Abstract
Background:
Chukar partridge (Alectoris chukar) is raised for its highly nutritional and relatively expensive meat.
Aims:
This study evaluates selected lymphoid organs of juvenile and peripubertal Chukar partridges using a histomorphometric approach.
Methods:
Thirty one-day-old male and female Chukar partridges were included in the study. Samples were taken from cloacal bursa, thymus, spleen and the caecal tonsil (CT) as well as oesophageal and pyloric tonsils at 80, 100, and 120 days (peripuberty) of age. Ten transverse sections were then taken from each sample of each bird and stained with haematoxylin and eosin (H&E) in order to measure plicae height and follicular width as well as thickness of follicular cortex and medulla in cloacal bursa, villus height and nodular unit (ND) width and height in CT, lobular width as well as lobular cortex and medullar width in thymus, white pulp width in spleen and follicular width in oesophageal and pyloric tonsils, a linear graticule was used, the number of follicles in each plicae in cloacal bursa and the number of follicles in each ND in CT were determined under a light microscope.
Results:
Drastic changes in histomorphometrical parameters of lymphoid tissues were observed in peripuberty, prominently in cloacal bursa and thymus as demonstrated by the significant decrease in all assayed parameters. Involutionary changes were also observed in oesophageal and pyloric tonsils.
Conclusion:
Involution occurs in bursa and thymus of partridges around puberty rather than their juvenile stages. These changes are also appreciable in CT, oesophageal and pyloric tonsils, whereas the spleen keeps the same histological features before and around puberty.
Key Words: Age, Chukar partridge, Histology, lymphoid organs
Introduction
Chukar partridge (Alectoris chukar) is a bird from the phasianidae family which is commonly known as a Eurasian upland game bird. The bird is also raised for its delicate, highly nutritional and relatively expensive meat in some areas of the world including Iran as a blossoming industry with a bright future and great outcome.
A sound and intact immune system can protect birds against infectious diseases with different etiological factors and subsequently reduce financial damages due to pharmacological management of the disease and bird loss as well as the possibility of drug residue violations in meat products.
Central (primary) and peripheral lymphoid organs produce lymphocytes which are highly important cells in the immune system. Thymus and cloacal bursa (bursa of Fabricius) are considered central lymphoid organs of birds and are involved in production, differentiation and maturation of T and B lymphocytes, respectively (Singh et al., 2010 ▶).
Spleen is considered a “peripheral or secondary” lymphoid tissue. Avian spleen consists of various functional cells in red and white pulps. The red pulp, which is a highly vascularized area, consists of B cells, macrophages and reticular cells. The white pulp, which is composed of the periarterial lymphatic sheath and the splenic nodule, mainly consists of T cells and B cells, respectively (Guo et al., 2015).
Caecal tonsils (CTs) which are lymphatic tissues in the caecal wall, act as an immunological surveillance against foreign micro organisms entering caeca. Caecal tonsils, which embrace nearly half of the lymph nodules of the avian lymphoid system, make an important immune structure in the avian cecum (Rezaian and Hamedi, 2007 ▶).
The oesophageal tonsil, which is located at the transition between the thoracic part of the oesophagus and the proventriculus (Sağsöz and Liman, 2009 ▶), is divided into up to eight tonsillar units that are all located in the lamina propria mucosae between two folds (Oláh et al., 2003 ▶; Sağsöz and Liman, 2009 ▶) where each tonsillar unit surrounds a crypt (Oláh et al., 2003 ▶).
Moreover, small secondary lymphoid follicles and inter follicular T cell regions are located in the wall of the proximal part of the duodenum near the pyloric sphincter (Arai et al., 1988 ▶; Nagy and Oláh, 2007 ▶). According to Nagy and Oláh (2007) ▶ this tonsil consists of 15 to 20 tonsillar units encapsulated by connective tissue. The pyloric tonsils may act as a compensatory mechanism for the absence of mesenteric lymph nodes in birds (Nagy and Oláh, 2007 ▶).
It has been clearly demonstrated that developmental changes in lymphoid organs of birds can be associated with a drastic change in their body functions (Ciriaco et al., 2003 ▶). However, contrary to mammalian species where a large body of knowledge exists regarding age-dependent changes in immune organs, data in avian species are relatively scarce. Recently, Zhao et al. (2016) ▶ showed that the structural development of cloacal bursa can affect the susceptibility of chickens to infectious bursal disease viruses. Age-related changes and the distribution of T cell markers and toll-like receptors in duck lymphoid organs have also been studied (Zhang et al., 2017 ▶).
Acquiring an insight into the physiology of the immune system requires comprehensive knowledge of basic structures of the lymphoid tissue and factors which may affect these structures such as age and developmental changes of the animal.
To the best of our knowledge, information about microstructural features of Chukar partridges' main lymphoid structures and their possible changes is very scarce. This study aims to evaluate selected lymphoid organs of these birds including the cloacal bursa, ceacal tonsil (CT), spleen, thymus, esophageal and pyloric tonsil of juvenile partridges as well as those close to physical puberty using a histomorphometric approach.
Materials and Methods
Birds
Thirty clinically healthy one-day-old male and female Chukar partridges were reared in similar environmental and nutritional conditions. The rearing condition was as follows: the chicks were kept at 32°C in the first week, after which temperature decreased 2 to 3°C per week until week 5. During the first 5 weeks, chicks had free access to water and feed that consisted of 28% crude protein (CP) and 3,200 (kcal/kg) metabolisable energy (ME). During the following weeks, chicks were fed with a ration that included 22.5% CP and 3,300 kcal/kg ME (Özcelik et al., 1995 ▶). A 24 h/d lighting program was used.
At 80, 100, and 120 days, birds were randomly selected and slaughtered by cervical dislocation (10 birds in each sampling time) and cloacal bursa, thymus, spleen, CT, as well as oesophageal and pyloric tonsils were immediately dissected.
All methods used in the study were in compliance with the Institutional Ethical Guidelines for use of animals in research.
Sampling and histological evaluation
Samples were prepared from cloacal bursa, CT, spleen, thymus, esophageal and pyloric tonsils and fixed in 10% buffered formalin. Routine histological laboratory methods were used and 6 μm-thick transverse sections were prepared using a rotary microtome. A total number of 10 sections were taken from each sample of each bird and stained with haematoxylin and eosin (H&E) in order to measure the height of plicae, follicular width, thickness of follicular cortex and medulla in cloacal bursa, villus height and nodular unit (ND) width and height in CT, lobular width, lobular cortex and medullar width in thymus, white pulp width in spleen and follicular width in pyloric tonsil a linear graticule was used, and the number of follicles in each plicae in the cloacal bursa and the number of follicle in each ND in CT was determined under a light microscope. The arithmetic mean of 15 measurements of each parameter per section was calculated.
Statistical analysis
Data were expressed as mean±SD. Data comparisons were performed by one-way ANOVA followed by Tukey’s multiple comparison test and the differences were considered statistically significant at P<0.05.
Results
Cloacal bursa
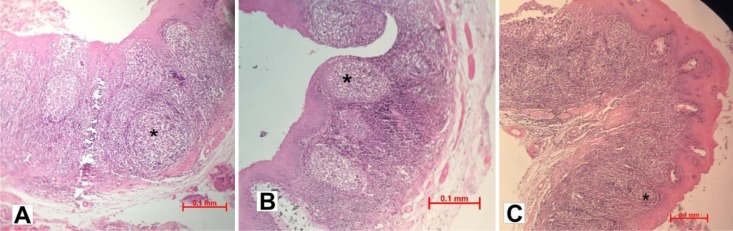
Cloacal bursa in Chukar partridge is a sac-like dorsal diverticulum of the proctodeal wall of the cloaca. Histology revealed that the bursa wall was composed of 3 tunics, the tunica mucosa, tunica muscularis and tunica serosa. The surface epithelium immediately overlying the follicle is the follicle associated epithelium (FAE) while the other surface epithelium is known as the interfollicular epithelium (Figs. 1A-C).
Fig. 1.
Histological section of Chukar partridge’s bursa of fabricius at the age of 80 (A), 100 (B), and 120 (C) days. The height of plicae and follicle (stars) numbers per pelica decreased from day 80 to 120, (H&E, scale bar, 0.1 mm)
As shown in Table 1, the height of plicae, follicular width, thickness of follicular cortex and medulla and follicle numbers per pelica decreased significantly (P<0.001) from day 80 to 120.
Table 1.
Histological measurements (mean±SD) of bursa of Fabricius of partridges at different ages
| Ages (day) | Parameters |
||||
|---|---|---|---|---|---|
| Pelica height (mm) | Follicular width (mm) | Follicular cortex thickness (mm) | Follicular medulla thickness (mm) | Number of follicles per pelica (mm) | |
| 80 | 1.25 ± 0.29a | 0.15 ± 0.02a | 0.02 ± 0.01a | 0.14 ± 0.01a | 19.10 ± 1.85a |
| 100 | 0.96 ± 0.28a, b | 0.13 ± 0.02b | 0.01 ± 0.00b | 0.09 ± 0.00b | 15.70 ± 1.83b |
| 120 | 0.93 ± 0.28b | 0.13 ± 0.03b | 0.01 ± 0.00b | 0.09 ± 0.00b | 16.60 ± 1.35b |
Different superscript letters are used to denote significant difference in a column (P<0.05)
Thymus
A connective tissue tunica covered the surface of the thymus that extended into the parenchyma to divide the parenchyma into several incomplete lobules. The lobule of the thymus included a cortex and a medulla comprised of epithelial reticular, lymphocytes, macrophages, etc. The cortex was enriched with lymphocytes. The medulla, which was located in the middle of the thymic parenchyma, had more epithelial reticular cells than lymphocytes. The medullary region was lightly stained revealing several Hassall’s corpuscles (Figs. 2A-C).
Fig. 2.
Histological section of Chukar partridge’s thymus at the age of 80 (A), 100 (B), and 120 (C) days. Lobular width as well as lobular cortex and medullar width decreased at the age of 100 and 120 days as compared to 80 day-old partridges, (H&E, scale bar, 0.1 mm)
As shown in Table 2 lobular width as well as lobular cortex and medullar width significantly decreased at the age of 100 and 120 days as compared to 80-day-old partridges (P<0.05).
Table 2.
Histological measurements (mean±SD) of thymus of partridge at different ages
| Ages (day) | Parameters |
||
|---|---|---|---|
| Lobular width (mm) | Lobular cortex width (mm) | Lobular medulla width (mm) | |
| 80 | 0.59 ± 0.08a | 0.35 ± 0.08a | 0.20 ± 0.05a |
| 100 | 0.46 ± 0.06b | 0.19 ± 0.03b | 0.14 ± 0.02b |
| 120 | 0.48 ± 0.03b | 0.19 ± 0.03b | 0.13 ± 0.01b |
Different superscript letters are used to denote significant difference in a column (P<0.05)
Spleen
In the present study, large arteries and veins in spleen hillus as well as reticular cells with euchromatin and prominent nucleoli were seen in 80 to 120-day-old partridges. The central artery was covered with a layer of squamous cells. Red pulp and white pulp were present in partridge spleen, and splenic cords were found in white pulp. The capsule was composed of dense irregular connective tissue enclosed by mesothelium cells. Billroth cords (splenic cords) were found in the red pulp. White pulp widths were 0.07 ± 0.00, 0.08 ± 0.00 and 0.07 ± 0.00 mm from 80 to 120 days, respectively and no significant difference was observed (Figs. 3A-C).
Fig. 3.
Histological section of Chukar partridge’s spleen at the age of 80 (A), 100 (B), and 120 (C) days. The white pulp (arrows) and Billroth (rectangles) showed no difference from 80 to 120 days, (H&E, scale bar, 0.1 mm)
Caecal tonsil
Mucosa in CT has two forms, one form which contains tonsils, may or may not be covered by short villi. The second form or the adjoining part contains no tonsils and resembles the small intestine. Villous covering epithelium contains simple columnar cells with striated borders (absorptive) and goblet cells. The caecal tonsil wall is thick and the lumen is almost completely closed by villi and NDs are prominent. Nodular units are tonsil like structures formed by a mucosal-sub mucosal prominence surrounded by a delicate layer of smooth muscle branching out of the aninner circular muscle layer and muscularis mucosa. Surface epithelium has made a deep crypt into the ND known as fossula. This fossula branches into several crypts that have direct connections with the caecal lumen. The crypts are lined by a lympho-epithelium that contains membranous (M) cells. Diffuse lymphatic tissue fills the laminapropria and the nodular lymphatic tissue remains deeper at lamina propria (Figs. 4A-D).
Fig. 4.
Histological section of Chukar partridge’s ceacal tonsils at the age of 80 (A), 100 (B), and 120 (C) days (stars show follicles and arrow shows fossula). Nodular units’ height decreased at 100 and 120 days, however villi became longer at 100, (H&E, scale bar, 0.1 mm), (D) Histological section of fossula with M cells in its epithelium (arrow), (H&E, scale bar, 0.02 mm)
Statistical results showed that ND’ height, follicular width and follicular number per ND significantly decreased with age, however the villi became longer at 120 days (P<0.05) (Table 3).
Table 3.
Histological measurements (mean±SD) of cecal tonsil of partridge at different ages
| Ages (day) | Parameters |
||||
|---|---|---|---|---|---|
| Villus height (mm) | Follicular width (mm) | ND width (mm) | ND height (mm) | Follicle number per ND | |
| 80 | 0.29 ± 0.04b | 0.11 ± 0.02a | 0.32 ± 0.05a | 0.88 ± 0.10a | 5.10 ± 1.60a |
| 100 | 0.33 ± 0.05b | 0.09 ± 0.03a, b | 0.33 ± 0.06a | 0.60 ± 0.06b | 4.00 ± 0.82b |
| 120 | 0.57 ± 0.07a | 0.07 ± 0.04b | 0.32 ± 0.04a | 0.55 ± 0.10b | 3.70 ± 0.82b |
Different superscript letters are used to denote significant difference in a column (P<0.05). ND: Nodular unit
Oesophageal and pyloric tonsils
The esophageal tonsil is anatomically located before the proventriculus. At this region, the tunica propria and the lymphoid tissue are covered by stratified squamous epithelium but the lymphoid tissue is associated with the bottom of the folds, where it forms isolated units. Although the lymphoid units appear circumferential in the wall of the esophagus, they do not form a continuous ring (Figs. 5A-C).
Fig. 5.
Histological section of Chukar partridge’s esophageal tonsil at the age of 80 (A), 100 (B), and 120 (C) days. The width of follicles (stars) showed decrease from day 80 to 120, (H&E, scale bar, 0.1 mm)
The follicular width of the oesophageal tonsils were 0.14 ± 0.02, 0.08 ± 0.01, and 0.07 ± 0.02 from 80 to 100 days, respectively. The widest follicles were observed at 80 days and were significantly different with other ages.
The lymphatic tissue of the pyloric region occupies the entire wall of the gastrointestinal tract. Crypts of Lieberkühn of the duodenum are transformed to tonsillar crypts and lined by the lymphoepithelium (LE). No “capsule” occurs around the aggregated follicles. The width of the follicles were 0.13 ± 0.00, 0.10 ± 0.00, and 0.01 ± 0.00 mm from 80 to 120 days, respectively, showing a significant decrease from day 80 to 120 (Figs. 6A-C).
Fig. 6.
Histological section of Chukar partridge’s pyloric tonsil at the age of 80 (A), 100 (B), and 120 (C) days. The width of follicles (stars) showed decrease from day 80 to 100, (H&E, scale bar, 0.1 mm)
Discussion
This study evaluates the histological features of selected immune organs of Chukar partridge during prepubertal and peripubertal periods using a histomorphometric approach. During the qualitative histological examination of the tissues, we observed close similarities of these immune organs to their counterparts in other avian species as described previously (Schat et al., 2014 ▶). For instance, M cells which are observed in the CT epithelium of chickens were present in the epithelia of proximal parts of the caecum of partridge during all sampling periods.
On the other hand, an age-dependent drastic change was observed in histomorphometrical parameters of each lymphoid tissue, which were most prominent in cloacal bursa and thymus as demonstrated by significant decreases in all assayed parameters in peripuberty as compared to the prepubertal period. In the Fabricius bursa, the most affected parameter was follicular cortex thickness that showed a 50% decrease in the peripubertal period compared to prepuberty. Interestingly, the lobular cortex width was the most affected parameter in thymus with a 46% decrease in the peripubertal period.
It has been demonstrated that follicular cortex has the highest rate of cell proliferation in juvenile bursa and most of the peripheral blood B cells are direct immigrants from the follicular cortex. However, when the bursa involutes, the spleen will work as a substitute (Schat et al., 2014 ▶). This phenomenon may explain our findings regarding a distinct decrease in follicular cortex thickness in the bursa after puberty where no appreciable difference was observed in the spleen white pulp width, for example, compared to the prepubertal period.
In avian species, the thymic cortex is loaded with CD 8+ cells, while CD 4+ cells are mostly found in the medulla. Therefore, age-dependent changes in the thymus of partridges may be more pronounced in CD 8+ cells, although we also observed a decrease in medullar width. Moreover, we cannot precisely differentiate whether the changes were due to the lower proliferation of cells or their higher migration.
In CTs we observed different changes; the villus height increased, the ND width remained the same and other parameters decreased with age.
The gut-associated lymphoid tissue (GALT) plays a pivotal role in confronting different pathogens. As important lymphoid tissues in the GALT, caecal tonsils are exposed to antigens from different origins and are able to participate in both cell-mediated and antibody-mediated immune responses (Befus et al., 1980 ▶). Moreover, CTs can produce pro-inflammatory and regulatory cytokines (Haghighi et al., 2008 ▶; Brisbin et al., 2010 ▶) as well as antimicrobial peptides (Akbari et al., 2008 ▶). Meshwork of the villi at the caecal entrance can exclude large particles from the colonic contents (Duke, 1986 ▶). On the other hand, longer villi have higher activated cell mitosis and more absorptive potential (Samanya and Yamauchi, 2002 ▶; Onderci et al., 2006 ▶). It seems that in partridges, these capabilities become more pronounced with puberty as shown by longer villi in CTs in the peripubertal period compared to juvenile birds. In a study by Rezaian and Hamedi (2007) ▶, the most prominent changes regarding the involution process of CTs in 4-6 month-old white leghorn chickens were a significant decline in the number of lymphatic nodules of the mucosal wall and the nodular evacuation at 6 months of age. We too observed similar histological features of CT changes with age in partridges, whereby most histomorphometrical characters decreased around the post-pubertal period. Similar to other parts of the GALT, involutionary changes were observed in oesophageal and pyloric tonsils of the partridges in our study. Our results could have been more comprehensive if sophisticated immunological evaluations and immunohistochemical examinations of different cell types were included.
In conclusion, it seems that age-dependent involution occurs in the bursa and thymus of partridges. These changes are also appreciable in caecal, oesophageal and pyloric tonsils, whereas the spleen keeps the same histological features in juvenile and peripubertal partridges.
Conflict of interest
The authors declare no conflicts of interest.
References
- Akbari MR, Haghighi HR, Chambers JR, Brisbin J, Read LR, Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enteric serovar typhimurium. Clin. Vaccine Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Hashimoto Y, Kitagawa H, Kon Y, Kudo N. Immunohistochemical study on the distribution of lymphoid tissues in the upper alimentary and respiratory tracts of chickens. Japan. J. Vet. Sci. 1988;50:183–192. doi: 10.1292/jvms1939.50.183. [DOI] [PubMed] [Google Scholar]
- Befus AD, Johnston N, Leslie GA, Bienenstock J. Gut-associated lymphoid tissue in the chicken. J. Immunol. 1980;125:2626–2632. [PubMed] [Google Scholar]
- Brisbin JT, Gong J, Parvizi P, Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010;17:1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriaco E, Píñera PP, Díaz-Esnal B, Laurà R. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius) Microsc. Res. Tech. 2003;62:482–487. doi: 10.1002/jemt.10416. [DOI] [PubMed] [Google Scholar]
- Duke GE. Alimentary canal: anatomy, regulation of feeding, and motility. In: Sturkie PD, editor. Avian physiology. New York: Springer-Verlag; pp. 269–288. [Google Scholar]
- Guo Q, Dong Y, Cao J, Wang Z, Zhang Z, Chen Y. Developmental changes of melatonin receptor expression in the spleen of the chicken, Gallus domesticus. Acta Histochem. 2015;117:559–565. doi: 10.1016/j.acthis.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 2008;126:225–233. doi: 10.1016/j.vetmic.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Nagy N, Oláh I. Pyloric tonsil as a novel gut-associated lymphoepithelial organ of the chicken. J. Anat. 2007;211:407–411. doi: 10.1111/j.1469-7580.2007.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh I, Nagy N, Magyar A, Palya V. Esophageal tonsil: a novel gut-associated lymphoid organ. Poult. Sci. 2003;82:767–770. doi: 10.1093/ps/82.5.767. [DOI] [PubMed] [Google Scholar]
- Onderci M, Sahin N, Sahin K, Cikim G, Aydin A, Ozercan I, Aydín S. Efficacy of supplementation of alpha-amylaseproducing bacterial culture on the performance, nutrient use and gut morphology of broiler chickens fed a corn-based diet. Poult. Sci. 2006;85:505–510. doi: 10.1093/ps/85.3.505. [DOI] [PubMed] [Google Scholar]
- Özcelik M. World of birds. Sci. Technol. 1995;328:66–73. [Google Scholar]
- Rezaian M, Hamedi S. Histological study of the caecal tonsil in the cecum of 4-6 months of age white leghorn chicks. America. J. Anim. Vet. Sci. 2007;2:50–54. [Google Scholar]
- Sağsöz H, Liman N. Structure of the oesophagus and morphometric, histochemical-immunohistochemical profiles of the oesophageal gland during the post-hatching period of Japanesequails (Coturnixcoturnix japonica) Anat. Histol. Embryol. 2009;38:330–340. doi: 10.1111/j.1439-0264.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- Samanya M, Yamauchi K. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var natto. Comp. Biochem. Physiol. Part A, Mol. Integr. Physiol. 2002;133:95–104. doi: 10.1016/s1095-6433(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Schat, KA, Kaspers, B, Kaiser, P. Avian immunology. 2nd Edn. MA, USA: Academic Press; 2014. [Google Scholar]
- Singh GK, Chauhan RS, Mishra UK. Histomorphological development of lymphoid organs in chicken: thymus and bursa of Fabricius. J. Immunol. Immunopathol. 2010;12:20–28. [Google Scholar]
- Zhang A, Xu J, Lai H, Huang W, Fang N, Chen R. Age-related changes and distribution of T cell markers (CD3 and CD4) and toll-like receptors (TLR2, TLR3, TLR4 and TLR7) in the duck lymphoid organs. Immunobiology. 2017;222:857–864. doi: 10.1016/j.imbio.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Zhao S, Jia Y, Han D, Ma H, Shah SZ, Ma Y, Teng K. Influence of the structural development of bursa on the susceptibility of chickens to infectious bursal disease virus. Poult. Sci. 2016;95:2786–2794. doi: 10.3382/ps/pew192. [DOI] [PubMed] [Google Scholar]