Abstract
Objective:
The current study used a sample of high-risk adolescents to examine cannabis withdrawal correlates, including assessments of other drug withdrawal and affective lability that may confound cannabis withdrawal measurement.
Method:
A total of 448 high-risk adolescents, living in the Southwest United States, were recruited from a juvenile detention center for a sexual risk–reduction intervention study (60% male; 67% Hispanic). Assessments were administered every 3 months for a year, resulting in five assessments of drug use and withdrawal (cannabis, alcohol, nicotine). Affective lability was also assessed.
Results:
Forty-two percent of participants endorsed cannabis withdrawal at baseline. Participants used cannabis, on average, 3.3 days/ week at baseline and 0.8–1.1 days/week at follow-ups. Cannabis use and withdrawal were only weakly to moderately correlated (r = .14–.32). Unexpectedly, alcohol withdrawal demonstrated strong correlations with cannabis withdrawal at all assessments (r = .41–.55). Furthermore, affective lability measures were related to cannabis withdrawal (r = .22–.32) but not with cannabis use (r = -.03–.09).
Conclusions:
Whereas cannabis withdrawal was only weakly to moderately related to cannabis use, it demonstrated strong associations with alcohol withdrawal across all assessments. In addition, affective lability measures were moderately correlated with cannabis withdrawal but not with cannabis use. Thus, other drug withdrawal and individual differences are essential to consider when assessing cannabis withdrawal.
Drug withdrawal is associated with many clinically relevant outcomes, including daily functioning, problematic use, and relapse (Budney & Hughes, 2006; Gelhorn et al., 2008; Piasecki et al., 2003; Piontek et al., 2011). Empirical evidence on cannabis withdrawal, however, has lagged behind that on other drug withdrawal. In fact, previous editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the International Classification of Diseases (ICD) highlighted uncertainties about the clinical significance and definition of cannabis withdrawal (American Psychiatric Association, 1994; Bonnet & Preuss, 2017; World Health Organization, 1992), and withdrawal was not included as a symptom of cannabis use disorder until the most recent DSM and ICD editions. As evidence increasingly supports the clinical significance of cannabis withdrawal, studies are needed to elucidate this construct, particularly in vulnerable populations.
Findings across the literature suggest that adolescents report higher rates of withdrawal from cannabis (65%–90% of treatment receivers) than they do from other drugs (10% of those with alcohol use disorder; 50% of cigarette smokers) (Colby et al., 2000; Cornelius et al., 2008; Crowley et al., 1998; Ershler et al., 1989; Preuss et al., 2010; Stewart & Brown, 1995; Vandrey et al., 2005). Furthermore, compared with adults, adolescents endorse similar rates of withdrawal from cannabis (50%–95% of adult treatment seekers) but lower rates of other drug withdrawal (alcohol = 45%–70%, nicotine = 85% in adults) (Budney & Hughes, 2006; Caetano et al., 1998; Gritz et al., 1991). Critically, while evidence suggests that cannabis withdrawal stands out from other drug withdrawal in adolescents, adolescent cannabis use has doubled (22%–36%), and perceived risk from regular use dropped by half among 12th graders from 1992 to 2016 (77% to 31%) (Monitoring the Future; Miech et al., 2018). The timeliness of understanding adolescent cannabis withdrawal is further highlighted by the increasing tetrahydrocannabinol (THC) potency of confiscated cannabis (from 4% to 12% THC, 1995–2012; ElSohly et al., 2000, 2016) and legal-market cannabis products (25+% flower, 80+% oils). The current study examined the correlates of cannabis withdrawal in adolescents, including drug use patterns and individual differences related to withdrawal.
The concept of drug withdrawal is based on opponent-process theory, which attributes withdrawal to system adaptations caused by repeated exposure to an external stimulus (Solomon, 1980; Solomon & Corbit, 1974). Following chronic exposure to a stimulus, such as a drug of abuse, its absence causes withdrawal symptoms that are counter to those caused by acute exposure. Drugs that acutely affect reward processes, as a consequence, often have withdrawal characterized by negative emotions (Koob, 2009). For example, cannabis withdrawal can include mood swings, irritability, anxiety, and depressed mood (Agrawal et al., 2008; Budney et al., 2003, 2004). Therefore, withdrawal underlies a shift to negative reinforcement as a primary motivation for drug use, thereby predicting relapse (Cornelius et al., 2008; Koob, 2009).
Given the role of negative reinforcement following protracted abstinence, negative emotional states may indicate withdrawal (Allsop et al., 2012). Trait negative emotionality, however, is also related to problematic substance use (Elkins et al., 2006; Rutledge & Sher, 2001). That is, negative emotional states may precede heavy use and be the result of withdrawal. In addition, other drug withdrawal may co-occur with cannabis withdrawal, particularly among individuals in sober environments. Thus, it may be important to assess affective lability, other drug withdrawal, and similar constructs that could confound measurements of cannabis withdrawal.
The current study examined cannabis withdrawal in high-risk adolescents, who were recruited after entering juvenile detention. This sample provides a unique window into adolescent withdrawal because heavy cannabis use was common among participants, but use was impossible immediately after recruitment. We examined associations between cannabis withdrawal and cannabis use (onset, frequency), affective lability, and other drug use/withdrawal. We hypothesized that affective lability and other drug withdrawal are associated with cannabis withdrawal, but cannabis use patterns (onset, frequency) would be particularly important.
Method
Participants and procedures
From 2010 to 2014, 448 adolescents (Mage = 15.8 years, SD = 1.2, range: 14–18; 40% female; 67% Hispanic) participated in a longitudinal study testing brief interventions to reduce risky sexual behavior. At recruitment, participants had recently entered a youth detention facility. Eligible youth had to (a) be 14–18 years old, (b) speak English, (c) have less than a month of juvenile detention remaining, and (d) sign a release form allowing access to their sexually transmitted infection test results (a primary outcome of the study) if they were tested at intake. After recruitment, participants completed a baseline assessment and were randomized to a 2-hour, group-based, sexual risk–reduction intervention (targeting sexual risk behavior alone or with alcohol/cannabis use). The interventions focused on reducing risky sexual behavior, including behavior following heavy drug use (for full details, see Callahan et al., 2013). The study was approved by the institutional review boards at the University of New Mexico and the University of Colorado. A federal Certificate of Confidentiality was issued to protect participants in this research.
After baseline, postintervention assessments were administered every 3 months for a year (ns = 414–422; 90%–92% participation). Group comparisons (t tests) suggested that attrition was unrelated to cannabis use (p = .62–.78) and withdrawal (p = .13–.45). Thus, data were determined to be missing at random.
Measures
Participants completed self-report questionnaires on a range of topics including drug use, drug-related problems (including withdrawal), and affective lability.
Cannabis use and withdrawal.
Cannabis use frequency was assessed by self-report response to “how often did you smoke marijuana?” in the last 3 months. Responses were on a 9-point scale and were recoded to a 0 to 90 scale, indicating the number of days participants had used cannabis in the last 90 days (e.g., never = 0, once a month = 3, every day = 90). At baseline, 337 participants reported any cannabis use (75%), and 172 reported daily use (38%). Age at cannabis onset was assessed with the item, “How old were you when you first used marijuana?”
The Marijuana Problems Index assessed the frequency of problems “while you were smoking marijuana or because of your marijuana use” in the last 3 months on a 5-point, Likert-type scale, from never to more than 10 times (Johnson & White, 1989; Simons & Carey, 2002). The Marijuana Dependence Scale assessed dependence symptoms “experienced with regard to marijuana use” in the last 3 months on a dichotomous scale (Stephens et al., 2000).
To assess withdrawal, two items were used from the Marijuana Problems Index (“had withdrawal symptoms, that is, felt sick because you stopped or cut down on smoking marijuana,” and “felt physically or physiologically dependent on marijuana”) and two items from the Marijuana Dependence Scale (“feeling what might be described as a ‘withdrawal symptom’ [e.g., sleep disturbance, irritability, etc. . . .],” and “feeling the need to smoke marijuana to avoid experiencing ‘withdrawal symptoms’”). Across these four items, 42% of participants endorsed some level of withdrawal (1–2 items endorsed = 30%, 3–4 items = 12%). Confirmatory factor analysis was conducted to accommodate the scaling across items, and factor scores were extracted. Factor loadings and thresholds were fixed for each item across assessments to ensure that factor scores represented the same construct across assessments. The fit of this model was adequate (root mean square error of approximation [RMSEA] = .053 [90% CI = .047, .059]; comparative fit index [CFI] = .96). At baseline, cannabis withdrawal factor scores ranged from -0.37 to 2.34 (M = 0.33).
Other drug use and withdrawal.
Measures similar to those for cannabis were used for alcohol and nicotine. Alcohol and nicotine use in the last 3 months was assessed on the same scale as that used for cannabis (White & Labouvie, 1989). Age at onset of alcohol and nicotine use was also assessed. Polysubstance use was assessed as the number of illicit drugs ever used (crack/cocaine, lysergic acid diethylamide (LSD)/ acid, mushrooms, Ecstasy, γ-hydroxybutyric acid (GHB), heroin, ketamine, crystal meth, “prescription drugs NOT prescribed to you”).
Alcohol withdrawal was assessed using two items from the Rutgers Alcohol Problem Index (i.e., “had withdrawal symptoms, that is, felt sick because you stopped or cut down on drinking,” and “felt physically or physiologically dependent on alcohol”) and one item from the Alcohol Use Disorders Identification Test (“How often during the past 3 months have you needed a first drink in the morning to get yourself going after a heavy drinking session?”) (Bohn et al., 1995; White & Labouvie, 1989). For consistency with cannabis withdrawal, confirmatory factor analysis was conducted, and factor scores were extracted (RMSEA = .038 [90% CI = .030, .047], CFI = .97).
Nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence (FTND), a well-validated seven-item questionnaire of dependence on nicotine that is associated with symptoms of nicotine withdrawal (Heatherton et al., 1991; Ríos-Bedoya et al., 2008). The FTND demonstrated internal consistencies below the preferred .70 threshold (α = .60–.67), consistent with prior studies using the FTND (Vink et al., 2005).
Affective lability.
Achenbach’s Youth Self-Report measure (YSR) was administered at the baseline assessment (Achenbach & Edelbrock, 1987). The YSR has been validated in general population and clinical samples to assess problems in youth (Ebesutani et al., 2011; Thurber & Hollingsworth, 1992; van Lang et al., 2005). Items assess characteristics that may describe the youth (e.g., “I am nervous or tense.”), with responses on a 3-point Likert-type scale (not true to very/often true). Affective lability was measured using the YSR anxious/depressed, withdrawn/depressed, and attention problems scales.
The State–Trait Anger Expression Inventory-2 (STAXI-2) was administered at the 6-, 9-, and 12-month assessments (Spielberger, 1999; Spielberger et al., 1999). The STAXI-2 consists of 35 items that query how participants “feel right now,” “usually feel,” and “feel or act . . . when you are angry.” Responses are on a three-point, Likert-type scale. Items demonstrated adequate internal consistency (α = .91–.96). A sum score of all 35 items was used to measure anger.
Analytic procedures
The relations among cannabis use and withdrawal, other drug use and withdrawal, and affective lability were evaluated via correlation estimates in R, using the cor.test function from the stats package (R Core Team, 2017).
Results
Descriptive statistics and correlation analyses
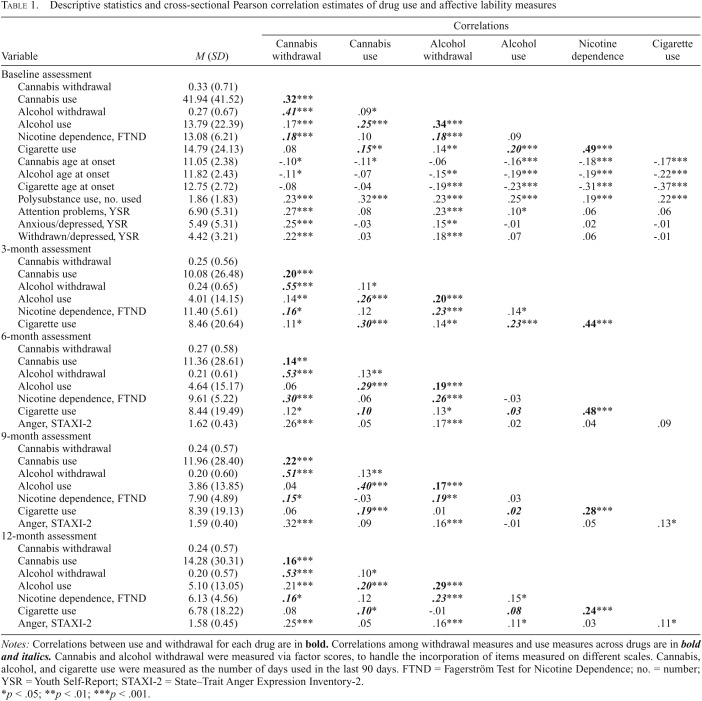
Table 1 displays means, standard deviations, and cross-sectional correlations of the relations between drug withdrawal, use, and affective lability. At baseline, participants reported using cannabis on approximately 42 days, alcohol on 14 days, and cigarettes on 15 days out of the last 90 days. Participants also reported using one to two other illicit drugs in their lifetime. At subsequent assessments, participants reported markedly less drug use.
Table 1.
Descriptive statistics and cross-sectional Pearson correlation estimates of drug use and affective lability measures
| Correlations |
|||||||
| Variable | M (SD) | Cannabis withdrawal | Cannabis use | Alcohol withdrawal | Alcohol use | Nicotine dependence | Cigarette use |
| Baseline assessment | |||||||
| Cannabis withdrawal | 0.33 (0.71) | ||||||
| Cannabis use | 41.94 (41.52) | .32*** | |||||
| Alcohol withdrawal | 0.27 (0.67) | .41*** | .09* | ||||
| Alcohol use | 13.79 (22.39) | .17*** | .25*** | .34*** | 13.79 (22.39) | .17*** | .25*** |
| Nicotine dependence, FTND | 13.08 (6.21) | .18*** | .10 | .18*** | .09 | ||
| Cigarette use | 14.79 (24.13) | .08 | .15** | .14** | .20*** | .49*** | |
| Cannabis age at onset | 11.05 (2.38) | -.10* | -.11* | -.06 | -.16*** | -.18*** | -.17*** |
| Alcohol age at onset | 11.82 (2.43) | -.11* | -.07 | -.15** | -.19*** | -.19*** | -.22*** |
| Cigarette age at onset | 12.75 (2.72) | -.08 | -.04 | -.19*** | -.23*** | -.31*** | -.37*** |
| Polysubstance use, no. used | 1.86 (1.83) | .23*** | .32*** | .23*** | .25*** | .19*** | .22*** |
| Attention problems, YSR | 6.90 (5.31) | .27*** | .08 | .23*** | .10* | .06 | .06 |
| Anxious/depressed, YSR | 5.49 (5.31) | .25*** | -.03 | .15** | -.01 | .02 | -.01 |
| Withdrawn/depressed, YSR | 4.42 (3.21) | .22*** | .03 | .18*** | .07 | .06 | -.01 |
| 3-month assessment | |||||||
| Cannabis withdrawal | 0.25 (0.56) | ||||||
| Cannabis use | 10.08 (26.48) | .20*** | |||||
| Alcohol withdrawal | 0.24 (0.65) | .55*** | .11* | ||||
| Alcohol use | 4.01 (14.15) | .14** | .26*** | .20*** | |||
| Nicotine dependence, FTND | 11.40 (5.61) | .16* | .12 | .23*** | .14* | ||
| Cigarette use | 8.46 (20.64) | .11* | .30*** | .14** | .23*** | .44*** | |
| 6-month assessment | |||||||
| Cannabis withdrawal | 0.27 (0.58) | ||||||
| Cannabis use | 11.36 (28.61) | .14** | |||||
| Alcohol withdrawal | 0.21 (0.61) | .53*** | .13** | ||||
| Alcohol use | 4.64 (15.17) | .06 | .29*** | .19*** | |||
| Nicotine dependence, FTND | 9.61 (5.22) | .30*** | .06 | .26*** | -.03 | ||
| Cigarette use | 8.44 (19.49) | .12* | .10 | .13* | .03 | .48*** | |
| Anger, STAXI-2 | 1.62 (0.43) | .26*** | .05 | .17*** | .02 | .04 | .09 |
| 9-month assessment | |||||||
| Cannabis withdrawal | 0.24 (0.57) | ||||||
| Cannabis use | 11.96 (28.40) | .22*** | |||||
| Alcohol withdrawal | 0.20 (0.60) | .51*** | .13** | ||||
| Alcohol use | 3.86 (13.85) | .04 | .40*** | .17*** | |||
| Nicotine dependence, FTND | 7.90 (4.89) | .15* | -.03 | .19** | .03 | ||
| Cigarette use | 8.39 (19.13) | .06 | .19*** | .01 | .02 | .28*** | |
| Anger, STAXI-2 | 1.59 (0.40) | .32*** | .09 | .16*** | -.01 | .05 | .13* |
| 12-month assessment | |||||||
| Cannabis withdrawal | 0.24 (0.57) | ||||||
| Cannabis use | 14.28 (30.31) | .16*** | |||||
| Alcohol withdrawal | 0.20 (0.57) | .53*** | .10* | ||||
| Alcohol use | 5.10 (13.05) | .21*** | .20*** | .29*** | |||
| Nicotine dependence, FTND | 6.13 (4.56) | .16* | .12 | .23*** | .15* | ||
| Cigarette use | 6.78 (18.22) | .08 | -.10* | -.01 | .08 | .24*** | |
| Anger, STAXI-2 | 1.58 (0.45) | .25*** | .05 | .16*** | .11* | .03 | .11* |
Notes: Correlations between use and withdrawal for each drug are in bold. Correlations among withdrawal measures and use measures across drugs are in bold and italics. Cannabis and alcohol withdrawal were measured via factor scores, to handle the incorporation of items measured on different scales. Cannabis, alcohol, and cigarette use were measured as the number of days used in the last 90 days. FTND = Fagerström Test for Nicotine Dependence; no. = number; YSR = Youth Self-Report; STAXI-2 = State–Trait Anger Expression Inventory-2.
p < .05; **p < .01; ***p < .001.
Cannabis use in the past 90 days was moderately correlated with cannabis withdrawal at baseline (r = .32) and only weakly correlated with withdrawal at subsequent assessments (r = .14–.22). For comparison, correlations between alcohol use and alcohol withdrawal were in the same range (.17–.34), but correlations between cigarette use and nicotine dependence were generally stronger (.24–.49). Similarly, correlations were weak for age at cannabis onset and withdrawal (r = -.10) and alcohol onset-withdrawal (r = -.15), but correlations were moderate for cigarette onset/nicotine dependence (r = -.31).
Unexpectedly, alcohol withdrawal was strongly correlated with cannabis withdrawal (r = .41–.55) but only weakly correlated with cannabis use (r = .09–.13). Nicotine dependence was moderately correlated with cannabis withdrawal (r = .15–.30) but not with cannabis use (r = -.03–.12). Furthermore, affective lability was moderately correlated with cannabis withdrawal (YSR = .22–.27, STAXI = .25–.32) but not with cannabis use (YSR = -.03–.08, STAXI = .05–.09). For comparison, affective lability was correlated with alcohol withdrawal (YSR = .15–.23; STAXI = .16–.17) but not with nicotine dependence (YSR = .02–.06; STAXI = .03–.05). Last, illicit polysubstance use was moderately correlated with both cannabis withdrawal (r = .23) and use (r = .32).
Discussion
This study examined the correlates of cannabis withdrawal in a longitudinal sample of high-risk adolescents. Nearly half (42%) of adolescents reported cannabis withdrawal at baseline, which is lower than the findings of studies of treatment-receiving adolescents (Cornelius et al., 2008; Crowley et al., 1998; Preuss et al., 2010; Vandrey et al., 2005). Cannabis withdrawal was moderately correlated with cannabis use at baseline, but correlations were weaker at subsequent assessments. Unexpectedly, our findings suggest that alcohol withdrawal and affective lability are at least as strongly related to cannabis withdrawal as cannabis use itself. These findings suggest that other drug withdrawal and affective lability may confound or predict adolescent cannabis withdrawal.
Many possible causes could underlie the links of alcohol withdrawal and affective lability to cannabis withdrawal. For example, alcohol withdrawal may exacerbate cannabis withdrawal symptoms, or withdrawal from one drug may obfuscate reports of withdrawal from the other drug. Of note, alcohol use in the present sample was markedly lower than would be expected to produce classic withdrawal. Thus, it is unclear whether participants experienced actual alcohol withdrawal or other phenomena. In addition, individuals high in affective lability may experience withdrawal more intensely, or negative affective states related to lability may be misinterpreted as withdrawal (e.g., irritability). Importantly, the associations between affective lability and cannabis withdrawal may be interpreted as a proxy for the relationship between depression/neuroticism and drug use (Cooper, 1994; Harder et al., 2006; Littlefield et al., 2011; Rutledge & Sher, 2001). However, affective lability measures were unrelated to cannabis use in the current study. That is, the associations between affective lability and cannabis withdrawal appear to be distinct from cannabis use.
The high rates of cannabis withdrawal demonstrated in the present study and in the literature may also indicate that adolescents are more sensitive to developing cannabis withdrawal than other drug withdrawal. Consistent with this possibility, some findings suggest that the adolescent brain is abundant in cannabinoid receptors, to which THC binds— resulting in greater susceptibility to the effects of cannabis (Bossong & Niesink, 2010; Chadwick et al., 2013). Of note, these receptors densely populate the amygdala, which is associated with emotional reactivity and may serve as a link to affective lability.
Limitations and future directions
The way cannabis withdrawal was measured in the present study should be considered when interpreting these findings. Although items were face valid (e.g., “feeling . . . a ‘withdrawal symptom’”) and confirmatory factor analyses suggested that the model adequately fit the data/items, more comprehensive measures of cannabis withdrawal have been developed. For example, the current measure used items that focus more on physical symptoms (e.g., felt sick) than psychological symptoms (e.g., irritability). Measures that assess multiple aspects of withdrawal can greatly extend this work, such as the Cannabis Withdrawal Scale (Allsop et al., 2012) that assesses attention problems (e.g., “The only thing I could think about was smoking some cannabis”), affective instability (e.g., “mood swings”), and other withdrawal domains.
In addition, longitudinal assessments of adolescent cannabis withdrawal and cannabinoid/metabolite levels could provide objective assessments of the time course of cannabis withdrawal. The time scale of assessments (once every 3 months) in the current study also limited the inferences that could be made about changes in cannabis use and withdrawal, and studies on changes in cannabis withdrawal should aim for fine-grained temporal resolution of assessments (e.g., weekly). Last, this study analyzed a high-risk adolescent sample, and these findings will best generalize to heavy-using populations, rather than covering the full spectrum of adolescent cannabis use.
Summary
This study provides insights into the correlates, and potential confounds, of cannabis withdrawal in adolescents. Whereas cannabis withdrawal in adolescents is associated with cannabis use, the current analyses suggested that alcohol withdrawal and affective lability also play an important role. The current data highlight but cannot disentangle these as possible confounds—suggesting the need for further research. Thus, longitudinal studies with frequent assessments (e.g., weekly) and objective measures of use and exposure (e.g., cannabinoids) will further help to advance the understanding of adolescent cannabis withdrawal.
Footnotes
This research was supported by grants from the National Institutes of Health to Angela D. Bryan (AA013844) Jarrod M. Ellingson (AA026635), L. Cinnamon Bidwell (DA033302, DA04413), Kent E. Hutchison (AA024632, DA039707), and Christian J. Hopfer (DA032555, DA035804, DA042755).
References
- Achenbach T. M., Edelbrock C. S. Manual for the youth self-report and profile. Burlington, VT: Department of Psychiatry, University of Vermont; 1987. [Google Scholar]
- Agrawal A., Pergadia M. L., Lynskey M. T. Is there evidence for symptoms of cannabis withdrawal in the National Epidemiologic Survey of Alcohol and Related Conditions? American Journal on Addictions. 2008;17:199–208. doi: 10.1080/10550490802019519. doi:10.1080/10550490802019519. [DOI] [PubMed] [Google Scholar]
- Allsop D. J., Copeland J., Norberg M. M., Fu S., Molnar A., Lewis J., Budney A. J. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7:e44864. doi: 10.1371/journal.pone.0044864. doi:10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bohn M. J., Babor T. F., Kranzler H. R. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. doi:10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Bonnet U., Preuss U. W. The cannabis withdrawal syndrome: Current insights. Substance Abuse and Rehabilitation. 2017;8:9–37. doi: 10.2147/SAR.S109576. doi:10.2147/SAR.S109576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong M. G., Niesink R. J. M. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Progress in Neurobiology. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. doi:10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Budney A. J., Hughes J. R. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. doi:10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney A. J., Hughes J. R., Moore B. A., Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. doi:10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney A. J., Moore B. A., Vandrey R. G., Hughes J. R. The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. doi:10.1037/0021-843X.112.3.393. [DOI] [PubMed] [Google Scholar]
- Caetano R., Clark C. L., Greenfield T. K. Prevalence, trends, and incidence of alcohol withdrawal symptoms: Analysis of general population and clinical samples. Alcohol Health and Research World. 1998;22:73–79. [PMC free article] [PubMed] [Google Scholar]
- Callahan T. J., Montanaro E., Magnan R. E., Bryan A. D. Project MARS: Design of a multi-behavior intervention trial for justice-involved youth. Translational Behavioral Medicine. 2013;3:122–130. doi: 10.1007/s13142-013-0192-5. doi:10.1007/s13142-013-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick B., Miller M. L., Hurd Y. L. Cannabis use during adolescent development: Susceptibility to psychiatric illness. Frontiers in Psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. doi:10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby S. M., Tiffany S. T., Shiffman S., Niaura R. S. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug and Alcohol Dependence, 59, Supplement 1. 2000:83–95. doi: 10.1016/s0376-8716(99)00166-0. doi:10.1016/S0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Cooper M. L. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment. 1994;6:117–128. doi:10.1037/1040-3590.6.2.117. [Google Scholar]
- Cornelius J. R., Chung T., Martin C., Wood D. S., Clark D. B. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addictive Behaviors. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. doi:10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. J., Macdonald M. J., Whitmore E. A., Mikulich S. K. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug and Alcohol Dependence. 1998;50:27–37. doi: 10.1016/s0376-8716(98)00003-9. doi:10.1016/S0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Ebesutani C., Bernstein A., Martinez J. I., Chorpita B. F., Weisz J. R. The youth self report: Applicability and validity across younger and older youths. Journal of Clinical Child & Adolescent Psychology. 2011;40:338–346. doi: 10.1080/15374416.2011.546041. doi:10.1080/15374416.2011.546041. [DOI] [PubMed] [Google Scholar]
- Elkins I. J., King S. M., McGue M., Iacono W. G. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: Prospective links from adolescence to young adulthood. Journal of Abnormal Psychology. 2006;115:26–39. doi: 10.1037/0021-843X.115.1.26. doi:10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- ElSohly M. A., Mehmedic Z., Foster S., Gon C., Chandra S., Church J. C. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry. 2016;79:613–619. doi: 10.1016/j.biopsych.2016.01.004. doi:10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly M. A., Ross S. A., Mehmedic Z., Arafat R., Yi B., Banahan B. F., III. Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980-1997. Journal of Forensic Sciences. 2000;45:24–30. doi:10.1520/JFS14636J. [PubMed] [Google Scholar]
- Ershler J., Leventhal H., Fleming R., Glynn K. The quitting experience for smokers in sixth through twelfth grades. Addictive Behaviors. 1989;14:365–378. doi: 10.1016/0306-4603(89)90024-5. doi:10.1016/0306-4603(89)90024-5. [DOI] [PubMed] [Google Scholar]
- Gelhorn H., Hartman C., Sakai J., Stallings M., Young S., Rhee S. H., Crowley T. Toward DSM-V: An item response theory analysis of the diagnostic process for DSM-IV alcohol abuse and dependence in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1329–1339. doi: 10.1097/CHI.0b013e318184ff2e. doi:10.1097/CHI.0b013e318184ff2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz E. R., Carr C. R., Marcus A. C. The tobacco withdrawal syndrome in unaided quitters. Addiction. 1991;86:57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. doi:10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Harder V. S., Morral A. R., Arkes J. Marijuana use and depression among adults: Testing for causal associations. Addiction. 2006;101:1463–1472. doi: 10.1111/j.1360-0443.2006.01545.x. doi:10.1111/j.1360-0443.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Johnson V., White H. R. An investigation of factors related to intoxicated driving behaviors among youth. Journal of Studies on Alcohol. 1989;50:320–330. doi: 10.15288/jsa.1989.50.320. doi:10.15288/jsa.1989.50.320. [DOI] [PubMed] [Google Scholar]
- Koob G. F. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Supplement 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. doi:10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield A. K., Agrawal A., Ellingson J. M., Kristjansson S., Madden P. A., Bucholz K. K., Sher K. J. Does variance in drinking motives explain the genetic overlap between personality and alcohol use disorder symptoms? A twin study of young women. Alcoholism: Clinical and Experimental Research. 2011;35:2242–2250. doi: 10.1111/j.1530-0277.2011.01574.x. doi:10.1111/j.1530-0277.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R. A., Johnston L. D., O’Malley P. M., Bachman J. G., Schulenberg J. E., Patrick M. E. Monitoring the Future national survey results on drug use, 1975–2017: Volume I, Secondary school students. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2018. [Google Scholar]
- Piasecki T. M., Jorenby D. E., Smith S. S., Fiore M. C., Baker T. B. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology. 2003;112:14–27. doi:10.1037/0021-843X.112.1.14. [PubMed] [Google Scholar]
- Piontek D., Kraus L., Legleye S., Bühringer G. The validity of DSM-IV cannabis abuse and dependence criteria in adolescents and the value of additional cannabis use indicators. Addiction. 2011;106:1137–1145. doi: 10.1111/j.1360-0443.2010.03359.x. doi:10.1111/j.1360-0443.2010.03359.x. [DOI] [PubMed] [Google Scholar]
- Preuss U. W., Watzke A. B., Zimmermann J., Wong J. W. M., Schmidt C. O. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug and Alcohol Dependence. 2010;106:133–141. doi: 10.1016/j.drugalcdep.2009.08.008. doi:10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Retrieved from https://www.R-project.org. [Google Scholar]
- Ríos-Bedoya C. F., Snedecor S. M., Pomerleau C. S., Pomerleau O. F. Association of withdrawal features with nicotine dependence as measured by the Fagerström Test for Nicotine Dependence (FTND) Addictive Behaviors. 2008;33:1086–1089. doi: 10.1016/j.addbeh.2008.04.005. doi:10.1016/j.addbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge P. C., Sher K. J. Heavy drinking from the freshman year into early young adulthood: The roles of stress, tension-reduction drinking motives, gender and personality. Journal of Studies on Alcohol. 2001;62:457–466. doi: 10.15288/jsa.2001.62.457. doi:10.15288/jsa.2001.62.457. [DOI] [PubMed] [Google Scholar]
- Simons J. S., Carey K. B. Risk and vulnerability for marijuana use problems: The role of affect dysregulation. Psychology of Addictive Behaviors. 2002;16:72–75. doi:10.1037/0893-164X.16.1.72. [PubMed] [Google Scholar]
- Solomon R. L. The opponent-process theory of acquired motivation: The costs of pleasure and the benefits of pain. The American Psychologist. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. doi:10.1037/0003-066X.35.8.691. [DOI] [PubMed] [Google Scholar]
- Solomon R. L., Corbit J. D. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. doi:10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spielberger C. D. State-Trait Anger Expression Inventory. Wiley Online Library; 1999. http://onlinelibrary.wiley.com/doi/10.1002/9780470479216.corpsy0942/full Retrieved from. [Google Scholar]
- Spielberger C. D., Sydeman S. J., Owen A. E., Marsh B. J. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI) In: Maruish M. E., editor. The use of psychological testing for treatment planning and outcomes assessment. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 993–1021. [Google Scholar]
- Stephens R. S., Roffman R. A., Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. doi:10.1037/0022-006X.68.5.898. [PubMed] [Google Scholar]
- Stewart D. G., Brown S. A. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. doi:10.1111/j.1360-0443.1995.tb02201.x. [DOI] [PubMed] [Google Scholar]
- Thurber S., Hollingsworth D. K. Validity of the Achenbach and Edelbrock Youth Self-Report with hospitalized adolescents. Journal of Clinical Child and Adolescent Psychology. 1992;21:249–254. doi:10.1207/s15374424jccp2103_6. [Google Scholar]
- Vandrey R., Budney A. J., Kamon J. L., Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug and Alcohol Dependence. 2005;78:205–210. doi: 10.1016/j.drugalcdep.2004.11.001. doi:10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lang N. D., Ferdinand R. F., Oldehinkel A. J., Ormel J., Verhulst F. C. Concurrent validity of the DSM-IV scales affective problems and anxiety problems of the youth self-report. Behaviour Research and Therapy. 2005;43:1485–1494. doi: 10.1016/j.brat.2004.11.005. doi:10.1016/j.brat.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Vink J. M., Willemsen G., Beem A. L., Boomsma D. I. The Fagerström Test for Nicotine Dependence in a Dutch sample of daily smokers and ex-smokers. Addictive Behaviors. 2005;30:575–579. doi: 10.1016/j.addbeh.2004.05.023. doi:10.1016/j.addbeh.2004.05.023. [DOI] [PubMed] [Google Scholar]
- White H. R., Labouvie E. W. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. doi:10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]