Abstract
Acute myeloid leukemia (AML) is an aggressive hematological malignancy with a globally poor outcome, especially in patients ineligible for intensive chemotherapy. Until recently, therapeutic options for these patients included low-dose cytarabine (LDAC) or the hypomethylating agents (HMA) azacitidine and decitabine, which have historically provided only short-lived and modest benefits. The oral B-cell lymphoma 2 inhibitor, venetoclax, Venetoclax, an oral B-cell lymphoma 2 (BCL2) inhibitor, is now approved by the USA Food and Drug Administration (FDA) in combination with LDAC or HMA in older AML patients ineligible for intensive chemotherapy. Is now approved by the US Food and Drug Administration for this indication. In the pivotal clinical trials evaluating venetoclax either in combination with LDAC or with HMA, the rates of complete remission (CR) plus CR with incomplete hematological recovery were 54% and 67%, respectively and the median overall survival (OS) was 10.4 months and 17.5 months, respectively, comparing favorably with outcomes in clinical trials evaluating single-agent LDAC or HMA. The most common adverse events with venetoclax combinations are gastrointestinal symptoms, which are primarily low grade and easily manageable, and myelosuppression, which may require delays between cycles, granulocyte colony-stimulating factor (G-CSF) administration, or decreased duration of venetoclax administration per cycle. A bone marrow assessment after the first cycle of treatment is critical to determine dosing and timing of subsequent cycles, as most patients will achieve their best response after one cycle. Appropriate prophylactic measures can reduce the risk of venetoclax-induced tumor lysis syndrome. In this review, we present clinical data from the pivotal trials evaluating venetoclax-based combinations in older patients ineligible for intensive chemotherapy, and provide practical recommendations for the prevention and management of adverse events associated with venetoclax.
Keywords: acute myeloid leukemia, older patients, treatment, venetoclax
Introduction
Acute myeloid leukemia (AML) is a hematopoietic stem-cell malignancy characterized by accumulation of clonal myeloblasts in the bone marrow, peripheral blood and extramedullary tissues.1 The median age at diagnosis is 68-years old and incidence increases with age.2 The standard curative treatment of AML consists of intensive chemotherapy aimed at achieving complete remission (CR) followed by consolidation with additional chemotherapy or allogeneic stem-cell transplantation (HSCT) to prevent relapse.3 However, intensive chemotherapy is not a suitable option for many older patients with significant comorbidities, baseline organ dysfunction, or poor performance status, in whom the risk of complications and treatment-related mortality is unacceptably high. In addition, older patients have a higher frequency of adverse-risk features, such as secondary AML, complex karyotype and TP53 mutation, which are associated with decreased responses to cytarabine-based intensive chemotherapy approaches. Therefore, older patients with AML are routinely treated with noncurative, low-intensity chemotherapy approaches, aimed at controlling the disease and maintaining an acceptable quality of life for an extended period. Low-intensity treatments for AML have historically included low-dose cytarabine (LDAC) or hypomethylating agents (HMA) azacitidine or decitabine (DAC), which prolong survival compared with best supportive care, but prognosis remains poor, with an expected survival of less than 12 months.4–6 In the past decade, multiple attempts with novel agents have failed to provide significant benefit over LDAC or HMA in older patients ineligible for intensive chemotherapy.4,7–10 For example, gemtuzumab ozogamicin, an anti-CD33 antibody–drug conjugate, or clofarabine added to LDAC, successfully increased the rate of CR, but these improvements did not translate into improved survival, and the polo-like kinase inhibitor, volasertib, plus LDAC, provided marginal improvement in survival at the expense of increased toxicity.7,8,10 Glasdegib, a hedgehog pathway inhibitor, is one of the only drugs now approved by the US Food and Drug Administration (FDA) in combination with LDAC for older AML patients ineligible for intensive chemotherapy. In the BRIGHT phase II randomized trial, the median overall survival (OS) was 8.8 months versus 4.9 months in the LDAC plus glasdegib and LDAC groups, respectively. The CR rate was 17% with LDAC plus glasdegib, and 2% with LDAC. The combination treatment was well tolerated with gastrointestinal symptoms, dysgeusia, muscle spasms, and fatigue reported as common nonhematological adverse events.11
Venetoclax is a BH3 mimetic and small molecule inhibitor of the antiapoptotic protein B-cell lymphoma 2 (BCL2). BCL2 is overexpressed in many myeloid and lymphoid malignancies as a mechanism of enhanced cell survival. Preclinical studies have demonstrated that AML cells, especially leukemic stem cells, are dependent on BCL2 for survival, and inhibition by venetoclax can lead to rapid initiation of apoptotic AML cell death.12,13 Based on this rationale, venetoclax was first evaluated in relapsed or refractory AML showing single-agent efficacy with an overall response rate (ORR) of 19% and a good safety profile.14 Despite modest results as a single agent in the relapsed/refractory setting, clear synergy with venetoclax and both hypomethylating agents and cytarabine was identified preclinically,15–18 leading to the multicenter phase I/II clinical trials of venetoclax in combination with either LDAC or HMA for newly diagnosed untreated AML patients ineligible for intensive chemotherapy.19,20 In these two pivotal clinical trials, the rates of CR plus CR with incomplete hematological recovery (CRi) were 54% and 67% in patients treated with venetoclax plus LDAC or HMA, respectively, and the median OS was 10.4 months and 17.5 months, representing significant improvement compared with historical cohorts treated with single-agent LDAC or HMA.4–6 The results of these nonrandomized clinical trials led to the accelerated approval of venetoclax by the FDA, for use in combination with LDAC or HMA for the treatment of AML in newly diagnosed patients older than 75 years, or with comorbidities that preclude intensive chemotherapy. These combination regimens produce notably different response kinetics compared with single-agent LDAC or HMA, as most patients on venetoclax combinations will achieve their best response after one cycle. It is also important to be aware that venetoclax may be associated with augmented or prolonged myelosuppression that can lead to infections or other cytopenia-related adverse events. Venetoclax can also cause tumor lysis syndrome (TLS), and appropriate preventive measures are required to avoid this complication. In this review, we will summarize the data from the pivotal clinical trials evaluating the venetoclax-based combination therapies in older patients ineligible for intensive chemotherapy, and provide practical recommendations to assist clinicians with the utilization of these regimens in daily clinical practice.
Venetoclax plus hypomethylating agents
The safety and efficacy of venetoclax in combination with either azacitidine or DAC was evaluated in a phase Ib/II clinical trial for patients with newly diagnosed untreated AML older than 65 years, considered unsuitable candidates for intensive chemotherapy.19 Patients who had previously received HMA therapy for an antecedent hematological disorder were ineligible. Patients were treated with either standard azacitidine 75 mg/m2 intravenously (IV) or subcutaneously (SC) for 7 days, or DAC 20 mg/m2 IV for 5 days, in combination with oral venetoclax at a dose of 400 mg, 800 mg, or 1200 mg daily in the phase Ib dose-finding period. All patients were hospitalized for TLS prophylaxis and monitoring during a 3–5-day dose ramp-up of the venetoclax in combination with initiation of the HMA therapy. All patients required initiation of allopurinol or other uric-acid-reducing agents prior to initiation of treatment, TLS biochemistry monitoring prior to, and 8 h after each new venetoclax dose, and appropriate oral or IV hydration. A total of 145 patients were enrolled in the dose escalation and expansion study with a median age of 74 (range 65–86) years and Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 in 84% of patients. A total of 71 (49%) patients had adverse-risk cytogenetics, and 36 (25%) patients had secondary AML.
The most common grade 3 or 4 adverse events were febrile neutropenia (43%), anemia (25%), thrombocytopenia (24%), neutropenia (17%), and pneumonia (13%). Gastrointestinal symptoms such as nausea, diarrhea, or constipation were reported in approximately half of patients; primarily grade 1 or 2, and typically manageable without dose interruptions or reductions of venetoclax. As expected in patients with AML, infections were a common adverse event observed in 74% of patients (grade 3 or 4 in 45% of patients), and 10 patients (7%) died from infectious complications. While no dose-limiting toxicity was observed, hematological and gastrointestinal adverse events seemed to be more frequent in patients receiving venetoclax at 1200 mg daily compared with the lower doses of 400 mg or 800 mg, and thus the 400 mg and 800 mg dose levels moved into subsequent expansion cohorts. Importantly, 68 (47%) patients required a dose interruption of venetoclax, most often due to persistent cytopenia without residual AML at the end of the first cycle’s bone marrow aspiration, requiring subsequent cycle delay to allow for count recovery.
Among 145 patients, the ORR [comprising CR, CRi and partial remission (PR)] was 68%, including 37% patients achieving CR and 30% patients achieving CRi (Table 1). Additionally, 21% of patients achieved a morphologic leukemia-free state (MLFS) for a total leukemia response rate of 83%, representing all patients who achieved a bone marrow morphologic remission with less than 5% blasts, regardless of hematological recovery. The ORR was not significantly different between a venetoclax dose of 400 mg and 800 mg (73% versus 68%, respectively), or between azacitidine and DAC (76% versus 71%, respectively). Responses were rapid with a median time to initial response of 1.2 months. With a median follow up of 15.1 months, the median OS was 17.5 months [95% confidence interval (CI), 12 months to not reached (NR)] and the median duration of response (DOR) was 11.3 months (95% CI, 8.9 months to NR). Measurable residual disease (MRD) negativity was assessed longitudinally in bone marrow aspirates by multiparameter flow cytometry detecting leukemia-associated immunophenotypes with a sensitivity of 10−3. MRD negativity was achieved in 28/97 (29%) patients in CR or CRi and median OS and DOR were NR in these patients. In return, patients in CR or CRi who did not achieve MRD negativity had a median DOR of 11.3 months and median OS was NR.
Table 1.
Summary of the clinical data for venetoclax in combination with low-dose cytarabine or hypomethylating agents.
| CR + CRi rate, n (%) | Median DOR, months (95% CI) | Median OS, months (95% CI) | |
|---|---|---|---|
| Venetoclax + LDAC | |||
| All patients | 44/82 (54) | 8.1 (5.3–14.9) | 10.1 (5.7–14.2) |
| Cytogenetic risk | |||
| Intermediate | 31/49 (63) | NA | 15.7 (7.0–NR) |
| Adverse | 11/26 (42) | NA | 4.8 (2.9–11.7) |
| AML | |||
| De novo | 30/42 (71) | NA | 16.9 (11.7–NR) |
| Secondary | 14/40 (35) | NA | 4.0 (3.0–6.5) |
| Mutations | |||
| FLT3 | 7/16 (44) | NA | 5.6 (3.0–14.3) |
| IDH1/2 | 13/18 (72) | NA | 19.4 (5.1–NR) |
| NPM1 | 8/9 (89) | NA | NR (0.5–NR) |
| TP53 | 3/10 (30) | NA | 3.7 (0.3–10.1) |
| Venetoclax + HMA | |||
| All patients | 97/145 (67) | 11.3 (8.9–NR) | 17.5 (12.3–NR) |
| Cytogenetic risk | |||
| Intermediate | 55/74 (74) | 12.9 (11.0–NR) | NR (17.5–NR) |
| Adverse | 42/71 (60) | 6.7 (4.1–9.4) | 9.6 (7.2–12.4) |
| AML | |||
| De novo | 73/109 (67) | 9.4 (7.2–11.7) | 12.5 (10.3–24.4) |
| Secondary | 24/36 (67) | NR (12.5–NR) | NR (14.6–NR) |
| Mutations | |||
| FLT3 | 13/18 (72) | 11.0 (6.5–NR) | NR (8.0–NR) |
| IDH1/2 | 25/35 (71) | NR (6.8–NR) | 24.4 (12.3–NR) |
| NPM1 | 21/23 (91) | NR (6.8–NR) | NR (11.0–NR) |
| TP53 | 17/36 (47) | 5.6 (1.2–9.4) | 7.2 (3.7–NR) |
CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete hematological recovery; DOR, duration of response (CR + CRi); HMA, hypomethylating agent; LDAC, low-dose cytarabine; NA, not available; NR, not reached; OS, overall survival.
Certain genomic features were confirmed to be of prognostic significance with HMA plus venetoclax therapy (Table 1). In patients with intermediate-risk and adverse-risk cytogenetics, the CR/CRi rates were 74% and 60% and the median OS were 12.9 months and 6.7 months, respectively. Patients with a TP53 mutation had lower CR/CRi rates of 47% and median OS of 7.2 months (95% CI 3.7 months to NR), whereas patients with IDH1/2 and NPM1 mutations had higher CR/CRi rates of 71% and 92% with a median OS of 24.4 months (95% CI, 12.3 months to NR) and not reached (95% CI, 11.0 months to NR), respectively.
The combination of venetoclax plus 10-day DAC was evaluated in an ongoing phase II clinical trial at the MD Anderson Cancer Center, enrolling newly diagnosed patients older than 60 years ineligible for intensive chemotherapy as well as relapsed/refractory AML.21 DAC was administered for 10 days per cycle until ORR was achieved, then was administered for 5 days on subsequent cycles. With the 10-day DAC regimen, venetoclax was administered for 21 days in cycle 1 then a bone marrow aspiration was performed on day 21 to guide the additional duration of venetoclax therapy. In patients with clearance of blasts (<5%), venetoclax was withheld, beginning on day 21, to allow count recovery, whereas in patients with persistent disease, the venetoclax was continued until day 28. Once in remission, the administration of venetoclax was kept at 21 days or reduced to 14 days for subsequent cycles, depending on hematological recovery. Data presented at the American Society of Hematology (ASH) 2018 annual meeting reviewed the first 24 newly diagnosed AML patients enrolled in the trial, including 6 patients (25%) with complex cytogenetics and 4 patients (17%) with TP53 mutations. The CR/CRi rate in these newly diagnosed patients was 92% (22/24), and among 21 evaluable responders, 11 patients (52%) achieved MRD negativity. Importantly, all four patients with TP53 mutation achieved CR. Although the adverse events were similar to DAC for 5 days plus venetoclax, myelosuppression was significant with the 10-day DAC induction with median time to neutrophil recovery above 0.5 × 109/l of 56 days and median time to platelet recovery above 50 × 109/l of 32 days, leading to the study modification to do a bone marrow aspiration and stop venetoclax on day 21. The potential for prolonged cytopenia observed with this regimen during induction underscores the critical importance of performing a bone marrow aspiration at day 21 to withhold venetoclax in early-responding patients and reduce the risk of cytopenia-related adverse events. Additional follow up from this cohort is anticipated later this year.
Venetoclax plus low-dose cytarabine
The combination of venetoclax with LDAC was evaluated in a phase Ib/II clinical trial in patients 60 years or older with previously untreated AML ineligible for intensive chemotherapy.20 Study eligibility was overall similar to the previous study with the notable exception that HMAs were allowed for the previous treatment of an antecedent hematologic disorder such as myelodysplastic syndrome. Patients were treated with LDAC at a dose of 20 mg/m2 by daily SC injection on days 1–10 per 28-day cycle, in addition to daily administration of oral venetoclax, which was initiated at a dose of 50 or 100 mg daily, and increased over 4–5 days up to the target dose. Hospitalization and TLS prophylaxis were mandated in the same manner as with the HMA-combination study during the initial ramp-up portion. During the phase Ib period of the study, no maximum tolerated dose was identified, but many patients receiving 800 mg daily of venetoclax experienced prolonged myelosuppression, requiring cycle interruptions to allow count recovery. Therefore, venetoclax 600 mg was selected as the phase II recommended dose and a total of 82 patients were enrolled at this dose. The median age of patients was 74 (range, 63–90) years, and 49% of patients had an antecedent hematological disorder, including 29% who had previously been treated with HMA. Most patients had a reasonable performance status (ECOG 0–1 in 71%) and 32% of patients had adverse-risk cytogenetics.
Similar to the experience with HMA plus venetoclax, the most common grade 3 or 4 adverse events were cytopenia, febrile neutropenia, and infections. Nausea, diarrhea, hypokalemia, and fatigue were common nonhematological adverse events. With the combination of LDAC and venetoclax 600 mg daily, the CR/CRi rate was 54% (95% CI, 42–65%) including CR and CRi in 26% and 28% patients, respectively (Table 1). Patients with de novo AML had a CR/CRi rate of 71%, compared with a CR/CRi rate of only 35% in patients with secondary AML. The CR/CRi rate was 62% in patients who never received HMA therapy and 33% in patients with prior receipt of HMA. Cytogenetic risk was again prognostic, with CR/CRi rates of 63% and 42% in patients with intermediate-risk and adverse-risk karyotype, respectively. Patients with mutations in NPM1 or IDH1/2 had particularly good responses to the venetoclax plus LDAC combination with CR/CRi rates of 89% and 72%, respectively, whereas patients with mutations in TP53 or FLT3 had lower responses with CR/CRi rates of 30% and 44%, respectively. The median DOR was 8.1 months (95% CI 5.3–14.9 months) among patients achieving CR/CRi, and the median OS was 10.1 months (95% CI, 5.7–14.2 months) in the global population (Table 1). In patients who were never exposed to HMA, the median OS was 13.5 months (95% CI, 7.0–18.4 months) compared to 4.1 months (95% CI, 2.9–10.1 months) in patients who had previously been treated with HMA.
Management of venetoclax-related adverse events
Management of cytopenia with venetoclax-based regimens
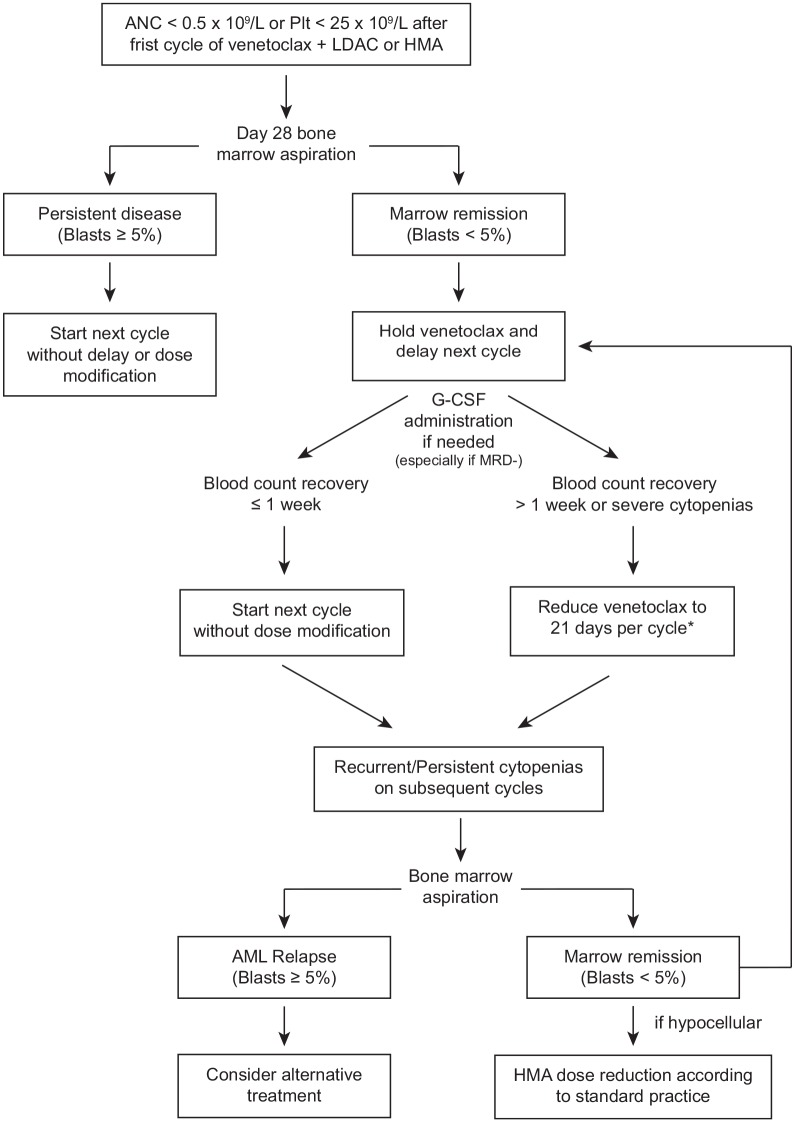
Cytopenia and infections are the most frequent grade 3 or 4 adverse events observed with the venetoclax-based low-intensity regimens. In the pivotal clinical trials evaluating venetoclax plus LDAC or HMA chemotherapy, treatment-emergent grade 3 or 4 neutropenia (<1.0 × 109/l) occurred in 17–27% of patients and grade 3 or 4 thrombocytopenia (<50 × 109/l) occurred in 24–38% of patients.19,20 Febrile neutropenia was reported in 42–43% of patients and infection of any grade occurred in 74% of patients treated with HMA plus venetoclax.19,20 In order to reduce the risk of infections and other cytopenia-related adverse events with the venetoclax-based combinations, delays between cycles, granulocyte colony-stimulating factor (G-CSF) administration, or shorter duration of venetoclax administration per cycle may be required. We provide herein some recommendations and an algorithm to assist clinicians in the management of cytopenia occurring in older patients treated with venetoclax-based low-intensity regimens (Figure 1).
Figure 1.
Algorithm for the management of cytopenia with venetoclax-based combination regimens.
AML, acute myeloid leukemia; ANC, absolute neutrophil count; G-CSF, granulocyte colony-stimulating factor; HMA, hypomethylating agents; LDAC, low-dose cytarabine; MRD−, minimal residual disease negative; Plt, platelets.
*Venetoclax can be reduced further to 14 days per cycle for persistent and/or recurrent cytopenias in subsequent cycles.
After the first cycle of therapy with a venetoclax-based combination, a bone marrow aspirate and biopsy should be performed to assess the response to treatment, including blast percentage and cellularity of the bone marrow. This should be performed between day 21 and 28 from the start of combination therapy and is particularly important for guiding the timing of the second cycle of therapy and inform on any appropriate dose adjustments. In patients with persistent disease after the first cycle of treatment, venetoclax should be continued without interruption, and the next cycle should start as scheduled, as any persisting cytopenias are related to the active AML and will not resolve until remission is achieved. In patients without morphologic evidence of leukemia (i.e. <5% blasts, or a therapy-induced aplastic marrow), persistent cytopenia is most likely secondary to combination therapy. In such cases, we recommend to withhold venetoclax and delay the start of the next cycle for up to 14 days to allow neutrophils to recover above 0.5 × 109/l and platelets to recover at least above 25 × 109/l (ideally above 50 × 109/l) before starting the next cycle of therapy. When more than 1 week is necessary for counts to recover prior to the second cycle of therapy, we recommend decreasing the duration of venetoclax administration to 21 days for the subsequent cycle. A repeat bone marrow aspiration may be required after 2–3 weeks if cytopenia persists with an empty or MLFS marrow at the end of cycle 1, to evaluate the status of disease. In subsequent cycles, if prolonged cytopenias reoccur and patients remain in remission, the duration of venetoclax administration can be further shortened to 14 days per cycle (or 21 days if the patient was still receiving 28 days per cycle), and G-CSF may also be given to help with the recovery of neutrophils. If patients tolerate G-CSF well with improvement in their neutrophil counts, then pegylated-G-CSF may be considered for subsequent cycles. G-CSF is often utilized at our institution for patients with venetoclax-related neutropenia, particularly once they have achieved MRD negativity in order to avoid any stimulation of blasts, which could bias the interpretation of later bone marrow aspirates. Additionally, in the setting of a hypocellular bone marrow and ongoing cytopenias without evidence of persistent disease, the dose of HMA may also be reduced or shortened according to standard practice. In these circumstances, we often recommend reducing the azacitidine to 5 days per cycle (instead of 7) and DAC to 3 or 4 days per cycle (instead of 5), which conveniently decreases the number of hospital visits for patients. Alternatively, the dose of azacitidine may be decreased to 50 mg/m2 (and further to 37.5 mg/m2 or 25 mg/m2) or DAC to 15 mg/m2 (and further to 10 mg/m2).
Venetoclax doses adjustments and drug interactions
Venetoclax is approved by the FDA at a dose of 600 mg in combination with LDAC and 400 mg, in combination with HMA. Higher doses of venetoclax in combination with LDAC or HMA are associated with a higher frequency of prolonged cytopenia and do not add clinical benefit. Importantly, because venetoclax is metabolized by the CYP3A4 cytochrome system, venetoclax dosing must be adjusted in the setting of concomitant administration of CYP3A4 inhibitors. Common CYP3A4 inhibitors utilized in AML patients include the strong inhibitors posaconazole and voriconazole and the moderate inhibitors fluconazole, isavuconazole, and ciprofloxacin (Table 2). Based on pharmacokinetic studies, the dose of venetoclax should be reduced by 50% with administration of a moderate CYP3A4 inhibitor (i.e. 200 mg of venetoclax + HMA), and should be reduced by 75% of the target dose with administration of a strong CYP3A4 inhibitor (i.e. 100 mg of venetoclax + HMA).22 Patients taking venetoclax should avoid intake of grapefruit products, as they contain CYP3A inhibitors, and moderate or strong CYP3A4 inducers should be avoided, as they may decrease the levels of venetoclax and impact its efficacy (Table 2).
Table 2.
Drug interactions and venetoclax dose adjustments.
| CYP3A4 inhibitors * | |
| Moderate CYP3A4 inhibitors: Azole antifungals: fluconazole, isavuconazole Protease inhibitors: amprenavir, atazanavir, darunavir/ritonavir Calcium-channel blockers: diltiazem, verapamil Others: aprepitant, ciprofloxacin, erythromycin |
Reduce dose of venetoclax by 50% |
| Strong CYP3A4 inhibitors: Azole antifungals: posaconazole, voriconazole Protease inhibitors: indinavir, lopinavir/ritonavir, telaprevir Others: clarithromycin, conivaptan, telithromycin |
Reduce dose of venetoclax by 75% |
| CYP3A4 inducers * | |
| Moderate CYP3A4 inducers: Bosentan, efavirenz, etravirine, modafinil, nafcillin |
Avoid concomitant administration Consider changing to alternative drugs |
| Strong CYP3A4 inducers: Avasimibe, carbamazepine, phenytoin, phenobarbital, rifampin, St. John’s wort |
|
These lists are not exhaustive and caution should be exercised when prescribing new medications to patients receiving venetoclax-based regimens with regards to potential drug–drug interactions.
Prevention of tumor lysis syndrome
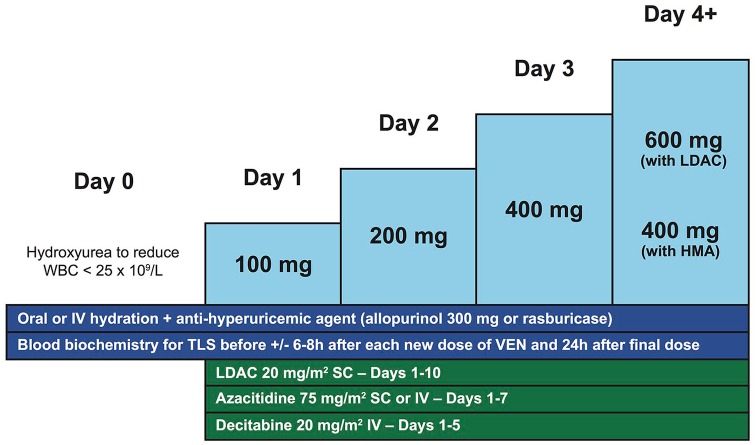
TLS may occur after initiation of venetoclax therapy due to the rapid initiation of apoptosis in leukemic cells caused by BCL2 inhibition. The risk of TLS is elevated in patients with certain risk factors such as elevated lactate dehydrogenase, white blood cell counts (WBC) above 25 × 109/l, uric acid above 7.5 mg/dl (446 µmol/l) and pre-existing renal insufficiency, such as a creatinine above 1.4 mg/dl (124 µmol/l).23 Careful monitoring and appropriate preventive measures with the initiation of venetoclax can minimize the risk of TLS, which appears to be much less in AML than what has been observed in chronic lymphocytic leukemia (CLL).24 Initiating venetoclax at a lower dose followed by a short daily ramp-up is recommended for achieving the target dose while reducing the risk of TLS in sensitive patients (Figure 2). Patients can start venetoclax at a dose of 100 mg on the first day of the cycle, in combination with LDAC or HMA, and the dose can thereafter be doubled every day until achieving the target dose (i.e. 100–200–400 mg with HMA and 100–200–400–600 mg with LDAC; Figure 2). In patients on CYP3A4 inhibitors, an adjusted daily ramp-up (e.g. 50–100–200 mg, with moderate CYP3A4 inhibitors and 20–50–100 mg with strong CYP3A4 inhibitors) is reasonable and appropriate. During the venetoclax dose ramp-up, patients should remain well hydrated with oral or IV fluids and they should receive uric-acid-lowering agents (allopurinol or rasburicase). Laboratory parameters of TLS should be monitored at least daily prior to each new dose of venetoclax and in patients at higher risk, we also recommend monitoring TLS biochemistry 6–8 h after administration of each new dose. Furthermore, it is recommended to lower WBC below 25 × 109/l with hydroxyurea before starting treatment with venetoclax, to decrease risk of TLS. With these preventive measures, no clinically significant TLS was reported in the clinical trials of venetoclax plus HMA and only two cases of laboratory (not clinical) TLS were reported with LDAC plus venetoclax. With these reassuring data, it is foreseeable that selected patients with no risk factors for TLS could start venetoclax combination therapy in an appropriate outpatient setting in which the above preventive measures and monitoring can be applied. In addition to standard risk factors for TLS, NPM1 and IDH1/2 mutations might be AML-specific risk factors, given the greater sensitivity of AML with these mutations to BCL2 inhibition, and anecdotal experience of clinically significant TLS has been observed in such cases.21
Figure 2.
Venetoclax dose ramp-up and tumor lysis syndrome preventive measures.
HMA, hypomethylating agents; IV, intravenously; LDAC, low-dose cytarabine; SC, subcutaneously; TLS, tumor lysis syndrome; VEN, venetoclax; WBC, white blood cell count.
Future directions
Based on the two independent nonrandomized studies showing safety and high efficacy of venetoclax in combination with LDAC or HMA for the treatment older patients with newly diagnosed AML ineligible for intensive chemotherapy, venetoclax-based regimens are now established treatment options for the treatment of newly diagnosed older or unfit patients.19,20 Ongoing phase III randomized clinical trials evaluating venetoclax versus placebo in combination with either LDAC or HMA have now completed accrual, and results are eagerly anticipated to confirm the added benefit of venetoclax to low-intensity therapy in older patients with AML ineligible for intensive chemotherapy [ClinicialTrials.gov identifiers: NCT02993523, NCT03069352].
To continue improving the outcome of older AML patients ineligible for intensive chemotherapy, several therapeutic avenues trying to circumvent mechanisms of primary or secondary resistance to venetoclax-based regimens are currently under investigation (Table 3). The most recognized and studied mechanism of resistance to venetoclax is overexpression of MCL1 or BCL-XL, two other antiapoptotic proteins from the BCL2 family, which may compensate BCL2 inhibition to promote leukemic-cell survival.25 Inhibition of MCL1 represents a more promising avenue than inhibition of BCL-XL, since severe thrombocytopenia was a dose-limiting toxicity with navitoclax, a nonselective BCL2 inhibitor also targeting BCL-XL, in patients with relapsed/refractory CLL.26 In preclinical models, downregulation of MCL1 by genetic knockdown or pharmacological inhibition was shown to overcome the resistance of AML cells to BCL2 inhibition, providing rationale for potential synergistic combination therapies of MCL1 inhibitors with venetoclax.27–30 Direct MCL1 inhibitors are therefore currently being evaluated in combination with venetoclax for patients with relapsed/refractory AML (Table 3). Downregulation of MCL1 can also be achieved indirectly by interfering with the MAPK, p53 and CDK9 pathways, which regulate the expression of MCL1. Cobimetinib, a mitogen-activated protein kinase (MEK) inhibitor suppressing the MAPK signaling pathway, downregulates MCL1 and acts synergistically with venetoclax in preclinical models of AML.31 Similarly, p53 activation with MDM2 inhibition promotes degradation of MCL1 and may overcome resistance to venetoclax in wild-type TP53 AML samples.32 These combination strategies are being tested in an ongoing phase Ib clinical trial evaluating combinations of venetoclax with idasanutlin (an MDM2 inhibitor) or cobimetinib (MEK inhibitor) in relapsed or refractory AML (Table 3). In preliminary results of this study presented at ASH 2017, the overall response rate (including CR, CRi and CRp) was 18% (4/22) and 20% (4/20) with venetoclax in combination with cobimetinib and idasanutlin, respectively.33 The most common adverse events were febrile neutropenia, and diarrhea that was specifically associated with the cobimetinib arm.33 Additionally, cyclin-dependent kinase 9 (CDK9) also regulates MCL1 protein levels and the CDK9 inhibitors dinaciclib and alvocidib have preclinical efficacy either alone or in combination with venetoclax.34,35 Clinical trials evaluating the combination of venetoclax with alvocidib or dinaciclib are currently recruiting patients (Table 3).
Table 3.
Ongoing clinical trials with venetoclax-based combinations for older patients with AML.
| Treatment | Trial phase | Clinical.Trials.gov identifier |
|---|---|---|
| Untreated AML | ||
| Azacitidine + venetoclax versus placebo | Phase III | NCT02993523 |
| Low-dose cytarabine + venetoclax versus placebo | Phase III | NCT03069352 |
| Azacitidine + venetoclax | Phase II | NCT03466294 |
| Decitabine 10 days + venetoclax | Phase II | NCT03404193 |
| LDAC + cladribine + venetoclax and azacitidine + venetoclax | Phase II | NCT03586609 |
| Azacitidine + venetoclax + pevonedistat (NEDD8-activating enzyme) | Phase I/II | NCT03862157 |
| Venetoclax + ivosidenib ± azacitidine | Phase I/II | NCT03471260 |
| Azacitidine + venetoclax + avelumab (PD-L1 inhibitor) | Phase I/II | NCT03390296 |
| Azacitidine + venetoclax + gemtuzumab ozogamicin | Phase I/II | NCT03390296 |
| Relapsed or refractory AML | ||
| Venetoclax + dinaciclib (CDK9 inhibitor) | Phase Ib | NCT03484520 |
| Venetoclax + alvocidib (CDK9 inhibitor) | Phase Ib | NCT03441555 |
| Venetoclax + ruxolitinib (JAK2 inhibitor) | Phase I | NCT03874052 |
| Venetoclax + gilteritinib (FLT3 inhibitor) | Phase I | NCT03625505 |
| Venetoclax + quizartinib (FLT3 inhibitor) | Phase Ib/II | NCT03735875 |
| Venetoclax + AMG-176 (MCL1 inhibitor) | Phase Ib | NCT03797261 |
| Venetoclax + S64315 (MCL1 inhibitor) | Phase I | NCT03672695 |
| Venetoclax + cobimetinib (MEK inhibitor) or Venetoclax + idasanutlin (MDM2 inhibitor) | Phase Ib/II | NCT02670044 |
| Venetoclax + lintuzumab-Ac225 (anti-CD33 monoclonal antibody) | Phase I/II | NCT03867682 |
| Azacitidine + venetoclax + Lintuzumab-Ac225 | Phase I/II | NCT03932318 |
| Venetoclax + HDM201 (MDM2 inhibitor) | Phase I | NCT03940352 |
| Venetoclax + selinexor (XPO1 inhibitor) | Phase I | NCT03955783 |
AML, acute myeloid leukemia; LDAC, low-dose cytarabine.
Based on the established efficacy of FLT3 and IDH1/2 inhibitors in the treatment of AML, the addition of targeted agents to venetoclax-based regimens represent an additional promising therapeutic avenue to further improve outcomes in older patients with FLT3- or IDH1/2-mutated AML (Table 3). FLT3 mutation is the most frequent molecular alteration in AML occurring in 25–30% of cases and is associated with a higher risk of death and relapse.36–38 In the study evaluating venetoclax plus LDAC, patients with FLT3 mutations had a lower CR/CRi of 44% and shorter median OS of 5.6 months, whereas responses and survival were similar to the global population in the study evaluating venetoclax plus HMA (Table 1).19,20 Addition of FLT3 inhibitors to these venetoclax-based regimens may increase the remission rates and survival, especially considering the added benefit of sorafenib to azacitidine demonstrated in patients with untreated or relapsed/refractory AML with FLT3-ITD mutation.39,40 In the ongoing study at MD Anderson evaluating 10-day DAC plus venetoclax in older AML patients ineligible for intensive chemotherapy, patients with FLT3 mutations may also receive a targeted FLT3 inhibitor [ClinicalTrials.gov identifier: NCT03404193]. Additional research is warranted to determine the benefit of the addition of FLT3 inhibitor to venetoclax-based combinations in untreated AML patients. In patients with relapsed/refractory FLT3-mutated AML, clinical trials evaluating venetoclax plus gilteritinib and venetoclax plus quizartinib are currently enrolling patients to improve upon the efficacy of single-agent FLT3 inhibitors and test the synergy of these combinations [ClinicalTrials.gov identifiers: NCT03625505, NCT03735875].
Recurrent mutations in IDH1 or IDH2 occur in 15–25% of AML. Ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) can successfully achieve remission in ~40% of patients with relapsed or refractory AML harboring these mutations.36–38,41,42 Both preclinical and clinical studies have demonstrated that IDH1/2-mutated AML has an increased sensitivity to venetoclax.19,20,43 In patients with IDH1/2-mutated AML enrolled in the pivotal trials of venetoclax-based combinations, the median OS was 19.4 months and 24.4 months, with LDAC and HMA, respectively, which is longer than in the global population of each study (Table 1).19,20 Based on the efficacy of IDH inhibitors and venetoclax in IDH1/2-mutated AML, it is likely that combinations of these drugs will act synergistically in patients harboring these mutations. This concept is currently being tested in a clinical trial at the MD Anderson Cancer Center with a combination of venetoclax and ivosidenib, with or without azacitidine in patients with IDH1-mutated AML [ClinicalTrials.gov identifier: NCT03471260]. In preliminary results available for 12 evaluable patients treated with ivosidenib and venetoclax, the remission rate (including CR, CR with partial hematologic recovery [CRh] or CRi) was 75% comparing favorably with response rates of ~40% with single-agent ivosidenib in relapsed/refractory IDH1-mutated AML.44 So far, no signal of significant added toxicity has been observed with this regimen, suggesting that triplet-drug combinations are likely to have a favorable risk–benefit profile. However, longer follow up with a higher number of patients is required to confirm this statement, which may also be dependent on which agents are combined together.
Other approaches currently under investigation to improve venetoclax-based combinations in older, untreated AML patients ineligible for intensive chemotherapy include the combination of azacitidine plus venetoclax with either gemtuzumab ozogamicin, an anti-CD33 antibody–drug conjugate, avelumab, a programmed-cell-death-ligand-1 inhibitor, or pevonedistat, a NEDD8-activating enzyme inhibitor, based on results from previous clinical trials evaluating these agents (or other agents of the same family) in combination with HMA. In relapsed/refractory AML, clinical trials are evaluating the safety and efficacy of venetoclax in combination with other experimental agents including, but not limited to, lintuzumab-Ac225, a CD33 monoclonal antibody, selinexor, a selective inhibitor of nuclear export specifically blocking XPO1, and HDM201, a MDM2 inhibitor activating p53 in AML with wild-type TP53 status (Table 3). Lastly, combination of venetoclax with panobinostat, a histone deacetylase inhibitor, showed promising TP53-independent activity in preclinical models of AML.45 A clinical trial evaluating the combination of venetoclax plus HDAC inhibitor is planned to open. This combination could be beneficial for patients with TP53-mutated AML who continue to have an adverse prognosis with venetoclax-based combination therapies.
Conclusion
The prognosis of older AML patients ineligible for intensive chemotherapy has been dismal for decades. The addition of venetoclax to LDAC and HMA therapies represent promising combination therapies with impressive remission rates and favorable OS, now FDA approved for the treatment of newly diagnosed and older or unfit AML patients. To reduce the risk of prolonged cytopenia and cytopenia-related complications such as febrile neutropenia and infections, while maintaining optimal antileukemic activity, proposed management guidelines are described in this review and encouraged for patients treated with venetoclax-based combinations. Awareness of venetoclax dose reductions in the setting of CYP3A4 inhibitors are essential to prevent toxicity, and preventive measures and appropriate monitoring for TLS are fundamental during the first week of treatment during the venetoclax dose ramp-up. Many clinical trials are currently ongoing evaluating how to further improve upon combinations of venetoclax plus LDAC or HMA in older AML patients ineligible for intensive chemotherapy.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: GR-C has no relevant conflict of interest to disclose. CDD discloses honoraria from Daiichi Sankyo, Jazz Pharmaceuticals and Syros, and consultant and advisory roles for Abbvie, Agios, Celgene and Notable Labs.
ORCID iD: Courtney D. DiNardo  https://orcid.org/0000-0001-9003-0390
https://orcid.org/0000-0001-9003-0390
Contributor Information
Guillaume Richard-Carpentier, Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Courtney D. DiNardo, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Box 428, Houston TX 77030, USA.
References
- 1. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer (IARC), 2017. [Google Scholar]
- 2. Surveillance, Epideiomolgy and End Results Program. Cancer stat facts: leukemia-acute myeloid leukemia (AML). National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/statfacts/html/amyl.html. (accessed 25 April 2018). [Google Scholar]
- 3. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129: 424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 2007; 109: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 5. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30: 2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015; 126: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013; 27: 75–81. [DOI] [PubMed] [Google Scholar]
- 8. Burnett AK, Russell NH, Hunter AE, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood 2013; 122: 1384–1394. [DOI] [PubMed] [Google Scholar]
- 9. Sekeres MA, Lancet JE, Wood BL, et al. Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia. Haematologica 2013; 98: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dohner H, Lubbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood 2014; 124: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019; 33: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013; 12: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 2014; 4: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 2016; 6: 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niu X, Zhao J, Ma J, et al. Binding of released bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML Cells. Clin Cancer Res 2016; 22: 4440–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teh TC, Nguyen NY, Moujalled DM, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia 2018; 32: 303–312. [DOI] [PubMed] [Google Scholar]
- 17. Tsao T, Shi Y, Kornblau S, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol 2012; 91: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bogenberger JM, Delman D, Hansen N, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma 2015; 56: 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019; 133: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei AH, Strickland SA, Jr, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol 2019: 37 : 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maiti A, DiNardo CD, Cortes JE, et al. Interim analysis of phase II study of venetoclax with 10-day decitabine (DEC10-VEN) in acute myeloid leukemia and myelodysplastic syndrome. Blood 2018; 132: 286. [Google Scholar]
- 22. Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther 2017; 39: 359–367. [DOI] [PubMed] [Google Scholar]
- 23. Montesinos P, Lorenzo I, Martin G, et al. Tumor lysis syndrome in patients with acute myeloid leukemia: identification of risk factors and development of a predictive model. Haematologica 2008; 93: 67–74. [DOI] [PubMed] [Google Scholar]
- 24. Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006; 10: 375–388. [DOI] [PubMed] [Google Scholar]
- 26. Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012; 30: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan R, Ruvolo VR, Wei J, et al. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-) BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood 2015; 126: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luedtke DA, Niu X, Pan Y, et al. Inhibition of Mcl-1 enhances cell death induced by the Bcl-2-selective inhibitor ABT-199 in acute myeloid leukemia cells. Signal Transduct Target Ther 2017; 2: 17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caenepeel S, Brown SP, Belmontes B, et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov 2018; 8: 1582–1597. [DOI] [PubMed] [Google Scholar]
- 30. Moujalled DM, Pomilio G, Ghiurau C, et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 2019; 33: 905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han L, Zhang Q, Dail M, et al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica 2019. pii: haematol.2018205534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan R, Ruvolo V, Mu H, et al. Synthetic lethality of combined Bcl-2 inhibition and p53 activation in AML: mechanisms and superior antileukemic efficacy. Cancer Cell 2017; 32: 748–760.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daver N, Pollyea DA, Yee KWL, et al. Preliminary results from a phase Ib study evaluating BCL-2 inhibitor venetoclax in combination with MEK inhibitor cobimetinib or MDM2 inhibitor idasanutlin in patients with relapsed or refractory (R/R) AML. Blood 2017; 130: 813. [Google Scholar]
- 34. Baker A, Gregory GP, Verbrugge I, et al. The CDK9 inhibitor dinaciclib exerts potent apoptotic and antitumor effects in preclinical models of MLL-rearranged acute myeloid leukemia. Cancer Res 2016; 76: 1158–1169. [DOI] [PubMed] [Google Scholar]
- 35. Bogenberger J, Whatcott C, Hansen N, et al. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget 2017; 8: 107206–107222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012; 366: 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cancer Genome Atlas Research Network, Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016; 374: 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohanian M, Garcia-Manero G, Levis M, et al. Sorafenib combined with 5-azacytidine in older patients with untreated FLT3-ITD mutated acute myeloid leukemia. Am J Hematol 2018; 93: 1136–1141. [DOI] [PubMed] [Google Scholar]
- 40. Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013; 121: 4655–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DiNardo CD, Stein EM, De Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 2018; 378: 2386–2398. [DOI] [PubMed] [Google Scholar]
- 42. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017; 130: 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan SM, Thomas D, Corces-Zimmerman MR, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med 2015; 21: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DiNardo CD, Takahashi K, Kadia T, et al. A phase 1b/2 clinical study of targeted IDH1 inhibition with ivosidenib, in combination with the BCL2 inhibitor venetoclax, for IDH1-mutated myeloid malignancies. In: 24th Annual Congress of the European Hematology Association, Amsterdam, Holland, 13–16 June 2019, abstract PF291, p. 97. [Google Scholar]
- 45. Salmon J, Pomilio G, Moujalled D, et al. Combined BCL-2 and HDAC targeting has potent and TP53 independent activity in AML. Exp Hematol 2018; 64: S99–S100. [Google Scholar]