Abstract
Many clinical studies have been conducted on ketamine-associated cystitis. However, the underlying mechanisms of ketamine-associated cystitis still remain unclear. Bladder tissues of rats were stained by Hematoxylin and Eosin (HE). The viability of human uroepithelial cells (SV-HUC-1 cells) was determined by cell counting kit-8 (CCK-8). Apoptosis and reactive oxygen species (ROS) were examined by flow cytometry. Additionally, the expressions of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β and IL-18 were respectively determined by reverse transcription quantitative (RTq)-PCR and enzyme-linked immunosorbent assay (ELISA). The mRNA and protein levels of B-cell lymphoma/leukemia-2 (Bcl2), Bcl-2-associated X protein (Bax), cleaved caspase 3, glucose-regulated protein 78 (GRP78), CCAAT/enhancer binding protein homologous protein (CHOP), NOD-like receptor 3 (NLRP3), thioredoxin-interacting protein (TXNIP), Catalase and MnSOD were examined by RT-qPCR and Western blot. Small interfering RNA target TXNIP transfection was performed using Lipofectamine™ 2000. We found that ketamine effectively damaged bladder tissues of rats and promoted apoptosis through regulating the expression levels of GRP78, CHOP, Bcl-2, Bax and cleaved Caspase-3 proteins in vivo and in vitro. NLRP3 inflammatory body and TXNIP were activated by ketamine, which was supported by the changes in TNF-α, IL-6, IL-1 and IL-18 in vivo and in vitro. Furthermore, knocking down TXNIP reversed the effects of ketamine on apoptosis and NLRP3 inflammatory body in SV-HUC-1 cells. Meanwhile, the changes of Catalase and MnSOD showed that ROS was enhanced by ketamine, however, such an effect was ameliorated by down-regulation of TXNIP in SV-HUC-1 cells. Ketamine promoted cell apoptosis and induced inflammation in vivo and in vitro by regulating NLRP3/TXNIP aix.
Keywords: apoptosis, Ketamine, NOD-like receptor 3/Thioredoxin-interacting protein, SV-HUC-1 cells
Introduction
Ketamine, which is a derivative of N-1-phenycyclohexy-piperidine (PCP), is a non-competitive antagonist of glutamate N-methyl-d-aspartate (NMDA) receptor. As ketamine inhibits the thalamic–neocortical system, selectively blocks pain and has an analgesic pharmacological effect, it is considered as an effective anesthetic [1–3]. Studies have shown that long-term abuse of ketamine often causes damage to acute urological and medical issues, and one of the most common complications is ketamine-associated cystitis [4–6]. However, the underlying mechanism of ketamine-associated cystitis progression remains to be elucidated.
Our previous research findings demonstrated that ketamine enhanced the production of reactive oxygen species (ROS) in human ureteral epithelial cells (SV-HUC-1) cells [7]. ROS overactivation activates the development of ERS and leads to the accumulation of unfolded proteins, which can be isolated from the three sensors of ERS to activate endoplasmic reticulum (ER) chaperone glucose-regulated protein 78 (GRP78) [8]. Therefore, a rapid up-regulation of GRP78 is considered as the most sensitive marker of ER stress. CCAAT/enhancer binding protein homologous protein (CHOP) is an important signaling molecule for pro-apoptosis, and ER stress-specific transcription factor, which has a low expression under normal conditions but a high expression under ER stress [9]. Thus, CHOP is considered as a marker of ER stress. Caspase could control the occurrence and progression of apoptosis by causing nuclear shrinkage and DNA segmentation to form apoptosis through cleaving protein kinases, nucleases and cytoskeleton [10,11]. Among them, caspase3 is the most important effector protease in the caspase cascade [12]. Bcl-2-associated X protein (Bax)/B-cell lymphoma/leukemia-2 (Bcl2) has been recognized as a protein associated with apoptosis [13]. Ketamine causes liver and neuronal apoptosis via Bax/Bcl2-mitochondrial-caspase protease pathway [14,15]. It is speculated that the Bax/Bcl2-mitochondrial-caspase protease pathway may be one of the related mechanisms underlying ketamine-induced apoptosis of bladder epithelial cells.
Recent researches have demonstrated that inflammation could induce ROS, leading to oxidative stress and may result in bladder dysfunction [16,17]. As an endogenous inhibitor of thioredoxin, thioredoxin-interacting protein (TXNIP) is an important antioxidant reductive protein and anti-apoptotic protein in cells [18]. Studies have shown that TXNIP activated NOD-like receptor 3 (NLRP3) inflammatory pathway under oxidative stress, thus causing the secretion of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 [19,20]. In recent years, it was found that NLRP3 inflammation was involved in the progression of cystitis [21,22], therefore, targeting the TXNIP/NLRP3 signal axis may be an effective therapeutic target for treating ketamine-associated cystitis.
Our study provides a better understanding ofn the underlying mechanisms of ketamine in ER stress and TXNIP/NLRP3 in vivo and in vitro as well as target strategy for treating ketamine-associated cystitis.
Materials and methods
Rats and ketamine treatment
Adult male Wistar rats (180–200 g) were purchased from the SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The rats were kept in a specific pathogen-free (SPF) environment at room temperature (25 ± 2°C) in 60–80% humidity under a 12-h/12-h light/dark cycle, and free access to food and water was provided. The rats were randomly divided into four groups, which were control group, saline group (NC), low-dose group (L-KET, 5 mg/kg) and high-dose group (H-KET, 50 mg/kg), with six rats in each group. The rats in the experimental group were intraperitoneally injected with ketamine or saline at the same volume at 3:00 p.m. everyday for 3 months. All animal experiments were carried out at The Affiliated Yantai Yuhuangding Hospital of Qingdao University and approved by Qingdao University Animal Ethics Committee (QD2573466).
Hematoxylin and Eosin staining
A small section was cut from bladder tissue of the rat by using microtome. The specimen was fixed with a 10% paraformaldehyde solution for more than 48 h, conventionally dehydrated and paraffin-embedded to prepare a 5-micron tissue section. The slices were baked in a 68°C incubator for 1–2 h and then placed in xylene for 30 min for three times to be dewaxed. Next, the sections were placed in 100, 95, 85 and 75% gradient alcohol for 5 min to be hydrated. After washing the slices for 2 min by tap water, the sections were stained by Hematoxylin for 10 min and by Eosin for 30 s. The sections were then infiltrated with xylene for 5 s, sealed by neutral gum and observed under a microscope (Olympus, Japan).
Cells and culture
SV-HUC-1 cells used in the present study were purchased from the American Type Culture Collection (ATCC, Wiltshire, U.S.A.) and were grown in RPMI 1640 medium containing 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, U.S.A.) in a humidified atmosphere with 5% CO2 at 37°C. The SV-HUC-1 cells were treated with 0.01, 0.1 and 1 mmol/l concentrations of ketamine and then the SV-HUC-1 cells were co-treated by 1 mmol/l ketamine with or without small interfering RNA of TXNIP or NC RNA.
Cell counting kit-8
Ketamines (0.01, 0.1 and 1 mmol/l) were used to treat the SV-HUC-1 cells for 24, 48 and 72 h, and cell viability was detected by cell counting kit-8 (CCK-8). To be more specific, the cells were digested with trypsin, adjusted to 1000 cells/well and placed in a 96-well culture plate at a volume of 100 μl/well. The medium in each well to be tested was washed away, and 10 μl of CCK-8 reagent was added to the well and incubated at 37°C with 5% CO2 for 2 h. A microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.) was used to determine the OD at an absorbance of 450 nm in each well in different cell groups.
Enzyme-linked immunosorbent assay
Cells were treated with ketamine (0.01, 0.1 and 1 mmol/l) or 1 mmol/l ketamine in combination with siRNA TXNIP or NC siRNA. Protein was isolated by RIPA (Cell Signaling Technology, Inc., Danvers, MA, U.S.A.), and BCA Protein Assay Kit (Pierce) was used to measure the concentrations of the proteins. The levels of IL-1β and IL-18 were determined using corresponding enzyme-linked immunosorbent assay (ELISA) (MD SpectraMax M5; Molecular Devices, U.S.A.) following the manufacturer’s instructions.
Apoptosis
Apoptosis detection kit (BD Biosciences Medical Devices Shanghai Co., Ltd., Shanghai, China) was used to detect SV-HUC-1 cells apoptosis. The SV-HUC-1 cells (5 × 105 cells/well) were seeded in six-well plates to reach 85% cell density. Ketamine at concentrations of 0.01, 0.1 and 1 mmol/l were used to treat SV-HUC-1 cells, and 1 mmol/l ketamine-treated SV-HUC-1 cells were treated by siTXNIP, cultured in serum-free medium for 5 h and the medium was replaced. After 48 h of culture, the cells were digested, centrifuged and washed twice with phosphate-buffered saline (PBS). A total of 100 μl of 1× Annexin-V Binding Buffer and 5 μl FITC-labeled Annexin-V (20 μg/ml) were added to the cells, which were kept at room temperature for 20 min. Next, the cells were added with 5 μl of PI (50 μg/ml), held for 5 min and 400 μl of binding buffer was then added. A flow cytometer (BD Bioscience, Shanghai, China) was used to analyze cell apoptosis.
Reverse transcription quantitative polymerase chain reaction
After the transfection, SV-HUC-1 cells were incubated in an incubator for 48 h, and total RNA from SV-HUC-1 cells was extracted using TRIzol® reagent (Thermo Fisher Scientific, Inc.). A NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific, Inc.) was conducted to quantify RNA (an A260/A280 ratio between 1.8 and 2.0 was required for generating cDNA). Oligo-dT or stem-loop reverse transcriptase primers (Takara Bio, Inc., Otsu, Japan) were used to obtain cDNA, and the reaction conditions were set as follows: at 42°C for 60 min, 70°C for 5 min and preserved at 4°C. qPCR was performed by the SYBR® Premix ExTaq™ II (Takara Bio Inc.) using real-time PCR Detection System (ABI 7500, Life Technology, U.S.A.). PCR conditions were set as follows: a pretreatment at 95°C for 10 min, followed by 40 cycles at 94°C for 15 s, at 60°C for 1 min, finally at 60°C for 1 min and preserved at 4°C. The 2−ΔΔCq method was used to process the data. The primers used in this experiment are listed in Table 1.
Table 1. Primers for RT-qPCR (R = Rat, H = Human).
| Genes | Forward | Reverse |
|---|---|---|
| GRP78 (H) | GTTTGCTGAGGAAGACAAAAAGCC | CACTTCCATAGAGTTTGCTGATATG |
| CHOP (H) | GGAAACAGAGTGGTCATTCCC | CTGCTTGAGCCGTTCATTCTC |
| Bax (H) | GAGCGAGTGTCTCCGGCGAATT | GCCACAAAGATGGTCACTGTCTGC |
| Bcl-2 (H) | TGGGATGCCTTTGTGGAACTAT | GCTGATTTGACCATTTGCCTGA |
| NLRP3 (H) | GATCTTCGCTGCGATCAACAG | CGTGCATTATCTGAACCCCAC |
| TXNIP (H) | GCCACACTTACCTTGCCA AT | TTGGATCCAGGAACGCTA AC |
| TNF-α (H) | ACCTCTCTCTAATCAGCCCTCT | GGGTTTGCTACAACATGGGCTA |
| IL-6 (H) | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG |
| MnSOD (H) | CGTGCTCCCACACATCAATC | TGAACGTCACCGAGGAGAAG |
| Catalase (H) | GCAGATACCTGTGAACTGTC | GTAGAATGTCCGCACCTGAG |
| GAPDH (H) | AACGTGTCAGTGGTGGACCTG | AGTGGGTGTCGCTGTTGAAGT |
| GRP78 (R) | CTGGGTACATTTGATCTGACTGG | GCATCCTGGTGGCTTTCCAGCCATC |
| CHOP (R) | CCAGCAGAGGTCACAAGCAC | CGCACTGACCACTCTGTTTC |
| TXNIP (R) | CTGATGGAGGCACAGTGAGA | CTCGGGTGGAGTGCTTAGAG |
| NLRP3 (R) | CAGACCTC CAAGACCACGACTG | CATCCGCAGCCAATGAACAGAG |
| Bax (R) | GCGAATTGGAGATGAACTGG | GTGAGCGAGGCGGTGAGGAC |
| Bcl-2 (R) | CTGGTGGACAACATCGCTCTG | GGTCTGCTGACCTCACTTGTG |
| GAPDH (R) | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
Western blot
SV-HUC-1 cells were treated with siTXNIP, TXNIP and corresponding control plasmid, and then cultured in an incubator for 48 h. Total proteins were collected by RIPA (Cell Signaling Technology, Inc., Danvers, MA, U.S.A.). BCA Protein Assay Kit (Pierce) was used to measure the concentration of proteins, which was adjusted to 6 μg/μl using 1× loading and DEPC water. Five microliters of the samples were separated by 10% SDS/PAGE gels and then transferred to polyvinylidene fluoride membrane (PVDF, Millipore, U.S.A.). After being blocked in 5% nonfat milk in PBST (0.1% Tween 20 in PBS) for 1 h, the membrane was probed with primary antibody overnight at 4°C, washed with PBST three times and then incubated with secondary antibody (horseradish peroxidase (HRP)–conjugated goat anti-mouse/rabbit IgG, 1:2000; sc-516102/sc-2357; Santa Cruz Biotechnology, Inc. Dallas, TX, U.S.A.) at room temperature. After 2 h of incubation, the membrane was washed by PBST three times. A developer (EZ-ECL kit; Biological Industries BI) was used for development, and gray value of the strips were analyzed and counted by ImageJ (version 5.0; Bio-Rad, Hercules, CA, U.S.A.). The antibodies used were anti-GAPDH (mouse; 1:1000; ab8245; abcam), anti-GRP78 (rabbit; 1:1000; ab21685; abcam), anti-CHOP (mouse; 1:1000; ab11419; abcam), anti-NLRP3 (rabbit; 1:1000; ab214185; abcam) anti-TXNIP (rabbit; 1:1000; ab188865; abcam), anti-Bax (rabbit; 1:1000; ab32503; abcam), anti-Bcl-2 (rabbit; 1:1000; ab59348; abcam), anti-Cleaved Caspase-3 (rabbit; ab2302; abcam), anti-Catalase (rabbit; ab16731; abcam) and anti-MnSOD (rabbit; 1:1000; ab13533; abcam).
Reagents
Ketamine and ELISA for determining IL-1β and IL-18 were purchased from Sigma, and small interfering TXNIP or NC siRNA were obtained from GenePharma (Shanghai, China).
Statistical analysis
Statistical analysis was performed using statistical software Prism 6 (GraphPad Software, Inc., San Diego, CA, U.S.A.). Data were presented as SEM. t test was used to detect the difference between two groups. P<0.05 indicated a significant difference.
Results
ER stress and NLRP3/TXNIP were activated by ketamine
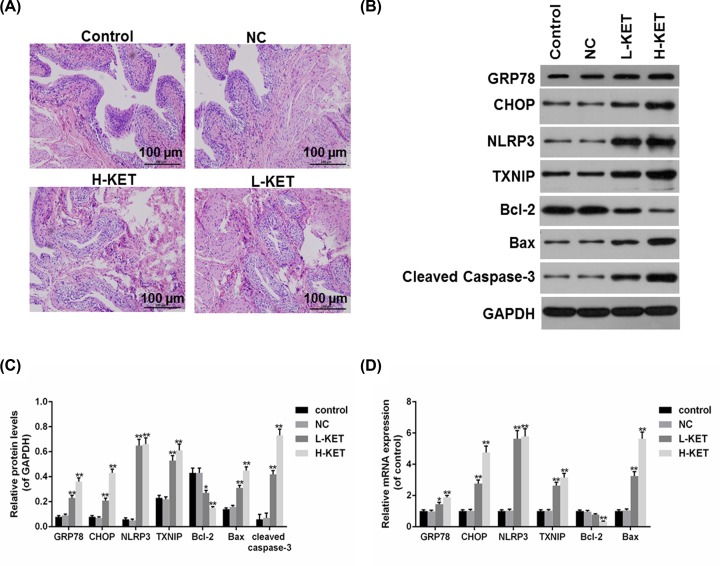
A rat model of ketamine-associated cystitis was established using intraperitoneal injection of ketamine (L-KET, 5 mg/kg and H-KET, 50 mg/kg). Hematoxylin and Eosin (HE) staining results showed that rats injected with ketamine exhibited obvious edema, vascular congestion and leukocyte infiltration in a dose-dependent manner (Figure 1A). Furthermore, the levels of ER stress-induced apoptosis-associated proteins and NLRP3/TXNIP were determined by RT-qPCR and Western blot, and cleaved Caspase-3 expression was detected by Western blot. The results of Western blot demonstrated that the protein levels of GRP78, CHOP, Bax, cleaved caspase-3, NLRP3 and TXNIP were significantly increased, however, Bcl-2 protein level was decreased by ketamine (Figure 1B,C). Consistent with the protein levels results, the mRNA levels of GRP78, CHOP, Bax, NLRP3 and TXNIP were as well enhanced, while Bcl-2 was reduced in ketamine groups, compared with control and NC groups (Figure 1D).
Figure 1. ERS-induced apoptosis and NLRP3/TXNIP were activated by ketamine.
(A) The bladder tissues of rats treated with lower and higher doses of ketamine were stained by HE staining. (B,C) The protein levels of GRP78, CHOP, Bax, Bcl-2, cleaved caspase-3, NLRP3 and TXNIP were determined (B) and quantified (C) by Western blot. (D) The mRNA levels of GRP78, CHOP, Bcl-2, Bax, NLRP3 and TXNIP were determined by RT-qPCR. *P<0.05, **P<0.01 vs. control.
Cell apoptosis and NLRP3/TXNIP were promoted by ketamine in SV-HUC-1 cells
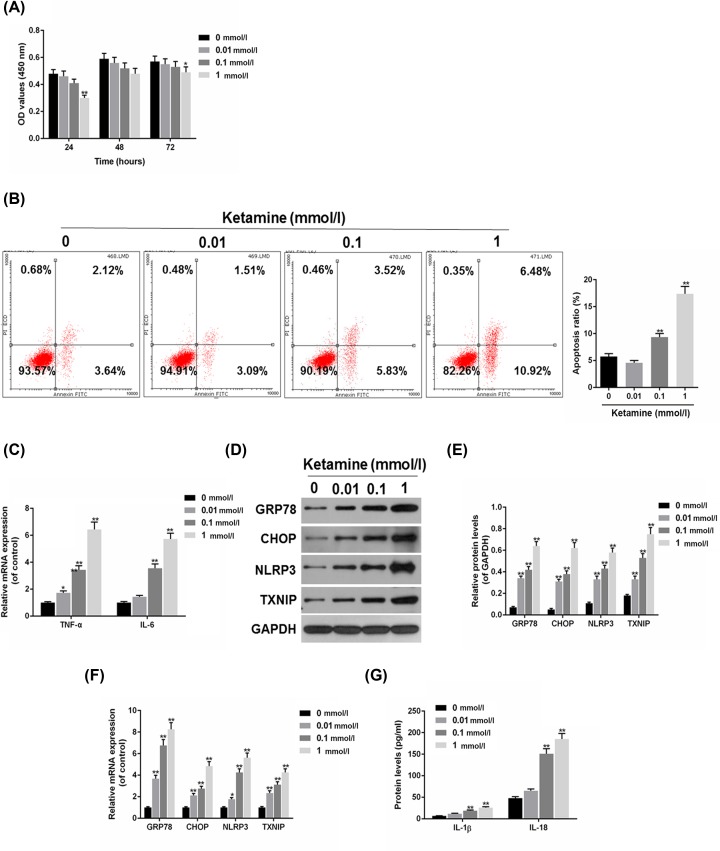
We found that ketamine could induce ERS-induced apoptosis and NLRP3/TXNIP in vitro. Furthermore, the role of ketamine in SV-HUC-1 cells was investigated. To study the effect of different concentrations of Ketamine on SV-HUC-1 cells viability, the SV-HUC-1 cells were respectively treated with 0.01, 0.1 and 1 mmol/l Ketamine for 24, 48 and 72 h. The results of CCK-8 assay indicated that cell viability was decreased by ketamine treatment in a dose-dependent manner, the viability of 1 mmol/l Ketamine treated cells decreased significantly (Figure 2A). Flow cytometry results revealed that SV-HUC-1 cells apoptosis was improved as the concentration of ketamine increased (Figure 2B). Inflammatory factors tumor necrosis factor-α (TNF-α) and IL-6 were detected using RT-qPCR, and the results showed that TNF-α and IL-6 mRNA levels were higher in ketamine groups than that in control group (Figure 2C). The expressions of GRP78, CHOP, NLRP3 and TXNIP found up-regulated in ketamine-treated SV-HUC-1 cells at protein (Figure 2D,E) and mRNA levels (Figure 2F), which were similar to the data in ketamine-treated rats (Figure 1B–D). Furthermore, ELISA found that the protein levels of IL-1β and IL-18 were improved by ketamine (Figure 2G).
Figure 2. Cell apoptosis and NLRP3/TXNIP were induced by ketamine in SV-HUC-1 cells.
(A) The viability of SV-HUC-1 cells treated with 0.01, 0.1 and 1 mmol/l ketamine for 24, 48 and 72 h were determined by CCK-8. (B) Ketamines at 0.01, 0.1 and 1 mmol/l was used to treat SV-HUC-1 cells apoptosis, which was analyzed by flow cytometry. (C) TNF-α and IL-6 mRNA levels were analyzed by RT-qPCR. (D,E) The protein levels of GRP78, CHOP, NLRP3 and TXNIP were determined (D) and quantified (E) by Western blot. (F) The mRNA levels of GRP78, CHOP, NLRP3 and TXNIP were determined by RT-qPCR. (G) The protein levels of IL-1β and IL-18 were measured by ELISA. *P<0.05, **P<0.01 vs. control.
Knockdown of TXNIP inhibited SV-HUC-1 cells apoptosis and inflammation
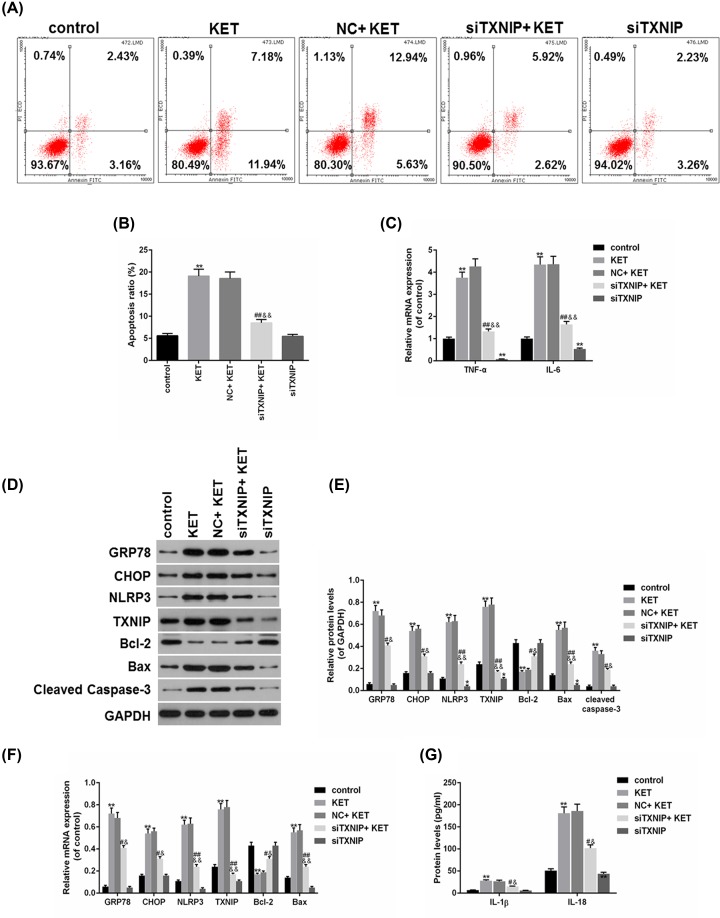
In order to investigate the role of TXNIP in ketamine-treated SV-HUC-1 cells, the expression of TXNIP was down-regulated by small interfering RNA. By performing functional experiments, we found that ketamine induced apoptosis, which could be reversed by down-regulating TXNIP (Figure 3A,B). Knockdown of SOX5 reversed the increase in TNF-α and IL-6 induced by ketamine (Figure 3C). In addition, the protein levels of GRP78, CHOP, Bax, cleaved caspase-3, NLRP3 and TXNIP were significantly lower, while Bcl-2 was higher in siTXNIP+KET group than that in NC+KET group (Figure 3D,E). Meanwhile, the data on mRNA levels of GRP78, CHOP, Bax, Bcl-2, NLRP3 and TXNIP were consistent with the Western blot results (Figure 3F). Moreover, down-regulation of TXNIP partially reversed the protein levels of IL-1β and IL-18 under the effect of ketamine (Figure 3G).
Figure 3. Knockdown of TXNIP inhibited SV-HUC-1 cells apoptosis and inflammation.
(A,B) SV-HUC-1 cells apoptosis was analyzed (A) and quantified (B) by flow cytometry. (C) TNF-α and IL-6 mRNA levels were analyzed by RT-qPCR. (D,E) The protein levels of GRP78, CHOP, Bax, Bcl2, cleaved caspase-3, NLRP3 and TXNIP were determined (D) and quantified (E) by Western blot. (F) The mRNA levels of GRP78, CHOP, Bax, Bcl2, NLRP3 and TXNIP were determined by RT-qPCR. (G) The protein levels of IL-1β and IL-18 were measured by ELISA. *P<0.05, **P<0.01 vs. control, #P<0.05, ##P<0.01 vs. KET, &P<0.05, &&P<0.01 vs. NC+KET.
Knockdown of TXNIP reversed ROS production caused by ketamine in SV-HUC-1 cells
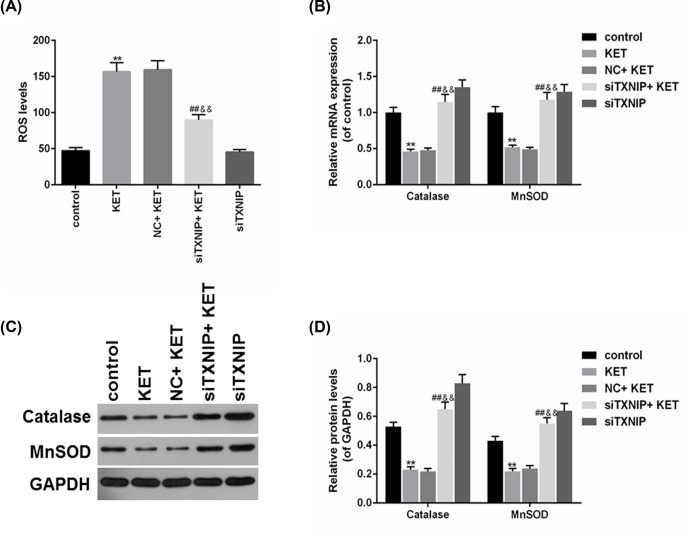
To study the effect of TXNIP on ROS production in Ketamine-treated SV-HUC-1 cells, ROS and antioxidant gene (Catalase and MnSOD) were detected using flow cytometry, RT-qPCR and Western blot, respectively. The results of flow cytometry showed that ROS production was obviously increased by ketamine, which could be ameliorated by knocking down TXNIP (Figure 4A). Furthermore, the Catalase and MnSOD protein levels were found increased in siTXNIP + KET in comparison with NC+KET group (Figure 4B,C), and the mRNA levels of Catalase and MnSOD were decreased by Ketamine, which could be reversed by down-regulating TXNIP (Figure 4D).
Figure 4. Knockdown of TXNIP reversed ROS production caused by ketamine in SV-HUC-1 cells.
(A) SV-HUC-1 cells ROS production was analyzed by flow cytometry. (B,C) The protein levels of Catalase and MnSOD were determined (B) and quantified (C) by Western blot. (D) The mRNA levels of Catalase and MnSOD were assessed by RT-qPCR. **P<0.01 vs. control, ##P<0.01 vs. KET, &&P<0.01 vs. NC+KET.
Discussion
In the present study, we investigated the underlying mechanisms of ketamine-associated cystitis in vivo and in vitro using Wistar rats and SV-HUC-1 cells. Our data suggested that ketamine damaged rat bladder tissues and induced ERS-related apoptosis and TXNIP/NLRP3 signal pathway in vivo. Furthermore, we also found that ketamine increased ERS-related apoptosis, ROS production and TXNIP/NLRP3 signal pathway in SV-HUC-1 cells. However, these phenomena by ketamine on SV-HUC-1 cells could be ameliorated when the SV-HUC-1 cells were transfected with small interfering RNA targeting at TXNIP.
Evidence showed that a long-term abuse of ketamine could affect urinary system and result in ketamine-associated cystitis [23–25]. For example, a study reported that severe urinary frequency was induced by a long-term abuse of ketamine in rats [26]. Kidney dysfunction including proteinuria, fibrosis of the urinary bladder and reduction in size of the urinary bladder leading to frequent urination were observed in patients who had a long history of ketamine use [23]. However, the pathogenesis of ketamine-associated cystitis is still poorly understood. In our study, we used Wistar rats and SV-HUC-1 cells to build a model of ketamine-associated cystitis by ketamine treatment. The results indicated that ketamine treatment caused significant damages to rat bladder tissues and reduced SV-HUC-1 cell viability, and these phenomena were similar to those in the patients and mice who had a long history of taking ketamine [27,28]. Those data suggested that the model of ketamine-associated cystitis in vivo and in vitro were successfully established and laid the foundation to further research on the underlying mechanisms.
It has been reported that ketamine could induce abnormal expression of ER stress-related proteins in nerve cells, liver cells and renal tubular epithelial cells [29,30]. ER stress will be activated by the increase in CHOP/GADD153 mRNA levels in adult neural stem cells in exposure to ketamine [30]. A study found that after ketamine treatment, the expressions of caspase-3, caspase-8, caspase-9, cytochrome c and PARP as well as TUNEL-positive cells were significantly increased in bladder tissue [31]. Consistent with above studies, we found that ER stress-related protein including GRP78 and CHOP were increased when the rats and the SV-HUC-1 cells were treated with ketamine. CHOP is a transcriptional regulator and an essential regulator of ER stress-mediated apoptosis pathway [32]. This study revealed that anti-apoptosis protein Bcl2 was down-regulated while pro-apoptosis proteins (Bax and cleaved caspase 3) were up-regulated in ketamine-treated rats. Meanwhile, SV-HUC-1 cells apoptosis was significantly enhanced after exposed SV-HUC-1 cells to ketamine. Those results indicated that ketamine-activated ER stress and thereby induced cell apoptosis, which may be one of the mechanisms of ketamine-associated cystitis.
It is recognized that overproduction of ROS and inflammation could impair cellular functions, resulting in cell death [33,34]. A study reported that the levels of NLRP3, IL-1β and IL-18 were significantly up-regulated after using multiple doses of ketamine to induce ketamine-related neurotoxicity in the hippocampus in neonatal and juvenile mouse [35]. It has been proved that TXNIP was related to ROS and NLRP3 inflammasome activation [36]. Recent studies suggested that ketamine increased the ROS production in primary hippocampal neurons [37,38]. Here, our results observed that ketamine increased TXNIP and NLRP3 levels in vivo and in vitro. Furthermore, the expressions of IL-1β, IL-18, TNF-α and IL-6 were elevated in ketamine-treated SV-HUC-1 cells, in which, ROS production was increased as well, accompanied with decreased Catalase and MnSOD levels. To investigate the important role of TXNIP in ketamine-associated cystitis, the expression of TXNIP was knocked down by small interfering RNA. By conducting functional experimentals, we discovered that the effects of ketamine on apoptosis, ER stress-correlated proteins and NLRP3 inflammasome and ROS in SV-HUC-1 cells were reversed by the down-regulation of TXNIP. These data demonstrated that TXNIP/NLRP3 aix and ROS participated in the progression of ketamine-associated cystitis at least in rats and SV-HUC-1 cells.
There are limitations in the present study, no in-depth study was conducted on the defined times of ketamine injection or treatment in rat and cells, considering that ketamine abusers would experience long duration of ketamine injection. Beyond that, there was also a lack of clinical data in our experiments, though clinical data have shown that ketamine abusers would suffer ketamine-associated cystitis. In addition, more elaborate studies will be necessary for further exploration of the role of ketamine on cystitis. Whether the activation of NLRP3 will affect the pyrogeny of SV-HUC-1 cells needs further discussion.
Conclusion
In conclusion, we revealed that in rat and cells models, ketamine-associated cystitis was associated with ER stress, apoptosis, TXNIP/NLRP3 aix and ROS. Our study provided a deeper understanding and a treatment strategy for ketamine-associated cystitis.
Abbreviations
- Bax
Bcl-2-associated X protein
- Bcl2
B-cell lymphoma/leukemia-2
- CCK-8
cell counting kit-8
- cDNA
complementary deoxyribonucleic acid
- CHOP
CCAAT/enhancer binding protein homologous protein
- DEPC
diethylpyrocarbonate
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- ERS
endoplasmic reticulum stress
- GADD153
growth arrest and DNA damage-inducible gene153
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GRP78
glucose-regulated protein 78
- IL
interleukin
- NC
negative control
- NLRP3
NOD-like receptor 3
- OD
optical density
- PARP
poly ADP-ribose polymerase
- PBS
phosphate-buffered saline
- PBST
0.1% Tween 20 in PBS
- PI
propidium iodide
- qPCR
quantitative polymerase chain reaction
- ROS
reactive oxygen species
- RT-qPCR
Real-time quantitative polymerase chain reaction
- TNF-α
tumor necrosis factor-α
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- TXNIP
thioredoxin-interacting protein
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
No humans were involved in this research.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81970659 & 81700664]; the Shandong Provincial Natural Science Foundation of China [grant number ZR2016HP38]; the Projects of Medical and Health Technology Development Program in Shandong Province [grant number 2016WS0714]; and the Science and Technology Project of Yantai [grant number 2016WS018].
Author Contribution
Substantial contributions to conception and design: L.C., X.J., C.Z., D.L.; Data acquisition, data analysis and interpretation: S.Y., Y.M., Z.S.; Drafting the article or critically revising it for important intellectual content: F.W., W.G. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: all authors.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Mathews M.J., Mead R.N.and Galizio M. (2018) Effects of N-Methyl-D-aspartate (NMDA) antagonists ketamine, methoxetamine, and phencyclidine on the odor span test of working memory in rats. Exp. Clin. Psychopharmacol. 26, 6–17 10.1037/pha0000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki H., Ideno S., Ishihara T., Watanabe K., Matsumoto M.and Morisaki H. (2018) Postoperative pain management in patients undergoing posterior spinal fusion for adolescent idiopathic scoliosis: a narrative review. Scoliosis Spinal Disord. 13, 17 10.1186/s13013-018-0165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan C.J.and Curran H.V. (2012) Ketamine use: a review. Addiction 107, 27–38 10.1111/j.1360-0443.2011.03576.x [DOI] [PubMed] [Google Scholar]
- 4.Jhang J.F., Birder L.A., Chancellor M.B.and Kuo H.C. (2017) Patient characteristics for different therapeutic strategies in the management ketamine cystitis. Neurourol. Urodyn. 36, 687–691 10.1002/nau.22996 [DOI] [PubMed] [Google Scholar]
- 5.Meng E., Chang H.Y., Chang S.Y., Sun G.H., Yu D.S.and Cha T.L. (2011) Involvement of purinergic neurotransmission in ketamine induced bladder dysfunction. J. Urol. 186, 1134–1141 10.1016/j.juro.2011.04.102 [DOI] [PubMed] [Google Scholar]
- 6.Wein A.J. (2016) Re: Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. J. Urol. 195, 222. [DOI] [PubMed] [Google Scholar]
- 7.Shan Z., Wei L., Yu S. et al. (2019) Ketamine induces reactive oxygen species and enhances autophagy in SV-HUC-1 human uroepithelial cells. J. Cell. Physiol. 234, 2778–2787 10.1002/jcp.27094 [DOI] [PubMed] [Google Scholar]
- 8.He Y., Zhou L., Fan Z., Liu S.and Fang W. (2018) Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by Nacetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress. Cell Death Dis. 9, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Yue Y., Fu S. et al. (2019) Icariside II prevents hypertensive heart disease by alleviating endoplasmic reticulum stress via the PERK/ATF-4/CHOP signalling pathway in spontaneously hypertensive rats. J. Pharm. Pharmacol. 71, 400–407 10.1111/jphp.13041 [DOI] [PubMed] [Google Scholar]
- 10.Kopeina G.S., Prokhorova E.A., Lavrik I.N.and Zhivotovsky B. (2018) Alterations in the nucleocytoplasmic transport in apoptosis: caspases lead the way. Cell Prolif. 51, e12467 10.1111/cpr.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldeeb M.A., Fahlman R.P., Esmaili M.and Ragheb M.A. (2018) Regulating apoptosis by degradation: the N-end rule-mediated regulation of apoptotic proteolytic fragments in mammalian cells. Int. J. Mol. Sci. 19, 3414 10.3390/ijms19113414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalilzadeh B., Shadjou N., Kanberoglu G.S. et al. (2018) Advances in nanomaterial based optical biosensing and bioimaging of apoptosis via caspase-3 activity: a review. Mikrochim. Acta 185, 434 10.1007/s00604-018-2980-6 [DOI] [PubMed] [Google Scholar]
- 13.Kashyap D., Kumar G., Sharma A., Sak K., Tuli H.S.and Mukherjee T.K. (2017) Mechanistic insight into carnosol-mediated pharmacological effects: recent trends and advancements. Life Sci. 169, 27–36 10.1016/j.lfs.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 14.Lee M.H., Han D.W., Hyon S.H.and Park J.C. (2011) Apoptosis of human fibrosarcoma HT-1080 cells by epigallocatechin-3-O-gallate via induction of p53 and caspases as well as suppression of Bcl-2 and phosphorylated nuclear factor-kappaB. Apoptosis 16, 75–85 10.1007/s10495-010-0548-y [DOI] [PubMed] [Google Scholar]
- 15.Oxley J.D., Cottrell A.M., Adams S.and Gillatt D. (2009) Ketamine cystitis as a mimic of carcinoma in situ. Histopathology 55, 705–708 10.1111/j.1365-2559.2009.03437.x [DOI] [PubMed] [Google Scholar]
- 16.Yu H.J., Lin B.R., Lee H.S. et al. (2005) Sympathetic vesicovascular reflex induced by acute urinary retention evokes proinflammatory and proapoptotic injury in rat liver. Am. J. Physiol. Renal Physiol. 288, F1005–F1014 10.1152/ajprenal.00223.2004 [DOI] [PubMed] [Google Scholar]
- 17.Chien C.T., Yu H.J., Lin T.B., Lai M.K.and Hsu S.M. (2003) Substance P via NK1 receptor facilitates hyperactive bladder afferent signaling via action of ROS. Am. J. Physiol. Renal Physiol. 284, F840–F851 10.1152/ajprenal.00187.2002 [DOI] [PubMed] [Google Scholar]
- 18.Han X., Wu Y.C., Meng M., Sun Q.S., Gao S.M.and Sun H. (2018) Linarin prevents LPSinduced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NFkappaB pathways. Int. J. Mol. Med. 42, 1460–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S., Zhao X., Yang S., Chen B.and Shi J. (2017) Salidroside alleviates high glucose-induced oxidative stress and extracellular matrix accumulation in rat glomerular mesangial cells by the TXNIP-NLRP3 inflammasome pathway. Chem. Biol. Interact. 278, 48–53 10.1016/j.cbi.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 20.Wen Y., Liu Y.R., Tang T.T. et al. (2018) mROS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKI. Int. J. Biochem. Cell Biol. 98, 43–53 10.1016/j.biocel.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 21.Hughes F.M. Jr, Kennis J.G., Youssef M.N., Lowe D.W., Shaner B.E.and Purves J.T. (2016) The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome mediates inflammation and voiding dysfunction in a lipopolysaccharide-induced rat model of cystitis. J. Clin. Cell Immunol. 7, 396 10.4172/2155-9899.1000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes F.M. Jr, Hill H.M., Wood C.M. et al. (2016) The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J. Urol. 195, 1598–1605 10.1016/j.juro.2015.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wai M.S., Luan P., Jiang Y. et al. (2013) Long term ketamine and ketamine plus alcohol toxicity - what can we learn from animal models? Mini Rev. Med. Chem. 13, 273–279 [DOI] [PubMed] [Google Scholar]
- 24.Gu D., Huang J., Shan Z., Yin Y., Zheng S.and Wu P. (2013) Effects of long-term ketamine administration on rat bladder protein levels: a proteomic investigation using two-dimensional difference gel electrophoresis system. Int. J. Urol. 20, 1024–1031 [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Peng B., Xu C. et al. (2016) BDNF-ERK1/2 signaling pathway in ketamine-associated lower urinary tract symptoms. Int. Urol. Nephrol. 48, 1387–1393 10.1007/s11255-016-1315-y [DOI] [PubMed] [Google Scholar]
- 26.Domiciano D.S., Figueiredo C.P., Lopes J.B. et al. (2013) Vertebral fracture assessment by dual X-ray absorptiometry: a valid tool to detect vertebral fractures in community-dwelling older adults in a population-based survey. Arthritis Care Res. (Hoboken) 65, 809–815 10.1002/acr.21905 [DOI] [PubMed] [Google Scholar]
- 27.Huang M.C., Chen L.Y., Chang H.M. et al. (2018) Decreased blood levels of oxytocin in ketamine-dependent patients during early abstinence. Front. Psychiatry 9, 633 10.3389/fpsyt.2018.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu C.T., Scheuing L., Liu G. et al. (2014) The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int. J. Neuropsychopharmacol. 18, pyu102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkan Y., Tomak Y., Altuner D. et al. (2014) Hepatic effects of ketamine administration for 2 weeks in rats. Human Exp. Toxicol. 33, 32–40 10.1177/0960327112472990 [DOI] [PubMed] [Google Scholar]
- 30.Mansouri S., Agartz I., Ogren S.O., Patrone C.and Lundberg M. (2017) PACAP protects adult neural stem cells from the neurotoxic effect of ketamine associated with decreased apoptosis, ER stress and mTOR pathway activation. PLoS ONE 12, e0170496 10.1371/journal.pone.0170496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K.M., Chuang S.M., Long C.Y. et al. (2015) Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am. J. Physiol. Renal Physiol. 309, F318–F331 10.1152/ajprenal.00607.2014 [DOI] [PubMed] [Google Scholar]
- 32.Nishitoh H. (2012) CHOP is a multifunctional transcription factor in the ER stress response. J. Biochem. (Tokyo) 151, 217–219 10.1093/jb/mvr143 [DOI] [PubMed] [Google Scholar]
- 33.Liu Z., Ren Z., Zhang J. et al. (2018) Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 9, 477 10.3389/fphys.2018.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvigneau J.C., Luis A., Gorman A.M. et al. (2018) Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine 1043–4666 10.1016/j.cyto.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 35.Ye Z., Li Q., Guo Q. et al. (2018) Ketamine induces hippocampal apoptosis through a mechanism associated with the caspase-1 dependent pyroptosis. Neuropharmacology 128, 63–75 10.1016/j.neuropharm.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou R., Tardivel A., Thorens B., Choi I.and Tschopp J. (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 10.1038/ni.1831 [DOI] [PubMed] [Google Scholar]
- 37.Liu F.F., Zhao S., Liu P.and Huo S.P. (2019) Influence of mTOR signaling pathway on ketamine-induced injuries in the hippocampal neurons of rats. Neurol. Res. 41, 77–86 10.1080/01616412.2018.1531203 [DOI] [PubMed] [Google Scholar]
- 38.Yan J., Huang Y., Lu Y., Chen J.and Jiang H. (2014) Repeated administration of ketamine can induce hippocampal neurodegeneration and long-term cognitive impairment via the ROS/HIF-1alpha pathway in developing rats. Cell. Physiol. Biochem. 33, 1715–1732 10.1159/000362953 [DOI] [PubMed] [Google Scholar]