Abstract
Idiopathic pulmonary arterial hypertension is a progressive disease with high mortality with an increasing burden of right ventricular. Right ventricular dyssynchrony was observed in idiopathic pulmonary arterial hypertension, but the association with mortality is unclear. This study aimed to investigate the impact of right ventricular dyssynchrony on the survival of idiopathic pulmonary arterial hypertension. A total of 116 patients with idiopathic pulmonary arterial hypertension were enrolled in this study. All these patients underwent comprehensive clinical evaluation. Right ventricular dyssynchrony was assessed by two-dimensional speckle-tracking echocardiography. The time to peak strain (Tpeak) of right ventricular segments were obtained. Right ventricular dyssynchrony was quantified by the standard deviation of the heart rate-corrected Tpeak of right ventricular four segments. All patients were followed up and the primary endpoint was all cause of death. Results found patients with significant right ventricular dyssynchrony present with advanced World Health Organization functional class, worse hemodynamic status and right ventricular function. Right ventricular dyssynchrony was an independent predictive factor for the survival of idiopathic pulmonary arterial hypertension. Kaplan–Meier survival curves showed patients with right ventricular dyssynchrony had worse prognosis. In conclusion, right ventricular dyssynchrony analyzed by speckle-tracking echocardiography provided added value to hemodynamic and echocardiographic parameters in evaluating the survival of patients with idiopathic pulmonary arterial hypertension.
Keywords: right ventricle, dyssynchrony, pulmonary arterial hypertension, prognosis
Introduction
Idiopathic pulmonary arterial hypertension (PAH) is considered as a rare, progressive and life-threatening disease with poor prognosis. Due to luminal obstruction of the distal pulmonary vasculature, pulmonary vascular resistance (PVR) and pulmonary arterial pressure are elevated, and finally leads to right ventricular (RV) failure and death.1–3 As described in left heart failure, RV adverse remodeling response to the increased afterload may also destroy its mechanical synchronicity. There are differences in the timing of contraction between the different myocardial segments.4 Recent study observed that the delayed contraction of basal and mid-RV free wall may be the main determinant of intraventricular dyssynchrony in patients with pulmonary hypertension.5,6 RV dyssynchrony even can be observed in patients with narrow QRS, and is strongly correlates with the extent of RV function impairment. Patients with higher RV dyssynchrony showed a more advanced WHO functional class (WHO-FC) and worse exercise tolerance and would predict clinical worsening in patients with PAH.7–9 However, the value of RV dyssynchrony to assess the mortality of idiopathic PAH has not been well defined. Therefore, we performed a study with a larger group of patients with idiopathic PAH, and extended the follow-up time to achieve their clinical outcomes to investigate correlations between RV dyssynchrony and the survival of idiopathic PAH.
Methods
Study population
This study included patients who were diagnosed as idiopathic PAH for the first time in our hospital from January 2010 to December 2014, who had underwent comprehensive clinical evaluation. Idiopathic PAH was established according to the guidelines10 by means of right heart catheterization (RHC). Other specific markers, including WHO-FC, 6-minute walking distance (6MWD) and standard transthoracic echocardiography parameters were sampled at the time of catheterization.11 Patients with wide QRS (QRS ≥120 ms), which may exert an electromechanical delay, were excluded.
Hemodynamics evaluation
RHC was performed using a standard protocol to measure the hemodynamic parameters, including the cardiac index (CI), pulmonary artery systolic pressure, pulmonary artery diastolic pressure (Pa dias), right ventricular systolic pressure, right ventricular end-diastolic pressure, mean right atrial pressure (MRAP), mixed venous oxygen saturation and pulmonary artery wedge pressure (PAWP). PVR was calculated as MPAP-PAWP, divided by cardiac output.
Standard echocardiographic evaluation
Standard transthoracic echocardiography was obtained with the Philips iE33 system, and images were analyzed offline after the procedure according to the recommendation.12 RV end-diastolic area (RVEDA) and RV end-systolic area (RVESA) were obtained from the apical four-chamber view. RV fractional area change (RVFAC) was defined as (RVEDA−RVESA)/RVEDA × 100%. Tricuspid annular plane systolic excursion (TAPSE) was acquired by M-mode image, and the cursor was placed through the lateral tricuspid annulus, the displacement of which from the end-diastole to the end-systole was measured. Right ventricular end-systolic volume (RVESV) and right ventricular end-diastolic volume (RVEDV) were detected by three-dimensional echocardiology, and right ventricular ejection fraction (RVEF) was calculated as (RVEDV−RVESV)/RVEDV × 100%.
A single experienced sonographer, who was blind of the clinical information of patients, performed image acquisition and analysis.
RV mechanical dyssynchrony analysis
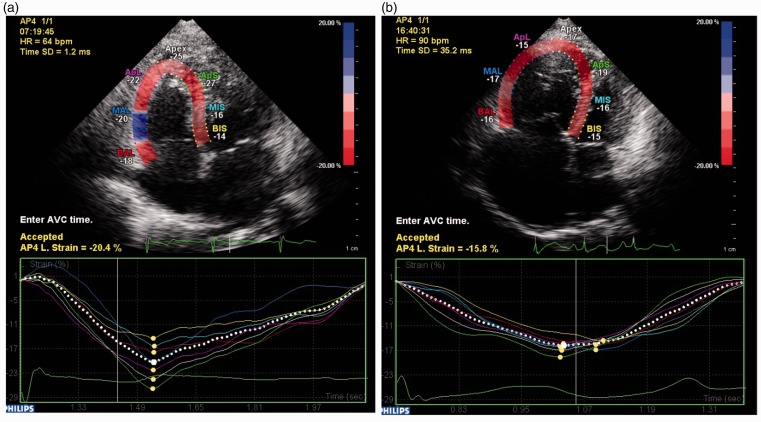
RV mechanical dyssynchrony was analyzed by conventional two-dimensional speckle-tracking echocardiography. RV longitudinal strain was assessed in the apical four-chamber images with a frame rate of 70–80 frames/s. Automated software (CMQ, Q-lab 10.5, Philips) divided the apical four-chamber image into six standard segments (apical, middle and basal of the RV free wall and interventricular septum). Longitudinal strain curves were obtained for six RV segments (shown in Fig. 1) and time to peak-systolic strain (Tpeak, from onset of QRS to the peak-systolic stain) of each segment was measured. As previous report and studies,7,9 time to peak for RV apical segments were more variable, so that we also use the four-segment RV (RV-SD4) model to calculate the synchronicity index. RV-SD4, the standard deviation (SD) of the heart rate–corrected Tpeak of 4 mid-basal RV segments, was used for qualify RV dyssynchrony.
Fig. 1.
Speckle-tracking strain imaging using the apical four-chamber view to assess RV dyssynchrony. The segmental (colored lines) strain curves represent the relative (percentage) shortening of the six regions of interest as a function of time (in milliseconds). The yellow dots in the segment strain curved represent the peak systolic strain of the segments. (a) A six-segment model of the right ventricle in a control subject; (b) An example of a patient with idiopathic PAH with RV-SD4 = 35 ms.
Endpoint and follow-up
All patients were followed up by outpatient clinic interview or telephone contact every six months from the date of referring to the hospital. The primary endpoint was all causes of mortality. Survival data were collected in a six-month interval.
Statistical analysis
All the analyses were performed using the Statistical Package for the Social Sciences software (SPSS, version 20.0). Continuous variables were presented as means ± SD. Student’s t-test and analysis of variance (ANOVA) were applied to compare the mean values of continuous variables, and Mann–Whitney U test for ordered variables. A Chi-squared statistics test was used to assess the differences between proportions. Correlations between RV dyssynchrony and hemodynamic variables were explored using Spearman/Pearson’s coefficient analysis. The prognostic value of RV dyssynchrony was tested by univariate and multivariate Cox proportional hazard regression analysis. Kaplan–Meier curves were used to illustrate the timing of endpoints during follow-up. The optimal cut-off value of RV dyssynchrony to predict mortality was determined by receiver operating characteristic (ROC) curve analysis. A p-value of ≤0.05 was considered statistically significant.
Results
Correlations of RV-SD4 with echocardiographic and hemodynamic parameters
Eleven patients were excluded because of poor image quality, and eventually 116 patients with idiopathic PAH were included in this study.
The mean age was 32 ± 10 years, and they are mainly female (75.8%). The baseline clinical, echocardiographic and hemodynamic characteristics of the study population are presented in Table 1.
Table 1.
The baseline clinical, echocardiographic and hemodynamic characteristic of the study population.
| Variables | Values |
|---|---|
| Age (years) | 32 ± 10 |
| Female (n, %) | 88 (75.9) |
| WHO-FC | |
| I, II (n, %) | 51 (44.0) |
| III (n, %) | 62 (53.4) |
| IV (n, %) | 3 (2.6) |
| Heart rate (beats/minute) | 74 ± 12 |
| Systolic blood pressure (mmHg) | 114 ± 15 |
| Diastolic blood pressure (mmHg) | 74 ± 12 |
| 6MWD (m) | 422 ± 82 |
| NT-proBNP (fmol/ml) | 1306.0 ± 821.4 |
| Echocardiographic parameters | |
| RVFAC (%) | 29.1 ± 8.3 |
| TAPSE (mm) | 16.2 ± 3.5 |
| LVEF (%) | 65.0 ± 6.8 |
| RVEDV (cm3) | 125.4 ± 54.8 |
| RVESV (cm3) | 90.4 ± 46.9 |
| RVEF (%) | 29.8 ± 9.8 |
| Tpeak of basal-RVFW (ms) | 429 ± 70 |
| Tpeak of mid-RVFW (ms) | 404 ± 72 |
| Tpeak of basal-IS (ms) | 414 ± 75 |
| Tpeak of mid-IS (ms) | 396 ± 68 |
| RV-SD4 (ms) | 31 ± 23 |
| Hemodynamic parameters | |
| MRAP (mmHg) | 6 ± 5 |
| MPAP (mmHg) | 59 ± 18 |
| PVR (wood units) | 14.9 ± 7.5 |
| CI (l/min/m2) | 2.7 ± 0.9 |
| Target therapy | |
| Sildenafil (n, %) | 70 (60.3) |
| Bosentan (n, %) | 14 (12.1) |
| Ambrisentan (n, %) | 6 (5.2) |
| Inhaled iloprost (n, %) | 7 (6.1) |
| Calcium channel blockers (n, %) | 15 (12.9) |
| None (n, %) | 4 (3.4) |
Note: Data are presented as n (%) and mean ± SD. WHO-FC: World Health Organization functional class; 6MWD: 6-minute walking distance; LVEF: left ventricular ejection fraction; RVFAC: right ventricular fractional area change; TAPSE: tricuspid annular plane systolic excursion; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; RVEF: right ventricular ejection fraction; Tpeak: time to peak-systolic strain; RVFW: right ventricular free wall; IS: interventricular septum; MRAP: mean right atrial pressure; MPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index.
Correlations between RV-SD4 with echocardiographic and hemodynamic parameters are detailed in Table 2. RV-SD4 had positive correlations with RVESV (r = 0.562, p < 0.001), RVEDV (r = 0.538, p < 0.001), PVR (r = 0.368, p < 0.001), and negative correlations with TAPSE (r = −0.375, p < 0.001), RVFAC (r = −0.411, p < 0.001), RVEF (r = −0.349, p < 0.001) and CI (r = −0.445, p < 0.001).
Table 2.
Correlations between RV-SD4 and echocardiographic, hemodynamic parameters.
| Variables | RV-SD4 |
|
|---|---|---|
| R | p-Value | |
| Echocardiographic parameters | ||
| TAPSE | −0.375 | <0.001 |
| RVESV | 0.562 | <0.001 |
| RVEDV | 0.538 | <0.001 |
| RVEF | −0.349 | <0.001 |
| LVEF | −0.230 | 0.018 |
| RVFAC | −0.411 | <0.001 |
| Hemodynamic parameters | ||
| MRAP | 0.202 | 0.032 |
| RVSP | 0.154 | 0.099 |
| RVEDP | 0.213 | 0.022 |
| MPAP | 0.171 | 0.067 |
| CI | −0.445 | <0.001 |
| PVR | 0.368 | <0.001 |
TAPSE: tricuspid annular plane systolic excursion; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; RVEF: right ventricular ejection fraction; LVEF: left ventricular ejection fraction; RVFAC: right ventricular fractional area change; MRAP: mean right atrial pressure; MPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index; RVSP: right ventricular systolic pressure; RVEDP: right ventricular end-diastolic pressure.
Clinical characteristics based on RV dyssynchrony
All the patients were divided into three tertiles based on the RV-SD4. Characteristics of the patients in the three groups are shown in Table 3. There was a significant gradient in severity of most variables among the three groups. Patients in the upper tertiles of RV dyssynchrony distribution experienced a more impaired WHO-FC, worse hemodynamic status and RV function. RV remodeling in the upper tertiles was also more significant, as RVEDV (165.2 ± 63.1 vs. 108.6 ± 40.8, 105.2 ± 37.0 cm3, p < 0.001) and RVESV (124.9 ± 55.7 vs. 78.6 ± 32.5, 70.2 ± 30.3 cm3, p < 0.001) were larger than the other groups. The plasma level of NT-proBNP of the intermediate and upper tertiles was higher than the lower tertile groups (1245.0 ± 734.6, 1628.4 ± 939.6 vs.1059.8 ± 691.1 fmol/ml, p = 0.008), which means worse RV function in these patients.
Table 3.
Comparison between patients with idiopathic PAH based on RV dyssynchrony.
| Variables | Lower tertiles (n = 39) | Intermediate tertiles (n = 39) | Upper tertiles (n = 38) | p-Value |
|---|---|---|---|---|
| Age (years) | 34 ± 10 | 31 ± 10 | 31 ± 11 | 0.402 |
| WHO-FC | 0.031 | |||
| I, II (n, %) | 23 (59.0) | 15 (38.5) | 13 (34.2) | |
| III, IV (n,%) | 16 (41.0) | 24 (61.5) | 25 (65.8) | |
| 6MWD (m) | 439 ± 77 | 415 ± 82 | 411 ± 87 | 0.306 |
| NT-proBNP (fmol/ml) | 1059.8 ± 691.1 | 1245.0 ± 734.6 | 1628.4 ± 939.6 | 0.008 |
| Echocardiographic parameters | ||||
| RVEDV (cm3) | 105.2 ± 37.0 | 108.6 ± 40.8 | 165.2 ± 63.1 | <0.001 |
| RVESV (cm3) | 70.2 ± 30.3 | 78.6 ± 32.5 | 124.9 ± 55.7 | <0.001 |
| RVEF (%) | 34.6 ± 9.4 | 28.0 ± 8.5 | 26.3 ± 9.6 | <0.001 |
| RVFAC (%) | 33.2 ± 9.8 | 29.1 ± 7.5 | 25.0 ± 4.9 | <0.001 |
| TAPSE (mm) | 17 ± 3 | 16 ± 3 | 15 ± 3 | 0.002 |
| LVEF (%) | 66.6 ± 6.3 | 63.9 ± 6.2 | 64.4 ± 7.7 | 0.202 |
| Hemodynamic parameters | ||||
| MRAP (mmHg) | 5 ± 4 | 6 ± 5 | 7 ± 5 | 0.191 |
| CI (l/min.m2) | 3.2 ± 1.0 | 2.7 ± 0.6 | 2.2 ± 0.6 | <0.001 |
| MPAP (mmHg) | 56 ± 18 | 58 ± 17 | 64 ± 17 | 0.131 |
| PVR (wood units) | 11.9 ± 6.1 | 14.1 ± 7.4 | 18.6 ± 7.5 | <0.001 |
Note: Data are presented as n (%) and mean ± SD. WHO-FC: World Health Organization functional class; 6MWD: 6-minute walking distance; TAPSE: tricuspid annular plane systolic excursion; LVEF: left ventricular ejection fraction; RVFAC: right ventricular fractional area change; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; RVEF: right ventricular ejection fraction; MRAP: mean right atrial pressure; MPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index.
Univariate and multivariate Cox proportional hazard analysis for parameters to predict mortality
The mean duration of follow-up was 41 ± 15 months. During this period, eight patients (7%) were lost, and 93% patients were followed up for at least three years or until death. There were 19 deaths (all causes) and most were died of right heart failure. The baseline clinical and hemodynamic characteristics of the deceased patients are described in Table 4. Among these patients, 18 were WHO-FC III at enrollment. Seventeen patients received PAH-specific drugs combined with conventional therapy as soon as the PAH diagnoses were confirmed. However, the other two patients only received conventional supportive therapy including diuretics, oxygen and digoxin because of financial issues. Compared with survivors, deceased patients had decreased 6MWD (386 ± 78 vs. 429 ± 82 m, p = 0.045), higher NT-proBNP (1728.0 ± 1148.6 vs. 1221.7 ± 717.8 fmol/ml, p = 0.014) and worse hemodynamics.
Table 4.
Baseline demographics, and clinical and hemodynamic characteristic of the survival and deceased patients.
| Variables | Survivors (n = 97) | Non-survivors (n = 19) | p-Value |
|---|---|---|---|
| Age (years) | 32 ± 10 | 31 ± 13 | 0.806 |
| Female (n, %) | 78 (80.4) | 10 (52.6) | <0.001 |
| WHO-FC | <0.001 | ||
| I/II (n, %) | 47 (48.5) | 4 (21.1) | |
| III/ IV (n, %) | 50 (51.5) | 15 (78.9) | |
| Heart rate (beats/minute) | 74 ± 12 | 79 ± 11 | 0.035 |
| Systolic blood pressure (mmHg) | 114 ± 14 | 111 ± 19 | 0.469 |
| Diastolic blood pressure (mmHg) | 74 ± 12 | 75 ± 13 | 0.655 |
| 6MWD (m) | 429 ± 82 | 386 ± 78 | 0.045 |
| NT-proBNP (fmol/ml) | 1221.7 ± 717.8 | 1728.0 ± 1148.6 | 0.014 |
| Echocardiographic parameters | |||
| TAPSE (mm) | 16.5 ± 3.5 | 14.6 ± 2.8 | 0.031 |
| RVEDV (cm3) | 115.0 ± 47.5 | 175.2 ± 61.1 | <0.001 |
| RVESV (cm3) | 81.8 ± 41.8 | 131.4 ± 49.3 | <0.001 |
| RVEF (%) | 30.7 ± 10.1 | 25.3 ± 6.1 | 0.027 |
| LVEF (%) | 65.4 ± 6.9 | 62.9 ± 5.9 | 0.182 |
| RV-SD4 (ms) | 25.9 ± 20.5 | 54.2 ± 22.9 | <0.001 |
| Hemodynamic parameters | |||
| MRAP (mmHg) | 5.0 ± 4.5 | 9.2 ± 4.2 | <0.001 |
| MPAP (mmHg) | 57.9 ± 17.4 | 66.3 ± 17.3 | 0.058 |
| PVR (wood units) | 13.8 ± 6.8 | 20.2 ± 9.1 | 0.001 |
| CI (l/min/m2) | 2.8 ± 0.9 | 2.2 ± 0.6 | 0.002 |
| Target therapy (yes) | 95 (97.9) | 17 (89.4) | 0.064 |
| Sildenafil (n, %) | 62 (63.9) | 8 (42.1) | |
| Bosentan (n, %) | 9 (9.3) | 5 (26.3) | |
| Ambrisentan (n, %) | 6 (6.2) | 0 (0) | |
| Inhaled iloprost (n, %) | 3 (3.1) | 4 (21.1) | |
| Calcium channel blockers (n, %) | 15 (15.4) | 0 (0) | |
| None (n, %) | 2 (2.1) | 2 (10.5) | |
Note: Data are presented as n (%) and mean ± SD. WHO-FC: World Health Organization functional class; 6MWD: 6-minute walking distance; LVEF: left ventricular ejection fraction; RVFAC: right ventricular fractional area change; TAPSE: tricuspid annular plane systolic excursion; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; RVEF: right ventricular ejection fraction; MRAP: mean right atrial pressure; MPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index.
Univariate Cox regression analysis determined that TAPSE, RVFAC, RVEDV, RVEF, MRAP, CI, PVR and RV-SD4 were predictive factors for death. In multivariate Cox proportional hazard analysis, the results showed that RV-SD4 still had the ability to independently predict the mortality after adjusted by other factors (HR = 1.425, p < 0.001) (Table 5).
Table 5.
The results of univariate and multivariate Cox regression analysis for 116 patients with idiopathic PAH.
| Variables | Univariate Cox regression |
Multivariate Cox regression |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-Value | Hazard ratio (95% CI) | p-Value | |
| Echocardiographic parameters | ||||
| TAPSE (mm) | 0.852 (0.740–0.982) | 0.026 | 1.066 (0.857–1.328) | 0.565 |
| RVFAC (%) | 0.932 (0.872–0.996) | 0.037 | 0.994 (0.905–1.091) | 0.893 |
| RVEDV (cm3) | 1.013 (1.006–1.021) | 0.001 | 1.005 (0.994–1.016) | 0.375 |
| RVEF (%) | 0.946 (0.897–0.998) | 0.043 | 1.025 (0.954–1.102) | 0.497 |
| RV-SD4 (ms) | 1.473 (1.237–1.755) | <0.001 | 1.425 (1.185–1.714) | <0.001 |
| Hemodynamic parameters | ||||
| MRAP (mmHg) | 1.115 (1.033–1.203) | 0.005 | 1.166 (1.058–1.286) | 0.002 |
| MPAP (mmHg) | 1.017 (0.996–1.039) | 0.111 | – | – |
| CI (l/min.m2) | 0.208 (0.077–0.567) | 0.002 | 1.068 (0.326–3.502) | 0.913 |
| PVR (wood units) | 1.075 (1.031–1.122) | 0.001 | 1.005 (0.994–1.016) | 0.078 |
WHO-FC: World Health Organization functional class; 6MWD: 6-minute walking distance; MRAP: mean right atrial pressure; MPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index. RVFAC: right ventricular fractional area change; TAPSE: tricuspid annular plane systolic excursion; RVESV: right ventricular end-systolic volume.
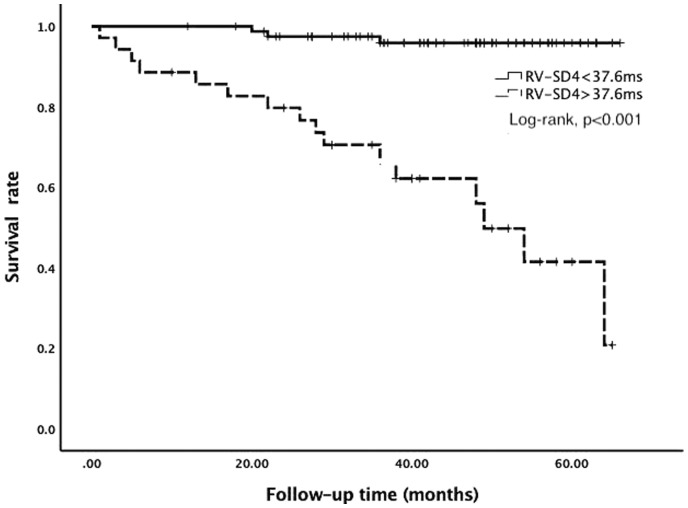
ROC curve analysis was performed to evaluate the utility of RV-SD4 as predictor for mortality. According to the ROC curve analysis, the cut-off value of RV-SD4 was 37.6 ms (AUC = 0.812, p < 0.001), with a sensitivity of 84.2% and a specificity of 80.4%. The Kaplan–Meier survival analysis showed that patients with RV-SD4 > 37.6 ms had worse prognosis (Log-rank, p < 0.001; Fig. 2).
Fig. 2.
Kaplan–Meier survival curve for the idiopathic PAH patients with RV-SD4 > 37.6 ms or RV-SD4 < 37.6 ms.
Reproducibility of RV-SD4
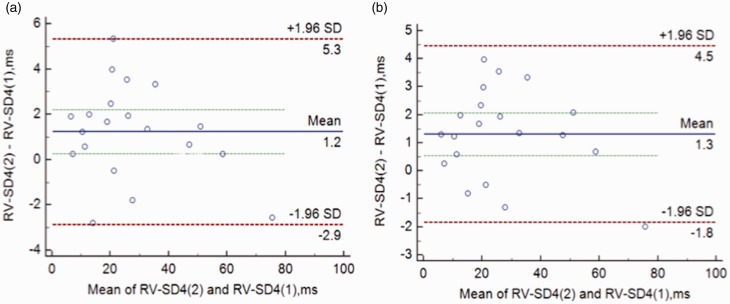
RV-SD4 was repeatedly measured in 20 randomly selected patients, and the interobserver and intraobserver variabilities are shown in Fig. 3, which was assessed by the Bland–Altman method. As a result, the reasonable reproducibility of intraventricular dyssynchrony evaluated by speckle-tracking echocardiography in this study was demonstrated.
Fig. 3.
Interobserver (a) and intraobserver (b) reproducibility Bland–Altman plots for the right ventricular dyssynchrony (RV-SD4).
Discussion
Idiopathic PAH is characterized by pulmonary vascular remodeling, which leads to elevated pulmonary artery pressure and RV failure. RV remodeling responses to chronic pulmonary hypertension is a major cause of RV failure,1,2,13–15 which often includes the adaptive stage and maladaptive stage. The former is associated with more concentric remodeling and preserved systolic function, whereas the latter is characterized by more eccentric hypertrophy and worse systolic function.16,17 Extensive investigations concerning left ventricular dyssynchrony suggest that exploration synchronicity of the RV may be a productive approach to understand RV dysfunction in PAH. Previous studies18–20 suggest that RV dyssynchrony is a marker of maladaptive remodeling and more severe dysfunction. In our study, we also found patients with greater RV dilatation had more significant RV dyssynchrony and poor pump function.
In contrast to the well-studied pathophysiology of LV dyssynchrony, the underlying mechanisms of dyssynchrony in the right ventricle remain largely unexplored. Recently, Gabrielli et al.21 showed that in patients with PAH iloprost inhalation induced an acute reduction in RV peak systolic strain dyssynchrony index together with a better RV performance. Iloprost inhalation has been widely used in patients with PAH and is proved to be able to improve their hemodynamics. In this study, patients with QRS ≥120 ms were excluded, so an acute RV afterload reduction with inhaled iloprost may explain these changes in RV synchronicity. In concordance with recent study, we also showed patients with higher pulmonary arterial pressure and resistance demonstrated significant RV dyssynchrony. The differential effect of elevated pressure overload on RV regional contractility might be attributed to the uneven distribution of mechanical properties across each RV segment caused by the complex structure and the distortion imposed by RV remodeling.
Outcome prediction in patients with idiopathic PAH has been extensively studied. One consistent finding among studies is that survival in idiopathic PAH is closely related to the status of RV rather than the increased pressure overload.22,23 Studies about hemodynamics had demonstrated the predictive value of MRAP and CI;24–26 magnetic resonance imaging studies had proved the value of stroke volume and RVEF.23,27,28 Echocardiographic studies found TAPSE and RVFAC had the predictive value in PAH.29,30 Because hemodynamics assessed by RHC is invasive and magnetic resonance imaging is more expensive, echocardiography is the most common and convenient method used to evaluate RV function. RV dyssynchrony had been described by speckle-tracking echocardiography. Researches by Murata et al.31 and Badagliacca et al.9 had also discovered the prognosis value of RV dyssynchrony. However, the prior study included a heterogeneous population of PH patients in terms of etiology, which are different in the natural progress and prognosis and may affect the results. In addition, in the two studies, the endpoint was complicated. For example, hospitalization and worsening in exercise capacity were regarded as primary outcome. As we know, we reported for the first time the association between RV dyssynchrony and the mortality of idiopathic PAH. Our study emphasized that the RV dyssynchrony is a predictor of survival in patients with idiopathic PAH, and after adjusting by MRAP, CI, TAPSE and RVFAC, RV dyssynchrony could independently predict survival.
This study had several limitations. Firstly, the main limitation is the two-dimensional approach. We assessed RV synchronicity only in the apical four-chamber view, and RV free wall is not seen in its full extent. However, the apical four-chamber view mainly encompasses the lateral portion of the free wall and interventricular septum, which account for a significant part of RV function through its longitudinal shortening according to the earlier study.32,33 Therefore, although far from conclusive, this approach might provide a relatively accurate model to evaluate RV synchronicity. Secondly, this was an observational study and was not able to provide information about the possible pathophysiologic mechanisms that underlie dyssynchrony in the right ventricle. Different from left heart failure, dilated right ventricle could also present with dyssynchrony in the absence of right bundle branch block. As in previous researches, RV dyssynchrony partners with adverse RV remodeling and dysfunction were seen in idiopathic PAH, but it is not clear whether RV dyssynchrony is the cause of RV dysfunction or just one of its marker. The mechanisms of RV dyssynchrony and its clinical use wait testing in the forge of additional research. At last, this study involved patients from a particular center. Therefore, we cannot exclude the possibility of biased estimates for the prevalence and degree of RV dyssynchrony in idiopathic PAH. Future studies with larger patient populations from multiple centers are necessary to verify the results.
In conclusion, RV dyssynchrony analyzed by speckle-tracking echocardiography provided added value to RHC and standard echocardiography in evaluating the survival of patients with idiopathic PAH.
Authors’ contribution
X-LC, B-YL: Study design, statistical analysis and manuscript editing; W-CW, HW: Echocardiographic image acquisition and analysis; WL, LH, TY: Literature research and follow-up of the patients; Z-HL: Manuscript revision; J-GH, C-MX: In charge of the entire study and manuscript final version approval.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This study was supported by the Capital Health Development and Scientific Research Projects, China (project number: 2016-2-4036); CAMS Initiative for Innovation Medicine (CAMS-I2M), China (project number: 2016-I2M-3-006) and Biomedicine and Life Sciences Innovation Cultivation Research Project, China (Z161100000116052).
Ethical approval
This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Fuwai Hospital (ethical approval no. 2018-1063).
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004; 351: 1655–1665. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Abman SH, Braun T, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S3–S9. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Davis M, Cornwell W. Pulmonary arterial hypertension. Curr Probl Cardiol 2011; 36: 461–517. [DOI] [PubMed] [Google Scholar]
- 4.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: D22–D33. [DOI] [PubMed] [Google Scholar]
- 5.Brili S, Stamatopoulos I, Misailidou M, et al. Longitudinal strain curves in the RV free wall differ in morphology in patients with pulmonary hypertension compared to controls. Int J Cardiol 2013; 167: 2753–2756. [DOI] [PubMed] [Google Scholar]
- 6.Kalogeropoulos AP, Georgiopoulou VV, Howell S, et al. Evaluation of right intraventricular dyssynchrony by two-dimensional strain echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr 2008; 21: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 7.Badagliacca R, Poscia R, Pezzuto B, et al. Right ventricular dyssynchrony in idiopathic pulmonary arterial hypertension: determinants and impact on pump function. J Heart Lung Transplant 2015; 34: 381–389. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Candales A, Dohi K, Rajagopalan N, et al. Right ventricular dyssynchrony in patients with pulmonary hypertension is associated with disease severity and functional class. Cardiovasc Ultrasound 2005; 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badagliacca R, Reali M, Poscia R, et al. Right intraventricular dyssynchrony in idiopathic, heritable, and anorexigen-induced pulmonary arterial hypertension: clinical impact and reversibility. JACC Cardiovasc Imaging 2015; 8: 642–652. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Car Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 12.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 13.Dell’Italia LJ. Anatomy and physiology of the right ventricle. Cardiol Clin 2012; 30: 167–187. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117: 1436–1448. [DOI] [PubMed] [Google Scholar]
- 15.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006; 114: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 16.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation 2009; 120: 992–1007. [DOI] [PubMed] [Google Scholar]
- 17.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 18.Handoko ML, Lamberts RR, Redout EM, et al. Right ventricular pacing improves right heart function in experimental pulmonary arterial hypertension: a study in the isolated heart. Am J Physiol Heart Circ Physiol 2009; 297: H1752–H1759. [DOI] [PubMed] [Google Scholar]
- 19.Dubin AM, Feinstein JA, Reddy VM, et al. Electrical resynchronization: a novel therapy for the failing right ventricle. Circulation 2003; 107: 2287–2289. [DOI] [PubMed] [Google Scholar]
- 20.Handoko ML, de Man FS, Allaart CP, et al. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: lessons from the left heart. Eur Respir Rev 2010; 19: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrielli L, Ocaranza MP, Sitges M, et al. Acute effect of iloprost inhalation on right atrial function and ventricular dyssynchrony in patients with pulmonary artery hypertension. Echocardiography 2017; 34: 53–60. [DOI] [PubMed] [Google Scholar]
- 22.Tuder RM, Archer SL, Dorfmüller P, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D4–D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 24.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 25.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 26.Thenappan T, Glassner C, Gomberg-Maitland M. Validation of the pulmonary hypertension connection equation for survival prediction in pulmonary arterial hypertension. Chest 2012; 141: 642–650. [DOI] [PubMed] [Google Scholar]
- 27.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 28.Vonk Noordegraaf A, Galie N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 2011; 20: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 30.Ghio S, Klersy C, Magrini G, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol 2010; 140: 272–278. [DOI] [PubMed] [Google Scholar]
- 31.Murata M, Tsugu T, Kawakami T, et al. Right ventricular dyssynchrony predicts clinical outcomes in patients with pulmonary hypertension. Int J Cardiol 2017; 228: 912–918. [DOI] [PubMed] [Google Scholar]
- 32.Rushmer RF, Thal N. The mechanics of ventricular contraction; a cinefluorographic study. Circulation 1951; 4: 219–228. [DOI] [PubMed] [Google Scholar]
- 33.Naito H, Arisawa J, Harada K, et al. Assessment of right ventricular regional contraction and comparison with the left ventricle in normal humans: a cine magnetic resonance study with presaturation myocardial tagging. Br Heart J 1995; 74: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]