Abstract
Background and aims
Lymphoma patients are frequently treated with cancer therapies that may increase the risk of adverse health outcomes later in life, including cardiovascular disease (CVD) mortality. We sought to investigate the long‐term risk of CVD incidence in this survivor population relative to the general population to quantify this health burden.
Methods
A systematic review and meta‐analysis was conducted using EMBASE, MEDLINE, and CINAHL databases, from date of inception to November 2016, with additional searches completed through June 2018. Included reports were observational studies assessing CVD incidence in patients of either Hodgkin or non‐Hodgkin lymphoma (HL, NHL) who survived for at least 5 years from the time of diagnosis or if the study had a median follow‐up of 10 years. Meta‐analyses were performed using random effects models, and subgroup analyses were conducted to determine the incidence of specific CVD subtypes (coronary heart disease, pericardial disease, valvular heart disease, myocardial disease, cardiac dysrhythmia, and cerebrovascular disease). Heterogeneity was assessed using I 2 statistics and prediction intervals.
Results
Of the 7734 studies identified, 22 studies were included in this review, representing 32 438 HL and NHL survivors. Relative to the general population, lymphoma survivors had statistically significant two to threefold increases in the risk for nearly all subtypes of CVD examined. Lymphoma survivors appeared to be particularly susceptible to pericardial diseases (HL: 10.67, 95% confidence interval (CI), 7.75‐14.69; NHL: 4.70, 95% CI, 2.08‐10.61) and valvular diseases (HL: 13.10, 95% CI, 7.41‐23.16; NHL: 3.76, 95% CI, 2.12‐6.66). Although the 95% CIs were suggestive of increased risks, the 95% prediction intervals often included the null, reflecting the high heterogeneity of the estimates.
Conclusion
Given the suggested increased risks of cardiovascular outcomes in lymphoma survivor populations relative to the general population, tailored screening and prevention programmes may be warranted to offset the future burden of disease.
Keywords: cardiovascular disease, Hodgkin, incidence, lymphoma, meta‐analysis, survivors
1. INTRODUCTION
Hodgkin Lymphoma (HL) and non‐Hodgkin Lymphoma (NHL) are solid tumours of the immune system common in both adults and children,1 accounting for an estimated 79 990 and 509 590 cases of cancer worldwide in 2018, respectively.2 Improvements in treatment and control strategies have resulted in an increased number of survivors, with 5‐year survival estimates of 86% and 70% for HL and NHL, respectively.3 Though many therapies have proven to be curative, there is increasing epidemiological evidence to suggest that individuals treated for cancer have an increased risk of adverse health outcomes, including fertility issues, cardiovascular diseases (CVD), and secondary cancers relative to the general population.4, 5, 6, 7, 8, 9, 10, 11, 12
In general, treatment for lymphoma involves chemotherapy alone or in combination with radiation, stem cell transplantation, or biologic therapies.3 The long‐term cardio‐toxic effects of these treatments, especially chemotherapy regimens utilizing anthracyclines and radiation therapy, have become more apparent in cancer survivors over the past decade.13, 14, 15, 16, 17, 18, 19 We previously conducted a meta‐analysis and found that the number of deaths due to CVDs within HL and NHL survivors were 7.31 (95% CI, 5.29‐10.10) and 5.35 (95% CI, 2.55‐11.24) times greater than the general population, respectively.20 In acknowledging that there is a substantially increased risk of mortality because of cardiovascular‐related events, we sought to further investigate if there is also an increased risk of CVD incidence within this population. It is possible that both the CVD incidence and mortality rates experienced by this survivor group relative to the general population are different because of cardio‐toxic effects of treatment and damage to the cardiovascular system. Additionally, given that HL and NHL account for 3.2% of all cancers globally,2, 21 there is a need to quantify the long‐term risk of CVD development among these survivors. Currently, international guidelines recommend lifelong follow‐up and surveillance of paediatric survivors treated with either high‐dose anthracyclines or high‐dose radiotherapy to the chest to decrease the burden of CVDs attributed to these treatments.22
To our knowledge, no meta‐analyses have previously examined the long‐term risk of CVD incidence among HL and NHL survivors compared with the general population. As such, in the current systematic review and meta‐analysis, we sought to examine the association of CVD development after treatment for HL and NHL, with particular emphasis on the type of CVD. We hypothesized that long‐term HL and NHL survivors will have an elevated risk of incident CVD events relative to the general population, and that the incidence would differ by type of CVD.
2. METHODS
2.1. Protocol and registration
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.23 The protocol was registered in PROSPERO (registration number: CRD42016052342).
2.2. Data sources and search strategy
We conducted a search of the EMBASE, MEDLINE, and CINAHL databases from their dates of inception up until November 22, 2016. The search strategy comprises four major themes related to our research question: (a) lymphoma, (b) long‐term survivor, (c) cardiovascular disease, and (d) observational study. There were no restrictions applied by geographical location, date, or language. Keywords, along with medical subject headings, were included in the search and have been previously published.20 Observational study design filters were adapted using keywords and subject headings from two previously designed search strategies.24, 25 Reference lists of included studies were hand‐searched to identify additional studies for inclusion. The search was rerun in MEDLINE on June 7, 2018 to ensure our results were up‐to‐date at the time of manuscript submission.
The initial screening was completed by two reviewers (D.J.B. and A.T.M.), who independently assessed articles in a two‐stage process. In the first stage, the title and abstract of each study were screened, and studies were then considered for full‐text assessment if they met the following criteria: (a) the study was published in a peer‐reviewed journal, (b) original data were presented, (c) human participants were under investigation, and (d) the article was relevant to the objectives of this review. In the second stage, studies were assessed in their entirety to determine whether or not they were eligible for inclusion into the systematic review. To be included in this review, all of the following criteria had to be met: (a) the population studied were patients with a diagnosis of and prior treatment for lymphoma; (b) the patients survived a minimum of 5 years after diagnosis, the study had a median follow‐up of at least 10 years from the time of diagnosis, or the study presented risk estimates specific to individuals who survived for 5 years or more after their diagnosis; (c) there was a comparator group that was representative of the general population; (d) the outcomes reported included risk, hazards, or odds ratios, or sufficient data were provided for their calculation; (e) the study was of a cohort, case‐control, nested case‐control, case‐cohort, or cross‐sectional design. A third reviewer updated the search utilizing the same two‐stage process (AM), consulting with C.R.S. and D.J.B. to ensure consistency.
At each stage of review, percent agreement and kappa (κ) statistics were used to quantify agreement between the two reviewers. Any disagreements were resolved by consensus between the reviewers. In cases where there were multiple studies using the same study population and assessing the same outcome, the study with the largest sample size was retained in the review and the study with the smaller sample size was excluded.
2.3. Data extraction and study quality assessment
A data extraction form to collect study information was created specifically for this review. Extracted variables included study population (ie, sex and median age at diagnosis), study characteristics (ie, design and comparator population), country, type(s) of CVD measured, median duration of follow‐up, treatment era, proportion receiving anthracycline chemotherapy, proportion treated with cardiac or mediastinal radiation, and mean cardiac radiation dose (Gy). Mean values were used when median values were not reported for relevant variables (ie, age at diagnosis and duration of follow‐up). Additionally, method of adjusting for confounders (modelling or matching), level of adjustment (crude, basic, extensive), study design, comparator group (expected, sibling or community controls), incidence or prevalence estimates (RR, SIR, HR, OR, or PR), and accompanying 95% confidence intervals (CIs) were extracted. For each study, we extracted incidence or prevalence estimates (RR, SIR, HR, OR, or PR) and 95% CIs. Estimates for population subgroups were extracted if overall estimates were not presented.
Clinical CVD endpoints were of interest in this review rather than subclinical endpoints. Estimates were categorized by cardiovascular disease type, as follows: (a) CVD reported without specification of type of disease; (b) coronary heart disease (CHD); (c) pericardial disease (PD); (d) valvular heart disease (VHD); (e) myocardial disease (MD); (f) cardiac dysrhythmia (CD); and (g) cerebrovascular disease (CBVD). If authors reported several incident outcomes that would be categorized into the same CVD subtype (eg, reporting estimates for two types of myocardial diseases: heart failure and cardiomyopathy), all relevant clinical study outcomes were extracted and included in the analysis, to maximize validity. It was assumed that reported outcomes would be largely independent from one another within a given publication (ie, few people would have developed multiple clinical CVD subtypes within a single study). The CVD groups are detailed in Table S1.
A single reviewer (A.M.) assessed study quality using the Newcastle‐Ottawa Scale for case‐control and cohort studies.26 This scale assessed the quality of included studies with scores ranging from 0 (indicating low quality studies) to 9 (indicating high quality studies). These scores came from three domains: selection (maximum of four points), comparability (maximum of two points), and outcome (maximum of three points).
2.4. Statistical analysis
Individual study results were pooled overall to derive a standardized incidence ratio (SIR) to estimate the risk of cardiovascular incidence among lymphoma survivors relative to the general population. All analyses were performed using Stata version 14.3. Meta‐analyses were conducted using a DerSimonian and Laird random‐effects model to acknowledge the clinical heterogeneity present in this body of literature.27 Meta‐analyses were stratified and conducted across CVD subtypes. Cumulative meta‐analyses were conducted within CVD subtypes to understand how the associations between lymphoma and types of CVD incidence changed over time.
Heterogeneity in the literature was assessed visually using forest plots, and statistically using I 2 statistics and prediction intervals using the Stata “rfdist” command.28, 29 To assess publication bias, we visually appraised funnel plots for asymmetry and also quantified asymmetry of funnel plots using Begg30 and Egger's31 regression tests. Trim‐and‐fill methods were applied to further assess publication bias where applicable.32
3. RESULTS
3.1. Literature search
We identified 7729 records in our database search and five from other sources (ie, reference list searches). After removing duplicates, 6282 records remained. Screening of titles/abstracts by two independent reviewers (A.T.M. and D.J.B.) resulted in 93.2% agreement on inclusion and exclusion (κ = 0.62). Nine hundred and thirty four records were eligible for full‐text screening. Full‐text screening resulted in 97.0% agreement on inclusion and exclusion criteria (κ = 0.73). After full‐text review, 22 records qualified for inclusion (Figure 1).33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54
Figure 1.
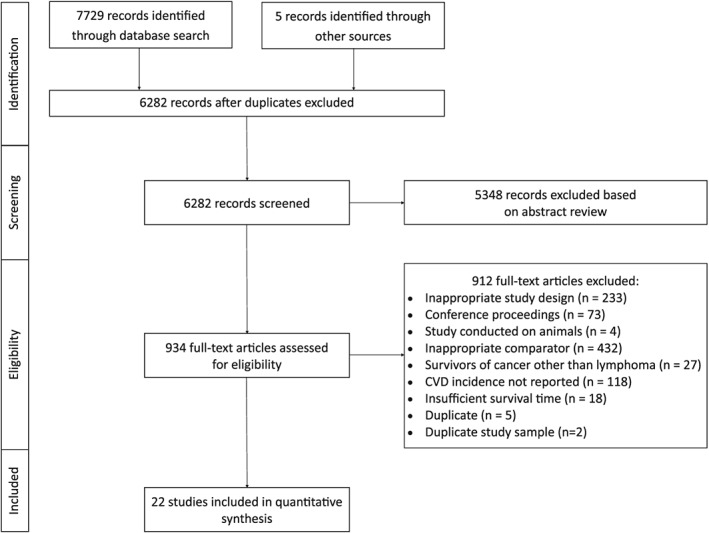
Flow of information through all phases of the systematic literature search
3.2. Study characteristics
The study characteristics for the 22 included reports are summarized in Table 1.33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Of the 22 articles included in this review, 13 (59%) originated from Europe and nine (41%) originated from North America. All studies had a cohort design, except for three,36, 49, 52 that were cross‐sectional studies. Thirteen studies presented standardized incidence ratios,33, 35, 37, 38, 39, 40, 42, 44, 47, 48, 50, 51, 54 two studies used hazard ratios,41, 46 three studies provided relative risk ratios,34, 43, 45 three studies used odds ratios,49, 52, 53 and one study presented a prevalence ratio.36 The average median duration of follow‐up for included studies was 14.7 years (range: 8.4 to 23.3; IQR: 13.6 to 18.0), median age at diagnosis was 27.1 years (range: 6 to 52; IQR: 19.7 to 33.8), and the median percentage of females present in the study populations was 46.4% (range: 38 to 64; IQR: 43.4 to 50.4).
Table 1.
Study characteristics of articles included in systematic review (n = 22)
| First Author (Year) | Cohort Designation | Country | Cancer Type | Sample Size (n) | Female (%) | Treatment Era (Years) | Agea (Years) | Follow Upb (Years) | Anthra‐cycline Exposure (%) | Mantle Field Radiation (%) | Outcomes Assessed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glanzmann (1998)33 | University Hospital of Zurich | Switzerland | HL | 352 | N/A | 1964‐1992 | 33.8 | 11.2 | 26.7 | 100.0 | CHD incidence |
| Reinders (1999)34 | Daniel den Hoed Cancer Center/Dijkzigt Hospital | Netherlands | HL | 258 | 47.7 | 1965‐1980 | 28 | 14.2 | 0.0 | 100.0 | CHD incidence |
| Hull (2003)35 | University of Florida Hospital | USA | HL | 415 | 39.0 | 1962‐1998 | 25.0 | 11.2 | 21.7 | 95.2 | CHD, VHD incidence |
| Ng (2005)36 | Brigham and Women's Hospital/Dana‐Farber Cancer Institute | USA | HL | 511 | 51.5 | 1969‐1996 | 44.0 | 15.0 | N/A | N/A | CVD prevalence |
| Moser (2006)37 | European Organization of Research and Treatment of Cancer Data Cohort | Netherlands and Belgium | NHL | 476 | 38.7 | 1980‐1999 | 49.0 | 8.4 | 35.7 | 0 | MD, CHD, CBVD incidence |
| Myrehaug (2008)38 | Princess Margaret Hospital/Toronto Sunnybrook Hospital | Canada | HL | 615 | 52.4 | 1988‐2000 | 29.0 | 11.8 | 62.4 | 81.6 | CVD, CHD, CVD, MD incidence |
| Andersson (2009)39 | Swedish Cancer Registry | Sweden | HL | 4635 | 41.4 | 1965‐1995 | 52.0 | 11.8 | N/A | N/A | CHD, CBVD, MD incidence |
| De Bruin (2009)40 | Netherland Hospitals | Netherlands | HL | 2201 | 44.0 | 1965‐1995 | 27.1 | 17.5 | 31.3 | 64.3 | CBVD incidence |
| Mulrooney (2009)41 | Childhood Cancer Survivor Study | USA | Both | 3008 | 46.3 | 1970‐1986 | 6.0 | 20.0 | 33.2 | N/A | CHD, MD, PD, VHD incidence |
| Galper (2011)42 | Harvard affiliated hospitals | USA | HL | 1279 | 46.4 | 1969‐1998 | 25.0 | 14.7 | 18.2 | 95.6 | CD, PD, CHD, VHD incidence |
| Lorenzi (2011)43 | British Columbia Cancer Registry | Canada | Both | 231 | 53.9 | 1981‐1995 | 9.0† | 12.0 | N/A | N/A | CVD incidence |
| Kurt (2012)44 | Childhood Cancer Survivor Study | USA | Both | 2136 | 48.0 | 1970‐1986 | 7.7 | 20.9 | 37.7 | N/A | CVD incidence |
| Mueller (2013)45 | Childhood Cancer Survivor Study | USA | Both | 2993 | 46.3 | 1970‐1986 | 7.8† | 23.3 | N/A | N/A | CBVD incidence |
| Kero (2014)46 | Finish Cancer Registry | Finland | Both | 2138 | 44.2 | 1975‐2004 | 21.4† | N/A | N/A | N/A | CBVD, CD, CHD, MD incidence |
| Rugbjerg (2014)47 | Danish Cancer Registry | Denmark | Both | 3459 | 60.2 | 1943‐2009 | 31.1† | 15.0 | N/A | N/A | CVD, CD, CHD, MD, VHD incidence |
| Gudmundsdottir (2015)48 | Adult Life after Childhood Cancer in Scandinavia (ALiCCS) | Nordic Countries5 | Both | 4138 | 46.4 | 1943‐2008 | 9.7† | 10.0 | N/A | N/A | CVD, CBVD, CD, CHD, MD, PD, VHD incidence |
| Murbraech (2015)49 | Norwegian Multicenter Study | Norway | Both | 274 | 38.0 | 1987‐2008 | 42.0 | 13.0 | 100.0 | N/A | MD prevalence |
| van Nimwegen (2015)50 | Netherland Hospitals | Netherlands | HL | 2524 | 45.7 | 1965‐1995 | 27.3 | 20.3 | N/A | 30.6 | CHD, MD incidence |
| Bhuller (2016)51 | British Columbia Cancer Registry | Canada | HL | 442 | 50.0 | 1970‐1999 | 19.7† | 19.6 | N/A | N/A | CVD incidence |
| Murbraech (2016)52 | Norwegian Multicenter Study | Norway | Both | 274 | 38.0 | 1987‐2008 | 42.0 | 13.0 | 100.0 | N/A | VHD prevalence |
| Bright (2017)54 | The Teenage and Young Adult Cancer Survivor Study (TYACSS) | England and Wales | Both | 23522 | N/A | 1971‐2006 | N/A | 11.3 | N/A | N/A | CBVD incidence |
| van Rosendael (2017)53 | Leiden University Medical Center | Netherlands | Both | 79 | 64 | 1980‐2005 | 26 | 10 | N/A | 41.8 | CHD prevalence |
Median age at diagnosis. Mean value reported when median not made available
Median follow‐up from time of diagnosis. Mean value reported when median not made available
Expected value calculated from available data. Mean dagger indicates that the information reported is not readily presented in the cited article, but rather that it has been calculated/derived based on available data from the cited article.
3.3. Study quality assessment
Attributes reflecting study quality are provided for all 22 studies in Table S2. Overall, the included studies were of high quality: six studies received 8 out of a possible 9 points on the Newcastle‐Ottawa Scale; 12 studies were scored at 7, three at 6, and only one study received a score of 5. All studies had a representative cohort of lymphoma survivors and a nonexposed comparator group drawn from the same community. Thirteen studies did not explicitly demonstrate that individuals with a history of CVD were excluded at baseline.33, 34, 35, 38, 39, 41, 42, 43, 44, 46, 51, 53, 54 All but three studies36, 37, 44 controlled or matched for age and sex, and four studies38, 41, 43, 46 controlled for additional factors. All studies had an adequate duration of follow‐up, which we defined as 5 years or more since time of diagnosis, or having a median follow‐up of 10 years or CVD incidence estimates exclusive to 5‐year survivors. Four investigations36, 41, 44, 45 relied on self‐reporting of CVD outcomes, however, all remaining studies measured the outcome objectively. Five studies did not describe the attrition of participants or had a loss‐to‐follow up greater than 20% with no description of lost participants.38, 39, 49, 52, 53
3.4. Meta‐analyses
Within HL survivors, there were statistically significant increased risks estimated for all seven CVD subtypes assessed relative to the general population, with the largest increases of risk seen for MD (3.95, 95% CI, 2.48‐6.27; I 2 = 97.1%), PD (10.67, 95% CI, 7.75‐14.69; I 2 = 63.3%), and VHD (13.10, 95% CI, 7.41‐23.16; I 2 = 96.2%) (Table 2). NHL survivors were also found to have statistically significant elevated risks for all CVD subtypes relative to the general population, with the exception of coronary heart diseases, for which the pooled effect estimate was 1.14 (95% CI, 0.95‐1.37). The largest increases of risk seen in NHL survivors were also MD (5.38; 95% CI, 3.35‐8.64; I 2 = 89.8%), PD (4.70; 95% CI, 2.08‐10.61), and VHD (3.76; 95% CI, 2.12‐6.66; I2 = 51.5%). When considering the 95% prediction intervals, nearly all results included the null value of 1 and were no longer statistically significant. Figures 2 and 3 depict forest plots for PD and VHD, respectively, sorted by median treatment era in ascending order, to allow visual inspection on how estimates have changed across time. Cumulative meta‐analyses conducted within CVD subtypes indicate that studies over time have consistently found statistically significant increases in risk and that the estimates are generally becoming more precise, as seen by narrowing CIs (Figures S1‐S13).
Table 2.
Preliminary analyses for pooled SIR of incident CVD in HL and NHL survivors (using random‐effects models)
| Outcome (CVD subtype) | Hodgkin Lymphoma | Non‐Hodgkin's Lymphoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of estimates | Pooled SIR estimate | 95% CI | I 2 | 95% Prediction Interval | No. of studies | No. of estimates | Pooled SIR estimate | 95% CI | I 2 | 95% Prediction interval | |
| CBVD | 7 | 10 | 1.99 | 1.56‐2.55 | 90.3% | 0.81‐2.55 | 5 | 5 | 1.66 | 1.46‐1.88 | 0.0% | 1.35‐2.04 |
| CD | 4 | 6 | 3.23 | 2.23‐4.68 | 93.0% | 0.86‐12.17 | 2 | 4 | 1.96 | 1.22‐3.13 | 72.3% | 0.27‐14.36 |
| CHD | 11 | 19 | 2.54 | 1.95‐3.31 | 97.2% | 0.80‐8.11 | 4 | 6 | 1.14 | 0.95‐1.37 | 0.0% | 0.88‐1.48 |
| CVD | 6 | 6 | 2.37 | 1.43‐3.91 | 98.1% | 0.38‐14.76 | 3 | 3 | 1.90 | 1.43‐2.52 | 86.7% | 0.06‐57.39 |
| MD | 7 | 12 | 3.95 | 2.48‐6.27 | 97.1% | 0.64‐24.50 | 5 | 7 | 5.38 | 3.35‐8.64 | 89.8% | 1.02‐28.33 |
| PD | 3 | 3 | 10.67 | 7.75‐14.69 | 63.3% | 0.33‐343.56 | 1 | 1 | 4.70 | 2.08‐10.61 | ‐ | ‐ |
| VHD | 5 | 5 | 13.10 | 7.41‐23.16 | 96.2% | 1.50‐114.72 | 2 | 2 | 3.76 | 2.12‐6.66 | 51.5% | ‐ |
Abbreviations: CBVD, cerebrovascular disease; CD, cardiac dysrhythmia; CHD, coronary heart disease; CVD, any cardiovascular disease; MD, myocardial disease; PD, pericardial disease; VHD, valvular heart disease.
Figure 2.
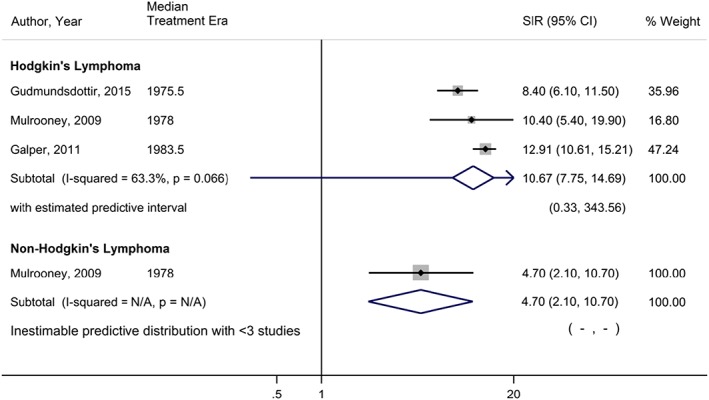
Forest plot of the risk of pericardial disease among lymphoma survivors, sorted in ascending order by median treatment era of each study
Figure 3.
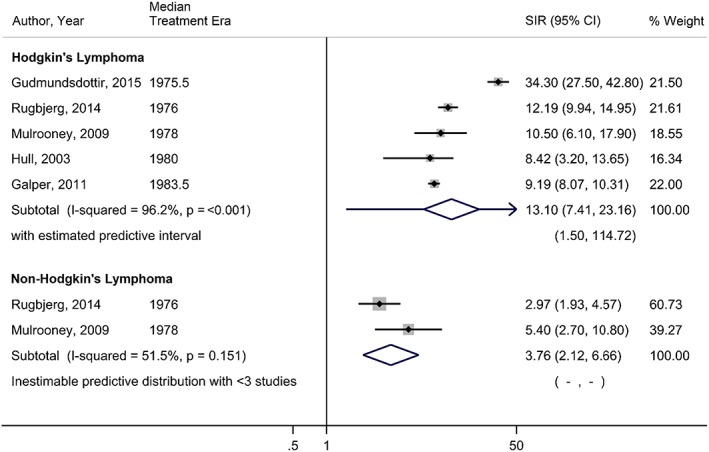
Forest plot of the risk of valvular heart disease among lymphoma survivors, sorted in ascending order by median treatment era of each study
3.5. Publication Bias
Publication bias was assessed within each CVD subtype among HL and NHL survivors. Within HL analyses, there was no evidence of publication bias according to either the Begg test or the Egger test, or inspection of funnel plots for CD, CHD, CVD, MD, PD, or VHD. Cerebrovascular disease, however, showed evidence of potential publication bias (Begg test P value = .032; Egger test P value = .060). Additionally, within NHL analyses, there was no evidence of statistically significant publication bias for CD, CVD, or MD, although both CBVD and CHD displayed conflicting evidence between the Begg test and the Egger tests (P values .462 and .034; and .260 and .030, respectively, for CBVD and CHD), indicating a possibility of publication bias present for these estimates. After applying trim‐and‐fill methods, the estimates were not changed significantly.
4. DISCUSSION
This systematic review and meta‐analysis suggests that, compared with the general population, lymphoma survivors are at an elevated risk of developing cardiovascular events. Together with the meta‐analysis that we previously completed, in which we investigated cardiovascular mortality in lymphoma survivors,20 it is apparent that both HL and NHL survivors have both a higher incidence and severity of cardiovascular events compared with the general population. Though there were high levels of unexplained heterogeneity present, a novel finding in our meta‐analysis is the differences in observed magnitude of increased risk between the various CVD subtypes, notably, the 10‐fold and 13‐fold increases in risk for PD and VHD, respectively, in HL. PD in lymphoma survivors may be more severe in magnitude compared with the general population because of the use of chemotherapeutic drugs including anthracyclines, as well as mediastinal radiation. It is possible that our inclusion criterion of results from studies of survivors who were at least 5 years post‐treatment could explain, in part, the higher incidence of pericardial disease that we observed. Delayed pericardial diseases can develop from 6 months post‐radiation treatment to 15‐years post treatment.55 Cardiac valves are not directly damaged by chemotherapeutic agents, however, radiation‐induced VHD is a relatively common side effect reported for lymphoma survivors.35 Interestingly, coronary heart disease in NHL was found to be the only cardiovascular subtype that did not have a statistically significant increased risk compared with the general population. In a consensus statement by Lancellotti et al, the authors state that coronary artery disease (which is captured within our CHD subtype), is latent until at least 10‐years after radiation exposure.56 This latency period may account for the nonapparent increased risk found in this subtype, since the patients included in our review may not have survived long enough to experience this outcome.
It is unlikely that the associations found in this meta‐analysis are spurious, for several reasons. First, temporality is evident, since all survivors must have been treated for HL or NHL to be subsequently assessed for CVD incidence within each included study. Second, the cumulative meta‐analyses performed demonstrated both consistency in the reporting of increased cardiovascular event incidence, as well as consistency in the overall strength and magnitude of the associations, with between 1.1 to 13.1 times increased risk of various cardiovascular conditions relative to the general population. Finally, there are hypothesized biologic mechanisms that could explain how lymphoma treatment may lead to increased risk of CVD. For example, anthracyclines are efficacious in the treatment of lymphomas, however, they generate reactive oxygen species and lipid perioxidation of the cell membrane, which can damage cardiomyocytes.55 These mechanisms and treatments have also been found to increase the risk of traditional cardiovascular risk factors such as hypertension, diabetes mellitus, dyslipidaemia, and obesity,57 which may further contribute to the increased incidence of CVD in lymphoma survivors relative to the general population. Several chronic inflammatory conditions might also be associated with increased CVD risk.58 We did not, however, directly examine whether or not lymphoma survivors have a risk that is similar or higher to other conditions that may be diagnosed in childhood, such as inflammatory bowel disease or juvenile rheumatoid arthritis. Furthermore, there may be an increased risk of CVD morbidity and mortality associated with childhood cancers. Consequently, there is a need to transition survivors of severe childhood illness carefully to adult care so that CVD screening can occur and adverse outcomes can be averted.
One of the limitations of this study is the level of heterogeneity found in pooled estimates. Though we presented the results separately by cancer type (HL and NHL) and by specific type of cardiovascular event, to assess the associations for these specific combinations of cancer and cardiovascular events, there were still high levels of heterogeneity that could not be explained, as there were not enough studies present within combinations of cancer and cardiovascular events to use meta‐regression techniques. The inclusion of prediction intervals aids in the clinical interpretation of the high heterogeneity found in our study, by estimating possible treatment effects that can be expected in future settings.59 Although the 95% CIs consistently suggested increased risks, the 95% prediction intervals included values consistent with a null effect or an effect in the opposite direction. Considering the high degree of heterogeneity in this evidence base, it is difficult to make any firm conclusions. Given the nature of observational studies, there is likely residual confounding that may introduce some bias to our pooled estimates. To address this concern, the most adjusted measure of risk/incidence was used in the meta‐analysis. Another limitation of this study was that we did not restrict to studies only looking at contemporary treatments, and there have been changes in treatments over time. Therefore, it is possible that the large effects found in our analyses may be overestimating the effects that truly occur in current practice with improved treatment modalities.60
In conclusion, this systematic review and meta‐analysis is the first to investigate the long‐term risks of CVD subtype incidence among HL and NHL survivors compared with the general population. Even if these risk estimates are overestimated because of uncontrolled confounding or heterogeneous studies, the overall magnitude of associations is strong enough to support the importance of utilizing cardiovascular screening, prevention, and surveillance programmes within this population of lymphoma survivors to potentially mitigate the future burden of CVD.
FUNDING
There was no specific funding source for this study.
CONFLICT OF INTEREST
Doreen M Rabi received travel reimbursement from Hypertension Canada. This funding did not influence the study design, collection, analysis and interpretation of data; writing of the manuscript; or the decision to submit for publication.
AUTHOR CONTRIBUTIONS
Conceptualization: Chelsea R Stone, Alexis T Mickle, Devon J Boyne, Doreen M Rabi, Darren R Brenner, Christine M Friedenreich
Formal analysis: Chelsea R Stone
Supervision: Doreen M Rabi, Darren R Brenner, Christine M Friedenreich
Writing – original draft: Aliya Mohamed, Chelsea Stone
Writing – review and editing: Chelsea R Stone, Alexis T Mickle, Devon J Boyne, Aliya Mohamed, Doreen M Rabi, Darren R Brenner, Christine M Friedenreich
All authors have read and approved the final version of the manuscript.
The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
The corresponding author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
Figure S1. Cumulative meta‐analysis for the incident cerebrovascular disease in Hodgkin's Lymphoma survivors.
Figure S2. Cumulative meta‐analysis for the incident cardiac dysrhythmia in Hodgkin's Lymphoma survivors.
Figure S3. Cumulative meta‐analysis for the incident coronary heart disease in Hodgkin's Lymphoma survivors.
Figure S4. Cumulative meta‐analysis for the incident cardiovascular disease in Hodgkin's Lymphoma survivors.
Figure S5. Cumulative meta‐analysis for the incident myocardial disease in Hodgkin's Lymphoma survivors.
Figure S6. Cumulative meta‐analysis for the incident pericardial disease in Hodgkin's Lymphoma survivors.
Figure S7. Cumulative meta‐analysis for the incident valvular heart disease in Hodgkin's Lymphoma survivors.
Figure S8. Cumulative meta‐analysis for the incident cerebrovascular disease in Non‐Hodgkin's Lymphoma survivors.
Figure S9. Cumulative meta‐analysis for the incident cardiac dysrhythmia in Non‐Hodgkin's Lymphoma survivors.
Figure S10. Cumulative meta‐analysis for the incident coronary heart disease in Non‐Hodgkin's Lymphoma survivors.
Figure S11. Cumulative meta‐analysis for the incident cardiovascular disease in Non‐Hodgkin's Lymphoma survivors.
Figure S12. Cumulative meta‐analysis for the incident myocardial disease in Non‐Hodgkin's Lymphoma survivors.
Figure S13. Cumulative meta‐analysis for the incident valvular heart disease in Non‐Hodgkin's Lymphoma survivors.
Table S1. Cardiovascular Disease Term Groupings
Supplemental Table 2. Newcastle‐Ottawa Scale for study quality assessment
Stone CR, Mickle AT, Boyne DJ, et al. Treatment for lymphoma and late cardiovascular disease risk: A systematic review and meta‐analysis. Health Sci Rep. 2019;2:e135 10.1002/hsr2.135
Protocol Registration Number: CRD42016052342
REFERENCES
- 1. Shankland KR, Armitage JO, Hancock BW. Non‐Hodgkin lymphoma. Lancet. 2012;380(9844):848‐857. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 3. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271‐289. [DOI] [PubMed] [Google Scholar]
- 4. Arden‐Close E, Eiser C, Pacey A. Sexual functioning in male survivors of lymphoma: a systematic review (CME). J Sex Med. 2011;8(7):1833‐1841. [DOI] [PubMed] [Google Scholar]
- 5. Bower JE. Cancer‐related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franco VI, Lipshultz SE. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol Young. 2015;25(Suppl 2):107‐116. [DOI] [PubMed] [Google Scholar]
- 7. Iyer NS, Balsamo LM, Bracken MB, Kadan‐Lottick NS. Chemotherapy‐only treatment effects on long‐term neurocognitive functioning in childhood ALL survivors: a review and meta‐analysis. Blood. 2015;126(3):346‐353. [DOI] [PubMed] [Google Scholar]
- 8. Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life‐long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300‐322. [DOI] [PubMed] [Google Scholar]
- 11. Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289‐301. [DOI] [PubMed] [Google Scholar]
- 12. Travis LB, Ng AK, Allan JM, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. Health Phys. 2014;106(2):229‐246. [DOI] [PubMed] [Google Scholar]
- 13. Valcovici M, Andrica F, Serban C, Dragan S. Cardiotoxicity of anthracycline therapy: current perspectives. Arch Med Sci. 2016;12(2):428‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61(23):2319‐2328. [DOI] [PubMed] [Google Scholar]
- 15. Haddy N, Diallo S, El‐Fayech C, et al. Cardiac diseases following childhood cancer treatment: cohort study. Circulation. 2016;133(1):31‐38. [DOI] [PubMed] [Google Scholar]
- 16. Svilaas T, Lefrandt JD, Gietema JA, Kamphuisen PW. Long‐term arterial complications of chemotherapy in patients with cancer. Thromb Res. 2016;140(Suppl 1):S109‐S118. [DOI] [PubMed] [Google Scholar]
- 17. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bini I, Asaftei SD, Riggi C, et al. Anthracycline‐induced cardiotoxicity in patients with paediatric bone sarcoma and soft tissue sarcoma. Cardiol Young. 2017;27(9):1815‐1822. [DOI] [PubMed] [Google Scholar]
- 19. Cheng YJ, Nie XY, Ji CC, et al. Long‐term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6(5):e005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyne DJ, Mickle AT, Brenner DR, et al. Long‐term risk of cardiovascular mortality in lymphoma survivors: a systematic review and meta‐analysis. Cancer Med. 2018;7(9):4801‐4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. [DOI] [PubMed] [Google Scholar]
- 22. Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123‐e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. University of Texas School of Public Health . Search filters for case‐control studies, cohort studies, cross‐ sectional studies, clinical trials, epidemiological studies. 2013.
- 25. Evidence BC. Study design search filters. 2012. p. 42‐43.
- 26. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2014.
- 27. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 28. Harris RJ, Deeks JJ, Altman DJ, Bradburn MJ, Harbord RM, Sterne JA. Metan: fixed and random‐effects meta‐analysis. Stata J. 2008;8(1):3‐28. [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer TM, Sutton AJ, Peters JL, Moreno SG. Contour‐enhanced funnel plots for meta‐analysis. Stata J. 2008;8(2):242‐254. [Google Scholar]
- 33. Glanzmann C, Kaufmann P, Jenni R, Hess OM, Huguenin P. Cardiac risk after mediastinal irradiation for Hodgkin's disease. Radiother Oncol. 1998;46(1):51‐62. [DOI] [PubMed] [Google Scholar]
- 34. Reinders JG, Heijmen BJ, Olofsen‐van Acht MJ, van Putten WL, Levendag PC. Ischemic heart disease after mantlefield irradiation for Hodgkin's disease in long‐term follow‐up. Radiother Oncol. 1999;51(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 35. Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290(21):2831‐2837. [DOI] [PubMed] [Google Scholar]
- 36. Ng AK, Li S, Recklitis C, et al. A comparison between long‐term survivors of Hodgkin's disease and their siblings on fatigue level and factors predicting for increased fatigue. Ann Oncol. 2005;16(12):1949‐1955. [DOI] [PubMed] [Google Scholar]
- 37. Moser EC, Noordijk EM, van Leeuwen FE, et al. Long‐term risk of cardiovascular disease after treatment for aggressive non‐Hodgkin lymphoma. Blood. 2006;107(7):2912‐2919. [DOI] [PubMed] [Google Scholar]
- 38. Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra‐additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49(8):1486‐1493. [DOI] [PubMed] [Google Scholar]
- 39. Andersson A, Naslund U, Tavelin B, Enblad G, Gustavsson A, Malmer B. Long‐term risk of cardiovascular disease in Hodgkin lymphoma survivors—retrospective cohort analyses and a concept for prospective intervention. Int J Cancer. 2009;124(8):1914‐1917. [DOI] [PubMed] [Google Scholar]
- 40. De Bruin ML, Dorresteijn LD, van't Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5‐year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101(13):928‐937. [DOI] [PubMed] [Google Scholar]
- 41. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339(dec08 1):b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412‐418. [DOI] [PubMed] [Google Scholar]
- 43. Lorenzi MF, Xie L, Rogers PC, Pritchard S, Goddard K, McBride ML. Hospital‐related morbidity among childhood cancer survivors in British Columbia, Canada: report of the childhood, adolescent, young adult cancer survivors (CAYACS) program. Int J Cancer. 2011;128(7):1624‐1631. [DOI] [PubMed] [Google Scholar]
- 44. Kurt BA, Nolan VG, Ness KK, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatr Blood Cancer. 2012;59(1):126‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kero AE, Jarvela LS, Arola M, et al. Cardiovascular morbidity in long‐term survivors of early‐onset cancer: a population‐based study. Int J Cancer. 2014;134(3):664‐673. [DOI] [PubMed] [Google Scholar]
- 47. Rugbjerg K, Mellemkjaer L, Boice JD, Kober L, Ewertz M, Olsen JH. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943‐2009. J Natl Cancer Inst. 2014;106(6):dju110. [DOI] [PubMed] [Google Scholar]
- 48. Gudmundsdottir T, Winther JF, de Fine LS, et al. Cardiovascular disease in adult life after childhood cancer in Scandinavia: a population‐based cohort study of 32308 one‐year survivors. Int J Cancer. 2015;137(5):1176‐1786. [DOI] [PubMed] [Google Scholar]
- 49. Murbraech K, Smeland KB, Holte H, et al. Heart failure and asymptomatic left ventricular systolic dysfunction in lymphoma survivors treated with autologous stem‐cell transplantation: a national cross‐sectional study. J Clin Oncol. 2015;33(24):2683‐2691. [DOI] [PubMed] [Google Scholar]
- 50. van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40‐year disease risk. JAMA Intern Med. 2015;175(6):1007‐1017. [DOI] [PubMed] [Google Scholar]
- 51. Bhuller KS, Zhang Y, Li D, et al. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol. 2016;172(5):757‐768. [DOI] [PubMed] [Google Scholar]
- 52. Murbraech K, Wethal T, Smeland KB, et al. Valvular dysfunction in lymphoma survivors treated with autologous stem cell transplantation: a national cross‐sectional study. JACC Cardiovasc Imaging. 2016;9(3):230‐239. [DOI] [PubMed] [Google Scholar]
- 53. van Rosendael AR, Daniels LA, Dimitriu‐Leen AC, et al. Different manifestation of irradiation induced coronary artery disease detected with coronary computed tomography compared with matched non‐irradiated controls. Radiother Oncol. 2017;125(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 54. Bright CJ, Hawkins MM, Guha J, et al. Risk of cerebrovascular events in 178 962 five‐year survivors of cancer diagnosed at 15 to 39 Years of Age: The TYACSS (Teenage and Young Adult Cancer Survivor Study). Circulation. 2017;135(13):1194‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768‐2801. [DOI] [PubMed] [Google Scholar]
- 56. Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi‐modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(9):1013‐1032. [DOI] [PubMed] [Google Scholar]
- 57. Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673‐3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu P, Jia F, Zhang B, Zhang P. Risk of cardiovascular disease in inflammatory bowel disease. Exp Ther Med. 2017;13(2):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koshy M, Fairchild A, Son CH, Mahmood U. Improved survival time trends in Hodgkin's lymphoma. Cancer Med. 2016;5(6):997‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cumulative meta‐analysis for the incident cerebrovascular disease in Hodgkin's Lymphoma survivors.
Figure S2. Cumulative meta‐analysis for the incident cardiac dysrhythmia in Hodgkin's Lymphoma survivors.
Figure S3. Cumulative meta‐analysis for the incident coronary heart disease in Hodgkin's Lymphoma survivors.
Figure S4. Cumulative meta‐analysis for the incident cardiovascular disease in Hodgkin's Lymphoma survivors.
Figure S5. Cumulative meta‐analysis for the incident myocardial disease in Hodgkin's Lymphoma survivors.
Figure S6. Cumulative meta‐analysis for the incident pericardial disease in Hodgkin's Lymphoma survivors.
Figure S7. Cumulative meta‐analysis for the incident valvular heart disease in Hodgkin's Lymphoma survivors.
Figure S8. Cumulative meta‐analysis for the incident cerebrovascular disease in Non‐Hodgkin's Lymphoma survivors.
Figure S9. Cumulative meta‐analysis for the incident cardiac dysrhythmia in Non‐Hodgkin's Lymphoma survivors.
Figure S10. Cumulative meta‐analysis for the incident coronary heart disease in Non‐Hodgkin's Lymphoma survivors.
Figure S11. Cumulative meta‐analysis for the incident cardiovascular disease in Non‐Hodgkin's Lymphoma survivors.
Figure S12. Cumulative meta‐analysis for the incident myocardial disease in Non‐Hodgkin's Lymphoma survivors.
Figure S13. Cumulative meta‐analysis for the incident valvular heart disease in Non‐Hodgkin's Lymphoma survivors.
Table S1. Cardiovascular Disease Term Groupings
Supplemental Table 2. Newcastle‐Ottawa Scale for study quality assessment
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


