Abstract
Identifying metastasis remains a challenge for death control and tailored therapy for nasopharyngeal carcinoma (NPC). Here, we addressed this by designing a nomogram-based Cox proportional regression model through integrating a panel of tumor biomarkers. A total of 147 locally patients with advanced NPC, derived from a randomized phase III clinical trial, were enrolled. We constructed the model by selecting the variables from 31 tumor biomarkers, including 6 pathological signaling pathway molecules and 3 Epstein-Barr virus-related serological variables. Through the least absolute shrinkage and selection operator (LASSO) Cox proportional regression analysis, a nomogram was designed to refine the metastasis risk of each NPC individuals. Using the LASSO Cox regression model, we constructed a 9 biomarkers-based prognostic nomogram: Beclin 1, Aurora-A, Cyclin D1, Ki-67, P27, Bcl-2, MMP-9, 14-3-3σ, and VCA-IgA. The time-dependence receiver operating characteristic analysis at 1, 3, and 5 years showed an appealing prognostic accuracy with the area under the curve of 0.830, 0.827, and 0.817, respectively. In the validation subset, the concordance index of this nomogram reached to 0.64 to identify the individual metastasis pattern. Supporting by this nomogram algorithm, the individual metastasis risk might be refined personally and potentially guiding the treatment decisions and target therapy against the related signaling pathways for patients with locally advanced NPC.
Keywords: nasopharyngeal carcinoma, tumor biomarker, nomogram algorithm, classifier, metastasis
Introduction
Nasopharyngeal carcinoma (NPC), an Epstein-Barr virus (EBV) infection-related head and neck squamous cell carcinoma, is highly prevalent in the Southeast Asian countries.1,2 Radiotherapy alone or concurrent chemoradiotherapy has been used as the primary curative treatment for early-stage NPC with a 5-year disease-free survival rising to 78%.3 However, although the introduction of neoadjuvant chemotherapy enhanced the local control rate, 20% to30% of patients still develop the distant metastasis, which is the main obstacle for improving the overall survival for NPC.4 Thus, recognizing the high-risk distant metastasis individuals is crucial to provide these particular patients a rational personalized therapy and follow-up.
Currently, the tumor–node–metastasis (TNM) staging system is the primary tool utilized to predict outcomes and contributes largely to the treatment planning for patients with NPC. However, the patients sharing the same TNM stage have the heterogeneous outcome, indicating that other factors might contribute to the determination of prognosis. For decades, tumor biomarkers were demonstrated to facilitate the TNM classification for risk definition and provide insights to develop target therapy to improve the survival in many cancers. For example, the breast cancer subset exhibiting high amplification of the human epidermal growth factor (HER2/neu) receptor had an inferior overall survival.5 Trastuzumab, a monoclonal antibody that was specifically designed to interfere with the HER2/neu receptor, was recommended to patients with HER2/neu overexpression, especially to those with metastatic disease.6 However, the single prognostic tumor biomarker or TNM prognostication system was also found to have the limitation of low sensitivity and specificity to predict tumor recurrence individually.7 For breast cancer, a 21-gene algorithm approach showed high predictive efficacy to stratify the individual patient as low risk and high risk to relapse (6.8% vs 30.5%) at the 10-year follow-up, validating a novel way to predict disease relapse risk individually. Importantly, this multigenes or tumor biomarkers (microRNA and proteins) integrated method had shown a powerful efficacy to predict individual prognosis precisely, not only for breast cancer but also for other cancers, such as colon cancer, nonsmall cell lung cancer, and NPC.8-10
Here, a nomogram model was developed to identify the individual risk to distant metastasis for patients with locally advanced NPC. Briefly, we integrated 3 EBV-related serological biomarkers and 31 clinicopathological tumor biomarkers in protein level. Further, the nomogram model was used to categorize the metastatic risk for each individual patients with NPC through Cox proportional hazards regression analysis and the least absolute shrinkage and selection operator (LASSO) method. Moreover, the predictive value between the nomogram model and current clinically used TNM staging system was compared to refine the individual metastatic risk for locally advanced NPC.
Materials and Methods
Patient Selection
A total of 147 diagnosed and pathologically confirmed patients with NPC, derived from a randomized phase III clinical trial, were enrolled in this study.11 Clinical stage was defined according to the NPC staging system of China.1,12 The patients received induction chemotherapy plus concurrent adjuvant chemoradiotherapy (IC/CCRT) or induction chemotherapy plus radiotherapy (IC/RT) randomly. The inclusion/exclusion criteria of this randomized trial have been reported previously.11,13 In brief, 147 patients were enrolled in this study; 7 patients were excluded, due to the history of previous anticancer therapy in 3 patients in IC/RT subgroup, and lost to 5-year follow-up in 4 patients (1 in IC/RT subgroup and 3 in IC/CCRT subgroup). In IC/CCRT subgroup, patients were treated with 2 cycles of floxuridine + carboplatin (floxuridine 750 mg/m2, day 1-5; carboplatin area under the curve [AUC] = 6) chemotherapy and 1 week later with radiotherapy and concurrent carboplatin (AUC = 6) chemotherapy on day 7, 28, and 49, respectively. In IC/RT subgroup, patients received 2 cycles of floxuridine + carboplatin (floxuridine 750 mg/m2, day 1-5; carboplatin AUC = 6) chemotherapy and underwent radiotherapy 1 week thereafter. In this study, a total radiation dose of 68 to 72 Gy with 2 Gy daily fractions and 5 days per week was conducted.
Immunohistochemical Staining and Measurements
Paraffin-embedded primary tumor tissues of the patients were collected to construct the tissue microassays according to the protocol published previously.14 The candidate biomarkers were all reported to be involved in NPC carcinogenesis or prognosis. In the present study, 31 biomarkers were tested: 6 pathological signaling pathway molecules, consisting of cell cycle: Cyclin D1, 14-3-3σ, Aurora-A, CENP-H, Stathmin, P21, CDC2, P27, ERK, p-ERK, and Ki-67; migration- and invasion-related molecules: E-Cadherin, β-Catenin, N-Cadherin, Snail, Twist, c-Met, and nm23; tumor microenvironment-related molecules: HIF-1α, COX2, MMP-2, MMP-9, and TIMP-2; apoptosis- and autophagy-related molecules: Bax, Bcl-2, Survivin, AKT 1, Pontin, and Beclin 1; epigenetic-related molecule: EZH2, and EBV-related molecule: LMP 1 were tested by immunohistochemical (IHC) staining following the procedures published previously.15,16,17 Here, we changed the specific primary antibody with non-immune serum immunoglobulins as a negative control (1:200 dilutions). Two independent observers blinded to the clinical information evaluated the IHC staining score of each biomarker. As previously reported, we semiquantitatively assessed each tumor biomarker expression level by measuring the staining intensity and extent.18 In brief, we graded staining intensity as follows: negative (score 0), bordering (score 1), weak (score 2), moderate (score 3), and strong (score 4). The staining extent was ranked into 4 parts according to the percentage of positive staining cells in the field: 0% to 25% (score 1), 26% to 50% (score 2), 51% to 75% (score 3), and 76% to 100% (score 4). The overall score was obtained by multiplying the staining intensity and extent. The scores were assessed by 2 independent pathologists blinded to the clinical follow-up. If there was any controversy about the score, a third observer would be intervened to make a final judgment. Here, the consistency of the overall score reached to 96%.
Epstein-Barr virus-Related Serological Antibodies Assays
The serums of each patient were obtained before the oncological treatment. Three EBV-related serological biomarkers associated with tumorigenesis of NPC, including EA-IgA, VCA-IgA, and anti-enzyme rate of EBV DNase-specific neutralizing antibody, were measured through enzyme-linked immunosorbent assay method.19
Patients Follow-Up
The patients in this study were followed up rigorously according to the follow-up protocol. In general, patients were observed at 3-month intervals during the first 3 years after therapy and at 6-month intervals thereafter. The routine follow-up examinations including history recording, physical examination, complete blood and biochemical examinations, fiberoptic nasopharyngoscopy, magnetic resonance imaging of the whole neck, cranial nerve examination, chest radiography, abdominal sonography, and whole-body bone scintigraphy were carried out when clinically indicated. The distant metastasis-free survival (DMFS) was defined as the time from diagnosis to the date of distant metastasis or when censored at the latest date.
Statistical Analysis
We used univariate Cox regression model to explore the association between DMFS and each biomarker. The LASSO multivariate Cox regression model was used to select the biomarkers, and the Cox regression coefficients were used to generate the nomograms. Discrimination was evaluated by analyzing the AUC of the receiver operating characteristic curve (ROC curve) as well as the concordance index (C-index). To alleviate the overfitting issue, we used 1000 bootstrap samples to correct the C-index. The average C-index of the models developed on the bootstrap sample and applied to the original sample were calculated. The difference between these 2 C-indexes was used to optimize the model based on the original model. Nomogram and bootstrap resampling were done with the rms package of R software, and all the other statistical tests were done with R software version 3.2.2. A 2-side P value of <.05 was considered as statistically significant.
Results
The clinical and pathological features are listed in Table 1, and the IHC staining of 31 biomarkers in the 147 patients with locally advanced NPC are shown in Figure S1. The median follow-up period was 60.8 months (range: 2.63-89.87 months) for all patients. Forty-two patients (28.6%) finally developed distant metastasis during the 5-year follow-up. The median DMFS was 62.4 and 60.4 months in IC/RT and IC/CCRT subgroups, respectively (P > .05). The 2-year and 5-year DMFS was 77.63% and 51.32% in the IC/RT subgroup, while 73.24% and 50.70% in the IC/CCRT subgroup, respectively (all P > .05).
Table 1.
Patient Characteristics and Correlation With Distant Metastasis-Free Survival.
| Characteristics (n = 147) | No (%) | Univariate, HR (95% CI) | P Value |
|---|---|---|---|
| Age, year (>43 vs ≤ 43) | 51.7 vs 48.3 | 1.01 (0.58-1.77) | .97 |
| Gender (male vs female) | 78.9 vs 21.1 | 1.05 (0.54-2.05) | .89 |
| T stage (T1-T2 vs T3-T4) | 16.3 vs 83.7 | 0.64 (0.27-1.52) | .31 |
| N stage (N0-N1 vs N2-N3) | 46.3 vs 53.7 | 0.79 (0.45-1.41) | .43 |
| Overall stage (III vs IVa) | 53.7 vs 46.3 | 0.93 (0.53-1.63) | .80 |
| Treatment (IC/RT vs IC/CRT) | 51.7 vs 48.3 | 1.10 (0.62-1.94) | .75 |
| 14-3-3σ a (>7.0 vs ≤ 7.0) | 33.3 vs 66.7 | 1.53 (0.81-2.89) | .19 |
| AKT1a (> 5.0 vs ≤ 5.0) | 44.2 vs 55.8 | 1.44 (0.79-2.62) | .23 |
| Aurora-Aa (≤6.0 vs > 6.0) | 51.0 vs 49.0 | 0.36 (0.20-0.66) | <.01 |
| Baxa (≤ 4.0 vs > 4.0) | 61.9 vs 38.1 | 0.75 (0.42-1.31) | .30 |
| Bcl-2a (>5.0 vs ≤ 5.0) | 54.4 vs 45.6 | 0.78 (0.44-1.38) | .40 |
| Beclin 1a (≤6.0 vs > 6.0) | 60.5 vs 39.5 | 0.59 (0.33-1.06) | .07 |
| β-Catenina (>6.0 vs ≤ 6.0) | 50.3 vs 49.7 | 0.79 (0.42-1.46) | .45 |
| CDC2a (≤5.0 vs >5.0) | 44.9 vs 55.1 | 0.71 (0.40-1.25) | .24 |
| CENP-Ha (≤5.0 vs > 5.0) | 53.1 vs 46.9 | 0.73 (0.42-1.28) | .28 |
| c-Meta (≤6.0 vs > 6.0) | 50.3 vs 49.7 | 0.89 (0.51-1.56) | .68 |
| COX2a (≤6.0 vs > 6.0) | 57.1 vs 42.9 | 0.83 (0.47-1.47) | .53 |
| Cyclin D1a (≤5.0 vs > 5.0) | 56.5 vs 43.5 | 0.64 (0.36-1.13) | .12 |
| E-Cadherina (>5.0 vs ≤ 5.0) | 56.5 vs 43.5 | 1.10 (0.62-1.92) | .75 |
| ERKa (≤5.0 vs > 5.0) | 53.1 vs 46.9 | 0.81 (0.46-1.43) | .47 |
| EZH2a (≤9.0 vs > 9.0) | 49.7 vs 50.3 | 0.82 (0.47-1.44) | .49 |
| HIF-1αa (≤7.0 vs > 7.0) | 58.5 vs 41.5 | 0.61 (0.34-1.07) | .08 |
| Ki-67a (≤5.0 vs > 5.0) | 49.6 vs 50.4 | 0.78 (0.44-1.36) | .37 |
| LMP1a (>4.0 vs ≤ 4.0) | 44.9 vs 55.1 | 0.94 (0.51-1.73) | .84 |
| MMP-2a (≤6.0 vs > 6.0) | 49.7 vs 50.3 | 0.64 (0.37-1.13) | .13 |
| MMP-9a (>2.0 vs ≤ 2.0) | 46.9 vs 53.1 | 1.60 (0.83-3.06) | .16 |
| N-Cadherina (≤5.0 vs > 5.0) | 53.7 vs 46.3 | 0.69 (0.39-1.21) | .19 |
| nm23a (≤ 5.0 vs >5.0) | 40.8 vs 59.2 | 0.62 (0.34-1.12) | .11 |
| P21a (≤4.0 vs > 4.0) | 58.5 vs 41.5 | 0.77 (0.44-1.35) | .35 |
| P27a (≤6.0 vs > 6.0) | 42.2 vs 57.8 | 0.49 (0.28-0.85) | .01 |
| p-ERKa (>3.0 vs ≤ 3.0) | 43.5 vs 56.5 | 1.12 (0.58-2.16) | .73 |
| Pontina (>3.0 vs ≤ 3.0) | 43.5 vs 56.5 | 0.86 (0.48-1.54) | .62 |
| Snaila (> 4.0 vs ≤ 4.0) | 54.4 vs 45.6 | 1.14 (0.65-2.00) | .65 |
| Stathmina (≤6.0 vs > 6.0) | 42.9 vs 57.1 | 0.69 (0.40-1.22) | .20 |
| Survivina (>3.0 vs ≤ 3.0) | 58.5 vs 41.5 | 1.10 (0.63-1.92) | .75 |
| TIMP-2a (>6.0 vs ≤ 6.0) | 46.9 vs 53.1 | 1.07 (0.61-1.88) | .81 |
| Twista (>3.0 vs ≤ 3.0) | 55.8 vs 44.2 | 0.96 (0.55-1.70) | .90 |
| EA-IgAc (≤1:40 vs > 1:40) | 65.3 vs 34.7 | 0.89 (0.49-1.63) | .71 |
| VCA-IgAc (≤1:160 vs > 1:160) | 39.5 vs 60.5 | 0.86 (0.48-1.56) | .63 |
| AERc (≤55.0% vs > 55.0%) | 38.8 vs 61.2 | 0.63 (0.35-1.13) | .12 |
Abbreviations: AER, antienzyme rate; HR, hazard ratio; CI, confidence interval.
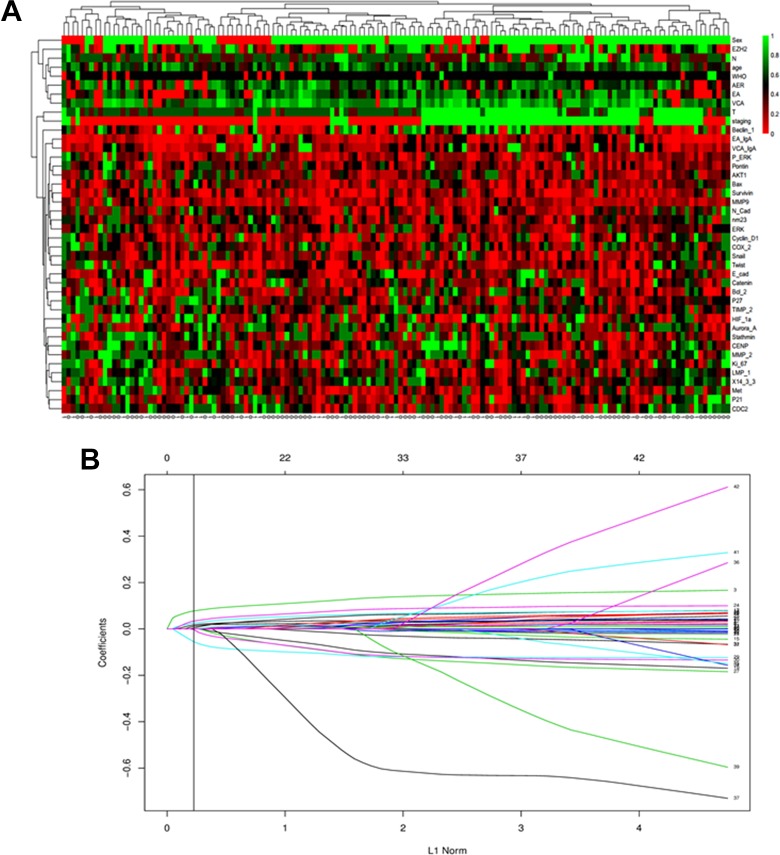
As shown in Figure 1a, there is no significant difference regarding to each clinicopathological variables between the subsets with and without distant metastasis. The univariate analysis indicated that Beclin 1, Aurora-A, Bcl-2, P27, 14-3-3σ, and VCA-IgA were associated with DMFS rate (Table S1). Further, the LASSO Cox regression model was used to construct a prognostic classifier, selecting 9 candidate variables from the 34 tumor-related molecular biomarkers and 8 clinical variables associated with DMFS (Table 2 and Figure 1b). To provide a clinically quantitative tool to predict the individual distant metastasis probability, a nomogram classifier was constructed to predict 1-, 3-, and 5-year DMFS using the 9 candidate biomarkers (Figure 2a). The nomogram can assign the probability of DMFS by adding up the scores of the selected factors shown on the point scale. Specifically, the “risk score” was used to identify the distant metastasis risk for each patient based on their individual 9 biomarker levels, where “risk score = 0.0524*Beclin 1 + 0.1156*Aurora-A + 0.0384*Cyclin D1 + 0.0569*Ki-67 + 0.0472*Bcl-2 + 0.0709*P27 − 0.1492*14-3-3σ − 0.1288*MMP-9 + 0.0004*VCA-IgA.”
Figure 1.
A, Heatmap of the 147 cancer tissues expressed 31 biomarkers. Every row represents an individual biomarker, and each column represents an individual sample. Pseudo colors indicate transcript levels from low to high on a scale from 0 to 1, ranging from a low association strength (bright, red) to high (bright, green). B, The least absolute shrinkage and selection operator coefficient profiles of 34 tumor-related molecular biomarkers and 8 clinicopathological variables associated with DMFS. A vertical line is drawn at the value chosen by 10-fold cross-validation. DMFS indicates distant metastasis-free survival.
Table 2.
Selected Variables According to the Cox Proportional Hazards Regression Model.
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| Beclin 1 | 1.05 | 1 to 1.11 | .051 |
| Aurora-A | 1.12 | 1.04 to 1.21 | .002 |
| Cyclin D1 | 1.04 | 0.98 to 1.11 | .236 |
| Ki-67 | 1.06 | 0.98 to 1.14 | .134 |
| Bcl-2 | 1.05 | 0.98 to 1.13 | .192 |
| P27 | 1.07 | 0.99 to 1.16 | .082 |
| 14-3-3σ | 0.86 | 0.78 to 0.95 | .002 |
| MMP-9 | 0.88 | 0.73 to 1.06 | .187 |
| VCA-IgA | 1.00 | 1 to 1 | .270 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
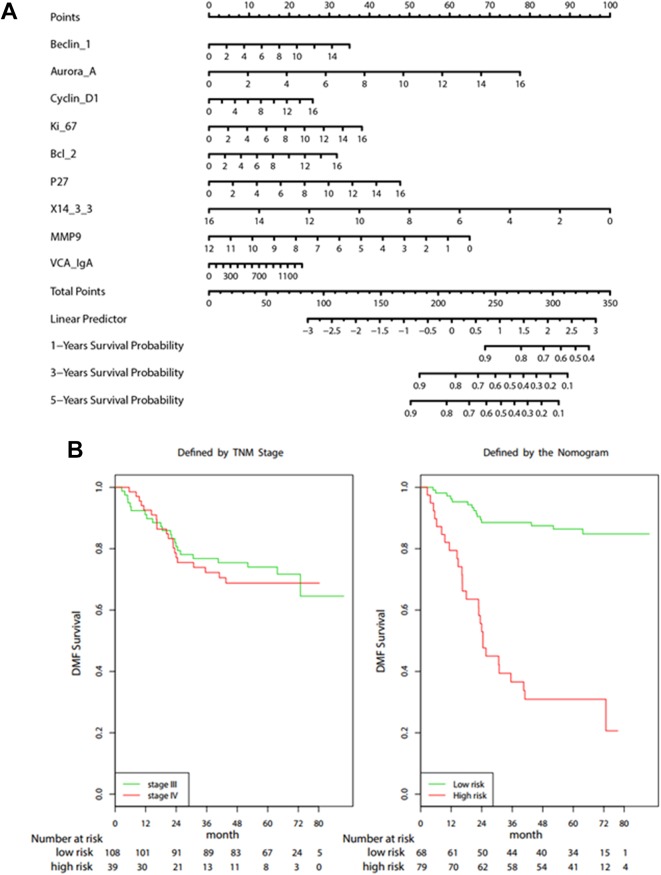
Figure 2.
A, Nomogram for predicting 1-, 3- and 5-year DMFS in patients with nasopharyngeal carcinoma. B, Kaplan-Meier survival curves of DMFS: TNM staging system and the Cox proportional hazards model. DMFS indicates distant metastasis-free survival; TNM, tumor–node–metastasis.
As shown in Table 2, except 14-3-3σ, the rest of the 8 variables were all positively associated with DMFS. The “rpart” procedure in R software was used to generate the optimum cut-off score, where those patients with a risk score of 0.491 or higher was classified as high risk of distant metastasis and those with a risk score lower than 0.491 was ranked as low risk of distant metastasis. Importantly, the distribution of the DMFS was varied significantly between the subsets identified as high-risk group and low-risk group, where the 3-year DMFS was 33.3% for the high-risk group, and 82.4% for the low-risk group (hazard ratio [HR]: 7.527, 95% confidence interval [CI]: 3.978-14.242, P < .0001). Conversely, the DMFS was indistinguishable between TNM stage III and stage IV (Figure 2b).
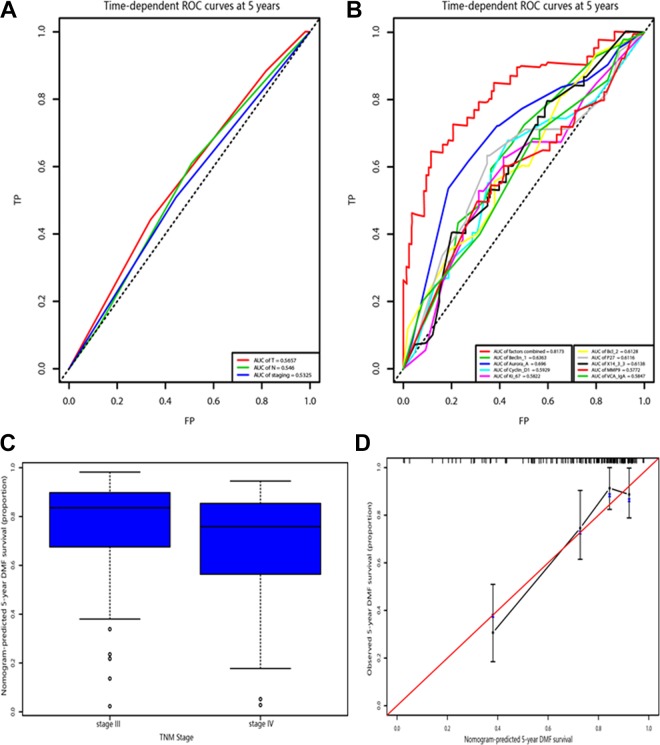
Individually, the predictive accuracy of this classifier to predict personal metastasis pattern was also characterized. The time-dependence ROC analysis at 1, 3, and 5 years proved that the AUC of this classifier could reach to 0.830, 0.827, and 0.817, respectively (Figure 3a and b). In addition, the C-index for classifier was 0.768. Accounting for the overfitting issue, the bootstrap approach was further utilized. With 1000 bootstrap resamples, the average C-statistics of the models developed on the original data, the training data, and the testing data were 0.81, 0.90, and 0.73, respectively. Thus, the expected C-index for the present study should be 0.64 when applied to validation data set.
Figure 3.
A, Receiver operating characteristic curve for the TNM stage. B, Receiver operating characteristic curve for the model and the selected biomarkers. C, The box plot represents the distribution of nomogram-predicted 5-year survival according the TNM staging system. D, Calibration of the nomogram. The x-axis represents the nomogram-predicted survival, and the y-axis represents actual survival and 95% CIs measured by Kaplan-Meier analysis. CI indicates confidence interval; DMFS, distant metastasis-free survival; TNM, tumor–node–metastasis.
Figure 3c demonstrates the 5-year survival predicted by the nomogram in stage III and stage IV of the NPC staging system of China. A wide range of predicted survival could be identified in stage III and stage IV. Besides, the range of predicted survival was wider for higher stages.
To further test the efficacy of the nomogram, the calibration curve of the nomogram 1 was plotted (Figure 3d). The y-axis was the observed DMFS estimated by the Kaplan-Meier method, and the x-axis was the predicted survival computed by the nomogram. As expected, the calibration plot showed a favorable agreement between the nomogram predicted DMFS and the actual observation. The tolerance of variation range was 10%.
Discussion
Nasopharyngeal carcinoma of the undifferentiated histological subtype has a remarkable racial and geographical distribution in Southeast Asia, especially in Cantonese area of China. The tremendous advances of overall prognosis and treatment outcomes during the past few decades have been attributed to the improvement of disease screening and diagnosis, diagnostic imaging, radiotherapy techniques, use of combination systemic therapy, and dedicated clinical and biomarker surveillance.20,21 Although the standard therapy for NPC has 78% 5-year survival rate, the metastasis hinders the achievement of a higher survival rate, the distant metastasis rate has remained high (occurring in approximately 30% of patients), and ultimately results in death. Therefore, more effective systemic therapy is needed to improve the overall survival for these patients.3,22,23 If metastasis was diagnosed at an earlier stage, the salvage treatment would achieve more favourable outcome. Therefore, identifying the high-risk distant metastasis individuals is crucial to provide these approximately 30% of remaining patients a rational personalized therapy and follow-up.
In clinical practice, the TNM staging system is the major tool for clinical decision-making and prognostic determinant for patients with tumor, including NPC. However, a great variation was also observed for the individuals sharing the same cancer stage, suggesting that the TNM staging system is not enough to determine patient outcome. On the other hand, recently, accumulated studies highlighted the use of single or integrated biomarkers to predict the outcome of patients with NPC. For example, enhanced expression of N-cadherin and β-catenin protein was positively correlated with NPC lymph node metastasis and predicted a poorer prognosis in patients with NPC. The impact of serum EBV antibodies on the prognosis of patients with nonmetastatic NPC from a population with a high prevalence of both EBV infection and NPC.24,25 However, the use of a single or several tumor biomarkers is limited due to its low predictive efficacy.26,27 Thus, it is reasonable to combine TNM staging system and tumor biomarkers to predict the prognosis of patients with tumor. Here, we explored a panel of molecular biomarkers integrated nomogram mathematic approach to identify individual metastasis risk for locally advanced NPC. By measuring the expression levels of 31 biomarkers related to 6 pathological signaling pathways at tumor specimen, and 3 EBV-related biomarkers at serum level, we constructed a simple graphical nomogram which might effectively define the risk of metastasis personally.
Indeed, our LASSO Cox proportional regression analysis allowed us to integrate multiple biomarkers into 1o nomogram, which had a higher predictive accuracy than the traditional TNM staging system, where the 3-year DMFS was 33.3% for the high-risk group, and 82.4% for the low-risk group (HR: 7.527, 95% CI: 3.978-14.242, P < .0001). Furthermore, patients with the same cancer stage were able to be stratified into different risk groups through this nomogram.28,29
Nasopharyngeal carcinoma is a heterogeneous disease since the outcomes vary between the patients with similar clinical and pathological features. And metastasis is a major hallmark for malignant tumors which accounts for the majority of cancer-related deaths including NPC.30,31 Application of this nomogram to recognize high- and low-risk metastatic population has a vast benefit. First, it complements and segregates the TNM staging system for prognostication instead of the predictive limitation seen in TNM staging system or biomarkers when used alone. Second, the risk stratification can guide treatment decision for patients to be treated by radiotherapy (RT) alone or RT with chemotherapy or IC/CCRT or other personalized therapy based on the risk definition. By this way, patients with high risk of metastasis could benefit from intensive therapy-like targeted therapy, intensity-modulated radiation therapy, whereas those with low risk could avoid unnecessary side effects.11,32,33 Although further large scale, prospective, multicenter studies are needed to test these strategies for clinical use. Third, the follow-up protocol could be improved to cut excess economic cost. Moreover, the methods for measuring biomarker expression and EBV-related antibodies are practicable and efficient making our result more adaptable to clinical practice.
While our proposed nomogram has the potential to precisely identify the individual metastasis risk, the method may pose some limitations. For example, our study is limited in terms of generalizability since all our data obtained from patients in China and the distribution of clinical characteristics may vary among other areas. Besides, there is no external validation of the model, additional sets of independent samples will be needed to confirm our findings.
In conclusion, our study demonstrated promising multibiomarkers integrated nomogram in predicting NPC metastasis risk and may potentially guide the treatment decisions and target therapy against the related signaling pathways for patients with locally advanced NPC.
Supplemental Material
Supplemental Material, Authorship_Change_Request_Form__CCX-19-0030 for Distant Metastasis Risk Definition by Tumor Biomarkers Integrated Nomogram Approach for Locally Advanced Nasopharyngeal Carcinoma by Xinjuan Fan, Ya Xie, Haiyang Chen, Xiaobo Guo, Yan Ma, Xiaolin Pang, Yan Huang, Fang He, Shuai Liu, Yizhen Yu, Minghuang Hong, Jian Xiao, Xiangbo Wan, Ming Li and Jian Zheng in Cancer Control
Supplemental Material
Supplemental Material, Figure_S1 for Distant Metastasis Risk Definition by Tumor Biomarkers Integrated Nomogram Approach for Locally Advanced Nasopharyngeal Carcinoma by Xinjuan Fan, Ya Xie, Haiyang Chen, Xiaobo Guo, Yan Ma, Xiaolin Pang, Yan Huang, Fang He, Shuai Liu, Yizhen Yu, Minghuang Hong, Jian Xiao, Xiangbo Wan, Ming Li and Jian Zheng in Cancer Control
Supplemental Material
Supplemental Material, Table_S for Distant Metastasis Risk Definition by Tumor Biomarkers Integrated Nomogram Approach for Locally Advanced Nasopharyngeal Carcinoma by Xinjuan Fan, Ya Xie, Haiyang Chen, Xiaobo Guo, Yan Ma, Xiaolin Pang, Yan Huang, Fang He, Shuai Liu, Yizhen Yu, Minghuang Hong, Jian Xiao, Xiangbo Wan, Ming Li and Jian Zheng in Cancer Control
Footnotes
Authors’ Note: The authors Xinjuan Fan, Ya Xie, Haiyang Chen, and Xiaobo Guo equally contributed to this work and are coprimary authors. X. J. F., Y. X., X. B. W., and J. Z. designed the study. H. Y. C., Y. M., X. L. P., Y. H., and M. L. drafted the manuscript. F. H., S. L., Y. Z. Y., M. H. H. and J. X. provided the oversight of provider accrual for the study. X. B. G. performed statistical analyses. All authors reviewed the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect tothe research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Collaborative Research Programme from International Centre for Genetic Engineering and Biotechnology (CRP—ICGEB), China (No. CRP/CHN16-04_EC to W. X. B.); Guangdong Natural Science Foundation for Distinguished Young Scholars, China (No. 2014A030306016 W. X. B.); Natural Science Foundation of Guangdong Province, China (No. 2016A030310187 to J. X.).
ORCID iD: Yan Ma, MS  https://orcid.org/0000-0002-3559-4393
https://orcid.org/0000-0002-3559-4393
Supplemental Material: Supplemental material for this article is available online.
Ethics Statement: The study was approved by the Sixth Affiliated Hospital of Sun Yat-sen University (Approval No. 2019ZSLYEC-111), and each participant signed an informed consent at their recruitment. The whole research was conducted in accordance with the approved guidelines.
References
- 1. Hong MH, Mai HQ, Min HQ, Ma J, Zhang EP, Cui NJ. A comparison of the Chinese 1992 and fifth-edition international union against cancer staging systems for staging nasopharyngeal carcinoma. Cancer. 2000;89(2):242–247. doi:10.1002/1097-0142(20000715)89:2. [DOI] [PubMed] [Google Scholar]
- 2. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi:10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 3. Wu F, Wang R, Lu H, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112(1):106–111. doi:10.1016/j.radonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 4. Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol. 2005;23(6):1118–1124.doi:10.1200/JCO.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 5. Baselga J, Cortés J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–3761.doi:10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 6. Daniels B, Kiely BE, Houssami N, et al. Survival outcomes for Australian women receiving trastuzumab for HER2-positive metastatic breast cancer following (neo) adjuvant trastuzumab: a national population-based observational study (2006-2014). Br J Cancer. 2018;118(3):441–447. doi:10.1038/bjc.2017.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2015;108(1). doi:10.1093/jnci/djv291. [DOI] [PubMed] [Google Scholar]
- 8. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869.doi:10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 9. Wen X, Tang X, Li Y, et al. Microarray expression profiling of long non-coding RNAs involved in nasopharyngeal carcinoma metastasis. Int J Mol Sci. 2016;17(11):1956–1968. doi:10.3390/ijms17111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang JX, Song W, Chen ZH, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14(13):1295–1306.doi:10.1016/S1470-2045(13)70491 -1. [DOI] [PubMed] [Google Scholar]
- 11. Huang PY, Zeng Q, Cao KJ, et al. Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: a single-institution experience from an endemic area. Eur J Cancer. 2015;51(13):1760–1770. doi:10.1016/j.ejca.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 12. Minsky BD, Cohen AM, Kemeny N, et al. Combined modality therapy of rectal cancer: decreased acute toxicity with the preoperative approach. J Clin Oncol. 1992;10(8):1218–1224. doi:10.1200/JCO.1992.10.8.1218. [DOI] [PubMed] [Google Scholar]
- 13. Huang PY, Cao KJ, Guo X, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48(10):1038–1044. doi:10.1016/j.oraloncology.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14. Fan XJ, Wan XB, Huang Y, et al. Epithelial-mesenchymal transition biomarkers and support vector machine guided model in preoperatively predicting regional lymph node metastasis for rectal cancer. Br J Cancer. 2012;106(11):1735–1741. doi:10.1038/bjc.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng HM, Zheng P, Wang XY, et al. Over-expression of Nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9(4):295–302.doi:10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 16. Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer. 2001;94(1):1–5.doi:10.1002/ijc.1385. [DOI] [PubMed] [Google Scholar]
- 17. Wan XB, Zhao Y, Fan XJ, et al. Molecular prognostic prediction for locally advanced nasopharyngeal carcinoma by support vector machine integrated approach. PLoS One. 2012;7(3):e31989 doi: 10.1371/journal.pone.0031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan XB, Fan XJ, Chen MY, et al. Elevated beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6(3):395–404. doi:10.4161/auto.6.3.11303. [DOI] [PubMed] [Google Scholar]
- 19. Xu J, Wan XB, Huang XF, et al. Serologic antienzyme rate of Epstein-Barr virus DNase-specific neutralizing antibody segregates TNM classification in nasopharyngeal carcinoma. J Clin Oncol. 2010;28(35):5202–5209. doi:10.1200/JCO.2009.25.6552. [DOI] [PubMed] [Google Scholar]
- 20. Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 21. Lee VH, Lam KO, Chang AT, et al. Management of nasopharyngeal carcinoma: Is adjuvant therapy needed? J Oncol Pract. 2018;14(10):594–602. doi: 10.1200/JOP.18.00219. [DOI] [PubMed] [Google Scholar]
- 22. Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol.2012;13(2):172–180.doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233–244.doi:10.1016/j.semradonc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 24. Sun H, Liu M, Wu X, et al. Overexpression of N-cadherin and β-catenin correlates with poor prognosis in patients with nasopharyngeal carcinoma. Oncol Lett. 2017;13(3):1725–1730.doi:10.3892/ol.2017.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao JJ, Lin L, Jin YN, et al. Prognostic value of serum Epstein-Barr virus antibodies in patients with nasopharyngeal carcinoma and undetectable pretreatment Epstein-Barr virus DNA. Cancer Sci. 2017;108(8):1640–1647.doi:10.1111/cas.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esselen KM, Cronin AM, Bixel K, et al. Use of Ca-125 tests and computed tomographic scans for surveillance in ovarian cancer. JAMA Oncol. 2016;2(11):1427–1433.doi:10.1001/jamaoncol.2016.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silva JP, Gorman RA, Berger NG, et al. The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J Surg Oncol. 2017;116(7):831–840.doi:10.1002/jso.24742. [DOI] [PubMed] [Google Scholar]
- 28. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28.doi:10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 29. Wan XB, Fan XJ, Huang PY, et al. Aurora-A activation, correlated with hypoxia-inducible factor-1α, promotes radiochemoresistance and predicts poor outcome for nasopharyngeal carcinoma. Cancer Sci. 2012;103(8):1586–1594.doi:10.1111/j.1349-7006.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong B, Lui VW, Hashiguchi M, Hui EP, Chan AT. Targeting tumor hypoxia in nasopharyngeal carcinoma. Head Neck. 2013;35(1):133–145.doi:10.1002/hed.21877. [DOI] [PubMed] [Google Scholar]
- 31. Xu T, Zhu G, He X, Ying H, Hu C. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral Oncol. 2014;50(2):71–76.doi:10.1016/j.oraloncology.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 32. Ma BB, Kam MK, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23(5):1287–1292. doi:10.1093/annonc/mdr401. [DOI] [PubMed] [Google Scholar]
- 33. Songthong AP, Kannarunimit D, Chakkabat C, Lertbutsayanukul C. A randomized phase II/III study of adverse events between sequential (SEQ) versus simultaneous integrated boost (SIB) intensity modulated radiation therapy (IMRT) in nasopharyngeal carcinoma; preliminary result on acute adverse events. Radiat Oncol. 2015;10:166–175. doi:10.1186/s13014-015-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Authorship_Change_Request_Form__CCX-19-0030 for Distant Metastasis Risk Definition by Tumor Biomarkers Integrated Nomogram Approach for Locally Advanced Nasopharyngeal Carcinoma by Xinjuan Fan, Ya Xie, Haiyang Chen, Xiaobo Guo, Yan Ma, Xiaolin Pang, Yan Huang, Fang He, Shuai Liu, Yizhen Yu, Minghuang Hong, Jian Xiao, Xiangbo Wan, Ming Li and Jian Zheng in Cancer Control
Supplemental Material, Figure_S1 for Distant Metastasis Risk Definition by Tumor Biomarkers Integrated Nomogram Approach for Locally Advanced Nasopharyngeal Carcinoma by Xinjuan Fan, Ya Xie, Haiyang Chen, Xiaobo Guo, Yan Ma, Xiaolin Pang, Yan Huang, Fang He, Shuai Liu, Yizhen Yu, Minghuang Hong, Jian Xiao, Xiangbo Wan, Ming Li and Jian Zheng in Cancer Control
Supplemental Material, Table_S for Distant Metastasis Risk Definition by Tumor Biomarkers Integrated Nomogram Approach for Locally Advanced Nasopharyngeal Carcinoma by Xinjuan Fan, Ya Xie, Haiyang Chen, Xiaobo Guo, Yan Ma, Xiaolin Pang, Yan Huang, Fang He, Shuai Liu, Yizhen Yu, Minghuang Hong, Jian Xiao, Xiangbo Wan, Ming Li and Jian Zheng in Cancer Control