Abstract
Activated platelets play a multifaceted role in tumorigenesis and progression. Platelet distribution width (PDW) is generally applied platelet parameters from routine blood test. Preoperative PDW has been considered a prognostic factor in many cancers. Nevertheless, the prognostic value of PDW in esophageal squamous cell carcinoma (ESCC) remains unknown. The study aimed to investigate whether preoperative PDW could serve as a prognostic factor in patients with ESCC. A total of 495 patients with ESCC undergoing curative surgery were enrolled. The relationship between PDW and clinical features in ESCC was analyzed using chi-square tests. Receiver operating characteristic (ROC) curve was used to determine the optimal cut-off value. Overall survival (OS) and disease-free survival (DFS) stratified by PDW were evaluated by Kaplan–Meier method and log-rank test. Univariate and multivariate Cox regression were used to evaluate the prognostic effect of PDW. Of the 495 patients, elevated PDW was observed in 241(48.7%) of the patients, respectively. An elevated PDW was correlated with depth of tumor (T stage, P = 0.031), nerve infiltration (P = 0.016), hospital time after operation (P = 0.020), platelet (P < 0.001), red cell distribution width (P < 0.001), and aspartate transaminase (P = 0.001). Moreover, elevated PDW (PDW ≥ 13.4 fL) predicted a worse OS and DFS in patients with ESCC (both P < 0.001). Multivariate analyses revealed that PDW was independently associated with OS (hazard ratios 1.194; 95% confidence interval 1.120–1.273; P < 0.001) and DFS (hazard ratios 2.562; 95% confidence interval 1.733–3.786; P < 0.001). Our findings indicated that elevated PDW could serve as an independent worse survival in ESCC.
Subject terms: Prognostic markers, Oesophageal cancer, Tumour biomarkers
Introduction
Esophageal cancer is the sixth and fourth cause of cancer-related mortality in the world and in China1,2, with ESCC accounting for 90% of all diagnosed esophageal cancer cases3. Although much progress has been achieved in the diagnosis and treatment, the prognosis of ESCC still remains unfavorable4–6. Currently, several factors are related to the outcome of ESCC including TNM stage and tumor differentiation. Nevertheless, even within the same staging category, there is disparate prognosis of ESCC because TNM stage could not reflect biological heterogeneity7. Therefore, identification of new and accurate prognosis biomarkers in patients with ESCC is of great importance. A growing number of studies have suggested that platelets play a vital role in tumor development, progression and metastasis8,9. Platelets take part in the different steps of angiogenesis including proliferation, migration, extracellular matrix degradation, and adhesion of endothelial cells10. Activated platelets are involved at cancer-associated thrombosis by releasing inflammatory information, and interacting with neutrophils and monocytes. In addition to activated platelets, an elevated platelet count that has been found in cancer patients seem to be related to a higher proportion of cancer-related venous thromboembolism11. Due to these mechanisms, platelets may serve as a potential therapeutic target12. Some platelet indices including the platelet count (PLT), platelet distribution width (PDW), and platelet-lymphocyte ratio (PLR), can be readily available and have been confirmed to be associated with the prognosis of various cancers, such as non-small cell lung cancer, pancreatic adenocarcinoma, cervical cancer, and colon cancer13–17.
Recently, some researches have showed that an increased pretreatment PLT or PLR could serve as an independent prognosis factor in patients with ESCC18,19. However, whether PDW is related to the prognosis in ESCC remains unknown. Therefore, the aim of this retrospective study was to evaluate the prognostic value of PDW in ESCC, and to investigate the relationship between PDW and the clinical-pathological features.
Results
Patient characteristics
After screening, 495 patients (428 male and 67 female) with complete follow-up data were enrolled in the final study. The median age at diagnosis was 62 years (Interquartile range: 55–67 years). 38 (7.8%) with well differentiated pathology grade, 326 (67.1%) with middle differentiated pathology grade, 121 (24.9%) with poorly differentiated pathology grade, and 1 (0.02%) with undifferentiated pathology grade. In addition, 223 (45.1%) had high- pathological stage (≥TNM3a-3c), 181 (36.6%) had middle- pathological stage (=TNM2a-2b), 91 (18.4%) early- pathological stage (=TNM1a-1b). 264 (53.3%) had lymph node invasion, 138 (27.9%) had vessel invasive, 169 (34.1%) had nerve infiltration, and 339 (68.5%) only received surgery. The median of hospital time after operation was 11(Interquartile range: 10–13), and the median of the PDW was 13.2(Interquartile range: 11.7–15.0). The clinical-pathological features are listed in Table 1.
Table 1.
Difference in PDW ratio according to clinical characteristics in ESCC patients.
| Variables | Cases | ||
|---|---|---|---|
| N | % | ||
| Sex | Male | 428 | 86.5 |
| Female | 67 | 13.5 | |
| Age at therapy initiation(years) | Median | 62 | |
| Interquartile range | (55–67) | ||
| Pathology grade | Well differentiated | 38 | 7.8 |
| middle differentiated | 326 | 67.1 | |
| Poorly differentiated | 121 | 24.9 | |
| Undifferentiated | 1 | 0.02 | |
| Depth of tumor | T1a–1b | 51 | 10.3 |
| T2 | 100 | 20.2 | |
| T3 | 344 | 69.5 | |
| Lymph node metastasis | N0 | 231 | 46.7 |
| N1 | 165 | 33.3 | |
| N2 | 74 | 14.9 | |
| N3 | 25 | 5.1 | |
| Pathological stage | 1a–1b | 91 | 18.4 |
| 2a–2b | 181 | 36.6 | |
| 3a–3c | 223 | 45.1 | |
| Vessel invasive | Yes | 138 | 27.9 |
| No | 357 | 72.1 | |
| Nerve infiltration | Yes | 169 | 34.1 |
| No | 326 | 65.9 | |
| Treatment regimen | S | 339 | 68.5 |
| S plus postoperative C | 111 | 22.4 | |
| S plus postoperative CRT | 45 | 9.1 | |
| Hospital time after operation(days) | Median | 11 | |
| Interquartile range | (10–13) | ||
| PDW | Median | 13.2 | |
| Interquartile range | (11.7–15.0) | ||
| Platelet | Median | 198.5 | |
| Interquartile range | (160.0–236.0) | ||
| Albumin | Median | 42.1 | |
| Interquartile range | (39.5–44.2) | ||
| RDW | Median | 12.8 | |
| Interquartile range | (12.3–13.3) | ||
| Aspartate transaminase | Median | 22 | |
| Interquartile range | (19.0–27.0) | ||
| Fibrinogen | Median | 3.73 | |
| Interquartile range | 3.19–4.34 | ||
| Hemoglobin | Median | 13.7 | |
| Interquartile range | (12.7–14.6) | ||
Abbreviations: S, surgery; C, chemotherapy; CRT, chemoradiotherapy; PDW, platelet distribution width; RDW, red cell distribution width.
High PDW is a predictor of adverse pathological features
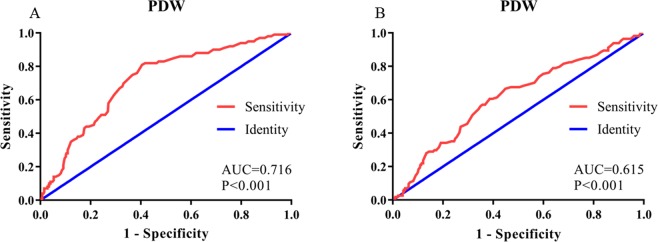
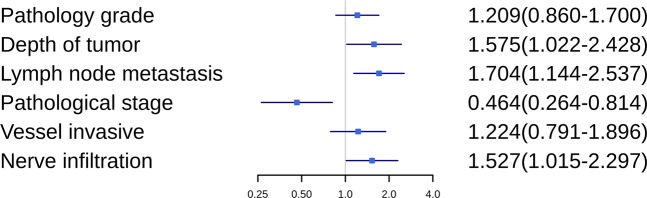
The areas under the ROC curves (AUCs) were 0.716 and 0.615 for OS and DFS, respectively (Fig. 1). The larger AUC of 0.716 acquired for OS was chose to be the optimal cut-off value of 13.4, with maximum specificity (81.0%) and sensitivity (59.49%) (Fig. 1A). According to the cut-off of PDW, 254 patients (51.3%) with PDW < 13.4 were grouped into the low PDW group, whereas the remaining 241 patients (48.7%) with PDW ≥ 13.4 were divided into the high PDW group. The association between PDW and clinical-pathological features are shown in Table 2. None of the clinical-pathological features was notably related to the PDW including gender, age at diagnosis, pathology grade, lymph node metastasis, pathological stage, vessel invasive, treatment regimen, albumin, fibrinogen, and hemoglobin. However, an elevated PDW was significantly associated with depth of tumor (P = 0.031), nerve infiltration (P = 0.016), hospital time after operation (P = 0.020), platelet (P < 0.001), red cell distribution width (P < 0.001), and aspartate transaminase (P = 0.001). Moreover, high PDW independently predicted depth of tumor (OR = 1.575, P = 0.040), lymph node metastasis (OR = 1.704, P = 0.009), pathological stage (OR = 0.464, P = 0.007), and nerve infiltration (OR = 1.527, P = 0.042) using logistic regression analysis (Table 3 and Fig. 2).
Figure 1.
ROC curves analysis of PDW for survival outcomes in patients with ESCC. (A) OS revealed the largest AUC (0.716), while PDW cutoff was set at 13.4 for the largest Youden Index (0.405) obtained (sensitivity, 81.0%; specificity, 59.5%). (B) DFS revealed the AUC (0.615). OS: overall survival; DFS: disease free survival; PDW: platelet distribution width; AUC: area under the ROC curve; ESCC: esophageal squamous cell carcinoma.
Table 2.
Relationship between preoperative PDW and clinical-pathological features in patients with ESCC.
| Characteristics | Total patients | PDW <13.4 (n = 254) | PDW ≥13.4 (n = 241) | P value |
|---|---|---|---|---|
| Sex | Male | 219 | 209 | 0.870 |
| Female | 35 | 32 | ||
| Age at therapy initiation(years) | ≤60 | 112 | 117 | 0.321 |
| >60 | 142 | 124 | ||
| Pathology grade | Well differentiated | 22 | 16 | 0.390 |
| middle differentiated | 170 | 156 | ||
| Poorly differentiated | 56 | 65 | ||
| Undifferentiated | 0 | 1 | ||
| Depth of tumor | T1a–1b | 34 | 17 | 0.031 |
| T2 | 44 | 56 | ||
| T3 | 176 | 168 | ||
| Lymph node metastasis | N0 | 123 | 108 | 0.260 |
| N1 | 89 | 76 | ||
| N2 | 32 | 42 | ||
| N3 | 10 | 15 | ||
| Pathological stage | 1a–1b | 49 | 42 | 0.844 |
| 2a–2b | 93 | 88 | ||
| 3a–3c | 112 | 111 | ||
| Vessel invasive | Yes | 63 | 75 | 0.117 |
| No | 191 | 166 | ||
| Nerve infiltration | Yes | 74 | 95 | 0.016 |
| No | 180 | 146 | ||
| Treatment regimen | S | 163 | 176 | 0.102 |
| S plus postoperative C | 64 | 47 | ||
| S plus postoperative CRT | 27 | 18 | ||
| Hospital time after operation(days) | ≤14 | 215 | 184 | 0.020 |
| >14 | 39 | 57 | ||
| Platelet | Median | 222.0 | 171.0 | <0.001 |
| Interquartile range | (190.0–257.0) | (142.0–206.0) | ||
| Albumin | Median | 42.1 | 41.9 | 0.992 |
| Interquartile range | (39.7–44.1) | (39.3–44.4) | ||
| RDW | Median | 12.7 | 12.9 | <0.001 |
| Interquartile range | (12.3–13.2) | (12.4–13.4) | ||
| Aspartate transaminase | Median | 21.0 | 23.0 | 0.001 |
| Interquartile range | (19.0–26.0) | (19.0–29.0) | ||
| Fibrinogen | Median | 3.8 | 3.7 | 0.108 |
| Interquartile range | (3.3–4.4) | (3.1–4.3) | ||
| Hemoglobin | Median | 13.8 | 13.7 | 0.169 |
| Interquartile range | (12.8–14.7) | (12.6–14.5) |
Abbreviations: S, surgery; C, chemotherapy; CRT, chemoradiotherapy; PDW, platelet distribution width; RDW, red cell distribution width.
Table 3.
Logistic regression analysis of PDW and its predictive value for adverse pathological outcomes.
| Adverse pathological outcomes | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Pathology grade | 1.209 | 0.860–1.7 | 0.275 |
| Depth of tumor | 1.575 | 1.022–2.428 | 0.040 |
| Lymph node metastasis | 1.704 | 1.144–2.537 | 0.009 |
| Pathological stage | 0.464 | 0.264–0.814 | 0.007 |
| Vessel invasive | 1.224 | 0.791–1.896 | 0.364 |
| Nerve infiltration | 1.527 | 1.015–2.297 | 0.042 |
Figure 2.
Forest map showing logistic regression analysis of PDW and its predictive value for adverse pathological outcomes.
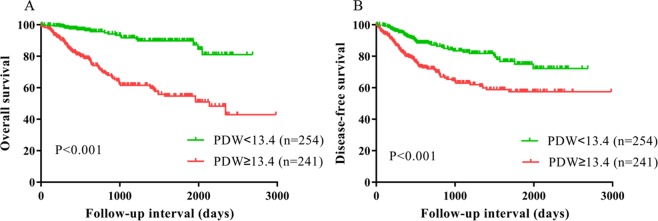
High PDW is related to poor OS and DFS
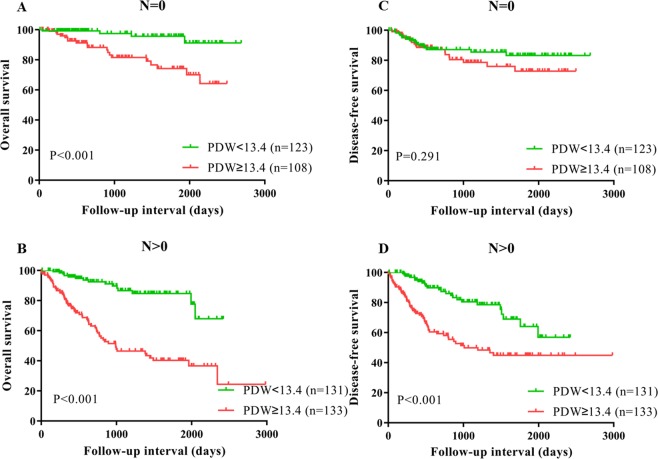
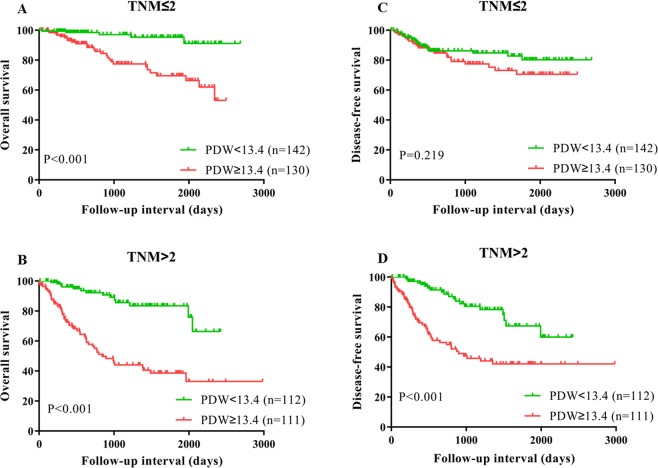
The Kaplan–Meier curves exhibited that patients with high PDW had a worse OS (P < 0.001, Fig. 3A) compared with low PDW group. In subgroup analysis according to lymph node metastasis and pathological stage, high PDW was related to worse OS for patients with or without lymph node metastasis (both P < 0.001) and less or more advanced stage (both P < 0.001) (Figs 4 and 5). In addition, univariate analysis shown that high PDW was correlated with worse OS (HR = 5.111, P < 0.001) (Table 4). Using multivariate analysis, high PDW (HR = 1.194, P < 0.001), lymph node metastasis (P < 0.05), nerve infiltration (P = 0.004), and hospital time (P = 0.009) were notable related to worse OS (Table 4).
Figure 3.
Kaplan–Meier curves for OS (A) and DFS (B) which was stratified according to PDW value (PDW <13.4 vs. PDW ≥13.4) for ESCC patients after surgery. The difference was evaluated by log-rank tests.
Figure 4.
Subgroup analysis based on lymph node metastasis, Kaplan–Meier curves for OS (A,B) and DFS (C,D), which was stratified according to PDW value (PDW <13.4 vs. PDW ≥13.4) for ESCC patients after surgery. The difference was evaluated by log-rank tests.
Figure 5.
Subgroup analysis based on pathological stage, Kaplan–Meier curves for OS (A,B) and DFS (C,D), which was stratified according to PDW value (PDW <13.4 vs. PDW ≥13.4) for ESCC patients after surgery. The difference was evaluated by log-rank tests.
Table 4.
Overall survival analyses according to preoperative PDW in 495 patients with ESCC.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| PDW (≥13.4 vs. <13.4) | 5.111 | 3.101–8.425 | <0.001 | 1.194 | 1.120–1.273 | <0.001 |
| Sex (male vs.female) | 1.676 | 0.845–3.326 | 0.139 | |||
| Age (>60 vs. ≤60) | 1.238 | 0.833–1.838 | 0.291 | |||
| Depth of tumor | ||||||
| T1a–1b | 0.296 | 0.093–0.937 | 0.038 | 0.447 | 0.116–1.722 | 0.242 |
| T2 | 0.607 | 0.355–1.038 | 0.607 | 0.435 | 0.135–1.399 | 0.162 |
| T3 | 1.000 | 1.000 | ||||
| Lymph node metastasis | ||||||
| N0 | 0.112 | 0.056–0.222 | <0.001 | 0.073 | 0.015–0.363 | 0.001 |
| N1 | 0.308 | 0.164–0.576 | <0.001 | 0.331 | 0.168–0.650 | 0.001 |
| N2 | 0.432 | 0.219–0.855 | 0.016 | 0.486 | 0.240–0.985 | 0.045 |
| N3 | 1.000 | 1.000 | ||||
| Pathological stage | ||||||
| 1a–1b | 0.194 | 0.084–0.447 | <0.001 | 2.384 | 0.184–30.799 | 0.506 |
| 2a–2b | 0.395 | 0.251–0.623 | <0.001 | 1.556 | 0.386–6.283 | 0.534 |
| 3a–3c | 1.000 | 1.000 | ||||
| Vessel invasive (absence vs. presence) | 1.793 | 1.197–2.686 | 0.005 | 1.098 | 0.704–1.713 | 0.681 |
| Nerve infiltration (absence vs. presence) | 1.990 | 1.343–2.948 | 0.001 | 1.855 | 1.214–2.836 | 0.004 |
| Treatment regimen | ||||||
| S | 1.425 | 0.656–3.099 | 0.371 | |||
| S plus postoperative C | 1.430 | 0.611–3.348 | 0.410 | |||
| S plus postoperative CRT | 1.000 | |||||
| Hospital time (days) (>14 vs. ≤14) | 1.811 | 1.169–2.803 | 0.008 | 1.828 | 1.159–2.881 | 0.009 |
| Platelet | 0.996 | 0.992–0.999 | 0.018 | 1.000 | 0.996–1.004 | 0.904 |
| Albumin | 0.931 | 0.884–0.981 | 0.007 | 0.947 | 0.892–1.006 | 0.076 |
| RDW | 1.258 | 1.016–1.557 | 0.035 | 1.072 | 0.838–1.370 | 0.579 |
| Aspartate transaminase | 0.995 | 0.972–1.019 | 0.709 | |||
| Fibrinogen | 1.137 | 0.909–1.422 | 0.262 | |||
| Hemoglobin | 0.831 | 0.729–0.948 | 0.006 | 0.853 | 0.726–1.002 | 0.053 |
Abbreviations: S, surgery; C, chemotherapy; CRT, chemoradiotherapy; PDW, platelet distribution width; RDW, red cell distribution width.
By Kaplan–Meier analysis, the DFS was poor in the high PDW group (P < 0.001, Fig. 3B). Similarly, based on subgroup analysis, with lymph node metastasis (P < 0.001) and advanced stage (P < 0.001) could serve as predictors for short DFS in patients with ESCC, which was not observed in patients without lymph node metastasis (P = 0.291) and less advanced stage (P = 0.219) (Figs 4 and 5). In the univariate analysis, high PDW was a significant predictor of unfavorable DFS (HR = 2.302, P < 0.001) (Table 5). After adjustment for confounders, high PDW (HR = 2.562, P < 0.001), lymph node metastasis (P < 0.05), and surgery (P = 0.047) were correlated with decreased DFS (Table 5). In a word, PDW was an independent prognostic factor for patients with ESCC undergoing surgery.
Table 5.
Disease-free survival analyses according to preoperative PDW in 495 patients with ESCC.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| PDW (≥13.4 vs. <13.4) | 2.302 | 1.567–3.383 | <0.001 | 2.562 | 1.733–3.786 | <0.001 |
| Sex (male vs.female) | 1.545 | 0.830–2.878 | 0.170 | |||
| Age (>60 vs. ≤60) | 0.881 | 0.610–1.273 | 0.501 | |||
| Depth of tumor | ||||||
| T1a–1b | 0.838 | 0.435–1.614 | 0.597 | |||
| T2 | 0.601 | 0.357–1.011 | 0.055 | |||
| T3 | 1.000 | |||||
| Lymph node metastasis | ||||||
| N0 | 0.160 | 0.084–0.303 | <0.001 | 0.205 | 0.074–0.569 | 0.002 |
| N1 | 0.266 | 0.141–0.500 | <0.001 | 0.265 | 0.136–0.515 | <0.001 |
| N2 | 0.471 | 0.243–0.915 | 0.026 | 0.424 | 0.217–0.827 | 0.012 |
| N3 | 1.000 | 1.000 | ||||
| Pathological stage | ||||||
| 1a–1b | 0.376 | 0.203–0.694 | 0.002 | 1.039 | 0.363–2.975 | 0.943 |
| 2a–2b | 0.511 | 0.337–0.775 | 0.002 | 1.082 | 0.517–2.261 | 0.835 |
| 3a–3c | 1.000 | 1.000 | ||||
| Vessel invasive (absence vs. presence) | 1.376 | 0.927–2.043 | 0.114 | |||
| Nerve infiltration (absence vs. presence) | 1.640 | 1.131–2.380 | 0.009 | 1.424 | 0.960–2.113 | 0.079 |
| Treatment regimen | ||||||
| S | 0.496 | 0.280–0.878 | 0.016 | 0.551 | 0.306–0.993 | 0.047 |
| S plus postoperative C | 1.344 | 0.748–2.416 | 0.323 | 1.304 | 0.719–2.364 | 0.382 |
| S plus postoperative CRT | 1.000 | |||||
| Hospital time (days) (>14 vs. ≤14) | 1.214 | 0.773–1.905 | 0.399 | |||
| Platelet | 0.998 | 0.995–1.001 | 0.285 | |||
| Albumin | 0.969 | 0.922–1.019 | 0.217 | |||
| RDW | 1.149 | 0.931–1.418 | 0.195 | |||
| Aspartate transaminase | 0.997 | 0.975–1.019 | 0.791 | |||
| Fibrinogen | 0.931 | 0.748–1.159 | 0.524 | |||
| Hemoglobin | 0.962 | 0.847–1.091 | 0.545 | |||
Abbreviations: S, surgery; C, chemotherapy; CRT, chemoradiotherapy; PDW, platelet distribution width; RDW, red cell distribution width.
Discussion
Numerous researches showed that platelet activation play an important part in cancer progression. Thrombocytosis is related to worse clinical outcome in patients with various cancers, including ovarian cancer, colorectal cancer, and pancreatic cancer20–22. The PDW that is one of the platelet indices not merely check platelet volume heterogeneity, but also reactive platelet activity. Recently, several studies revealed that a high PDW is an unfavorable prognosis factor in melanoma patients, laryngeal cancer, and gastric cancer23–25. To the best of our knowledge, the prognostic value of the preoperative PDW in ESCC patients remains unknown.
This was the first retrospective research revealed that a PDW with a cut-off 13.4 fL was an independent prognostic factor for the OS and DFS in ESCC patients. Our findings reported that an elevated PDW was correlated with depth of tumor, nerve infiltration, and hospital time after operation. Moreover, high PDW was an independent predictor for ESCC patients with lymph node metastasis according to further subgroup analyses.
Nevertheless, the potential mechanism by which PDW have an effect on cancer progression is unclear. One possible cause is that platelets facilitate the hypercoagulability in tumor. Activated platelets produce a procoagulant micro-environment and aggregate with tumor cell. Platelet-derived growth factor (PDGF) family members including PDGF-A, PDGF-B, PDGF-C and PDGF-D, play a vital role in cancer cell proliferation, apoptosis, transformation, invasion, metastasis and angiogenesis26–31. In esophageal cancer, PDGF-D expression is associated with clinical-pathological features and worse survival. Moreover, platelet-derived growth factor-D contributes to proliferation and invasion of esophageal squamous cell carcinoma by up-regulating NF-κB signaling pathways32. Consistent with previous studies, our findings indirectly suggested anti-platelet could serve as one part of cancer adjuvant therapy33.
Another possible mechanism is that bone marrow cells malfunction may be associated with the lower PDW. PDW reflects platelet heterogeneity, which is caused by heterogeneous demarcation of megakaryocytes34. Cytokines, including interleukin-6 (IL-6), macrophage colony stimulating factor (M-CSF), and granulocytes colony stimulating factor (G-CSF), have an effect on megakaryocytic maturation, platelet production, and platelet size35. IL-6 facilitates cancer cell proliferation, invasion, and metastasis. IL-6 is correlated with the prognosis and depression of cancer patients and is considered to the therapy target36–38. Moreover, G-CSF stimulates megakaryopoiesis and constrains tumor to proliferation. M-CSF was an important factor in the cancer microenvironment, involving in the interactions between tumor-infiltrated macrophages and tumor cells39–41. Those reports are in accord with the point that activated platelets participate in the pathogenesis of esophageal cancer.
There were several limitations of our study: first, this was the single-center design and retrospective study, which might have selection bias. Second, the biological mechanism of PDW affecting prognosis need to explored. Third, a controversial cut-off value determined by different ways, such as mean, ROC curve, and C index, could be the optimal predictor of clinical outcome in ESCC patients. In this study, we chose ROC curve to determine the cut-off value. Future studies with multi-center design and prospective trials are necessary to validate the prognostic value of PDW in ESCC patients.
An elevated preoperative PDW indicates a worse OS and DFS of patients with newly diagnosed ESCC undergoing surgery. Our finding may contribute to assess the prognosis of ESCC.
Methods
Patient recruitment and data collection
This retrospective study was approved by the Ethics Committee of Zhejiang Cancer Hospital, and included 590 ESCC patients who were newly diagnosed between 2008 and 2013. 95 patients who met the following standard were excluded from the study: neoadjuvant chemotherapy or radiotherapy before surgery; loss to follow-up; data missing; concomitant disease that could interfere with platelet, including autoimmune disease, splenic disease, severe hypertension, and a history of blood transfusion; other factors that could affect the PDW, including megaloblastic anemia, acute myeloid leukemia, splenectomy, giant platelet syndrome, and thrombotic disease. The enrolled 495 patients completed written informed consent.
The pretreatment peripheral blood cell count was checked via a SYSMEX XE-2100 (Sysmex, Kobe, Japan) Automatic Blood Cell Analyzer. The PDW measurement is the first time of admission.
Follow-up strategy
After surgery, patients were followed up every three months for the first year, six months during the second year and 12 months thereafter. Physical examination, blood routine examination, and medical history were achieved conventionally. Bone scans, chest/abdominal CT/MRI, and chest radiography were acquired when in cases of suspicious metastasis or recurrence.
Statistical analysis
The PDW was analyzed as continuous variables and the clinical-pathological features were counted as categorical variables. The optimal cut-off value of PDW for predicting survival was determined by the ROC curve analysis. The relationship between PDW and clinical-pathological features in ESCC was analyzed by chi-square tests. The Kaplan-Meier method and the log-rank test were used for the overall survival (OS) and disease-free survival (DFS) analyses. The association between PDW and clinical-pathological features were investigated by logistic regression analysis. Clinical-pathological features with P < 0.01 were selected to be the subgroup factor. Subgroup analysis was based on lymph node metastasis and pathological stage. Whether the OS and DFS was an independent prognosis factor was determined by Cox proportional hazards regression models. Risk factors with P < 0.01 in univariate analysis were chosen to multivariate analyses. The SPSS software version 19.0 (IBM SPSS, Chicago, IL, USA) was utilized for statistical analysis.
Ethics approval and consent to participate
All procedures in the present study were performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. The study approval was obtained from ethics committee at Zhejiang Cancer Hospital and informed consents were informed from all participants.
Acknowledgements
We thank the included patients and all the investigators, including the clinicians and laboratory technicians in our study. This study was funded by National Natural Science Foundation of China (contract/grant number: 81602615) and General research program of Health Department of Zhejiang Province (contract/grant number: 2016KYB048) and Zhejiang Youth Talents Project (contract/grant number: 2019RC026).
Author contributions
Q.S. and S.W. contributed to conception and analysis of data; J.W. contributed to data acquisition; Q.S. and S.W. contributed to study design, manuscript preparation;W.C. contributed to conception and follow-up. All authors read and approved the final manuscript.
Data availability
The data and materials can be found from the first author and corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Advances in nutrition. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Tran GD, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. International journal of cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 4.Gertler R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Annals of surgery. 2011;253:689–698. doi: 10.1097/SLA.0b013e31821111b5. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, et al. Cancer statistics, 2007. CA: a cancer journal for clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 7.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Annals of surgical oncology. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 8.Mezouar S, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thrombosis research. 2016;139:65–76. doi: 10.1016/j.thromres.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. International journal of cancer. 2012;130:2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 10.Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discovery medicine. 2009;8:237–241. [PubMed] [Google Scholar]
- 11.Simanek R, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Journal of thrombosis and haemostasis: JTH. 2010;8:114–120. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 12.Mege D, et al. Involvement of Platelets in Cancers. Seminars in thrombosis and hemostasis. 2019;45:569–575. doi: 10.1055/s-0039-1693475. [DOI] [PubMed] [Google Scholar]
- 13.Cui MM, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Scientific reports. 2017;7:3456. doi: 10.1038/s41598-017-03772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Sheng L, Du X, Qiu G, Su D. Elevated platelet count is a strong predictor of poor prognosis in stage I non-small cell lung cancer patients. Platelets. 2015;26:138–142. doi: 10.3109/09537104.2014.888547. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Zhu Y, Liu L. Elevated pretreatment plasma D-dimer levels and platelet counts predict poor prognosis in pancreatic adenocarcinoma. OncoTargets and therapy. 2015;8:1335–1340. doi: 10.2147/OTT.S82329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu M, et al. Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer. Clinica chimica acta; international journal of clinical chemistry. 2018;483:296–302. doi: 10.1016/j.cca.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Eyuboglu M. Predictive Value of Combination of Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio for Prognosis. Angiology. 2016;67:195. doi: 10.1177/0003319715593224. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, et al. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:9323–9331. doi: 10.1007/s13277-015-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X, Luo KJ, Hu Y, Wang JY, Chen J. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2016;29:79–85. doi: 10.1111/dote.12296. [DOI] [PubMed] [Google Scholar]
- 20.Ye Q, Cheng J, Ye M, Liu D, Zhang Y. Association of pretreatment thrombocytosis with prognosis in ovarian cancer: a systematic review and meta-analysis. Journal of gynecologic oncology. 2019;30:e5. doi: 10.3802/jgo.2019.30.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YH, et al. The pretreatment thrombocytosis may predict prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Biomarkers in medicine. 2017;11:195–210. doi: 10.2217/bmm-2016-0214. [DOI] [PubMed] [Google Scholar]
- 22.Chadha AS, et al. Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta oncologica. 2015;54:971–978. doi: 10.3109/0284186X.2014.1000466. [DOI] [PubMed] [Google Scholar]
- 23.Li N, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Scientific reports. 2017;7:2970. doi: 10.1038/s41598-017-03212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget. 2017;8:48138–48144. doi: 10.18632/oncotarget.18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng S, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC gastroenterology. 2017;17:163. doi: 10.1186/s12876-017-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiological reviews. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 27.Heldin CH, Hammacher A, Nister M, Westermark B. Structural and functional aspects of platelet-derived growth factor. British journal of cancer. 1988;57:591–593. doi: 10.1038/bjc.1988.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. Journal of biochemistry and molecular biology. 2003;36:49–59. doi: 10.5483/bmbrep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Kong D, Li Y, Sarkar FH. PDGF-D signaling: a novel target in cancer therapy. Current drug targets. 2009;10:38–41. doi: 10.2174/138945009787122914. [DOI] [PubMed] [Google Scholar]
- 30.Ustach CV, et al. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer research. 2004;64:1722–1729. doi: 10.1158/0008-5472.CAN-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Tong R, Cochran DM, Jain RK. Blocking platelet-derived growth factor-D/platelet-derived growth factor receptor beta signaling inhibits human renal cell carcinoma progression in an orthotopic mouse model. Cancer research. 2005;65:5711–5719. doi: 10.1158/0008-5472.CAN-04-4313. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, et al. Down-regulation of platelet-derived growth factor-D expression blockades NF-kappaB pathway to inhibit cell proliferation and invasion as well as induce apoptosis in esophageal squamous cell carcinoma. Molecular biology reports. 2013;40:2473–2483. doi: 10.1007/s11033-012-2328-y. [DOI] [PubMed] [Google Scholar]
- 33.Mitrugno A, et al. The role of coagulation and platelets in colon cancer-associated thrombosis. American journal of physiology. Cell physiology. 2019;316:C264–C273. doi: 10.1152/ajpcell.00367.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulus JM. Recent advances in the story of megakaryocyte physiology. Pathologie-biologie. 1981;29:133–135. [PubMed] [Google Scholar]
- 35.Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood. 1998;92:345–344. doi: 10.1182/blood.V92.2.345. [DOI] [PubMed] [Google Scholar]
- 36.Lippitz BE, Harris RA. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology. 2016;5:e1093722. doi: 10.1080/2162402X.2015.1093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacina L, Brabek J, Kral V, Kodet O, Smetana K., Jr. Interleukin-6: a molecule with complex biological impact in cancer. Histology and histopathology. 2019;34:125–136. doi: 10.14670/HH-18-033. [DOI] [PubMed] [Google Scholar]
- 38.Kampan NC, et al. Immunotherapeutic Interleukin-6 or Interleukin-6 Receptor Blockade in Cancer: Challenges and Opportunities. Current medicinal chemistry. 2018;25:4785–4806. doi: 10.2174/0929867324666170712160621. [DOI] [PubMed] [Google Scholar]
- 39.Dobrenis K, Gauthier LR, Barroca V, Magnon C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. International journal of cancer. 2015;136:982–988. doi: 10.1002/ijc.29046. [DOI] [PubMed] [Google Scholar]
- 40.Ao JY, et al. Colony-Stimulating Factor 1 Receptor Blockade Inhibits Tumor Growth by Altering the Polarization of Tumor-Associated Macrophages in Hepatocellular Carcinoma. Molecular cancer therapeutics. 2017;16:1544–1554. doi: 10.1158/1535-7163.MCT-16-0866. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, et al. Interactions between colon cancer cells and tumor-infiltrated macrophages depending on cancer cell-derived colony stimulating factor 1. Oncoimmunology. 2016;5:e1122157. doi: 10.1080/2162402X.2015.1122157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials can be found from the first author and corresponding author.