Abstract
Essential oils (EO) of several plant species have the potential to combat plant and fungal diseases. However, the effects of Achillea millefolium EO on the development of common bean (Phaseolus vulgaris L.), is still unknown. Moreover, its effect on N2-fixing bacteria, and in general on soil properties has not been studied yet. A greenhouse trial was set up to evaluate both the influence that Achillea millefolium EO and the inoculation with three different Rhizobium strains have on the bean plant and on the chemical and microbiological properties of an agriculturally used Cambisol. Non-inoculated pots were used as control. Our findings showed a decrease in bacterial colony forming units due to EO application and an increase following the Rhizobium inoculation compared to the control. The EO application decreased soil basal respiration and activities of dehydrogenase, urease, β-glucosidase and acid phosphatase. Such effects were stronger with higher oil concentrations. Moreover, the treatments combining Rhizobium inoculation with EO showed a positive effect on nodulation and plant height. Overall, the combined application of Achillea millefolium EO and rhizobia works as an efficient biocide that could be applied in organic agriculture without hampering the activity of nodule-forming N-fixing bacteria and the development of common bean.
Subject terms: Plant sciences, Environmental sciences
Introduction
It is expected that the world population will reach 9.8 billion people by 2050, and 11.2 billion in 2100 according to the United Nations1, and thus global agriculture will be intensified to feed the increasing population. Enhancing the productivity of agricultural systems while substantially reducing environmental impact is a worldwide responsibility in order to ensure food security for future generations.
Common bean (Phaseolus vulgaris L.) is one of the most important legumes in the world2. In areas of Eastern Africa and Latin America, this plant contributes to 65% of the total protein consumed and 32% of the energy intake3. Moreover, it has been estimated that approximately 26 million metric tons of beans were produced worldwide in 20164, being a crucial crop in developing countries5. In developing areas, a sustainable practice to enhance common bean production consists in their inoculation with symbiotic and associative bacteria to enhance plant growth6.
Plant oils have been used for thousands of years for several purposes7 and are in ever increasing demand nowadays as an alternative medicine. Previous scientific studies have related essential oil compounds to their medical effectivity8. Another use for plant-essential oils is in the food industry due to their antibacterial, antifungal, antiviral, and antioxidant properties9. However, in agriculture, their application is connected to plant protection10 and for their further use as eco-friendly pesticides11–13. The essential oils of Achillea species have been the subject of several studies in which their therapeutic, cosmetic, and fragrant properties have been demonstrated. Işcan et al.14 studied the antimicrobial properties of several plants of the Achillea genus and found that the Achillea aleppica subsp. aleppica had moderate antimicrobial activity, similar to Achillea millefolium. Along these lines, Stojanovic et al.15 evaluated the in vitro antimicrobial effect of four different Achillea species confirming the antibacterial and fungicidal effect of Achillea millefolium. Despite the proven effect of Achillea essential oils in the medical field, their potential application in agriculture has not been studied yet, potentially opening a promising door for future applications, especially in biologically and dynamically managed agricultural fields.
It is widely known that diazotrophic bacteria, among them rhizobia, promote biological nitrogen (N) fixation resulting in better plant development16,17. Moreover, previous studies dealing with the effect of Rhizobium inoculation on plants have focused on soil and soil mineral N content18, nodulation19, nitrogen fixation20, plant height21 and plant growth22. All of these parameters are crucial to assess if the soil-microbe-plant interaction shall lead to enhanced plant performance.
The use of bacteria from the genus Rhizobium has been shown to be an environment-friendly alternative to mineral nitrogen fertilizers, due to their positive effects on plant nodulation, biological N-fixation and consequently N-availability. Although the fixed nitrogen is not readily available to the plant, inoculation ensures long-term N-availability in soil, thus, being an ecologically viable option for amelioration of agricultural soils23. In line with this, inoculation of Rhizobium sp. BARIRGm901 in gray terrace soils led to higher nitrogen fixation and yield of soybean (Glycine max)24. Moreover, Rhizobium inoculation does not only affect the plant performance but also the soil microbiota; indeed, Fall et al.25 found that Rhizobium inoculation on a Gum Arabic production area with natural trees of Senegalia senegal (L.) Britton, (synonym: Acacia senegal) led to an increase in soil microbial biomass and respiration as well as mineral nitrogen content; and positive effect on soil microbial functional diversity was also observed25. Numerous studies have examined the influence of plant essential oils with regard to plant protection as biological control agents, either alone or in combination with alternative fungicides. However, there is still scarce information on how soil productivity and plant growth are affected by the application of essential oils alone or in combination with Rhizobium, and if these affect the microbiota in an antagonistic or synergistic way.
The aims of the present study were to investigate the effects of Achillea millefolium essential oil and inoculation with different Rhizobium strains on soil biological properties as well as on the growth of bean (Phaseolus vulgaris L.) and its nutrient content. For this purpose, we determined the effects on soil enzyme activities, soil microbial biomass and respiration, plant biomass and plant nutrient content (N), together with soil nutrient content (e.g. mineral N) and plant root nodulation.
Material and Methods
Experimental setup
Soil sampling
In the present study, a factorial greenhouse pot experiment was set up in July, 2013 in the Helmholtz Zentrum München - German Research Center for Environmental Health (Neuherberg, Germany). The soil used was collected on a research farm in the municipality of Scheyern (Germany, 48°29′54.1″N 11°26′21.3″E). It was classified as Cambisol26, and its main physiochemical properties are shown in Table 1. After the sampling, the soil was air-dried, sieved (Ø < 2 mm), and sterilized by autoclaving three consecutive times at 121 °C for 30 min, prior to its distribution in sterile pots. After autoclaving, one kg sterile soil was placed in sterile pots (20 cm in diameter and 18 cm in height).
Table 1.
Physiochemical properties of the soil used in this study.
| Parameters | Units | Values |
|---|---|---|
| Sand | % | 21.7 ± 1.0 |
| Silt | % | 46.4 ± 0.9 |
| Clay | % | 31.9 ± 1.9 |
| Organic Matter | % | 3.28 ± 0.1 |
| CaCO3 | % | 0.47 ± 0.1 |
| pH | — | 6.50 ± 0.1 |
| ECa | dS m−1 | 0.52 ± 0.1 |
| CECb | cmolc kg−1 | 35.7 ± 0.8 |
| Phosphorus | mg kg−1 | 9.78 ± 0.1 |
| Total Nitrogen | mg kg−1 | 250.3 ± 9.5 |
| N-Ammonium | mg kg−1 | 19.1 ± 1.2 |
| N-Nitrate | mg kg−1 | 29.9 ± 0.6 |
Values are given on a dry weight basis as an average of n = 3 ± standard deviation.
aElectrical conductivity.
bCation exchange capacity.
Essential plant oil preparation
Leaves of Achillea millefolium plants were collected from the research campus at Helmholtz Zentrum München - for Environmental Health (Neuherberg, Germany) in June 2013. Freshly collected leaves were shade-dried at room temperature (22 ± 1 °C) for 10–15 days. Afterwards, the dried leaves were ground into a fine powder with a pestle and mortar. 50 gr of the sample was subjected to hydro distillation for 3 h in 500 mL distilled water using a Clevenger type apparatus for 4 h in order to produce essential oil. After distillation, the EO concentration was determined by gas chromatography-mass spectrometry (GC-MS). Concentrated EO (1500 ppm) were then diluted using distilled water to the following concentrations: 0 ppm: 0 ml EO 100 ml−1, 100 ppm: 6.66 EO 100 ml−1, and 1000 ppm: 66.6 EO ml 100 ml−1.
Rhizobium inoculum preparations
Rhizobium leguminosarum biovar phaseoli cultures were obtained from the laboratory stock culture collection and prepared by sub-culturing in three mL of YMA (yeast extract–mannitol agar) and incubated at 28 °C for 5 days. Rhizobial inocula (Rhizobium leguminosarum biovar phaseoli F7, Rhizobium leguminosarum biovar phaseoli F83, Rhizobium leguminosarum biovar phaseoli Ciat 899) were incubated at 30 °C for three days in YMA, and were posterior used as material for inoculation in this experiment27. In order to have equal numbers of bacteria as inoculation material, absorbance values at 540 nm wavelength were measured spectrophotometrically. To achieve an equal bacterial density, the lowest number of bacteria after 24 h was taken as a baseline. Where appropriate, bacterial suspensions were diluted to the same absorbance value with sterile physiological saline solution (PSS)27.
Greenhouse set up
A total of twelve treatments resulted from combining three different Achillea millefolium essential oil doses (E0 (control), E1 (100 mg kg−1) and E2 (1000 mg kg−1)) per kg of pot soils with three different Rhizobium inocula and a non-inoculated control (R0: No inoculation; R1: Rhizobium leguminosarum biovar phaseoli F7; R2: Rhizobium leguminosarum biovar phaseoli F83; R3: Rhizobium leguminosarum biovar phaseoli Ciat 889). All treatments were done in triplicate, with a total of 36 pots.
R0 E0 No inoculation/No essential oil
R0 E1 No inoculation/essential oil (100 mg kg−1)
R0 E2 No inoculation/essential oil (1000 mg kg−1)
R1 E0 Rhizobium leguminosarum biovar phaseoli F7/No essential oil
R1 E1 Rhizobium leguminosarum biovar phaseoli F7/essential oil (100 mg kg−1)
R1 E2 Rhizobium leguminosarum biovar phaseoli F7/essential oil (1000 mg kg−1)
R2 E0 Rhizobium leguminosarum biovar phaseoli F83/No essential oil
R2 E1 Rhizobium leguminosarum biovar phaseoli F83/essential oil (100 mg kg−1)
R2 E2 Rhizobium leguminosarum biovar phaseoli F83/essential oil (1000 mg kg−1)
R3 E0 Rhizobium leguminosarum biovar phaseoli Ciat 889/No essential oil
R3 E1 Rhizobium leguminosarum biovar phaseoli Ciat 889/essential oil (100 mg kg−1)
R3 E2 Rhizobium leguminosarum biovar phaseoli Ciat 889/essential oil (1000 mg kg−1)
Phaseolus vulgarıs L seeds were surface-sterilized in 5% H2O2 for 15 minutes and rinsed 5 times with sterile distilled water. After the soils were potted, each pot was planted with three sterile seeds. After seedling, the two seedlings showing the weakest growth were removed 7 days after planting. The essential oils were diluted (0, 100 and 1000 ppm) with 10 ml ethyl alcohol and were slowly surface-sprayed. Seven days after planting, each seedling was inoculated with one mL of log phase bacterial culture with the help of a sterile syringe on the soil surface in the root area. During the 45-day incubation period, soil moisture content was kept at field capacity. The pots were irrigated with water containing bacterial culture suspensions (diluted to 1 × 109 cells in each pot) to 55–60% of the soil water-holding capacity. Soils were maintained at this moisture level by watering them to weight every 2–3 days. After the incubation period, plants were removed from the pots and soil samples were collected and kept at 4 °C prior to analysis.
Soil physico-chemical analyses
Soil electrical conductivity (EC) and pH were determined in 10 g of soil in combination with 25 mL A.d. at a ratio of 1:2.5. Soil texture was analyzed according to Bouyoucos28. Soil cation exchange capacity (CEC) and organic matter (OM) were analyzed as described in Sumner and Miller29 and Nelson and Sommers30 respectively. Soil inorganic nitrogen (N-NH4+ and N-NO3−) was determined in 2 M KCl extracts according to Bremmer and Keeney31. To determine extractable inorganic P, soil samples were extracted with 0.5 M NaHCO3 (pH 8.5) for 30 min, and P was measured following the method proposed by Olsen and Sommers32.
Soil biological analyses
Soil bacterial and fungal abundance
Bacterial and fungal abundance in the soil samples were assessed by determining colony forming units (CFU). One g of soil was extracted in 9 mL phosphate buffered saline (PBS) in a rotation shaker for 30 min. Samples were diluted 10−6 and 10−7, and 100 μL of each sample was then plated on soil extract medium and dextrose-peptone agar for bacteria and fungi, respectively. Bacterial plates were incubated at 30 °C, and fungal ones at 25 °C for a total of seven days33. In the case of bacterial colonies, the average CFUs per gram of oven-dried equivalent (ODE) of field-moist soil was calculated by using an automated colony counter34,35. Fungal colonies growing in agar were observed under a dissecting microscope. They were then marked and recorded in the surface agar. Total culturable fungi were calculated by viable fungal spore g−1 of oven dry soil36. It has to be addressed that this culture‐dependent method is biased because it generally underestimates the total cell number by a factor of 10–10037.
Soil microbial activity
Soil basal respiration (BR) was determined according to Islam and Weil38. Briefly, the CO2 released from 20–25 g moist soil sample previously incubated at 25 ± 1 °C for 24 h was then trapped in 20 mL of 0.5 M NaOH solution. Afterwards, BaCl2 was added to precipitate the CO2 as BaCO3, followed by a titration with 0.5 M HCl.
Soil enzyme activities
Five soil enzyme activities were measured: acid phosphatase (AcdP) and alkaline phosphatase (AlkP) activities were assessed by using pNPP (para-Nitrophenyl phosphate) as substrate and expressed as µg pNP g DM−1 h−1 following the method of Tabatabai39. Soil urease (UE) activity was determined using the steam distillation methods as described by Tabatabai and Bremner40 using urea as substrate and expressed as µg NH4-N g DM−1 2 h−1. β-glucosidase activity (β-Glu) was assayed using β-PNPGLU (p-Nitrophenyl-β-D-glucopyranoside) as substrate and expressed as µg PNP g DM−1 h−1 39. Dehydrogenase (DH) activity was determined with the TTC (triphenyl tetrazolium chloride) method39 and expressed as µg TPF g DM−1 24 h−1.
Plant analyses
The growth response (plant height), total plant biomass, nodule number, and plant total N-content were measured in this experiment, as these parameters are well-known to show a quick response when plants are inoculated with nodule-forming N-fixing bacteria. Both the aboveground (leaf and stem) and root biomass were collected separately from the experimental pots and washed. Roots were oven-dried at 65 °C for 72 h, while leaves and stems were dried for 48 h to determine their dry matter content. After the plant harvest, the numbers of nodules per plant were determined in the roots. Total plant nitrogen contents were determined by the Kjeldahl method after salicylic-sulfuric acid digestion41.
Statistical analyses
Statistical analyses were performed using the SPSS 23 Software (2015, IBM Corp. Armonk, NY, USA). Data were analyzed using a two-way analysis of variance (ANOVA). When significant differences were obtained, an analysis with Tukey’s HSD (honestly significant difference) comparison procedure was further performed as a post hoc test. Prior to analysis, data were tested for normality and transformed to meet this condition when necessary. When data did not meet the normality assumption, they were subjected to non-parametric tests for several independent samples (Kruskal–Wallis test) and pair-wise comparisons were performed using the non-parametric Friedman test.
Results
Influence of essential oil and Rhizobium inoculation on soil chemical properties
The inoculation of soil with different Rhizobium strains and the application of different doses of Achillea millefolium essential oil as well as their interaction did not induce significant effects (p > 0.05) on different soil N-pools (nitrate, ammonium and total N) (Tables 2 and 4). Despite there were no significant effects of the different treatments on the soil ammonium content, soils inoculated with R2 and R3 had higher ammonium contents than the remaining soils. A similar trend was recorded for the soil nitrate content for which no significant differences were recorded among treatments (Tables 2 and 4). In particular, for soils inoculated with Rhizobium species (R1, R2, and R3) the application of E1 induced an increase in the nitrate content, while no such increase was observed for E2.
Table 2.
Effects of different rates of essential oil (E0: No essential oil; E1: essential oil (100 mg kg−1), E2: essential oil (1000 mg kg−1)) and different Rhizobium inocula (R0: No inoculation, R1: Rhizobium leguminosarum biovar phaseoli F7, R2: Rhizobium leguminosarum biovar phaseoli F83, R3: Rhizobium leguminosarum biovar phaseoli Ciat899) on soil chemical and microbial properties.
| Treatments | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Inoculation | Essential Oil | Total N (%) |
NO3− (µg N g−1) |
NH4± (µg N g−1) |
Bacterial abundance (CFU g−1) |
Fungal abundance (CFU g−1) |
Basal respiration (µg C g−1 h−1) |
|
No inoculation (R0) |
E0 | 0.19 ± 0.01 | 55.1 ± 18.0 | 15.3 ± 0.6 | 3.34 · 107 ± 8.91 · 106 ab | 5.50 · 106 ± 0.12 · 106 a | 24.9 ± 0.4 a |
| E1 | 0.20 ± 0.01 | 43.3 ± 6.9 | 12.8 ± 3.3 | 3.78 · 107 ± 7.55 · 106 a | 5.53 · 106 ± 3.73 · 106 a | 17.0 ± 3.0 b | |
| E2 | 0.21 ± 0.01 | 34.1 ± 6.3 | 17.0 ± 11.8 | 1.35 · 107 ± 1.38 · 106 b | 3.16 · 106 ± 1.38 · 106 b | 13.7 ± 2.8 b | |
| R1 | E0 | 0.20 ± 0.02 | 46.8 ± 20.5 | 16.6 ± 6.4 | 2.65 · 107 ± 2.38 · 106 a | 6.06 · 106 ± 0.21 · 106 b | 26.5 ± 2.8 a |
| E1 | 0.18 ± 0.06 | 67.6 ± 29.8 | 17.5 ± 4.2 | 0.74 · 107 ± 4.17 · 106 b | 9.73 · 106 ± 4.17 · 106 a | 17.2 ± 3.4 b | |
| E2 | 0.18 ± 0.03 | 60.1 ± 26.1 | 12.7 ± 2.7 | 0.82 · 107 ± 0.71 · 106 b | 4.16 · 106 ± 0.71 · 106 c | 13.3 ± 5.4 b | |
| R2 | E0 | 0.20 ± 0.01 | 42.0 ± 17.5 | 21.0 ± 3.6 | 1.43 · 107 ± 9.37 · 106 a | 3.73 · 106 ± 1.37 · 106 c | 15.5 ± 4.8 a |
| E1 | 0.19 ± 0.02 | 55.6 ± 23.3 | 14.6 ± 1.5 | 0.69 · 107 ± 1.82 · 106 c | 4.63 · 106 ± 1.82 · 106 b | 17.3 ± 1.6 a | |
| E2 | 0.20 ± 0.01 | 43.9 ± 9.4 | 11.5 ± 0.8 | 1.32 · 107 ± 0.53 · 106 b | 16.1 · 106 ± 2.20 · 106 a | 15.4 ± 1.5 a | |
| R3 | E0 | 0.19 ± 0.03 | 48.9 ± 4.0 | 15.7 ± 2.4 | 7.60 · 107 ± 1.83 · 106 a | 7.60 · 106 ± 3.16 · 106 a | 14.5 ± 2.8 b |
| E1 | 0.20 ± 0.02 | 52.1 ± 8.5 | 24.3 ± 11.6 | 2.65 · 107 ± 2.40 · 106 b | 2.65 · 106 ± 1.08 · 106 c | 13.5 ± 1.45 c | |
| E2 | 0.22 ± 0.02 | 34.3 ± 7.1 | 15.7 ± 1.2 | 1.39 · 107 ± 2.19 · 106 c | 5.90 · 106 ± 3.67 · 106 b | 23.7 ± 7.2 a | |
Different letters indicate significant differences followed by Tukey post- hoc test considering essential oil application for each inoculation treatment and interaction. Values are given as an average for n = 3 on a dry weight basis ± standard deviation.
Table 4.
Two-way analysis of variance (ANOVA) for the soil chemical and microbiological parameters of the soil treated with rhizobial inocula and essential oils as source of variance.
| Parameters | Inoculation treatment | Essential oil treatment | Inoculation × Essential oil | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Total N | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| NH4+ | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| NO3− | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Bacterial abundance | 27.8 | p < 0.001 | 26.6 | p < 0.001 | 7.74 | p < 0.001 |
| Fungal abundance | n.s. | n.s. | n.s. | n.s. | 0.28 | p < 0.001 |
| Basal respiration | n.s. | n.s. | 4.90 | p < 0.05 | 6.93 | p < 0.001 |
n.s.: Not significant.
Influence of essential oil and Rhizobium inoculation on soil biological properties
The application of essential oil doses to the soils significantly decreased bacterial abundance (Tables 2 and 4; p < 0.001), as did the inoculation with different Rhizobium strains (p < 0.001). Moreover, a significant interaction between the application of essential oil and Rhizobium strains (p < 0.001) was also found (Table 4). The bacterial abundance increased for R3 strain, but not for R1 and R2 strains, compared to the control pots where no essential oil was added (E0). The addition of essential oils to the soils induced a significant decrease in bacterial CFUs. When oil at a concentration of 100 ppm was added (E1), a reduction of approximately 30% in bacterial CFUs was observed in all the inoculation treatments (R1, R2, R3), but not for the non-inoculated one (Table 2). The application of a dose 10 times higher (E2) only induce a further decrease for R2 and R3 treatments. Contrarily to what was observed for bacterial CFUs, only the interaction between the application of essential oil and Rhizobium strains (p < 0.001) was found to affect soil fungal CFUs (Table 4). Each combination of oil treatments x Inoculation affected the soil fungal abundance differently (Table 2). For the control soils, only high oil doses of 1000 ppm (E2) induced a decrease in the fungal abundance. In the case of R1 and R2 treatments, less concentrated oil application (E1) enhanced the fungal CFUs, while higher concentrations (E2) significantly decreased the number of culturable fungi. Contrarily, a sharp decrease in the fungal CFUs was observed for soil inoculated with the strain R3, ranging from 7.6·106 to 2.65·106, when applying a lower dose of essential oil (E1). A lower reduction was found for higher oil concentrations (E2).
As described above for the microbial CFUs, BR was negatively affected by the application of essential oils (p < 0.05). In general, the application of the oil extracts decreased of soil basal respiration (Table 2). A significant interaction between both, EO application and inoculation, was also observed (p < 0.001; Table 4). Both oil doses, E1 and E2, reduced BR, from 24.9 to 17.0 and 13.7 mg C kg−1 h−1, respectively. A decrease from 26.5 to 17.2 and 13.2 mg C kg−1 h−1 was observed for non-inoculated soils and R1 treatment, respectively. In contrast, when soil was inoculated with R3 BR increased up to 23.7 mg C kg−1 h−1 with regard to the higher oil doses (E2: 1000 ppm) (Table 4).
Regarding the effects of Achillea millefolium L. oil extracts on the activity of the different soil enzyme activities, no effects were observed, except for β-Glu (p < 0.05) for which a reduction was detected compared to the control.
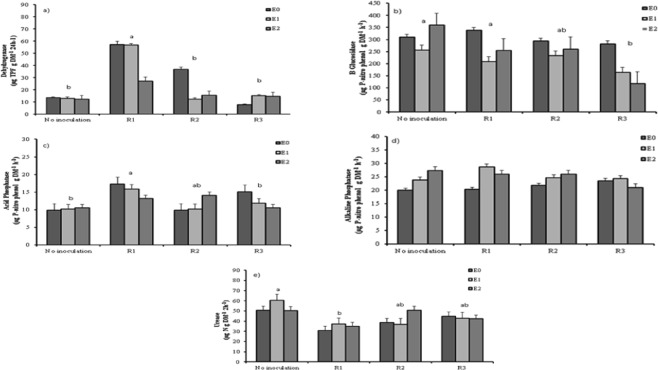
Four of the studied enzymes, that is DH, β-Glu, AcdP and urease (UE) showed a significant decline in their activity (p < 0.001, for all enzymes), when soil was inoculated with the different Rhizobium strains (Fig. 1). Interestingly, neither the application of oils nor the Rhizobium inoculation affected AlkP. However, AcdP activity reached its maximum value with R1 treatment (15.5 ± 1.2 µg pNP g DM−1 h−1), while the minimum AcdP activity (10.2 ± 0.5 µg pNP g DM−1 h−1) was observed in the non-inoculated treatment (Fig. 1c). The highest DH activity was found in the R1 treatment (57.2 ± 7.4 µg TPF g DM−1 24−1) that showed double the activity in comparison with the other treatments (Fig. 1a).
Figure 1.
Effects of Rhizobium inoculation on (a) dehydrogenase enzyme activity, (b) β-Glucosidase enzyme activity, (c) acid phosphatase enzyme activity, (d) acid phosphatase enzyme activity, and (e) urease enzyme activity of soils amended with essential oil (E0: No essential oil; E1: essential oil (100 mg kg−1), E2: essential oil (1000 mg kg−1) and Rhizobium inoculation (R1: Rhizobium leguminosarum biovar phaseoli F7; R2: Rhizobium leguminosarum biovar phaseoli F83; R3: Rhizobium leguminosarum biovar phaseoli Ciat 889). Values are means ± SE. Different letters indicate significant differences at p < 0.001. Post doc comparisons have been done between the different inoculation treatments.
Contrarily to the effect observed for AcdP and DH, the highest UE activity (53.9 ± 6.9 µg NH4-N g DM−1 2 h−1) was observed in the non-inoculated treatment, while the minimum UE activity (34.4 ± 3.7 µg NH4-N g DM−1 2 h−1) was measured in R1 (Fig. 1e). β-Glu activity was the only soil enzyme activity significantly affected by both the application of essential oil and the inoculation with different Rhizobium strains (p < 0.001 for both factors). The maximum β-Glu activity (358 ± 29 µg PNP g DM−1 h−1) was observed in the non-inoculated treatment, while the lowest was found in R3 (188 ± 27 µg PNP g DM−1 h−1) (Fig. 1b). In line with this, the application of essential oils also induced a reduction of the β-Glu activity; higher values of this enzyme activity were observed in the E0 treatment than in E1 and E2.
Influence of essential oil and Rhizobium inoculation on plant properties
The effects of the application of different Rhizobium inoculation treatments on plant height are displayed in (Table 5). Plant height slightly increased with the application of Rhizobium strains; however, for R1 and R2 in combination with the lower essential oil dose (E1), a significant decrease was observed (Tables 3 and 5). Contrarily, for R3 a strong increase in plant height of almost 10 cm was observed compared to the other treatments (non-inoculated, R1, R2). However, we found reduced growth with the lower essential oils dose (E1) in comparison to E0 and E2. As expected, the number of nodules measured was influenced by the different inoculation treatments (p < 0.001). A higher average number of nodules was observed for R2 and R3 (44.3 and 50.4, respectively for both treatments), compared to R1 (29.5 nodules) and the non-inoculated control, for which no visible nodules were observed. Moreover, significant interaction between both treatments (Inoculation × Essential oil; p < 0.05) was observed for the number of nodules (Tables 3 and 5) in the case of plants inoculated with R3, being the treatment R3 E1 (Table 3) the one presenting the highest nodule number. Although no significant effects of any of the applied treatments were observed for plant biomass (Table 3), it can be noticed that, on average, plants inoculated with Rhizobium strains (R1, R2, and R3) yielded a higher biomass than the control one (Table 5). Similarly, inoculation did not affect plant N-content (Table 5); however, plants grown in those soils inoculated with R1, R2 and R3 showed higher nitrogen contents (2.4, 2.2, and 2.7 g kg−1 respectively) than the non-inoculated control (approx. 1.8 g kg−1).
Table 5.
Two-way analyses of variance (ANOVA) for plant parameters of Phaseolus vulgaris L. treated with rhizobial inocula and essential oil as the source of variance.
| Parameters | Inoculation treatment | Essential oil treatment | Inoculation × Essential oil | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Nodules | 217.3 | p < 0.001 | n.s. | n.s. | 3.18 | p < 0.05 |
| Plant height | 9.7 | p < 0.001 | n.s. | n.s. | n.s. | n.s. |
| Total plant biomass | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Plant N content | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
n.s.: Not significant.
Table 3.
Effects of different rates of essential oil (E0: No essential oil; E1: essential oil (100 mg kg−1), E2: essential oil (1000 mg kg−1)) and different Rhizobium inocula (R0: No Inoculation, R1: Rhizobium leguminosarum biovar phaseoli F7, R2: Rhizobium leguminosarum biovar phaseoli F83, R3: Rhizobium leguminosarum biovar phaseoli Ciat899) on nodules, plant height, total plant biomass and plant N content.
| Treatments | Plant parameters of Phaseolus vulgaris L. | ||||
|---|---|---|---|---|---|
| Inoculation | Essential Oil | Nodules | Plant height (cm) | Total plant biomass (g DW) | Plant N content (g kg−1) |
| No inoculation (R0) | E0 | 0 ± 0.0 a | 35.3 ± 1.5 | 2.96 ± 0.6 | 1.70 ± 0.5 |
| E1 | 0 ± 0.0 a | 34.3 ± 1.5 | 2.87 ± 0.4 | 1.80 ± 1.1 | |
| E2 | 0 ± 0.0 a | 27.0 ± 2.0 | 2.78 ± 0.7 | 1.98 ± 0.5 | |
| R1 | E0 | 22.7 ± 6.6 a | 36.3 ± 9.5 a | 3.31 ± 0.8 | 2.24 ± 0.8 |
| E1 | 36.7 ± 9.0 b | 28.3 ± 1.5 b | 3.22 ± 0.9 | 2.42 ± 0.5 | |
| E2 | 29.0 ± 1.7 ab | 39.0 ± 9.2 a | 3.39 ± 0.7 | 2.49 ± 1.4 | |
| R2 | E0 | 48.3 ± 2.8 a | 31.0 ± 10.5a | 3.81 ± 0.8 | 1.66 ± 0.8 |
| E1 | 40.7 ± 9.3 b | 26.0 ± 3.6 b | 3.63 ± 0.8 | 2.17 ± 0.9 | |
| E2 | 44.0 ± 3.6 ab | 35.0 ± 7.5 a | 3.49 ± 1.7 | 2.69 ± 1.4 | |
| R3 | E0 | 46.3 ± 2.9 a | 48.0 ± 1.0 a | 3.94 ± 1.0 | 2.58 ± 1.0 |
| E1 | 54.0 ± 3.0 b | 42.0 ± 6.2 b | 3.89 ± 0.9 | 2.45 ± 1.3 | |
| E2 | 51.0 ± 1.00 ab | 47.0 ± 2.6 a | 3.54 ± 0.9 | 3.05 ± 1.7 | |
Different letters indicate significant differences followed by Tukey post- hoc test considering essential oil application for each inoculation treatment. Values are given as an average for n = 3 on a dry weight basis ± standard deviation.
Discussion
The presence of plant growth promoting bacteria (PGPB) in agricultural soils and their dynamics with plants has gained increasing interest among the scientific community thanks to the abilities of PGPB to solubilize nutrients42–44. In fact, the application of Rhizobia and plant extracts in agriculture could be considered as a potential strategy for their application in organically managed and ecologically oriented farming systems. This is also true for the combined effect of Achillea millefolium essential oil and Rhizobium inoculation based on their potential influence on soil chemical, biological and plant growth parameters. Moreover, to our knowledge, there are no studies yet that have considered these factors.
For all of the combinations of Rhizobium with essential oil, a reduced abundance of both bacterial and fungal CFUs was measured in the present study. However, in the case of the R0E0 treatment, the bacterial CFUs were significantly higher than in the remaining treatments. Oils are known to have inhibitory effects against microbial populations and, in particular essential oil extracted from Achillea millefolium L has shown antimicrobial and antifungal properties15. Although the antifungal effect in the treatments containing oil was significant, such effect was not as strong as that registered for bacteria.
In our study, it has been observed that even a small amount of essential oil had a positive effect in combination with Rhizobium to suppress both the bacterial and the fungal abundances. However, the specific oil component responsible for this suppression, and the underlying mechanisms behind this effect are not clear yet.
In line with our results, where we observed that the inoculation with 2 of the 3 strains led to a reduction in bacterial abundance, Christensen and Jakobsen45 also recorded a decrease in the bacterial abundance in cucumber plants after four weeks following inoculation with vesicular-arbuscular mycorrhizal fungi (AMF). Also, a pronounced antibacterial response of oil extract from Achillea millefolium on Staphylococcus species was reported46. Moreover, the inhibitory effect of essential oils on several plant pathogens has been observed in previous studies47–49. However, other studies reported an enhanced soil metabolism and microbial activity following the application of essential oils50–52. In addition, it has been demonstrated that small amounts of Lavandula stoechas L. essential oil have a positive effect on mycorrhiza development53. Thus, the utilization of plants containing this essential oil in agriculture can be beneficial due to the natural origin of such oils, and their efficient action against pathogens based on the fact that they contain multiple active compounds, which hampers pathogen resistance development54.
Soil microbial activity, measured as basal respiration in this study, reached higher values when no essential oil was applied for R0 and R1; and decreased with the addition of essential oil except for the R3E2 treatment. Several researchers have documented an enhancement of soil respiration after the application of essential oils and their constituents50,55. A plausible explanation could be that the application of this type of oils induced a shift in the soil microbial community favouring the appearance of bacterial strains that are resistant and/or capable of catabolizing such components51. Assuming this, we would expect that some biologically active secondary metabolites derived from the extracted essential oil of Achillea millefolium L might be able to alter soil microbial communities and promote the beneficial microbes involved in a rapid colonization of rhizosphere. However, the short duration of our experiment (45 days) made it impossible to confirm such observations.
In this experiment, the inoculation with different rhizobial strains induced a decrease in most of the measured soil enzyme activities, in particular DH, which can be linked to the soil microbial biomass since its activity serves as an indicator for the physiologically active soil microbiota56,57. Indeed, some essential oils and the individual constituents do not cause an immediate effect on the soil microbiota, but rather seem to require a certain period of time, between 4 and 16 weeks depending on the studies, before increasing soil microbial abundance and basal respiration50,55. However, it seems that R1 bacteria are more resistant than R2 and R3 bacteria to the effect resulting from the application of Achillea millefolium essential oil. Contrary to AlkP, AcdP activity reached its maximum level following R1 inoculation, while the lowest AcdP activity was observed in the no inoculation treatment. Although alkaline phosphatase is an enzyme produced during microbe-plant interactions58, it showed a positive response to the application of all Rhizobium inoculation and essential oil combinations compared to the no inoculation treatment. Nevertheless, numerous studies have demonstrated that the activity of alkaline phosphatase can remain unchanged after several treatments like those resulting from silvicultural activities59.
DH activity showed a significant decline depending on the bacterial species. In addition, DH activity which is primarily involved in the carbon cycle has a pivotal role in the enzyme system of all microorganisms57 that are linked with soil microbial respiration. The abovementioned activity decrease occurred after both inoculation and essential oil treatments. Both treatments also induced a reduction in soil microbial abundance that could explain the decrease of β-Glu activity, which seems to be highly related to the reduction in microbial carbon60. As UE activity was strongly affected by the inoculation treatments used in our study, the decrease in this enzyme activity could be due to nitrogen utilization by bacteria and reduction in soil N available forms61. In addition62, reported that the amendment with different natural products may act as an inhibitor of UE activity.
In contrast to our expectations, Rhizobium inoculation and the treatment with essential oil doses neither affected NH4+ N, NO3− N nor total N contents in soils, despite the increased number of root nodules in the inoculated treatments. However, since in the present study the whole plant was collected, the additional N accumulated in nodules was not added to the soil because it is released only during the decomposition of legume biomass. The rotating cropping system considers the release of N in situ either of the whole biomass, or of the roots and nodules63. Another plausible explanation for the non-significant effect could be related to the short duration of the experiment. Nevertheless, it is important to highlight that plants amended with both treatments showed similar NH4+ N, NO3− N, and total N contents than those from the treatments with no essential oil doses. This indicates that the application of these extracts affects soil microbiota in the short term rather than soil nutrient contents.
We observed that Rhizobium inoculation in combination with all of the rates of essential oil showed a positive response in terms of nodule number and plant height. Nevertheless, inoculating common bean and amending with essential oil did not result in a significant increase in plant biomass and plant N content. Previous studies have reported that Rhizobia are considered a main agent for N fixation in plants, root nodulation and crop growth64–66. Boddey et al.67 found that inoculation of Cowpea with the Rhizobium strain BR 3299 significantly increased grain yield. Furthermore, as stated in Fan et al.68, inoculation of tomato plants with Plant Growth Promoting Rhizobacteria enhanced N and P uptake in the shoots. Contrary to these authors, and in line with our observations, Chekanai et al.69 observed that inoculation with Rhizobium did not induce a positive response in plant biomass or grain yield in common bean (Phaseolus vulgaris L). However, it is known that essential oils are proven to be a thousand times less toxic than standard insecticides, and many of their constituents are not persistent in freshwater and can be metabolized by soil-borne microorganisms10.
Conclusions
In summary, we conclude that the application of Achillea millefolium essential oils alone or in combination with Rhizobium bacteria had an influence on soil productivity and the development of common bean (Phaseolus vulgaris L.). The biocide effect of the application of these essential oils was reflected in a decrease in bacterial and fungal CFUs as well as in basal respiration and soil enzymes activities. Moreover, such effects were stronger with higher oil concentrations. However, the applied essential oil did not seem to harm the effectivity of the used Rhizobium strains, in fact, its joint application increased the number of nodules and the plant height in the studied bean plants. Such effects were not accompanied by an increase in neither inorganic nor total N content in soil, which could be due to the duration of the study. In order to corroborate the positive results of this study, it would be necessary to perform further long-term studies under field conditions to determine the upscale application of Achillea millefolium essential oils, and their potential as additives suitable for organic farming and biologically managed soils.
Acknowledgements
The authors thank Dr. Lyudmila Lyubenova from Helmholtz Zentrum München for their valuable help during the greenhouse and laboratory work. Veysel Turan has been supported by a postdoctoral research grant from The Scientific and Technological Research Council of Turkey (TUBITAK, grant number, 1059B191601133) and a doctoral grant from The Council of Higher Education, Turkey (CoHE, grant number: 82444403-207.04/26187).
Author contributions
All authors (V.T., P.S., S.B., H.I. and M.F.D.J.) contributed jointly to the reported work in the manuscript. V.T. performed most of the experiments. Data analysis was performed by V.T. and M.F.D.J. P.S. and S.B. co-supervised V.T. in this research. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables (2017).
- 2.Myers, J. R. & Kmiecik, K. Common Bean: Economic Importance and Relevance to Biological Science Research. In The Common Bean Genome (eds Pérez de la Vega, M., Santalla, M. & Marsolais, F.) 1–20 (2017).
- 3.Petry N, Boy E, Wirth JP, Hurrell RF. Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification. Nutrients. 2015;7:1144–1173. doi: 10.3390/nu7021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FAOSTAT. Food and Agriculture Organization of the United Nations, FAOSTAT. Food and agriculture data (2018).
- 5.McConnell M, et al. Syntenic relationships among legumes revealed using a gene-based genetic linkage map of common bean (Phaseolus vulgaris L.) Theor Appl Genet. 2010;121:1103–1116. doi: 10.1007/s00122-010-1375-9. [DOI] [PubMed] [Google Scholar]
- 6.de Souza JEB, de Brito Ferreira EP. Improving sustainability of common bean production systems by co-inoculating rhizobia and azospirilla. Agric Ecosyst Environm. 2017;237:250–257. doi: 10.1016/j.agee.2016.12.040. [DOI] [Google Scholar]
- 7.Jones FA. Herbs: useful plants. J Roy Soc Med. 1996;89:717–719. doi: 10.1177/014107689608901219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao LK, et al. Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. J Agric Food Chem. 2005;53:7274–8. doi: 10.1021/jf051151u. [DOI] [PubMed] [Google Scholar]
- 9.Lv F, Liang H, Yuan Q, Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res Intern. 2011;44:3057–3064. doi: 10.1016/j.foodres.2011.07.030. [DOI] [Google Scholar]
- 10.Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- 11.Batish DR, Singh HP, Kohli SK, Kaur S. Eucalyptus essential oil as a natural pesticide. Forest Ecol Manage. 2008;256:2166–2174. doi: 10.1016/j.foreco.2008.08.008. [DOI] [Google Scholar]
- 12.Lopez-Reyes JG, Spadaro D, Prelle A, Garibaldi A, Gullino ML. Efficacy of Plant Essential Oils on Postharvest Control of Rots Caused by Fungi on Different Stone Fruits In Vivo. J Food Protect. 2013;76:631–639. doi: 10.4315/0362-028X.JFP-12-342. [DOI] [PubMed] [Google Scholar]
- 13.Dunn LL, et al. Essential Oil Emulsions as Postharvest Sanitizers To Mitigate Salmonella Cross-Contamination on Peppers. J Food Protect. 2019;82:159–163. doi: 10.4315/0362-028X.JFP-18-190. [DOI] [PubMed] [Google Scholar]
- 14.İşcan G, et al. Biological Activity and Composition of the Essential Oils of Achillea schischkinii Sosn. and Achillea aleppica DC. subsp. aleppica. J Agricult Food Chem. 2006;54:170–173. doi: 10.1021/jf051644z. [DOI] [PubMed] [Google Scholar]
- 15.Stojanovic G, Radulovic N, Hashimoto T, Palic R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol. 2005;101:185–190. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Knoth JL, Kim SH, Ettl GJ, Doty SL. Effects of cross host species inoculation of nitrogen-fixing endophytes on growth and leaf physiology of maize. GCB Bioenergy. 2013;5:408–418. doi: 10.1111/gcbb.12006. [DOI] [Google Scholar]
- 17.Knoth JL, Kim SH, Ettl GJ, Doty SL. Biological nitrogen fixation and biomass accumulation within poplar clones as a result of inoculations with diazotrophic endophyte consortia. New Phytol. 2014;201:599–609. doi: 10.1111/nph.12536. [DOI] [PubMed] [Google Scholar]
- 18.Erman M, Ari E, Togay Y, Cig F. Response of Field Pea (Pisum sativum sp Arvense L.) to Rhizobium Inoculation and Nitrogen Application in Eastern Anotolia. J Anim Vet Adv. 2009;8:612–616. [Google Scholar]
- 19.Lu, J. K. et al. Co-existence of Rhizobia and Diverse Non-rhizobial Bacteria in the Rhizosphere and Nodules of Dalbergia odorifera Seedlings Inoculated with Bradyrhizobium elkanii, Rhizobium multihospitium-Like and Burkholderia pyrrocinia-Like Strains. Front Microbiol8 (2017). [DOI] [PMC free article] [PubMed]
- 20.Schutz, L. et al. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization-A Global Meta-analysis. Front Plant Sci8 (2018). [DOI] [PMC free article] [PubMed]
- 21.Gopalakrishnan S, et al. Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech. 2015;5:355–377. doi: 10.1007/s13205-014-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Heerwaarden J, et al. Soyabean response to rhizobium inoculation across sub-Saharan Africa: Patterns of variation and the role of promiscuity. Agric, Ecosyst & Environm. 2018;261:211–218. doi: 10.1016/j.agee.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korir, H., Mungai, N. W., Thuita, M., Hamba, Y. & Masso, C. Co-inoculation Effect of Rhizobia and Plant Growth Promoting Rhizobacteria on Common Bean Growth in a Low Phosphorus Soil. Front Plant Sci8 (2017). [DOI] [PMC free article] [PubMed]
- 24.Alam F, et al. Effect of Rhizobium sp. BARIRGm901 inoculation on nodulation, nitrogen fixation and yield of soybean (Glycine max) genotypes in gray terrace soil. Biosci Biotech Bioch. 2015;79:1660–1668. doi: 10.1080/09168451.2015.1044931. [DOI] [PubMed] [Google Scholar]
- 25.Fall, D. et al. Rhizobial Inoculation Increases Soil Microbial Functioning and Gum Arabic Production of 13-Year-Old Senegalia senegal (L.) Britton, Trees in the North Part of Senegal. Front Plant Sci7 (2016). [DOI] [PMC free article] [PubMed]
- 26.IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. 192 (2015).
- 27.Somasegaran, P. & Hoben, H. J. Handbook for Rhizobia: Methods in Legumes-Rhizobium. Springer, Heidelberg (1994).
- 28.Bouyoucos GJ. A Recalibration of the Hydrometer Method for Making Mechanical Analysis of Soils. Agron J. 1951;43:434–438. doi: 10.2134/agronj1951.00021962004300090005x. [DOI] [Google Scholar]
- 29.Sumner, M. E. & Miller, W. P. Cation Exchange Capacity and Exchange Coefficients. In Methods of soil analysis, part 3. Chemical Methods (ed. Sparks, D. L.) 1201–1230 (1996).
- 30.Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon and organic matter. In Methods of soil analysis, part 2. Chemical and Microbiological Properties (eds Miller, R. H. & Keeney, D. R.) 539–579 (1982).
- 31.Bremmer JM, Keeney DR. Steam distillation methods for determination of ammonium, nitrate and nitrite. Analytica Chimica Acta. 1965;32:485–495. doi: 10.1016/S0003-2670(00)88973-4. [DOI] [Google Scholar]
- 32.Olsen, S. R. & Sommers, L. E. Phosphorus. In Methods of soil analysis, part 2. Chemical and microbiological properties (eds Miller, R. H. & Keeney, D. R.) 403–430 (1982).
- 33.Wollum, A. G. Cultural methods for soil microorganisms, In: Miller, R. H. & Keeney, D. R. (Eds). In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties 781–802 (Am. Soc. Agron, 1982).
- 34.Madigan, M. T., Martinko, J. M. & Brock, R. D. Culture-dependen analyses of microbial communities. Biology of microorganisms (2006).
- 35.Canbolat MY, Bilen S, Cakmakci R, Sahin F, Aydin A. Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol Fert Soils. 2006;42:350–357. doi: 10.1007/s00374-005-0034-9. [DOI] [Google Scholar]
- 36.Pratt RG. Fungal population levels in soils of commercial swine waste disposal sites and relationships to soil nutrient concentrations. Appl Soil Ecol. 2008;38:223–229. doi: 10.1016/j.apsoil.2007.10.013. [DOI] [Google Scholar]
- 37.Vestergård M, et al. The relative importance of the bacterial pathway and soil inorganic nitrogen increase across an extreme wood-ash application gradient. GCB Bioenergy. 2018;10:320–334. doi: 10.1111/gcbb.12494. [DOI] [Google Scholar]
- 38.Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agr Ecosyst Environ. 2000;79:9–16. doi: 10.1016/S0167-8809(99)00145-0. [DOI] [Google Scholar]
- 39.Tabatabai, M. A. Methods of soil analysis, part 2. Microbiological and biochemical properties. In (ed. Weaver, R. W. et al.) 801–834 (1994).
- 40.Tabatabai MA, Bremner JM. Assay of urease activity in soils. Soil Biol Biochem. 1972;4:479–487. doi: 10.1016/0038-0717(72)90064-8. [DOI] [Google Scholar]
- 41.Jones, J. B. Laboratory guide for conducting soil tests and plant analysis. In Conducting soil tests and plant analysis 363 (CRC Press, 2001).
- 42.Rajkumar M, Ae N, Prasad MNV, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Minaxi, Nain L, Yadav RC, Saxena J. Characterization of multifaceted Bacillus sp RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol. 2012;59:124–135. doi: 10.1016/j.apsoil.2011.08.001. [DOI] [Google Scholar]
- 44.Oteino, N. et al. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol6 (2015). [DOI] [PMC free article] [PubMed]
- 45.Christensen H, Jakobsen I. Reduction of Bacterial-Growth by a Vesicular-Arbuscular Mycorrhizal Fungus in the Rhizosphere of Cucumber (Cucumis-Sativus L) Biol Fert Soils. 1993;15:253–258. doi: 10.1007/BF00337209. [DOI] [Google Scholar]
- 46.Issabeagloo E, Taghizadieh M, Abri B. Antimicrobial effects of yarrow (Achillea millefolium) essential oils against Staphylococcus spp. African J Pharm Pharmacol. 2012;6:2895–2899. doi: 10.5897/AJPP12.397. [DOI] [Google Scholar]
- 47.Karamanoli K, Vokou D, Menkissoglu U, Constantinidou H-I. Bacterial Colonization of Phyllosphere of Mediterranean Aromatic Plants. J Chem Ecol. 2000;26:2035–2048. doi: 10.1023/A:1005556013314. [DOI] [Google Scholar]
- 48.Daferera DJ, Ziogas BN, Polissiou MG. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp and Clavibacter michiganensis subsp michiganensis. Crop Prot. 2003;22:39–44. doi: 10.1016/S0261-2194(02)00095-9. [DOI] [Google Scholar]
- 49.Kadoglidou K, et al. Inhibitory and stimulatory effects of essential oils and individual monoterpenoids on growth and sporulation of four soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum, Penicillium expansum, and Verticillium dahliae. Eur J Plant Pathol. 2011;130:297–309. doi: 10.1007/s10658-011-9754-x. [DOI] [Google Scholar]
- 50.Vokou D, Liotiri S. Stimulation of soil microbial activity by essential oils. Chemoecology. 1999;9:41–45. doi: 10.1007/s000490050032. [DOI] [Google Scholar]
- 51.Vokou D, Chalkos D, Karamanlidou G, Yiangou M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J Chem Ecol. 2002;28:755–768. doi: 10.1023/A:1015236709767. [DOI] [PubMed] [Google Scholar]
- 52.Owen SM, Clark S, Pompe M, Semple KT. Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. Fems Microbiol Lett. 2007;268:34–39. doi: 10.1111/j.1574-6968.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 53.Hassiotis CN, Orfanoudakis M. The impact of Lavandula stoechas L. degradation on arbuscular mycorrhizal fungi, in a Mediterranean ecosystem. Appl Soil Ecol. 2018;126:182–188. doi: 10.1016/j.apsoil.2018.02.025. [DOI] [Google Scholar]
- 54.Chalkos D, et al. Mentha spicata and Salvia fruticosa composts as soil amendments in tomato cultivation. Plant Soil. 2010;332:495–509. doi: 10.1007/s11104-010-0315-4. [DOI] [Google Scholar]
- 55.Vokou D, Margaris NS. Decomposition of Terpenes by Soil-Microorganisms. Pedobiologia. 1988;31:413–419. [Google Scholar]
- 56.Wolińska, A. & Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases (ed. Prof. Rosa Angela Canuto) 183–210 (InTech, 2012).
- 57.Subhani A, Changyong H, Zhengmiao X, Min L, El-ghamry AM. Impact of Soil Environment and Agronomic Practices on Microbial/Dehydrogenase Enzyme Activity in Soil. A Review. Pakistan J Biol Sci. 2001;4:333–338. doi: 10.3923/pjbs.2001.333.338. [DOI] [Google Scholar]
- 58.Nakas JP, Gould WD, Klein DA. Origin and Expression of Phosphatase-Activity in a Semiarid Grassland Soil. Soil Biol Biochem. 1987;19:13–18. doi: 10.1016/0038-0717(87)90118-0. [DOI] [Google Scholar]
- 59.Perie C, Munson AD. Ten-year responses of soil quality and conifer growth to silvicultural treatments. Soil Sci Soc Am J. 2000;64:1815–1826. doi: 10.2136/sssaj2000.6451815x. [DOI] [Google Scholar]
- 60.Turner BL, Hopkins DW, Haygarth PM, Ostle N. β-Glucosidase activity in pasture soils. Appl Soil Ecol. 2002;20:157–162. doi: 10.1016/S0929-1393(02)00020-3. [DOI] [Google Scholar]
- 61.Zhang Q, et al. Effects of fomesafen on soil enzyme activity, microbial population, and bacterial community composition. Environ Monit Assess. 2014;186:2801–2812. doi: 10.1007/s10661-013-3581-9. [DOI] [PubMed] [Google Scholar]
- 62.Patra DD, Kiran U, Pande P. Urease and nitrification retardation properties in natural essential oils and their by-products. Commun Soil Sci Plan. 2006;37:1663–1673. doi: 10.1080/00103620600710306. [DOI] [Google Scholar]
- 63.Peoples MB, Craswell ET. Biological nitrogen fixation: Investments, expectations and actual contributions to agriculture. Plant Soil. 1992;141:13–39. doi: 10.1007/BF00011308. [DOI] [Google Scholar]
- 64.Graham PH, Vance CP. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003;131:872. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 66.Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60:579–598. doi: 10.1007/s13213-010-0117-1. [DOI] [Google Scholar]
- 67.Boddey RM, et al. Cowpea (Vigna Unguiculata) Crops in Africa Can Respond to Inoculation with Rhizorium. Exp Agr. 2017;53:578–587. doi: 10.1017/S0014479716000594. [DOI] [Google Scholar]
- 68.Fan XH, et al. Effects of Plant Growth-Promoting Rhizobacteria and N Source on Plant Growth and N and P Uptake by Tomato Grown on Calcareous Soils. Pedosphere. 2017;27:1027–1036. doi: 10.1016/S1002-0160(17)60379-5. [DOI] [Google Scholar]
- 69.Chekanai V, Chikowo R, Vanlauwe B. Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric Ecosyst Environ. 2018;266:167–173. doi: 10.1016/j.agee.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]