Abstract
Exposure to tobacco smoke has been associated with heightened endothelial cell activities associated with cardiovascular diseases (CVD). Conversely, the exposure to nicotine both activates and inhibits particular endothelial cell functions. However, which constituent(s) of tobacco smoke is responsible for these changes is unknown, since toxic gases and fine particulate matter cannot be isolated. Electronic cigarette vapor allows us to isolate these constituents, providing us the ability to evaluate individual constituents. Here, we used e-cigarettes to (1) identify which constituents of tobacco products are most responsible for altered CVD functions and (2) elucidate the underlying risk of e-cigarette exposure. To accomplish this goal, endothelial cells were exposed to extracts produced from tobacco cigarettes or e-cigarettes. Endothelial cell inflammatory processes, viability, density and metabolic activity were observed. In general, a significant increase in complement deposition, the expression of the receptors for C1q, coupled with a decrease in cell proliferation and metabolic activity was observed. These results were independent of nicotine and the exposure to e-vapor was just as harmful as tobacco smoke extracts. Thus, the exposure to fine particulate matter and not toxic combustion gases or nicotine may be the most critical for regulating CVD progression.
Keywords: Electronic cigarettes, Cardiovascular diseases, Cardiovascular risk factors, Complement
Introduction
It has been well established that exposure to tobacco smoke, in both mainstream (smoke directly inhaled from the cigarette by the smoker) and sidestream (smoke primarily from the smoldering end of the cigarette) formulations, is a significant risk factor for the initiation of cardiovascular diseases (CVDs).12,21,29 However, it has proven difficult to identify which of the three main constituents of tobacco smoke (toxic gases produced from combustion, particulate matter with a hydrodynamic diameter of less than 2.5 μm and exposure to psychoactive compounds) are most responsible for these observed changes. In traditional tobacco smoke formulations, toxic combustion products and fine particulate matter cannot be separated. Conversely, the aerosolization of e-juice produces only fine particulate matter at similar concentrations as traditional tobacco products.3,4,7 This provides us with a unique opportunity to evaluate the individual constituents of tobacco products, since nicotine concentration in e-juice can be varied from zero to normal concentrations found in tobacco products. Furthermore, the effects of e-cigarettes on cardiovascular health are currently unknown, even though these products are increasing in popularity. Thus, our goal was two-fold (1) to identify which constituents of tobacco cigarettes are most deleterious for cardiovascular health and (2) to evaluate the risk associated with e-vapor exposure.
The role that endothelial cells play in CVD development is well established. Endothelial cells regulate the migration of cells and compounds out of the vascular space that can instigate CVDs.23 This primarily occurs through altered adhesion receptor expression and altered permeability. However, endothelial cells also regulate hemostasis and play a very important role in adaptive immunity.17,24,26 Specifically, complement activity on endothelial cells can initiate both local and systemic inflammatory responses that lead to CVD progression. Tobacco smoke extracts have shown to induce C3a and C5a production.18 Furthermore, work from our group has shown that exposure to tobacco smoke enhances C1q, C3b and C4d deposition onto endothelial cells, gC1qR expression and complement inhibitor production.26,27 Importantly, the exposure to tobacco smoke predisposed endothelial cells to altered shear induced activation, illustrating that the combination of cardiovascular risk factors is especially detrimental to cardiovascular health. Furthermore, although the “smoker’s paradox” has been shown to exist, a long-term exposure to tobacco products is harmful for cardiovascular health.
Complement products play a role in both the physiological clearance of apoptotic cells preventing local necrosis and pathological adaptive immune reactions. Complement products have been found within atherosclerotic lesions.15 This appears to be regulated by endothelial cells which have been previously shown to express various complement receptors including gC1qR.6 gC1qR has been shown to directly activate the classical complement pathway on various cell lines, including endothelial cells, platelets and Kupffer cells.16,20,26 Thus, there is a strong link between heightened complement activation and the progression of various diseases.
In the present study, we explored the effects of electronic cigarette extract exposure on endothelial cell complement activation as a marker for enhanced vascular inflammation. Additionally, by using electronic cigarettes, we can evaluate which of the three main tobacco smoke constituents are most responsible for the altered endothelial cell functions (e.g. toxic combustion products, fine particulate matter or nicotine). To accomplish this, endothelial cells were exposed to electronic cigarette products and the expression of gC1qR, cC1qR and various complement inhibitors, the deposition of multiple complement products and general endothelial cell culture parameters was investigated. We hypothesized that the exposure to electronic cigarette extracts would enhance endothelial cell inflammatory processes associated with CVD development.
Methods
E-Vapor Extraction/Nicotine
Tobacco smoke extracts and e-vapor extracts were prepared as previously described.20 – 22 Briefly, tobacco cigarettes (Marlboro 100 s, 16 mg tar, 1.2 mg nicotine, Philip Morris) and e-cigarettes (NJoy, OneJoy Traditional Flavor with 1.2% nicotine or 1.8% nicotine by volume, which is equivalent to 12 and 18 mg/mL, respectively, and eGo OKC Vapes, Desert Sands Flavor with 0, 12 or 18 mg/mL nicotine) were placed in the extraction apparatus for 5 s with a metered flow rate of 500 mL/min. This was followed by a 25 s period where the flow rate was set to 200 mL/min (the e-cigarette was removed from the apparatus) to mimic puffing. This procedure was followed for 5 min to standardize our extracts at 1 cigarette/100 mL. Smoke or vapor was distributed into a ~15 cm high column of a HEPES buffer20 via a step-down manifold consisting of 6 0.9 mm capillary tubes. Extracts were aliquoted and stored at −20 °C until use. All parts of the extraction apparatus and the e-cigarette were extensively cleaned between uses.
For some experiments, pure nicotine was added to cells at a final concentration of 50 nM, which approximates the blood concentration of a smoker after smoking one cigarette.1 Nicotine and all other chemicals (unless noted otherwise) were purchased from Sigma-Aldrich.
Endothelial Cells
Human umbilical vein endothelial cells (HUVECs) were purchased from ScienCell Research Laboratories (Carlsbad, CA) and used between passages 2 and 6 for all experiments. HUVECs were maintained in endothelial cell basal medium supplemented with 5% fetal bovine serum, 1x endothelial cell growth supplement, 10 U/mL penicillin and 10 μg/mL streptomycin (all from ScienCell).10,11 HUVECs were passaged at confluence with trypsin. 24 h after passing cell samples were randomly incubated with tobacco smoke extracts or e-vapor extracts at a final concentration of 1 cigarette/5 L or 50 nM nicotine. Some endothelial cell samples were incubated without exogenous conditions or with 1 μg/mL lipopolysaccharide as daily internal controls.20 For statistical purposes, the endothelial cell seeding density was maintained at ~10000 cells/cm 21 for all experimental conditions. Note that for this experiment, endothelial cells were not cultured on a substrate (e.g. collagen).
Enzyme Linked Immunosorbent Assay
After exposure to mainstream/sidestream tobacco smoke extracts, e-vapor extracts or pure nicotine, the activation/deposition of complement proteins onto endothelial cells was quantified, as a means to monitor the progression of innate immune responses. For reference, C1q, C4d and C3b are the deposited activation products of C1, C4 and C3, respectively, and were quantified here. Additionally, deposition of C5b-9, the final product of the complement cascade, was also observed. Endothelial cells were fixed with 0.5% glutaraldehyde (15 min, 37 °C, pH 7.4), washed (2x, TBS, pH 7.4), and then neutralized with 100 mM glycine-0.1% BSA (30 min, 37 °C) after the particular exposure conditions. Briefly, to quantify complement protein deposition, endothelial cell monolayers were exposed to normal human platelet poor plasma for 1 h at 37 °C.16,20 Using a solid-phase ELISA approach, the deposition of complement proteins was assessed with appropriate primary antibodies (Quidel Corp., San Diego, CA, 1:200 dilution), as previously reported by us without modifications.16,20 Appropriate alkaline phosphatase conjugated secondary antibodies were used to detect primary binding. Color development was achieved by addition of pNPP and the absorbance was read spectrophotometrically at 405 nm. Note that all appropriate controls were included within each independent plate (e.g. no primary antibody, etc.) and that we included a wash step (2x, TBS) between every ELISA step highlighted. In parallel, endothelial cell surface expression of cC1qR and gC1qR was assessed using ELISA. All details are the same as reported for the complement protein deposition experiments (above) with the following differences. Experiments were conducted without the exposure to normal human platelet poor plasma and appropriate monoclonal primary antibodies and alkaline phosphatase conjugated secondary antibodies were used to assess the expression.20,27,28 Note that we make use of two antibodies that recognize different structural and functional domains of gC1qR.5,14 Finally, the endothelial cell surface expression of complement inhibitors was also assessed using ELISA methods. Using similar methods as described to assess the expression of the C1qRs, the expression of CR1 (CD35), DAF (CD55) and Protectin (MAC-IP, CD59) was assessed. Briefly, cells were washed, fixed and neutralized as previously described. Specific primary monoclonal antibodies targeting one of the complement regulatory proteins were incubated with endothelial cell samples for 1 h (1:200 dilution, all antibodies from Ancell Corp., Bayport, MN). Appropriate alkaline phosphatase secondary antibodies were used to assess primary binding and then this was assessed spectrophotometrically at 405 nm.27
Endothelial Cell Viability, Density and Metabolic Activity
To assess the alteration to basic endothelial cell culture conditions, we evaluated endothelial cell viability, density and metabolic activity after exposure to mainstream and sidestream tobacco smoke extracts, e-vapor extracts and pure nicotine. Briefly, to assess viability and density, endothelial cells were incubated with 2 μM calcein and 4 μM ethidium (Invitrogen, Carlsbad, CA) for approximately 10 min and then imaged immediately on a Nikon inverted microscope.19 We define cell viability as the percentage of calcein positive cells (live cells) divided by the total number of cells (calcein plus ethidium positive cells). Furthermore, cell density is defined as the total number of live cells divided by the imaging area, which has been calibrated for our objective/camera combination. Metabolic activity was quantified using a standard 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. Endothelial cells were incubated with reconstituted MTT reagent for 3 h. Formed formazan crystals were dissolved in 10% Triton-X and 0.1 M HCl (in anhydrous isopropanol). The resulting solution was gently mixed and absorbance at 630 nm was quantified. This assay measures metabolic activity through the actions of mitochondrial dehydrogenase.
Statistics
The data associated with endothelial cell viability and density is reported as a quantity live and percent live divided by imaging area as described elsewhere.19 The data from each independent ELISA or metabolic activity experiment was normalized to the endothelial cell control sample (incubated with standard culture media without tobacco smoke or e-cigarette vapor) with background subtraction as appropriate. Additionally, since we observed a significant decrease in cell density, ELISA and metabolic activity data were normalized by this difference in cell area. This second normalization was conducted since both ELISA and our metabolic activity data are dependent on the percent coverage of cells and thus these changes need to be considered when reporting data. Note that if there were multiple dependent wells within one experiment, the normalized data was averaged first to provide a single independent data point. Normalized data from a minimum of 5 independent experiments are shown and were used for statistical procedures (the exact sample size are reported in the appropriate figure legends). Statistical analysis was carried out using SAS (V9.3, SAS Institute, Cary, NC). For all conditions, an ANOVA procedure was used to assess the differences within the normalized data. Significant differences between pre-planned comparisons were found based on the least squares means values from the ANOVA results.
Results
Endothelial Cell Viability and Density
Endothelial cell viability and density were observed after exposure to mainstream and sidestream tobacco smoke extracts, e-vapor extracts and pure nicotine. We observed a significant decrease in endothelial cell viability for the majority of endothelial cells exposed to tobacco or e-cigarette products but not nicotine (Fig. 1). In contrast, there was an approximate 25% significant reduction in cell density for endothelial cells exposed to tobacco smoke, e-vapor products or pure nicotine for 48 h. Interestingly, while endothelial cell density was sensitive to the presence of tobacco smoke, e-vapor extracts or pure nicotine, endothelial cells were not sensitive to (1) the concentration of nicotine in the formulation or (2) the presence of toxic gases (since there is no difference between tobacco smoke extracts and e-vapor extracts).
Figure 1.
Endothelial cell viability and density after the exposure to tobacco cigarette and electronic cigarette extracts for 48 h. All data are reported as the mean + standard error of the mean for a minimum of 12 independent experiments (range 12–20). * Significantly different than endothelial cell negative control (ANOVA, P < 0.05), + significantly different than exposure to 50 nM nicotine (ANOVA, P < 0.05). EC endothelial cell negative control, LPS lipopolysaccharide positive control.
Endothelial Cell Metabolic Activity
Endothelial cell metabolic activity was assessed after exposure to tobacco smoke and e-vapor extracts of various compositions or pure nicotine (Fig. 2). For the majority of e-vapor extracts and for all of the tobacco smoke extracts the metabolic activity of endothelial cells was significantly reduced after the exposure conditions. Interestingly, endothelial cell metabolic activity was sensitive to the presence of the extracts but metabolic activity was not sensitive to the exact formulation of the extract. Furthermore, the exposure to pure nicotine did not alter endothelial cell metabolic activity, as compared with the control conditions. The majority of e-vapor/tobacco smoke formulations that induced a reduction in metabolic activity as compared with control conditions also induced a significant reduction in metabolic activity as compared with the exposure to pure nicotine. Thus, endothelial cell metabolic activity is not sensitive to nicotine concentration within the extract and/or the presence of toxic combustion products (since there were no differences between the exposures to the two tobacco products or between the tobacco products and e-vapor extracts).
Figure 2.
Endothelial cell metabolic activity after the exposure to tobacco cigarette and electronic cigarette extracts for 48 h. All data are reported as the mean + standard error of the mean for a minimum of 6 independent experiments (range 6–11). * Significantly different than endothelial cell negative control (ANOVA, P < 0.05), + significantly different than exposure to 50 nM nicotine (ANOVA, P < 0.05). EC endothelial cell negative control, LPS lipopolysaccharide positive control.
Complement Deposition onto Endothelial Cells
Complement deposition onto endothelial cells can precede CVDs and has been observed within atherosclerotic lesions. Thus, we quantified the deposition of C1q (Fig. 3a), C3b (Fig. 3b), C4d (Fig. 3c) and C5b-9 (Fig. 3d) onto endothelial cells after exposure to tobacco extracts and e-vapor extracts. In general, the deposition of C1q and C5b-9 (Figs. 3a, 3d) onto endothelial cells after exposure to tobacco smoke and e-vapor products was significantly enhanced as compared with control endothelial cells. This was typically independent of the exact formulation of the extract. Interestingly, the deposition of C3b and C4d (Figs. 3b and 3c), the major deposition products of the alternative pathway, was generally not enhanced after these exposure conditions as compared with the control conditions, although there were some significantly different conditions. Furthermore, in terms of C4d deposition, it appears that the exposure to tobacco smoke, in any formulation, is more detrimental than the exposure to e-vapor. Thus, the activation of the classical complement cascade, but not the alternative complement cascade, appears to be sensitive to the presence of fine particulate matter only. In contrast, the activation of the alternative pathway (C4d) appears to be sensitive to multiple components of tobacco smoke.
Figure 3.
Deposition of complement product C1q (a), C3b (b), C4d (c) and C5b-9 (d) onto endothelial cells after the exposure to tobacco cigarette and electronic cigarette extracts for 48 h. All data are reported as the mean + standard error of the mean for a minimum of 10 independent experiments (range 10–21). * Significantly different than endothelial cell negative control (ANOVA, P < 0.05), + significantly different than exposure to 50 nM nicotine (ANOVA, P < 0.05). EC endothelial cell negative control, LPS lipopolysaccharide positive control.
gC1qR and cC1qR Expression
Since we observed a heightened deposition of C1q onto endothelial cells after exposure to tobacco smoke and e-vapor extracts, we investigated the expression of gC1qR and cC1qR (Figs. 4a–4c). A heightened expression of either of these receptors could provide a mechanism for altered C1q deposition. In general, there was a significantly enhanced expression of gC1qR (Figs. 4a, 4b) after exposure to e-vapor, tobacco smoke or pure nicotine as compared with the control endothelial cells, but this enhancement was independent of the exact formulation of the extract. In contrast, the expression of cC1qR (Fig. 4c) was significantly enhanced only after exposure to tobacco or e-vapor products as compared with control endothelial cells or pure nicotine. The enhancement of cC1qR was also independent of the exact formulation of the tobacco or e-vapor product.
Figure 4.
Expression of endothelial cell gC1qR (a, b) or cC1qR (c) after the exposure to tobacco cigarette and electronic cigarette extracts for 48 h. All data are reported as the mean + standard error of the mean for a minimum of 5 independent experiments (range 5–11). * Significantly different than endothelial cell negative control (ANOVA, P < 0.05), + significantly different than exposure to 50 nM nicotine (ANOVA, P < 0.05). EC endothelial cell negative control, LPS lipopolysaccharide positive control.
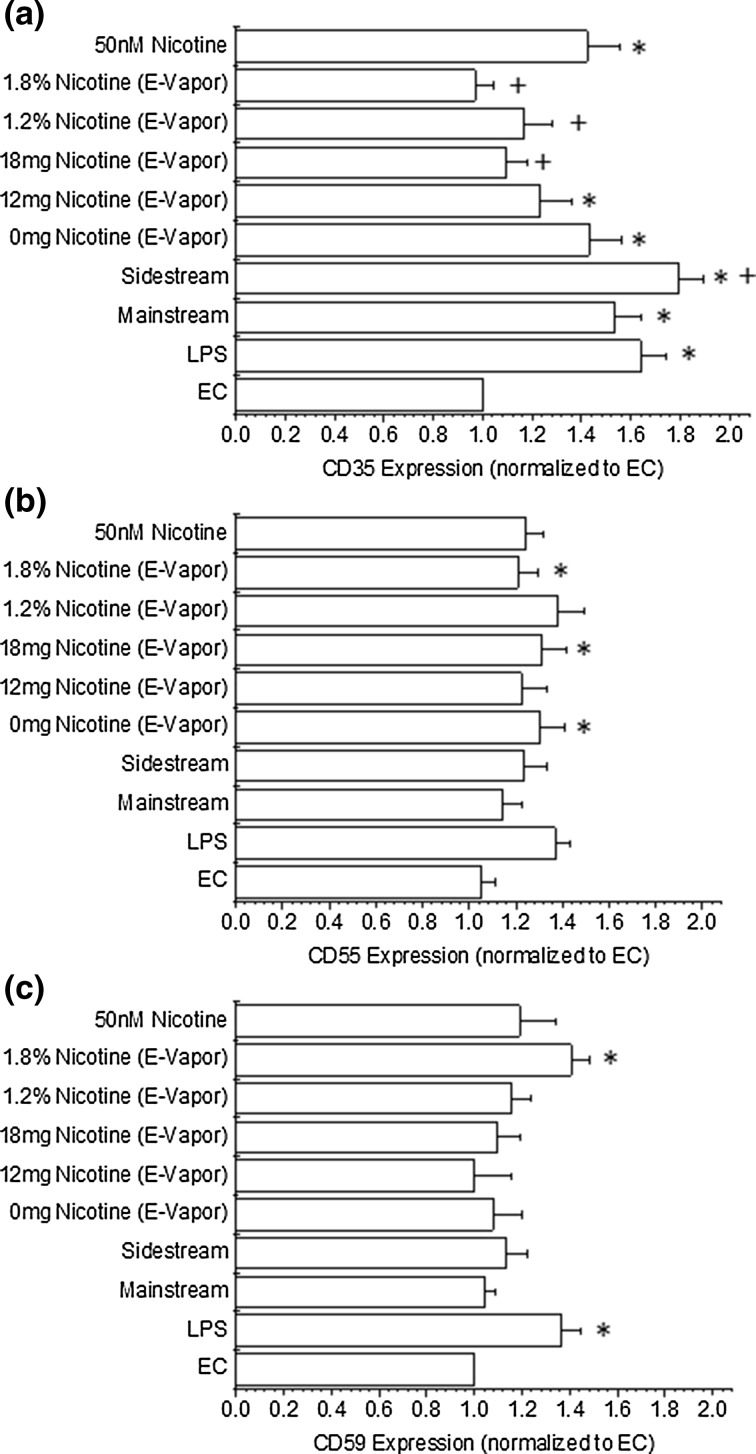
Complement Inhibitor/Regulator Expression
Since we observed a heightened C1q and C5b-9 deposition and no change in C4d or C3b deposition, we investigated the expression of complement inhibitors/regulators after exposure to tobacco smoke/e-vapor (Fig. 5). CD35 (complement receptor 1, Fig. 5a) can regulate the deposition of C3b and C4b to initiate the alternative complement pathway. Interestingly, we observed a significant increase in the expression of CD35 for all conditions, as compared with control endothelial cells, that did not translate to a heightened deposition of complement products onto endothelial cells. We also investigated the expression of CD55 (complement decay accelerating factor, Fig. 5b), which can reduce the formation of C3 convertase in either the classical or alternative complement pathway, thereby reducing the formation of the membrane attack complex (C5b-9). We observed a marginal increase in CD55 expression under all conditions that was sometimes significantly different after exposure to e-vapor products than control conditions. Similarly, we observed the expression of CD59 (protectin, Fig. 5c), which can prevent the formation of the membrane attack complex by interfering with C9 activities, after exposure to tobacco/e-vapor products. CD59 expression was also marginally increased after the exposure, but was only significantly enhanced for one of the experimental conditions, as compared with the control conditions.
Figure 5.
Expression of endothelial cell CD35 (a), CD55 (b) or CD59 (c) after the exposure to tobacco cigarette and electronic cigarette extracts for 48 h. All data are reported as the mean + standard error of the mean for a minimum of 9 independent experiments (range 9–14). * Significantly different than endothelial cell negative control (ANOVA, P < 0.05), + significantly different than exposure to 50 nM nicotine (ANOVA, P < 0.05). EC endothelial cell negative control, LPS lipopolysaccharide positive control.
Discussion
The goal of this work was to evaluate the effects of e-vapor on endothelial cell functions associated with CVD progression and to use e-vapor to elucidate which of the three main constituents of tobacco smoke are most responsible for CVD progression. Importantly, e-vapor provides us with the unique ability to separate toxic combustion products from fine particulate matter, while modulating the nicotine concentration within the extract.
Endothelial Cell Viability and Density
Endothelial cell viability and density were quantified as a means to evaluate the underlying changes to the endothelial cell culture conditions after exposure to tobacco smoke, pure nicotine or e-vapor extracts (Fig. 1). We observed a slight reduction in viability for most of the exposure conditions and these reductions were independent of nicotine concentration or the exact formulation of the extract. Similarly, for all exposures there was a significant reduction in cell density. This was independent of nicotine concentration or the exact formulation of the extract. Thus, in terms of cell viability it appears that fine particulate matter was most responsible for the reduced cell viability and with an increasing nicotine concentration this reduction was abolished. Our data is in agreement with our previous work and the work of others that have shown that endothelial cell viability is somewhat impaired after tobacco smoke exposure.8,22 In terms of cell density, it appears that the presence of nicotine was sufficient to impair cell growth and the addition of fine particulate matter and/or toxic combustion products had little additive effect in addition to nicotine exposure. Similarly, we and others have shown that there is marked impairment in endothelial cell growth after exposure to tobacco products.22,25 Importantly, none of these previous studies were able to differentiate between the effects of toxic combustion products or fine particulate matter on endothelial cell health. In contrast, nicotine has been shown to have a differential effect on endothelial cells; sometimes eliciting pro-inflammatory/pro-thrombotic responses and under different exposures endothelial cells are maintained in a quiescent state.2,13 Here we show that endothelial cell viability and density appear to be regulated by different constituents of tobacco products.
Endothelial Cell Metabolic Activity
We also confirmed the effects of e-vapor exposure on endothelial cell metabolic activity as a means to determine which constituents of tobacco products alters endothelial cell functions (Fig. 2). We show that in general there was a significant decrease in metabolic activity for cells that were exposed to tobacco smoke or e-vapor extracts independent of the formulation of the extract. Additionally, the exposure to pure nicotine did not alter endothelial cell metabolic activity. Our finding that tobacco smoke reduces metabolic activity of endothelial is in agreement with prior work,8 however to the best of our knowledge no one has characterized which constituent of tobacco smoke is most responsible for these changes. Our findings suggest that the exposure to fine particulate matter is sufficient to reduce endothelial cell metabolic activity, but it is possible that exposure to sufficient concentrations of nicotine can counter the alterations induced by fine particulate matter.
Complement Deposition onto Endothelial Cells
We also investigated the deposition of complement products onto endothelial cells after exposure to e-vapor extracts (Fig. 3). In general, we observed a significant increase in the deposition of C1q and C5b-9, C3b to a lesser extent but not C4d. In earlier work from our group we showed that additional complement products can deposit onto endothelial cells after exposure to tobacco products but that the combination of disturbed shear and tobacco product exposure significantly enhances complement deposition.27 This finding is confirmed here, but importantly our data begins to illustrate which constituents of tobacco smoke are most detrimental for complement activation. The classical complement pathway appears to be antagonized by the presence of fine particulate matter, and not nicotine and toxic combustion products (C1q activation). However, the classical pathway is potentially inhibited at the level of C4d. It is possible that the alternative pathway is activated by tobacco smoke and/or e-vapor and our data supports the role of fine particulate matter in this activation, however, toxic combustion products cannot be ruled out at this point (C3b). Our data suggest that nicotine does not play a significant role in the activation of the alternative pathway. Interestingly, it appears that the amplification within the complement system cannot be inhibited fully during these exposures since we observed significant deposition of C5b-9 after exposure to tobacco products and some of the e-cigarette products. This finding also supports the role of fine particulate matter in the activation of complement but the role of toxic combustion products cannot be conclusively evaluated from our findings.
gC1qR and cC1qR Expression
Since we observed a heightened C1q deposition onto endothelial cells, we wanted to evaluate whether or not C1qR (both the receptor for the globular head of C1q; gC1qR, and the receptor for the collagen region of C1q; cC1qR) expression was altered after exposure to e-vapor. We observed a significant enhancement of gC1qR (in particular for the 74.5.2 clone) and cC1qR after these exposure conditions (Fig. 4). Previously, we showed an increased expression of C1qR’s that was perpetuated by the exposure to shear stress.27 Here we show that nicotine is sufficient to enhance the expression of gC1qR (74.5.2), and that there is little difference between the exposure to tobacco products or e-vapor products. cC1qR expression appears to be most sensitive to the exposure to fine particulate matter. Thus, our new findings show that there may be multiple pathways for tobacco products and/or e-cigarette products to antagonize the complement system.
Complement Inhibitor/Regulator Expression
Since there appeared to be a differential expression within the complement cascade and between different pathways within the complement cascade, we investigated the expression of various complement inhibitors/regulators (Fig. 5). In general, we observed a significant up-regulation of CD35 and a slight but non-significant enhancement in the expression of CD55 and CD59. CD35 can regulate C3 and C4 activation. Our data showed a marginal increase in C3b and C4d deposition and this may have been induced by the heightened activity of CD35. Likewise, we observed an enhancement in C5b-9 deposition coupled with marginal changes to CD55/CD59 expression. CD55/CD59 expression inhibits the deposition of C5b-9 (through different mechanisms) and no change in the expression of CD55/CD59 would suggest that C5b-9 would be able to deposit onto cells if the complement cascade is active. We are aware of one report which investigated complement inhibitors/regulators and their expression after tobacco smoke exposure. Our work agrees with this previous finding in terms of an enhanced CD35 expression and no change in CD55/CD59 expression after exposure to tobacco smoke.26 In terms of CD35 expression our work further suggests that toxic combustion products and/or nicotine may be inducing the enhanced expression, however, fine particulate matter cannot be ruled out. Additionally, endothelial cell CD55 and CD59 appears to not be sensitive to any of the constituents of tobacco products.
Summary/Limitations
The goal of the current report was to elucidate which tobacco smoke constituent is most responsible for changes in endothelial cell inflammation and basic culture conditions. In contrast to platelets9 and Kupffer cells,20 which appear to be most sensitive to fine particulate matter for all observed responses, endothelial cells appear to be sensitive to different constituents for different responses. In general, endothelial cell pro-inflammatory enhancements seem to be most susceptible to the presence of fine particulate matter, however, the receptors and regulators of innate inflammatory processes seem to be most sensitive to nicotine or some combination of tobacco smoke constituents. Furthermore, the reduction in endothelial cell metabolic activity and density appear to be most sensitive to fine particulate matter and nicotine, respectively. Thus, our work further evaluates the role of tobacco smoke constituents on endothelial cell functions that have been associated with CVD progression. Finally, with these conclusions there are some limitations that must be considered. Our extraction method may not represent lung extraction and blood dilution accurately. Additionally, we have not considered blood flow or other hemostatic regulators in our system. However, even with these limitations, it is interesting to note that we can begin to delineate which tobacco smoke constituent appears to regulate various endothelial cell functions associated with CVD progression. Finally, it has been previously shown that trace concentrations of LPS (on the order of 12 × 10−15 M) are found in tobacco smoke. It is likely that some LPS is found in our tobacco extracts and the effects of this cannot be excluded since our LPS positive control (~6 × 10−8 M) elicits robust responses. However, it has not been confirmed whether or not LPS is found in e-vapor.
Acknowledgments
Conflict of interest
Dr. Rubenstein, Ms. Barber, Dr. Yin and Dr. Ghebrehiwet report no conflict of interest.
Ethical Considerations
All authors have read and approved the submission of this manuscript and have agreed to be named as authors. Additionally, each author has contributed significantly to the work presented, by designing the experiments, conducting the experiments, analyzing/summarizing the results and/or critically analyzing the manuscript. All work of others has been properly acknowledged. All data are true and accurate to the knowledge of the authors. None of the authors have any potential competing interest with the publication of this manuscript. This manuscript is not under consideration for publication in any other journal and has not been previously published in any other format and is original. This work does not involve human subjects, vertebrae animals, human embryonic stem cells and only makes use of cell obtained commercially, thus it was not necessary for our local IRB/IACUC to review/approve this work.
Abbreviations
- gC1qR
Receptor for the globular head of C1q
- cC1qR
Receptor for the collagen region of C1q
References
- 1.Benowitz NL, Kuyt F, Jacob P., 3rd Influence of nicotine on cardiovascular and hormonal effects of cigarette smoking. Clin. Pharmacol. Ther. 1984;36:74–81. doi: 10.1038/clpt.1984.142. [DOI] [PubMed] [Google Scholar]
- 2.Cucina A, Corvino V, Sapienza P, Borrelli V, Lucarelli M, Scarpa S, Strom R, Santoro-D’Angelo L, Cavallaro A. Nicotine regulates basic fibroblastic growth factor and transforming growth factor beta1 production in endothelial cells. Biochem. Biophys. Res. Commun. 1999;257:306–312. doi: 10.1006/bbrc.1999.0478. [DOI] [PubMed] [Google Scholar]
- 3.Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob. Res. 2014;16:655–662. doi: 10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Ghebrehiwet B, Jesty J, Peerschke EI. Gc1q-r/p33: structure-function predictions from the crystal structure. Immunobiology. 2002;205:421–432. doi: 10.1078/0171-2985-00143. [DOI] [PubMed] [Google Scholar]
- 6.Ghebrehiwet B, Peerschke EI. Cc1q-r (calreticulin) and gc1q-r/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol. Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarino F, Cantarella G, Caruso M, Russo C, Mancuso S, Arcidiacono G, Cacciola RR, Bernardini R, Polosa R. Endothelial activation and injury by cigarette smoke exposure. J. Biol. Regul. Homeost. Agents. 2011;25:259–268. [PubMed] [Google Scholar]
- 9.Hom, S., L. Chen, T. Wang, B. Ghebrehiwet, W. Yin, and D. A. Rubenstein. Platelet activation, adhesion, inflammation and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentration. Platelets in press. [DOI] [PubMed]
- 10.Maria Z, Yin W, Rubenstein DA. Glycated albumin and pathological shear stress alters endothelial cell thrombogenic potential, pro-inflammatory state and cytoskeletal dynamics. J. Diabetes Metab. 2011;S4:003. [Google Scholar]
- 11.Maria Z, Yin W, Rubenstein DA. Combined effects of physiologically relevant disturbed wall shear stress and glycated albumin on endothelial cell functions associated with inflammation, thrombosis and cytoskeletal dynamics. J. Diabetes Investig. 2014;5:372–381. doi: 10.1111/jdi.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Shields MS, Deedwania P, Mehta LS, Phan BA, Benowitz NL. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the American College of Cardiology. J. Am. Coll. Cardiol. 2015;66:1378–1391. doi: 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Patil AJ, Gramajo AL, Sharma A, Seigel GM, Kuppermann BD, Kenney MC. Differential effects of nicotine on retinal and vascular cells in vitro. Toxicology. 2009;259:69–76. doi: 10.1016/j.tox.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Peerschke EI, Bayer AS, Ghebrehiwet B, Xiong YQ. Gc1qr/p33 blockade reduces staphylococcus aureus colonization of target tissues in an animal model of infective endocarditis. Infect. Immun. 2006;74:4418–4423. doi: 10.1128/IAI.01794-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peerschke EI, Minta JO, Zhou SZ, Bini A, Gotlieb A, Colman RW, Ghebrehiwet B. Expression of gc1q-r/p33 and its major ligands in human atherosclerotic lesions. Mol. Immunol. 2004;41:759–766. doi: 10.1016/j.molimm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Peerschke EI, Yin W, Ghebrehiwet B. Platelet mediated complement activation. Adv. Exp. Med. Biol. 2008;632:81–91. doi: 10.1007/978-0-387-78952-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira IA, Borba EF. The role of inflammation, humoral and cell mediated autoimmunity in the pathogenesis of atherosclerosis. Swiss Med. Wkly. 2008;138:534–539. doi: 10.4414/smw.2008.12287. [DOI] [PubMed] [Google Scholar]
- 18.Robbins RA, Nelson KJ, Gossman GL, Koyama S, Rennard SI. Complement activation by cigarette smoke. Am. J. Physiol. 1991;260:L254–L259. doi: 10.1152/ajplung.1991.260.4.L254. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein D, Han D, Goldgraben S, El-Gendi H, Gouma PI, Frame MD. Bioassay chamber for angiogenesis with perfused explanted arteries and electrospun scaffolding. Microcirculation. 2007;14:723–737. doi: 10.1080/10739680701410173. [DOI] [PubMed] [Google Scholar]
- 20.Rubenstein DA, Hom S, Ghebrehiwet B, Yin W. Tobacco and e-cigarette products initiate kupffer cell inflammatory responses. Mol. Immunol. 2015;67:652–660. doi: 10.1016/j.molimm.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein D, Jesty J, Bluestein D. Differences between mainstream and sidestream cigarette smoke extracts and nicotine in the activation of platelets under static and flow conditions. Circulation. 2004;109:78–83. doi: 10.1161/01.CIR.0000108395.12766.25. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein DA, Morton BE, Yin W. The combined effects of sidestream smoke extracts and glycated serum albumin on endothelial cells and platelets. Cardiovasc. Diabetol. 2010;9:28. doi: 10.1186/1475-2840-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt MM, Fraemohs L, Hackeng TM, Weber C, Koenen RR. Atherogenic mononuclear cell recruitment is facilitated by oxidized lipoprotein-induced endothelial junctional adhesion molecule-a redistribution. Atherosclerosis. 2014;234:254–264. doi: 10.1016/j.atherosclerosis.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Schulte DJ, Yilmaz A, Shimada K, Fishbein MC, Lowe EL, Chen S, Wong M, Doherty TM, Lehman T, Crother TR, Sorrentino R, Arditi M. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking kawasaki disease. J. Immunol. 2009;183:5311–5318. doi: 10.4049/jimmunol.0901395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YM, Liu GT. Damaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanism. Biomed. Environ. Sci. 2004;17:121–134. [PubMed] [Google Scholar]
- 26.Yin W, Ghebrehiwet B, Weksler B, Peerschke EI. Regulated complement deposition on the surface of human endothelial cells: effect of tobacco smoke and shear stress. Thromb. Res. 2008;122:221–228. doi: 10.1016/j.thromres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin W, Ngwe EC, Ghebrehiwet B, Rubenstein DA. The combined effect of sidestream smoke and dynamic shear stress on endothelial cell inflammatory responses. Thromb. Res. 2015;135:362–367. doi: 10.1016/j.thromres.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Yin W, Rubenstein DA. Dose effect of shear stress on platelet complement activation in a cone and plate shearing device. Cell. Mol. Bioeng. 2009;2:274–280. doi: 10.1007/s12195-009-0055-9. [DOI] [Google Scholar]
- 29.Yin W, Rubenstein DA. Differences between mainstream and sidestream tobacco smoke extracts and nicotine in the activation and aggregation of platelets subjected to cardiovascular conditions in diabetes. Diabetes Vasc. Dis. Res. 2013;10:57–64. doi: 10.1177/1479164112445282. [DOI] [PubMed] [Google Scholar]