can lead to reduced migration and invasion properties (Sun et al., 2013) | Hypermethylation (Anwar et al., 2013) | Downregulation |
Data in Table 4 shows some consistent pattern between the role of different miRNAs and their methylation patterns. To illustrate, miRNAs that act as tumor suppressors are hypermethylated while oncomiR are hypomethylated, which finally leads to HCC progression. On the other hand, in HCC, some miRNAs showed an opposite pattern. MiR-10a, miR-10b, miR-9, and miR-196b have been shown to be hypermethylated and their hypermethylation state would lead to reduced tumorigenesis. Therefore, further studies are needed to investigate other roles for these specific miRNAs, and especially the function of miR-596 in HCC progression.
Conclusion
Several studies showed how tumor microenvironment enhances cancer development and progression (Whiteside, 2008; Wang et al., 2017; Klymenko and Nephew, 2018). Studies from our laboratory have shown that soluble HCC factors play a vital role in the induction of chemoresistance properties in human bone marrow (hBM)-MSCs and trigger their transformation into CSC-like cells (El-Badawy et al., 2017). However, the mechanism of this transformation remains unclear. Although the initiation of HCC is known to be preceded by cirrhosis, the initiation mechanism itself is thought to involve CSCs. CSCs were proposed to be responsible for chemoresistance and relapse in most cancers. Previous data reported by our research group highlighted the role of CSCs in HCC initiation and progression (El-Badawy et al., 2017). Studies also demonstrated that liver CSCs are associated with liver cancer metastasis and relapse and that CSCs play a substantial role in the resistance of liver cancer to conventional treatment (Xu et al., 2009). These data indicate that targeting CSCs as a potential therapeutic approach for liver cancer holds huge promise for improving the treatment outcomes (Lou et al., 2018). Since reprogramming events responsible for the transformation and initiation processes are mainly controlled by epigenetic modifications, determining the role of such epigenetic fingerprints, including miRNAs and their methylation, in initiation, relapse, and chemoresistance in HCC and CSCs is central to understanding tumor biology and developing effective therapies.
Herein, we are presenting growing evidence supporting the central role of miRNAs in many biological processes (Brennecke et al., 2003; Ambros, 2004). In addition, miRNA dysfunction causes the development of diverse cancers (Iorio and Croce, 2009; Negrini et al., 2009). Recent studies show that HCC is associated with altered levels of miRNAs (Murakami et al., 2006; Jiang et al., 2008; Wong et al., 2008). Moreover, several miRNAs, such as miR-195, miR-122, miR-101, and miR-121, have been reported to regulate cell invasion, migration, apoptosis and growth (Fornari et al., 2008; Wang et al., 2008; Su et al., 2009). These findings suggest that dysregulation of miRNA may be attributed to hepatocarcinogenesis (Xiong et al., 2010).
Accumulating data on the expression profiles, roles, and regulation of miRNAs are essential for designing effective stem cell therapies for HCC. In this review, we highlighted the dysregulated expression of relevant miRNAs in HCC and CSCs. Based on the classification of miRNAs as mutually upregulated, mutually downregulated, or mutually dysregulated, we proposed their roles in cancer progression. Despite the lack of data on miRNA expression in MSCs, four miRNAs have dysregulated expression in HCC, CSCs, and MSCs. Based on their functions in MSCs, these miRNAs have been shown to mainly affect differentiation. It is clear, however, that more research is required on the expression profiles of miRNAs in MSCs under physiological conditions and from different tissue sources. Such studies are essential for determining how MSCs regulate and interact with cancer cells and CSCs in HCC.
In the context of clinical applications, miRNAs could represent an opportunity to develop safe strategies for achieving early diagnosis, monitoring disease status, and improving the effectiveness of non-invasive HCC treatment (Valeri et al., 2009). Several studies have shown the effect of miRNAs on enhancing the sensitivity of liver CSCs to treatment. Many dysregulated miRNAs in liver CSCs exert their roles by binding to specific target genes that are key molecules in many pathways. Targeting these miRNAs, their targeted genes, or respective pathways may thus effectively target CSCs, and disturb their role in metastasis, recurrence, and resistance to therapy (Lou et al., 2018). Together with conventional treatment, targeting specific miRNAs involved in tumor progression in combination therapy represent an attractive approach to multifactorial effective therapy to liver cancer (Tao et al., 2018). Although no miRNAs-based drugs are available in current clinical practice (Lou et al., 2018), the antitumor efficiency of modern anticancer drugs like sorafenib on HCC was significantly increased in vivo upon delivery of miR-122-exosome to the tumor (Blechacz and Gores, 2008; Lou et al., 2015). Such enhancement is promising to patients of unresectable HCC whose treatment with sorafenib was of limited efficacy, by prolonging survival for only 3 months (Blechacz and Gores, 2008; Kane et al., 2009). Investigating the expression signature of those candidate miRNAs in HCC diagnosis, prognosis, metastasis, and recurrence and determining how these miRNAs genetically and epigenetically regulate the transformation of somatic stem cells to a more chemoresistant phenotype is needed for translation to clinical practice (Valeri et al., 2009; El-Badawy et al., 2017).
Author Contributions
Each author has substantially contributed to conducting this study and drafting this manuscript. MN and RS wrote the manuscript. MN, ME, and SE analyzed data and moderate figures and tables. While, NE-B contributed in writing and editing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was funded by the Egyptian Science and Technology Development Fund (STDF Grant ID: 5300).
References
- Ali Hosseini Rad S. M., Bavarsad M. S., Arefian E., Jaseb K., Shahjahani M., Saki N. (2013). The role of microRNAs in stemness of cancer stem cells. Oncol. Rev. 7, e8. 10.4081/oncol.2013.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G., Glaser S. S., Zhang J. P., Francis H., Han Y., Gong J., et al. (2011). Regulation of placenta growth factor by microRNA-125b in hepatocellular cancer. J. Hepatol. 55, 1339–1345. 10.1016/j.jhep.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. (2004). The functions of animal microRNAs. Nature 431:350. 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- Amr K. S., Ezzat W. M., Elhosary Y. A., Hegazy A. E., Fahim H. H., Kamel R. R. (2016). The potential role of miRNAs 21 and 199-a in early diagnosis of hepatocellular carcinoma. Gene 575, 66–70. 10.1016/j.gene.2015.08.038 [DOI] [PubMed] [Google Scholar]
- Anwar S. L., Albat C., Krech T., Hasemeier B., Schipper E., Schweitzer N., et al. (2013). Concordant hypermethylation of intergenic microRNA genes in human hepatocellular carcinoma as new diagnostic and prognostic marker. Int. J. Cancer. 133, 660–670. 10.1002/ijc.28068 [DOI] [PubMed] [Google Scholar]
- Ashmawy A. M., Elgeshy K. M., Abdel Salam E. T., Ghareeb M., Kobaisi M. H., Amin H. A. A., et al. (2017). Crosstalk between liver-related microRNAs and Wnt/β-catenin pathway in hepatocellular carcinoma patients. Arab J. Gastroenterol. 18, 144–150. 10.1016/j.ajg.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Assal R. A., El Tayebi H. M., Hosny K. A., Esmat G., Abdelaziz A. I. (2015). A pleiotropic effect of the single clustered hepatic metastamiRs miR-96-5p and miR-182-5p on insulin-like growth factor II, insulin-like growth factor-1 receptor and insulin-like growth factor-binding protein-3 in hepatocellular carcinoma. Mol. Med. Rep. 12, 645–650. 10.3892/mmr.2015.3382 [DOI] [PubMed] [Google Scholar]
- Bao B., Ali S., Ahmad A., Azmi A. S., Li Y., Banerjee S., et al. (2012). Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6, and miR-21, which can be attenuated by CDF treatment. PLoS ONE 7:e50165. 10.1371/journal.pone.0050165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Zhao J., Dai X., Wang Y., Ma R., Su Y., et al. (2014). Correlation between miR-23a and onset of hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 38, 318–330. 10.1016/j.clinre.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Bartel D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Baskerville S., Bartel D. P. (2005). Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11, 241–247. 10.1261/rna.7240905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B., Blanpain C. (2013). Unravelling cancer stem cell potential. Nat. Rev. Cancer 13, 727–738. 10.1038/nrc3597 [DOI] [PubMed] [Google Scholar]
- Bedard P. L., Hansen A. R., Ratain M. J., Siu L. L. (2013). Tumour heterogeneity in the clinic. Nature 501, 355–364. 10.1038/nature12627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessède E., Staedel C., Acuña Amador L. A., Nguyen P. H., Chambonnier L., Hatakeyama M., et al. (2014). Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene 33, 4123–4131. 10.1038/onc.2013.380 [DOI] [PubMed] [Google Scholar]
- Blechacz B., Gores G. J. (2008). Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 48, 308–321. 10.1002/hep.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel F., Konstantinova P., Jansen P. L. (2012). Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J. Hepatol. 56, 1371–1383. 10.1016/j.jhep.2011.11.026 [DOI] [PubMed] [Google Scholar]
- Brennecke J., Hipfner D. R., Stark A., Russell R. B., Cohen S. M. (2003). Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36. 10.1016/S0092-8674(03)00231-9 [DOI] [PubMed] [Google Scholar]
- Bu Y., Cao D. (2012). The origin of cancer stem cells. Front. Biosci. 4, 819–830. [DOI] [PubMed] [Google Scholar]
- Budhu A., Jia H. L., Forgues M., Liu C. G., Goldstein D., Lam A., et al. (2008). Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 47, 897–907. 10.1002/hep.22160 [DOI] [PubMed] [Google Scholar]
- Bushati N., Cohen S. M. (2007). microRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175–205. 10.1146/annurev.cellbio.23.090506.123406 [DOI] [PubMed] [Google Scholar]
- Cabrera R., Nelson D. R. (2010). Review article: the management of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 31, 461–476. 10.1111/j.1365-2036.2009.04200.x [DOI] [PubMed] [Google Scholar]
- Calin G. A., Croce C. M. (2006). MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866. 10.1038/nrc1997 [DOI] [PubMed] [Google Scholar]
- Callegari E., Elamin B. K., Sabbioni S., Gramantieri L., Negrini M. (2013). Role of microRNAs in hepatocellular carcinoma: a clinical perspective. Onco. Targets. Ther. 6, 1167–1178. 10.2147/OTT.S36161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Qiu J., Wang X., Lu Z., Wang D., Feng H., et al. (2018). Identification of microRNA-124 in regulation of Hepatocellular carcinoma through BIRC3 and the NF-κB pathway. J. Cancer. 9, 3006–3015. 10.7150/jca.25956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S., Bazan V., Rolfo C., Insalaco L., Fanale D., Bronte G., et al. (2012). MicroRNAs in colorectal cancer stem cells: new regulators of cancer stemness? Oncogenesis 1:e32. 10.1038/oncsis.2012.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. M., Yang H., Fang F., Xu J. F., Yang L. Y. (2014). MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology 60, 1251–1263. 10.1002/hep.27221 [DOI] [PubMed] [Google Scholar]
- Chang Y., Yan W., He X., Zhang L., Li C., Huang H., et al. (2012). miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 143, 177–87 e8. 10.1053/j.gastro.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Chapnik E., Rivkin N., Mildner A., Beck G., Pasvolsky R., Metzl-Raz E., et al. (2014). miR-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. Elife 3:e01964. 10.7554/eLife.01964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Huang Y. H., Chen J. L. (2013). Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol. Sin. 34:732–740. 10.1038/aps.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chu F., Cao Y., Shao J., Wang F. (2015). Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumor Biol. 36, 7439–7447. 10.1007/s13277-015-3430-2 [DOI] [PubMed] [Google Scholar]
- Chen S. Y., Ma D. N., Chen Q. D., Zhang J. J., Tian Y. R., Wang Z. C., et al. (2017). MicroRNA-200a inhibits cell growth and metastasis by targeting Foxa2 in hepatocellular carcinoma. J. Cancer 8:617. 10.7150/jca.17394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Kim J. K., Nam J. S., Wang H. J., Lee J. H., Kim B. W., et al. (2015). High circulating microRNA-122 expression is a poor prognostic marker in patients with hepatitis B virus-related hepatocellular carcinoma who undergo radiofrequency ablation. Clin. Biochem. 48, 1073–1078. 10.1016/j.clinbiochem.2015.06.019 [DOI] [PubMed] [Google Scholar]
- Cholankeril G., Patel R., Khurana S., Satapathy S. K. (2017). Hepatocellular carcinoma in non-alcoholic steatohepatitis: current knowledge and implications for management. World J. Hepatol. 9:533–543. 10.4254/wjh.v9.i11.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Fan W., Guo W., Zhang Y., Wang L., Guo L., et al. (2017). miR-1247-5p functions as a tumor suppressor in human hepatocellular carcinoma by targeting Wnt3. Oncol. Rep. 38, 343–351. 10.3892/or.2017.5702 [DOI] [PubMed] [Google Scholar]
- Chuang J. C., Jones P. A. (2007). Epigenetics and microRNAs. Pediatr. Res. 61(5 Pt 2), 24R−29R. 10.1203/pdr.0b013e3180457684 [DOI] [PubMed] [Google Scholar]
- Connolly E., Melegari M., Landgraf P., Tchaikovskaya T., Tennant B. C., Slagle B. L., et al. (2008). Elevated expression of the miR-17-92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am. J. Pathol. 173, 856–864. 10.2353/ajpath.2008.080096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins J. M., Velculescu V. E. (2006). Implications of micro-RNA profiling for cancer diagnosis. Oncogene 25, 6220–6227. 10.1038/sj.onc.1209914 [DOI] [PubMed] [Google Scholar]
- Dambal S., Shah M., Mihelich B., Nonn L. (2015). The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res. 43, 7173–7188. 10.1093/nar/gkv703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta J., Kutay H., Nasser M. W., Nuovo G. J., Wang B., Majumder S., et al. (2008). Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 68, 5049–5058. 10.1158/0008-5472.CAN-07-6655 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dhanasekaran R., Limaye A., Cabrera R. (2012). Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat. Med. 4:19–37. 10.2147/HMER.S16316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhayat S. A., Mardin W. A., Köhler G., Bahde R., Vowinkel T., Wolters H., et al. (2014). The microRNA-200 family—a potential diagnostic marker in hepatocellular carcinoma? J. Surg. Oncol. 110, 430–438. 10.1002/jso.23668 [DOI] [PubMed] [Google Scholar]
- Duursma A. M., Kedde M., Schrier M., le Sage C., Agami R. (2008). miR-148 targets human DNMT3b protein coding region. RNA 14, 872–877. 10.1261/rna.972008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves C. J., Humphries R. K. (2010). Acute myeloid leukemia and the WNT pathway. N. Engl. J. Med. 362, 2326–2327. 10.1056/NEJMcibr1003522 [DOI] [PubMed] [Google Scholar]
- El Tayebi H. M., Hosny K. A., Esmat G., Breuhahn K., Abdelaziz A. I. (2012). miR-615-5p is restrictedly expressed in cirrhotic and cancerous liver tissues and its overexpression alleviates the tumorigenic effects in hepatocellular carcinoma. FEBS Lett. 586, 3309–3316. 10.1016/j.febslet.2012.06.054 [DOI] [PubMed] [Google Scholar]
- El Tayebi H. M., Waly A. A., Assal R. A., Hosny K. A., Esmat G., Abdelaziz A. I. (2015). Transcriptional activation of the IGF-II/IGF-1R axis and inhibition of IGFBP-3 by miR-155 in hepatocellular carcinoma. Oncol. Lett. 10, 3206–3212. 10.3892/ol.2015.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Abd N. E., Fawzy N. A., El-Sheikh S. M., Soliman M. E. (2015). Circulating miRNA-122, miRNA-199a, and miRNA-16 as biomarkers for early detection of hepatocellular carcinoma in Egyptian patients with chronic hepatitis C virus infection. Mol. Diagn. Ther. 19, 213–220. 10.1007/s40291-015-0148-1 [DOI] [PubMed] [Google Scholar]
- El-Badawy A., Ghoneim M. A., Gabr M. M., Salah R. A., Mohamed I. K., Amer M., et al. (2017). Cancer cell-soluble factors reprogram mesenchymal stromal cells to slow cycling, chemoresistant cells with a more stem-like state. Stem Cell Res. Ther. 8, 254. 10.1186/s13287-017-0709-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Halawany M. S., Ismail H. M., Zeeneldin A. A., Elfiky A., Tantawy M., Kobaisi M. H., et al. (2015). Investigating the pretreatment miRNA expression patterns of advanced hepatocellular carcinoma patients in association with response to TACE treatment. Biomed. Res. Int. 2015:649750. 10.1155/2015/649750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H. B. (2002). Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 36(5 Suppl. 1), S74–83. 10.1053/jhep.2002.36807 [DOI] [PubMed] [Google Scholar]
- Eshelman M. A., Yochum G. S. (2016). The Wnt/β-catenin pathway is activated by miR-1246 in liver cancer stem cells. Transl. Cancer Res. S 1457–S1460. 10.21037/tcr.2016.12.57 [DOI] [Google Scholar]
- Espada J., Calvo M. B., Díaz-Prado S., Medina V. (2009). Wnt signalling and cancer stem cells. Clin. Transl. Oncol. 11, 411–427. 10.1007/s12094-009-0380-4 [DOI] [PubMed] [Google Scholar]
- Fawzy I. O., Hamza M. T., Hosny K. A., Esmat G., Abdelaziz A. I. (2016). Abrogating the interplay between IGF2BP1, 2 and 3 and IGF1R by let-7i arrests hepatocellular carcinoma growth. Growth Factors. 34, 42–50. 10.3109/08977194.2016.1169532 [DOI] [PubMed] [Google Scholar]
- Fornari F., Gramantieri L., Ferracin M., Veronese A., Sabbioni S., Calin G. A., et al. (2008). MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 27, 5651–5661. 10.1038/onc.2008.178 [DOI] [PubMed] [Google Scholar]
- Fregni G., Quinodoz M., Möller E., Vuille J., Galland S., Fusco C., et al. (2018). Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMed. 29, 128–145. 10.1016/j.ebiom.2018.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M., Kozaki K., Tanimoto K., Tanaka S., Arii S., Shimamura T., et al. (2013). The tumor-suppressive miR-497-195 cluster targets multiple cell-cycle regulators in hepatocellular carcinoma. PLoS ONE 8:e60155. 10.1371/journal.pone.0060155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M., Kozaki K. I., Tanaka S., Arii S., Imoto I., Inazawa J., et al. (2010). miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis 31, 766–776. 10.1093/carcin/bgp250 [DOI] [PubMed] [Google Scholar]
- Gailhouste L., Gomez-Santos L., Hagiwara K., Hatada I., Kitagawa N., Kawaharada K., et al. (2013). miR-148a plays a pivotal role in the liver by promoting the hepatospecific phenotype and suppressing the invasiveness of transformed cells. Hepatology 58, 1153–1165. 10.1002/hep.26422 [DOI] [PubMed] [Google Scholar]
- Gao H., Priebe W., Glod J., Banerjee D. (2009). Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27, 857–865. 10.1002/stem.23 [DOI] [PubMed] [Google Scholar]
- Garg M. (2015). Emerging role of microRNAs in cancer stem cells: implications in cancer therapy. World J. Stem Cells 7, 1078–1089. 10.4252/wjsc.v7.i8.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M., Di Leva G., Romano G., Nuovo G., Suh S. S., Ngankeu A., et al. (2009). miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 16, 498–509. 10.1016/j.ccr.2009.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- George G. P., Mittal R. D. (2010). MicroRNAs: potential biomarkers in cancer. Indian J. Clin. Biochem. 25, 4–14. 10.1007/s12291-010-0008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi A., Abtahi S. (2018). Mesenchymal stem cells: miraculous healers or dormant killers? Stem Cell Rev. Rep. 14, 722–733. 10.1007/s12015-018-9824-y [DOI] [PubMed] [Google Scholar]
- Gramantieri L., Ferracin M., Fornari F., Veronese A., Sabbioni S., Liu C. G., et al. (2007). Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 67, 6092–6099. 10.1158/0008-5472.CAN-06-4607 [DOI] [PubMed] [Google Scholar]
- Gregory P. A., Bert A. G., Paterson E. L., Barry S. C., Tsykin A., Farshid G., et al. (2008). The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10, 593–601. 10.1038/ncb1722 [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S., Grocock R. J., van Dongen S., Bateman A., Enright A. J. (2006). miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–D144. 10.1093/nar/gkj112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T., Hämmerle M., Pazaitis N., Bley N., Fiskin E., Uckelmann H., et al. (2014). Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is an important protumorigenic factor in hepatocellular carcinoma. Hepatology 59, 1900–1911. 10.1002/hep.26997 [DOI] [PubMed] [Google Scholar]
- Guzman G., Brunt E. M., Petrovic L. M., Chejfec G., Layden T. J., Cotler S. J., et al. (2008). Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch. Pathol. Lab. Med. 132, 1761–1766. 10.1043/1543-2165-132.11.1761 [DOI] [PubMed] [Google Scholar]
- Habashy D. A., El Tayebi H. M., Fawzy I. O., Hosny K. A., Esmat G., Abdelaziz A. I. (2016). Interplay between microRNA-17-5p, insulin-like growth factor-II through binding protein-3 in hepatocellular carcinoma. World J. Hepatol. 8, 976–984. 10.4254/wjh.v8.i23.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Li J., Zhao H., Liang P., Huang X., Zheng L., et al. (2014). Identification of the typical miRNAs and target genes in hepatocellular carcinoma. Mol. Med. Rep. 10, 229–235. 10.3892/mmr.2014.2194 [DOI] [PubMed] [Google Scholar]
- Han Z. B., Chen H. Y., Fan J. W., Wu J. Y., Peng Z. H., Wang Z. W. (2013). Expression and survival prediction of microRNA-155 in hepatocellular carcinoma after liver transplantation. Zhonghua Yi Xue Za Zhi. 93, 884–887. [PubMed] [Google Scholar]
- Hassan M. Q., Gordon J. A., Beloti M. M., Croce C. M., van Wijnen A. J., Stein J. L., et al. (2010). A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc. Natl. Acad. Sci. U.S.A. 107, 19879–19884. 10.1073/pnas.1007698107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X. X., Chang Y., Meng F. Y., Wang M. Y., Xie Q. H., Tang F., et al. (2012). MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene 31, 3357–3369. 10.1038/onc.2011.500 [DOI] [PubMed] [Google Scholar]
- He X. X., Kuang S. Z., Liao J. Z., Xu C. R., Chang Y., Wu Y. L., et al. (2015). The regulation of microRNA expression by DNA methylation in hepatocellular carcinoma. Mol. Biosyst. 11, 532–539. 10.1039/C4MB00563E [DOI] [PubMed] [Google Scholar]
- He Y., Cui Y., Wang W., Gu J., Guo S., Ma K., et al. (2011). Hypomethylation of the hsa-miR-191 locus causes high expression of hsa-mir-191 and promotes the epithelial-to-mesenchymal transition in hepatocellular carcinoma. Neoplasia 13, 841–853. 10.1593/neo.11698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo I., Joo C., Cho J., Ha M., Han J., Kim V. N. (2008). Lin28 mediates the terminal uridylation of let-7 precursor MicroRN. Mol. Cell 32, 276–284. 10.1016/j.molcel.2008.09.014 [DOI] [PubMed] [Google Scholar]
- Higashi T., Hayashi H., Ishimoto T., Takeyama H., Kaida T., Arima K., et al. (2015). miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br. J. Cancer 113, 252–258. 10.1038/bjc.2015.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. S., Pelagalli A., Passaro N., Zannetti A. (2017). Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 8, 73296–73311. 10.18632/oncotarget.20265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Shen S. Q., Cui Z. H., Chen Z. B., Li W. (2015). Effect of microRNA-1 on hepatocellular carcinoma tumor endothelial cells. World J. Gastroenterol. 21, 5884–5892. 10.3748/wjg.v21.i19.5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., He X., Ding J., Liang L., Zhao Y., Zhang Z., et al. (2008). Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-beta-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int. J. Cancer 123, 972–978. 10.1002/ijc.23580 [DOI] [PubMed] [Google Scholar]
- Huang W., Li J., Guo X., Zhao Y., Yuan X. (2016). miR-663a inhibits hepatocellular carcinoma cell proliferation and invasion by targeting HMGA2. Biomed. Pharmacother. 81, 431–438. 10.1016/j.biopha.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Huang X. B., Li J., Zheng L., Zuo G. H., Han K. Q., Li H. Y., et al. (2013). Bioinformatics analysis reveals potential candidate drugs for HCC. Pathol. Oncol. Res. 19, 251–258. 10.1007/s12253-012-9576-y [DOI] [PubMed] [Google Scholar]
- Huang X. H., Wang Q., Chen J. S., Fu X. H., Chen X. L., Chen L. Z., et al. (2009). Bead-based microarray analysis of microRNA expression in hepatocellular carcinoma: miR-338 is downregulated. Hepatol. Res. 39, 786–794. 10.1111/j.1872-034X.2009.00502.x [DOI] [PubMed] [Google Scholar]
- Hung C. H., Hu T. H., Lu S. N., Kuo F. Y., Chen C. H., Wang J. H., et al. (2016). Circulating micro RNAs as biomarkers for diagnosis of early hepatocellular carcinoma associated with hepatitis B virus. Int. J. Cancer 138, 714–720. 10.1002/ijc.29802 [DOI] [PubMed] [Google Scholar]
- Hwang-Verslues W. W., Chang P. H., Wei P. C., Yang C. Y., Huang C. K., Kuo W. H., et al. (2011). miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene 30, 2463–2474. 10.1038/onc.2010.618 [DOI] [PubMed] [Google Scholar]
- Iorio M. V., Croce C. M. (2009). MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 27, 5848–5856. 10.1200/JCO.2009.24.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip B., Wang X.-D. (2014). Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention. Nutrients 6, 124–162. 10.3390/nu6010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Nozawa Y., Akao Y. (2009). MicroRNA-141 and−200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J. Biol. Chem. 284, 19272–19279. 10.1074/jbc.M109.014001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C., Liu H., Yin Q., Li H., Gao H. (2017). miR-93 enhances hepatocellular carcinoma invasion and metastasis by EMT via targeting PDCD4. Biotechnol. Lett. 39, 1621–1629. 10.1007/s10529-017-2403-5 [DOI] [PubMed] [Google Scholar]
- Ji J., Shi J., Budhu A., Yu Z., Forgues M., Roessler S., et al. (2009a). MicroRNA expression, survival, and response to interferon in liver cancer. N. Engl. J. Med. 361, 1437–1447. 10.1056/NEJMoa0901282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Yamashita T., Budhu A., Forgues M., Jia H. L., Li C., et al. (2009b). Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 50, 472–480. 10.1002/hep.22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Gusev Y., Aderca I., Mettler T. A., Nagorney D. M., Brackett D. J., et al. (2008). Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin. Cancer Res. 14, 419–427. 10.1158/1078-0432.CCR-07-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Cheng Q., Zhang B. H., Zhang M. Z. (2015). Circulating microRNAs as biomarkers in hepatocellular carcinoma screening: a validation set from China. Medicine. 94:e13434. 10.1097/MD.0000000000000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., et al. (2005). RAS is regulated by the let-7 microRNA family. Cell 120, 635–647. 10.1016/j.cell.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Kane R. C., Farrell A. T., Madabushi R., Booth B., Chattopadhyay S., Sridhara R., et al. (2009). Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 14, 95–100. 10.1634/theoncologist.2008-0185 [DOI] [PubMed] [Google Scholar]
- Kawai T., Yasuchika K., Ishii T., Katayama H., Yoshitoshi E. Y., Ogiso S., et al. (2015). Keratin 19, a cancer stem cell marker in human hepatocellular carcinoma. Clin. Cancer Res. 21, 3081–3091. 10.1158/1078-0432.CCR-14-1936 [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Hwang S. H., Cho H. H., Shin K. K., Bae Y. C., Jung J. S., et al. (2012). MicroRNA 21 regulates the proliferation of human adipose tissue-derived mesenchymal stem cells and high-fat diet-induced obesity alters microRNA 21 expression in white adipose tissues. J. Cell. Physiol. 227, 183–193. 10.1002/jcp.22716 [DOI] [PubMed] [Google Scholar]
- Klein C. A. (2013). Selection and adaptation during metastatic cancer progression. Nature 501, 365–372. 10.1038/nature12628 [DOI] [PubMed] [Google Scholar]
- Klymenko Y., Nephew K. (2018). Epigenetic crosstalk between the tumor microenvironment and ovarian cancer cells: a therapeutic road less traveled. Cancers 10:E295. 10.3390/cancers10090295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köberle V., Kronenberger B., Pleli T., Trojan J., Imelmann E., Peveling-Oberhag J., et al. (2013). Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur. J. Cancer. 49, 3442–3449. 10.1016/j.ejca.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Krol J., Loedige I., Filipowicz W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610. 10.1038/nrg2843 [DOI] [PubMed] [Google Scholar]
- Lashine Y. A., Seoudi A. M., Salah S., Abdelaziz A. I. (2011). Expression signature of microRNA-181-a reveals its crucial role in the pathogenesis of paediatric systemic lupus erythematosus. Clin. Exp. Rheumatol. 29, 351–357. [PubMed] [Google Scholar]
- Lau W. Y., Lai E. C. (2008). Hepatocellular carcinoma: current management and recent advances. HBPD INT 7, 237–257. [PubMed] [Google Scholar]
- Law P. T., Ching A. K., Chan A. W., Wong Q. W., Wong C. K., To K. F., et al. (2012). MiR-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma. Carcinogenesis 33, 1134–1141. 10.1093/carcin/bgs130 [DOI] [PubMed] [Google Scholar]
- Leal J. A., Lleonart M. E. (2013). MicroRNAs and cancer stem cells: therapeutic approaches and future perspectives. Cancer Lett. 338, 174–183. 10.1016/j.canlet.2012.04.020 [DOI] [PubMed] [Google Scholar]
- Leung W. K., He M., Chan A. W., Law P. T., Wong N. (2015). Wnt/beta-catenin activates MiR-183/96/182 expression in hepatocellular carcinoma that promotes cell invasion. Cancer Lett. 362, 97–105. 10.1016/j.canlet.2015.03.023 [DOI] [PubMed] [Google Scholar]
- Lewis B. P., Burge C. B., Bartel D. P. (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20. 10.1016/j.cell.2004.12.035 [DOI] [PubMed] [Google Scholar]
- Li B., Huang P., Qiu J., Liao Y., Hong J., Yuan Y. (2014). MicroRNA-130a is down-regulated in hepatocellular carcinoma and associates with poor prognosis. Med. Oncol. 31:230. 10.1007/s12032-014-0230-2 [DOI] [PubMed] [Google Scholar]
- Li B., Lu Y., Yu L., Han X., Wang H., Mao J., et al. (2017). miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem. Biol. Interact. 277, 33–42. 10.1016/j.cbi.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Li J., Fang L., Yu W., Wang Y. (2015). MicroRNA-125b suppresses the migration and invasion of hepatocellular carcinoma cells by targeting transcriptional coactivator with PDZ-binding motif. Oncol. Lett. 9, 1971–1975. 10.3892/ol.2015.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Fu H., Xu C., Tie Y., Xing R., Zhu J., et al. (2010). miR-183 inhibits TGF-β1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer. 10:354. 10.1186/1471-2407-10-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Tang J., Zhang B., Yang W., LiuGao M., Wang R., et al. (2015). Epigenetic modification of MiR-429 promotes liver tumour-initiating cell properties by targeting Rb binding protein 4. Gut 64, 156–167. 10.1136/gutjnl-2013-305715 [DOI] [PubMed] [Google Scholar]
- Li L. H., Gao Q., Wang X. Y., Guo Z. J. (2013). [miR-378 suppresses HBV-related hepatocellular carcinoma tumor growth by directly targeting the insulin-like growth factor 1 receptor]. Zhonghua Gan Zang Bing Za Zhi. 21, 609–613. 10.3760/cma.j.issn.1007-3418.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Li N., Fu H., Tie Y., Hu Z., Kong W., Wu Y., et al. (2009). miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 275, 44–53. 10.1016/j.canlet.2008.09.035 [DOI] [PubMed] [Google Scholar]
- Li S., Li J., Fei B. Y., Shao D., Pan Y., Mo Z. H., et al. (2015). MiR-27a promotes hepatocellular carcinoma cell proliferation through suppression of its target gene peroxisome proliferator-activated receptor γ. Chinese Med. J. 128, 941–947. 10.4103/0366-6999.154302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Yin J., Yuan L., Wang S., Yang L., Du X., et al. (2014). Downregulation of microRNA-139 is associated with hepatocellular carcinoma risk and short-term survival. Oncol. Rep. 31, 1699–1706. 10.3892/or.2014.3032 [DOI] [PubMed] [Google Scholar]
- Li Y., Tan W., Neo T. W., Aung M. O., Wasser S., Lim S. G., et al. (2009). Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 100, 1234–1242. 10.1111/j.1349-7006.2009.01164.x [DOI] [PubMed] [Google Scholar]
- Liao C. G., Kong L. M., Zhou P., Yang X. L., Huang J. G., Zhang H. L., et al. (2014). miR-10b is overexpressed in hepatocellular carcinoma and promotes cell proliferation, migration and invasion through RhoC, uPAR and MMPs. J. Transl. Med. 12, 234–234. 10.1186/s12967-014-0234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. L., Miller J. D., Ying S. Y. (2006). Intronic microRNA (miRNA). J. Biomed. Biotechnol. 2006:26818. 10.1155/JBB/2006/26818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li J., Cairns M. J. (2014). Identifying miRNAs, targets and functions. Brief. Bioinformatics 15, 1–19. 10.1093/bib/bbs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Wu J., Liu M., Yin H., He J., Zhang B. (2015). Downregulation of miRNA-30c and miR-203a is associated with hepatitis C virus core protein-induced epithelial–mesenchymal transition in normal hepatocytes and hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 464, 1215–1221. 10.1016/j.bbrc.2015.07.107 [DOI] [PubMed] [Google Scholar]
- Liu D. Z., Zhang H. Y., Long X. L., Zou S. L., Zhang X. Y., Han G. Y., et al. (2015). MIR-150 promotes prostate cancer stem cell development via suppressing p27Kip1. Eur. Rev. Med. Pharmacol. Sci. 19, 4344–4352. [PubMed] [Google Scholar]
- Liu F., Kong X., Lv L., Gao J. (2015). TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 359, 288–298. 10.1016/j.canlet.2015.01.030 [DOI] [PubMed] [Google Scholar]
- Liu J., Stevens P. D., Gao T. (2011). mTOR-dependent regulation of PHLPP expression controls the rapamycin sensitivity in cancer cells. J. Biol. Chem. 286, 6510–6520. 10.1074/jbc.M110.183087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. L., Jiang L., Lin Q. X., Deng C. Y., Mai L. P., Zhu J. N., et al. (2012). MicroRNA 16 enhances differentiation of human bone marrow mesenchymal stem cells in a cardiac niche toward myogenic phenotypes in vitro. Life Sci. 90, 1020–1026. 10.1016/j.lfs.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Liu L., Cai X., Liu E., Tian X., Tian C. (2017). MicroRNA-18a promotes proliferation and metastasis in hepatocellular carcinoma via targeting KLF4. Oncotarget 8, 68263–68269. 10.18632/oncotarget.19293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu W., Hu C., Xue Z., Wang G., Ding B., et al. (2011). MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells 29, 1804–1816. 10.1002/stem.728 [DOI] [PubMed] [Google Scholar]
- Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., et al. (2015). Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 8:122. 10.1186/s13045-015-0220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou W., Liu J., Gao Y., Zhong G., Ding B., Xu L., et al. (2018). MicroRNA regulation of liver cancer stem cells. Am. J. Cancer Res. 8, 1126–1141. [PMC free article] [PubMed] [Google Scholar]
- Lu C. Y., Lin K. Y., Tien M. T., Wu C. T., Uen Y. H., Tseng T. L. (2013). Frequent DNA methylation of MiR-129-2 and its potential clinical implication in hepatocellular carcinoma. Genes Chromosomes Cancer 52, 636–643. 10.1002/gcc.22059 [DOI] [PubMed] [Google Scholar]
- Lu J., Getz G., Miska E. A., Alvarez-Saavedra E., Lamb J., Peck D., et al. (2005). MicroRNA expression profiles classify human cancers. Nature 435, 834–838. 10.1038/nature03702 [DOI] [PubMed] [Google Scholar]
- Luo J., Chen M., Huang H., Yuan T., Zhang M., Zhang K., et al. (2013). Circulating microRNA-122a as a diagnostic marker for hepatocellular carcinoma. Onco. Targets. Ther. 6, 577–583. 10.2147/OTT.S44215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Tang K. H., Chan Y. P., Lee T. K., Kwan P. S., Castilho A., et al. (2010). miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 7, 694–707. 10.1016/j.stem.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Malaguarnera G., Cataudella E., Giordano M., Nunnari G., Chisari G., Malaguarnera M. (2012). Toxic hepatitis in occupational exposure to solvents. World J. Gastroenterol. 18, 2756–2766. 10.3748/wjg.v18.i22.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero J. A., Fontana R. J., Su G. L., Conjeevaram H. S., Emick D. M., Lok A. S. (2002). NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 36, 1349–1354. 10.1002/hep.1840360609 [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchez A., Dudek K. A., Murphy C. L. (2012). Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J. Biol. Chem. 287, 916–924. 10.1074/jbc.M111.302430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham C. E., Morrison S. J. (2013). Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337. 10.1038/nature12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y., Bian C., Li J., Du Z., Zhou H., Yang Z., et al. (2013). miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J. Cell. Biochem. 114, 1374–1384. 10.1002/jcb.24479 [DOI] [PubMed] [Google Scholar]
- Meng F.-L., Wang W., Jia D.- W. (2014). Diagnostic and prognostic significance of serum miR-24-3p in HBV-related hepatocellular carcinoma. Med. Oncol. 31:177. 10.1007/s12032-014-0177-3 [DOI] [PubMed] [Google Scholar]
- Mens M. M. J., Ghanbari M. (2018). Cell cycle regulation of stem cells by MicroRNAs. Stem Cell Rev. Rep. 14, 309–322. 10.1007/s12015-018-9808-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K. C., Huynh T., Tay Y., Ang Y. S., Tam W. L., Thomson A. M., et al. (2006). A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 126, 1203–1217. 10.1016/j.cell.2006.07.031 [DOI] [PubMed] [Google Scholar]
- Mohammed M. K., Shao C., Wang J., Wei Q., Wang X., Collier Z., et al. (2016). Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 3, 11–40. 10.1016/j.gendis.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Tamori A., Itami S., Tanahashi T., Toyoda H., Tanaka M., et al. (2013). The expression level of miR-18b in hepatocellular carcinoma is associated with the grade of malignancy and prognosis. BMC Cancer 13:566. 10.1186/1471-2407-13-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Yasuda T., Saigo K., Urashima T., Toyoda H., Okanoue T., et al. (2006). Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 25, 2537–2545. 10.1038/sj.onc.1209283 [DOI] [PubMed] [Google Scholar]
- Negrini M., Gramantieri L., Sabbioni S., Croce C. M. (2011). microRNA involvement in hepatocellular carcinoma. Anticancer Agents Med. Chem. 11, 500–521. 10.2174/187152011796011037 [DOI] [PubMed] [Google Scholar]
- Negrini M., Nicoloso M. S., Calin G. A. (2009). MicroRNAs and cancer—new paradigms in molecular oncology. Curr. Opin. Cell Biol. 21, 470–479. 10.1016/j.ceb.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Nie Y., Han B. M., Liu X. B., Yang J. J., Wang F., Cong X. F., et al. (2011). Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int. J. Biol. Sci. 7, 762–768. 10.7150/ijbs.7.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C. A., Pollett A., Gallinger S., Dick J. E. (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110. 10.1038/nature05372 [DOI] [PubMed] [Google Scholar]
- Pan L., Huang S., He R., Rong M., Dang Y., Chen G., et al. (2014). Decreased expression and clinical significance of miR-148a in hepatocellular carcinoma tissues. Eur. J. Med. Res. 19, 68–68. 10.1186/s40001-014-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Zhu S., Yuan M., Cui H., Wang L., Luo X., et al. (2010). MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 184, 6773–6781. 10.4049/jimmunol.0904060 [DOI] [PubMed] [Google Scholar]
- Peter M. E. (2009). Let-7 and miR-200 microRNAs: Guardians against pluripotency and cancer progression. Cell Cycle 8, 843–852. 10.4161/cc.8.6.7907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuget S., Bonacci T., Soubeyran P., Iovanna J., Dusetti N. J. (2014). Oxidative stress-induced p53 activity is enhanced by a redox-sensitive TP53INP1 SUMOylation. Cell Death Differ. 21, 1107–1118. 10.1038/cdd.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peveling-Oberhag J., Seiz A., Döring C., Hartmann S., Köberle V., Liese J., et al. (2014). MicroRNA profiling of laser-microdissected hepatocellular carcinoma reveals an oncogenic phenotype of the tumor capsule. Transl. Oncol. 7, 672–680. 10.1016/j.tranon.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pode-Shakked N., Shukrun R., Mark-Danieli M., Tsvetkov P., Bahar S., Pri-Chen S., et al. (2013). The isolation and characterization of renal cancer initiating cells from human Wilms' tumour xenografts unveils new therapeutic targets. EMBO Mol. Med. 5, 18–37. 10.1002/emmm.201201516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova A., Albat C., Hasemeier B., Haeussler K., Lamprecht S., Suerbaum S., et al. (2011). Systematic cross-validation of 454 sequencing and pyrosequencing for the exact quantification of DNA methylation patterns with single CpG resolution. BMC Biotechnol. 11:6. 10.1186/1472-6750-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C. C., Cheng H. H., Tewari M. (2012). MicroRNA profiling: approaches and considerations. Nat. Rev. Genet. 13, 358–369. 10.1038/nrg3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S. K., Steel J. L., Chen H. W., DeMateo D. J., Cardinal J., Behari J., et al. (2012). Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 55, 1809–1819. 10.1002/hep.25536 [DOI] [PubMed] [Google Scholar]
- Ren F. H., Yang H., He R. Q., Lu J. N., Lin X. G., Liang H. W., et al. (2018). Analysis of microarrays of miR-34a and its identification of prospective target gene signature in hepatocellular carcinoma. BMC Cancer 18:12. 10.1186/s12885-017-3941-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong M., He R., Dang Y., Chen G. (2014). Expression and clinicopathological significance of miR-146a in hepatocellular carcinoma tissues. Ups. J. Med. Sci. 119, 19–24. 10.3109/03009734.2013.856970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscigno G., Puoti I., Giordano I., Donnarumma E., Russo V., Affinito A., et al. (2017). MiR-24 induces chemotherapy resistance and hypoxic advantage in breast cancer. Oncotarget 8, 19507–19521. 10.18632/oncotarget.14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolmeesters A., Eklund T., Leake D., Vermeulen A., Smith Q., Force Aldred S., et al. (2009). Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS ONE 4:e5605. 10.1371/journal.pone.0005605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraivogel D., Weinmann L., Beier D., Tabatabai G., Eichner A., Zhu J. Y., et al. (2011). CAMTA1 is a novel tumour suppressor regulated by miR-9/9* in glioblastoma stem cells. EMBO J. 30, 4309–4322. 10.1038/emboj.2011.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaalan Y. M., Handoussa H., Youness R. A., Assal R. A., El-Khatib A. H., Linscheid M. W., et al. (2018). Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 32, 2217–2220. 10.1080/14786419.2017.1366478 [DOI] [PubMed] [Google Scholar]
- Shariff M. I., Cox I. J., Gomaa A. I., Khan S. A., Gedroyc W., Taylor-Robinson S. D. (2009). Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev. Gastroenterol. Hepatol. 3, 353–367. 10.1586/egh.09.35 [DOI] [PubMed] [Google Scholar]
- Shen J., Wang S., Zhang Y. J., Kappil M. A., Chen Wu H., Kibriya M. G., et al. (2012). Genome-wide aberrant DNA methylation of microRNA host genes in hepatocellular carcinoma. Epigenetics 7, 1230–1237. 10.4161/epi.22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Lin Y., Yuan X., Shen L., Chen J., Chen L., et al. (2016). Biomarker MicroRNAs for diagnosis, prognosis and treatment of hepatocellular carcinoma: a functional survey and comparison. Sci. Rep. 6:38311. 10.1038/srep38311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Zhang Z., Yang B., Guo H., Jing L., Liu T., et al. (2017). Overexpression of microRNA let-7 correlates with disease progression and poor prognosis in hepatocellular carcinoma. Medicine 96, e7764–e7764. 10.1097/MD.0000000000007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono Y., Mukohyama J., Nakamura S., Minami H. (2015). MicroRNA regulation of human breast cancer stem cells. J. Clin. Med. 5:E2. 10.3390/jcm5010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono Y., Zabala M., Cho R. W., Lobo N., Dalerba P., Qian D., et al. (2009). Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138, 592–603. 10.1016/j.cell.2009.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skårn M., Namløs H. M., Noordhuis P., Wang M. Y., Meza-Zepeda L. A., Myklebost O., et al. (2012). Adipocyte differentiation of human bone marrow-derived stromal cells is modulated by microRNA-155, microRNA-221, and microRNA-222. Stem Cells Dev. 21, 873–883. 10.1089/scd.2010.0503 [DOI] [PubMed] [Google Scholar]
- Song B., Wang Y., Titmus M. A., Botchkina G., Formentini A., Kornmann M., et al. (2010). Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol. Cancer 9:96. 10.1186/1476-4598-9-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Ouyang Y., Che J., Li X., Zhao Y., Yang K., et al. (2017). Potential value of miR-221/222 as diagnostic, prognostic, and therapeutic biomarkers for diseases. Front. Immunol. 8:56. 10.3389/fimmu.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler B., Ivanovska I., Mehta K., Song S., Nelson A., Tan Y., et al. (2010). Characterization of microRNAs involved in embryonic stem cell states. Stem Cells Dev. 19, 935–950. 10.1089/scd.2009.0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yang J. R., Xu T., Huang J., Xu L., Yuan Y., et al. (2009). MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 69, 1135–1142. 10.1158/0008-5472.CAN-08-2886 [DOI] [PubMed] [Google Scholar]
- Sun C., Yao X., Jiang Q., Sun X. (2018). miR-106b targets DAB2 to promote hepatocellular carcinoma cell proliferation and metastasis. Oncol. Lett. 16, 3063–3069. 10.3892/ol.2018.8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Wang J., Pan Q., Yu Y., Zhang Y., Wan Y., et al. (2009). Characterization of function and regulation of miR-24-1 and miR-31. Biochem. Biophys. Res. Commun. 380, 660–665. 10.1016/j.bbrc.2009.01.161 [DOI] [PubMed] [Google Scholar]
- Sun T. Y., Xie H. J., Li Z., Kong L. F., Gou X. N., Li D. J., et al. (2017). miR-34a regulates HDAC1 expression to affect the proliferation and apoptosis of hepatocellular carcinoma. Am. J. Transl. Res. 9, 103–114. [PMC free article] [PubMed] [Google Scholar]
- Sun X., Liu J., Xu C., Tang S. C., Ren H. (2016). The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J. Cell. Mol. Med. 20, 1779–1788. 10.1111/jcmm.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Han Q., Zhou N., Wang S., Lu S., Bai C., et al. (2013). MicroRNA-9 enhances migration and invasion through KLF17 in hepatocellular carcinoma. Mol. Oncol. 7, 884–894. 10.1016/j.molonc.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. Y., Liao C. H., Wu H. P., Hsiao W. C., Wu I. H., Jinpu, et al. (2013). Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PLoS ONE 8:e71637. 10.1371/journal.pone.0071637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szotek P. P., Pieretti-Vanmarcke R., Masiakos P. T., Dinulescu D. M., Connolly D., Foster R., et al. (2006). Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc. Natl. Acad. Sci. U.S.A. 103, 11154–11159. 10.1073/pnas.0603672103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R.-,u., Miyazaki H., Ochiya T. (2014). The role of microRNAs in the regulation of cancer stem cells. Front. Genet. 4:295. 10.3389/fgene.2013.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata A., Otsuka M., Yoshikawa T., Kishikawa T., Ohno M., Koike K. (2013). MicroRNAs and liver function. Minerva Gastroenterol. Dietol. 59, 187–203. [PubMed] [Google Scholar]
- Tan Y., Ge G., Pan T., Wen D., Chen L., Yu X., et al. (2014). A serum microRNA panel as potential biomarkers for hepatocellular carcinoma related with hepatitis B virus. PLoS ONE 9:e107986. 10.1371/journal.pone.0107986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. (2013). miR-10a regulates epithelial-mesenchymal transition and adhesion and angiogenesis in hepatoma. FASEB J. 27(1_supple), lb153–lb153. 10.1371/journal.pone.0064273 [DOI] [Google Scholar]
- Tang T., Yang Z., Zhu Q., Wu Y., Sun K., Alahdal M., et al. (2018). Up-regulation of miR-210 induced by a hypoxic microenvironment promotes breast cancer stem cell metastasis, proliferation, and self-renewal by targeting E-cadherin. FASEB J. 32, 6965–6981. 10.1096/fj.201801013R [DOI] [PubMed] [Google Scholar]
- Tao J., Jiang L., Chen X. (2018). Roles of micro RNA in liver cancer. Liver Res. 2, 61–72. 10.1016/j.livres.2018.06.002 [DOI] [Google Scholar]
- Taube J. H., Malouf G. G., Lu E., Sphyris N., Vijay V., Ramachandran P. P., et al. (2013). Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci. Rep. 3:2687. 10.1038/srep02687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnherr T., Mah W. C., Lei Z., Jin Y., Rozen S. G., Lee C. G. (2016). Differentially expressed miRNAs in hepatocellular carcinoma target genes in the genetic information processing and metabolism pathways. Sci. Rep. 6:20065. 10.1038/srep20065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y., Xu W., Han H., Chen Y., Yang J., Qiao H., et al. (2011). Tanshinone IIA increases recruitment of bone marrow mesenchymal stem cells to infarct region via up-regulating stromal cell-derived factor-1/CXC chemokine receptor 4 axis in a myocardial ischemia model. Phytomedicine 18, 443–450. 10.1016/j.phymed.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Tsai S. C., Lin C. C., Shih T. C., Tseng R. J., Yu M. C., Lin Y. J., et al. (2017). The miR-200b–ZEB1 circuit regulates diverse stemness of human hepatocellular carcinoma. Mol. Carcinog. 56, 2035–2047. 10.1002/mc.22657 [DOI] [PubMed] [Google Scholar]
- Tsang F. H., Au V., Lu W. J., Shek F. H., Liu A. M., Luk J. M., et al. (2014). Prognostic marker microRNA-125b inhibits tumorigenic properties of hepatocellular carcinoma cells via suppressing tumorigenic molecule eIF5A2. Dig. Dis. Sci. 59, 2477–2487. 10.1007/s10620-014-3184-5 [DOI] [PubMed] [Google Scholar]
- Tu H., Wei G., Cai Q., Chen X., Sun Z., Cheng C., et al. (2015). MicroRNA-212 inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting FOXA1. Onco. Targets. Ther. 8, 2227–2235. 10.2147/OTT.S87976 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Turchi L., Debruyne D. N., Almairac F., Virolle V., Fareh M., Neirijnck Y., et al. (2013). Tumorigenic potential of miR-18A* in Glioma initiating cells requires NOTCH-1 signaling. Stem Cells. 31, 1252–1265. 10.1002/stem.1373 [DOI] [PubMed] [Google Scholar]
- Ullmann P., Nurmik M., Schmitz M., Rodriguez F., Weiler J., Qureshi-Baig K., et al. (2019). Tumor suppressor miR-215 counteracts hypoxia-induced colon cancer stem cell activity. Cancer Lett. 450, 32–41. 10.1016/j.canlet.2019.02.030 [DOI] [PubMed] [Google Scholar]
- Valeri N., Vannini I., Fanini F., Calore F., Adair B., Fabbri M. (2009). Epigenetics, miRNAs, and human cancer: a new chapter in human gene regulation. Mamm Genome. 20, 573–580. 10.1007/s00335-009-9206-5 [DOI] [PubMed] [Google Scholar]
- Wahid F., Shehzad A., Khan T., Kim Y. Y. (2010). MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta. 1803, 1231–1243. 10.1016/j.bbamcr.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Wang C., Wang X., Su Z., Fei H., Liu X., Pan Q., et al. (2015). MiR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1. Oncotarget 6, 36231–36244. 10.18632/oncotarget.4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li J., Wang X., Zheng C., Ma W. (2013). Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem. Biophys. Res. Commun. 439, 47–53. 10.1016/j.bbrc.2013.08.032 [DOI] [PubMed] [Google Scholar]
- Wang L.-J., He C. C., Sui X., Cai M. J., Zhou C. Y., Ma J. L., et al. (2015). MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTE. Oncotarget 6, 5932–5946. 10.18632/oncotarget.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Zhang J., Tong L., Ma X., Qiu X. (2015). MiR-195 is a key negative regulator of hepatocellular carcinoma metastasis by targeting FGF2 and VEGFA. Int. J. Clin. Exp. Pathol. 8, 14110–14120. [PMC free article] [PubMed] [Google Scholar]
- Wang M., Zhao J., Zhang L., Wei F., Lian Y., Wu Y., et al. (2017). Role of tumor microenvironment in tumorigenesis. J. Cancer. 8, 761–773. 10.7150/jca.17648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yin J., Li T., Yuan L., Wang D., He J., et al. (2015). Upregulated circulating miR-150 is associated with the risk of intrahepatic cholangiocarcinoma. Oncol. Rep. 33, 819–825. 10.3892/or.2014.3641 [DOI] [PubMed] [Google Scholar]
- Wang W. Y., Zhang H. F., Wang L., Ma Y. P., Gao F., Zhang S. J., et al. (2014). miR-21 expression predicts prognosis in hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 38, 715–719. 10.1016/j.clinre.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang W., Sun X., Lin Y., Chen W. (2018). Cancer-associated fibroblasts induce epithelial-mesenchymal transition through secreted cytokines in endometrial cancer cells. Oncol. Lett. 15, 5694–5702. 10.3892/ol.2018.8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan H., Zhao G., Liu D., Du L., Wang Z., et al. (2012). miR-16 inhibits the proliferation and angiogenesis-regulating potential of mesenchymal stem cells in severe pre-eclampsia. FEBS J. 279, 4510–4524. 10.1111/febs.12037 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lee A. T., Ma J. Z., Wang J., Ren J., Yang Y., et al. (2008). Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J. Biol. Chem. 283, 13205–13215. 10.1074/jbc.M707629200 [DOI] [PubMed] [Google Scholar]
- Wei W., Wanjun L., Hui S., Dongyue C., Xinjun Y., Jisheng Z., et al. (2013). miR-203 inhibits proliferation of HCC cells by targeting survivin. Cell Biochem. Funct. 31, 82–85. 10.1002/cbf.2863 [DOI] [PubMed] [Google Scholar]
- Wei X., Tan C., Tang C., Ren G., Xiang T., Qiu Z., et al. (2013). Epigenetic repression of miR-132 expression by the hepatitis B virus x protein in hepatitis B virus-related hepatocellular carcinoma. Cell. Signal. 25, 1037–1043. 10.1016/j.cellsig.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Wheeler B. M., Heimberg A. M., Moy V. N., Sperling E. A., Holstein T. W., Heber S., et al. (2009). The deep evolution of metazoan microRNAs. Evol. Dev. 11, 50–68. 10.1111/j.1525-142X.2008.00302.x [DOI] [PubMed] [Google Scholar]
- White D. L., Kanwal F., El–Serag H. B. (2012). Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 10, 1342–1359. e2. 10.1016/j.cgh.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. (2008). The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912. 10.1038/onc.2008.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Q. W., Lung R. W., Law P. T., Lai P. B., Chan K. Y., To K. F., et al. (2008). MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 135, 257–269. 10.1053/j.gastro.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Xia C., Shui L., Lou G., Ye B., Zhu W., Wang J., et al. (2017). 0404 inhibits hepatocellular carcinoma through a p53/miR-34a/SIRT1 positive feedback loop. Sci. Rep. 7:4396. 10.1038/s41598-017-04487-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., Yuan P., Wang D., Jin H., Chen H. (2017). Expression and prognostic significance of miR-375 and miR-221 in liver cancer. Oncol. Lett. 14, 2305–2309. 10.3892/ol.2017.6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F., Yuan Y., Xie L., Ran P., Xiang X., Huang Q., et al. (2017). miRNA-320a inhibits tumor proliferation and invasion by targeting c-Myc in human hepatocellular carcinoma. Onco. Targets. Ther. 10, 885–894. 10.2147/OTT.S122992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Yao Q., Butt A. M., Guo J., Tian Z., Bao X., et al. (2014). Expression profiling of serum microRNA-101 in HBV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cancer Biol. Ther. 15, 1248–1255. 10.4161/cbt.29688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Fang J. H., Yun J. P., Yang J., Zhang Y., Jia W. H., et al. (2010). Effects of MicroRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51, 836–845. 10.1002/hep.23380 [DOI] [PubMed] [Google Scholar]
- Xu C., Ren G., Cao G., Chen Q., Shou P., Zheng C., et al. (2013). miR-155 regulates immune modulatory properties of mesenchymal stem cells by targeting TAK1-binding protein 2. J. Biol. Chem. 288, 11074–11079. 10.1074/jbc.M112.414862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Kang Y., Liao W. M., Yu L. (2012). MiR-194 regulates chondrogenic differentiation of human adipose-derived stem cells by targeting Sox5. PLoS ONE 7:e31861. 10.1371/journal.pone.0031861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Zhang M., Tu J., Pang L., Cai W., Liu X. (2015). MicroRNA-122 affects cell aggressiveness and apoptosis by targeting PKM2 in human hepatocellular carcinoma. Oncol. Rep. 34, 2054–2064. 10.3892/or.2015.4175 [DOI] [PubMed] [Google Scholar]
- Xu W., Ji J., Xu Y., Liu Y., Shi L., Liu Y., et al. (2015). MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol. Carc. 54 (Suppl. 1), E148–E161. 10.1002/mc.22221 [DOI] [PubMed] [Google Scholar]
- Xu X. L., Xing B. C., Han H. B., Zhao W., Hu M. H., Xu Z. L., et al. (2009). The properties of tumor-initiating cells from a hepatocellular carcinoma patient's primary and recurrent tumor. Carcinogenesis 31, 167–174. 10.1093/carcin/bgp232 [DOI] [PubMed] [Google Scholar]
- Xu Y., An Y., Wang Y., Zhang C., Zhang H., Huang C., et al. (2013). miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 29, 2019–2024. 10.3892/or.2013.2338 [DOI] [PubMed] [Google Scholar]
- Xu Y., Bu X., Dai C., Shang C. (2015). High serum microRNA-122 level is independently associated with higher overall survival rate in hepatocellular carcinoma patients. Tumor Biol. 36, 4773–4776. 10.1007/s13277-015-3128-5 [DOI] [PubMed] [Google Scholar]
- Yacoub R. A., Fawzy I. O., Assal R. A., Hosny K. A., Zekri A. N., Esmat G., et al. (2016). miR-34a: multiple opposing targets and one destiny in hepatocellular carcinoma. J. Clin. Transl. Hepatol. 4, 300–305. 10.14218/JCTH.2016.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. J., Zhang Y. N., Liao J. Z., Ke K. P., Chang Y., Li P. Y., et al. (2015). MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget 6, 29527–29542. 10.18632/oncotarget.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Zhang L., Wang F., Wang Y., Huo X. S., Yin Y. X., et al. (2011). Modulation of the unfolded protein response is the core of microRNA-122-involved sensitivity to chemotherapy in hepatocellular carcinoma. Neoplasia 13, 590–600. 10.1593/neo.11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Cho M. E., Li T. W., Peng H., Ko K. S., Mato J. M., et al. (2013). MicroRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. J. Clin. Invest. 123, 285–298. 10.1172/JCI63861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. D., Roberts L. R. (2010). Epidemiology and management of hepatocellular carcinoma. Infect. Dis. Clin. North Am. 24, 899–919. 10.1016/j.idc.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang J., Xia T., Li G., Tian T., Wang M., et al. (2016). MicroRNA-210 promotes cancer angiogenesis by targeting fibroblast growth factor receptor-like 1 in hepatocellular carcinoma. Oncol. Rep. 36, 2553–2562. 10.3892/or.2016.5129 [DOI] [PubMed] [Google Scholar]
- Yata K., Beder L. B., Tamagawa S., Hotomi M., Hirohashi Y., Grenman R., et al. (2015). MicroRNA expression profiles of cancer stem cells in head and neck squamous cell carcinoma. Int. J. Oncol. 47, 1249–1256. 10.3892/ijo.2015.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Hou P., Wu Z., Wang T., Nie Y. (2015). Circulating miR-375 and miR-199a-3p as potential biomarkers for the diagnosis of hepatocellular carcinoma. Tumor Biol. 36, 4501–4507. 10.1007/s13277-015-3092-0 [DOI] [PubMed] [Google Scholar]
- Youness R. A., El-Tayebi H. M., Assal R. A., Hosny K., Esmat G., Abdelaziz A. I. (2016). MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol. Lett. 12, 2567–2573. 10.3892/ol.2016.4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Lu Z., Chen B., Dong P., Zheng J. (2015). microRNA-150: a promising novel biomarker for hepatitis B virus-related hepatocellular carcinoma. Diagn. Pathol. 10:129. 10.1186/s13000-015-0369-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Gong X., Sun L., Zhou Q., Lu B., Zhu L., et al. (2016). The circular RNA Cdr1as act as an oncogene in hepatocellular carcinoma through targeting miR-7 expression. PLoS ONE 11:e0158347. 10.1371/journal.pone.0158347 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yu Y., Nangia-Makker P., Farhana L. G, Rajendra S., Levi E., Majumdar A. P., et al. (2015). miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol. Cancer. 14:98. 10.1186/s12943-015-0372-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Lin X., Tian M., Chang W. (2018). microRNA196b promotes cell migration and invasion by targeting FOXP2 in hepatocellular carcinoma. Oncol. Rep. 39, 731–738. 10.3892/or.2017.6130 [DOI] [PubMed] [Google Scholar]
- Yunqiao L., Vanke H., Jun X., Tangmeng G. (2014). MicroRNA-206, down-regulated in hepatocellular carcinoma, suppresses cell proliferation and promotes apoptosis. Hepatogastroenterology 61, 1302–1307. [PubMed] [Google Scholar]
- Zhang H., Cai K., Wang J., Wang X., Cheng K., Shi F., et al. (2014). MiR-7, inhibited indirectly by lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells. 32, 2858–2868. 10.1002/stem.1795 [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Fu W. M., He M. L., Xie W. D., Lv Q., Wan G., et al. (2011). MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 8, 829–838. 10.4161/rna.8.5.16043 [DOI] [PubMed] [Google Scholar]
- Zhang L., Huang J., Yang N., Greshock J., Megraw M. S., Giannakakis A., et al. (2006). microRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl. Acad. Sci. U.S.A. 103:9136 10.1073/pnas.0508889103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. C., Chin T. M., Yang H., Nga M. E., Lunny D. P., Lim E. K., et al. (2016). Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat. Commun. 7:11702. 10.1038/ncomms11702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guo X., Xiong L., Kong X., Xu Y., Liu C., et al. (2012). MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 586, 4362–4370. 10.1016/j.febslet.2012.10.053 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Jia H. L., Zhou H. J., Dong Q. Z., Fu L. Y., Yan Z. W., et al. (2009). [Identification of metastasis-related microRNAs of hepatocellular carcinoma in hepatocellular carcinoma cell lines by quantitative real time PCR]. Zhonghua Gan Zang Bing Za Zhi. 17, 526–530. [PubMed] [Google Scholar]
- Zheng J., Dong P., Gao S., Wang N., Yu F. (2013). High expression of serum miR-17-5p associated with poor prognosis in patients with hepatocellular carcinoma. Hepatogastroenterology 60, 549–552. 10.5754/hge12754 [DOI] [PubMed] [Google Scholar]
- Zhou C., Liu J., Li Y., Liu L., Zhang X., Ma C. Y., et al. (2011). microRNA-1274a, a modulator of sorafenib induced a disintegrin and metalloproteinase 9 (ADAM9) down-regulation in hepatocellular carcinoma. FEBS Lett. 585, 1828–1834. 10.1016/j.febslet.2011.04.040 [DOI] [PubMed] [Google Scholar]
- Zhou J., Lu S., Yang S., Chen H., Shi H., Miao M., et al. (2014). MicroRNA-127 post-transcriptionally downregulates Sept7 and suppresses cell growth in hepatocellular carcinoma cells. Cell. Physiol. Biochem. 33, 1537–1546. 10.1159/000358717 [DOI] [PubMed] [Google Scholar]
- Zhou J., Yu L., Gao X., Hu J., Wang J., Dai Z., et al. (2011). Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J. Clin. Oncol. 29, 4781–4788. 10.1200/JCO.2011.38.2697 [DOI] [PubMed] [Google Scholar]
- Zhou X., Yue Y., Wang R., Gong B., Duan Z. (2017). MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer stem cells. Int. J. Oncol. 50, 853–862. 10.3892/ijo.2017.3857 [DOI] [PubMed] [Google Scholar]
- Zhu H. T., Dong Q. Z., Sheng Y. Y., Wei J. W., Wang G., Zhou H. J., et al. (2012). MicroRNA-29a-5p is a novel predictor for early recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. PLoS ONE 7:e52393. 10.1371/journal.pone.0052393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Gong L., Wang J., Tu Q., Yao L., Zhang J. R., et al. (2016). miR-10b exerts oncogenic activity in human hepatocellular carcinoma cells by targeting expression of CUB and sushi multiple domains 1 (CSMD1). BMC Cancer 16, 806–806. 10.1186/s12885-016-2801-4 [DOI] [PMC free article] [PubMed] [Google Scholar]