Summary
Identified 50 years ago, mesenchymal stromal/stem cells (MSCs) immediately generated a substantial interest among the scientific community because of their differentiation plasticity and hematopoietic supportive function. Early investigations provided evidence of a relatively low engraftment rate and a transient benefit for challenging congenital and acquired diseases. The reasons for these poor therapeutic benefits forced the entire field to reconsider MSC mechanisms of action together with their ex vivo manipulation procedures. This phase resulted in advances in MSCs processing and the hypothesis that MSC‐tissue supportive functions may be prevailing their differentiation plasticity, broadening the spectrum of MSCs therapeutic potential far beyond their lineage‐restricted commitments. Consequently, an increasing number of studies have been conducted for a variety of clinical indications, revealing additional challenges and suggesting that MSCs are still lagging behind for a solid clinical translation. For this reason, our aim was to dissect the current challenges in the development of still promising cell types that, after more than half a century, still need to reach their maturity. stem cells translational medicine 2019;8:1135–1148
Significance Statement.
This article highlights mesenchymal stromal/stem cell (MSC) strengths and overall weaknesses in order to contribute to the achievement of a better awareness of challenges related to MSC developments that should be taken into account in future MSC‐based therapeutics.
Mesenchymal Stromal/Stem Cells in Last Century
Mesenchymal stromal/stem cells (MSCs) were identified more than 50 years ago, and these originally elusive progenitor cells present in bone marrow (BM) immediately attracted the interest of scientists attempting to determine the existence of multifunctional cells in a tissue known to possess hematopoietic stem cells (HSCs). That was the case for Friedenstein, whose group, in 1966, reported the existence of BM cells capable of generating hematopoietic cells, fibroblastic reticular cells and bone in vivo 1. In the beginning, the nature of those differentiating cells was unclear. However, a few years later, the same authors described a rare population of BM cells that adhered to plastic after in vitro seeding, creating a heterogeneous multitude of spindle‐shaped cells. In those days, cells were grown without any specific nutritional requirements except for the addition of fetal bovine serum (FBS). The definition of that time was “mechanocytes” due to the tendency of these cells to generate bone when mechanically stimulated, suggesting that a physical stimulus could induce differentiation 2.
In parallel, Dexter et al. observed that human BM cells, after in vitro seeding, formed a heterogeneous adherent cell layer composed of macrophages, endothelial cells, and fibroblasts often characterized by preadipocytic features 3. They further determined the functional character of these cells in an in vitro environment, revealing their capacity to support and maintain HSCs, and for this reason, they defined this population as stromal cells. It was in 1988 that Owen and, once again, Friedenstein demonstrated that these stromal elements were capable of producing colonies with self‐renewal and multipotency 4. They additionally described that factors added to the medium were able to activate specific differentiation pathways, in that case, toward a bone commitment. Thus, stromal function and stemness could overlap, at least according to those early in vitro experiments.
Around the same years, Caplan proposed MSCs as mesenchymal progenitors capable of generating mesodermal restricted lineages 5, and later on, several groups published relevant data on the differentiation capacity of MSCs providing evidence of their plasticity even across ontogenic lineages 6, 7. With these data in mind, clinicians and scientists moved ahead, and the differentiation capacity of MSCs was challenged in vivo in preclinical models and in early clinical studies of tissue regeneration 8. A much higher level of stemness has then been attributed to MSCs, up to pluripotency, with almost infinite proliferation and differentiation capacity, with a terminology shift to multipotent adult progenitor cells (MAPCs) 9, 10. Further in vivo investigations in a variety of regenerative medicine approaches have revealed a suboptimal performance with a low engraftment rate that was still associated with some therapeutic impact 11. Thus, investigators wondered whether the word “stem” would still be fitting with MSCs biological properties and how MSCs would exert (lasting) therapeutic benefits in vivo 12. In this context, scientists have been more focused on biological functions more attributable to stromal functions than to multipotency 13, 14.
Essentially, we could summarize the relationship between stemness and “stromalness” in a sort of diagram (Fig. 1). In the late 1960s and 1970s there was a progressive increase in data regarding the proliferation and differentiation capacity of MSCs with the stemness concept that reached maturity in the 1980s. This advancement peaked around 2000 and then decreased. This left more room for MSCs “stromalness” to grow, which started with the hematopoietic supportive stromal cells described by Dexter et al., has progressively increased involving the release of a variety of growth factors, chemokines, cytokines, and more recently extracellular vesicles (EVs) 3, 15, 16, 17, 18 The capacity to release molecules has been further empowered by the provocative theory that MSCs could actually be Medicinal Signaling Cells, keeping the acronym but changing a paradigm 19. Nowadays, we may state that stemness and “stromalness” coexist at different levels, influencing each other in a reciprocal manner. The challenge is to understand the extent of the two in MSC preparations accounting for variability in tissue sources, in donor‐related issues (including age, disease, gender, lifestyle), in the ex vivo culture conditions (isolation tools, culture media, population doublings [PD], dissociation reagents) and in the delivery strategies (fresh versus frozen product, transportation buffer, injection routes, and injection tools).
Figure 1.
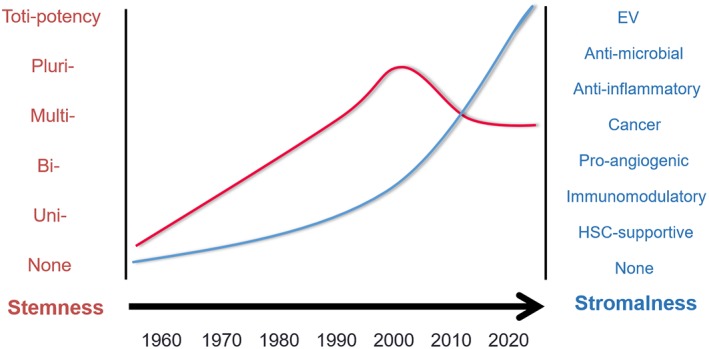
Relationship between stemness and “stromalness.” Mesenchymal stromal/stem cell (MSC) stemness has been supported by a progressive accumulation of data regarding their proliferation and differentiation capacity since the late 1960s, reaching its peak around 2000. This feature then decreased slightly, leaving more room for another MSC feature, here defined as “stromalness,” based on tissue‐supportive functions (such as hematopoietic stem cell‐supportive function), which progressively increased involving the release of a variety of growth factors, chemokines, cytokines and, more recently, extracellular vesicles.
Learning from More Than 50 Years of MSC Investigations
Several properties have been attributed to MSCs in the last 50 years of research, even if basic concepts originally developed by Friedenstein and Dexter still have pivotal roles. MSCs can now be isolated from different sources, and MSC populations have been described in a variety of sources including adipose tissue (AT), skin, cord blood, Wharton jelly, placenta, amniotic fluid, liver, bile ducts, lung, and teeth 20. This wide distribution of MSCs throughout the body has been quite surprising, and it is supported by the concept indicating that MSC‐like populations can virtually reside in all organs, since they are connected to vessels as pericyte populations 21.
The multiplicity of sources of MSCs has been accompanied by the emergence of novel tools for their ex vivo isolation and the following in vitro expansion and manipulation for therapeutic purposes. These tools include isolation reagents and devices, specific culture media, and ancillary reagents whose introduction both at the academic and industrial level have introduced innovative protocols on how to efficiently isolate MSCs, allowing the transfer of this technology to large‐scale expansion and facilitating the translation of preclinical findings into the clinic. For example, focusing on BM‐derived MSCs, it is now established that starting from 20 ml of marrow aspirate shipped overnight from the clinic to the cell manufacturing site, MSCs can be isolated and amplified to up to hundreds of millions of cells in less than 1 month. Cells can then be shipped back to the operating room for surgical implantation alone or even in combination with biomaterials for skeletal disorders 22, 23.
New isolation methods have also been changing the paradigm of plastic‐adherent MSCs, and a source of MSCs has also been described in the nonadherent culture fraction, called nonadherent mesenchymal progenitors (NAMP) 24, 25, 26. This cell fraction, dependent on basic fibroblast growth factor (bFGF) action, can be expanded as a cell suspension, possibly representing a specific population of early progenitors with a less committed phenotype compared with the plastic‐adherent MSCs 27, 28. According to these protocols, using both BM and fat as resources, it is possible to isolate from NAMP an adherent fraction with similar proliferation and differentiation potential as MSCs 29. Although these protocols need to be standardized, these data indicate how cell culture conditions could isolate/rescue progenitor cells with different potential, virtually representing a complementary cell source in clinical applications.
Beside the matter of quantity, in the last decades, MSCs were better characterized from several points of view. Although MSCs still lack a single specific surface marker, panels of markers guiding their identification have been proposed 30, 31, 32. Other features have been described and are now shared by the scientific community, such as genomic, epigenetic, secretomic, and proteomic profiling 33, 34, 35, 36. More recently, the release of exosomes, a smaller EV fraction, has been outlined as a relevant biological mechanism in driving tissue regeneration 37. Although these properties are sufficient for defining a correlation between phenotype and stromal/stem cell functions it still has to be convincingly demonstrated; however, it represents a foundation on which more specific criteria to better identify MSC properties could be developed.
Cell availability and characterization now allow investigators to introduce MSCs in various disease models with promising although sometimes disappointing outcomes, not much related to the safety of the procedures rather than the efficacy endpoints to be reached. Essentially, we found ourselves in paradoxical situation of being able to amplify billions of MSCs and infuse them in a variety of clinical applications together with the difficulties in dissecting what is/are the precise mechanism/s of action (MoA) for a defined clinical indication 38. The task to identify MSCs MoA is itself a challenge and, despite being compared with “classic” drugs, these cells are a living entity with metabolic activity, receptors, and the capacity to respond to environmental stimuli, influencing their therapeutic actions. This so‐called “plasticity” has the predicted value to make MSCs a sort of “chameleon” in different contexts, with the capacity to adapt their ability to pathological conditions and thereby exert a therapeutic benefit 14.
However, this plasticity is difficult to follow in the laboratory, and the complexity of mimicking those pathological conditions ex vivo is the most critical aspect in predicting the precise MoA of MSCs in vivo and therefore in the development of effective potency assays 39. This difficulty is also related to the preclinical animal models that have been introduced to demonstrate the efficacy of MSC‐based strategy. Generally, animal models are developed to resemble human diseases, but they have limitations in the different immunological and serological (chemokines, cytokines) contexts, which may not be ideal in triggering the desired MSCs phenotype in vivo, not only under xenogeneic conditions but also in syngeneic models, due to the difference between human and animal MSCs 40.
Tissue Source Matters
The early origin of MSCs has been limited to BM for a long time, despite that adipose‐derived progenitors have been described since the 60s 41, 42. Nowadays, MSC‐like populations can be isolated from a variety of sources and each has its peculiarity with early comparative analyses reporting differences based on the tissue of origin as summarized in Table 1 43.
Table 1.
Comparison of mesenchymal stromal/stem cells (MSCs) from different sources with reference to different parameters: bone marrow‐derived MSCs, adipose tissue‐derived MSCs, umbilical cord‐derived MSCs, dental pulp stem cells, and endometrial MSCs
| BM‐MSC | AT‐MSC | UC‐MSC | DPSC | eMSC | |
|---|---|---|---|---|---|
| Tissue availability |


|



|



|

|



|
| Cell yield |

|


|


|


|


|
| Cell shape uniformity |

|


|



|


|



|
| Proliferation |


|


|



|



|



|
| In vitro senescence |



|


|

|


|

|
| Differentiation ability |



|


|


|


|


|
| Stromal function |



|



|


|


|


|
| Autologous use |



|



|

|

|


|
| Allogenic use |



|



|



|



|



|
 , low;
, low; 
 , medium;
, medium; 

 , high.
, high.
BM was long regarded as the major source of MSCs 1. However, BM collection requires an invasive bone harvest procedure that may also be accompanied by pain and risk of infection 44. In addition, since MSC precursors account for only 0.001% to 0.01% of the overall cell population, allowing the isolation of only 60–600 cells per ml of BM aspirate, there is a need to harvest a large amount of raw material along with extensive ex vivo culture to obtain sufficient cell numbers for clinical applications in humans 7. BM‐derived MSCs retain substantial multilineage differentiation, although mesodermal restricted and coupled with a low proliferation capacity compared with other MSC types 43. BM‐MSCs have a longer doubling time (DT, approximately 60 hours) compared with such cells from other tissue sources 45. This is also associated with a quite heterogeneous cell size with large flat cells together with small round cells and spindle‐shaped cells (Fig. 2) and the appearance of senescence landmarks, since relatively early passages are involved, even as of passage 7 46, 47. Thus, MSCs aging has an important impact on the effectiveness of BM cells, influencing their number, maximal lifespan and differentiation potential. These properties make cells certainly useful for autologous transplantation, where immunity should not interfere with the regenerative properties of the cells and considering that allogeneic use may be associated with very relevant ex vivo expansion to reach a billion‐cell level, which may be difficult in this case.
Figure 2.
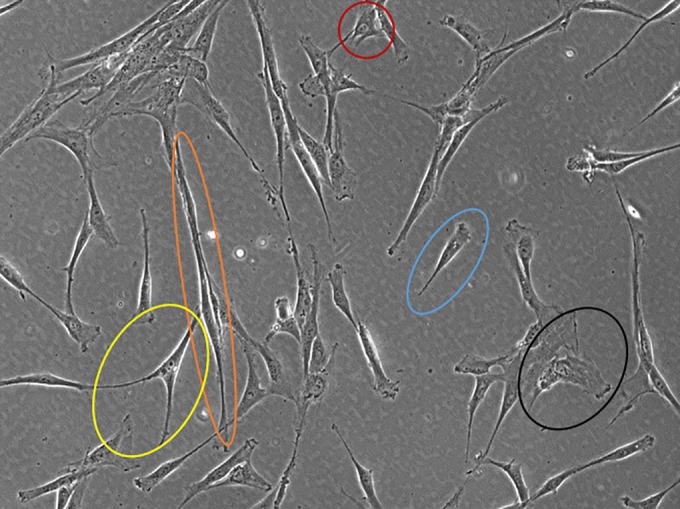
Mesenchymal stromal/stem cells (MSCs) heterogeneity. Representative photomicrograph showing the variety of cell sizes and shapes in bone marrow MSCs. There are tripolar‐shaped cells (yellow), long spindle‐shaped cells (orange), small round cells (red), short spindle‐shaped cells (blue), and flattened enlarged cells (black). Contrast Ph1 ×100 magnification, observer A1 (Zeiss).
However, autologous use is suffering from conditions associated with marrow toxicity, such as during major silver sulfadiazine toxicity, as a result of sepsis, during chemo‐ or radiotherapy 48, 49, 50. In view of the clinical application of MSCs, accumulated evidence suggests that the bioactive secretion of MSCs is the main reason for their therapeutic benefits, improving the angiogenic activity of endothelial cells and promoting blood restoration. Numerous putative angiogenic proteins have recently been identified in EVs derived from MSCs that contribute to cell‐to‐cell communication to control angiogenesis during wound healing. BM‐MSCs are characterized by a significant paracrine function and could be preferred in clinical applications for therapeutic angiogenesis due to the angiogenic factors, growth factors, and cytokines discovered in their secretome 51, 52, 53.
Over time, a number of other tissues have been identified as alternative sources of human MSCs. Nowadays, MSCs can be isolated from multiple tissues, including AT, perinatal tissue, dental tissue and decidual endometrial blood 54.
AT‐MSCs were identified early in the 1960s, which were then characterized by Zuk et al. in 2001 55, 56. AT‐MSCs started to be used in regenerative medicine (RM), as an alternative or in addition to BM‐MSCs 31. Subcutaneous AT represents an abundant source of AT‐MSCs 57, and the average increase in body mass index (BMI) easily allowing to find this material, normally discarded 58. Compared with BM‐MSCs, they are superior candidate cells for autologous cell transplantation, as they can be isolated from the biological material harvested by less invasive procedures as the liposuction, lipoplasty, or lipectomy 59. AT is not only easier to harvest, but it also has a better yield with 500 times more MSCs precursor cells than from an equivalent amount of BM 60, 61. For these reasons AT‐MSCs are attractive candidates for therapeutic use. Like BM‐MSCs, AT‐MSCs can be easily expanded in culture, and they have higher proliferation capacity, showing shorter DT by approximately 20 hours. Although still related to the body region from where they originate and unlike BM‐MSCs, AT‐MSCs can be cultivated up to passage 8 without any sign of senescence 45, 46, even if passages may not precisely reflect the replicative age of MSCs 62. There is evidence that adipocyte progenitors vary on the basis of individual adipose depots and depending on the age, sex, and body weight of the individual, location and type of fat tissue, tissue collection method, and culture conditions 63, 64, 65. Furthermore, recent studies suggest that the heterogeneous mixture of cells obtained after digestion of AT, contains subpopulations of stem cells and more committed progenitor cells, such as dedifferentiated endothelial cells, smooth muscle cells, and pericytes. However, the heterogeneity of cultured AT‐MSCs seems to be limited after plastic adhesion, when they appear morphologically smaller than BM cells 63. Although AT‐MSCs may represent a valid alternative to BM‐MSCs, able to solve issues related to the cell quantity required for therapies, their “innate” tendency for adipose differentiation rather than skeletal tissue development has to be seriously considered 66, 67. Furthermore, AT‐MSCs show a significantly lower secretion of proangiogenic molecules and cytokines compared with BM‐MSCs, with a possible impact on their therapeutic benefit, such as the reduction of inflammation 68, 69. This may also be related to the different in vivo environments of the respective tissue of origin: BM is abundant with a variety of hematopoietic and endothelial cells, whereas AT is predominantly filled with adipocytes.
MSCs can also be isolated from birth‐associated tissues, including placenta, amnion, umbilical cord (UC), and umbilical cord blood (UCB). They are readily available, thus avoiding invasive procedures or ethical issues and offer an advantage not found in adult tissue because their harvest avoids the potential problems related to age. Moreover, they show improved proliferation ability, lifespan, and differentiation potential compared with BM‐MSCs and AT‐MSCs 43, 70. More interestingly, the possibility to have a large amount of cord allows a rapid isolation of the MSCs population for allogeneic cell‐based regenerative medicine applications 71. UCB‐MSCs from a single donor are sufficient to generate 1 × 109 cells within passage 3–4 in a cell factory system, and furthermore, they can be expanded over 16 passages with retention of normal karyotype and without any sign of senescence or variation in cell morphology 72, 73. However, there are some drawbacks linked to the successful isolation and expansion of UCB‐MSCs, mainly due to the low frequency of MSC clones in particular when compared with UC, a rich source of highly proliferative MSCs characterized by: a higher isolation yield (5 × 104–5 × 105 cells from 1 cm3 of UC tissue), higher frequency of colony‐forming unit (CFU)‐F and shorter DT (24 hours) compared with BM‐MSCs and a homogenous phenotype of adherent, spindle‐shaped fibroblast‐like cells in primary culture 74, 75, 76. The therapeutic effect of the secretome from UC‐MSCs is currently under investigation, and it appears to be rich in angiogenic factors that make it similar and competitive compared with the BM‐MSC secretome 77. Furthermore, stem cell banks offer the opportunity to cryopreserve cord tissue as a possible source of MSCs for future autologous uses, pending the possibility to isolate cells after long‐time freezing. However, there are controversial opinions approximately cryopreserved cord tissue. On one side, it could circumvent early MSCs isolation and related manipulations before freezing 71, on the other the isolation of living MSCs from thawed cord tissue is not always successful, due to the differences in exposure to cryoprotectant between the outer and inner parts of the tissue, resulting in cell death 78, 79.
MSCs can be easily isolated from various dental tissues including dental pulp, apical papilla, dental follicle, human exfoliated deciduous teeth, periodontal ligament, gingival, and tooth germ‐derived cells 80. Dental pulp stem cells (DPSC) are increasingly being recognized as a viable cell source for regenerative medicine. Stromal cells represent roughly 10% of dental pulp cells and display multipotency, and interestingly, they exhibit a higher proliferation rate in vitro compared with BM‐MSCs 81. Current studies indicate that they maintain their high rate of proliferation even after extensive subculture with a DT of 30 hours 82. Their proliferative and multilineage capacity as well as accessibility of sources makes them attractive for therapeutic purposes. However, they show some limitations due to the intrinsic nature of dental tissue that does not undergo continuous remodeling as shown in bone tissue. Therefore, dental tissue‐derived MSCs may be more committed or restricted in their differentiation potency in comparison to BM‐MSCs, as demonstrated by their weaker chondrogenic and adipogenic potential 83. MSC populations in dental pulp are heterogeneous and demonstrate differences in proliferation rate and differentiation capacity, depending on donor age, also detected between DPSC populations from donors within a similar age range and even between cell populations from the same patient. At early passage (7–8 PD), all DPSC appear morphologically similar, with long spindle shape, whereas at later stages, cells appear larger and more stellate with prominent stress fibers, but the onset of the senescent phenotype is highly variable 84. Although DPSC show an efficient secretion of bioactive molecules, the secretion of proangiogenic factors is lower compared with BM‐MSCs and AT‐MSCs. However, they secrete significantly larger amounts of chemokines and neurotrophins implicated in neuroprotection and neural supportive properties in response to injuries and pathologies of the nervous system 85, 86. Autologous transplantation remains the most common practice for dental MSC applications, even if it shows serious limitations because it requires the extraction of remaining teeth. Furthermore, donor age should also be taken in consideration, since proliferation, migration, and differentiation capacity of these cells decrease with donor age 87. Thus, one of the main challenges in autologous clinical applications of this MSC type is the limited cell availability, where paradoxically, the patient would need more of these cells. Tooth banking could ensure the cryopreservation of cells and tissues for future need in autologous transplantation, but it is not currently a popular practice.
Since 2004, a small population of clonogenic stromal cells with typical adult stem cell properties of self‐renewal, high proliferative potential, and multilineage differentiation capacity, although less robust than BM‐MSCs, was identified in endometrial MSCs (eMSCs) 88. They are normally obtained from hysterectomy tissue and uterine biopsy or more easily from menstrual blood, which is considered an attractive source of MSCs for regenerative medicine because of its relative ease of acquisition with minimal morbidity. Obtaining eMSCs from menstrual blood uses body waste and requires no invasive procedures using a menstrual cup over several hours on days 2–3 of the menstrual period, providing sufficient cell number 89. Isolation yield is variable, but 2–4 × 106 nucleated cells per milliliter of initial blood can be isolated, of which approximately 0.2%–0.3% are colony‐forming cells expressing standard mesenchymal markers 90. Adherent cells cultured from menstrual blood rapidly expand with a DT of 27.6 hours, undergoing 25–30 PD without any sign of senescence until passage 26 91, 92, 93. They show the typical mesenchymal appearance, being fibroblastoid‐like, spindle‐shaped cells, although some endothelial‐like cells can appear in the very early passages, disappearing at passage 3 when the cell population becomes rather homogeneous 89, 94. The secretome of eMSCs shows an immunomodulatory activity and when compared with the exosome fractions of different human MSC sources, eMSC exosomes show superior effects on neuritic outgrowth, while their proangiogenic effect is not well documented 95. Despite that eMSCs can be isolated from women of all ages irrespective of hormonal status or treatments, they represent a gender‐related source, and the woman's age may influence cell proliferation, impacting their potential autologous use 96, 97. Although there is some evidence that BM cells contribute to a small population of endometrial epithelial and stromal cells, eMSCs are a novel and still not completely understood population of mesenchymal progenitors, which still lack specific markers.
Thus, despite similarities, MSCs derived from such a wide variety of human tissues have different functional properties 70, 72. More than 10 years ago, under the umbrella of the International Society for Cellular Therapy (ISCT), we proposed a series of basic criteria with the aim to better define MSCs in the context of preclinical investigations 30. Although the simplicity of the approach favored the recognition of these minimal criteria, even beyond the original scope of the article, the increasing complexity of the MSCs field is now calling for updated criteria to define these cells. Although basic features (fluorescence‐activated cell sorting [FACS] analyses, morphology and basic differentiation in vitro) may help in distinguishing MSCs from other cell types, they are certainly unable to snapshot the current predicted in vitro/in vivo performance, calling for novel ways to characterize MSCs. Theoretically, this has to be achieved for each specific MSC type considering the originating tissue and addressing macroscopic features, phenotypic markers, defined differentiation properties, and specific molecular fingerprints as well as specific secretory potential either as released soluble molecules or as exosomes. An attempt toward an updated MSC characterization in a tissue‐specific manner has been already proposed, and ISCT in collaboration with other societies has dissected adipose‐derived‐MSC properties, combining a variety of phenotypical markers, functions, and differentiation‐related genes 31.
MSC Donor‐Related Variability Matters
An autologous MSC source is widely used in cell therapy 98. However, several studies have revealed donor‐related variations, caused by gender, BMI, donor site, age, and diseases. The recognition of the variability in biological function has a relevant impact either in an autologous setting, where MSCs implantation would not be followed by the desired benefit or most importantly in an allogeneic context where apparently healthy and functional MSCs may be actually dysfunctional for yet unclear reasons. Although the identification of these donor‐related variations appears complex, their investigation and discovery could be key in identifying specific MSC donors for particular diseases to ultimately avoid failure in both early and late clinical trials. In the attempt to identify these factors, we have recently reported the development of an assay capable of predicting bone formation 99. According to this approach, a gene signature has been identified in relationship with in vitro and in vivo functional assays to be ultimately compared with the outcome of clinical investigations.
A study performed in a rat model revealed the superiority of female MSCs in reducing lung inflammation compared with male MSCs 100. Women are more exposed to hormonal variations, such as a decrease in circulating estrogen levels in menopausal status, which has been shown to be responsible for loss of osteogenic potential of stem cells 101. Gender variability was also confirmed for AT‐MSCs by Ogawa et al. who showed a greater level of Peroxisome Proliferator Activated Receptor‐ϒ2 (PPAR‐ϒ2), a marker of adipogenesis, in cells harvested from female mice compared with male mice 102.
Although there are conflicting reports approximately the effect of aging on MSCs performance, disparate studies have reported age‐related changes in MSCs, such as loss of proliferation and differentiation potential, increase in senescence and loss of in vivo bone formation 103. MSCs from older patients show a reduction in superoxide dismutase activity and an increase in nitric oxide and reactive oxygen species, which lead to oxidative damage in MSCs and consequently apoptosis and senescence. Moreover, aged MSCs are characterized by an upregulation of both p53 and p21, well known for their pro‐apoptotic activity, and a decline of Notch‐1 receptor, probably involved in bone differentiation 103. A substantial influence of donor age is also reported for CFU‐f number, which decreases in older patients, and for molecular markers. In particular, Candini et al. identified seven miRNAs differentially expressed in young and old groups, among which miR‐196a was upregulated in aged MSCs, whose level correlated inversely with ki67. The substantial interest shown in these data is due to the involvement of miR‐196a in the regulation of the homeobox (HOX) gene and the essential role of the HOX family in skeletal formation 36, 104, 105.
As MSCs are the basis of tissue regeneration therapies, altered cell functionality in elderly patients may compromise the efficacy of autologous approaches. With an increase in the aging population, older patients are becoming the most common target for cell therapies, and it is important to investigate how much donor age is a critical factor in achieving expected results 106. Alternative strategies are necessary, such as a younger MSCs bank for later use, and preserving progenitor cells maintaining their regenerative potential. The previous association between HOX expression and aging has been confirmed by the lower levels observed in adult groups. When MSCs are modified to overexpress HOXB7, there is an increased level of ki67 and bFGF, which is involved in progenitor self‐renewal, proliferation, differentiation, and osteogenesis 107. These proof‐of‐concept findings can open the way to novel strategies, particularly for skeletal disorders, and offer new explanations for tissue engineering failures.
The efficacy of autologous cell therapy in the treatment of several diseases has to deal with the intrinsic impact that the diseases themselves may have on MSCs regenerative potential. Systemic and more focal diseases can affect the MSCs compartment in multiple ways, possibly restricting the effectiveness of autologous treatments. Systemic diseases may affect autologous cell‐based treatment, such as diabetes or cardiovascular diseases (CVDs).
In particular, type 2 diabetes (DM2) is a chronic metabolic disease that represents a major risk factor for the development of vascular disease, causing a high rate of mortalities globally. It has been reported that DM2 has adverse effects on MSCs function, inhibiting the angiogenic ability of MSCs through the downregulation of pro‐angiogenic factors. Furthermore, BM‐MSCs from diabetic patients show hampered paracrine secretion and an increased propensity to differentiate into adipocytes 108, 109.
Another global burden is represented by CVDs, one of the leading causes of death in the Western world 110. Aging is the main driver of CVD progression, inducing vascular changes and reducing regenerative potential of stem cells. In turn, CVDs also affect the progenitor cell compartment, causing increased senescence, reduced proliferation, and regenerative potential in MSCs 111. This could represent a limitation for autologous cell therapy in CVDs, which may be solved by using allogeneic MSCs, selected on the basis of age and comorbidities.
Furthermore, BMI has been reported to have an effect on the differentiation and proliferation abilities of adipocytes 112. In obese individuals, these potentials and DNA telomere length as well are compromised, suggesting a decreased self‐renewal capacity and early apoptosis. Interestingly, after massive weight loss, there is reduced DNA damage and an improvement of cell viability and replicative lifespan 113. Obesity characterized by high BMI negatively affects not only AT‐MSCs but also BM‐MSCs, showing severely impaired osteogenic and diminished adipogenic differentiation, decreased proliferation rates, increased senescence, and elevated expression of endoplasmic reticulum stress–related genes. This may have a direct impact on their use, particularly in the field of regenerative medicine, where these cells could be potentially used for the treatment of orthopedic issues. It is conceivable that stem cells, obtained from very overweight donors, may display severe differentiation and proliferation defects, resulting in an isolated stem cell product of poor quality and decreased regenerative potential in vivo 114. An abnormal increase in fat mass results in dysfunctional AT, and consequently, AT‐MSCs are defective in differentiation, pro‐angiogenesis, motility, immunomodulation, and development of primary cilia, which are shortened and unable to properly respond to stimuli 115, 116. AT can also be affected by lipodystrophic syndromes, rare and heterogeneous disorders characterized by an anomalous distribution of body fat or a generalized loss of AT and variable degrees of insulin resistance. In particular, in lamin‐linked lipodystrophies, adipose MSCs show a reduced differentiation and proliferation capacity, premature senescence and an altered gene expression profile related to lipid metabolism 117, 118. However, maternal obesity can also affect UC‐MSCs, altering the metabolic environment in utero. Thus, UC‐MSCs from obese donors show slower PD and a stronger immunosuppressive activity, which is critical for heterologous clinical applications 119.
In addition, MSCs can be impaired due to more local/focused disorders targeting a particular organ from where the cells are harvested, impacting cell performance and compromising their regenerative ability in an autologous and also allogeneic setting. BM is currently the most used source of MSCs; however, various diseases could affect this tissue, such as severe aplastic anemia, a rare but potentially life‐threatening disease, which is a paradigm of BM failure syndromes 120. The immune‐mediated destruction of HSCs is considered a key factor, but MSCs also display a poor potential for proliferation and adipogenic and osteogenic differentiation. BM‐MSCs are also implicated in the biology of splenic marginal zone lymphoma, where BM is frequently infiltrated by the malignant cell population. Cells show impaired growth potential and an increased DT, which could be attributed to increased apoptosis 121, 122. Another rare pathological condition that affects BM is Shwachman‐Diamond syndrome (SDS), a rare multi‐organ recessive disease mainly characterized by pancreatic insufficiency, skeletal defects, short stature and BM failure. MSCs derived from SDS patients show a defective ability to form capillary tubes and vessels and display a marked decrease in Vascular Endothelial Growth Factor A expression, together with an inability to recreate a functional BM niche 123.
Teeth‐derived MSCs could represent an alternative source of autologous cells after the extraction of a compromised tooth, considering the impact of the related condition. In the case of inflamed periodontal ligaments of periodontally compromised teeth, MSCs show an increased proliferation capacity and migration potential; however, they are characterized by a decreased capacity for osteogenic differentiation 80. Similarly, inflamed dental pulp causes a decreased PD in DPSC, so inflammation may affect the total number of cell divisions 124.
Similarly, eMSCd are influenced by endometriosis condition. Menstrual blood‐derived stromal cells from woman with endometriosis are morphologically different from healthy patient‐derived MSCs and show a higher proliferation and invasion capacity and a greater potential to direct the inflammatory responses in their favor, thereby facilitating the development of endometriosis 125. This makes them similar to cancer cells; in fact, endometriosis patients have been reported to be more prone to the development of ovarian cancer.
There may also be donor‐related MSCs variability based on on‐going treatments. The administration of immunosuppressive drugs, such as tacrolimus, is fundamental after organ transplantation, but it can compromise the viability and proliferative ability of progenitors in a dose‐dependent manner 126. Similarly, tamoxifen, an antitumor drug used in breast cancer, shows a dose‐ and time‐dependent influence on in vitro adipose‐derived progenitors, resulting in apoptosis, inhibition of proliferation and differentiation 127. Other chemotherapeutic agents may have a conflicting impact on MSCs 128, 129, 130. Similarly, radiotherapy is used to treat various malignancies. However, it can also adversely impact normal tissues and MSCs, causing premature senescence or accelerated terminal differentiation 131. Collectively, these data indicate that an autologous‐based strategy in oncology may be extremely challenging, particularly when there is a demand for a substantial number of stem cells.
Similarly, the prolonged use of morphine therapeutically administered to relieve pain, impairs angiogenesis and the activation of endothelial progenitor cells. Furthermore, morphine affects MSCs proliferation and differentiation in a dose‐dependent manner and causes changes in the phenotype, growth properties and secretory functions of MSCs, having a negative impact on wound repair 132.
All these variabilities suggest the need for developing a personalized approach for patients, considering their age and comorbidities, even with pretreatment or modifications of stem cells. Furthermore, cell dose or cell delivery may be adapted to state of illness, type of MSCs and condition of the patient 133.
Isolation Procedures Matter
The challenges relative to MSCs may also be related to different protocols for MSCs isolation. Accordingly, standardization is difficult to be achieved, also accounting for the different tissue sources and variability in the amount of starting raw material and in relation to protection of intellectual property rights. However, each of the early isolation steps can have an impact on quantity and quality of ex vivo isolated cells. A conventional procedure to select MSCs is based on their capacity to adhere to plastic after an appropriate isolation method, whose choice is still a critical step in obtaining MSC preparations for both preclinical and clinical uses. Isolation methods are classified into four categories: enzymatic, mechanical, explant culture and density‐gradient centrifugation methods.
The most common approach is the digestion of tissue by enzymes, such as collagenase, trypsin, and dispase, which consists in incubation at 37°C for at least 1 hour, generally with mixing. Depending on the tissue to be digested and the protocol adopted, differences can be found in the number of washing steps, enzyme concentration, centrifugation parameters, erythrocyte lysis methods to reduce hematopoietic contamination and final filtration to remove tissue fragments 134, 135. Sutradhar et al. showed that trypsin–EDTA, used to detach cells from the plastic surface, causes loss of membrane integrity and a decrease in cell viability in a time‐dependent manner 136. For more reasons, digestion time periods longer than 5 minutes can affect cell surface antigens 137, cell surface topography, cytoskeletal components and distribution of intramembranous particles 138 and compromise cell isolation success. Since the use of enzymes is characterized also by high costs and an impact on vitality and cell yield, mechanical methods have been investigated, using shear force, centrifugal force, radiation force, and pressure. However, these non‐enzymatic approaches are similarly variable, according to protocols and methods 135.
Over the last years, several mechanical approaches have been introduced to standardize the isolation process of MSC precursors, particularly from AT. For example, new technology guarantees shorter procedure times in a rapid and disposable device, where AT‐MSC precursors can be isolated 139, 140.
On the other hand, explant culture methods show several advantages compared with enzymatic and mechanical isolation, such as reduced cost and risk of biological contamination, a more homogenous cell population and less cell damage, with a consequent higher proliferation rate of MSCs. Cells are transferred from in vivo to in vitro conditions with a fragment of tissue without the separation stress on cells 141. Despite the cost advantage, the success of this method depends mainly on the manual skills of operator and quantity of starting material, making it not always reproducible. In addition, undesired cellular elements may be carried over in culture, generating a contamination that could become an important issue for MSCs preparation release.
One of the ways to better purify the starting MSC products and the common method used for BM‐MSCs isolation remains the density‐gradient centrifugation method. Most preclinical and clinical studies use Ficoll‐Paque as density medium to enrich the mononuclear cell population and the rare progenitor cells. However, Percoll density medium is alternatively used because of its lower cytotoxicity. Still, an important limitation of these density‐based methods is the lack of a high resolution in distinguishing stem cells and nonstem cells, where their size difference is not absolute 142. Nevertheless, given the low concentration of stem cells within a given tissue, it is generally possible to distinguish them from the other cell populations, using specific cell markers on their surface. Obviously, some markers are common between more cell types, so a combination of different markers is the best choice to isolate the cell population of interest. Also, little difference in density may affect the quality of MSCs type, as we have previously seen in comparing Ficoll‐Paque of 1.073 versus 1.077 g/ml 143.
Magnetic‐activated cell sorting, MACS, is the most commonly used method of sorting cells by magnetic forces. Magnetic beads are generally coated with a specific antibody for desired cells and passing through a magnetic field, only antibody‐bound cells are retained. Cells are finally recovered by enzymatic detachment from the magnetic beads 144. Another purification method is flow cytometry using FACS instruments, based on the positive identification of cell surface markers expressed by MSCs. However, the lack of knowledge of unique markers for different cell types is an important limitation for this method, together with the possibility of cell contamination during the sorting phases and physical stress caused by probes, laser damage and osmotic stress on cells 145. Both techniques are based on adherent cell purification and their detachment from the plastic surface is a critical step, considering that enzymatic dissociation can lead to proteolytic damage of cell surface proteins.
Once MSCs are isolated using different approaches, the number of cells from the primary culture is often insufficient for clinical application, especially when more systemic infusions are necessary. For this reason, cell expansion is essential to reach an appropriate number of MSCs. Cell culture variables include medium formulation (basal media and supplements), culture surface substrate, cell seeding density, physiochemical environment (dissolved oxygen and carbon dioxide concentrations, temperature, pH, osmolarity, and buffer system), along with subculture protocols 146.
The literature suggests that seeding density has an impact on cell proliferation rate. Both and collaborators described a faster proliferation rate when MSCs were seeded at lower densities 147. However, the recommended seeding density for BM‐MSCs is 5,000 to 10,000 cells per cm2.
Cell expansion requires enzyme dissociation and cell subculture. The evaluation of the optimal cell confluence is operator‐dependent, although 70%–80% confluence is recommended to be reached before detachment. Furthermore, repeated cell passages are necessary to obtain a sufficient number of cells and the optimal passage to use depends on the intrinsic proliferative ability of the cell sample. Wagner et al. reported that when expansion is protracted for 43–77 days of cultivation, MSCs show senescence aspects with morphological abnormalities, enlargement, attenuated expression of specific surface markers, arrested proliferation, and also the loss of differentiation capacity 148. In addition, telomere length decreases 149 as well as their migratory capacity 150.
To achieve a cell dose‐dependent efficacy of the treatment in a clinical trial, a minimum of 1.5–6 × 107 cells per single dose is required 151, 152. Therefore, large production processes to obtain this number of MSCs are needed, given that the increase in incubator occupancy, handling time for culture monitoring, and risk of contamination make two‐dimensional supports inadequate. The success of the final product is related to the robustness, reproducibility, and efficacy of the Good Manufacturing Practice (GMP) process 153. Different bioreactors with automated controls can guarantee a homogeneous distribution of nutrients in the culture medium and meet the challenge of extensive ex vivo cell expansion. Bioreactors enable the monitoring of various parameters (pH, pO2, pCO2) and, hence, allow ensured cultivation under well‐controlled and reproducible conditions. MSCs expansion in the rotation bed bioreactor provides a high number of cells, maintaining their stem cell properties such as specific surface markers, proliferation capacity and differentiation potential 154.
Among cell culture variables, the choice of a well‐formulated culture medium for both isolation and expansion of MSCs is a critical aspect. Cells require a basal medium supplemented with proteins, growth factors, and enzymes to support cell attachment and proliferation. Human MSCs can be grown in a variety of medium formulations, such as Dulbecco's modified Eagle's medium (DMEM) or alpha minimal essential medium (α‐MEM). DMEM is commonly used by most groups for MSCs expansion, and furthermore, over the last years, some studies have also demonstrated that the use of α‐MEM as basal medium has better performance in MSCs expansion and osteogenic differentiation, compared with DMEM basal medium 147.
Basal medium is typically supplemented with 10% to 20% FBS, which is considered essential for good proliferation of all fibroblast cultures including BM‐derived stromal cells. However, the use of animal‐derived serum is not the best choice for clinical applications because of the risk of the transmission of infections, associated with pathogens such as viruses, prions, mycoplasma or other zoonotic agents. Furthermore, the high content of xenogeneic proteins could be associated with immune reactions in patients in MSCs translation to the clinic and with respect to GMP, the high degree of batch‐to‐batch variation is not compatible with the need for reproducibility 146. To overcome these regulatory concerns, human autologous serum has been introduced in MSCs expansion 155, although it is difficult to obtain a sufficient amount for supporting the growth of great numbers of MSCs, and its beneficial effect may decrease with age, becoming nonapplicable in elderly patients.
More recently, many studies have reported the use of human platelet lysate (PL), prepared by lysis of the platelet membrane, as an alternative source of growth factors and a valid substitute for FBS. It is easily obtained from autologous peripheral blood with a large quantity and minimal donor site morbidity 156. PL has been reported to have a positive influence on MSCs proliferation and osteogenic differentiation, being a natural reservoir of cytokines and growth factors 157, helpful in tissue engineering approaches.
In addition, several serum‐free media have been developed, but the omission of serum, which is a complex fluid rich in biological factors, nutrients, growth factors, antitoxins and much more, requires many supplements for cell survival and proliferation 146. The optimization of defined serum‐free medium for a specific cell type is very difficult and influenced by multiple variables regarding cell characteristics. Serum functions can be replaced by supplements such as binding proteins (i.e., albumin, transferrin), additional nutrients (i.e., lipids, vitamins, amino acids), physiochemical reagent (i.e., buffer), hormones (i.e., insulin), growth factors (i.e., Platelet‐Derived Growth Factor, Epidermal Growth Factor, Fibroblast Growth Factor) and attachment factors 158, 159.
Why MSC Functions Do Not Solidly Shift Them Toward Clinic Yet?
The enthusiasm around MSC properties, together with ease of isolation and ex vivo expansion, reinvigorated a global interest in MSCs with an increasing number of clinical studies. In January 2019, a search on the public website clinicaltrial.gov, using as keywords “MSC,” “mesenchymal cells,” “stromal cells,” resulted in more than 900 registered studies worldwide. A more careful look revealed that most of these MSC‐based trials are still in early phases I or II, while only a small portion of them (less than 50) are in phase III 98, and currently, only nine products (approximately 1%) have been approved worldwide for market authorization 160. This should be seen as unexpected for a 50 year‐old cell type, particularly considering that both local and intravascular MSC infusions have been considered relatively safe procedures 161.
One of the possibilities to explain this is related to the type of disease that is meant to be cure by MSCs. In several cases these cells are transplanted for rare incurable congenital disorders that may not have the numbers to fit the requirement of phase III studies 162. On the other hand, when the number of patients might be suitable, the results may not fully meet the endpoints due to inclusion criteria that may deal with extremely advanced diseases where the MSC therapeutic effects may not be fully manifested due to improper microenvironmental stimuli or just because the potential benefit is unbalanced versus the severity of the conditions.
Finally, there are human diseases where the responsible pathophysiological event is yet unclear and researchers have attempted to introduce MSCs on the basis of a working hypothesis. That is the case with neurodegenerative disorders that are dramatically widespread and include multiple sclerosis, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, spinal cord injury, and Alzheimer disease. MSC‐based therapy is actually considered one of the most promising treatment for these disabilities; however, the observed effects might be only marginally associated with MSCs differentiation into functional neural cells 163, 164, 165. These early beneficial effects of MSCs reported in pilot clinical studies may be derived from their stromal action, through the secretion of neural growth factors, although the precise mechanism of neural tissue repair is still unclear.
These controversial successes in neurodegenerative disease treatment might have also arisen from the way in which MSCs are delivered 166, 167. When possible, local implantation with direct application into the injured site may be preferable for tissue defects. However, most MSCs target diseases are systemic, and intravenous administration of MSCs is then required. This is linked to cell biodistribution and to the questions regarding the number of cells reaching the injured site. It was originally suggested that the majority of intravenously administered MSCs are trapped in the lungs, as the first organs encountered, and subsequently in other tissues, consequently resulting in a reduced therapeutic profile 168, 169. Other investigations very recently described how pulmonary trapping is followed by phagocytosis of MSCs by monocytes, which subsequently migrate from the lungs to other body sites, modulating cells of the adaptive immune system and still providing a therapeutic impact 170. Along the same line, it has been demonstrated that infused apoptotic MSCs can act together with monocytes to promote immunosuppression in an animal model of graft versus host disease 171. Collectively, MSC‐based pharmacological developments are still missing robust pharmacodynamic and pharmacokinetic models to be applied in the different clinical situations 172, 173. This surely calls for ad hoc investigations able to reflect the complexity of these cell types, their interactions with host cells and diseases in which they are applied.
Systemic administration of MSCs is one of the proposed treatments in regenerating different damaged tissues, such as myocardium 174, 175, basing its action on the ability of MSCs to home to the injured site, driven by the interaction with chemokines and cytokines. The ease and low invasiveness of the approach represents an advantage in the treatment of different dysfunctions which require repeated injections of stem cells to obtain a therapeutic effect 176. A remarkable clinical interest has been developed over the years with reference to cardiac dysfunctions. The excessive immune/inflammatory responses, which contribute to the progression of acute myocardial infarction and ischemic cardiomyopathy, can be controlled by the anti‐inflammatory effects of MSCs 175. The exact mechanism of action is not clear, but the idea that paracrine signals could play a key role in cardioprotection has taken hold, especially in myocardial infarction, where the increase in neoangiogenesis and vascularization is decisive 177. Differently from the brain, the heart is well known and studied, and numerous data have been collected from animal models. The promising results with infarcted murine hearts, where injected MSCs became functionally integrated with the surrounding native myocardium, have contributed to the increased expectations for MSCs differentiation into cardiomyocytes. Despite the numerous preclinical and clinical studies conducted, the contradictory data approximately the efficacy of cell therapy in cardiac function improvement require a better understanding of MSCs source, delivery manner and cell doses to overcome the limitations of the therapeutic approach 178. Their positive effect may occur via a paracrine action thanks to numerous angiogenic, arteriogenic, chemotactic, and antiapoptotic growth factors.
Site‐directed delivery might be more feasible in tissue engineering, where cells can be locally implanted in association with biomaterials, avoiding the first passage time distribution via blood circulation. This significantly enhances homing of MSCs to the injury site compared with distant intravenous injection. One example of this approach is related to skeletal regeneration where MSCs can be applied with or without supportive biomaterial. In the field of orthopedic regeneration, there are data that support the beneficial effect of MSCs treatment in meniscus, intervertebral disc, ligament, and tendon or muscle injuries 179. The particular interest around bone defects, which are often associated with a substantial morbidity, has led to a clinical implementation of bone regeneration approaches, such as for osteoarthritis 180, osteonecrosis, nonunion bone defects 181, infantile hypophosphatasia 182, osteoporosis 183, and bone fractures 184. This has been also happening for rare and very severe skeletal diseases such as for osteogenesis imperfecta (OI) even with intravenous injection. OI is a prenatal genetic deadly disorder characterized by bone fragility, bone deformity, and frequent fractures mainly caused by a defect of collagen genes. OI treatment with intravenously delivered MSCs has been pioneeristically performed by Horwitz group indicating feasibility although associated with transient efficacy 185, 186 that opened novel hypothesis on how MSCs may repair skeletal tissues after systemic injection 187.
Business, Costs, and MSCs Clinical Translation
Despite the development and the interest in MSC‐based therapy and its clinical potentials, few human MSC‐based products have been authorized for market and used in clinics worldwide 160. Many challenges remain, including the need for more harmonized regulatory frames among countries to facilitate from early developments to large multinational trials.
Clinical translation cannot be realized without also considering investor expectations in relation to the product potential and its possible therapeutic impact. For this reason economic factors have been also influencing the field: when an MSC‐based product has been showing a valid safety and therapeutic profile, corporate investments raise making product available for many 188. However, when product retains uncertainties that are not challenged within adequate preclinical models followed by well‐designed clinical trials, venture capital and big pharma may be generally reluctant to invest. Thus, it is key to reach a comfort zone in the R&D package since the early steps of development to be properly validated before transferring tasks to a clinical‐grade development. This calls also for attention related to the transition between preclinical and clinical developments in terms of reagent use and procedures. If applicable, cGMP implementation has to be considered during preclinical product characterization to avoid being trapped in the translation process. In addition, we feel that possible massive corporate investments in MSC‐based therapies have been also hindered by the fear that similar academic‐based products can be used in patients as part of a consolidated clinical practice and/or can be produced within hospital‐based manufacturing facilities impacting on the possible revenues. In this sense a clearer interaction in the relationship between public hospital/academia and private sector is requested under the umbrella of regulatory agencies within defined reimbursements policy and in the respect of intellectual property protection.
A strong MSCs therapy clinical translation cannot be realized without considering the relevant aspects of the product cycle and its related cost. Currently, the costs of MSCs manufacturing vary between 15,000 and 30,000 $/€ per 1–5 million cGMP‐MSCs per kilogram 189, 190. These manufacturing costs then have to be added to the expenses related to the clinical delivery and the follow up. In addition, when a gene vector has to be included in the manufacturing, numbers considerably increase. Although for a life‐saving cell‐based strategies a cost over 100,000 $/€ may be well tolerated 191, for treatments counteracting diseases with an impact in quality of life these number may be questionable by either private or public reimbursement bodies. This has been an aspect that additionally impacted on the solid translation of MSCs toward clinic. Therefore, efforts have to be made in minimizing the cost per unit of cells and ultimately the cost per treatments, while maintaining product quality. This could have a disruptive impact on the clinical need in particular for allogeneic MSCs therapy, which is a more commercially attractive option because it provides cells ready‐to‐use for many patients 192. In this scenario, the mentioned MSCs biological factors (i.e., tissue source, donor variability) have to be accounted since they may have a profound impact on manufacturing processes due to the expansion potential of the cells that is directly tied to the cost of production. Many efforts have been focused on the development of novel expansion technologies, to meet the needs of substantial cell numbers and the creation of a robust cell bank to supply cells for large number of recipients 193. The introduction of bioreactors providing billions of cells with consistent product quality and reproducibility has been recently proposed with promising results. Furthermore, clinically approved alternatives to animal sera for cell culture also based on blood‐derived materials, such as PL, and recombinant media have been successfully introduced. However, this may not be enough and a significant reduction of manufacturing costs will come from the decrease in quality control testing expenses conceiving novel assays that would maintain product quality while reducing the price and time for execution.
Conclusions and Future Perspectives
The excitement around MSCs research increased since their early identification generating a relatively rapid clinical translation. This promising race, that sounded like a possible solution for many diseases, clashed with evidence of a suboptimal performance. Future MSC‐based preclinical and clinical investigations should take into account a variety of issues that we here wanted to emphasize. In particular, considering (a) donor‐related features (age and comorbidities) that may impact on the quantity and quality of obtainable cells; (b) a better identification of the precise beneficial mechanism/s of action calling for ad hoc investigations also able to dissect MSCs complexity (i.e., stromal versus stem) and their interactions with host tissues; (c) the development of more robust pharmacodynamic and pharmacokinetic models to be applied in the different clinical situations; and (d) a selection of reagents and procedures that may be applied from preclinical to clinical developments aimed to maintain cell consistency and ultimately reduce manufacturing costs. To conclude, this review points out MSC strengths and weaknesses in order to generate a better awareness on the challenges that should be taken into account for more performing MSC‐based therapeutics.
Author Contributions
IM, EMF, AM, OC, AVM, GG, EV, EMH and MD participated in literature search, wrote the manuscript and prepared the tables; IM and MD conceived the manuscript concept.
Disclosure of Potential Conflicts of Interest
M.D. declared patent holder in the field of cell and gene therapy, a consultancy role, research funding, and stock ownership with Rigenerand srl. The other authors indicated no potential conflicts of interest.
Acknowledgments
This work is supported in parts by H2020 project Orthounion (Grant 733288), by “Progetto Dipartimenti Eccellenti 2017” from Ministero Istruzione Università Ricerca (MIUR).
References
- 1. Friedenstein AJ, Piatetzky‐Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966;16:381–390. [PubMed] [Google Scholar]
- 2. Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol 1976;47:327–359. [DOI] [PubMed] [Google Scholar]
- 3. Lanotte M, Metcalf D, Dexter TM. Production of monocyte/macrophage colony‐stimulating factor by preadipocyte cell lines derived from murine marrow stroma. J Cell Physiol 1982;112:123–127. [DOI] [PubMed] [Google Scholar]
- 4. Owen M, Friedenstein AJ. Stromal stem cells: Marrow‐derived osteogenic precursors. Ciba Found Symp 1988;136:42–60. [DOI] [PubMed] [Google Scholar]
- 5. Caplan AI. Mesenchymal stem cells. J Orthop Res 1991;9:641–650. [DOI] [PubMed] [Google Scholar]
- 6. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–74. [DOI] [PubMed] [Google Scholar]
- 7. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 8. D'souza N, Burns JS, Grisendi G et al. MSC and tumors: Homing, differentiation, and secretion influence therapeutic potential. Adv Biochem Eng Biotechnol 2013;130:209–266. [DOI] [PubMed] [Google Scholar]
- 9. Reyes M, Lund T, Lenvik T et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001;98:2615–2625. [DOI] [PubMed] [Google Scholar]
- 10. Jiang Y, Jahagirdar BN, Reinhardt RL et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 11. D'souza N, Rossignoli F, Golinelli G et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med 2015;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells 2007;25:2896–2902. [DOI] [PubMed] [Google Scholar]
- 13. Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy 2008;10:771–774. [DOI] [PubMed] [Google Scholar]
- 14. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witwer KW, Van Balkom BWM, Bruno S et al. Defining mesenchymal stromal cell (MSC)‐derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles 2019;8:1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spano C, Grisendi G, Golinelli G et al. Soluble TRAIL armed human MSC as gene therapy for pancreatic cancer. Sci Rep 2019;9:1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matthay MA, Pati S, Lee JW. Concise review: Mesenchymal stem (stromal) cells: Biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells 2017;35:316–324. [DOI] [PubMed] [Google Scholar]
- 18. Lykhmus O, Koval L, Voytenko L et al. Intravenously injected mesenchymal stem cells penetrate the brain and treat inflammation‐induced brain damage and memory impairment in mice. Front Pharmacol 2019;10:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 2011;9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mushahary D, Spittler A, Kasper C et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018;93:19–31. [DOI] [PubMed] [Google Scholar]
- 21. Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 22. Veronesi E, Murgia A, Caselli A et al. Transportation conditions for prompt use of ex vivo expanded and freshly harvested clinical‐grade bone marrow mesenchymal stromal/stem cells for bone regeneration. Tissue Eng Part C Methods 2014;20:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gómez‐Barrena E, Rosset P, Gebhard F et al. Feasibility and safety of treating non‐unions in tibia, femur and humerus with autologous, expanded, bone marrow‐derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non‐comparative trial. Biomaterials 2018;196:100–108. [DOI] [PubMed] [Google Scholar]
- 24. Wan C, He Q, McCaigue M et al. Nonadherent cell population of human marrow culture is a complementary source of mesenchymal stem cells (MSCs). J Orthop Res 2006;24:21–28. [DOI] [PubMed] [Google Scholar]
- 25. Leonardi E, Ciapetti G, Baglìo SR et al. Osteogenic properties of late adherent subpopulations of human bone marrow stromal cells. Histochem Cell Biol 2009;132:547–557. [DOI] [PubMed] [Google Scholar]
- 26. Di Maggio N, Mehrkens A, Papadimitropoulos A et al. Fibroblast growth factor‐2 maintains a niche‐dependent population of self‐renewing highly potent non‐adherent mesenchymal progenitors through FGFR2c. Stem Cells 2012;30:1455–1464. [DOI] [PubMed] [Google Scholar]
- 27. Baksh D, Davies JE, Zandstra PW. Adult human bone marrow‐derived mesenchymal progenitor cells are capable of adhesion‐independent survival and expansion. Exp Hematol 2003;31:723–732. [DOI] [PubMed] [Google Scholar]
- 28. Baksh D, Zandstra PW, Davies JE. A non‐contact suspension culture approach to the culture of osteogenic cells derived from a CD49elow subpopulation of human bone marrow‐derived cells. Biotechnol Bioeng 2007;98:1195–1208. [DOI] [PubMed] [Google Scholar]
- 29. Mehrkens A, Di Maggio N, Gueven S et al. Non‐adherent mesenchymal progenitors from adipose tissue stromal vascular fraction. Tissue Eng Part A 2014;20:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 31. Bourin P, Bunnell BA, Casteilla L et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mabuchi Y, Matsuzaki Y. Prospective isolation of resident adult human mesenchymal stem cell population from multiple organs. Int J Hematol 2016;103:138–144. [DOI] [PubMed] [Google Scholar]
- 33. Foster LJ, Zeemann PA, Li C et al. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells 2005;23:1367–1377. [DOI] [PubMed] [Google Scholar]
- 34. Leuning DG, Beijer NRM, du Fossé NA et al. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci Rep 2018;8:7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. da Silva ML, de Deus Wagatsuma VM, Malta TM et al. The gene expression profile of non‐cultured, highly purified human adipose tissue pericytes: Transcriptomic evidence that pericytes are stem cells in human adipose tissue. Exp Cell Res 2016;349:239–254. [DOI] [PubMed] [Google Scholar]
- 36. Candini O, Spano C, Murgia A et al. Mesenchymal progenitors aging highlights a miR‐196 switch targeting HOXB7 as master regulator of proliferation and osteogenesis. Stem Cells 2015;33:939–950. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen DC, Lewis HC, Joyner C et al. Extracellular vesicles from bone marrow‐derived mesenchymal stromal cells support ex vivo survival of human antibody secreting cells. J Extracell Vesicles 2018;7:1463778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christ B, Franquesa M, Najimi M et al. Cellular and molecular mechanisms of mesenchymal stem cell actions. Stem Cells Int 2017;2017:2489041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy 2015;17:125–127. [DOI] [PubMed] [Google Scholar]
- 40. Li J, Ezzelarab MB, Cooper DK. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation 2012;19:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hollenberg CH, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest 1969;47:2485–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest 1976;58:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hass R, Kasper C, Böhm S et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue‐derived MSC. Cell Commun Signal 2011;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abbas OL, Özatik O, Gönen ZB et al. Comparative analysis of mesenchymal stem cells from bone marrow, adipose tissue, and dental pulp as sources of cell therapy for zone of stasis burns. J Invest Surg 2018;14:1–14. [DOI] [PubMed] [Google Scholar]
- 45. Peng L, Jia Z, Yin X et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 2008;17:761–773. [DOI] [PubMed] [Google Scholar]
- 46. Kern S, Eichler H, Stoeve J et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 47. Stenderup K, Justesen J, Clausen C et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003;33:919–926. [DOI] [PubMed] [Google Scholar]
- 48. Gamelli RL, Paxton TP, O'Reilly M. Bone marrow toxicity by silver sulfadiazine. Surg Gynecol Obstet 1993;177:115–120. [PubMed] [Google Scholar]
- 49. Shoup M, Weisenberger JM, Wang JL et al. Mechanisms of neutropenia involving myeloid maturation arrest in burn sepsis. Ann Surg 1998;228:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nurgalieva Z, Liu CC, Du XL. Chemotherapy use and risk of bone marrow suppression in a large population‐based cohort of older women with breast and ovarian cancer. Med Oncol 2011;28:716–725. [DOI] [PubMed] [Google Scholar]
- 51. Bronckaers A, Hilkens P, Martens W et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther 2014;143:181–196. [DOI] [PubMed] [Google Scholar]
- 52. Kwon HM, Hur SM, Park KY et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol 2014;63:19–28. [DOI] [PubMed] [Google Scholar]
- 53. Anderson JD, Johansson HJ, Graham CS et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor‐kappab signaling. Stem Cells 2016;34:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lisini D, Nava S, Pogliani S et al. Adipose tissue‐derived mesenchymal stromal cells for clinical application: An efficient isolation approach. Curr Res Transl Med 2019;67:20–27. [DOI] [PubMed] [Google Scholar]
- 55. Kitagawa YKM, Korobi M, Toriyama K et al. History of discovery of human adipose‐derived stem cells and their clinical application. Jpn J Plast Reconstr Surg 2006;49:1097–1104. [Google Scholar]
- 56. Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 57. Gimble JM, Katz AJ, Bunnell BA. Adipose‐derived stem cells for regenerative medicine. Circ Res 2007;100:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katz AJ, Llull R, Hedrick MH et al. Emerging approaches to the tissue engineering of fat. Clin Plast Surg 1999;26:587–603. [PubMed] [Google Scholar]
- 59. Kuhbier JW, Weyand B, Radtke C et al. Isolation, characterization, differentiation, and application of adipose‐derived stem cells. Adv Biochem Eng Biotechnol 2010;123:55–105. [DOI] [PubMed] [Google Scholar]
- 60. Fraser JK, Wulur I, Alfonso Z et al. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006;24:150–154. [DOI] [PubMed] [Google Scholar]
- 61. Sabol RA, Bowles AC, Côté A et al. Therapeutic potential of adipose stem cells. Adv Exp Med Biol 2018. 10.1007/5584_2018_248. [DOI] [PubMed] [Google Scholar]
- 62. Sotiropoulou PA, Perez SA, Salagianni M et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006;24:462–471. [DOI] [PubMed] [Google Scholar]
- 63. Baer PC, Geiger H. Adipose‐derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int 2012;2012:812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol 2018;221:jeb162958. [DOI] [PubMed] [Google Scholar]
- 65. Pérez LM, Bernal A, de Lucas B et al. Altered metabolic and stemness capacity of adipose tissue‐derived stem cells from obese mouse and human. PLoS One 2015;10:e0123397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu L, Liu Y, Sun Y et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther 2017;8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fennema EM, Tchang LAH, Yuan H et al. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med 2018;12:e150–e158. [DOI] [PubMed] [Google Scholar]
- 68. Du WJ, Chi Y, Yang ZX et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther 2016;7:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Elman JS, Li M, Wang F et al. A comparison of adipose and bone marrow‐derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jin HJ, Bae YK, Kim M et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci 2013;14:17986–18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Choudhery MS, Badowski M, Muise A et al. Utility of cryopreserved umbilical cord tissue for regenerative medicine. Curr Stem Cell Res Ther 2013;8:370–380. [DOI] [PubMed] [Google Scholar]
- 72. Zhang X, Hirai M, Cantero S et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem 2011;112:1206–1218. [DOI] [PubMed] [Google Scholar]
- 73. Avanzini MA, Bernardo ME, Cometa AM et al. Generation of mesenchymal stromal cells in the presence of platelet lysate: A phenotypic and functional comparison of umbilical cord blood‐ and bone marrow‐derived progenitors. Haematologica 2009;94:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Capelli C, Gotti E, Morigi M et al. Minimally manipulated whole human umbilical cord is a rich source of clinical‐grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy 2011;13:786–801. [DOI] [PubMed] [Google Scholar]
- 75. Lu LL, Liu YJ, Yang SG et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis‐supportive function and other potentials. Haematologica 2006;91:1017–1026. [PubMed] [Google Scholar]
- 76. Pirjali T, Azarpira N, Ayatollahi M et al. Isolation and characterization of human mesenchymal stem cells derived from human umbilical cord Wharton's jelly and amniotic membrane. Int J Organ Transplant Med 2013;4:111–116. [PMC free article] [PubMed] [Google Scholar]
- 77. Hsieh JY, Wang HW, Chang SJ et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One 2013;8:e72604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chatzistamatiou TK, Papassavas AC, Michalopoulos E et al. Optimizing isolation culture and freezing methods to preserve Wharton's jelly's mesenchymal stem cell (MSC) properties: An MSC banking protocol validation for the Hellenic Cord Blood Bank. Transfusion 2014;54:3108–3120. [DOI] [PubMed] [Google Scholar]
- 79. Fong CY, Subramanian A, Biswas A et al. Freezing of fresh Wharton's Jelly from human umbilical cords yields high post‐thaw mesenchymal stem cell numbers for cell‐based therapies. J Cell Biochem 2016;117:815–827. [DOI] [PubMed] [Google Scholar]
- 80. Ercal P, Pekozer GG, Kose GT. Dental stem cells in bone tissue engineering: Current overview and challenges. Adv Exp Med Biol 2018;1107:113–127. [DOI] [PubMed] [Google Scholar]
- 81. d'Aquino R, Graziano A, Sampaolesi M et al. Human postnatal dental pulp cells co‐differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ 2007;14:1162–1171. [DOI] [PubMed] [Google Scholar]
- 82. Perry BC, Zhou D, Wu X et al. Collection, cryopreservation, and characterization of human dental pulp‐derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods 2008;14:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gronthos S, Mankani M, Brahim J et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2005;97:13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alraies A, Alaidaroos NY, Waddington RJ et al. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol 2017;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mead B, Logan A, Berry M et al. Paracrine‐mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose‐derived mesenchymal stem cells. PLoS One 2014;9:e109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yu S, Zhao Y, Ma Y et al. Profiling the secretome of human stem cells from dental apical papilla. Stem Cells Dev 2016;25:499–508. [DOI] [PubMed] [Google Scholar]
- 87. Zhang J, An Y, Gao LN et al. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012;33:6974–6986. [DOI] [PubMed] [Google Scholar]
- 88. Khanmohammadi M, Khanjani S, Edalatkhah H et al. Modified protocol for improvement of differentiation potential of menstrual blood‐derived stem cells into adipogenic lineage. Cell Prolif 2014;47:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rossignoli F, Caselli A, Grisendi G et al. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013;2013:901821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meng X, Ichim TE, Zhong J et al. Endometrial regenerative cells: A novel stem cell population. J Transl Med 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell‐based therapies. Expert Opin Biol Ther 2013;13:1387–1400. [DOI] [PubMed] [Google Scholar]
- 92. Patel AN, Park E, Kuzman M et al. Multipotent menstrual blood stromal stem cells: Isolation, characterization, and differentiation. Cell Transplant 2008;17:303–311. (Erratum in: Cell Transplant. 2008;17(6):721. Cell Transplant. 2008;17(7):875). [DOI] [PubMed] [Google Scholar]
- 93. Hida N, Nishiyama N, Miyoshi S et al. Novel cardiac precursor‐like cells from human menstrual blood‐derived mesenchymal cells. Stem Cells 2008;26:1695–1704. [DOI] [PubMed] [Google Scholar]
- 94. Zlatska AV, Rodnichenko AE, Gubar OS et al. Endometrial stromal cells: Isolation, expansion, morphological and functional properties. Exp Oncol 2017;39:197–202. [PubMed] [Google Scholar]
- 95. Lopez‐Verrilli MA, Caviedes A, Cabrera A et al. Mesenchymal stem cell‐derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016;320:129–139. [DOI] [PubMed] [Google Scholar]
- 96. Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril 2005;84:1124–1130. [DOI] [PubMed] [Google Scholar]
- 97. Chen J, Du X, Chen Q et al. Effects of donors' age and passage number on the biological characteristics of menstrual blood‐derived stem cells. Int J Clin Exp Pathol 2015;8:14584–14595. [PMC free article] [PubMed] [Google Scholar]
- 98. Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015;17:11–22. [DOI] [PubMed] [Google Scholar]
- 99. Murgia A, Veronesi E, Candini O et al. Potency biomarker signature genes from multiparametric osteogenesis assays: Will cGMP human bone marrow mesenchymal stromal cells make bone? PLoS One 2016;11:e0163629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sammour I, Somashekar S, Huang J et al. The effect of gender on mesenchymal stem cell (MSC) efficacy in neonatal hyperoxia‐induced lung injury. PLoS One 2016;11:e0164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhu M, Kohan E, Bradley J et al. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose‐derived stem cells. J Tissue Eng Regen Med 2009;3:290–301. [DOI] [PubMed] [Google Scholar]
- 102. Ogawa R, Mizuno H, Hyakusoku H et al. Chondrogenic and osteogenic differentiation of adipose‐derived stem cells isolated from GFP transgenic mice. J Nippon Med Sch 2004;71:240–241. [DOI] [PubMed] [Google Scholar]
- 103. Stolzing A, Jones E, McGonagle D et al. Age‐related changes in human bone marrow‐derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev 2008;129:163–173. [DOI] [PubMed] [Google Scholar]
- 104. Gersch RP, Lombardo F, McGovern SC et al. Reactivation of Hox gene expression during bone regeneration. J Orthop Res 2005;23:882–890. [DOI] [PubMed] [Google Scholar]
- 105. Leucht P, Kim JB, Amasha R et al. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 2008;135:2845–2854. [DOI] [PubMed] [Google Scholar]
- 106. Choudhery MS, Badowski M, Muise A et al. Donor age negatively impacts adipose tissue‐derived mesenchymal stem cell expansion and differentiation. J Transl Med 2014;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Coutu DL, Galipeau J. Roles of FGF signaling in stem cell self‐renewal, senescence and aging. Aging 2011;3:920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ferland‐McCollough D, Maselli D, Spinetti G et al. MCP‐1 feedback loop between adipocytes and mesenchymal stromal cells causes fat accumulation and contributes to hematopoietic stem cell rarefaction in the bone marrow of patients with diabetes. Diabetes 2018;67:1380–1394. [DOI] [PubMed] [Google Scholar]
- 109. Rezabakhsh A, Cheraghi O, Nourazarian A et al. Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over‐activity of the autophagic pathway. J Cell Biochem 2017;118:1518–1530. [DOI] [PubMed] [Google Scholar]
- 110. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van Rhijn‐Brouwer FCC, Gremmels H, Fledderus JO et al. Mesenchymal stromal cell characteristics and regenerative potential in cardiovascular disease: Implications for cellular therapy. Cell Transplant 2018;27:765–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Geissler PJ, Davis K, Roostaeian J et al. Improving fat transfer viability: The role of aging, body mass index, and harvest site. Plast Reconstr Surg 2014;134:227–232. [DOI] [PubMed] [Google Scholar]
- 113. Mitterberger MC, Mattesich M, Zwerschke W. Bariatric surgery and diet‐induced long‐term caloric restriction protect subcutaneous adipose‐derived stromal/progenitor cells and prolong their life span in formerly obese humans. Exp Gerontol 2014;56:106–113. [DOI] [PubMed] [Google Scholar]
- 114. Ulum B, Teker HT, Sarikaya A et al. Bone marrow mesenchymal stem cell donors with a high body mass index display elevated endoplasmic reticulum stress and are functionally impaired. J Cell Physiol 2018;233:8429–8436. [DOI] [PubMed] [Google Scholar]
- 115. Pérez LM, Bernal A, San Martín N et al. Obese‐derived ASCs show impaired migration and angiogenesis properties. Arch Physiol Biochem 2013;119:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ritter A, Louwen F, Yuan J. Deficient primary cilia in obese adipose‐derived mesenchymal stem cells: Obesity, a secondary ciliopathy? Obes Rev 2018;19:1317–1328. [DOI] [PubMed] [Google Scholar]
- 117. Bermeo S, Vidal C, Zhou H et al. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/β‐catenin pathway. J Cell Biochem 2015;116:2344–2353. [DOI] [PubMed] [Google Scholar]
- 118. Ruiz de Eguino G, Infante A, Schlangen K et al. Sp1 transcription factor interaction with accumulated prelamin a impairs adipose lineage differentiation in human mesenchymal stem cells: Essential role of sp1 in the integrity of lipid vesicles. Stem Cells Translational Medicine 2012;1:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Badraiq H, Cvoro A, Galleu A et al. Effects of maternal obesity on Wharton's jelly mesenchymal stromal cells. Sci Rep 2017;7:17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chao YH, Lin CW, Pan HH et al. Increased apoptosis and peripheral blood mononuclear cell suppression of bone marrow mesenchymal stem cells in severe aplastic anemia. Pediatr Blood Cancer 2018;65:e27247. [DOI] [PubMed] [Google Scholar]
- 121. Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: From genetics to management. Blood 2016;127:2072–2081. [DOI] [PubMed] [Google Scholar]
- 122. Pontikoglou C, Kalyva A, Kalpadakis C et al. Bone marrow‐derived mesenchymal stem/stromal cells from patients with splenic marginal zone lymphoma are intrinsically impaired and influence the malignant B‐cells. Leuk Lymphoma 2018;3:1–3. [DOI] [PubMed] [Google Scholar]
- 123. Bardelli D, Dander E, Bugarin C et al. Mesenchymal stromal cells from Shwachman‐Diamond syndrome patients fail to recreate a bone marrow niche in vivo and exhibit impaired angiogenesis. Br J Haematol 2018;182:114–124. [DOI] [PubMed] [Google Scholar]
- 124. Alongi DJ, Yamaza T, Song Y et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 2010;5:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nikoo S, Ebtekar M, Jeddi‐Tehrani M et al. Menstrual blood‐derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol Hum Reprod 2014;20:905–918. [DOI] [PubMed] [Google Scholar]
- 126. Tsuji W, Schnider JT, McLaughlin MM et al. Effects of immunosuppressive drugs on viability and susceptibility of adipose‐ and bone marrow‐derived mesenchymal stem cells. Front Immunol 2015;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pike S, Zhang P, Wei Z et al. In vitro effects of tamoxifen on adipose‐derived stem cells. Wound Repair Regen 2015;23:728–736. [DOI] [PubMed] [Google Scholar]
- 128. Varghese J, Griffin M, Mosahebi A et al. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res Ther 2017;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wu X, Wang X, Qien X et al. Four years' experience with the treatment of all‐trans retinoic acid in acute promielocitic leukemia. Am J Hematol 1993;43:183–189. [DOI] [PubMed] [Google Scholar]
- 130. Carlo‐Stella C, Tabilio A, Regazzi E et al. Effect of chemotherapy for acute myelogenous leukemia on hematopoietic and fibroblast marrow progenitors. BMT 1997;20:465–471. [DOI] [PubMed] [Google Scholar]
- 131. Poglio S, Galvani S, Bour S et al. Adipose tissue sensitivity to radiation exposure. Am J Pathol 2009;174:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Holan V, Cechova K, Zajicova A et al. The impact of morphine on the characteristics and function properties of human mesenchymal stem cells. Stem Cell Rev 2018;14:801–811. [DOI] [PubMed] [Google Scholar]
- 133. Efimenko AY, Kochegura TN, Akopyan ZA et al. Autologous stem cell therapy: How aging and chronic diseases affect stem and progenitor cells. Biores Open Access 2015;4:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Aguena M, Fanganiello RD, Tissiani LA et al. Optimization of parameters for a more efficient use of adipose‐derived stem cells in regenerative medicine therapies. Stem Cells Int 2012;2012:303610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Oberbauer E, Steffenhagen C, Wurzer C et al. Enzymatic and non‐enzymatic isolation systems for adipose tissue‐derived cells: Current state of the art. Cell Regen 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sutradhar BC, Park J, Hong G et al. Effects of trypsinization on viability of equine chondrocytes in cell culture. Park Vet J 2010;30:232–238. [Google Scholar]
- 137. Fakhri O, Tan RS, Short communications . The effect of trypsin on cell surface antigens. Cell Immunol 1975;15:452–456. [DOI] [PubMed] [Google Scholar]
- 138. Furcht LT, Wendelschafer‐Crabb G. Trypsin‐induced coordinate alterations in cell shape, cytoskeleton, and intrinsic membrane structure of contact‐inhibited cells. Exp Cell Res 1978;114:1–14. [DOI] [PubMed] [Google Scholar]
- 139. Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: State of the art and lipogems® technology development. Curr Stem Cell Rep 2016;2:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bianchi F, Maioli M, Leonardi E et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte‐like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013;22:2063–2077. [DOI] [PubMed] [Google Scholar]
- 141. Hendijani F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif 2017;50:e12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhu B, Murthy SK. Stem cell separation technologies. Curr Opin Chem Eng 2013;2:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Grisendi G, Annerén C, Cafarelli L et al. GMP‐manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion. Cytotherapy 2010;12:466–477. [DOI] [PubMed] [Google Scholar]
- 144. Müller W, Weichel W, Radbruch A et al. High gradient magnetic cell separation with MACS. Cytometry 1990;11:231–238. [DOI] [PubMed] [Google Scholar]
- 145. Chippendale TWE, Coopman K, Rafiq Q et al. Isolation of mesenchymal stem cells from bone marrow aspirate. Comprehensive Biotechnology: Volume 5, Medical Biotechnology and Healthcare. 2nd Edition Pergamon 2011:115–123. [Google Scholar]
- 146. Jung S, Panchalingam KM, Rosenberg L et al. Ex vivo expansion of human mesenchymal stem cells in defined serum‐free media. Stem Cells Int 2012;2012:123030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Both SK, van der Muijsenberg AJ, van Blitterswijk CA et al. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng 2007;13:3–9. [DOI] [PubMed] [Google Scholar]
- 148. Wagner W, Bork S, Horn P et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One 2009;4:e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Egermann M, Heil P, Tami A et al. Assessment of in vitro aging of mesenchymal stem cell. J Orthop Res 2010;28:798–804. [DOI] [PubMed] [Google Scholar]
- 150. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009;4:206–216. [DOI] [PubMed] [Google Scholar]
- 151. Jung S, Panchalingam KM, Wuerth RD et al. Large‐scale production of human mesenchymal stem cells for clinical applications. Biotechnol Appl Biochem 2012;59:106–120. [DOI] [PubMed] [Google Scholar]
- 152. Chen AK, Reuveny S, Oh SK. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnol Adv 2013;31:1032–1046. [DOI] [PubMed] [Google Scholar]
- 153. Elseberg CL, Salzig D, Czermak P. Bioreactor expansion of human mesenchymal stem cells according to GMP requirements. Methods Mol Biol 2015;1283:199–218. [DOI] [PubMed] [Google Scholar]
- 154. Neumann A, Lavrentieva A, Egger D et al. Approaches for automized expansion and differentiation of human MSC in specialized bioreactors. BMC Proc 2013;7:P47. [Google Scholar]
- 155. Tateishi K, Ando W, Higuchi C et al. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: Potential feasibility for clinical applications. Cell Transplant 2008;17:549–557. [DOI] [PubMed] [Google Scholar]
- 156. Watatani S, Terashi H, Saigo K et al. Autologous platelet‐rich plasma (PRP) is more useful than fetal calf serum (FCS) in adipose‐derived stem cells (ADSCS) culture for bone regeneration. Int J Oral Maxillofac Surg 2005;34:123. [Google Scholar]
- 157. Lee JY, Nam H, Park YJ et al. The effects of platelet‐rich plasma derived from human umbilical cord blood on the osteogenic differentiation of human dental stem cells. In Vitro Cell Dev Biol Anim 2011;47:157–164. [DOI] [PubMed] [Google Scholar]
- 158. Solchaga LA, Penick K, Goldberg VM et al. Fibroblast growth factor‐2 enhances proliferation and delays loss of chondrogenic potential in human adult bone‐marrow‐derived mesenchymal stem cells. Tissue Eng 2010;16:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol 2010;2010:795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cuende N, Rasko JEJ, Koh MBC et al. Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy 2018;20:1401–1413. [DOI] [PubMed] [Google Scholar]
- 161. Lalu MM, McIntyre L, Pugliese C et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta‐analysis of clinical trials. PLoS One 2012;7:e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. García‐Castro J, Singeç I. Prospects of pluripotent and adult stem cells for rare diseases. Adv Exp Med Biol 2017;1031:371–386. [DOI] [PubMed] [Google Scholar]
- 163. Karussis D, Karageorgiou C, Vaknin‐Dembinsky A et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010;67:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yamout B, Hourani R, Salti H et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J Neuroimmunol 2010;227:185–189. [DOI] [PubMed] [Google Scholar]
- 165. Dulamea A. Mesenchymal stem cells in multiple sclerosis—Translation to clinical trials. J Med Life 2015;8:24–27. [PMC free article] [PubMed] [Google Scholar]
- 166. Osaka M, Honmou O, Murakami T et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res 2010;1343:226–235. [DOI] [PubMed] [Google Scholar]
- 167. Harting MT, Jimenez F, Xue H et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 2009;110:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gao J, Dennis JE, Muzic RF et al. The dynamic in vivo distribution of bone marrow‐derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001;169:12–20. [DOI] [PubMed] [Google Scholar]
- 169. Fischer UM, Harting MT, Jimenez F et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first‐pass effect. Stem Cells Dev 2009;18:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. de Witte SFH, Luk F, Sierra Parraga JM et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 2018;36:602–615. [DOI] [PubMed] [Google Scholar]
- 171. Galleu A, Riffo‐Vasquez Y, Trento C et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient‐mediated immunomodulation. Sci Transl Med 2017;9:416. [DOI] [PubMed] [Google Scholar]
- 172. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 2010;12:87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Salvadori M, Cesari N, Murgia A, Puccini P, Riccardi B, Dominici M Dissecting the pharmacodynamics and pharmacokinetics of multipotent mesenchymal stromal/stem cells to overcome limitations in their clinical translations. Mol Ther Methods Clin Dev 2019;14: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Barbash IM, Chouraqui P, Baron J et al. Systemic delivery of bone marrow‐derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003;108:863–868. [DOI] [PubMed] [Google Scholar]
- 175. Luger D, Lipinski MJ, Westman PC et al. Intravenously delivered mesenchymal stem cells: Systemic anti‐inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res 2017;120:1598–1613. [DOI] [PubMed] [Google Scholar]
- 176. Sheng CC, Zhou L, Hao J. Current stem cell delivery methods for myocardial repair. Biomed Res Int 2013;2013:547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhang J, Wu Y, Chen A et al. Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cell Physiol Biochem 2015;35:1219–1229. [DOI] [PubMed] [Google Scholar]
- 178. Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res Ther 2016;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Steinert AF, Rackwitz L, Gilbert F et al. Concise review: The clinical application of mesenchymal stem cells for musculoskeletal regeneration: Current status and perspectives. Stem Cells Translational Medicine 2012;1:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Centeno C, Pitts J, Al‐Sayegh H et al. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int 2014;2014:370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Quarto R, Mastrogiacomo M, Cancedda R et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001;344:385–386. [DOI] [PubMed] [Google Scholar]
- 182. Whyte MP, Kurtzberg J, McAlister WH et al. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res 2003;18:624–636. [DOI] [PubMed] [Google Scholar]
- 183. http://www.clinicaltrials.gov/
- 184. Watson L, Elliman SJ, Coleman CM. From isolation to implantation: A concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res Ther 2014;5:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Horwitz EM, Gordon PL, Koo WK et al. Isolated allogeneic bone marrow‐derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA 2002;99:8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Otsuru S, Gordon PL, Shimono K et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012;120:1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Otsuru S, Desbourdes L, Guess AJ et al. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy 2018;20:62–73. [DOI] [PubMed] [Google Scholar]
- 188. Panés J, García‐Olmo D, Van Assche G et al. Long‐term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn's disease. Gastroenterology 2018;154:1334.e4–1342.e4. [DOI] [PubMed] [Google Scholar]
- 189. Lipsitz YY, Milligan WD, Fitzpatrick I et al. A roadmap for cost‐of‐goods planning to guide economic production of cell therapy products. Cytotherapy 2017;19:1383–1391. [DOI] [PubMed] [Google Scholar]
- 190. Bandeiras C, Koc JR, Ma Y et al. Cost effectiveness analysis of allogeneic, just‐in‐time expansion of mesenchymal stem cells with plustm human platelet lysate for a clinical trial. Cytotherapy 2018;20:S60. [Google Scholar]
- 191. Sarkar RR, Gloude NJ, Schiff D et al. Cost‐effectiveness of chimeric antigen receptor T‐cell therapy in pediatric relapsed/refractory B‐cell acute lymphoblastic leukemia. J Natl Cancer Inst 2018;111:djy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jossen V, van den Bos C, Eibl R et al. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl Microbiol Biotechnol 2018;102:3981–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Harrison RP, Medcalf N, Rafiq QA. Cell therapy‐processing economics: Small‐scale microfactories as a stepping stone toward large‐scale macrofactories. Regen Med 2018;13:159–173. [DOI] [PubMed] [Google Scholar]


