Abstract
Early diagnosis and proper treatment of pyogenic vertebral osteomyelitis (PVO) in patients with cirrhosis is challenging to clinicians, and the mortality rate is expected to be high. A retrospective study was conducted to investigate the treatment outcome in PVO patients with cirrhosis and to identify the predictors of their mortality. Mortality was divided into two categories, 30-day and 90-day mortality. A stepwise multivariate logistic regression model was used to identify predictors of mortality. Eighty-five patients were identified after initial exclusion. The patients’ mean age was 60.5 years, and 50 patients were male. The early mortality rates within 30 and 90 days were 17.6% and 36.5%, respectively. Multivariate analysis revealed that increased age, CTP class C, and bacteremia at the time of PVO diagnosis were predictors of 30-day mortality, while higher MELD score, presence of combined infection, and multiple spinal lesions were predictors of 90-day mortality. Attention should be paid to the high mortality between 30 and 90 days after PVO diagnosis (18.8%), which was higher than the 30-day mortality. Liver function was consistently a strong predictor of mortality in PVO patients with cirrhosis. The high-risk patients should be targeted for an aggressive diagnostic approach, using spinal MRI and intensive monitoring and treatment strategies.
Subject terms: Bacterial infection, Risk factors
Introduction
The mortality in liver cirrhosis was reported to be greater than that in the five major cancers1. Infection further increases the mortality of patients with cirrhosis by fourfold2, and infection is directly responsible for 30–50% of deaths in patients with cirrhosis3,4. Considering the greatly increased mortality from infection in patients with cirrhosis2, early diagnosis and prompt treatments should be compulsory to save patients’ lives. However, adherence to such a basic principle for patients with cirrhosis is not easy for clinicians engaged in the treatment of pyogenic vertebral osteomyelitis (PVO).
A retrospective study reported that 77% of patients with end-stage liver disease had bodily pain, in the abdomen, back, head/neck, and upper and lower extremities, within 24 hours of the evaluation, and most patients (90%) received various analgesics5. Such a high prevalence of bodily pain in patients with cirrhosis prevents the use of the clinical symptoms of PVO as an indicator for early diagnosis. In addition, bacterial infections are common in immunocompromised patients with cirrhosis6, and patients with cirrhosis have a fivefold greater risk of developing infection than the general population7. Such high prevalence of bacterial infection in patients with cirrhosis also prevents the use of infection markers as indicators for early diagnosis of PVO. According to the guideline of the Infectious Diseases Society of America, spine magnetic resonance imaging is recommended for patients with suspected PVO who have new or worsening back pain and elevated erythrocyte sedimentation rate or C-reactive protein level8. However, such an approach is considered to have limitations in PVO patients with cirrhosis.
Treatment of PVO in patients with cirrhosis is challenging for clinicians. Attenuated liver function by bacterial infection threatens the life of patients with cirrhosis through variceal rupture9 and multiorgan failure10. Treatment failure or recurrence is expected to be high, owing to cirrhosis-associated immune dysfunction11 and the high prevalence of multidrug resistant organisms12. The strictly required long-term intravenous antibiotics8 to reduce the recurrence of PVO potentially can paradoxically cause Clostridium difficile infection, which is associated with higher mortality13. Decreased bone mineral density with deteriorated bony microarchitecture in patients with cirrhosis14, disuse osteoporosis caused by immobilization, and long-term hospitalization aggravates skeletal destruction by the pyogenic organism, and can easily cause neurological and structural instabilities that require surgical treatment. However, the basic principles in the surgical treatment of PVO15, including sufficient removal of paraspinal abscesses and firm spinal instrumentation, are technically challenging in patients with cirrhosis who have poor bone quality and bleeding tendency with coagulopathy.
As a result, difficulty in early diagnosis and prompt treatments in PVO patients with cirrhosis is expected to be related to poorer clinical outcomes, including higher mortality. However, to our knowledge, no reports have described the treatment outcome in this patient group. In addition, under the expected higher mortality, prognostic studies to identify high-risk patients, on whom intensive monitoring and treatment strategies should be concentrated, are essential for the improvement of clinical outcome. We performed a retrospective study to investigate the treatment outcome in PVO patients with cirrhosis, and to identify the predictors of their mortality.
Methods
Study design and ethics
A retrospective case review was performed in patients with cirrhosis who received treatment for PVO in our institution between January 2000 and March 2018. This study was designed and conducted using the format recommended by STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines16. This study was approved by the institutional review board of Hallym University Sacred Heart Hospital. The institutional review board waived the informed consent for this study. All methods were carried out in accordance with the relevant guidelines and regulations.
Study patients
Our university medical center is one of the largest medical institutions in our country, consisting of six general hospitals. This study was performed in the main institute among the six general hospitals. As the main institute of our medical center, our hospital serves as a tertiary referral center for not only the other five general hospitals but also numerous local hospitals.
Patients with cirrhosis who received treatment for PVO were eligible for inclusion. Cirrhosis was diagnosed by liver pathological examination or a combination of laboratory biochemical, radiological, and endoscopic findings, if a liver biopsy result was not available14. PVO was defined using the following criteria: suggestive clinical symptoms, accompanying typical radiological features on MRI, and microbiological identification15. Microbiological confirmation included isolation from blood culture, CT-guided needle biopsy, or surgical biopsy. Patients were excluded if their medical records indicated that they had received a previous liver transplantation before the diagnosis of PVO. Patients were also excluded if they had received a previous spine surgery using instrumentation at the same site of the PVO. Other reasons for exclusion were incomplete medical records or imaging data.
Data collection
Data were retrieved from electronic medical records using a standardized collection form. Demographic, laboratory, and other clinical data at the time of PVO diagnosis were ascertained. Medical history was retrieved from the records, and the Charlson comorbidity index was calculated to assess comorbid medical conditions17. The presence of ascites, encephalopathy, and gastrointestinal (GI) bleeding at the time of PVO diagnosis were retrieved from the records, and laboratory data at the time of PVO diagnosis were retrieved. Then, liver function was determined using the Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP) class, and CTP scores. The severity of infection at the time of PVO diagnosis was retrieved using a validated classification system by Pola et al.18, which divided pyogenic spondylodiscitis into three types as follows: 1) type A, cases without biomechanical instability, neither acute neurological impairment nor epidural abscesses; 2) type B, cases with radiological evidence of significant bone destruction or biomechanical instability without acute neurological impairment or epidural abscesses; and 3) type C, cases with epidural abscesses or acute neurological impairment.
Definitions
The presence of combined infection was retrospectively retrieved from the medical records and classified as follows:
Intra-abdominal infection: Spontaneous bacterial peritonitis was diagnosed on the basis of an ascitic fluid neutrophil count of >250/mm3 or a positive bacteriological culture of the ascitic fluid19. Infectious enterocolitis was diagnosed in patients with diarrhea and leukocytes in stool or positive stool culture for pathogens, including Salmonella, Shigella, Yersinia, Campylobacter, and pathogenic Escherichia coli, or a positive Clostridium difficile stool assay7.
Urinary tract infection: Urinary tract infection included both laboratory-confirmed UTI defined by the presence of pyuria (>10 white blood cells/mm3 per high-power field) and bacteria (urinary pathogen of ≥105 colony-forming units per mL)20,21, and asymptomatic bacteriuria defined by the presence of 1 or more species of bacteria growing in the urine at specified quantitative counts (≥105 colony-forming units [CFU]/mL or ≥108 CFU/L) irrespective of the presence of pyuria22.
Cardiac infection: Infective endocarditis was diagnosed using the modified Duke criteria23.
Pneumonia: At least one of the respiratory symptoms with one of the following: rales and/or crepitation on auscultation; at least one sign of infection in the absence of antibiotics; presence of pulmonary infiltrate on radiological imaging; or positive sputum culture7.
Other musculoskeletal infections: Septic arthritis was diagnosed on the basis of a synovial fluid leukocyte count of >50,000 cells/μL or positive synovial fluid culture21. Osteomyelitis was diagnosed on the basis of typical radiological findings on MRI or positive culture results24–26.
Multiple spinal lesions were defined when the spinal involvement presented beyond two vertebral bodies on MRI, with at least one completely uninvolved vertebral body between the involved vertebral bodies. Early surgery was defined as a surgical treatment performed under general anesthesia within 30 days after PVO diagnosis.
Outcomes
The mortality of the patients was divided into two categories, early and late mortality. Early mortality within 30 or 90 days after PVO diagnosis were investigated. The clinical outcomes were investigated in patients with at least a 90-day survival. Recurrence was defined as having recurrent symptoms and signs after the completion of antibiotics and receiving a second course of intravenous antibiotics15.
Statistical analyses
Continuous variables were presented as the mean ± standard deviation and compared using an independent t test. Categorical variables were presented by frequency (%) and compared using the Pearson chi-square test, Fisher exact test, or linear-by-linear association.
Predictors of 30- and 90-day mortality were analyzed using the logistic regression model, and all variables identified as significant in the univariate analysis (p < 0.05) were included in the stepwise multivariate logistic regression model. The Kaplan-Meier survival curve was used to display the cumulative probability of late survival in patients, and the log-rank test was used to compare survival curves between the two groups.
The statistical tests were two-tailed, and a p value of <0.05 was considered to indicate statistical significance. All the analyses were performed using SPSS 24 (SPSS Inc., Chicago, Illinois, USA).
Results
Baseline Patient Characteristics
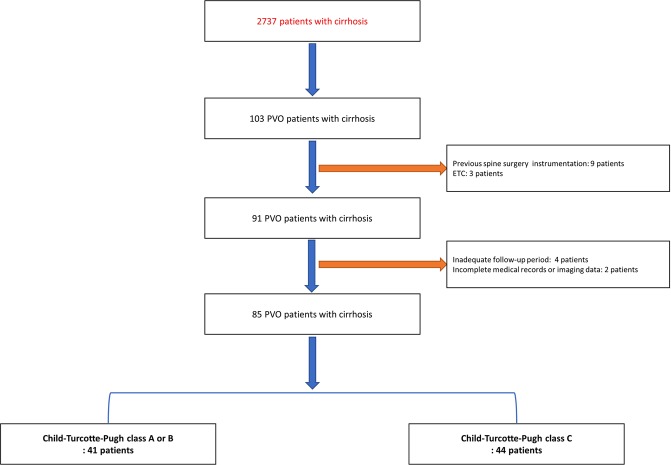
Eighty-five patients were identified after the initial exclusion (Fig. 1). The patients’ mean age was 60.5 years, and 50 patients (58.9%) were male (Table 1). The most common etiology of cirrhosis was viral hepatitis (55.3%). Ascites, encephalopathy, and GI bleeding were present at the time of PVO diagnosis in 28 (32.9%), 20 (23.5%), and 16 patients (18.8%), respectively. Seven, 34, and 44 patients had CTP class A, B, and C cirrhosis, respectively. Hepatocellular carcinoma was identified in 16 patients (18.8%).
Figure 1.
Flowchart of patients included in the study. Abbreviations: PVO, pyogenic vertebral osteomyelitis.
Table 1.
Baseline patient characteristics.
| Variables | Categories of variables | All patients | Child-Turcotte-Pugh class A or B | Child-Turcotte-Pugh class C | p-value |
|---|---|---|---|---|---|
| Number of patients | 85 | 41 | 44 | ||
| Age | 60.5 ± 8.7 | 58.7 ± 8.6 | 62.1 ± 8.6 | 0.068 | |
| Sex ratio (F: M) | 35: 50 | 18: 23 | 17: 27 | 0.622 | |
| BMI (kg/m2) | 24.4 ± 2.9 | 24.2 ± 2.9 | 24.5 ± 3.0 | 0.574 | |
| Etiology of cirrhosis | Viral | 47 (55.3) | 19 | 28 | 0.289 |
| Alcoholic | 29 (34.1) | 18 | 11 | ||
| others | 9 (10.6) | 4 | 5 | ||
| Charlson comorbidity index score | 5.8 ± 3.2 | 5.1 ± 2.8 | 6.4 ± 3.4 | 0.066 | |
| Medical history | Coronary artery disease | 10 (11.8) | 4 | 6 | 0.740 |
| End stage renal disease | 12 (14.1) | 3 | 9 | 0.120 | |
| Diabetes Mellitus | 41 (48.2) | 12 | 29 | 0.002 | |
| Overall malignancy | 23 (27.1) | 11 | 12 | 0.963 | |
| Hepatocellular carcinoma | 16 (18.8) | 4 | 12 | 0.053 | |
| Child-Turcotte-Pugh class (A/B/C) | 7/34/44 | 7/34/0 | 0/0/44 | ||
| Child-Turcotte-Pugh score | 9.6 ± 2.5 | 7.3 ± 1.0 | 11.8 ± 1.3 | <0.001 | |
| MELD score | 23.0 ± 12.1 | 12.9 ± 5.4 | 32.3 ± 8.4 | <0.001 | |
| Morbidity related with cirrhosis | Ascites | 28 (32.9) | 0 | 28 | <0.001 |
| Encephalopathy | 20 (23.5) | 0 | 20 | <0.001 | |
| GI bleeding | 16 (18.8) | 0 | 16 | <0.001 | |
| Laboratory data | WBC (×103/μL) | 9.8 ± 3.9 | 9.4 ± 3.7 | 10.1 ± 4.1 | 0.424 |
| Platelet (×103/μL) | 10.4 ± 7.1 | 138 ± 82 | 72 ± 37 | <0.001 | |
| Serum albumin (g/dl) | 2.4 ± 0.6 | 2.5 ± 0.6 | 2.4 ± 0.6 | 0.400 | |
| Total bilirubin (mg/dl) | 6.3 ± 5.3 | 2.5 ± 2.0 | 9.9 ± 5.0 | <0.001 | |
| Prothrombin time (INR) | 2.1 ± 1.1 | 1.2 ± 0.3 | 2.9 ± 0.9 | <0.001 | |
| Serum creatinine (mg/dl) | 2.0 ± 1.7 | 1.4 ± 1.2 | 2.5 ± 2.0 | 0.003 | |
| C-reactive protein (CRP, mg/L) | 75 ± 35 | 79 ± 38 | 70 ± 32 | 0.214 | |
| Erythrocyte sedimentation rate (ESR, mm/h) | 60 ± 27 | 62 ± 30 | 59 ± 26 | 0.627 | |
| Neurologic deficit by ASIA grade | A | 0 | 0 | 0 | 0.906 |
| B | 5 (5.9) | 3 | 2 | ||
| C | 12 (14.1) | 7 | 5 | ||
| D | 43 (50.6) | 17 | 26 | ||
| E | 25 (29.4) | 14 | 11 | ||
| Bacteremia | 55 (64.7) | 23 | 32 | 0.109 | |
| Combined infection | Presence of combined infection | 48 (56.5) | 18 | 30 | 0.024 |
| Intraabdominal | 7 (8.2) | 1 | 6 | 0.110 | |
| Urinary tract | 26 (30.6) | 10 | 16 | 0.231 | |
| Cardiac | 4 (4.7) | 3 | 1 | 0.349 | |
| Pneumonia | 19 (22.4) | 5 | 14 | 0.038 | |
| Other musculoskeletal | 12 (14.1) | 3 | 9 | 0.120 | |
| Others | 8 (9.4) | 4 | 4 | 1.000 | |
| Spinal anatomical involvement | Single | 56 (65.9) | 30 | 26 | 0.171 |
| mainly cervical | 4 (4.7) | 3 | 1 | ||
| mainly thoracic | 13 (15.3) | 10 | 3 | ||
| mainly lumbosacrum | 39 (45.9) | 17 | 22 | ||
| Multiple | 29 (34.1) | 11 | 18 | ||
| Number of infected vertebral bodies | within 3 levels | 33 (38.3) | 23 | 10 | 0.002 |
| over 3 levels | 52 (61.2) | 18 | 34 | ||
| Severity of infection by Pola et al. | Type A | 5 (5.9) | 5 | 0 | 0.031 |
| Type B | 8 (9.4) | 4 | 4 | ||
| Type C | 72 (84.7) | 32 | 40 | ||
| Causative organism of PVO | Staphylococcus aureus | 42 (49.4) | 19 | 23 | 0.560 |
| Methicillin resistant | 21 (24.7) | 9 | 12 | ||
| Methicillin sensitive | 21 (24.7) | 10 | 11 | ||
| Other gram positive bacteria | 15 (17.6) | 7 | 8 | ||
| Enterobacteriaceae | 18 (21.2) | 10 | 8 | ||
| Others | 10 (11.8) | 5 | 5 |
Data were presented by number (%) of patients or mean ± standard deviation.
Bacteremia was present in 55 patients (64.7%; Table 1). Combined infection was present in 48 patients (56.5%), and urinary tract infection was the most common (26 patients, 30.6%). Most of the patients (56 patients, 65.9%) had a single spinal lesion, but multiple spinal lesions were observed in 29 patients (34.1%). The number of infected vertebral bodies was >3 levels in 52 patients (61.2%), and most patients had type C infection according to the classification of Pola et al. (72 patients, 84.7%). The most common causative organism was Staphylococcus aureus (42 patients, 49.4%), and it was methicillin resistant in half of the patients (21/42 patients).
CTP class C patients had a significantly increased number of infected vertebral bodies (p = 0.002) and severe types of infection according to the classification of Pola et al. (p = 0.031) when compared to the CTP class A or B patients (Table 1). Combined infection was more frequent in the CTP class C patients (p = 0.024).
Surgical treatment was performed in 10.6% (9 of 85 patients) within one week of PVO diagnosis, and 29.4% between one and four weeks after PVO diagnosis (25 of 85 patients).
Mortality of PVO patients with cirrhosis
The early mortality rates within 30 and 90 days were 17.6% (15/85 patients) and 36.5% (31/85 patients), respectively (Table 2). The CTP class C patients had greatly increased 30-day (31.8% vs 2.4%, p < 0.001) and 90-day mortality (54.5% vs 17.1%, p < 0.001) when compared to the CTP class A or B patients (Fig. 2).
Table 2.
Mortality of PVO patients with cirrhosis.
| Categories of mortality | All patients | Child-Turcotte-Pugh class A or B | Child-Turcotte-Pugh class C | p-value | |
|---|---|---|---|---|---|
| Early mortality | 30-day mortality | 15 (17.6%) | 1 (2.4%) | 14 (31.8%) | <0.001 |
| 90-day mortality | 31 (36.5%) | 7 (17.1%) | 24 (54.5%) | <0.001 | |
| Late survival | Mean survival (days) | 1474 ± 1743 | 1514 ± 1431 | 1422 ± 2114 | 0.315 |
| Interquartile range (days) | (137, 2215) | (459, 2035) | (67, 2459) | 0.990 | |
Data were presented by number (%) of patients or mean ± standard deviation.
Figure 2.
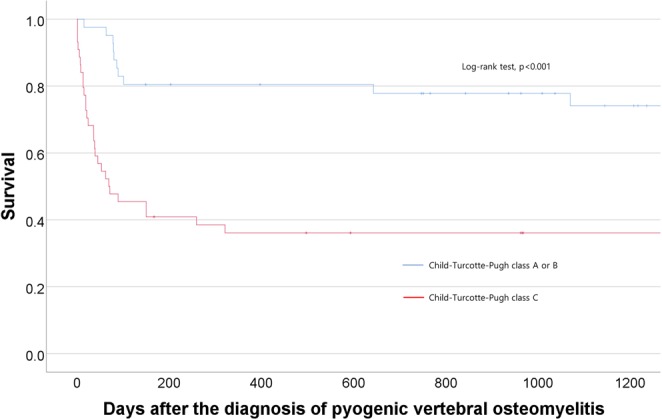
Cumulative probability of survival for PVO patients with cirrhosis according to Child-Turcotte-Pugh class.
Predictors related with 30- or 90-day mortality: logistic regression analysis
In the stepwise multivariate analysis (Table 3), increased age (odds ratio, 1.102; p = 0.019), CTP class C (odds ratio, 18.707; p = 0.009), and bacteremia (odds ratio, 12.956; p = 0.025) at the time of PVO diagnosis were identified as predictors of 30-day mortality, and higher MELD score (odds ratio, 1.079; p = 0.003), presence of combined infection (odds ratio, 6.264; p = 0.003), and multiple spinal lesions (odds ratio, 3.838; p = 0.023) were identified as predictors of 90-day mortality.
Table 3.
Predictors related with 30- or 90-day mortality: logistic regression analysis.
| Variables | Category | 30-day mortality | 90-day mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable (Stepwise) | Univariate | Multivariate (Stepwise) | ||||||||||
| odds ratio | 95% confidence interval | p-value | odds ratio | 95% confidence interval | p-value | odds ratio | 95% confidence interval | p-value | odds ratio | 95% confidence interval | p-value | ||
| Age | 1.087 | (1.016, 1.162) | 0.015 | 1.102 | (1.016, 1.196) | 0.019 | |||||||
| Charlson comorbidity index | |||||||||||||
| Child-Turcotte-Pugh class | A and B | — | — | — | — | — | — | — | — | — | — | — | — |
| C | 18.667 | (2.324, 149.903) | 0.006 | 18.707 | (2.053, 170.421) | 0.009 | 5.829 | (2.129, 15.954) | 0.001 | ||||
| Child-Turcotte-Pugh score | 1.496 | (1.140, 1.962) | 0.004 | 1.380 | (1.131, 1.685) | 0.002 | |||||||
| MELD score | 1.069 | (1.017, 1.123) | 0.009 | 1.084 | (1.036, 1.135) | <0.001 | 1.079 | (1.026, 1.135) | 0.003 | ||||
| Hepatocellular carcinoma | 4.000 | (1.168, 13.698) | 0.027 | ||||||||||
| Ascites | |||||||||||||
| Encephalopathy | 3.632 | (1.281, 10.296) | 0.015 | ||||||||||
| GI bleeding | 5.931 | (1.729, 20.338) | 0.005 | 5.390 | (1.659, 17.508) | 0.005 | |||||||
| Platelet (×103/μL) | 0.989 | (0.980, 0.998) | 0.016 | ||||||||||
| Total bilirubin (mg/dl) | 1.140 | (1.041, 1.248) | 0.005 | ||||||||||
| Prothrombin time (INR) | 1.903 | (1.138, 3.182) | 0.014 | 2.342 | (1.437, 3.817) | 0.001 | |||||||
| Serum creatinine (mg/dl) | 1.402 | (1.043, 1.885) | 0.025 | ||||||||||
| Bacteremia | 9.902 | (1.232, 79.561) | 0.031 | 12.956 | (1.383, 121.346) | 0.025 | 4.483 | (1.498, 13.419) | 0.007 | ||||
| Presence of combined infection | 6.500 | (1.365, 30.954) | 0.019 | 5.616 | (1.982, 15.913) | 0.001 | 6.264 | (1.883, 20.842) | 0.003 | ||||
| Other musculoskeletal infection | 4.500 | (1.193, 16.972) | 0.026 | 6.955 | (1.717, 28.174) | 0.007 | |||||||
| Urinary tract infection | 3.665 | (1.394, 9.637) | 0.009 | ||||||||||
| Multiple spinal lesion | 3.750 | (1.180, 11.913) | 0.025 | 2.679 | (1.056, 6.795) | 0.038 | 3.838 | (1.201, 12.263) | 0.023 | ||||
Effect of early surgery on 30- or 90-day mortality: logistic regression analysis
Early surgery was performed in 34 patients (40.0%), of whom 9 (26.5%) underwent spinal instrumentation. The multivariate logistic regression analysis revealed that early surgical treatment was not associated with a statistically significant improvement in 30- or 90-day mortality (Table 4). A model (model 2 in Table 4) adjusted for all significant variables in the univariate analysis (Table 3) only showed a significantly lower odds ratio (0.005) for 30-day mortality in the patients who had an early surgery (p = 0.012).
Table 4.
Effect of early surgery on 30-day or 90-day mortality: logistic regression analysis.
| Categories of mortality | Method of adjustment | odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|---|---|
| 30-day mortality | Non-adjusted | None | 0.707 | (0.218, 2.287) | 0.563 |
| Adjusted | Model 1 | 0.394 | (0.083, 1.867) | 0.241 | |
| Model 2 | 0.002 | (<0.001, 0.347) | 0.018 | ||
| 90-day mortality | Non-adjusted | None | 0.741 | (0.298, 1.846) | 0.520 |
| Adjusted | Model 3 | 0.588 | (0.180, 1.922) | 0.379 | |
| Model 4 | 0.194 | (0.026, 1.436) | 0.108 | ||
Model 1: adjusted for age, Child-Turcotte-Pugh, and bacteremia.
Model 2: adjusted for age, Child-Turcotte-Pugh class, Child-Turcotte-Pugh score, MELD score, hepatocellular carcinoma, GI bleeding, prothrombin time (INR), sepsis, urinary tract infection, other musculoskeletal infection, and multiple spinal lesion.
Model 3: adjusted for MELD score, presence of combined infection, and multiple spinal lesion.
Model 4: adjusted for age, Child-Turcotte-Pugh class, Child-Turcotte-Pugh score, MELD score, hepatocellular carcinoma, GI bleeding, platelet, bilirubin, prothrombin time (INR), creatinine, sepsis, presence of combined infection, urinary tract infection, other musculoskeletal infection, and multiple spinal lesion.
Treatment outcomes in patients with at least 90-day survival
Surgical treatment was performed in 51.9% (28 of 54 patients) of the survivors, and instrumentation was performed in 37% (20 of 54 patients) of the survivors (Table 5). The duration of antibiotic treatment and the length of hospital stay (from the PVO diagnosis) was longer in CTP C patients, however they were statistically insignificant (Table 5). Recurrence of PVO was identified in 11 patients (20.4%) and was more common in CTP C patients (p = 0.028) (Table 5).
Table 5.
Treatment outcomes in patients with at least 90-day survival.
| All patients | Child-Turcotte-Pugh class A or B | Child-Turcotte-Pugh class C | p-value | ||
|---|---|---|---|---|---|
| Number of patients | 54 | 34 | 20 | ||
| Presence of surgical treatment | 28 (51.9) | 19 (55.9) | 9 (45.0) | 0.440 | |
| Timing of initial surgery | Within 1 week | 3 (5.6) | 1 (2.9) | 2 (10.0) | |
| Between 1 and 4 weeks | 20 (37.0) | 14 (41.2) | 6 (30.0) | ||
| After 4 weeks | 5 (9.3) | 4 (11.8) | 1 (5.0) | ||
| None | 24 (44.4) | 15 (44.1) | 11 (55.0) | ||
| Presence of spinal instrumentation | 20 (37.0) | 14 (41.2) | 6 (30.0) | 0.411 | |
| Surgery related complication | Instrument failure | 7 (13.0) | 2 (5.9) | 5 (25.0) | 0.087 |
| Wound problem | 5 (9.3) | 4 (11.8) | 3 (15.0) | 0.347 | |
| Duration of antibiotics (days) | 77.1 ± 28.3 | 71.2 ± 24.6 | 87.1 ± 31.9 | 0.065 | |
| Hospital stay (days) | 80.4 ± 28.0 | 74.3 ± 24.3 | 90.6 ± 31.4 | 0.055 | |
| Recurrence | 11 (20.4) | 4 (11.8) | 7 (35.0) | 0.028 |
Data were presented by number (%) of patients or mean ± standard deviation.
Discussion
As the first study to investigate the treatment outcome of PVO patients with cirrhosis, our study demonstrated that the 30- and 90-day mortality rates were 17.6% and 36.5%, respectively (Table 2). Multivariate analysis revealed increased age, CTP class C, and bacteremia at the time of PVO diagnosis as predictors of 30-day mortality, whereas higher MELD score, presence of combined infection, and multiple spinal lesions were predictors for 90-day mortality (Table 3). Early surgery did not lead to meaningful differences in the survival of the PVO patients with cirrhosis with respect to early mortality (Table 4).
Previous studies reported the early mortality of PVO patients, including in-hospital mortality or 90-day mortality ranging from 2.8% to 16.8%27–32. A recent study investigating the clinical outcome of PVO patients with hemodialysis reported an in-hospital mortality of 14.9% and 1-year mortality of 22.4%15. Compared with the results of previous studies, the mortality in our PVO patients with cirrhosis was considerably higher. The remarkable finding was the increased mortality observed between 30 and 90 days after PVO diagnosis (18.8%, 16/85 patients; Table 2), which was higher than the 30-day mortality (17.6%; Table 2). Although, inferring the cause of the higher mortality between 30 and 90 days after PVO diagnosis is beyond the scope of our study, we could explain the cause as follows: first, PVO patients generally require long-term hospitalization for the administration of intravenous antibiotics for at least 6 weeks8, which paradoxically increases the risk of hospital-acquired infections such as Clostridium difficile infection or the risk of recurrence by multidrug-resistant organisms. In addition, long-term intravenous antibiotics can attenuate liver or kidney function, which negatively influences the survival of patients. Second, pain and disability from the spinal structural instability negatively influences survival. During PVO treatment, significant bone loss occurs directly by causative organisms and indirectly by disuse-type bone loss33–35. Such bone loss can induce structural instability, which leads to neurological deficit, spinal deformity, and even death36,37. Therefore, permanent, and extensive stabilization using spinal instrumentation is often required after debridement or neural decompression. However, such long instrumentation is technically demanding in patients with cirrhosis, and it even fails in such patients with osteoporosis and progressive bone loss38.
The factors related with liver function were consistently significant predictors of PVO patients’ survival (Table 3) (Fig. 2), and these results are in line with the results of other types of infection in patients with cirrhosis39,40. The multivariate analysis identified CTP class and MELD score as significant predictors of 30-day and 90-day mortality, respectively (Table 3). Within 30 days after PVO diagnosis, only one of the patients with CTP class A or B died (2.4%, Table 2). However, one third of the patients with CTP class C died within 30 days (31.8%, Table 2). The Charlson comorbidity index score did not show a significant association with 30-day (p = 0.125; odds ratio, 1.138), 90-day mortality (p = 0.258; odds ratio, 1.083), and late mortality (p = 0.931, odds ratio, 0.994).
In addition to liver function, the significant predictor of mortality in PVO patients with cirrhosis was the gross extent of infection indicated by the presence of multiple spinal lesions and combined infection (Table 3). The diagnosis of PVO is frequently delayed in clinical practice41. Unfortunately, such delayed diagnosis of PVO is believed to cause extensive musculoskeletal involvement of the spine and neurological and structural instabilities in patients with cirrhosis. Approximately one-third of the cohort (34.1%; Table 1) had multiple spinal lesions, and two-thirds of the cohort (61.2%; Table 2) had extensive spinal involvement beyond 3 vertebral bodies. According to the classification of Pola et al., 94.1% of the cohort (80/85 patients) had structural instability (type B) or neurological compromise (type C), which theoretically requires surgical treatment18. Compared with the results of previous reports15,42, our results showed that PVO patients with cirrhosis are considered to have an even more extensive spinal involvement than other groups of PVO patients. We hypothesized that immune dysfunction11 and impaired bony microarchitecture14 contributes to aggressive infection.
Combined bacterial infection is common in patients with cirrhosis7 and reported to be closely related to high short-term mortality7,43. Therefore, combined infection should be considered in studies about infection-related treatment outcome in patients with cirrhosis. In our study, combined infection presented in more than half of the patients (56.5%; Table 1), and the most common combined infection was urinary tract infection (30.6%; Table 1). The multivariate analysis confirmed that combined infection is closely related with the mortality of PVO patients with cirrhosis (Table 3). In this respect, clinicians should pay great attention to the presence of combined infection in PVO patients with cirrhosis. If PVO patients with cirrhosis are considered to have combined infection in other organs or if patients with cirrhosis are receiving treatment for infection in other organs show symptoms or signs of PVO, clinicians should be aware that such a combined infection is closely related to patient survival.
The establishment of prognostic factors related to mortality should be connected to early treatment strategies. In our study, aged patients with advanced cirrhosis who had combined infection or multiple spinal lesions were identified to have high mortality rates. Therefore, this group of patients should be targeted for an aggressive diagnostic approach using spinal MRI and intensive monitoring and treatment strategies. If the diagnosis is established, broad-spectrum antibiotics should be administered as early as possible; this is a prerequisite to decrease the burden of infection and to prevent early mortality in PVO patients with cirrhosis. In this respect, early surgical drainage can be theoretically suggested as a possible treatment option to rapidly remove epidural and intraosseous abscesses, which occur in relatively avascular areas where antibiotics cannot easily reach and require long-term intravenous antibiotic administration8. However, early surgical treatment did not show a statistically significant outcome in our study (Table 4). The survival of the PVO patients with cirrhosis was strongly influenced by their liver function (Table 1), and early surgical treatment was believed to be insufficient for a clinically significant decrease in infection burden in these patients with such wide extent of combined or multiple spine infection (Table 1). However, a multivariate analysis revealed a significantly lower odds ratio for 30-day mortality in patients with early surgery (odds ratio, 0.002, p = 0.018, model 2 in Table 4). A large-scale multicenter study is required to confirm the effect of early surgery on the survival of PVO patients with cirrhosis.
The main limitation of our study is its retrospective design, and some unidentified confounders may have influenced the clinical outcomes of our patients. Precise clinical factors including the method of antibiotic treatment, method of surgical treatment including spinal instrumentation, surgery-related complications may have influenced the treatment outcomes, especially mortality, of our cohort. However, owing to the high early mortality in our cohort and small sample size, inclusion of such various clinical factors to estimate their association with clinical outcome was difficult. Next, due to limited population of our cohorts, we only investigated association between the presence of combined infection and the mortality of PVO patients. Further large-sized studies are required to investigate the individual impact of each type of combined infection on the mortality of PVO patients with cirrhosis.
In conclusion, the 30- and 90-day mortality rates of the PVO patients with cirrhosis were 17.6% and 36.5%, respectively. Attention should be paid to the high mortality between 30 and 90 days after PVO diagnosis (18.9%), which was higher than the 30-day mortality. Liver function was consistently a strong predictor of mortality in PVO patients with cirrhosis. We also identified increased age and bacteremia at the time of PVO diagnosis as predictors of 30-day mortality; and the presence of combined infection, and multiple spinal lesions as predictors of 90-day mortality. This group of patients should be targeted for an aggressive diagnostic approach using spinal MRI and intensive monitoring and treatment strategies.
Author contributions
Jihye Kim: study design, data analysis, data interpretation, drafting manuscript. Ho Suk Kang: study design, data analysis, data interpretation, Jeoung Woo Kim: data collection and data analysis. Seok Woo Kim, Jae-Keun Oh, Young-Woo Kim, and Moon Soo Park: revision of manuscript. Tae-Hwan Kim: study design, data analysis, data interpretation, drafting manuscript, approving final version of manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung W, Jo C, Chung WJ, Kim DJ. Liver cirrhosis and cancer: comparison of mortality. Hepatology international. 2018;12:269–276. doi: 10.1007/s12072-018-9850-5. [DOI] [PubMed] [Google Scholar]
- 2.Arvaniti, V. et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology139, 1246–1256, 1256.e1241–1245 (2010). [DOI] [PubMed]
- 3.Borzio M, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2001;33:41–48. doi: 10.1016/S1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 4.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. Journal of hepatology. 1993;18:353–358. doi: 10.1016/S0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 5.Madan A, et al. Chronic pain among liver transplant candidates. Progress in transplantation (Aliso Viejo, Calif.) 2012;22:379–384. doi: 10.7182/pit2012535. [DOI] [PubMed] [Google Scholar]
- 6.Jalan R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. Journal of hepatology. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, et al. A model predicting short-term mortality in patients with advanced liver cirrhosis and concomitant infection. Medicine. 2018;97:e12758. doi: 10.1097/MD.0000000000012758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berbari EF, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61:e26–46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 9.Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet (London, England) 1999;353:139–142. doi: 10.1016/S0140-6736(98)06020-6. [DOI] [PubMed] [Google Scholar]
- 10.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Seminars in liver disease. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 11.Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. Journal of hepatology. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Addo Smith JN, et al. Bacteremia in Patients With Liver Cirrhosis: Prevalence and Predictors of Multidrug Resistant Organisms. Journal of clinical gastroenterology. 2018;52:648–654. doi: 10.1097/MCG.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj JS, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. The American journal of gastroenterology. 2010;105:106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 14.Wakolbinger, R. et al. Bone microarchitecture and bone turnover in hepatic cirrhosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA (2019). [DOI] [PMC free article] [PubMed]
- 15.Kim J, et al. The outcome following spinal instrumentation in haemodialyzed patients with pyogenic spondylodiscitis. The bone & joint journal. 2019;101-b:75–82. doi: 10.1302/0301-620X.101B1.BJJ-2018-0869.R1. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Pola E, et al. New classification for the treatment of pyogenic spondylodiscitis: validation study on a population of 250 patients with a follow-up of 2 years. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2017;26:479–488. doi: 10.1007/s00586-017-5043-5. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of hepatology53, 397–417 (2010). [DOI] [PubMed]
- 20.Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infectious disease clinics of North America. 2014;28:75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.High KP, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:149–171. doi: 10.1086/595683. [DOI] [PubMed] [Google Scholar]
- 22.Nicolle LE, et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of Americaa. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2019;68:1611–1615. doi: 10.1093/cid/ciz021. [DOI] [PubMed] [Google Scholar]
- 23.Li JS, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 24.Simpfendorfer CS. Radiologic Approach to Musculoskeletal Infections. Infectious disease clinics of North America. 2017;31:299–324. doi: 10.1016/j.idc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt SK. Osteomyelitis. Infectious disease clinics of North America. 2017;31:325–338. doi: 10.1016/j.idc.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Miller JM, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2018;67:e1–e94. doi: 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grammatico L, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiology and infection. 2008;136:653–660. doi: 10.1017/S0950268807008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990–95. Scandinavian journal of infectious diseases. 2001;33:527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 29.Akiyama, T. et al. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ open3 (2013). [DOI] [PMC free article] [PubMed]
- 30.Aguilar Company J, et al. Native vertebral osteomyelitis in aged patients: distinctive features. An observational cohort study. Infection. 2018;46:679–686. doi: 10.1007/s15010-018-1177-6. [DOI] [PubMed] [Google Scholar]
- 31.Brummerstedt M, Bangstrup M, Barfod TS. High mortality from pyogenic vertebral osteomyelitis: a retrospective cohort study. Spinal cord series and cases. 2018;4:59. doi: 10.1038/s41394-018-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehrer M, Pedersen C, Jensen TG, Hallas J, Lassen AT. Increased short- and long-term mortality among patients with infectious spondylodiscitis compared with a reference population. The spine journal: official journal of the North American Spine Society. 2015;15:1233–1240. doi: 10.1016/j.spinee.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 33.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clinical Infectious Diseases. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 34.Nasto LA, et al. Is posterior percutaneous screw-rod instrumentation a safe and effective alternative approach to TLSO rigid bracing for single-level pyogenic spondylodiscitis? Results of a retrospective cohort analysis. The spine journal. 2014;14:1139–1146. doi: 10.1016/j.spinee.2013.07.479. [DOI] [PubMed] [Google Scholar]
- 35.Wood KB, Li W, Lebl DR, Ploumis A. Management of thoracolumbar spine fractures. The spine journal. 2014;14:145–164. doi: 10.1016/j.spinee.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 36.Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. British Medical Bulletin. 2016;117:121–138. doi: 10.1093/bmb/ldw003. [DOI] [PubMed] [Google Scholar]
- 37.Pourtaheri S, et al. Comparison of Instrumented and Noninstrumented Surgical Treatment of Severe Vertebral Osteomyelitis. Orthopedics. 2016;39:e504–e508. doi: 10.3928/01477447-20160427-07. [DOI] [PubMed] [Google Scholar]
- 38.Bydon M, et al. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World neurosurgery. 2014;82:e807–e814. doi: 10.1016/j.wneu.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Ning NZ, et al. Clinical and bacteriological features and prognosis of ascitic fluid infection in Chinese patients with cirrhosis. BMC infectious diseases. 2018;18:253. doi: 10.1186/s12879-018-3101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allaire M, et al. Infectious endocarditis in the case of cirrhosis: where do we stand? European journal of gastroenterology & hepatology. 2018;30:1406–1410. doi: 10.1097/MEG.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 41.Jean M, et al. Diagnostic delay of pyogenic vertebral osteomyelitis and its associated factors. Scandinavian journal of rheumatology. 2017;46:64–68. doi: 10.3109/03009742.2016.1158314. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Jang SB, Kim SW, Oh JK, Kim TH. Clinical effect of early bisphosphonate treatment for pyogenic vertebral osteomyelitis with osteoporosis: An analysis by the Cox proportional hazard model. The spine journal: official journal of the North American Spine Society. 2019;19:418–429. doi: 10.1016/j.spinee.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Yuan LT, et al. Multiple bacterial infections increase the risk of hepatic encephalopathy in patients with cirrhosis. PloS one. 2018;13:e0197127. doi: 10.1371/journal.pone.0197127. [DOI] [PMC free article] [PubMed] [Google Scholar]