Abstract
This study aimed to determine whether autologous orthobiologic tissue source affects pain and functional outcomes in patients with symptomatic knee osteoarthritis (OA) who received microfragmented adipose tissue (MFAT) or bone marrow aspirate concentrate (BMAC) injection. We retrospectively reviewed prospectively collected data from patients who received BMAC or MFAT injection for symptomatic knee OA. Patients completed baseline and follow‐up surveys. Each survey included the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire, Emory Quality of Life (EQOL) questionnaire, and Visual Analog Scale (VAS) for pain. The follow‐up responses were compared with baseline for all patients and between BMAC and MFAT groups. A total of 110 patients met inclusion criteria, with 76 patients (BMAC 41, MFAT 35) and 106 knees (BMAC 58, MFAT 48) having appropriate follow‐up data. The BMAC group included 17 females and 24 males, with a mean age of 59 ± 11 years. The MFAT group included 23 females and 12 males, with a mean age of 63 ± 11 years. Minimum follow‐up time was 0.5 years. Mean follow‐up time was 1.80 ± 0.88 years for BMAC and 1.09 ± 0.49 years for MFAT. Both groups had significant improvement in EQOL, VAS, and all KOOS parameters preprocedure versus postprocedure (p < .001). There was not a significant difference when comparing postprocedure scores between groups (p = .09, .38, .63, .94, .17, .15, .70, respectively). These data demonstrate significant improvement in pain and function with both MFAT and BMAC injections in patients with symptomatic knee OA without a significant difference in improvement when comparing the two autologous tissue sources. stem cells translational medicine 2019;8:1149–1156
Keywords: Adipose stem cells, Adipose, Adult stem cells, Bone marrow, Mesenchymal stem cells, Stem cells, Arthritis
Lessons Learned.
There were significant improvements in pain and function with both microfragmented adipose tissue and bone marrow aspirate concentrate injections in patients with symptomatic knee osteoarthritis (OA), without a significant difference in improvement when comparing the two autologous tissue sources.
The results are important in that they will help us provide outcome knowledge to future patients.
This will help guide patients toward the best treatment possible for symptomatic knee OA.
Significance Statement.
This is the first study of its kind to compare clinical outcomes between autologous orthobiologic tissue sources regardless of orthopedic condition. Furthermore, most nonoperative treatments for knee osteoarthritis provide only short‐term improvement. These data show that both microfragmented adipose tissue and bone marrow aspirate concentrate injections provide prolonged improvement (>1 year) in pain and function without a difference in the extent of improvement when comparing tissue source.
Introduction
Osteoarthritis (OA) is a chronic degenerative disease of articular cartilage that is the leading cause of joint disease in the United States. The Centers for Disease Control currently estimates that OA affects over 30 million U.S. adults 1, with an associated treatment cost of $185.5 billion per year 2. Its incidence has doubled in women and tripled in men over the last several decades 3. Knee OA accounts for over 80% of the diseases total burden 4 and affects at least 19% of the U.S. population aged 45 or older 5. Risk factors for OA include age, obesity, trauma, genetics, muscle weakness, prior surgery, and repetitive use among others 6, 7, 8, 9. Age and obesity are consistently correlated with the prevalence of OA, with one epidemiological study reporting that evidence of OA can be found in 44% and 42.6% of males and females over the age of 30, respectively 10. Furthermore, Goulston et al. found that elevated body mass index (BMI) was an independent risk factor for knee pain at baseline and 15 year follow‐up 11. Given this information, treatment options for OA will become more coveted as obesity rates continue to rise and as the baby boomer generation, that reached a mean age of 65 years in 2011, continues to age in the United States 12.
At this time, conventional conservative interventions are often not effective in preventing the progression of OA or providing long‐term improvements in pain and function 13. Traditional treatment recommendations for patients with knee OA include weight loss, physical therapy, acetaminophen, non‐steroidal anti inflammatory drug (NSAIDs), corticosteroid injections, hyaluronic acid injections, bracing, and orthotics among others. Recent studies have examined the effect size (ES) of some of these treatments. ES is a reporting measure to quantify treatment efficacy. The closer the ES is to 1, the greater the efficacy. The ES for various conservative therapies for knee OA is 0.1 for therapeutic ultrasound, 0.2 for acetaminophen and arthroscopic debridement/lavage, 0.3 for NSAIDs and knee strengthening exercises, and 0.6 for short‐term relief with intra‐articular hyaluronic acid injections 14. Therefore, there is a definitive treatment gap for those with symptomatic knee OA who have failed these short‐term conservative measures and who either refuse or are not appropriate candidates for total knee arthroplasty. It is estimated that 3.6 million Americans are in this treatment gap at any given time and that for each patient, this gap endures for an average of 20 years. This prolonged treatment gap, where the patient suffers significant pain and spends significant economic resources, highlights the need for other nonsurgical options that can provide longer‐term relief and can slow the progression of OA. These options need to have excellent safety profiles and high patient acceptance 15. Recent advances in cellular based therapies provide a promising treatment option to fill in this gap moving forward.
Orthobiologic injections that include mesenchymal stem cells (MSCs) as effector cells have recently been applied for the treatment of OA. Autologous tissue sources have traditionally been the referred source for orthopedic use, with the most common sources being bone marrow (BM) and adipose tissue (AT) given ease of accessibility. The use of autologous orthobiologic therapies in the treatment of OA has been determined to be safe in a number of different studies 16, with a large multicenter prospective analysis demonstrating no increased risk of neoplasm 17.
The investigation of intra‐articular autologous bone marrow aspirate concentrate (BMAC) and microfragmented AT (MFAT) injections for the treatment of OA has increased in recent years, yet there remains a tremendous need for further data and well‐conducted trials. Furthermore, there are no published studies that directly compare outcomes between these two autologous tissue sources. Therefore, the purpose of this study is to determine whether autologous BMAC or MFAT injections provide significant pain and functional improvements in patients with symptomatic knee OA and whether outcomes differ between tissue sources. We expect that both treatments will provide significant improvements in pain and function.
Materials and Methods
We retrospectively reviewed prospectively collected data from patients who received BMAC or MFAT injections for symptomatic knee OA. Patients completed baseline and follow‐up surveys. Surveys included the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire, Emory Quality of Life (EQOL) questionnaire, and Visual Analog Pain Scale (VAS). Follow‐up responses were compared with baseline in all patients. Follow‐up responses were also compared between BMAC and MFAT groups. Baseline x‐ray (XR) or magnetic resonance imaging (MRI) imaging was evaluated for severity using the Kellgren–Lawrence (KL) grading criteria.
The BMAC procedure protocol was as follows: during the preoperative period, the patient again reviewed the procedure and gave verbal and written consent. After sterile prep with betadine/chloraprep and a sterile drape applied, the posterior superior iliac spine (PSIS) was anesthetized with 10 cc of lidocaine 1% and 10 cc of Marcaine 0.25%. Following this, 60 cc of bone marrow aspirate (BMA) was harvested from the patient's unilateral PSIS using ultrasound guidance. Three separate sites on the PSIS were used with 10 cc syringes (20 cc from each site). The BMA was then processed on location in an Emcyte centrifuge, and approximately 8 cc of pure BMAC was obtained. The patient was then positioned supine on the table and the knee to be treated was prepped in a sterile manner using betadine/chloraprep. A minimal amount of local anesthesia, ropivacaine 0.2%, was used superficially and down to the joint capsule only as necessary. The BM concentrate was then injected to the patient's knee joint using ultrasound guidance to ensure proper placement. The procedure took 60 minutes to complete, including the harvesting and creation of the BM concentrate.
The MFAT procedure protocol was as follows: the patient was evaluated in the supine position to determine the harvest site, which was then marked. The patient remained in the supine position with attention to the lower abdomen. The skin was cleansed using betadine/chloraprep. A 25 g × 2 in. needle was used to anesthetize the superficial skin using 5–10 cc of 1% lidocaine. A small incision was then made using a #11 scalpel. A blunt tip anesthesia cannula was used with a 60‐cc syringe to infiltrate 120 cc of tumescent anesthesia to the AT of the abdomen through the previously made incision. The cannula was directed toward the umbilicus and then, if necessary, posteriorly toward the flank through the same incision site. The lipoaspirate cannula was then placed through the incision site and 30 cc of lipoaspirate was obtained through low‐pressure vacuum. Care was taken to avoid any air in the syringe. Approximately 30 cc of lipoaspirate in a syringe was placed in a sterile cup to decant. The tumescent anesthesia was removed and care was used to avoid any air to the graft. The lipoaspirate was then transferred to the Lipogems manual processing device to wash and mechanically breakdown to allow injection. The final product was placed in 3‐cc syringes for the treatment. The puncture site was dressed with 4 × 4 sterile gauze and Tegaderm. Attention was then turned to the treatment. A minimal amount of local anesthesia, ropivacaine 0.2%, was used superficially and down to the joint capsule only as necessary. Under sterile condition, 9 cc of MFAT was injected into the knee joint. Ultrasound guidance was used to ensure proper placement. The procedure took 60 minutes to complete, including the harvesting and creation of MFAT.
A repeated‐measures analysis of VAS pain score was performed with a means model via the SAS MIXED Procedure (version 9.4; SAS Institute, Cary, NC), providing separate estimates of the means by time on study (preprocedure and postprocedure) and treatment group (BMAC or MFAT). The statistical model included three predictors (treatment arm, time on study [categorical as preprocedure and postprocedure] and the statistical interaction between treatment arm and time on study). A compound‐symmetric variance–covariance form in repeated measurements was assumed for VAS pain score and robust estimates of the standard errors of parameters were used to perform statistical tests and construct 95% confidence intervals 18. The model‐based means are unbiased with unbalanced and missing data, so long as the missing data are noninformative (missing at random). A p‐value ≤.05 was considered statistically significant for the main effects (treatment and time on study) and for the treatment by time on study interaction effect from the repeated‐measures analysis.
The statistical test for interaction between time on study and treatment was used as the overall hypothesis test to determine whether pain scores in the two study groups changed in significantly different ways during follow‐up (i.e., different temporal patterns over time). If mean pain scores in the two treatment groups were consistently different or similar over time (i.e., no statistical interaction) then the main effect test for treatment was used as the primary hypothesis test to compare the two treatment groups (i.e., the time‐averaged differences between the two treatment groups). The primary study results from this model were the mean VAS pain score and 95% confidence interval for each of the two treatment groups and the treatment mean difference and 95% confidence intervals. If a significant interaction was detected, then t tests were used to compare the differences between the model‐based treatment means at each time point and to compare differences over time within each treatment arm. Specific statistical tests were done within the framework of the mixed effects linear model. All statistical tests were two‐sided and unadjusted for multiple comparisons. For multivariable analysis, the VAS pain score repeated‐measures model was refitted including the following baseline covariates: treatment, time on study, the interaction between treatment and time on study, location of pain (left or right), KL grading scale (grades 1–4), the interaction between KL grading scale and treatment, sex, and age (above or below the median age).
Similar analysis plans were implemented for the five KOOS subscales and the EQOL outcome scores.
Results
A total of 110 patients met inclusion criteria, with 76 patients (BMAC 41, MFAT 35) and 106 knees (BMAC 58, MFAT 48) having appropriate follow‐up data. BMAC group included 17 females and 24 males with a mean age of 59 ± 1. The MFAT group included 23 females and 12 males with a mean age of 63 ± 11. Minimum follow‐up time was 0.5 years in both groups. Mean follow‐up time was 1.80 ± 0.88 years for BMAC and 1.09 ± 0.49 years for MFAT.
Appropriate preprocedure imaging (MRI or XR) was available for 101 of the 106 treated knees upon chart review. KL grading was as follows: grade 1: 5 knees, grade 2: 23 knees, grade 3: 55 knees, and grade 4: 18 knees. We then further identified each knee as a “responder” or “nonresponder.” A “responder” was defined as a knee with at least a 25% improvement in VAS pain score at final follow‐up post procedure. Peerbooms et al. previously used the 25% responder rate in the orthobiologics literature 19. The responder rate by KL grade was as follows: grade 1: 100% (5/5), grade 2: 73.9% (17/23), grade 3: 47.2% (26/55), and grade 4: 55.6% (10/18; Fig. 1). In the responder cohort, the average percent improvement for the BMAC group was 78% and 69% for MFAT. The combined reduction in VAS for the responder cohort was 73%.
Figure 1.
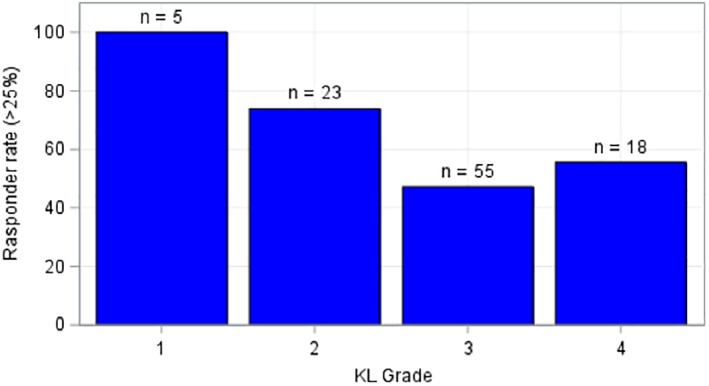
Responder rate by Kellgren–Lawrence grade.
Knee VAS pain scores in the two treatment groups changed in similar ways (similar temporal patterns of reduced pain over time) during follow‐up (p = .89, test for interaction between time on study and treatment group). Mean VAS in the two treatment groups was similar (p = .38 for testing the time‐averaged differences between the two treatment groups). The overall decrease during follow‐up in VAS pain scores was significant (p < .001, the two treatment groups improved in VAS pain scores over time). The decrease in mean VAS pain score was 1.5 points (mean = 4.1 and 2.6 at preprocedure and postprocedure). Both treatment groups (BMAC and MFAT) demonstrated significant improvement pre to postprocedure in VAS pain scores (p < .001; Table 1). Previously, it has been reported that a change of 8–10 points represents the minimal perceptible clinical improvement for all KOOS parameters 20.
Table 1.
Univariable repeated‐measures analyses: Longitudinal change in functional outcomes by treatment group for symptomatic knee osteoarthritis patients
| Treatment | Preprocedure | Postprocedure | p values | Change (pre–post) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | n | Mean ± SEM | Treat | Time | Treat by time | Mean change (95% CI) | p | ||
| VAS for pain | ||||||||||
| Pain | BMAC | 41 | 3.9 ± 0.355 | 40 | 2.5 ± 0.351 | .38 | <.01 | .89 | 1.502 (0.780, 2.223) | <.01 |
| MFAT | 35 | 4.3 ± 0.385 | 35 | 2.8 ± 0.376 | – | – | – | 1.411 (0.635, 2.186) | <.01 | |
| Emory quality of life | ||||||||||
| Mobility | BMAC | 39 | 1.694 ± 0.074 | 39 | 1.407 ± 0.080 | .45 | <.01 | .65 | 0.287 (0.103, 0.470) | <.01 |
| MFAT | 32 | 1.732 ± 0.082 | 32 | 1.508 ± 0.088 | – | – | – | 0.223 (0.019, 0.428) | .03 | |
| Self‐care | BMAC | 39 | 1.187 ± 0.057 | 39 | 1.050 ± 0.037 | .50 | .06 | .30 | 0.137 (0.015, 0.259) | .03 |
| MFAT | 32 | 1.102 ± 0.062 | 32 | 1.061 ± 0.041 | – | – | – | 0.041 (‑0.094, 0.176) | .55 | |
| Usual activities | BMAC | 39 | 1.646 ± 0.083 | 38 | 1.494 ± 0.094 | .05 | .02 | .65 | 0.152 (‑0.071, 0.375) | .18 |
| MFAT | 32 | 1.887 ± 0.091 | 32 | 1.659 ± 0.102 | – | – | – | 0.228 (‑0.016, 0.473) | .07 | |
| Pain/discomfort | BMAC | 39 | 2.056 ± 0.079 | 38 | 1.735 ± 0.089 | .28 | <.01 | .51 | 0.321 (0.129, 0.512) | <.01 |
| MFAT | 32 | 2.215 ± 0.087 | 32 | 1.799 ± 0.097 | – | – | – | 0.416 (0.205, 0.627) | <.01 | |
| Anxiety | BMAC | 39 | 1.210 ± 0.070 | 37 | 1.164 ± 0.079 | .24 | .49 | .98 | 0.047 (‑0.129, 0.222) | .60 |
| MFAT | 32 | 1.314 ± 0.077 | 32 | 1.271 ± 0.085 | – | – | – | 0.043 (‑0.148, 0.235) | .65 | |
| Composite | BMAC | 39 | 0.727 ± 0.027 | 37 | 0.835 ± 0.027 | .09 | <.01 | .98 | ‑0.108 (‑0.162, ‑0.054) | <.01 |
| MFAT | 32 | 0.667 ± 0.030 | 32 | 0.774 ± 0.029 | – | – | – | ‑0.107 (‑0.166, ‑0.047) | <.01 | |
| Knee Injury and Osteoarthritis Score | ||||||||||
| Pain | BMAC | 39 | 54.6 ± 2.674 | 40 | 70.6 ± 3.313 | .63 | <.01 | .56 | ‑16.0 (‑23.1, ‑8.9) | <.01 |
| MFAT | 34 | 51.4 ± 2.867 | 32 | 70.4 ± 3.692 | – | – | – | ‑19.1 (‑26.9, ‑11.2) | <.01 | |
| Symptoms | BMAC | 39 | 53.7 ± 2.992 | 40 | 69.4 ± 3.702 | .94 | <.01 | .61 | ‑15.7 (‑23.2, ‑8.1) | <.01 |
| MFAT | 34 | 54.9 ± 3.209 | 32 | 67.6 ± 4.120 | – | – | – | ‑12.7 (‑21.1, ‑4.4) | <.01 | |
| ADL | BMAC | 39 | 63.6 ± 2.946 | 40 | 79.2 ± 3.053 | .17 | <.01 | .58 | ‑5.5 (‑22.5, ‑8.6) | <.01 |
| MFAT | 33 | 57.2 ± 3.202 | 32 | 75.6 ± 3.401 | – | – | – | ‑18.4 (‑26.1, ‑10.7) | <.01 | |
| Sport/recreation | BMAC | 29 | 28.6 ± 4.224 | 26 | 56.1 ± 5.831 | .15 | <.01 | .79 | ‑27.5 (‑40.2, ‑14.8) | <.01 |
| MFAT | 24 | 21.3 ± 4.634 | 22 | 46.3 ± 6.333 | – | – | – | ‑25.0 (‑39.0, ‑11.0) | <.01 | |
| QOL | BMAC | 39 | 28.6 ± 2.995 | 40 | 52.0 ± 3.858 | .70 | <.01 | .45 | ‑23.4 (‑31.7, ‑15.1) | <.01 |
| MFAT | 32 | 29.4 ± 3.302 | 32 | 48.0 ± 4.303 | – | – | – | ‑18.6 (‑27.9, ‑9.3) | <.01 | |
Abbreviations: ADL, activities of daily living; BMAC, bone marrow aspirate concentrate; CI, confidence interval; MFAT, microfragmented adipose tissue; QOL, quality of life; VAS, Visual Analog Scale.
Knee KOOS pain scores in the two treatment groups changed in similar ways (similar temporal patterns reduced pain over time) during follow‐up (p = .56, test for interaction between time on study and treatment group). Mean KOOS pain scores in the two treatment groups were similar (p = .63 for testing the time‐averaged differences between the two treatment groups). The overall improvement during follow‐up in KOOS pain scores was significant (p < .001, the two treatment groups improved in KOOS pain scores over time). The mean KOOS pain score improvement was 17.5 points (mean = 53.0 and 70.5 at preprocedure and postprocedure). Both treatment groups demonstrated significant improvement preprocedure to postprocedure in KOOS pain scores (p < .001; Table 1, Fig. 2).
Figure 2.
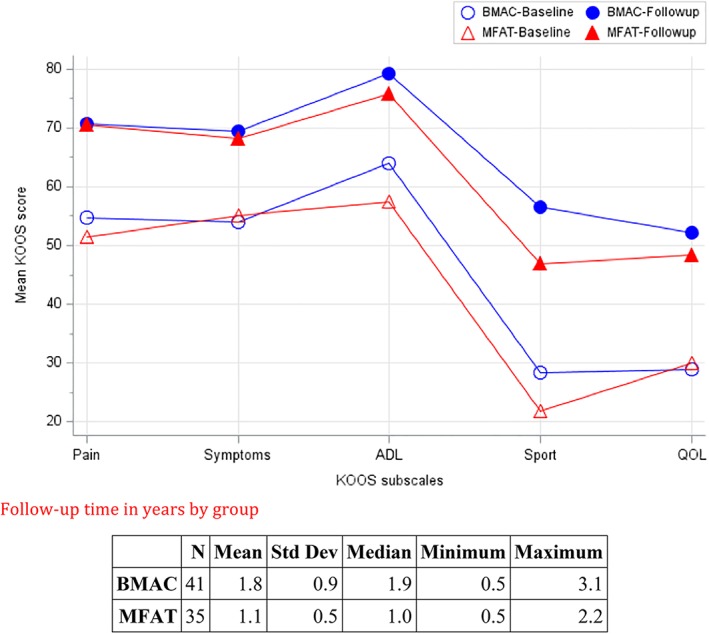
Knee Injury and Osteoarthritis Outcome Score at baseline and follow‐up.
Knee KOOS other symptom scores in the two treatment groups changed in similar ways (similar temporal patterns of reduced other symptoms over time) during follow‐up (p = .61, test for interaction between time on study and treatment group). Mean KOOS symptom scores in the two treatment groups were similar (p = .94 for testing the time‐averaged differences between the two treatment groups). The overall decrease during follow‐up in KOOS symptom scores was significant (p < .001, the two treatment groups improved in KOOS symptom scores over time). The mean KOOS symptom score improvement was 14.0 points (mean = 54.0 and 68.0 at preprocedure and postprocedure). Both treatment groups demonstrated significant improvement preprocedure to postprocedure in KOOS symptom scores (p < .001 for BMAC and p = .003 for MFAT; Table 1, Fig. 2).
KOOS activities of daily living (ADL) scores in the two treatment groups changed in similar ways (similar temporal patterns of improved ADL over time) during follow‐up (p = .58, test for interaction between time on study and treatment group). Mean KOOS ADL scores in the two treatment groups were similar (p = .17 for testing the time‐averaged differences between the two treatment groups). The overall improvement during follow‐up in KOOS ADL scores was significant (p < .001, the two treatment groups improved in KOOS ADL scores over time). The mean KOOS ADL score improvement was 17.0 points (mean = 60.4 and 77.4 at preprocedure and postprocedure). Both treatment groups demonstrated significant improvement preprocedure to postprocedure in KOOS ADL scores (p < .001 for BMAC and p = .001 for MFAT; Table 1, Fig. 2).
KOOS sport and recreation scores in the two treatment groups changed in similar ways (similar temporal patterns of improvement over time) during follow‐up (p = .79, test for interaction between time on study and treatment group). Mean KOOS sport and recreation scores in the two treatment groups were similar (p = .15 for testing the time‐averaged differences between the two treatment groups). The overall improvement during follow‐up in KOOS sport and recreation scores was significant (p < .001, the two treatment groups improved in KOOS sport and recreation scores over time). The mean KOOS sport and recreation score improvement was 26.3 points (mean = 24.9 and 51.2 at preprocedure and postprocedure). Both treatment groups demonstrated significant improvement preprocedure to postprocedure in KOOS sport scores (p < .001; Table 1, Fig. 2).
KOOS knee‐related quality of life (QOL) scores in the two treatment groups changed in similar ways (similar temporal patterns of improvement over time) during follow‐up (p = .45, test for interaction between time on study and treatment group). Mean KOOS QOL scores in the two treatment groups were similar (p = .70 for testing the time‐averaged differences between the two treatment groups). The overall improvement during follow‐up in KOOS QOL scores was significant (p < .001, the two treatment groups improved in KOOS QOL scores over time). The mean KOOS QOL score improvement was 21.0 points (mean = 29.0 and 50.0 at preprocedure and postprocedure). Both treatment groups demonstrated significant improvement preprocedure to postprocedure in KOOS QOL scores (p < .001; Table 1, Fig. 2).
EQOL scores in the two treatment groups changed in similar ways (similar temporal patterns over time) during follow‐up (p = .98, test for interaction between time on study and treatment group). Mean EQOL in the two treatment groups were similar (p = .09 for testing the time‐averaged differences between the two treatment groups). The overall increase during follow‐up in EQOL scores was significant (p < .001, the two treatment groups improved in EQOL scores over time). The mean EQOL score improvement was 0.10 points (mean = 0.70 and 0.80 at preprocedure and postprocedure).
Both treatment groups demonstrated significant improvement pre to post in EQOL (p < .001; Table 1).
Discussion
The purpose of this study was to compare outcomes after MFAT or BMAC injections in individuals with symptomatic knee osteoarthritis. Regardless of tissue source, our data demonstrate that there are statistically significant improvements in pain and function after autologous orthobiologic injection for knee OA. For the purpose of this discussion and to fully review the history of these injections, we have included data from a breadth of BM and AT‐derived orthobiologic literature, much of it from culture‐expanded MSC products. It should be noted that the preparations we used are, at best, MSC enriched and it is difficult to directly compare our data with that of culture‐expanded populations.
Centeno et al. first examined autologous BM‐derived orthobiologics for knee OA in two case studies conducted in 2008 21, 22. Since that time, multiple investigators have further studied the safety and efficacy of autologous BM‐derived injections for knee OA 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33. The study from Shapiro et al. is particularly noteworthy in that it is the first prospective, placebo‐controlled trial of BMAC treatment for knee OA against a proposed saline placebo. Safety outcomes, pain relief, and function were measured at 1 week, 3 months, and 6 months postintervention. Results revealed substantial pain relief in both BMAC and saline treated knees; however, there was no statistically significant difference between the groups. This study is often cited as evidence against the use of autologous BMAC; however, limitations to this study include short follow‐up period, low number of patients, high variability in MSCs and effector cells that were injected, possible systemic effects of MSCs that may have affected the saline injected knee, as well as the fact that normal saline is not a true placebo as multiple level I studies have described the therapeutic effects of intra‐articular normal saline injections for knee OA 34. The Shapiro study does further expand the research demonstrating that autologous BM‐derived orthobiologics are safe and can provide significant pain relief in knee OA, but highlights the need for further studies in the field.
Korean research groups have been at the forefront of adipose‐derived orthobiologic use. Koh et al. published two papers in 2012 and 2013 that studied the use of adipose‐derived MSCs for the treatment of knee OA 28, 29. The results revealed a significant reduction in pain and increased QOL, with some improvements extending beyond 2 years. Moreover, they showed a positive correlation between the number of cells injected and improvements in pain. Jo et al. also reported similar results with injected adipose‐derived MSCs for knee OA. Their data showed a significant dose‐dependent improvement in pain and function for up to 2 years 35. Furthermore, two randomized, controlled clinical trials have recently been published with both demonstrating significant improvements in pain and function in knee OA patients after injection of autologous AT‐derived MSCs when compared with controls 36, 37. However, once again, it should be noted that these were culture‐expanded cells, which were not used in the current study and cannot be used in the United States due to Federal Drug Administration minimal manipulation regulations.
As mentioned previously, although here we have discussed MSCs, the BMAC and MFAT injections used in the current study are not bona fide MSC treatments as would be expected if we were able to use culture expansion. Previous data shows that MSCs collected from BMA make up only a small percentage of mononuclear cells, approximately 0.001% to 0.02% 38, 39. While this is fewer than what is obtained through culture expansion, MSCs are present in BMAC and the number of MSCs needed for clinical effect has not yet been determined. Thus, MSCs are likely exerting some of the clinical benefit observed with BMAC injections. Other cytokines and growth factors are also at work with BMAC treatments. These include vascular endothelial growth factor, transforming growth factor‐β, platelet derived growth factor (PDGF), and interleukin (IL)1‐RA 40. Furthermore, BMAC has a high concentration of platelets that can decrease pain through peripheral endocannabinoid pathways, the NF‐κB pathway, and through enhancing the production of endogenous hyaluronic acid 41, 42, 43.
MFAT treatments also include MSCs, but the total number of MSCs present has not yet been estimated. Ceserani et al. note that estimation of MSC number per milliliter of MFAT has proven to be technically difficult 44. However, MFAT is rich in microvessels and pericytes, which are immature MSC progenitors 44, 45. Multiple investigators have clearly documented that pericytes give rise to MSCs 46, 47. Thus, the thought is that pericytes in MFAT preparations differentiate into MSCs specific to the pathological microenvironment after injection, although this is difficult to prove in situ. Previous data have shown that the majority of MSCs cultured from MFAT are of pericyte origin 44. In addition, MFAT is also rich in angiogenic, anti‐inflammatory, and immunomodulatory growth factors and cytokines such as IL1‐RA, placental growth factor, PDGF, dipeptidyl peptidase 4, Ang‐1, Ang‐2, matrix metallopeptidase (MMP)‐7, MMP‐9, adiponectin, and HGF 44, 45. MFAT has further anti‐inflammatory properties through blocking monocyte inflammatory functions 44.
Thus, in regard to both BMAC and MFAT injections, they are not true MSC treatments since they are not isolated and culture‐expanded. Their clinical effects are most likely exerted through some concentration of MSCs in combination with angiogenic, anti‐inflammatory, and immune‐modulatory cytokines and growth factors.
To date, there are no published studies comparing the efficacy of BM and adipose‐derived orthobiologic treatments, making it impossible to draw a reliable conclusion about which is the superior candidate for treating knee OA. Therefore, these are unique data from a prospective comparison and outcome analysis of patients who received either an MFAT or a BMAC injection. It is noteworthy that both sources resulted in statistically significant improvements in pain and function without a significant difference when the outcomes between the two tissue sources were compared. Given that the safety and efficacy of injectable autologous BM and adipose‐derived orthobiologic injections have already been evaluated independently for use in knee OA, most notable here is our finding that improvements in pain and function after autologous BMAC or MFAT injection for knee OA do not significantly differ between the two tissue sources.
It will likely be determined that the decision of autologous orthobiologic tissue source depends on a large variety of factors including but not limited to patient age, BMI, comorbidities, OA severity, and preference. Soler et al. excluded patients older than 65 and with severe OA, and found that WOMAC and Lequesne scores decreased in parallel to VAS scores, ultimately leading authors to surmise that younger patients with mild to moderate OA are the ideal candidates for orthobiologic treatment. Other investigators have arrived at a similar conclusion that high prognostic value lies in appropriate patient selection (based on age and OA severity) and earlier intervention 48, 49. Specifically, Kim et al. discovered that when classified according to the KL grading scale, the VAS scores at 3‐, 6‐, and 12‐month follow‐ups in the grade 4 group were significantly poorer than in the grade 1–3 groups 49. Our data also show that the likelihood of achieving meaningful improvements in pain and function was greater with earlier OA changes based on the KL scale (grades 1 and 2) as opposed to more advanced knee OA (grades 3 and 4; Fig. 1). This is an important clinical point that should be addressed when counseling patients on what they might expect following orthobiologic procedures, especially those with advanced knee OA trying to delay or avoid total knee arthroplasty.
It is worth mentioning that since we did not have multiple follow‐up points in our study, the responder rate (those with at least 25% improvement in VAS) may have been higher if we had analyzed responses at various time points. Some who may have been early responders may have experienced waning improvement at 1–1.5 year follow‐up. Also of note, the average percent improvement in VAS in the responder cohort was 73%, which is in line with previous findings (71.4%) presented by Sampson et al. 43.
The limitations of this study are significant. Patients were not randomized into BMAC or MFAT groups for the purpose of this study. Furthermore, outcomes with BMAC and MFAT procedures were not analyzed against a placebo or a more economical injection such as corticosteroid, saline, or hyaluronic acid. Without a control group or randomization process, it is difficult to know for sure if the results can be partially explained by a placebo effect or preexisting characteristics of the subjects that could influence the response. Furthermore, BMAC and MFAT were not quantified for contents (MSCs, platelets, cytokines, etc.) prior to injection. Therefore, it is possible that nonresponders were injected with a lesser stimulus. In order to further advance the data in this emerging field, future clinical trials should focus on more rigid study designs with randomized and controlled head‐to‐head comparisons of autologous orthobiologic tissue sources, tissue analysis, the use of multiple injections, and the use of varying MSC doses for patients with knee OA and other orthopedic conditions.
Conclusion
This is a novel study that compares outcomes between BM and AT‐derived orthobiologic injections for symptomatic knee OA. Based on our data, autologous tissue source does not affect outcomes as both BMAC and MFAT groups had significantly improved pain and function compared with their baseline without a significant difference in improvements between the two groups.
Author Contributions
K.M.: conception and design, provision of study material or patients, collection and/or assembly of data, manuscript writing, final approval of manuscript; R.B.: conception and design, provision of study material or patients, collection and/or assembly of data, manuscript writing, final approval of manuscript; K.E.: data analysis and interpretation, manuscript writing; Z.F. and R.R.: collection and/or assembly of data.
Disclosure of Potential Conflicts of Interest
K.M. currently receives book royalties from Elsevier and McGraw‐Hill, and is a minor investor in Tenex Health. The other authors indicated no potential conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Osteoarthritis (OA; CDC website). Available at https://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed April 3, 2018.
- 2. Kotlarz H, Gunnarsson CL, Fang H, et al. Insurer and out‐of‐pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum 2009;60:3546–3553. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen US, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: Survey and cohort data. Ann Intern Med 2011;155:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullough PG. The geometry of diarthrodial joints, its physiologic maintenance, and the possible significance of age‐related changes in geometry‐to‐load distribution and the development of osteoarthritis. Clin Orthop Relat Res 1981;156:61–66. [PubMed] [Google Scholar]
- 7. Aigner T, Rose J, Martin J, et al. Aging theories of primary osteoarthritis: From epidemiology to molecular biology. Rejuvenation Res 2004;7:134–145. [DOI] [PubMed] [Google Scholar]
- 8. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br 1961;43‐B:752–757. [DOI] [PubMed] [Google Scholar]
- 9. Zgoda M, Paczek L, Bartlomiejczyk I, et al. Age‐related decrease in the activity of collagenase in the femoral head in patients with hip osteoarthritis. Clin Rheumatol 2007;26(2):284 [DOI] [PubMed] [Google Scholar]
- 10. Peyron JG. Epidemiologic and etiologic approach of osteoarthritis. Semin Arthritis Rheum 1979;8:288–306. [DOI] [PubMed] [Google Scholar]
- 11. Goulston LM, Kiran A, Javaid MK, et al. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis Care Res 2011;63:1398–1406. [DOI] [PubMed] [Google Scholar]
- 12. Kurtz SM, Lau E, Ong K, et al. Future young patient demand for primary and revision joint replacement: National projections from 2010 to 2030. Clin Orthop Relat Res 2009;467:2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr Cartil 2007;15:981–1000. [DOI] [PubMed] [Google Scholar]
- 14. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part III: Changes in evidence following systematic cumulative update of research published through 2009. Osteoarthr Cartil 2010;18:476–499. [DOI] [PubMed] [Google Scholar]
- 15. London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypoth 2011;76:887–892. [DOI] [PubMed] [Google Scholar]
- 16. Yubo M, Yanyan L, Li L, et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta‐analysis. PLoS One 2017;12:e0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centeno CJ, Al‐Sayegh H, Freeman MD, et al. A multi‐center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop 2016;40:1755–1765. [DOI] [PubMed] [Google Scholar]
- 18. Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, UK: Clarendon Press, 1994:68–69. [Google Scholar]
- 19. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double‐blind randomized controlled trial: Platelet‐rich plasma versus corticosteroid injection with a 1‐year follow‐up. Am J Sports Med 2010;38:255–262. [DOI] [PubMed] [Google Scholar]
- 20. Roos EM, Lohmander LS. The Knee Injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centeno CJ, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted autologous mesenchymal stem cells. Pain Phys 2008;11:343–353. [PubMed] [Google Scholar]
- 22. Centeno CJ, Busse D, Kisiday J, et al. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypoth 2008;71:900–908. [DOI] [PubMed] [Google Scholar]
- 23. Centeno CJ , Schultz JR, Cheever M, et al. Safety and complications reporting on the re‐implantation of culture‐expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther 2011;6:368–378. [DOI] [PubMed] [Google Scholar]
- 24. Emadedin M, Aghdami N, Taghiyar L, et al. Intra‐articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med 2012;15:422–428. [PubMed] [Google Scholar]
- 25. Emadedin M, Ghorbani Liastani M, Fazeli R, et al. Long‐term follow‐up of intra‐articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med 2015;18:336–344. [PubMed] [Google Scholar]
- 26. Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 2011;14:211–215. [DOI] [PubMed] [Google Scholar]
- 27. Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow‐up of three patients. Int J Rheum Dis 2015;19:219–225. [DOI] [PubMed] [Google Scholar]
- 28. Koh YG, Choi YJ. Infrapatellar fat pad‐derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012;19:902–907. [DOI] [PubMed] [Google Scholar]
- 29. Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013;29:748–755. [DOI] [PubMed] [Google Scholar]
- 30. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013;95:1535–1541. [DOI] [PubMed] [Google Scholar]
- 31. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two‐year follow‐up results. Transplantation 2014;97:e66–e68. [DOI] [PubMed] [Google Scholar]
- 32. Soler R, Orozco L, Munar A, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016;23:647–654. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro SA, Kazmerchak SE, Heckman MG, et al. A prospective, single‐blind, placebo‐controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med 2017;45:82–90. [DOI] [PubMed] [Google Scholar]
- 34. Saltzman BM , Leroux T, Meyer MA, et al. The therapeutic effect of intra‐articular normal saline injections for knee osteoarthritis: A meta‐analysis of evidence level 1 studies. Am J Sports Med 2017;45:2647–2653. [DOI] [PubMed] [Google Scholar]
- 35. Jo CH, Chai JW, Jeong EC, et al. Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A 2‐year follow‐up study. Am J Sports Med 2017;45:2774–2783. [DOI] [PubMed] [Google Scholar]
- 36. Lee WS, Kim HJ, Kim KI, et al. Intra‐articular injection of autologous adipose tissue‐derived mesenchymal stem cells for the treatment of knee osteoarthritis: A phase IIb, randomized, placebo‐controlled clinical trial. Stem Cells Translational Medicine 2019;8:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freitag J, Bates D, Wickham J, et al. Adipose‐derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen Med 2019;14:213–230. [DOI] [PubMed] [Google Scholar]
- 38. Alvarez‐Viejo M, Menendez‐Menendez Y, Blanco‐Gelaz MA, et al. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc 2013;45:434–439. [DOI] [PubMed] [Google Scholar]
- 39. Peng L, Jia Z, Yin X, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 2008;17:761–773. [DOI] [PubMed] [Google Scholar]
- 40. Cassano JM, Kennedy JG, Ross KA, et al. Bone marrow concentrate and platelet‐rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc 2018;26:333–342. [DOI] [PubMed] [Google Scholar]
- 41. Descalzi F, Ulivi V, Cancedda R, et al. Platelet‐rich plasma exerts antinociceptive activity by a peripheral endocannabinoid‐related mechanism. Tissue Eng Part A 2013;19:2120–2129. [DOI] [PubMed] [Google Scholar]
- 42. Sundman EA, Cole BJ, Karas V, et al. The anti‐inflammatory and matrix restorative mechanisms of platelet‐rich plasma in osteoarthritis. Am J Sports Med 2014;42:35–41. [DOI] [PubMed] [Google Scholar]
- 43. Sampson S, Smith J, Vincent H, et al. Intra‐articular bone marrow concentrate injection protocol: Short‐term efficacy in osteoarthritis. Regen Med 2016;11:511–520. [DOI] [PubMed] [Google Scholar]
- 44. Ceserani V, Ferri A, Berenzi A, et al. Angiogenic and anti‐inflammatory properties of micro‐fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell 2016;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vezzani B , Shaw I, Lesme H, et al. Higher pericyte content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Translational Medicine 2018;7:876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caplan AI. All MSCs are pericytes? Cell Stem Cell 2008;3:229–230. [DOI] [PubMed] [Google Scholar]
- 47. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 48. Vanlauwe J, Saris DB, Victor J, et al. Five‐year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: Early treatment matters. Am J Sports Med 2011;39:2566–2574. [DOI] [PubMed] [Google Scholar]
- 49. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol 2014;24:1505–1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


