Abstract
Oil palm decanter cake (OPDC) in the current study was converted to valuable products as laccase and manganese peroxidase (MnP) by an undescribed strain of the white-rot fungus, Pseudolagarobasidium sp. PP17-33. The optimization to enhance the production of enzymes through solid-state fermentation was performed using Plackett–Burman design and response surface methodology. The highest observed laccase was 5.841 U/gds and observed MnP was 5.156 U/gds, which enhanced yield by 2.59-fold and 1.94-fold from the non-optimization. The optimized medium (mg/g of OPDC) consisted of 0.852 mg CuSO4·5H2O, 13.512 mg glucose, 2 mg yeast extract, 0.2 mg KH2PO4, 1.5 mg MgSO4·7H2O, 0.01 mg FeSO4·7H2O, 0.15 mg MnSO4·H2O, 0.01 mg ZnSO4·7H2O and 0.3 mg Tween 80 (pH 5.0) when incubated at 30 °C for 7 days. The most significant variables of laccase and MnP productions were CuSO4·5H2O and glucose concentrations. This study is the first to report on the production of ligninolytic enzymes from OPDC waste using white-rot fungi. In addition, five different white-rot fungi, Coriolopsis aspera, C. retropicta, Dentipellis parmastoi, Nigroporus vinosus and Tyromyces xuchilensis, are newly observed producers of ligninolytic enzymes in Thailand. The results obtained from this study are significant not only for agro-industrial waste management but also for value-added enzyme production.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1945-8) contains supplementary material, which is available to authorized users.
Keywords: Laccase, Manganese peroxidase, Oil palm decanter cake, Pseudolagarobasidium, Response surface methodology
Introduction
Palm oil is one of the major agricultural commodities in the world and is, therefore, one of the largest agricultural industries. In Thailand, the production of palm oil has become one of the most important agro-industries during the last 20 years, mainly in the eastern and southern regions of the country (Rongwang et al. 2017). A typical palm oil mill generates a substantial mass of waste for every ton of fresh fruit bunches, empty fruit bunches, oil palm decanter cake (OPDC) and palm mesocarp fibre. Many researchers have studied the suitability of OPDC as animal feed, fertiliser and composting material due to its high nutrient content and its ability to be a source of biogas production (Kaosol and Sohgrathok 2012). However, its economic value is low and it is, therefore, not considered to possess an attractive potential for development. As the major components of OPDC are lignin (30.66%), cellulose (21.61%) and hemicellulose (3.94%) (Rasak et al. 2012), it can be utilized as an alternate low-cost substrate for ligninolytic enzyme production that acts corporately to degrade the components of lignocellulose. Moreover, OPDC contains organic matters and nutrients that are essential for the growth of fungi during ligninolytic enzyme production.
White-rot fungi are well known for their essential role in the naturally occurring degradation of lignocellulose, which is enabled by secretion of extracellular ligninolytic enzymes such as laccase, manganese peroxidase (MnP) and lignin peroxidase (LiP). The non-specificity of these enzymes makes them very attractive for different industrial and biotechnological applications, such as the production of biofuel from plant biomass, biopulping and biobleaching. At present, organic wastes from agro-industries are a major source of pollution. Therefore, the utilization of agro-industrial wastes and effluents through recycling and clean technology has been widely investigated (Silva et al. 2014; Akpinar and Urek 2017; Ajayi and Femi-Ola 2019). Thailand is known to be a major centre for global biodiversity, comprising of many different types of habitats. In tropical countries, there are an enormous number of potential sites for fungi in every locality and there are at least 31 ecological niches meriting study and requiring different techniques and expertise to explore this magnitude (Hawksworth 2001). Fungi are also considered to have an increasing importance in medicine and biotechnology resulting from their unique biosynthetic abilities and metabolic products. In Thailand, the study of ligninolytic enzymes from white-rot fungi is currently limited not only because of the lack of knowledge on their taxonomy and genetic diversity but also by the limited interest in their potential use in treating agro-industrial waste (Vaithanomsat et al. 2015; Ghebreslasie et al. 2016).
Amongst the processes used for enzyme production, solid-state fermentation (SSF) using agro-industrial wastes is an attractive and cost-effective option because it represents higher productivity with simpler operation when compared with submerged fermentation. The production of ligninolytic enzymes is affected by medium components and types of substrates, such as carbon, nitrogen and metal ions (Liu et al. 2009). Thus, it is very important to optimize enzyme production when investigating new substrates and/or fungal strains to identify suitable components in the medium for enzyme production. Statistical experimental designs are useful tools for optimization with a significant impact on enzyme production. Plackett–Burman design (PBD) and response surface methodology (RSM) have been successfully used to improve product yield and reduce development time and overall process cost (Thakur and Gupte 2015; Venkateswarulu et al. 2017; Senthivelan et al. 2019). Therefore, the aims of this study were to select the active strains of white-rot fungi that produce ligninolytic enzymes using agro-industrial waste OPDC as a substrate in the SSF system and to statistically optimize the medium components for ligninolytic enzyme production using a potentially novel strain.
Materials and methods
Isolation and screening of white-rot fungi
The fruiting bodies of basidiomycete fungi found on decayed or fallen wood were collected from various parts of Thailand, e.g., provinces of Chaiyaphum, Nakhon Phanom, Sakon Nakhon and Udon Thani. The collections were then isolated by culturing on potato dextrose agar (PDA) plates with incubation at 30 °C for 5–7 days. The pure cultures of fungi were preserved on PDA slants at 4 °C. They are maintained in the culture collection of the Department of Microbiology, Faculty of Science, Srinakharinwirot University. The screening of all fungal isolates for ligninolytic enzyme production was performed using an agar plate assay. Remazol brilliant blue R (RBBR) dye, 2,2′-azino-bis (3-ethylbenzthiazoline)-6-sulfonate (ABTS) and guaiacol (Sigma-Aldrich Chemical Co., St. Louis, USA) were used as indicators. An active fungal mycelial plug of 6-mm diameter taken from a 7-day-old culture was placed in the centre of a 9-cm diameter Petri dish of glucose asparagine agar (10 g/L glucose, 1 g/L l-asparagine, 0.5 g/L yeast extract, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·H2O, 0.01 g/L FeSO4·H2O and 15 g/L agar) containing different concentrations of RBBR (0.01% w/v), ABTS (200 mg/L) or guaiacol (0.56 mL/L) (Kalmis et al. 2007). Triplicate plates were incubated at 30 °C in the dark for 7 days. RBBR decolorization or ABTS/guaiacol halo formation of each isolate was determined by measuring their respective diameters.
Preparation of substrate and screening of ligninolytic enzyme production
OPDC from Suksomboon Palm Oil Co., Ltd., Chonburi Province, Thailand, was dried in a hot-air oven at 60 °C for 7 days and then autoclaved at 121 °C for 20 min. The components of lignin, cellulose and hemicellulose of OPDC were analysed at Kasetsart Agricultural and Agro-Industrial Product Improvement Institute in Thailand. The fungal inoculum was grown on basal medium agar modified from Téllez-Téllez et al. (2008), which contained (in gram per litre): 5.0 g glucose, 2.5 g yeast extract, 0.4 g KH2PO4, 0.5 g MgSO4·7H2O, 0.01 g FeSO4·7H2O, 0.05 g MnSO4·H2O, 0.25 g CuSO4·5H2O, 0.01 g ZnSO4·7H2O, and 0.1 ml Tween-80 (pH 5). The cultures were incubated at 30 °C for 7 days. Positive isolates from the agar plate assay were selected to produce laccase, MnP and LiP under SSF using OPDC as the substrate. Ten grams of OPDC was adjusted to a moisture content of 65% humidity with 20 ml of liquid basal medium (pH 5) in 250-mL Erlenmeyer flasks and autoclaved at 121 °C for 15 min. A plug of fungal mycelial (8 mm in diameter) was transferred to a flask and incubated at 30 °C in static conditions for 7 days. The mycelial growth was determined by measuring the colony diameter. Crude enzymes were extracted by adding 50 mL of 100 mM sodium acetate buffer (pH 5) and shaking at 150 rpm, 20 °C, for 90 min. The crude enzyme was filtered through Whatman No. 1 filter paper, and the supernatant was harvested by centrifugation at 8000 rpm at 4 °C for 20 min.
Laccase, MnP and LiP activity assay
Laccase activity was determined by monitoring the oxidation of ABTS and the reaction was monitored by measuring the increase in absorbance at 420 nm (ε = 36,000 M−1 cm−1) (Machado and Matheus 2006). MnP activity was determined by monitoring the oxidation of 2,6-dimethoxyphenol (DMP) as the oxidation of Mn2+ to Mn3+ by following the formation of the Mn3+-tartrate complex at 469 nm (ε = 10,000 M−1 cm−1) (Silva et al. 2014). LiP activity was determined by following the oxidation of veratryl alcohol (3,4-dimethoxybenzyl alcohol) to veratraldehyde in the presence of H2O2 and the increase in absorbance at 310 nm (ε = 9300 M−1 cm−1) was recorded (Tien and Kirk 1998). One unit (U) of the activity of all ligninolytic enzymes is defined as the amount of enzyme that transforms 1 µmol of substrate per minute.
White-rot fungal identification and nucleotide sequence analysis
The isolated ligninolytic enzyme-producing fungi were identified based on their morphological characteristics and the nucleotide sequences of their internal transcribed spacer (ITS). Fresh mycelia of pure culture were extracted for genomic DNA using the Plant Genomic DNA extraction kit (Favorgen, Taiwan) and ITS fragments were amplified using the polymerase chain reaction (PCR) with primers ITS1/ITS4 (White et al. 1990). The reaction was performed according to the previous study (Suwannasai et al. 2013). The PCR products were purified using a PCR purification kit (Favorgen, Taiwan) and sequenced at the 1st BASE (Malaysia). The results obtained were manually checked using Chromas version 1.45 (Queensland, Australia) and compared with nucleotide sequences available in GenBank using the BLAST program. ITS sequences obtained from this study were deposited in the GenBank database.
Cultivation conditions for laccase and manganese peroxidase production under SSF
Ten grams of dried OPDC was added to 250-mL Erlenmeyer flasks and adjusted to a moisture content of 65% with basal medium broth. After sterilization, five mycelial plugs (8 mm in diameter) were used to inoculate the flasks and these were incubated at 30 °C under static conditions for 7 days. Crude enzymes were extracted by adding 100 mM sodium acetate buffer (pH 5) according to the method described above.
Experimental design and statistical analysis
Single parameter optimization of the nitrogen source (peptone, yeast extract and sodium nitrate) at 0.25% was employed for laccase and MnP activities by adding 2.5 g/L of basal medium instead of a nitrogen source. Then, a suitable nitrogen source was selected for further optimization with other important medium components using PBD and then central composite design (CCD). All experiments were conducted in triplicate. The experimental results obtained were expressed as the means and standard deviations.
Screening of important medium components by PBD
PBD was used as a primary step in the optimization (Plackett and Burman 1946). It was applied to determine the most important components that influence overall laccase and MnP production in the system. For each enzyme, the optimization was performed using the same methodology for both enzyme activity and its corresponding production. In this study, nine factors (glucose, yeast extract, KH2PO4, MgSO4·7H2O, FeSO4·7H2O, MnSO4·H2O, CuSO4·5H2O, ZnSO4·7H2O and Tween 80) were selected for testing. A set of 12 experiments was designed for the nine medium components, namely, X1–X9 using the Minitab package. Each variable was tested at high (+ 1) and low (− 1) levels (Table 1). All experiments were carried out in triplicate, and the results are the means ± SD of triplicate experiments. The variables with a confidence level greater than 95% were considered more significant for enzyme production, and the response was measured as laccase and MnP activities. The measured data are the means of enzyme activity.
Table 1.
PBD for optimization of independent variables used in trials
| Variables | Medium component | Low values (− 1) (g/L) | High values (+ 1) (g/L) |
|---|---|---|---|
| X1 | Glucose | 2 | 8 |
| X2 | Yeast extract | 1 | 4 |
| X3 | KH2PO4 | 0.1 | 0.7 |
| X4 | MgSO4·7H2O | 0.25 | 0.75 |
| X5 | FeSO4·7H2O | 0.005 | 0.015 |
| X6 | MnSO4·H2O | 0.025 | 0.075 |
| X7 | CuSO4·5H2O | 0.125 | 0.375 |
| X8 | ZnSO4·7H2O | 0.005 | 0.015 |
| X9 | Tween 80 | 0.05 | 0.15 |
Optimization of significant medium components by CCD
To enhance the production of laccase and MnP, CCD was selected to optimize the most significant factors obtained by PBD. The two independent factors selected were CuSO4·5H2O and glucose. Each variable was set at five different levels (− 1.414, − 1, 0, + 1 and + 1.414). The other variables in the study were maintained at a constant level, which gave maximum activity in the PBD experiments. A total of 13 experiments were designed using the Design-Expert software for implementing the CCD design. The mean response (enzyme activity) is the combined effects of the two independent factors studied in a defined range. The experimental results of response surface methodology (RSM) were fitted with the response surface regression procedure using the two significant variables and can be approximately plotted by the following quadratic model equation:
where Y is the predicted response, β0 is the intercept term, β1 and β2 are linear coefficients, β11 and β22 are quadratic coefficients, β12 is an interaction coefficient and X1 and X2 are coded independent variables.
Statistical analysis
The results obtained from the single parameter of nitrogen source optimization were analysed by one-way analysis of variance (ANOVA). The ligninolytic enzyme production in SSF was carried out with response surface methodology using the statistical software package Minitab® 18 (Minitab, Inc., PA, USA) and Design-Expert® software version 11 (Stat-Ease Inc., USA).
Results and discussion
Fungal isolation and primary screening of enzyme activity
One hundred and fifty isolates of white-rot fungi from 264 samples of the fruiting bodies were successfully cultured on PDA. Since many samples were dried and/or spoiled by insects, only 57% of all collections were successfully isolated into pure culture. Among all isolates tested, 63 isolates (approximately 42%) exhibited positive results with varying degrees of degradation against the three indicators RBBR, ABTS and guaiacol on the agar plate assay. The isolates performed high levels of RBBR decolorization, and high extracellular oxidation activity producing the dark green (ABTS) and brown (guaiacol) colouration within 7 days (Table S1). Although the use of coloured indicators helps identify ligninolytic enzyme activity, it is not specific for enzyme type. The 63 isolates were, therefore, screened for the ligninolytic enzyme activity of laccase, MnP and LiP using OPDC as a substrate under the SSF system. Some isolates grew slowly on OPDC, and as a result, only 42 isolates were identified as having ligninolytic enzyme activity. Most isolates produced laccase and MnP, whereas only a few isolates produced LiP, which was at a low level or was undetectable. Some isolates showed mycelial growth on OPDC, but no ligninolytic enzymes were detected. These fungi possibly require different culture conditions for their lignin degradation capabilities. Table S2 shows the 25 most promising positive isolates regarding laccase, MnP and LiP production. The varying enzyme activity values of the isolates indicated that their ability to produce enzymes varies according to either isolate or strain. This result is in agreement with several reports that some white-rot fungi contain all three ligninolytic enzymes, while others contain only one or two of these enzymes (Pelaez et al. 1995). Generally, laccase and MnP are more widely distributed among white-rot fungi than LiP (Pelaez et al. 1995). Some white-rot fungi may be restricted by the culturing method and types of substrate utilized. Kinnunen et al. (2017) reported that LiP activity was rather rare; fungi perform this activity under nutrient- and nitrogen-limited conditions, and the activity was detected when the assay was performed in very acidic conditions (pH 3). Therefore, in the present study, only the two enzymes laccase and MnP were further investigated.
White-rot fungal identification and nucleotide sequence analysis
White-rot fungal isolates producing laccase and MnP using OPDC as substrates were classified and identified based on morphological characteristics and nucleotide sequences. Most of the isolates could be identified to the species level, which was supported by the high identity of ITS sequences (> 97% identity) when compared with nucleotide sequences in the GenBank database. The morphological characteristics of positive isolates confirmed the species descriptions according to previous reports. Based on ITS sequences, ligninolytic enzyme-producing white-rot fungi found in this study belonged to 6 families, 10 genera and 16 species. The BLAST results and GenBank accession numbers (MK589268-MK589292) of the 25 positive isolates are listed in Table S3. Most of them were basidiomycetes, and only one isolate, Pestalotiopsis theae PP17-19, was an ascomycete. White-rot basidiomycetes are generally good candidates for ligninolytic enzyme production compared with ascomycetes (Bodke et al. 2012). Ganoderma consisted of the greatest number of different species based on ITS sequence analysis. These species are difficult to distinguish based solely on morphological features or ITS sequences. Trametes is another well-known genus with the most efficient lignin-degraders, which can be attributed to a well-developed ligninolytic enzyme system (Cupul et al. 2014). In our study, three different species of Trametes, T. elegans, T. hirsuta and T. sanguinea were identified, which exhibited moderate levels of laccase and MnP activities. Considering the genus Microporus, there have only been limited ligninolytic enzyme studies at the present time. Our study found two unidentified species that produced both laccase and MnP with moderate growth on OPDC (Table S2). Various isolates produced different combinations of ligninolytic enzymes, even among the same species because of the diversified ability of fungal strains to degrade substrate, which is related to their strategies for lignin biodegradation in nature. However, our present study is the first report of white-rot fungi producing ligninolytic enzymes from OPDC waste. In addition, five identified species, C. aspera, C. retropicta, D. parmastoi, N. vinosus and T. xuchilensis, were also noted for the first time in a ligninolytic enzyme study. Fungal strains obtained from this study will be good candidates for ligninolytic enzyme production, lignin degradation and suitable for biotechnological applications in the future.
The isolate PP17-33 showed high activities of both laccase and MnP in the primary screening with a fast growth rate on OPDC waste. Therefore, it was selected for optimization of enzyme production by statistical methods. Moreover, the species identification of PP17-33 based on ITS sequences revealed a close relationship with Pseudolagarobasidium belizense (GenBank accession number NR120036.1) with 96% identity. Then, the morphological characteristics of PP17-33 were carefully examined according to the description of Nakasone and Lindner (2012) (Fig. S1). In addition, the morphological characteristics of PP17-33 are completely different from those of P. belizense, including the colour and shape of fruiting bodies and the size of basidiospores. The fruiting body of PP17-33 is resupinate and is light cream-coloured with brown aculei, is colourless in KOH, and has small basidiospores of (2.7-)3-4(-4.5) × 2-3 mm (length × width). Notably, PP17-33 is considered an unidentified species, Pseudolagarobasidium sp. PP17-33 (GenBank accession number MK589289), and it may prove to be a new species. However, it is important to carefully examine the holotype material before the fungus is described as a new species. In addition, only one species of P. acaciicola AGST3 to date has been studied for laccase production and application for dye decolorization (Thakur and Gupte 2015). Later, Adak et al. (2016) reported that only laccase activity was detected in P. acaciicola LA1, and no activities of MnP and LiP were detected in this isolate. Until now, there has been no information from ligninolytic enzyme studies of other species of Pseudolagarobasidium. This study represents new information on ligninolytic enzyme production from the novel strain Pseudolagarobasidium sp. PP17-33.
Screening of important medium components for laccase and manganese production according to PBD
The substrate used in this study was OPDC, which contained 30.62% lignin, 14.71% hemicellulose and 22.39% cellulose. The maximum laccase and MnP activities of Pseudolagarobasidium sp. PP17-33 were preliminarily screened, and the highest activities were detected on the 7th day of a 12-day cultivation period (unpublished reports). The results of laccase and MnP production demonstrated that yeast extract was a suitable nitrogen source for both enzymes produced by Pseudolagarobasidium sp. PP17-33 (Fig. S2). This result is in agreement with several studies indicating that yeast extract is a suitable nitrogen source for laccase production of Agaricus bisporus, Schizophyllum commune and Ganoderma sp. (Adejoye and Fasidi 2010; Othman et al. 2018) and for MnP production by G. lucidum and T. versicolor (Asgher and Iqbal 2011; Xu et al. 2017). However, other nitrogen sources, such as peptone and ammonium chloride, were found to be the optimum nitrogen source for laccase production by Aspergillus flavus (Kumar et al. 2016) and Pleurotus ostreatus (Stajic et al. 2006), respectively. The role of nitrogen source in the regulation of enzyme synthesis depends not only on the physiology of fungi but also on the medium composition, especially in the presence of lignocellulose substrate.
Then, the enzyme optimization was first screened with PBD to select the most significant variables for laccase and MnP production by Pseudolagarobasidium sp. PP17-33. Table 2 shows the PBD matrix of 12 trials and the corresponding laccase and MnP production in terms of units per gram of dry substrate (U/gds). The effect, coefficient estimate, mean square, F value and P value for each component are presented in Table 3. The coefficient of determination, R2, provides information about the goodness-of-fit of the model. For a good statistical model, an R2 value greater than 0.75 indicates the explanatory variable and the suitability of the model. According to the Pareto analysis, the variable that had significant effects on laccase production at the confidence level of 95% was CuSO4·5H2O (R2 = 0.7681), while MnP production was significantly affected by CuSO4·5H2O and glucose concentrations (R2 = 0.8512) (Fig. S3). The effect of CuSO4·5H2O on the production of both enzymes was positive, which indicated the requirement of a high concentration for enzyme production. During the PBD experiment, a negative effect of glucose concentration on MnP production was observed, indicating a low amount is required. Cu2+ is an inducer of enzyme production, especially ligninolytic enzymes and several studies have shown that laccase production is regulated by metal ions such as Cu2+ and Fe3+ through gene expression induction or translational or posttranslational regulation (Fonseca et al. 2010). Although Cu2+ is essential for inducing laccase production by basidiomycetes, there is an optimum amount required that is species-specific. Glucose had a positive effect on the production of most enzymes because it is readily utilizable and efficiently metabolized by the microorganism, resulting in high levels of enzyme production. The study conducted by Schneider et al. (2018) with Marasmiellus palmivorus showed that glucose was the best carbon source for laccase and MnP production. However, a high concentration of glucose represses laccase synthesis in fungi.
Table 2.
Screening of medium components for laccase and MnP production by PBD
| Exp. no. | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | Lac activity (U/gds) | MnP activity (U/gds) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | – | + | + | + | – | – | – | 1.410 | 1.990 |
| 2 | + | – | + | + | + | – | – | – | + | 1.764 | 2.216 |
| 3 | – | + | + | + | – | – | – | + | – | 1.572 | 2.893 |
| 4 | + | + | + | – | – | – | + | – | + | 2.422 | 2.973 |
| 5 | + | + | – | – | – | + | – | + | + | 1.687 | 2.156 |
| 6 | + | – | – | – | + | – | + | + | – | 2.555 | 2.570 |
| 7 | – | – | – | + | – | + | + | – | + | 3.053 | 3.313 |
| 8 | – | – | + | – | + | + | – | + | + | 2.048 | 2.966 |
| 9 | – | + | – | + | + | – | + | + | + | 2.140 | 2.516 |
| 10 | + | – | + | + | – | + | + | + | – | 2.581 | 3.198 |
| 11 | – | + | + | – | + | + | + | – | – | 1.892 | 3.080 |
| 12 | – | – | – | – | – | – | – | – | – | 1.898 | 2.786 |
X1 = glucose, X2 = yeast extract, X3 = KH2PO4, X4 = MgSO4·7H2O, X5 = FeSO4·7H2O, X6 = MnSO4·H2O, X7 = CuSO4·5H2O, X8 = ZnSO4·7H2O and X9 = Tween 80
Table 3.
Statistical analysis of PBD results on laccase and MnP production
| Variables | Medium component | Coefficient estimate | Mean square | F value | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lac | MnP | Lac | MnP | Lac | MnP | Lac | MnP | ||
| Corrected model | 0.250 | 0.199 | 4.98 | 7.87 | 0.179 | 0.118 | |||
| X1 | Glucose | − 0.005 | − 0.068 | 0.003 | 0.500 | 0.06 | 19.78 | 0.835 | 0.047 |
| X2 | Yeast extract | − 0.154 | − 0.080 | 0.642 | 0.173 | 12.78 | 6.84 | 0.070 | 0.120 |
| X3 | KH2PO4 | − 0.129 | 0.554 | 0.018 | 0.332 | 0.36 | 13.10 | 0.611 | 0.069 |
| X4 | MgSO4·7H2O | 0.006 | − 0.135 | 0.000 | 0.014 | 0.00 | 0.54 | 0.984 | 0.539 |
| X5 | FeSO4·7H2O | − 23.4 | − 33.02 | 0.164 | 0.327 | 3.27 | 12.92 | 0.212 | 0.069 |
| X6 | MnSO4·H2O | 1.07 | 2.50 | 0.009 | 0.047 | 0.17 | 1.85 | 0.720 | 0.307 |
| X7 | CuSO4·5H2O | 2.843 | 1.762 | 1.515 | 0.582 | 30.15 | 23.00 | 0.032 | 0.041 |
| X8 | ZnSO4·7H2O | 2.4 | − 0.98 | 0.002 | 0.000 | 0.03 | 0.01 | 0.870 | 0.925 |
| X9 | Tween 80 | 2.01 | − 0.628 | 0.121 | 0.012 | 2.41 | 0.47 | 0.261 | 0.565 |
R squared = 0.9614 (laccase; Lac), 0.9752 (MnP) and R squared (adjustment) = 0.7681 (laccase), 0.8512 (MnP)
Optimization of screened medium components by RSM
After PBD analysis, the significant variables for laccase and MnP production were subsequently optimized using response surface methodology by CCD. Thirteen experiments with the variables of CuSO4·5H2O and glucose concentration were performed. The experimental design, statistical results and predicted enzyme values are presented in Tables 4 and 5. The F values of laccase (13.86) and MnP (30.57) production demonstrated high significance in the regression models (Table 6). The P values of laccase and MnP production were 0.002 and 0.000, respectively, indicating that the model terms are significant. The ANOVA of the optimization study indicated that the model terms A, A2 and B2 were significant for laccase production, while A, A2, B2 and AB were significant for MnP production (P < 0.05). The linear effects of CuSO4·5H2O (P < 0.001) were determined to be more significant than the effects of the other variables. These results indicate that the concentration of CuSO4·5H2O is directly related to the production of both enzymes. The models of laccase and MnP production resulting from the analysis of the data in Table 6 are expressed below:
Table 4.
Ranges of the independent variables used in RSM
| Variables | Medium component | Levels (g/L) | ||||
|---|---|---|---|---|---|---|
| − 1.414 | − 1 | 0 | + 1 | + 1.414 | ||
| A | CuSO4·5H2O | 0.073 | 0.125 | 0.25 | 0.375 | 0.426 |
| B | Glucose | 0.758 | 2 | 5 | 8 | 9.242 |
Table 5.
CCD of factors in coded levels with enzyme activity as response
| Run no. | Variables | Laccase activity (U/gds) | Manganese peroxidase activity (U/gds) | |||
|---|---|---|---|---|---|---|
| CuSO4·5H2O | Glucose | Experiment | Prediction | Experiment | Prediction | |
| 1 | − 1 | − 1 | 3.971 | 4.221 | 4.233 | 4.176 |
| 2 | 1 | − 1 | 5.031 | 5.029 | 5.400 | 5.303 |
| 3 | − 1 | 1 | 4.211 | 4.099 | 4.553 | 4.646 |
| 4 | 1 | 1 | 6.183 | 5.818 | 4.980 | 5.033 |
| 5 | − 1.414 | 0 | 4.116 | 3.994 | 4.546 | 4.519 |
| 6 | 1.414 | 0 | 5.546 | 5.781 | 5.560 | 5.590 |
| 7 | 0 | − 1.414 | 4.660 | 4.460 | 4.346 | 4.453 |
| 8 | 0 | 1.414 | 4.620 | 4.932 | 4.700 | 4.595 |
| 9 | 0 | 0 | 5.315 | 5.656 | 4.850 | 4.858 |
| 10 | 0 | 0 | 5.820 | 5.656 | 4.920 | 4.858 |
| 11 | 0 | 0 | 5.570 | 5.656 | 4.740 | 4.858 |
| 12 | 0 | 0 | 5.810 | 5.656 | 4.840 | 4.858 |
| 13 | 0 | 0 | 5.765 | 5.656 | 4.940 | 4.858 |
Table 6.
Analysis of variance (ANOVA) for response surface quadratic model for the production of laccase and MnP
| Source | Coefficient factor | Sum of squares | DF | F value | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lac | MnP | Lac | MnP | Lac | MnP | Lac | MnP | Lac | MnP | |
| Model | 5.9562 | 1.5983 | 5 | 5 | 13.86 | 30.57 | 0.002 | 0.000 | ||
| Linear | 3.4162 | 1.1662 | 2 | 2 | 19.87 | 55.76 | 0.001 | 0.000 | ||
| A—CuSO4·5H2O | 0.632 | 0.379 | 3.1933 | 1.1461 | 1 | 1 | 37.15 | 109.60 | 0.000 | 0.000 |
| B—Glucose | 0.167 | 0.050 | 0.2229 | 0.0201 | 1 | 1 | 2.59 | 1.92 | 0.151 | 0.209 |
| AB | 0.228 | − 0.185 | 0.2079 | 0.1369 | 1 | 1 | 2.42 | 13.09 | 0.164 | 0.009 |
| A2 | − 0.384 | 0.098 | 1.0264 | 0.0673 | 1 | 1 | 11.94 | 6.44 | 0.011 | 0.039 |
| B2 | − 0.480 | − 0.167 | 1.6003 | 0.1931 | 1 | 1 | 18.62 | 18.47 | 0.004 | 0.004 |
| Lack of fit | 0.4155 | 0.0483 | 3 | 3 | 2.98 | 2.59 | 0.160 | 0.190 | ||
| Pure error | 0.1862 | 0.02488 | 4 | 4 | ||||||
| Total | 6.5579 | 1.6715 | 12 | 12 | ||||||
A = CuSO4·5H2O, B = Glucose; laccase (Lac), R squared = 0.9083, R squared (adjustment) = 0.8427 and R squared (prediction) = 0.5051; MnP, R squared = 0.9562, R squared (adjustment) = 0.9249 and R squared (prediction) = 0.7712
The experiments for verifying the predicted models revealed correlation coefficients (R2) of 0.8427 and 0.9249 for laccase and MnP production, respectively, which were considered to be high correlations. The present R2 value reflected a very good fit between the observed and predicted responses. These results implied that the present models are reliable for laccase and MnP production. The lack of fit of both models (laccase; P value = 0.1598 and MnP; P value = 0.1903) were non-significant, which confirmed that the designed models are good. The adequate precision values of the present models were 9161 for laccase production and 20,352 for MnP production, which suggested that the models can be used to navigate the design space. The adequate precision value is an index of the signal-to-noise ratio, and values of higher than 4 are essential prerequisites for the model to be a good fit.
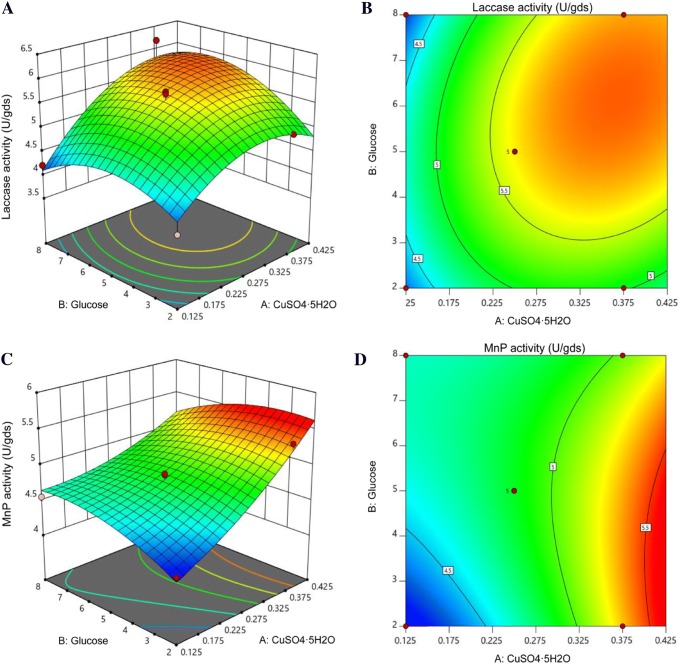
To determine the optimal levels of each variable for maximum laccase and MnP production, three-dimensional response surface plots (Fig. 1a, c) and contour plots (Fig. 1b, d) were constructed against two independent variables. The interaction between CuSO4·5H2O and glucose concentrations for laccase production indicated that laccase production was maximum at a high concentration of CuSO4·5H2O (0.366 g/L) and an intermediate concentration of glucose (6.157 g/L) (Fig. 1a, b). The condition for optimum MnP production can be verified in the response surface plot (Fig. 1c). The axial points of the experimental design, not the central point, are associated with the highest MnP production. The highest concentration of CuSO4·5H2O (0.426 g/L), which was associated with a slightly low concentration of glucose (3.072 g/L) (Fig. 1c, d), led to maximum MnP production. As a result of the highest glucose concentration in this experimental design, both laccase and MnP production were slightly decreased. The decrease in enzyme production with high glucose concentrations can be explained by carbon catabolite repression. This is an important mechanism commonly found in many fungi and helps them to save energy by controlling transcription. The fungi assimilate glucose and highly favourable sugars before switching to less-favoured sources of carbon. The regulatory role of carbon catabolite repression in fungi has been extensively studied in Aspergillus spp. The significant transcription factor, CreA, represses the genes encoding ligninolytic enzymes in A. nidulans for utilizing secondary carbon sources such as lignocellulose when glucose is presented (Ries et al. 2016).
Fig. 1.
Response surface plots of laccase (a) and MnP (c) production, and contour curve plots of laccase (b) and MnP (d) production showing the interactive effect of CuSO4·5H2O and glucose concentration. Colour scale: blue (< low enzyme activity) to red (> high enzyme activity)
Validation of the model
The validation of the statistical model and regression equation were conducted for both laccase and MnP production under the same conditions and this is because it is proposed to use the enzyme mixture in several biotechnological applications. The prediction model of the maximum production of laccase (5.905 U/gds) and MnP (5.406 U/gds) was obtained in the presence of 0.426 g/L CuSO4·5H2O and 6.756 g/L glucose. The observed experimental values of laccase and MnP production were 5.841 U/gds and 5.156 U/gds, respectively. These results confirmed the validity of the model, and the experimental values were determined to be quite close to the predicted values. This statistical design could increase laccase and MnP production by 2.59-fold and 1.94-fold from non-optimized production, respectively. Although the optimization of laccase and MnP production from different white-rot fungi has been achieved using statistical methods, the present study is the first report of the culture conditions for laccase and MnP production from the novel strain Pseudolagarobasidium sp. PP17-33 on OPDC as substrate by SSF. The major effectors of enzyme production were concentrations of CuSO4.5H2O and glucose, which depended on the fungal strain and composition of the substrates used.
Conclusion
Oil palm wastes are potential substrates for microbial conversion via solid substrate fermentation into value-added products from ligninolytic enzymes. The current investigation has identified a potentially promising isolate of the novel strain Pseudolagarobasidium sp. PP17-33, which is worthy of further experimentation and may become commercially viable for laccase and MnP production from oil palm waste. ITS sequences confirmed the distinguishing characteristics of PP17-33 and they have been submitted to GenBank database as MK589289. The statistical optimization successfully increased the laccase and MnP production to 5.841 U/gds and 5.156 U/gds, respectively, at 30 °C for 7 days. These activities were 2.59 and 1.94 times greater than the initial activities. The concentrations of CuSO4·5H2O and glucose significantly affected production of both enzymes. These enzymes will be applied in further applications of synthetic dye decolorization, bioremediation, biofuel and lignocellulose pretreatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the Biodiversity-based Economy Development Office (BEDO-Thailand) for funding the research activities (Grant no. 37/2561) and The King Mongkut’s University of Technology North Bangkok (Grant no. KMUTNB-62-KNOW-07). We would like to thank Assistant Professor Dr. Rungpetch Khaengraeng for the supporting of fungal collections.
Author contributions
PT, CP, NP and NS designed the experiments and drafted the manuscript. PT, CP and NS collected the fungal samples. PT, CP, NS and AJSW classified and identified the fungal species. PT, SK and NS analysed the data using statistical methods. AJSW edited and modified the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest.
References
- Adak A, Tiwari R, Singh S, Sharma S, Nain L. Laccase production by a novel white-rot fungus Pseudolagarobasidium acaciicola LA1 through fermentation of Parthenium biomass and its application in dyes decolorization. Waste Biomass Valor. 2016;7(6):1427–1435. doi: 10.1007/s12649-016-9550-0. [DOI] [Google Scholar]
- Adejoye OD, Fasidi IO. Effect of cultural conditions on biomass and laccase production in submerged medium by Schizophyllum commune (FR.), a Nigerian edible mushroom. Electron J Environ Agric Food Chem. 2010;9:600–609. [Google Scholar]
- Ajayi OO, Femi-Ola TO. Evaluation of lignocellulosic enzymes profile of Pleurotus sajor-caju grown on selected agro-industrial wastes. Am J Microbiol Res. 2019;7(1):1–11. [Google Scholar]
- Akpinar M, Urek RO. Induction of fungal laccase production under solid state bioprocessing of new agroindustrial waste and its application on dye decolorization. 3 Biotech. 2017;7:98. doi: 10.1007/s13205-017-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgher M, Iqbal HMN. Characterization of a novel manganese peroxidase purified from solid state culture of Trametes versicolor IBL-04. BioResources. 2011;6(4):1–14. [Google Scholar]
- Bodke PM, Senthilarasu G, Raghukumar S. Screening diverse fungi for laccases of varying properties. Indian J Microbiol. 2012;52(2):247–250. doi: 10.1007/s12088-011-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupul WC, Abarca GH, Carrera DM, Vazquez RR. Enhancement of ligninolytic enzyme activities in a Trametes maxima–Paecilomyces carneus co-culture: key factors revealed after screening using a Plackett-Burman experimental design. Electron J Biotechnol. 2014;17(3):114–121. doi: 10.1016/j.ejbt.2014.04.007. [DOI] [Google Scholar]
- Fonseca MI, Shimizu E, Zapata PD, Villalba LL. Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina) Enzyme Microb Technol. 2010;46(6):534–539. doi: 10.1016/j.enzmictec.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Ghebreslasie Z, Premjet D, Permjet S. Screening of fungi producing ligninolytic enzymes. KKU Res J. 2016;21(2):200–209. [Google Scholar]
- Hawksworth DL. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res. 2001;105(12):1422–1432. doi: 10.1017/S0953756201004725. [DOI] [Google Scholar]
- Kalmis E, Azbar N, Kalyoncu F. Agar plate screening for textile dye decolorisation by white rot fungi Pleurotus species (Pleurotus cornucopiae var. citrinopileatus, P. djamor, P. eryngii, P. ostreatus and P. sajor-caju) Fresen Environ Bull. 2007;16:1309–1314. [Google Scholar]
- Kaosol T, Sohgrathok N. Enhancement of biogas production potential for anaerobic co-digestion of wastewater using decanter cake. Am J Agric Biol Sci. 2012;7(4):494–502. doi: 10.3844/ajabssp.2012.494.502. [DOI] [Google Scholar]
- Kinnunen AJ, Maijala PM, Järvinen PP, Hatakka AI. Improved efficiency in screening for lignin-modifying peroxidases and laccases of basidiomycetes. Curr Biotechnol. 2017;6(2):105–115. doi: 10.2174/2211550105666160330205138. [DOI] [Google Scholar]
- Kumar R, Kaur J, Jain S, Kumar A. Optimization of laccase production from Aspergillus flavus by design of experiment technique: partial purification and characterization. J Genet Eng Biotechnol. 2016;14:125–131. doi: 10.1016/j.jgeb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Shi S, Gao Y, Qin M. Fiber modification of kraft pulp with laccase in presence of methyl syringate. Enzyme Microb Technol. 2009;44:89–95. doi: 10.1016/j.enzmictec.2008.10.014. [DOI] [Google Scholar]
- Machado KMG, Matheus DR. Biodegradation of remazol brilliant blue R by ligninolytic enzymatic complex produced by Pleurotus ostreatus. Braz J Microbiol. 2006;37:468–473. doi: 10.1590/S1517-83822006000400013. [DOI] [Google Scholar]
- Nakasone KK, Lindner DL. Taxonomy of Pseudolagarobasidium (Polyporales, Basidiomycota) Fungal Divers. 2012;55:155–169. doi: 10.1007/s13225-012-0161-1. [DOI] [Google Scholar]
- Othman AM, Elsayed MA, Elshafei AM, Hassan MM. Application of central composite design as a strategy to maximize the productivity of Agaricus bisporus CU13 laccase and its application in dye decolorization. Biocatal Agric Biotechnol. 2018;14:72–79. doi: 10.1016/j.bcab.2018.02.008. [DOI] [Google Scholar]
- Pelaez F, Martinez MJ, Martinez AT. Screening of 68 species of basidiomycetes for enzymes involved in lignin degradation. Mycol Res. 1995;99:37–42. doi: 10.1016/S0953-7562(09)80313-4. [DOI] [Google Scholar]
- Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–325. doi: 10.1093/biomet/33.4.305. [DOI] [Google Scholar]
- Rasak MNA, Ibrahim MF, Yee PL, Hassan MA, Abd-Aziz S. Utilization of oil palm decanter cake for cellulose and polyoses production. Biotechnol Bioprocess Eng. 2012;17:547–555. doi: 10.1007/s12257-011-0590-9. [DOI] [Google Scholar]
- Ries LN, Beattie SR, Espeso EA, Cramer RA, Goldman GH. Diverse regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics. 2016;203:335–352. doi: 10.1534/genetics.116.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongwang C, Polprasert S, Kanchanasuta S. Effect of partial ozonation and thermal pretreatment on biogas production from palm oil decanter cake. Chem Eng Trans. 2017;57:1987–1992. [Google Scholar]
- Schneider WDH, Fontana RC, Mendonca S, de Siqueira FG, Dillon AJP, Camassola M. High level production of laccases and peroxidases from the newly isolated white-rot basidiomycete Marasmiellus palmivorus VE111 in a stirred-tank bioreactor in response to different carbon and nitrogen sources. Process Biochem. 2018;69:1–11. doi: 10.1016/j.procbio.2018.03.005. [DOI] [Google Scholar]
- Senthivelan T, Kanagaraj J, Panda RC, Narayani T. Screening and production of a potential extracellular fungal laccase from Penicillium chrysogenum: media optimization by response surface methodology (RSM) and central composite rotatable design (CCRD) Biotechnol Rep. 2019;23:e00344. doi: 10.1016/j.btre.2019.e00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MLC, Souza VB, Santos VS, Kamida HM, Vasconcellos-Neto JRT, Goes-Neto A, Koblitz MGB. Production of manganese peroxidase by Trametes villosa on unexpensive substrate and its application in the removal of lignin from agricultural wastes. Adv Biosci Biotechnol. 2014;5:1067–1077. doi: 10.4236/abb.2014.514122. [DOI] [Google Scholar]
- Stajic M, Persky L, Friesem D, Hadar Y, Wasser SP, Nevo E. Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzyme Microb Technol. 2006;38:65–73. doi: 10.1016/j.enzmictec.2005.03.026. [DOI] [Google Scholar]
- Suwannasai N, Martín MP, Phosri C, Sihanonth P, Whalley AJS, Spouge JL. Fungi in Thailand: a case study of the efficacy of an ITS barcode for automatically identifying species within the Annulohypoxylon and Hypoxylon genera. PLoS One. 2013;8(2):e54529. doi: 10.1371/journal.pone.0054529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téllez-Téllez M, Fernández FJ, Montiel-González AM, Sánchez C, Díaz-Godínez G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl Microbiol Biotechnol. 2008;81:675–679. doi: 10.1007/s00253-008-1628-6. [DOI] [PubMed] [Google Scholar]
- Thakur S, Gupte A. Optimization and hyper production of laccase from novel agaricomycete Pseudolagarobasidium acaciicola AGST3 and its application in in vitro decolorization of dyes. Ann Microbiol. 2015;65:185–196. doi: 10.1007/s13213-014-0849-4. [DOI] [Google Scholar]
- Tien M, Kirk TK. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1998;161:238–249. doi: 10.1016/0076-6879(88)61025-1. [DOI] [Google Scholar]
- Vaithanomsat P, Sangnam A, Boonpratuang T, Choeyklin R, Promkiam-on P, Chuntranuluck S, Kreetachat T. Wood degradation and optimized laccase production by resupinate white-rot fungi in northern Thailand. BioResources. 2015;8(4):6342–6360. [Google Scholar]
- Venkateswarulu TC, Prabhakar KV, Kumar RB, Krupanidhi S. Modeling and optimization of fermentation variables for enhanced production of lactase by isolated Bacillus subtilis strain VUVD001 using artificial neural networking and response surface methodology. 3 Biotech. 2017;7:186. doi: 10.1007/s13205-017-0802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Xu H, Gua M-Y, Gao Y-H, Bai X-H, Zhou X-W. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol. 2017;17:19. doi: 10.1186/s12896-017-0338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.