Abstract
The bone morphogenetic protein receptor II (BMPRII) signaling pathway is impaired in pulmonary arterial hypertension and mutations in the BMPR2 gene have been observed in both heritable and idiopathic pulmonary arterial hypertension. However, all BMPR2 mutation carriers do not develop pulmonary arterial hypertension, and inflammation could trigger the development of the disease in BMPR2 mutation carriers. Circulating levels and/or lung tissue expression of cytokines such as tumor necrosis factor-α or interleukin-18 are elevated in patients with pulmonary arterial hypertension and could be involved in the pathogenesis of pulmonary arterial hypertension. We consequently hypothesized that cytokines could trigger endothelial dysfunction in addition to impaired BMPRII signaling. Our aim was to determine whether impairment of BMPRII signaling might affect endothelium barrier function and adhesiveness to monocytes, in response to cytokines. BMPR2 was silenced in human lung microvascular endothelial cells (HLMVECs) using lentiviral vectors encoding microRNA-based hairpins. Effects of tumor necrosis factor-α and interleukin-18 on HLMVEC adhesiveness to the human monocyte cell line THP-1, adhesion molecule expression, endothelial barrier function and activation of P38MAPK were investigated in vitro. Stable BMPR2 silencing in HLMVECs resulted in impaired endothelial barrier function and constitutive activation of P38MAPK. Adhesiveness of BMPR2-silenced HLMVECs to THP-1 cells was enhanced by tumor necrosis factor-α and interleukin-18 through ICAM-1 adhesion molecule. Interestingly, tumor necrosis factor-α induced activation of P38MAPK and disrupted endothelial barrier function in BMPR2-silenced HLMVECs. Altogether, our findings showed that stable BMPR2 silencing resulted in impaired endothelial barrier function and activation of P38MAPK in HLMVECs. In BMPR2-silenced HLMVECs, cytokines enhanced adhesiveness capacities, activation of P38MAPK and impaired endothelial barrier function suggesting that cytokines could trigger the development of pulmonary arterial hypertension in a context of impaired BMPRII signaling pathway.
Keywords: pulmonary arterial hypertension, inflammation, BMPR2 mutation, signaling, cell adhesion molecules
Introduction
Pulmonary arterial hypertension (PAH) is a severe and life-threatening disease resulting in right heart failure and need for lung transplantation in many patients. Despite the recent development of several specific therapies for PAH, there is still no cure and the prognosis of the disease remains poor.1 Current PAH-specific therapies mainly substitute pulmonary arterial endothelium-derived vasodilatory mediators, but do not reverse pulmonary vascular remodeling characterized by endothelium dysfunction, loss of pre-capillary pulmonary arteries and proliferation of pulmonary vascular cells resulting in obstruction of the vessel lumen. Among patients harboring a familial form of PAH, 70% carry an autosomal dominant mutation resulting in haploinsufficiency or loss-of-function of bone morphogenetic protein receptor II (BMPRII). BMPR2 gene mutations are also present in 20% of sporadic cases of idiopathic PAH2; mutations in other receptors of the transforming growth factor β (TGF-β) family such as activin receptor-like kinase-type 1 3 and endoglin,4 and affecting BMP signaling including SMAD9,5 caveolin-16 and potassium channel subfamily K member 3 (KCNK3)7 have been also identified in patients with PAH. However, BMPR2 mutation displays a low penetrance since only 20% of the BMPR2 mutation carriers develop clinical symptoms of PAH. In addition, this low penetrance suggests that BMPR2 mutation carriers may harbor enhanced susceptibility to an inflammatory insult, for instance.8
Endothelium dysfunction plays a major role in the initiation and the progression of PAH and is associated with impaired BMP signaling as illustrated by decreased pulmonary arterial endothelial cells (PAECs) survival in response to injury,9 impaired adhesion and migration,10,11 enhanced adhesiveness for monocytes in response to inflammatory mediators12 and disordered angiogenesis.13 Reduced expression of BMPRII results in impaired canonical BMP signaling including SMAD1/5/8 and Id proteins as well as in the implementation of compensatory alternative pathways including P38MAPK pathway.14–17 Accordingly, inflammatory mediators may contribute to P38MAPK activation in a context of impaired BMPRII function.18
Considering the potential role of inflammatory cytokines and chemokines including IL1,19 IL6,20 IL8,21 CCL2,22 CXCL10,23 CCL5 24,25 and fraktalkine26 in PAH and the recent demonstration of IL6 production by PAECs from BMPR2 mutation carriers in response to inflammatory mediators,12 we hypothesized that cytokines could negatively affect the endothelial function in a context of impaired BMPRII signaling pathway.
Interestingly, interleukin-18 (IL18), a pro-inflammatory cytokine, implicated in the pathogenesis of atherosclerotic disease,27 may display adverse effects on endothelium function by inducing apoptosis and increasing permeability of pulmonary microvascular endothelial cells (ECs),28 increasing the expression of adhesion molecules in human dermal microvascular ECs29 or inducing the expression of cytokines by ECs.30 In addition, plasma levels of IL18 and its downstream chemokine, CXCL10, are increased in PAH patients, and medial pulmonary smooth muscle cells (SMCs) are a source of IL18 and its receptor, IL18R, in PAH patients.31 Moreover, IL18 disruption has been recently shown to suppress hypoxia-induced PAH in mice.32 Eventually, tumor necrosis factor-α (TNFα), another pro-inflammatory cytokine, is involved in the pathogenesis of PAH,33 noteworthy by potentially contributing to endothelial dysfunction through P38MAPK.34
Due to the limited access to native PAECs from PAH patients, we knocked down BMPR2 in human lung microvascular endothelial cells (HLMVECs) to obtain a stable BMPR2-silenced HLMVEC lineage, in which we investigated the effects of cytokines, including IL18 and TNFα, on endothelium function and on activation of the P38MAPK pathway.
Materials and methods
Tissue collection
Lung parenchyma was collected at the time of lung transplantation from patients with idiopathic PAH (n = 8), from control subjects at the time of lobectomy or pneumonectomy for suspected localized lung tumor (n = 8) and from an unused donor lung (n = 1). After collection, lung tissue was immediately snap-frozen and stored at − 80℃ until use. The study protocol was approved by the Institutional Ethics Committee of the University of Leuven and participants gave written informed consent. Demographic characteristics of patients and control subjects are briefly described in Table 1.
Table 1.
Characteristics of control subjects and IPAH patients.
| Controls (n = 9) | IPAH (n = 8) | |
|---|---|---|
| Age (years) | 66 ± 12 | 46 ± 17 |
| Gender (M/F) | 5/4 | 4/4 |
Generation of lentiviral transfer plasmids and lentiviral vector production
Short interfering RNA (siRNA) sequence targeting human BMPR2 mRNA at positions 1920, 4277 and 7048 were developed using siSearch software (http://www.dharmacon.com/DesignCenter/DesignCenterPage.aspx). The number refers to the respective positions in the reference human BMPR2 mRNA (NM_001204.6) that are recognized by the siRNA. Based on this sequence, simian immunodeficiency virus (SIV)-based lentiviral vectors, gift of D. Nègre (ENS Lyon, France) and encoding microRNA 30 (miR30)-based knockdown hairpins derived from the aforementioned siRNA, were generated to allow stable knockdown as previously described35 (referred to as LV_miR_BMPR2_1920, LV_miR_BMPR2_4277 or LV_miR_BMPR2_7048, respectively), in addition to the control hairpins directed against the firefly luciferase (fLuc; LV_miR_fLuc). The transfer plasmid constructs, pGAE SIV SFFV-eGFP-P2A-zeo-miRNA-HsBMPR2-WPRE and pGAE-SFFV-eGFP-P2A-zeo-miRNA-fLuc-WPRE contain a zeocin resistance cassette (zeo) driven from an SFFV (spleen focus forming virus) long terminal repeat promoter, followed by the respective miRs and the WPRE (woodchuck hepatitis virus posttranscriptional regulatory element). In addition, the constructs contain the cDNA for enhanced green fluorescent protein (eGFP) cassette as a reporter gene that allows monitoring of the transduced cells by flow cytometry. The eGFP reporter cDNA and the ZeoR cDNA are connected by a peptide2A sequence, which allows equimolar expression of both proteins from the same mRNA transcript.36 All cloning steps were sequence verified. All lentiviral vector plasmids were designed and cloned at the Leuven Viral Vector Core and vector production was performed as previously described.36 Briefly, vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped lentiviral vector particles were produced by triple transient polyethylenimine transfection in HEK293T cells using pMDG.2, which encodes the vesicular stomatitis virus glycoprotein envelope, pAD_SIV3+, packaging plasmid and the transfer plasmid pGAE-SFFV-eGFP-P2A-zeo-miRNA-HsBMPR2-WPRE, to generate LV_miR_BMPR2_1920; _4277; _7048. In parallel, a control vector was produced with miR-based hairpins which target the mRNA of fLuc, resulting in LV_miR_fLuc. The latter vector will be referred to as “control” throughout the text. Quality control tests for lentiviral vector production were conducted: transduction units/mL were assessed on 293T cells and p24 concentrations in pg/mL was determined by p24/p27 ELISA (Innotest HIV Ag mAb 480T, Innogenetics-Fujirebio).
Lentiviral vector transduction efficiency in HLMVEC
Stable BMPR2 knockdown and controls in HLMVEC were generated following lentiviral transduction. Briefly, HLMVECs were seeded in a T75 flask at a density of 200,000 cells. When HLMVECs were 40% confluent, cells were transduced with a serial dilution series of lentiviral vectors LV_miR_BMPR2_1920, LV_miR_BMPR2_4277, LV_miR_BMPR2_7048 and LV_miR_fLuc, as control. After 48 h, the medium was replaced with growth medium containing 200 µg/mL zeocin to select transduced cells.
Transduction efficiency was evaluated by flow cytometry for eGFP expression. Transduction efficiencies in HLMVECs (% eGFP positive cells in the population) were similar for the different lentiviral vectors, LV_miR_BMPR2_7048, LV_miR_BMPR2_4277, LV_miR_BMPR2_1920, for the respective dilutions (Figure S1A). Expression of eGFP was slightly lower in HLMVECs transduced with the control vector (1/20: 90.7%; 1/40: 71.7%; 1/80: 62.2%). Consequently, we opted for a 1/40 dilution of the lentiviral vectors and determined the relative BMPR2 mRNA expression at this dilution. Relative BMPR2 mRNA expression was significantly lower (ANOVA, p<0.01) in HLMVECs transduced with LV_miR_BMPR2_4277 (7.5 × 10−4 ± 2.0 × 10−4) or LV_miR_BMPR2_1920 (2.5 × 10−4 ± 7.0 × 10−5) compared with LV_miR_BMPR2_7048 (2.2 × 10−3 ± 6.0 × 10−4) or control vector (2.5 × −3 ± 7.0 × 10−5) (Figure S1B). The viability of HLMVECs transduced with the control vector was 95.3 ± 1.6% and with LV_miR_BMPR2_1920 was 97.0 ± 0.64%. The percentage of HLMVECs expressing eGFP were 78.8 ± 10.4% for the control vector and 89.2 ± 9.4% for LV_miR_BMPR2_1920 (Figure S1C). Consequently, LV_miR_BMPR2_1920 was chosen and named as BMPR2-KD.
Phenotyping of HLMVECs
Lentiviral vector-transduced HLMVECs were phenotyped by labeling cells with Dil-Ac-LDL and by immunofluorescence using antibodies against human CD31, VE-cadherin and von Willebrand factor (vWF), as previously described.37 To quantify immunofluorescence staining, five images from non-overlapping fields on each slide were captured at 40 × magnification. After having separated the different channels, red staining was measured using the ImageJ software and expressed as arbitrary units (AU). In addition, CD31 positive, viable HLMVECs were quantified by flow cytometry (FacsCanto II, Becton Dickinson) as described elsewhere.12
Cell adhesiveness assay
HLMVECs were seeded at a density of 12,500 cells/cm2 on gelatin-coated 12 well-plates. Subconfluent HLMVECs were starved in starving medium for 24 h. The cells were stimulated with human recombinant TNFα (10 ng/mL) or IL18 (10 ng/mL) for 3 h. Human monocytic THP-1 cells were radio-labeled with 1 μCi [3H]-thymidine per 106 cells for 48 h and added (5 × 105 per well) to the endothelial cell monolayer for 3 h at 37℃. Non-adherent cells were washed out. Radioactivity incorporated into monocytes in suspension and attached to the EC monolayer was quantified as previously described.38 Data are expressed as percentage of adhering cells over cells initially added.
Assessment of HLMVEC permeability
HLMVECs were seeded onto 24-well Transwell® inserts (6.5 mm diameter, 0.4 µm pore size, Corning) at full confluence (100,000 cells/insert) to ensure formation of endothelial monolayer. After 24 h, HLMVECs were starved for 18 h. The HLMVEC monolayer was then stimulated with TNFα (10 ng/mL) or IL18 (10 ng/mL) for 4 h, washed twice with starvation medium, and 200 µL fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA; 0.1 mg/mL) in starvation medium was added to the upper chamber and 1 mL of starving medium was added to the lower chamber. Leakage of FITC-labeled BSA into the lower chamber was assessed by collecting 50 µL of sample from the lower chamber at baseline, after 30 min, 1, 2 and 4 h. Absorbance was measured at 450 nm using a FLUOstar Omega microplate reader (BMG Labtech). Concentrations of BSA were calculated and plotted vs. time; area under the curve (AUC) was calculated for each conditions.
Adhesion molecule expression
Subconfluent HLMVECs seeded onto fibronectin-coated chamber slides were starved for 18 h and further stimulated with 10 µg/mL TNFα or IL18 for 3 h at 37℃. HLMVECs were not permeabilized in order to detect adhesion molecules expressed only at the cell surface. ICAM-1 and VCAM-1 expression was detected by immunofluorescence using antibodies against ICAM-1 and VCAM-1 as previously described.12 Quantification of immunofluorescence staining was performed as mentioned above.
BMPR2, IL18 and IL18R mRNA expression
Total RNA was extracted from pulmonary tissue and HLMVECs; mRNA expression levels of BMPR2, IL18, IL18 receptor (IL18R) and the housekeeping gene β-actin were determined by qrtPCR as previously described.12
Western blotting
HLMVECs were grown to 90% confluence in six well-plates and starved in starving medium for 24 h and further stimulated with 10 µg/mL TNFα or IL18 for 1 h at 37℃. HLMVECs were washed twice in ice-cold phosphate-buffered saline, pH 7.4 and lysed for 30 min at 4℃ in ice-cold RIPA buffer. The samples were centrifuged at 12,000 g for 15 min and protein concentrations were determined using the Bradford method. Proteins were separated by SDS-PAGE and transferred to polyvinylidenefluoride filters by electroblotting. Filters were blocked in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-Tween) and either 3% BSA or 5% nonfat dry milk for 1 h at room temperature (RT). Filters were incubated with primary antibodies overnight at 4℃ in TBS-Tween containing either 3% BSA or 5% non-fat dry milk. Horseradish peroxidase-conjugated secondary antibodies were incubated in TBS-Tween for 1 h at RT and peroxidase staining was revealed by chemiluminescence, imaged and analyzed.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.03 (GraphPad Software Inc., La Jolla, California). Differences between groups were analyzed using Student’s t-test, one-way or two-way ANOVA followed by a post-hoc Tukey test. All p-values are for two-sided tests. A value of p < 0.05 was considered statistically significant. Continuous and normally distributed values are expressed as mean ± SD.
A detailed method section is available in the online supplemental data.
Results
Phenotyping of lentiviral vector-transduced HLMVECs
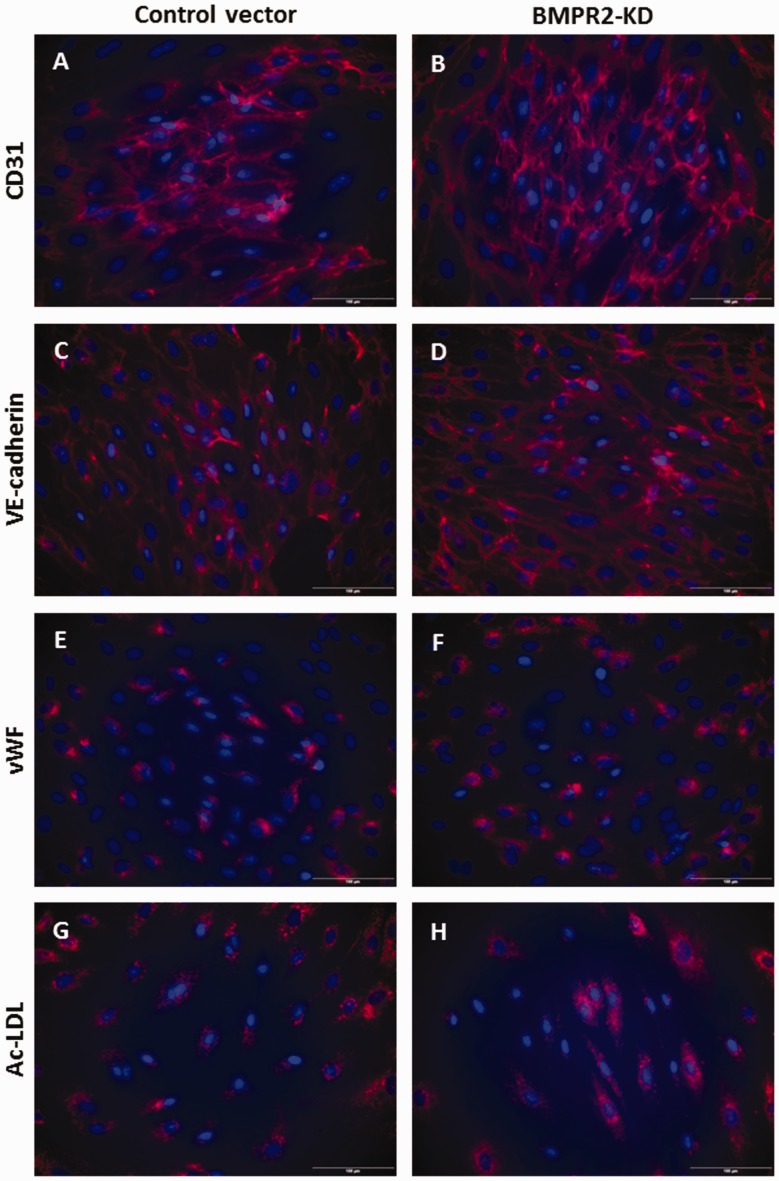
HLMVECs transduced with the control vector and BMPR2-silenced HLMVECs both expressed CD31 (Fig. 1a and b) and VE-cadherin (Fig. 1c and d) at their surface, contained vWF in Weibel-Palade bodies (Fig. 1e and f) and demonstrated Ac-LDL uptake (Fig. 1g and h). Immunofluorescence staining was further quantified and did not show any significant difference in the expression of specific endothelial markers, e.g. CD31, VE-cadherin, vWF and Ac-LDL uptake, between HLMVECs transduced with the control vector and BMPR2-silenced HLMVECs (Figure S2).
Fig. 1.
Phenotyping of HLMVECs knocked down for BMPRII. HLMVECs transduced with control or BMPR2-KD lentiviral vectors were immuno-labeled with antibodies raised against human CD31 (a, b), VE-cadherin (c, d), vWF (e, f) and stained with DiI-Ac-LDL (g, h). Nuclei were counterstained using DAPI (blue). Experiments were performed in HLMVECs at passage 5. Scale: 100 µm.
BMPR2 silencing affects BMP signaling
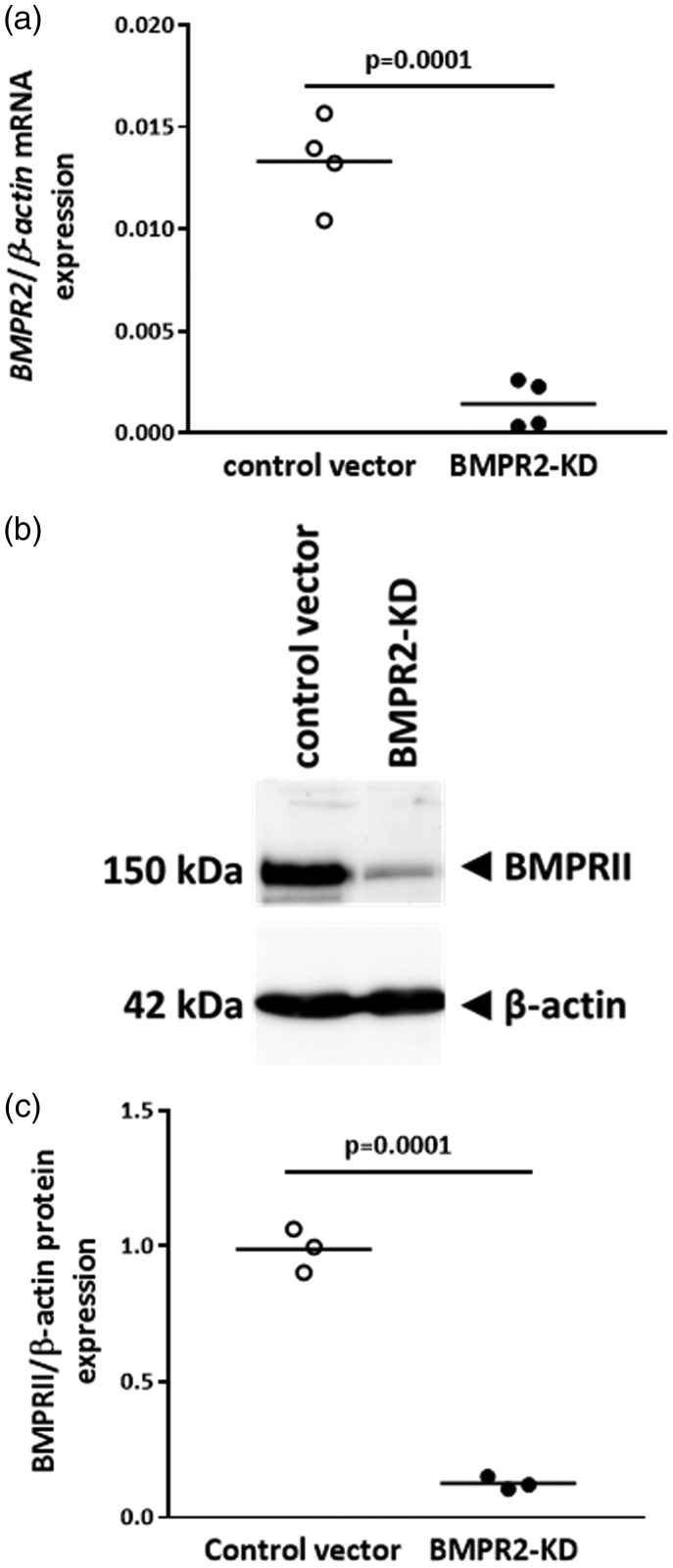
Lentiviral vector transduction of HLMVECs with BMPR2-KD vector resulted in an 89% decrease in BMPR2 mRNA expression (p = 0.0001; Fig. 2a) and an 87% decrease in BMPRII protein expression (p = 0.0001; Fig. 2b and c), compared to cell transduced with control vector.
Fig. 2.
BMPRII expression in HLMVECs transduced with control or BMPR2-KD lentiviral vectors. (a) Relative BMPR2 mRNA expression. Independent experiments were performed in quadruplicate for HLMVECs between passages 4 and 7. (b) Representative Western blot of BMPRII and β-actin protein expression. (c) Quantitative expression of BMPRII protein. Results are presented as relative protein expression ratio of BMPRII over β-actin. Independent experiments were performed in triplicate in HLMVECs between passages 4 and 7.
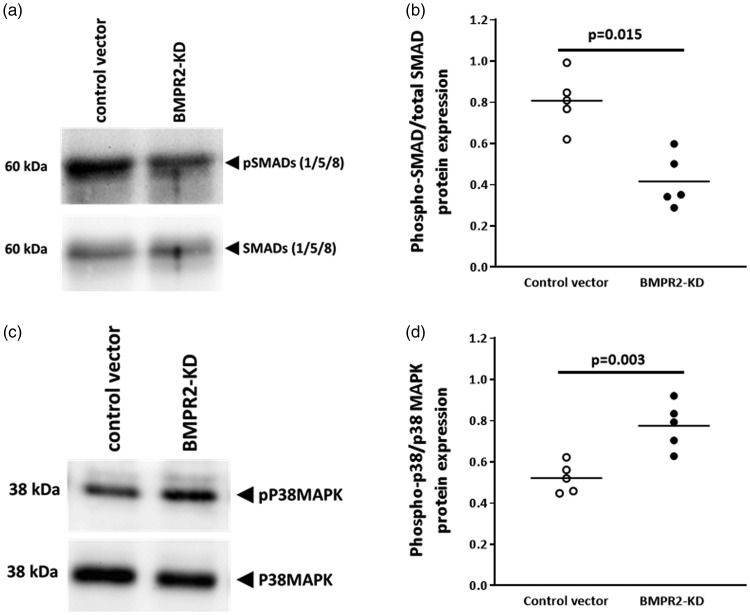
We further observed that in resting HLMVECs knocked down for BMPR2, SMAD1/5/8 phosphorylation (0.42 ± 0.13) was significantly lower (p = 0.015) compared with resting control HLMVECs (0.81 ± 0.13; Fig. 3a and b).
Fig. 3.
Activation of SMAD (1/5/8) and P38MAPK proteins in HLMVECs transduced with control or BMPR2-KD lentiviral vectors. Representative Western blot of phosphorylated and total SMAD (1/5/8) proteins (a) and P38MAPK (b). Quantitative expression of phosphorylated vs. total SMAD (1/5/8) proteins (c) and P38MAPK (d). Results are presented as relative protein expression ratio of phospho-SMAD/P38MAPK over total-SMAD/P38MAPK. Independent experiments were performed in quintuplicate for HLMVECs between passages 4 and 7.
In addition, we found that silencing of BMPR2 in resting HLMVECs resulted in significantly higher phosphorylation levels of P38MAPK compared with resting control HLMVECs (0.78 ± 0.11 vs. 0.52 ± 0.07; p = 0.003; Fig. 3c and d).
BMPR2 silencing negatively affects endothelium function
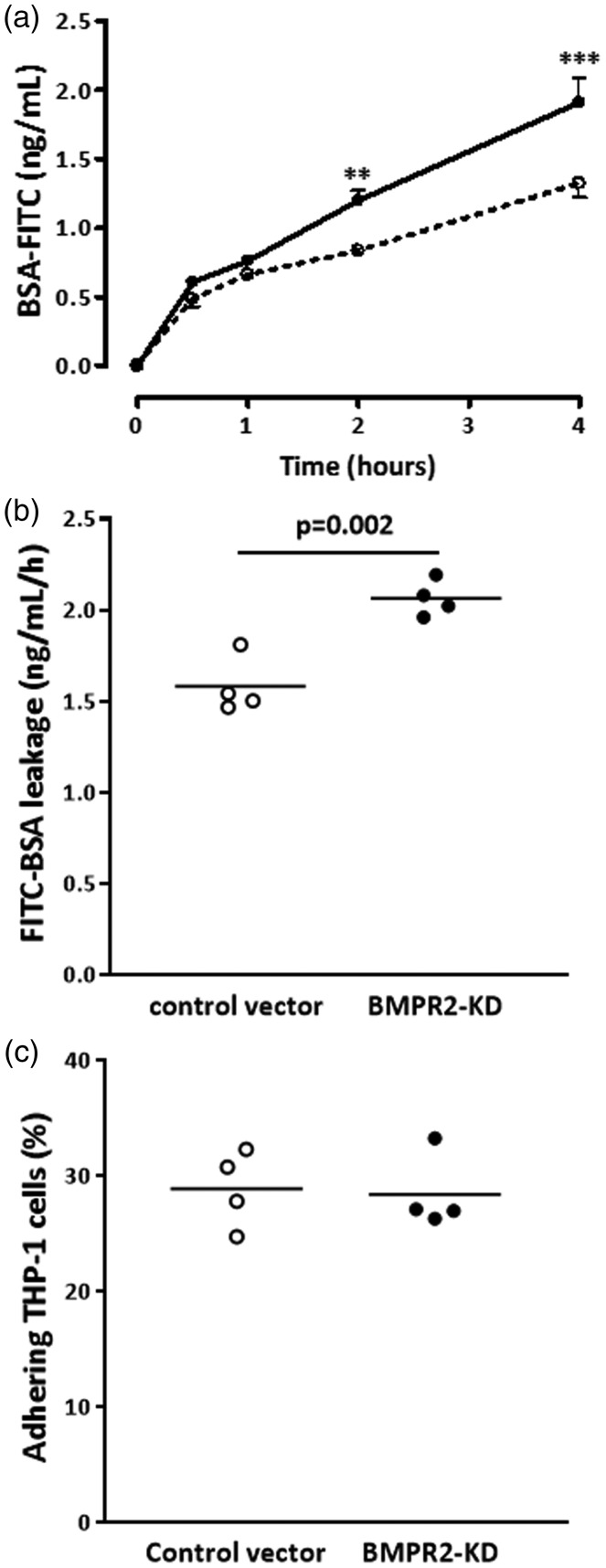
Next, we determined the effect of BMPR2 silencing on HLMVEC barrier function. We assessed the leakage of FITC-labeled BSA through the monolayer of HLMVECs transduced with either control or BMPR2-KD vectors over time. The increase in FITC-labeled BSA leakage through the monolayer of resting BMPR2-silenced HLMVECs was significantly higher compared to resting control HLMVECs over time (Fig. 4a). After a 4-h period, BSA-FITC leakage was significantly higher (p = 0.002) through BMPR2-silenced HLMVECs compared to control HLMVECs (2.07 ± 0.10 vs. 1.58 ± 0.16; Fig. 4b).
Fig. 4.
Barrier function and adhesiveness to monocytes of HLMVECs. Leakage of FITC-labeled bovine serum albumin (BSA) through HLMVEC monolayers seeded onto Transwell® inserts, transduced with control or BMPR2-KD lentiviral vectors, was assessed after 30 min, 1, 2 and 4 h in resting HLMVECs. (a) BSA-FITC leakage over time in one independent experiment performed in triplicate; (dotted line, control vector; full line, BMPR2-KD); **p < 0.001, ***p < 0.0001 vs. control vector. (b) BSA-FITC leakage after 4 h in three independent experiments performed in triplicate or quadruplicate in HLMVECs between passages 4 and 7. Results are expressed as BSA-FITC leakage (ng/mL/h). (c) Adhesiveness of resting HLMVECs, transduced with control or BMPR2-KD lentiviral vectors, to the monocytic cell line THP-1 in four independent experiments performed in triplicate in HLMVECs between passages 4 and 7.
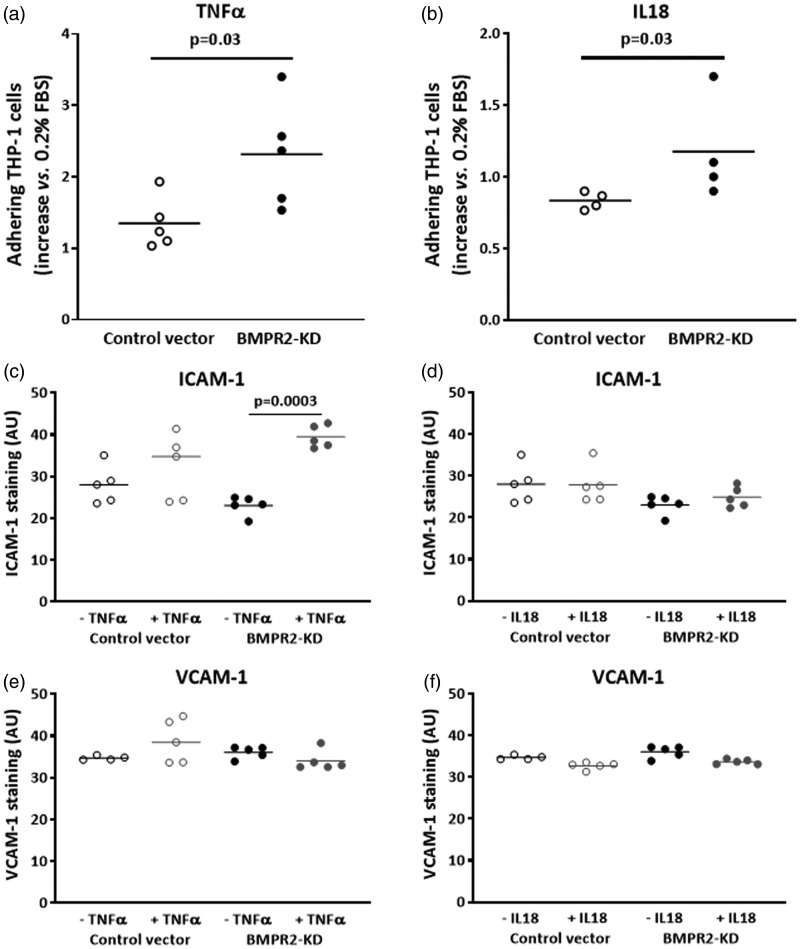
Considering that activated endothelium may influence the attraction and infiltration of inflammatory cells, we investigated whether impaired BMPRII may affect the adhesiveness capacities of HLMVECs to monocytes. We did not observe any difference in the amount of adhering human monocytic cells (THP-1) between resting BMPR2-silenced HLMVECs and control HLMVECs (28.9 ± 3.4% vs. 28.4 ± 3.3%; Fig. 4c). Accordingly, we did not find any differential expression in adhesion molecules ICAM-1 and VCAM-1 at the surface of resting BMPR2-silenced HLMVECs and control HLMVECs (Figs. 5a, d, g, j; 6c–f).
Fig. 5.
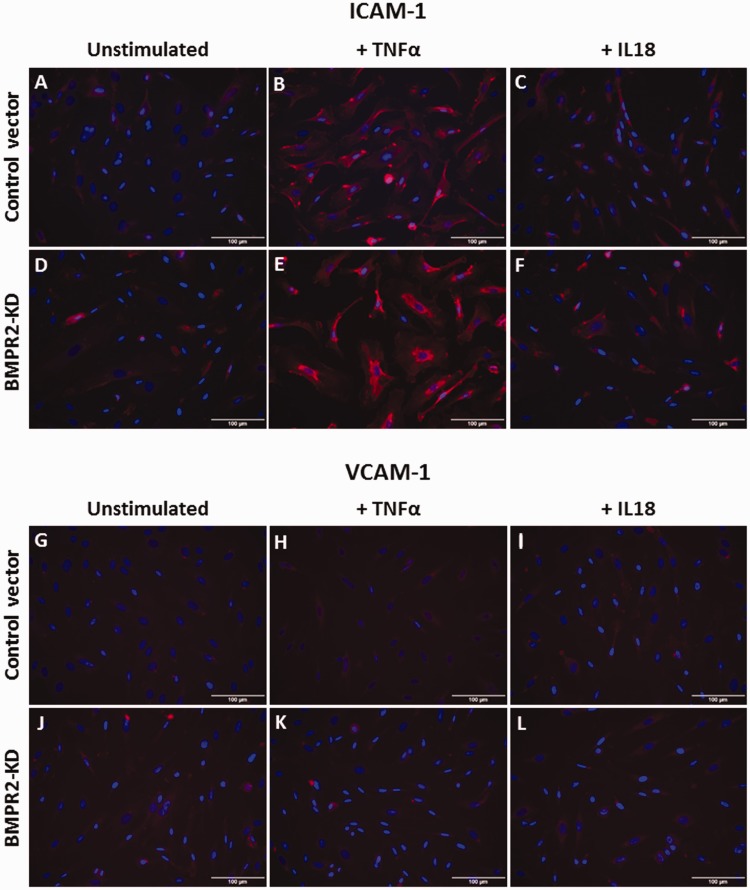
Effect of TNFα and IL18 on adhesion molecule expression in HLMVECs. Expression of ICAM-1 (a–f) and VCAM-1 (g–l) in HLMVECs transduced with control (a–c; g–i) or BMPR2-KD lentiviral vectors (d–f; j–l), at rest (a, d, g, j), in response to TNFα (b, e, h, k) or IL18 (c, f, i, l). Experiments were performed in HLMVECs between passages 4 and 7. Scale: 100 µm.
Fig. 6.
Effect of TNFα and IL18 on adhesiveness to monocytes and quantitative expression of adhesion molecules ICAM-1 and VCAM-1 in HLMVECs. Adhesiveness of HLMVECs, transduced with control or BMPR2-KD lentiviral vectors, to the monocytic cell line THP-1 in response to TNFα (a) or IL18 (b). Results were expressed as fold-increase vs. resting HLMVECs (0.2% FBS); four to five independent experiments were performed in triplicate in HLMVECs between passages 4 and 7. Quantification of the expression of ICAM-1 (c, d) and VCAM-1 (e, f), in response to TNFα (c, e) or IL18 (d, f) performed on five independent experiments.
TNFα enhances P38MAPK activation in BMPR2-silenced HLMVECs
We found that expression of IL18 mRNA was 1.6-fold higher in pulmonary tissue from patients with IPAH compared with controls (p = 0.02; Table 2). By contrast, expression of IL18 receptor mRNA was similar in pulmonary tissue from patients with IPAH compared with controls (Table 2). Moreover, considering that the non-canonical P38MAPK pathway is involved in the inflammatory process mediated by cytokines such as TNFα,33 we investigated whether IL18 and TNFα could further activate P38MAPK in BMPR2-silenced HLMVECs. We observed that IL18 did not induce any significant increase in P38MAPK activation both in control (0.52 ± 0.07 vs. 0.58 ± 0.15; p = 0.39) and BMPR2-silenced HLMVECs (0.77 ± 011 vs. 0.85 ± 0.11; p = 0.30), (Fig. 7b and d).
Table 2.
Expression of IL18 and IL18R mRNAs in human pulmonary tissue samples.
| Controls | IPAH | p-Value | |
|---|---|---|---|
| IL18 | 7.5 × 10−3 ± 2.8 × 10 −3 | 12.4 × 10−3 ± 4.7 × 10−3* | 0.02 |
| IL18R | 4.3 × 10−3 ± 2.8 × 10−3 | 8.1 × 10−3 ± 5.9 × 10−3 | 0.10 |
Fig. 7.
Effect of TNFα and IL18 on activation of P38MAPK proteins in HLMVECs. Representative Western blot of phosphorylated and total P38MAPK proteins in HLMVECs transduced with control or BMPR2-KD lentiviral vectors stimulated with TNFα (a) or IL18 (b). Quantitative expression of phosphorylated vs. total P38MAPK proteins in HLMVECs stimulated with TNFα (c) or IL18 (d). Results are presented as relative protein expression ratio of phospho-P38MAPK and total-P38MAPK. Independent experiments were performed in quintuplicate for HLMVECs between passages 4 and 7.
By contrast, TNFα induced a significant increase in P38MAPK phosphorylation in BMPR2-silenced HLMVECs (0.77 ± 0.11 vs. 1.01 ± 0.17; p = 0.03), but not in control HLMVECs (0.52 ± 0.07 vs. 0.69 ± 0.23; p = 0.10; Fig. 7a and c).
TNFα and IL18 enhance adhesiveness of BMPR2-silenced HLMVECs
Adhesiveness of BMPR2-silenced HLMVECs to THP-1 cells was significantly higher compared to that of control HLMVECs in the presence of IL18, (fold-increase: 1.18 ± 0.35 vs. 0.83 ± 0.06, p = 0.03; Fig. 6b) as well as in the presence of TNFα (fold-increase: 2.31 ± 0.75 vs. 1.35 ± 0.36, p = 0.03; Fig. 6a). In resting control HLMVECs or BMPR2-silenced HLMVECs, both ICAM-1 and VCAM-1 adhesion molecules were poorly expressed at the cell surface (Fig. 5a, d, g, j). TNFα significantly induced the expression of ICAM-1 at the surface of BMPR2-silenced HLMVECs (Figs. 5d, e and 6c), but not at the surface of control HLMVECs (Figs. 5a, b and 6c). IL18 did not induce the expression of ICAM-1 at the surface of both control and BMPR2-silenced HLMVECs (Figs. 5a–f and 6d). By contrast, neither IL18 nor TNFα induced VCAM-1 expression at the surface of control and BMPR2-silenced HLMVECs (Figs. 5g–l and 6e, f). These results suggest that TNFα-induced adhesiveness of BMPR2-silenced HLMVECs could be attributed to enhanced ICAM-1 expression at their surface.
TNFα impairs endothelial barrier function in BMPR2-silenced HLMVECs
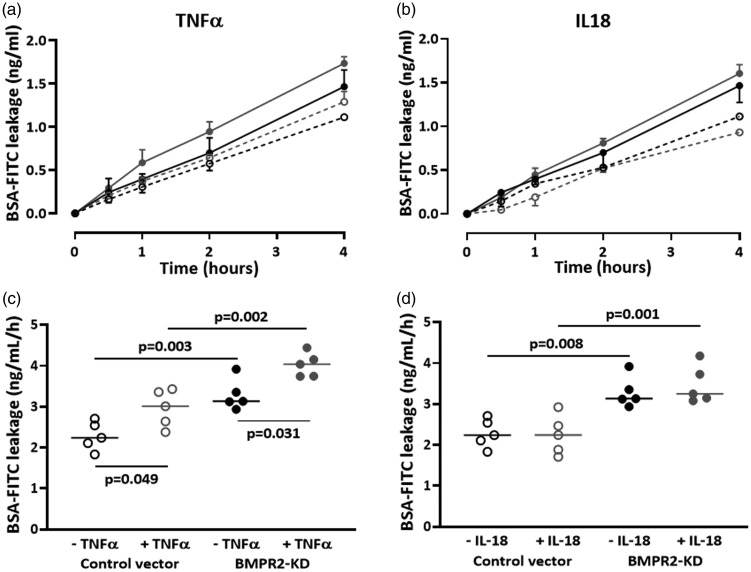
TNFα significantly reduced endothelium barrier function in both control and BMPR2-silenced HLMVECs (AUC: 2.29 ± 0.16 vs. 2.96 ± 0.20, p = 0.049 and 3.29 ± 0.13 vs. 4.02 ± 0.13 ng/ml/h, p = 0.031; Fig. 8a and c). The TNFα-induced loss of endothelium barrier function was similar in BMPR2-silenced and control HLMVECs (31 ± 9% vs. 23 ± 3% increase, p = 0.42).
Fig. 8.
Effect of TNFα and IL18 on barrier function of HLMVECs. Leakage of
BSA-FITC through HLMVEC monolayers seeded onto Transwell® inserts,
transduced with control or BMPR2-KD lentiviral vectors, was assessed
after 30 min, 1, 2 and 4 h. BSA-FITC leakage over time in one
independent experiment performed in triplicate, in response to TNFα
(a) or IL18 (b); ( control vector;
control vector;
 BMPR2-KD;
BMPR2-KD;  control
vector + TNFα/IL18;
control
vector + TNFα/IL18;  BMPR2-KD + TNFα/IL18). BSA-FITC leakage after 4 h in five
independent experiments performed in triplicate or quadruplicate in
HLMVECs between passages 4 and 7, in response to TNFα (c) and IL18
(d). Results are expressed as BSA-FITC leakage (ng/mL/h).
BMPR2-KD + TNFα/IL18). BSA-FITC leakage after 4 h in five
independent experiments performed in triplicate or quadruplicate in
HLMVECs between passages 4 and 7, in response to TNFα (c) and IL18
(d). Results are expressed as BSA-FITC leakage (ng/mL/h).
IL18 did not display any effect on endothelium barrier function both in control HLMVECs or BMPR2-silenced HLMVECs (AUC: 2.29 ± 0.16 vs. 2.24 ± 0.21 and 3.29 ± 0.13 vs. 3.48 ± 0.213 ng/ml/h; Fig. 8b and d).
Discussion
In the present study, stable silencing of BMPR2 in HLMVECs resulted in reduced expression of BMPRII protein, decreased phosphorylation of SMAD1/5/8 proteins, loss of endothelium barrier function and activation of P38MAPK. Additionally, cytokines such as IL18 and TNFα induced adhesiveness of BMPR2-silenced HLMVECs to THP-1 monocytic cells, presumably through the adhesion molecule ICAM-1. Finally, TNFα-induced loss of endothelium barrier function in BMPR2-silenced HLMVECs was accompanied by P38MAPK activation. Altogether, these results suggest that in the absence of functional BMPRII signaling, cytokines such as IL18 and TNFα impair endothelial function, and concomitantly activate P38MAPK.
Effects of reduced BMPRII expression on pulmonary endothelial function
We found that stable silencing of BMPR2 in HLMVECs resulted in significantly decreased activation of the canonical downstream effectors of BMPRII, namely SMAD 1/5/8 proteins, similar to the decrease in SMAD protein activation observed in pulmonary arterial smooth muscle cells (PASMCs) from IPAH patients,39 whereas transient BMPR2 silencing in HLMVECs or PASMCs did not alter SMAD1/5/8 protein phosphorylation.14,40 Interestingly, we observed that impairment of BMPRII signaling resulted in a loss of the endothelium barrier function. Accordingly, transient BMPR2 silencing resulted in increased permeability of human PAEC monolayer41; in addition, an increase in pulmonary vascular reactivity has been observed in vivo in heterozygous BMPR2-deficient mice.21,42 These findings, together with the demonstration that SRC-dependent caveolar dysfunction may contribute to endothelial barrier dysfunction of PAECs from heterozygous null Bmpr2+/− mutant mice,42 suggest that BMPRII plays a role in maintaining pulmonary endothelial barrier function. This is also consistent with a previous study whereby (i) BMP9 prevented lipopolysaccharide (LPS)-induced loss of endothelial barrier function of blood outgrowth EC monolayers from PAH patients with BMPR2 mutations and (ii) silencing of SMAD1/5/8 eliminated the capacity of BMP9 to enhance BMPRII expression in PAECs.43 In addition, our current findings showing a concomitant loss of endothelial barrier function and decreased in SMAD protein activation in BMPR2-silenced HLMVECs confirms that canonical SMAD signaling is important in maintaining PAEC barrier function.
Surprisingly, BMPR2 silencing did not affect either HLMVEC adhesiveness properties to monocytic cells, or the expression of the adhesion molecules ICAM-1 and VCAM-1, whereas we previously found increased adhesiveness to monocytic cells concomitant with increased expression of ICAM-1 mRNA in HLMVECs isolated from BMPR2 mutation carriers PAH patients.12 Discrepancies between HLMVECs from PAH patients with a BMPR2 mutation and BMPR2-silenced HLMVECs could be attributed to the use of siRNA to knockdown BMPR2. Whereas siRNA targets cellular mRNAs, mutations in patients are permanent alterations in the structure of the BMPR2 gene, which can be responsible for alterations in the trafficking of BMPRII to the cell surface.44 In this respect, cysteine substitution in the ligand-binding or kinase domains results in reduced trafficking of mutant BMPRII to the cell surface, whereas variants carrying non-cysteine mutations in the kinase domain reach the cell surface, but fail in activating SMAD signaling pathway.16
In parallel to a decrease in canonical SMAD-dependent BMPRII signaling pathway, we also observed that BMPR2 silencing resulted in increased activation of non-canonical SMAD-independent P38MAPK. Accordingly, increased phosphorylation of P38MAPK has been observed in hypoxia- and monocrotaline-induced PH in rats45,46 as well as in heterozygous BMPR2 deficient and mutant mice.47 In addition, a loss of BMPRII in PASMCs results in activation of P38MAPK.48 These results suggest that impaired BMPRII function may result in abnormal activation of alternative pathways such as the P38MAPK pathway.
BMPRII silencing and cytokine-induced endothelial dysfunction
Considering that effects of the cytokines IL18 or TNFα on the endothelium are potentially mediated by activation of P38MAPK,49,50 we consequently aimed to investigate whether IL18 and TNFα would (i) worsen endothelial dysfunction in addition to silencing of BMPRII and (ii) activate the non-canonical P38MAPK pathway.
Despite higher levels of IL18 mRNA observed in lung parenchyma from PAH patients, IL18 failed to further increase BMPRII silencing-induced P38MAPK activation in HLMVECs. By contrast, a synergic effect of TNFα and BMPR2 silencing on P38MAPK activation was observed in HLMVECs. These results indicate that TNFα, but not IL18, is able to potentiate the non-canonical SMAD-independent P38MAPK pathway, in a context of a loss of BMPRII in HLMVECs. Accordingly, loss of BMPRII induced prolonged phosphorylation of P38MAPK in response to TNFα in human PAECs.18
In parallel, we observed that TNFα was able to potentiate the effects of a loss of BMPRII on the pulmonary endothelium barrier function. Endothelial barrier compromise is accompanied by an activation of the P38MAPK cascade in human PAECs;51 in rats with hypoxia-induced PH, whereby BMPRII expression is reduced in the lungs, P38MAPK inhibitor reversed impaired endothelium-dependent relaxation in isolated pulmonary artery rings.52 In addition, TNFα has been previously shown to induce endothelium permeability, concomitantly with an activation of P38MAPK;49 treatment with TNFα resulted in increased permeability of BMPR2 knockdown human PAEC monolayers.41 Although IL18 has been previously shown to induce vascular SMC proliferation and migration,53,54 and increase permeability of rat PMVEC monolayers,28 we did not observe any additional effects of IL18 on endothelial barrier function in HLMVECs with a loss of BMPRII.
Whereas silencing of BMPRII had no effect on adhesiveness capacities of HLMVECs to monocytic cells, we observed that both IL18 and TNFα induced adhesion of BMPRII-silenced HLMVECs to THP-1. Accordingly, we previously found that CRP and TNFα increased adhesion of U937 monocytic cells to PMVECs isolated from PAH patients with a BMPR2 mutation.12 Interestingly, lentiviral vector-mediated overexpression of BMPRII in HLMVECs has been recently shown to suppress LPS-induced neutrophil-endothelial adhesion.55 TNFα-induced adhesiveness was mainly attributable to induced expression of ICAM-1. In addition, we have also observed that PMVECs isolated from PAH with a BMPR2 mutation displayed increased mRNA or protein expression of ICAM-1 in response to CRP and TNFα.12 Inversely, when BMPRII is overexpressed in HLMVECs, LPS-induced ICAM-1 expression was dramatically reduced.55
Eventually, our results are in line with the coincidence of BMPR2 heterozygosity with an inflammatory insult required to develop PAH.56
Limitations
Findings of the present study are mostly based on in vitro investigation, using ECs in which BMPR2 has been depleted. Silencing does not exactly reproduce the pathological situation of BMPR2 mutations and in vitro cellular assays only allow to investigate some mechanistic aspects of the consequences of a loss of BMPRII. However, considering that access to microvascular pulmonary ECs from PAH patients is extremely limited and only possible at the time of lung transplantation, silencing of BMPRII in HLMVECs is a rather fair alternative to overcome this challenge and further investigate the consequences of a loss of BMPRII on the pathobiology of pulmonary vascular cells.
Conclusion
In the present study, we observed that a loss of BMPRII in HLMVECs resulted in increased permeability and activation of p38 MAP kinase. In addition, we found that TNFα enhanced the effects of a loss of BMPRII on the pulmonary endothelium barrier function, and concomitantly on the activation of P38MAPK. Finally, TNFα-induced adhesion capacities of BMPR2-silenced HLMVECs were partially attributable to enhanced expression of ICAM-1 at the cell surface. Altogether, our results suggest that cytokines such as TNFα or IL18 could trigger the development of PAH, in addition to a loss of BMPRII.
Supplemental Material
Supplemental material, PUL883607 Supplemental Material for Cytokines trigger disruption of endothelium barrier function and p38 MAP kinase activation in BMPR2-silenced human lung microvascular endothelial cells by Birger Tielemans, Leanda Stoian, Rik Gijsbers, Annelies Michiels, Allard Wagenaar, Ricard Farre Marti, Catharina Belge, Marion Delcroix and Rozenn Quarck in Pulmonary Circulation
Authors’ contribution
MD and RQ conceived and designed the study; LS, BT and AW collected the data; RG, AM, RFM contributed to data and analysis tool; LS, BT and RQ performed the analysis and interpretation of the data; LS and RQ wrote the manuscript; BT, RG, CB and MD reviewed the manuscript; all authors approved the final version.
Conflict of interest
MD is holder of the Actelion Chair for Pulmonary Hypertension at the KU Leuven – University of Leuven.
Ethical approval
The study was approved by the Institutional Ethics Committee of the University of Leuven.
Funding
The authors would like to thank the Belgian Association of Patients for Pulmonary Hypertension (Belgische Pulmonale Hypertensie Patiëntenvereniging) for its financial support.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.McLaughlin V, Bacchetta M, Badesch D, et al. Update on pulmonary arterial hypertension research: proceedings from a meeting of experts. Curr Med Res Opin 2018; 34: 263–273. [DOI] [PubMed] [Google Scholar]
- 2.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 2000; 26: 81–84. [DOI] [PubMed] [Google Scholar]
- 3.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 2003; 40: 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaouat A, Coulet F, Favre C, et al. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax 2004; 59: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake KM, Dunmore BJ, McNelly LN, et al. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2013; 49: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin ED, Ma L, LeDuc C, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 2012; 5: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 2013; 369: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Jones JE, Beppu H, et al. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 2005; 112: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jesus Perez VA, Alastalo TP, Wu JC, et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-β-catenin and Wnt-Rho-Rac1 pathways. J Cell Biol 2009; 184: 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes CJ, Im H, Cao A, et al. RNA Sequencing Analysis Detection of a Novel Pathway of Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2015; 192: 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jesus Perez VA, Yuan K, Orcholski ME, et al. Loss of adenomatous poliposis coli-α3 integrin interaction promotes endothelial apoptosis in mice and humans. Circ Res 2012; 111: 1551–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vengethasamy L, Hautefort A, Tielemans B, et al. BMPRII influences the response of pulmonary microvascular endothelial cells to inflammatory mediators. Pflugers Arch 2016; 468: 1969–1983. [DOI] [PubMed] [Google Scholar]
- 13.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension: a unique process in a unique disease. Antioxid Redox Signal 2002; 4: 833–843. [DOI] [PubMed] [Google Scholar]
- 14.Davies RJ, Holmes AM, Deighton J, et al. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-beta in pulmonary artery smooth muscle cells: role of proinflammatory cytokines. Am J Physiol-Lung Cell Mol Physiol 2012; 302: L604–L615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagna G, Nguyen PH, Ni W, et al. BMP-dependent activation of caspase-9 and caspase-8 mediates apoptosis in pulmonary artery smooth muscle cells. Am J Physiol-Lung Cell Mol Physiol 2006; 291: L1059. [DOI] [PubMed] [Google Scholar]
- 16.Rudarakanchana N, Flanagan JA, Chen H, et al. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 2002; 11: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Long L, Southwood M, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 2005; 96: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 18.Sawada H, Saito T, Nickel NP, et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med 2014; 211: 263–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 20.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 21.Burton VJ, Holmes AM, Ciuclan LI, et al. Attenuation of leukocyte recruitment via CXCR1/2 inhibition stops the progression of PAH in mice with genetic ablation of endothelial BMPR-II. Blood 2011; 118: 4750–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez O, Marcos E, Perros F, et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2007; 176: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 23.Heresi GA, Aytekin M, Hammel JP, et al. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J 2014; 43: 912. [DOI] [PubMed] [Google Scholar]
- 24.Amsellem V, Lipskaia L, Abid S, et al. CCR5 as a treatment target in pulmonary arterial hypertension. Circulation 2014; 130: 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorfmuller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 534–539. [DOI] [PubMed] [Google Scholar]
- 26.Balabanian K, Foussat A, Dorfmuller P, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JL, Carlquist JF. Cytokines, interleukin-18, and the genetic determinants of vascular inflammation. Circulation 2005; 112: 620. [DOI] [PubMed] [Google Scholar]
- 28.Kratzer A, Salys J, Nold-Petry C, et al. Role of IL-18 in second-hand smoke-induced emphysema. Am J Respir Cell Mol Biol 2013; 48: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel JCM, Park CC, Woods JM, et al. A novel role for interleukin-18 in adhesion molecule induction through NFκB and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem 2001; 276: 37069–37075. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes N, Sukhova GK, Libby P, et al. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages. J Exp Med 2002; 195: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross DJ, Strieter RM, Fishbein MC, et al. Type I immune response cytokine-chemokine cascade is associated with pulmonary arterial hypertension. J Heart Lung Transpl 2012; 31: 865–873. [DOI] [PubMed] [Google Scholar]
- 32.Morisawa D, Hirotani S, Oboshi M, et al. Interleukin-18 disruption suppresses hypoxia-induced pulmonary artery hypertension in mice. Int J Cardiol 2016; 202: 522–524. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Zuo X, Wang Y, et al. Monocrotaline-induced pulmonary arterial hypertension is attenuated by TNF-α antagonists via the suppression of TNF-α expression and NF-κB pathway in rats. Vascul Pharmacol 2013; 58: 71–77. [DOI] [PubMed] [Google Scholar]
- 34.Liang Y, Li X, Zhang X, et al. Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock 2014; 41: 275–281. [DOI] [PubMed] [Google Scholar]
- 35.Schrijvers R, De Rijck J, Demeulemeester J, et al. LEDGF/p75-independent HIV-1 replication demonstrates a role for HRP-2 and remains sensitive to inhibition by LEDGINs. PLOS Pathog 2012; 3: e1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahimi A, Vande Velde G, Reumers V, et al. Highly efficient multicistronic lentiviral vectors with peptide 2A sequences. Hum Gene Ther 2009; 20: 845–860. [DOI] [PubMed] [Google Scholar]
- 37.Quarck R, Wynants M, Ronisz A, et al. Characterization of proximal pulmonary arterial cells from chronic thromboembolic pulmonary hypertension patients. Respir Res 2012; 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynants M, Quarck R, Ronisz A, et al. Effects of C-reactive protein on human pulmonary vascular cells in chronic thromboembolic pulmonary hypertension. Eur Respir J 2012; 40: 886–894. [DOI] [PubMed] [Google Scholar]
- 39.Barnes JW, Kucera ET, Tian L, et al. Bone morphogenic protein type 2 receptor mutation-independent mechanisms of disrupted bone morphogenetic protein signaling in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2016; 55: 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Star GP, Giovinazzo M, Langleben D. ALK2 and BMPR2 knockdown and endothelin-1 production by pulmonary microvascular endothelial cells. Microvasc Res 2013; 85: 46–53. [DOI] [PubMed] [Google Scholar]
- 41.Burton VJ, Ciuclan LI, Holmes AM, et al. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood 2011; 117: 333–341. [DOI] [PubMed] [Google Scholar]
- 42.Prewitt AR, Ghose S, Frump AL, et al. Heterozygous null bone morphogenetic protein receptor type 2 mutations promote SRC kinase-dependent caveolar trafficking defects and endothelial dysfunction in pulmonary arterial hypertension. J Biol Chem 2015; 290: 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long L, Ormiston ML, Yang X, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 2015; 21: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frump AL, Lowery JW, Hamid R, et al. Abnormal trafficking of endogenously expressed BMPR2 mutant allelic products in patients with heritable pulmonary arterial hypertension. PLOS One 2013; 8: e80319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Church AC, Martin DH, Wadsworth R, et al. The reversal of pulmonary vascular remodeling through inhibition of p38 MAPK-alpha: a potential novel anti-inflammatory strategy in pulmonary hypertension. Am J Physiol-Lung Cell Mol Physiol 2015; 309: L333–L347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi H, Goto N, Kojima Y, et al. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol-Lung Cell Mol Physiol 2006; 290: L450–L458. [DOI] [PubMed] [Google Scholar]
- 47.West J, Harral J, Lane K, et al. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol-Lung Cell Mol Physiol 2008; 295: L744–L755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagen M, Fagan K, Steudel W, et al. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol-Lung Cell Mol Physiol 2007; 292: L1473–L1479. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Hu J, He T, et al. P38/MAPK contributes to endothelial barrier dysfunction via MAP4 phosphorylation-dependent microtubule disassembly in inflammation-induced acute lung injury. Sci Rep 2015; 5: 8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Cheon S, Jung MK, et al. Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Biophys Res Commun 2015; 459: 379–386. [DOI] [PubMed] [Google Scholar]
- 51.Birukova AA, Birukov KG, Gorshkov B, et al. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol-Lung Cell Mol Physiol 2005; 289: L75–L84. [DOI] [PubMed] [Google Scholar]
- 52.Weerackody RP, Welsh DJ, Wadsworth RM, et al. Inhibition of p38 MAPK reverses hypoxia-induced pulmonary artery endothelial dysfunction. Am J Physiol-Heart Circ Physiol 2009; 296: H1312–H1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandrasekar B, Mummidi S, Mahimainathan L, et al. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-κB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem 2006; 281: 15099–15109. [DOI] [PubMed] [Google Scholar]
- 54.Chandrasekar B, Mummidi S, Valente AJ, et al. The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J Biol Chem 2005; 280: 26263–26277. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Xu J, Zhao X, et al. Fasudil inhibits neutrophil-endothelial cell interactions by regulating the expressions of GRP78 and BMPR2. Exp Cell Res 2018; 365: 97–105. [DOI] [PubMed] [Google Scholar]
- 56.Tielemans B, Delcroix M, Belge C, et al. TGFβ and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov Today 2019; 24: 703–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL883607 Supplemental Material for Cytokines trigger disruption of endothelium barrier function and p38 MAP kinase activation in BMPR2-silenced human lung microvascular endothelial cells by Birger Tielemans, Leanda Stoian, Rik Gijsbers, Annelies Michiels, Allard Wagenaar, Ricard Farre Marti, Catharina Belge, Marion Delcroix and Rozenn Quarck in Pulmonary Circulation