Abstract
Persons with schizophrenia exhibit sensitivity to stress and negative affect (NA), both strongly correlated with poor functional outcome. This theoretical review suggests that NA reflects a “fragile brain,” ie, vulnerable to stress, including events not experienced as stressful by healthy individuals. Based on postmortem evidence of altered gamma-aminobutyric acid (GABA) function in parvalbumin positive interneurons (PVI), animal models of PVI abnormalities and neuroimaging data with GABAergic challenge, it is suggested that GABAergic disruptions weaken cortical regions, which leads to stress vulnerability and excessive NA. Neurocircuits that respond to stressful and salient environmental stimuli, such as the hypothalamic-pituitary-adrenal axis and the amygdala, are highly dysregulated in schizophrenia, exhibiting hypo- and hyper-activity. PVI abnormalities in lateral prefrontal cortex and hippocampus have been hypothesized to affect cognitive function and positive symptoms, respectively; in the medial frontal cortex (dorsal anterior cingulate cortex and dorsal medial prefrontal cortex), these abnormalities may lead to vulnerability to stress, NA and dysregulation of stress responsive systems. Given that postmortem PVI disruptions have been identified in other conditions, such as bipolar disorder and autism, stress vulnerability may reflect a transdiagnostic dimension of psychopathology.
Keywords: schizophrenia, emotion, anterior cingulate cortex, benzodiazepine, amygdala, HPA axis
Introduction
Clinicians working with schizophrenia inevitably notice that their patients often exhibit emotional fragility and sensitivity to minor stresses. These negative affective states appear in contrast to the emotional deficits first noted by Kraepelin and now incorporated into the concept of negative symptom or deficit schizophrenia.1,2 Overactive emotions were also noted by Kraepelin, but particularly emphasized by Bleuler, who described “an over-sensitivity, so that the patients consciously and deliberately isolate themselves in order to avoid everything that may arouse affects.”3 In the mid-twentieth century, Meehl described the tendency toward negative affective states (aversive drift) as a fundamental process in the schizophrenia prodrome.4 Deficits in emotional expression are a core phenomenological construct of negative symptoms,5 but contemporary work recognizes that the absence of external expression of emotion sometimes conceals the presence of negative affective (NA) states.6–8 The purpose of this review is to address this phenomenon of “stress sensitivity” in schizophrenia, focusing on the intrinsic vulnerability to stress and the tendency to exhibit NA in settings not ordinarily considered stressful. In what follows, we will review studies and put forth the hypothesis that inhibitory, gamma-aminobutyric acid (GABA)ergic interneurons play critical roles in determining stress vulnerability and the tendency to experience NA in schizophrenia.
Vulnerability and the Tendency to Negative Affect
Stress-Vulnerability in Schizophrenia
The stress-vulnerability, or “stress-diathesis,” framework was first formulated for schizophrenia over 40 years ago.9,10 Biologically, stress is usually conceptualized as an “allostatic load,” where “allostasis” refers to the adaptive process of maintaining stable autonomic, immune, and hypothalamic-pituitary-adrenal (HPA) axis function in response to environmental challenges.11,12 Schizophrenia patients are highly sensitive to stress,13–22 and this review will build upon this prior work. Our focus will be on the vulnerability itself, which we define as the tendency to experience NA, and which we attribute to a low threshold for what is experienced as stressful.
While the stress-vulnerability framework has been very useful, it may place an undue emphasis on antecedent events as potential triggers of the disorder. It is important to distinguish schizophrenia from post-traumatic stress disorder (PTSD), although there is comorbidity of the 2 diagnoses,23 and some evidence suggests that psychosis can emerge secondary to PTSD.24,25 However, careful examination of reported life events has shown that rather than having more adverse life events, schizophrenia patients experience events as more stressful than healthy subjects.13,26 In clinical high risk groups, the evidence for stressful events preceding the new onset of psychosis is weak to nonexistent, according to a recent review.27 On the other hand, in-the-moment studies of patient experience, using experience sampling methodologies (ESM), show that, through the course of the day, patients experience more negative emotions, and less positive emotions, than control subjects.28–30 A recent meta-analysis found, relative to healthy controls, robust mean effect sizes of 0.84 for greater NA, and −0.75 for less positive affect.28 Importantly, first-degree relatives of patients also show more NA,31 suggesting that this tendency to NA may have a genetic component. Trait personality measures of persons with schizophrenia show high levels of NA, such as high scores on neuroticism scales, in addition to reduced levels of positive affectivity.32–34 In a laboratory setting, schizophrenia patients tend to rate positive and neutral stimuli as being more negative, whereas they give largely equivalent ratings of negative stimuli as control subjects,7 indicating an intact capacity to appraise stimuli,6,35–39 although some have suggested that a subtype of patients may have a more negative experience of negative stimuli.40 Taken together, the data demonstrate that persons with schizophrenia have more experience of NA than healthy persons.
The concept of a tendency to experience NA takes several forms. It is similar to Meehl’s notion of “aversive drift”—“intensely negative affective states not clinically identifiable as variants of the commonly recognized aversive emotions.”41 A patient account of living with schizophrenia illustrates the emergence of NA through the course of daily life, wherein vulnerability can be seen as a limited capacity to engage with the environment:
People like my husband who are rich with mental health are blessed with 100 pennies, while I, having a thought disorder, only get 20 pennies…. Before I commit myself to almost any sort of action, I often silently calculate: how much will this activity drain me? Every sundry task costs…. If I am not careful, I can spend all my pennies by 12 noon…. The world turns dark and threatening, and all my earthly delights and good fortune are forgotten.42
This writer, in noting that “Every sundry task costs,” captures an alternative way to think about stress vulnerability—that it reflects a reduced capacity for engaging with the environment. With reduced capacity, the threshold for what makes an event stressful is lowered. Why NA emerges when a limited processing capacity is exceeded is a critical question for future research, which this review seeks to stimulate (table 1).
Table 1.
Questions for Future Research
| Questions | Possible Methods of Investigation |
|---|---|
| 1) If stress vulnerability can be thought of as reduced capacity to handle events, why does NA emerge when this capacity is exceeded? Are there are other signs that a limited capacity has been exceeded, besides NA? What are the neurocircuits which carry this load? | Animal models, neuroimaging studies, stress and emotion challenges, experience sampling methodologies, computational modeling |
| 2) What are the developmentally sensitive periods for the emergence of NA associated with the development of psychosis? | Longitudinal studies including critical developmental periods, animal models |
| 3) What is the relationship between vulnerability and cognition? Between vulnerability and negative symptoms? | Clinical phenotyping and analytics, eg, network analysis, structural equation modeling, causal modeling, etc. |
| 4) If cognitive function is improved, can NA be properly regulated? | Cognitive behavior therapy for psychosis, cognitive training, pharmacologic cognitive enhancers |
| 5) How can we separate NA related to a GABAergic (PVI) deficit from other causes of NA? | Multimodal neuroimaging and EEG with pharmacologic challenge; animal models |
| 6) How much of the vulnerability in schizophrenia is shared across other disorders? | Transdiagnostic studies (including postmortem samples), genetic markers (polygenic risk scores) |
A Model of Vulnerability and Negative Affect
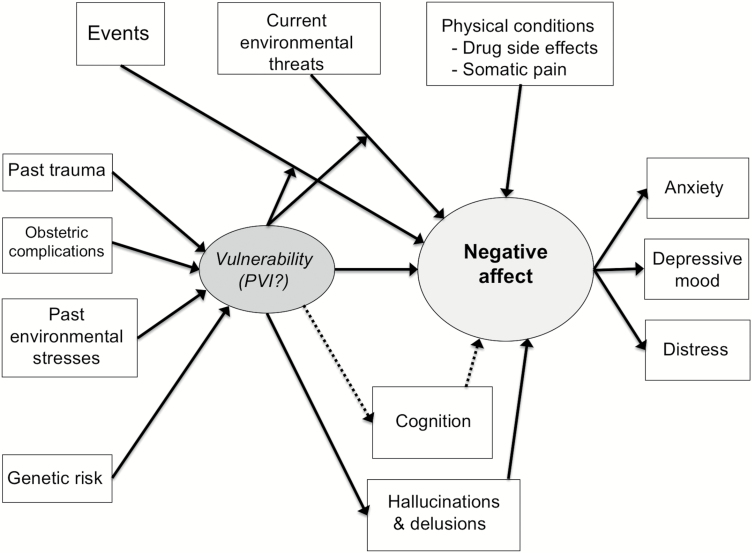
Our conceptual model is depicted in figure 1. Stress vulnerability, or simply “vulnerability,” is conceptualized as a latent construct, driving NA, itself a latent construct comprised of the measurable phenotypes of anxiety, depressive mood, and general distress. NA might arise in the absence of external triggers, although it is difficult to distinguish spontaneously arising NA from NA triggered by an event. Some events are stressful enough that they cause NA, regardless of an underlying vulnerability, whereas other events, eg, involving novelty or requiring adaptation, strain a limited capacity to process information, and lead to NA, moderated by vulnerability. Not all NA reflects vulnerability, especially in physical conditions (somatic pain, drug side effects). Current environmental threats, such as poverty and discrimination, may directly cause NA, and this causal relationship may be moderated by the vulnerability.
Fig. 1.
Relationship between negative affect (NA) and stress vulnerability. Solid lines indicate positive relationships, while dotted lines indicate negative relationships. Vulnerability moderates the influence of events and environmental threats to produce NA. An event, which could be a benign social interaction, could turn into a stressful event through this moderation. Cognition and hallucinations/delusions are also posited in a causal relationship with the underlying GABAergic mechanism, but through different neurocircuitry than NA (figure 2). PVI, parvalbumin positive interneurons.
The model also includes etiological factors associated with schizophrenia, which may cause deficits in GABAergic interneurons (discussed below). Environmental stresses in early life and adolescence, such as social deprivation, urbanicity, and toxin exposures,19,21,43,44 as well as obstetric complications,44–46 have been linked to schizophrenia and the risk of developing a psychosis. Among environmental stresses, childhood trauma stands out as a risk factor, occurring in up to one-third of patients with schizophrenia,47–50 and childhood trauma has been linked to NA and emotional problems in adolescence and adulthood.51,52 Some stresses may be chronic and more subtle, but persistent enough to wear down adaptive mechanisms (inducing vulnerability) and directly causing negative affect.53 Genetics is another possible etiological factor. Polygenic risk scores for schizophrenia are associated with NA in adolescence and adulthood54–56; and genome-wide association studies57 and analysis of copy number variants58 have strongly implicated GABAergic signaling in schizophrenia. It is beyond the scope of this review to go into each one of these potential causative factors, and we do not mean to imply that these factors do not act on the clinical phenotype through other pathways, leading to symptoms besides NA. Our emphasis here is on NA.
Specifically, we hypothesize that this vulnerability is linked to abnormalities in GABAergic, parvalbumin positive interneurons (PVI), in conjunction with their relationship to excitatory pyramidal cells. It has been suggested that damage to these vulnerable, fast-spiking interneurons is a final common pathway of many mental illnesses besides schizophrenia, including bipolar disorder, autism, and depression, mediated by converging influences of peri-natal stress and inflammation, all triggering oxidative stress.59 Here, our model becomes trans-diagnostic, as implied in the previous paragraph, where risk factors for schizophrenia, such as early life stress, are also risk factors for other psychiatric disorders.51,52 In addition to their vulnerability to a variety of insults, PVI neurons mature later in life,60 and they are highly sensitive to stress early in life,61 which might be one explanation for developmental periods when traumatic events can have more of an adverse impact on emotion regulation in adulthood.62,63
For the purposes of this review, we will limit our focus to schizophrenia. Many of the studies we cite include schizoaffective patients, but there has been little attempt to differentiate NA in schizoaffective disorder and schizophrenia, aside of the obvious higher incidence of NA (depressive symptoms) in schizoaffective disorder.64 Although we see the proposed GABAergic mechanisms of vulnerability and NA as transdiagnostic, most of the relevant research has occurred in schizophrenia; hence, our focus.
Negative Affect and Schizophrenia Symptoms
A diagnosis of schizophrenia does not require the presence of anxiety or depressive symptoms, but NA is frequently present. Factor analytic studies of clinician-administered rating scales, such as the Positive and Negative Symptom Scale (PANSS), consistently show the emergence of a depression/anxiety factor, along with positive symptoms, negative symptoms, disorganization, and excitement.65–67 Depression occurs in 30%–50% of schizophrenia patients,68,69 and social phobia in 13%–36% of schizophrenia cases,70–72 with as many as 60% of patients experiencing some form of social anxiety.73 NA is associated with poor social functioning and poor quality of life, after controlling for positive and negative symptoms,32,64,74,75 suggesting that it is an important target for interventions.
In our model, we indicate that this GABAergic vulnerability is also linked to cognitive dysfunction and positive symptoms (hallucinations and delusions). While NA appears as an independent component of the clinical phenotype, it is also true that positive symptoms, eg, persecutory delusions causing fear, can directly contribute to NA. The causality may go both ways, as NA/vulnerability could also exacerbate positive symptoms. Stressful events have been linked with variation in positive symptoms,13,76 although sample sizes have been small and most studies have been cross-sectional. Another possibility is that PVI deficits may directly lead to positive symptoms, as suggested by animal models and theoretical considerations, which we discuss below.
The question of to what degree NA might reflect cognitive deficits and a failure to inhibit NA remains an unsettled area of study. Limited work has been done in this area. An inverse relationship between neurocognition and stress sensitivity was reported in one study,77 whereas another group found that poor memory performance was directly related to more NA.78 Studies have generally found that NA is an independent predictor of poor functional outcome, after controlling for neurocognition,64,75,79,80 but this does not preclude that cognition might interact with NA, as some have found.79 Indeed, as we suggest, PVI dysfunction may be a “third variable” that could also cause the association between impaired cognition and NA.
Negative symptoms have a more complicated relationship with NA, which is not yet worked out sufficiently to be included in figure 1. As mentioned above, factor analytic studies have pulled out NA measures distinct from negative symptoms.65–67 A recent categorial formulation distinguished a distress subtype, distinct from the deficit (enduring negative symptom) subtype in chronic schizophrenia patients,81 and a network analysis of negative symptoms found an inverse relationship with NA.82 Using continuous measures, others have found higher negative symptoms associated with reduced NA29,83 and less reactivity to negative stimuli,84,85 raising the intriguing possibility that affective flattening and amotivation might be an adaptation to NA. On the other hand, studies of anhedonia have suggested that NA might contribute to anhedonia by reducing pleasure seeking behavior,86 which could reflect a form of secondary negative symptoms, and that ratings of anhedonia, specifically anticipatory pleasure, is reduced by NA.87,88 As with cognition, the relationship between NA and negative symptoms, primary and secondary, is an area for future research (table 1).
Evidence for Disruption of PVI in Schizophrenia
Postmortem Findings in GABAergic Systems
Postmortem studies have demonstrated abnormalities in GABAergic systems, prominently implicating PVI. Findings have included reductions in parvalbumin expression,89 reductions in the synthetic enzyme for GABA, glutamic acid decarboxylase (GAD67),90–93 alterations in pre- and postsynaptic terminals,94 and reduction of the peri-neuronal nets surrounding PVI.95 Reductions in mRNA levels of GABAergic-related neuropeptides have also been noted, such as neuropeptide Y, somatostatin, and cholecystokinin.96 Abnormal PVI findings have been observed throughout the cortex97–99 and hippocampus.100,101 PVI cell counts are not reduced in the cortex,102–104 while some have found reduced cell counts of PVI in hippocampus105,106 and layers II/III of the anterior cingulate gyrus.107 Evidence suggests that the changes are not due to extraneous factors, such as age, sex, or medication. Lastly, consistent with our transdiagnostic formulation, GAD67/PVI deficits have also been found in bipolar disorder,92,108,109 although not as often in unipolar depression.92,110
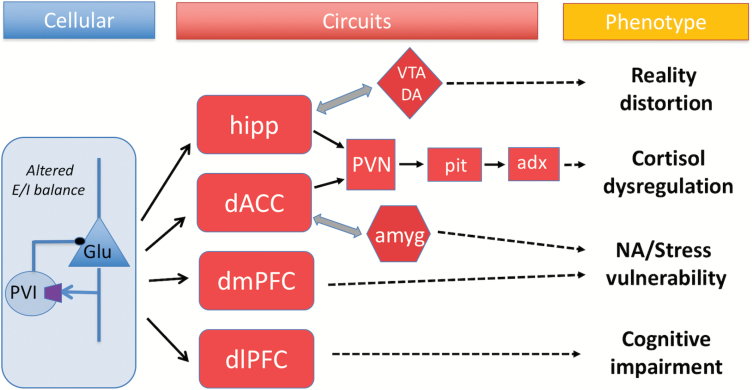
The PVI findings have generated several pathophysiological models for schizophrenia. One type of fast-spiking PVI, the basket cells (PVBC), which synapse on the cell bodies of layer III, excitatory pyramidal cells, have been implicated in cortical gamma oscillations.111–113 These oscillations are associated with integrative cortical functions such as attention and working memory in prefrontal cortex,114–116 and they are abnormal in schizophrenia, suggesting a possible pathogenic mechanism of cognitive deficits.117,118 One of the challenges in the field has been sorting out primary from secondary and compensatory changes. A primary deficit in PVBC might lead to disinhibition of pyramidal cell firing; alternatively, PVBC changes might be secondary. For example, a reduction in the dendritic spine density of pyramidal neurons could lead to reduced excitation, followed by compensatory adjustments to re-set the balance between excitation and inhibition, eg, reduced GAD67 to reduce GABA release, followed by increased postsynaptic GABA receptors (GABAR).91,119 GABAergic abnormalities have also been linked to hypofunction of the excitatory N-methyl-d-aspartic acid glutamate receptors (NMDAR), located on GABAergic interneurons.120 For PVBC synapsing on hippocampal pyramidal cells, this could lead to hippocampal hyperactivity, increased dopaminergic activity and the positive symptoms of psychosis.119,121 One intriguing idea around the re-setting of excitatory/inhibitory (E/I) balance is that the overall capacity of the system to process information would be reduced.91 Importantly, the PVI findings have been noted across diverse areas of cortex, as well as subcortical structures, potentially implicating diverse behaviors (figure 2).
Fig. 2.
Depending upon the cortical or subcortical structure affected, diverse phenotypic manifestations of GABAergic abnormalities in parvalbumin positive interneurons (PVI), working in concert with excitatory neurons to maintain proper excitatory/inhibitory (E/I) balance, may occur. hipp, hippocampus; VTA DA, ventral tegmental dopamine; PVN, paraventricular nucleus of hypothalamus; Pit, pituitary; Adx, adrenal cortex; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; dACC, dorsal anterior cingulate cortex; amyg, amygdala; ctx, cortex; Glu, glutamate.
Animal Models of PVI Deficits
Prior theoretical work has focused on cognitive or positive symptoms, but there has been a surprising neglect of NA in the literature, in spite of the fact that animal models have implicated affect dysregulation. PVI disruption occurs in several developmental rodent models, including neonatal ventral hippocampal lesions,122 maternal immune activation,123–125 and prenatal infusion of methylazoxymethanol (MAM).126,127 Reduced PVI immunoreactivity has been demonstrated in isolation-reared rats,128 and in adult rats exposed to chronic stress through social isolation.129,130 Mice exposed to immune activation in utero exhibit increased anxiety behaviors, as well as impaired set shifting behavior, but intact working memory.123 MAM-treated rats exhibit heightened anxiety-related behavior as adults, increased firing of basolateral amygdala (BLA) neurons and larger increase in BLA theta power in response to conditioning stimuli, as well as reduced gamma-band response to a conditioned stimulus in the medial PFC.131 Importantly, peripubertal administration of diazepam, which potentiates GABA receptors, reduces the anxiety, normalizes the BLA neuron firing rates and reduces the abnormally high theta power increase in the BLA seen by conditioning stimuli.127 MAM-treated rats also exhibit increased spontaneous DA firing, which is also reduced by diazepam.
Another approach to modeling GABA dysfunction has directly targeted GAD1 (the enzyme which produces the GAD67 protein), and these models also show affective dysregulation. A knock-down mouse targeting PV+ neurons showed impaired fear extinction, along with impaired sensori-motor gating and increased novelty seeking.132 With a different GAD1 knock-down mouse model, another group reported increased anxiety-related behavior in the open field test and light/dark box, as well as reduced effort-based behaviors, but intact spatial working memory and normal sensorimotor gating.133 Overall, the behavioral phenotypes are not entirely consistent,134,135 which may reflect different populations of GAD1-containing neurons besides PV+ interneurons, eg, cholecystokinin or somatostatin. While rodent models cannot be expected to capture all aspects of schizophrenia, it is notable that when PV+ neurons are targeted in a way that parallels the postmortem findings in the psychosis spectrum, an affective phenotype emerges.
Neurocircuits Implicated in Stress and Affect in Schizophrenia
Stress Response Systems in Schizophrenia
A considerable amount of research has focused on the physiological stress response in schizophrenia. Brain responses to stress-inducing stimuli activate several key systems in the brain, including the hypothalamic-pituitary-adrenal (HPA) axis and the sympatho-adrenal medullary system.136,137 The latter is more associated with fast, short-term responses to stress, whereas the former has been the focus of most of the stress research in schizophrenia.22,138–143 The HPA axis involves multiple levels of control and feedback, most easily studied by measuring the major secretagogue of the adrenal cortex, cortisol. Schizophrenia shows a complex picture of a dysregulated system. Baseline cortisol levels appear to be elevated in early schizophrenia, particularly in unmedicated cohorts, and possibly in at-risk subjects.22,141–143 Studies of the cortisol awakening response (CAR) are fewer and show more inconsistencies, but a recent meta-analysis concluded that the CAR is blunted in schizophrenia, although not different in at-risk subjects.139 Similarly, challenge studies with psychosocial stressors report a blunted cortisol response,142 although a more recent review reached the opposite conclusion, noting similar levels of subjective stress responses in psychosocial challenge studies, except for patients early in the illness.138 While cortisol measures have generally not shown consistent associations with symptoms and equivocal evidence for predicting conversion in high risk subjects,22 a recent systematic review found somewhat consistent correlates with anxiety and stress-intolerance measures in at-risk subjects.140 In general, the complexity of the findings likely reflects multiple, difficult-to-control factors, such as heterogeneity in patient samples, differences in measurement, variable medication status and the multiple upstream pathways that regulate the HPA axis.
Given these complexities, it is important to consider these upstream influences. The paraventricular nucleus (PVN) of the hypothalamus releases corticotrophin releasing hormone (CRH), which triggers the release of adrenocorticotropin releasing hormone in the pituitary, in turn triggering cortisol release by the adrenal cortex. This stress cascade is modulated by projections to the PVN from the hippocampus, amygdaloid nuclei, and medial frontal cortex,137 all regions implicated in emotion.144 Relatively little work in schizophrenia has addressed whether or not pathology exists at level of the hypothalamus, pituitary or adrenal gland, but an abundance of data demonstrates abnormalities in these limbic and cortical structures, as we discuss below.
Another neural system relevant for the stress response and centrally implicated in the pathophysiology of schizophrenia is dopamine. Contemporary accounts of the dopamine hypothesis increasingly suggest that dopamine dysregulation is a downstream consequence of dysregulation in other systems, eg, falsely signaling salient states and triggering dopamine release.145,146 Stress elevates striatal dopamine release in animals147 and humans,148and an impaired ability to regulate psychosocial stress has been suggested as one of the upstream triggers leading to hyper-dopaminergia and positive symptoms in schizophrenia.149,150 One possible locus of this failure is the hippocampus, which is structurally and functionally abnormal in schizophrenia.151–155 It is possible that hypercortisolemia causes the volume loss observed in the hippocampi of schizophrenia, leading to hippocampal dysfunction and dopamine dysregulation.156 Animal models suggest that hippocampal GABA-glutamatergic circuits may send aberrant signals to midbrain dopaminergic neurons, leading to hyperactivity.157,158 While such a mechanism could contribute to NA and positive symptoms, the fact that these 2 clinical phenomena are dissociable suggests that another mechanism might be implicated. For example, the medial frontal cortex, which also regulates the HPA axis,137 may be another critical upstream structure responsible for stress vulnerability.
Affective Neurocircuits in Schizophrenia
Neuroimaging studies examining brain responses to salient emotional stimuli provide clues about neurocircuits relevant for stress vulnerability. A large body of work has implicated amygdala dysfunction in schizophrenia.159–161 There is heterogeneity in the findings, and a pattern of dysregulation is more apparent than simple reductions or increases in reactivity. For example, while meta-analytic studies have repeatedly identified reduced activation to emotional face stimuli,160,161 the amygdala may be hyperactive at baseline and activated by neutral stimuli that evoke less response from healthy subjects.160 Measures of cerebral perfusion—resting activity not measurable by fMRI blood oxygenation level dependent (BOLD)—show increased amygdala activity in the absence of an emotional challenge.162–164 The findings of reduced activity change with BOLD may reflect reduced dynamic range of measurable signal change, or a shift in the response of the amygdala to salient stimuli, in general.
The amygdala has strong connectivity with medial frontal structures, including the dorsal anterior cingulate cortex (dACC), rostral ACC, and ventromedial prefrontal cortex (vmPFC), as well as, to a lesser extent, the dorsal medial prefrontal cortex (dmPFC).165,166 Several studies have found reduced connectivity in schizophrenia between the amygdala and medial frontal regions while processing emotional stimuli.167–169 Neuroimaging studies show that medial frontal regions are associated with HPA axis activity,170 regulate emotional responses and process social information, particularly around agency and theory of mind in the dmPFC.144,171,172 Interestingly, when a GABA antagonist is infused in the prelimbic cortex (PL) of rats, homologous to the dACC in humans, the rats respond to cues not previously paired with an aversive stimulus as if those cues predicted the aversive stimulus.173 PL is also associated with turning down the HPA axis, and disrupted PL is associated with HPA axis dysregulation.137 Given that NA very frequently emerges in social situations, impaired medial frontal-amygdala connectivity could be one of the neurocircuit substrates of stress vulnerability, including dysregulation of the HPA axis.
It is noteworthy that both the HPA axis and the amygdala, neural systems that are tuned to detect salient stimuli and mobilize appropriate responses, have been observed as both hypo- and hyper-responsive in schizophrenia. We have suggested this reflects not primary deficits in these systems, but instead, dysregulation by the upstream, cortical and subcortical circuitry that regulate them, particularly in the mPFC and dACC (figure 2). A meta-analysis of emotion perception in schizophrenia showed reduced activation during emotion perception in the dACC, medial prefrontal cortex (mPFC), visual processing areas, dorsolateral prefrontal cortex (dlPFC), thalamus and caudate, along with increased activation in posterior regions not typically activated by emotion probes.161 A meta-analytic study of theory of mind showed hypoactivation in the mPFC,174 and 2 large meta-analyses have identified prominent structural deficits in mPFC and dACC.175,176 Thus, these cortical regions stand out as possible loci of dysfunction involved in vulnerability.
Functional Studies of GABA in Schizophrenia
A dearth of in vivo studies of GABA function in humans has limited the understanding of GABAergic activity and behavior. Neuroimaging studies of GABAR binding and GABA levels have been equivocal and difficult to interpret, and a full discussion of these area is beyond the scope of the present review (see refs.177,178).
Pharmacologic challenge studies can provide insight into dynamic GABA function.178,179 For instance, we gave schizophrenia patients and healthy comparison subjects intravenous bolus infusions of lorazepam (LRZ) and saline (SAL) in a within-subjects, cross-over design to probe GABAergic activity with fMRI while subjects viewed salient emotional stimuli.178 In addition to an expected effect of reduced BOLD signal in occipital cortex, we found group by drug interactions in the fusiform gyrus, right superior frontal gyrus and dmPFC. Schizophrenia patients showed an increased BOLD signal to LRZ challenge, rather than the decreased signal exhibited by the comparison group, and more positive BOLD change after LRZ directly associated with greater NA. By potentiating GABA receptors with LRZ, the challenge paradigm revealed dysregulated dynamics of GABA receptors and the excitatory pyramidal cells with which they interact in the cortex, a dysregulation that might reflect the PVI disruptions found in postmortem data. Of course, the relatively crude BOLD signal does not enable the cellular resolution necessary to test this hypothesis, but it does show an abnormality consistent with these postmortem findings. It also shows the link between trait-level NA and GABA dynamics. Localization of this effect to dmPFC and occipital cortex is consistent with neuroimaging findings of abnormal emotion processing in schizophrenia.161,164,180–182
Treatment Implications
Treatment With GABAergic Agents
Suggestions to use GABAergic drugs to treat schizophrenia are not new.183–187 Benzodiazepines (BDZ) and valproic acid, which both potentiate GABA transmission, have been used clinically, usually as adjunctive agents to manage NA and agitation.188–190 Although a recent Cochrane review found no support for BDZ as effective augmentation agents in schizophrenia,191,192 the level of evidence was generally poor, and the authors could not conclude that BDZ did not help. In fact, they concluded there was support for the use of BDZ for acute agitation and anxiety in schizophrenia. Anxiety is a form of NA, and the fact that BDZ have utility in the treatment of schizophrenia, at all, is an important clue about underlying mechanisms. While BDZ are generally not regarded as antipsychotics, diazepam has been shown to reduce psychotic relapse in schizophrenia patients in a randomized clinical trial.193 Iomazenil, a partial inverse agonist of the benzodiazepine receptor, increases psychotic symptoms in schizophrenia patients,194 further implicating GABA mechanisms.
An intriguing clinical linkage between GABA and therapeutics can be seen in catatonic states, which often respond dramatically and rapidly to benzodiazepines.195,196 Catatonia patients often show very strong NA, sometimes described as being “immobilized by anxiety,”197,198 and reduced GABAergic binding has been identified in the right orbitofrontal cortex of patients in catatonic states.199 Northoff et al assessed the subjective experience of 22 patients with catatonia, after they had recovered from the acute state. They rated their experience of anxiety and feeling overwhelmed as comparable to patients with major depression. Catatonic patients who had an immediate response to lorazepam, a benzodiazepine, described more subjective distress than those who did not have an immediate response to lorazepam. Lastly, the fact that catatonia is often more characteristic of bipolar disorder than schizophrenia200 is very much in line with our formulation of stress vulnerability as a transdiagnostic facet of psychiatric phenomenology.
Studies of New GABAergic Agents
What are the implications of our model for new treatments? Benzodiazepines have limited efficacy and many downsides, such as poor selectivity for GABAR subtypes and high liability for abuse. The fact that they are not more effective in the treatment of schizophrenia likely reflects the complexity of GABA and its regulation, with multiple points of modulation. Subtype selective agents for GABAR have been examined. A study with an α–2/3 selective subtype for the GABAR initially showed promise, including the ability to enhance gamma activity linked to PVBC,201 only to fail in a larger, controlled trial.202 However, relative to chlorodiazepoxide, this compound only had 11% efficacy at α-2 GABAR.186 Other possibilities include α-5 selective drugs, which have shown promise in reducing DA hyperactivity in a rodent model.203 Another target is the Kv3.1 family of potassium channel,204 highly localized to PV+ neurons, and found to be deficient in neocortex of schizophrenia patients in postmortem work.205 Most importantly, if, as we argue, NA is a relevant clinical phenotype of GABAergic dysfunction, then pharmacologic strategies targeting GABA and PVI would do well to screen for effects on NA, in addition to the usual assays typically used for schizophrenia drugs. There is a growing recognition in the field that managing susceptibility to stress and reducing NA is a critical part of treating schizophrenia patients, particularly in the early phase of the illness and for people at high risk of psychosis.206,207
Conclusion
We have presented a model of stress vulnerability in schizophrenia, connecting this trait vulnerability with an overall tendency to experience NA. NA represents an independent symptom dimension of schizophrenia, although it interacts with other symptoms, and it is strongly associated with poor functional outcome. Our model proposes that GABAergic dysfunction, specifically of PVI, may be one of the root causes of the vulnerability. This hypothesis builds upon postmortem findings and animal models, but we recognize that a large gulf exists between these evidence domains and the clinical phenotype, and no data has yet made a direct link between PVI abnormalities observed in postmortem data and any clinical condition. As we mention at the outset, the postmortem findings cut across diagnoses, and we predict the relationship between PVI, vulnerability and NA is a transdiagnostic one. For example, there is a significant literature implicating GABAergic mechanisms in depression,208–210 and transdiagnostic studies will be required to determine how much overlap these mechanisms have with NA in schizophrenia. With emerging technologies and focused hypotheses, linkages between the molecular-cellular, circuit-level, and clinical phenotype should be able to parse PVI vulnerability from other forms of NA in schizophrenia, and other major psychiatric disorders.
Funding
This work was supported by the National Institute of Mental Health (MH101676[SFT], MH086701[SFT], MH108823[IFT], and MH082784[VE]) and the Boledovich Schizophrenia Research Fund.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–583. [DOI] [PubMed] [Google Scholar]
- 2. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 3. Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International University Press; 1911/1950. [Google Scholar]
- 4. Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 5. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol. 1993;102:507–517. [DOI] [PubMed] [Google Scholar]
- 7. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. 2013;9:409–433. [DOI] [PubMed] [Google Scholar]
- 9. Zubin J, Spring B. Vulnerability—a new view of schizophrenia. J Abnorm Psychol. 1977;86:103–126. [DOI] [PubMed] [Google Scholar]
- 10. Rosenthal D. Genetic Theory and Abnormal Behavior. New York: McGraw Hill; 1970. [Google Scholar]
- 11. McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 12. Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, eds. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. [Google Scholar]
- 13. Norman RM, Malla AK. Stressful life events and schizophrenia. I: a review of the research. Br J Psychiatry. 1993;162:161–166. [DOI] [PubMed] [Google Scholar]
- 14. Malla AK, Norman RM. Relationship of major life events and daily stressors to symptomatology in schizophrenia. J Nerv Ment Dis. 1992;180:664–667. [PubMed] [Google Scholar]
- 15. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. [DOI] [PubMed] [Google Scholar]
- 16. Walker EF, Diforio D. Schizophrenia: a neural diathesis–stress model. Psychol Rev. 1997;104:667–685. [DOI] [PubMed] [Google Scholar]
- 17. Jones SR, Fernyhough C. A new look at the neural diathesis–stress model of schizophrenia: the primacy of social-evaluative and uncontrollable situations. Schizophr Bull. 2007;33:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. [DOI] [PubMed] [Google Scholar]
- 19. Yuii K, Suzuki M, Kurachi M. Stress sensitization in schizophrenia. Ann N Y Acad Sci. 2007;1113:276–290. [DOI] [PubMed] [Google Scholar]
- 20. Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27:307–317. [DOI] [PubMed] [Google Scholar]
- 21. Mizrahi R. Social stress and psychosis risk: common neurochemical substrates? Neuropsychopharmacology. 2016;41:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191–218. [DOI] [PubMed] [Google Scholar]
- 23. Achim AM, Maziade M, Raymond E, Olivier D, Merette C, Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Compean E, Hamner M. Posttraumatic stress disorder with secondary psychotic features (PTSD-SP): diagnostic and treatment challenges. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braakman MH, Kortmann FA, van den Brink W. Validity of ‘post-traumatic stress disorder with secondary psychotic features’: a review of the evidence. Acta Psychiatr Scand. 2009;119:15–24. [DOI] [PubMed] [Google Scholar]
- 26. Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophr Res. 2005;75:363–374. [DOI] [PubMed] [Google Scholar]
- 27. Mayo D, Corey S, Kelly LH, et al. The role of trauma and stressful life events among individuals at clinical high risk for psychosis: a review. Front Psychiatry. 2017;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho H, Gonzalez R, Lavaysse LM, Pence S, Fulford D, Gard DE. Do people with schizophrenia experience more negative emotion and less positive emotion in their daily lives? A meta-analysis of experience sampling studies. Schizophr Res. 2017;183:49–55. [DOI] [PubMed] [Google Scholar]
- 29. Oorschot M, Lataster T, Thewissen V, et al. Emotional experience in negative symptoms of schizophrenia—no evidence for a generalized hedonic deficit. Schizophr Bull. 2013;39:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Myin-Germeys I, Delespaul PA, deVries MW. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull. 2000;26:847–854. [DOI] [PubMed] [Google Scholar]
- 31. Myin-Germeys I, Delespaul P, van Os J. Behavioural sensitization to daily life stress in psychosis. Psychol Med. 2005;35:733–741. [DOI] [PubMed] [Google Scholar]
- 32. Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull. 1998;24:413–424. [DOI] [PubMed] [Google Scholar]
- 33. Horan WP, Blanchard JJ. Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophr Res. 2003;65:125–137. [DOI] [PubMed] [Google Scholar]
- 34. Horan WP, Blanchard JJ. Emotional responses to psychosocial stress in schizophrenia: the role of individual differences in affective traits and coping. Schizophr Res. 2003;60:271–283. [DOI] [PubMed] [Google Scholar]
- 35. Herbener ES, Song W, Khine TT, Sweeney JA. What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res. 2008;98:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116:30–42. [DOI] [PubMed] [Google Scholar]
- 37. Quirk SW, Strauss ME, Sloan DM. Emotional response as a function of symptoms in schizophrenia. Schizophr Res. 1998;32:31–39. [DOI] [PubMed] [Google Scholar]
- 38. Sison CE, Alpert M, Fudge R, Stern RM. Constricted expressiveness and psychophysiological reactivity in schizophrenia. J Nerv Ment Dis. 1996;184:589–597. [DOI] [PubMed] [Google Scholar]
- 39. Earnst KS, Kring AM. Emotional responding in deficit and non-deficit schizophrenia. Psychiatry Res. 1999;88:191–207. [DOI] [PubMed] [Google Scholar]
- 40. Strauss GP, Herbener ES. Patterns of emotional experience in schizophrenia: differences in emotional response to visual stimuli are associated with clinical presentation and functional outcome. Schizophr Res. 2011;128:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meehl PE. Toward an integrated theory of schizotaxia, schizotypy, and schizophrenia. J Personal Disord. 1990;4:1–99. [Google Scholar]
- 42. Blair K. Ability and disability. Schizophr Bull. 2007;33:1260–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 44. Fusar-Poli P, Tantardini M, De Simone S, et al. Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65–75. [DOI] [PubMed] [Google Scholar]
- 45. Belbasis L, Köhler CA, Stefanis N, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137:88–97. [DOI] [PubMed] [Google Scholar]
- 46. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. [DOI] [PubMed] [Google Scholar]
- 47. Ruby E, Polito S, McMahon K, Gorovitz M, Corcoran C, Malaspina D. Pathways associating childhood trauma to the neurobiology of schizophrenia. Front Psychol Behav Sci. 2014;3:1–17. [PMC free article] [PubMed] [Google Scholar]
- 48. Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma—a critical review. Schizophr Bull. 2007;33:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonoldi I, Simeone E, Rocchetti M, et al. Prevalence of self-reported childhood abuse in psychosis: a meta-analysis of retrospective studies. Psychiatry Res. 2013;210:8–15. [DOI] [PubMed] [Google Scholar]
- 50. Misiak B, Krefft M, Bielawski T, Moustafa AA, Sąsiadek MM, Frydecka D. Toward a unified theory of childhood trauma and psychosis: a comprehensive review of epidemiological, clinical, neuropsychological and biological findings. Neurosci Biobehav Rev. 2017;75:393–406. [DOI] [PubMed] [Google Scholar]
- 51. Björkenstam E, Vinnerljung B, Hjern A. Impact of childhood adversities on depression in early adulthood: a longitudinal cohort study of 478,141 individuals in Sweden. J Affect Disord. 2017;223:95–100. [DOI] [PubMed] [Google Scholar]
- 52. Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. [DOI] [PubMed] [Google Scholar]
- 53. Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Br J Psychiatry. 2005;187:101–102. [DOI] [PubMed] [Google Scholar]
- 54. Sengupta SM, MacDonald K, Fathalli F, et al. Polygenic risk score associated with specific symptom dimensions in first-episode psychosis. Schizophr Res. 2017;184:116–121. [DOI] [PubMed] [Google Scholar]
- 55. Riglin L, Collishaw S, Richards A, et al. The impact of schizophrenia and mood disorder risk alleles on emotional problems: investigating change from childhood to middle age. Psychol Med. 2018;48:2153–2158. [DOI] [PubMed] [Google Scholar]
- 56. Riglin L, Collishaw S, Richards A, et al. Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: a population-based cohort study. Lancet Psychiatry. 2017;4:57–62. [DOI] [PubMed] [Google Scholar]
- 57. Devor A, Andreassen OA, Wang Y, et al. Genetic evidence for role of integration of fast and slow neurotransmission in schizophrenia. Mol Psychiatry. 2017;22:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pocklington AJ, Rees E, Walters JT, et al. Novel findings from CNVs implicate inhibitory and excitatory signaling complexes in schizophrenia. Neuron. 2015;86:1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steullet P, Cabungcal JH, Coyle J, et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang JM, Zhang J, Yu YQ, Duan S, Li XM. Postnatal development of 2 microcircuits involving fast-spiking interneurons in the mouse prefrontal cortex. Cereb Cortex. 2014;24:98–109. [DOI] [PubMed] [Google Scholar]
- 61. O’Donnell P. Cortical interneurons, immune factors and oxidative stress as early targets for schizophrenia. Eur J Neurosci. 2012;35:1866–1870. [DOI] [PubMed] [Google Scholar]
- 62. Alameda L, Golay P, Baumann PS, et al. Mild depressive symptoms mediate the impact of childhood trauma on long-term functional outcome in early psychosis patients. Schizophr Bull. 2017;43:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McGrath JJ, McLaughlin KA, Saha S, et al. The association between childhood adversities and subsequent first onset of psychotic experiences: a cross-national analysis of 23 998 respondents from 17 countries. Psychol Med. 2017;47:1230–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grove TB, Tso IF, Chun J, et al. Negative affect predicts social functioning across schizophrenia and bipolar disorder: findings from an integrated data analysis. Psychiatry Res. 2016;243:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peralta V, Cuesta MJ. Psychometric properties of the Positive and Negative Syndrome Scale (PANSS) in schizophrenia. Psychiatry Res. 1994;53:31–40. [DOI] [PubMed] [Google Scholar]
- 66. Lindenmayer JP, Grochowski S, Hyman RB. Five factor model of schizophrenia: replication across samples. Schizophr Res. 1995;14:229–234. [DOI] [PubMed] [Google Scholar]
- 67. Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophr Bull. 1990;16:537–545. [DOI] [PubMed] [Google Scholar]
- 68. Conley RR, Ascher-Svanum H, Zhu B, Faries DE, Kinon BJ. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr Res. 2007;90:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siris SG, Addington D, Azorin JM, Falloon IR, Gerlach J, Hirsch SR. Depression in schizophrenia: recognition and management in the USA. Schizophr Res. 2001;47:185–197. [DOI] [PubMed] [Google Scholar]
- 70. Kendler KS, McGuire M, Gruenberg AM, Walsh D. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry. 1995;152:755–764. [DOI] [PubMed] [Google Scholar]
- 71. Bermanzohn PC, Porto L, Arlow PB, Pollack S, Stronger R, Siris SG. Hierarchical diagnosis in chronic schizophrenia: a clinical study of co-occurring syndromes. Schizophr Bull. 2000;26:517–525. [DOI] [PubMed] [Google Scholar]
- 72. Pallanti S, Quercioli L, Hollander E. Social anxiety in outpatients with schizophrenia: a relevant cause of disability. Am J Psychiatry. 2004;161:53–58. [DOI] [PubMed] [Google Scholar]
- 73. Voges M, Addington J. The association between social anxiety and social functioning in first episode psychosis. Schizophr Res. 2005;76:287–292. [DOI] [PubMed] [Google Scholar]
- 74. Huppert JD, Weiss KA, Lim R, Pratt S, Smith TE. Quality of life in schizophrenia: contributions of anxiety and depression. Schizophr Res. 2001;51:171–180. [DOI] [PubMed] [Google Scholar]
- 75. Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL. Subjective and objective quality of life in schizophrenia. Schizophr Res. 2008;98:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C. Life events and psychosis: a review and meta-analysis. Schizophr Bull. 2013;39:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Myin-Germeys I, Krabbendam L, Jolles J, Delespaul PA, van Os J. Are cognitive impairments associated with sensitivity to stress in schizophrenia? An experience sampling study. Am J Psychiatry. 2002;159:443–449. [DOI] [PubMed] [Google Scholar]
- 78. Lysaker PH, Bell MD, Greig TC, Bryson GJ. Emotional discomfort and impairments in verbal memory in schizophrenia. Psychiatry Res. 2000;97:51–59. [DOI] [PubMed] [Google Scholar]
- 79. Nugent KL, Chiappelli J, Rowland LM, Daughters SB, Hong LE. Distress intolerance and clinical functioning in persons with schizophrenia. Psychiatry Res. 2014;220:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tso IF, Grove TB, Taylor SF. Emotional experience predicts social adjustment independent of neurocognition and social cognition in schizophrenia. Schizophr Res. 2010;122:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dickinson D, Pratt DN, Giangrande EJ, Grunnagle M, Orel J, Weinberger DR, Callicott JH, Berman KF. Attacking heterogeneity in schizophrenia by deriving clinical subgroups from widely available symptom data. Schizophr Bull. 2018;44:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Strauss GP, Esfahlani FZ, Galderisi S, et al. Network analysis reveals the latent structure of negative symptoms in schizophrenia. Schizophr Bull. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cohen AS, Docherty NM, Nienow T, Dinzeo T. Self-reported stress and the deficit syndrome of schizophrenia. Psychiatry. 2003;66:308–316. [DOI] [PubMed] [Google Scholar]
- 84. Docherty NM. Affective reactivity of symptoms as a process discriminator in schizophrenia. J Nerv Ment Dis. 1996;184:535–541. [DOI] [PubMed] [Google Scholar]
- 85. Cohen AS, Docherty NM. Affective reactivity of speech and emotional experience in patients with schizophrenia. Schizophr Res. 2004;69:7–14. [DOI] [PubMed] [Google Scholar]
- 86. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Buck B, Lysaker PH. Consummatory and anticipatory anhedonia in schizophrenia: stability, and associations with emotional distress and social function over six months. Psychiatry Res. 2013;205:30–35. [DOI] [PubMed] [Google Scholar]
- 89. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. [DOI] [PubMed] [Google Scholar]
- 90. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 2015;40:190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. [DOI] [PubMed] [Google Scholar]
- 93. Curley AA, Arion D, Volk DW, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fatemi SH, Folsom TD. GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr Res. 2015;167:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015;167:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. [DOI] [PubMed] [Google Scholar]
- 98. Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. [DOI] [PubMed] [Google Scholar]
- 99. Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620, 544. [DOI] [PubMed] [Google Scholar]
- 102. Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. [DOI] [PubMed] [Google Scholar]
- 103. Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. [DOI] [PubMed] [Google Scholar]
- 105. Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. [DOI] [PubMed] [Google Scholar]
- 106. Konradi C, Yang CK, Zimmerman EI, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. [DOI] [PubMed] [Google Scholar]
- 108. Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. [DOI] [PubMed] [Google Scholar]
- 109. Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fuchs EC, Zivkovic AR, Cunningham MO, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. [DOI] [PubMed] [Google Scholar]
- 113. Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. [DOI] [PubMed] [Google Scholar]
- 114. Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. [DOI] [PubMed] [Google Scholar]
- 115. Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. [DOI] [PubMed] [Google Scholar]
- 116. Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. [DOI] [PubMed] [Google Scholar]
- 119. Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. [DOI] [PubMed] [Google Scholar]
- 121. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. [DOI] [PubMed] [Google Scholar]
- 122. Cabungcal JH, Counotte DS, Lewis E, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Canetta S, Bolkan S, Padilla-Coreano N, et al. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry. 2016;21:956–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang Z, van Praag H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav Immun. 2015;45:60–70. [DOI] [PubMed] [Google Scholar]
- 125. Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307–315. [DOI] [PubMed] [Google Scholar]
- 126. Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Du Y, Grace AA. Amygdala hyperactivity in MAM model of schizophrenia is normalized by peripubertal diazepam administration. Neuropsychopharmacology. 2016;41:2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm (Vienna). 2007;114:893–898. [DOI] [PubMed] [Google Scholar]
- 129. Schiavone S, Sorce S, Dubois-Dauphin M, et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. [DOI] [PubMed] [Google Scholar]
- 130. Filipović D, Zlatković J, Gass P, Inta D. The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience. 2013;236:47–54. [DOI] [PubMed] [Google Scholar]
- 131. Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Brown JA, Ramikie TS, Schmidt MJ, et al. Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol Psychiatry. 2015;20:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kolata SM, Nakao K, Jeevakumar V, et al. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and hippocampus. Neuropsychopharmacology. 2018;43:1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fujihara K, Miwa H, Kakizaki T, et al. Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology. 2015;40:2475–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Georgiev D, Yoshihara T, Kawabata R, et al. Cortical gene expression after a conditional knockout of 67 kDa glutamic acid decarboxylase in parvalbumin neurons. Schizophr Bull. 2016;42:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lange C, Deutschenbaur L, Borgwardt S, Lang UE, Walter M, Huber CG. Experimentally induced psychosocial stress in schizophrenia spectrum disorders: a systematic review. Schizophr Res. 2017;182:4–12. [DOI] [PubMed] [Google Scholar]
- 139. Berger M, Kraeuter AK, Romanik D, Malouf P, Amminger GP, Sarnyai Z. Cortisol awakening response in patients with psychosis: systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;68:157–166. [DOI] [PubMed] [Google Scholar]
- 140. Karanikas E, Garyfallos G. Role of cortisol in patients at risk for psychosis mental state and psychopathological correlates: a systematic review. Psychiatry Clin Neurosci. 2015;69:268–282. [DOI] [PubMed] [Google Scholar]
- 141. Shah JL, Malla AK. Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr Res. 2015;162:253–260. [DOI] [PubMed] [Google Scholar]
- 142. Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38:603–611. [DOI] [PubMed] [Google Scholar]
- 143. Girshkin L, Matheson SL, Shepherd AM, Green MJ. Morning cortisol levels in schizophrenia and bipolar disorder: a meta-analysis. Psychoneuroendocrinology. 2014;49:187–206. [DOI] [PubMed] [Google Scholar]
- 144. Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry. 2017;81:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY. Stress and the mesocorticolimbic dopamine systems. Ann N Y Acad Sci. 1988;537:138–147. [DOI] [PubMed] [Google Scholar]
- 148. Mizrahi R, Addington J, Rusjan PM, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–567. [DOI] [PubMed] [Google Scholar]
- 149. Gomes FV, Grace AA. Adolescent stress as a driving factor for schizophrenia development—a basic science perspective. Schizophr Bull. 2017;43:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kircher T, Whitney C, Krings T, Huber W, Weis S. Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res. 2008;101:242–255. [DOI] [PubMed] [Google Scholar]
- 152. Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55:668–675. [DOI] [PubMed] [Google Scholar]
- 153. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Whitworth AB, Kemmler G, Honeder M, et al. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res. 2005;140:225–237. [DOI] [PubMed] [Google Scholar]
- 155. Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. [DOI] [PubMed] [Google Scholar]
- 156. Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 157. Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2013;43:553–569. [DOI] [PubMed] [Google Scholar]
- 160. Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. 2012;38:608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pinkham AE, Liu P, Lu H, Kriegsman M, Simpson C, Tamminga C. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172:784–792. [DOI] [PubMed] [Google Scholar]
- 163. Fernandez-Egea E, Parellada E, Lomeña F, et al. 18FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:69–76. [DOI] [PubMed] [Google Scholar]
- 164. Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. [DOI] [PubMed] [Google Scholar]
- 165. Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bjorkquist OA, Olsen EK, Nelson BD, Herbener ES. Altered amygdala-prefrontal connectivity during emotion perception in schizophrenia. Schizophr Res. 2016;175:35–41. [DOI] [PubMed] [Google Scholar]
- 168. Das P, Kemp AH, Flynn G, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. [DOI] [PubMed] [Google Scholar]
- 169. Mukherjee P, Sabharwal A, Kotov R, et al. Disconnection between amygdala and medial prefrontal cortex in psychotic disorders. Schizophr Bull. 2016;42:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, Taylor SF. Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. Am J Psychiatry. 2007;164:1250–1258. [DOI] [PubMed] [Google Scholar]
- 171. de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J Neurosci. 2016;36:6553–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. [DOI] [PubMed] [Google Scholar]
- 173. Piantadosi PT, Floresco SB. Prefrontal cortical GABA transmission modulates discrimination and latent inhibition of conditioned fear: relevance for schizophrenia. Neuropsychopharmacology. 2014;39:2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6:e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gupta CN, Calhoun VD, Rachakonda S, et al. Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull. 2015;41:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. [DOI] [PubMed] [Google Scholar]
- 177. Schur RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of H-MRS studies. Hum Brain Mapp. 2016;37:3337–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Taylor SF, Tso IF. GABA abnormalities in schizophrenia: a methodological review of in vivo studies. Schizophr Res. 2015;167:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Frankle WG, Cho RY, Prasad KM, et al. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry. 2015;172:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Williams LM, Das P, Liddell BJ, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155:29–44. [DOI] [PubMed] [Google Scholar]
- 182. Lee KH, Brown WH, Egleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–1933. [DOI] [PubMed] [Google Scholar]
- 183. Costa E, Davis JM, Dong E, et al. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol. 2004;16:1–23. [DOI] [PubMed] [Google Scholar]
- 184. Gill KM, Grace AA. The role of α5 GABAA receptor agonists in the treatment of cognitive deficits in schizophrenia. Curr Pharm Des. 2014;20:5069–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Guidotti A, Auta J, Davis JM, et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl). 2005;180:191–205. [DOI] [PubMed] [Google Scholar]
- 186. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Rudolph U, Möhler H. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22:27–47. [DOI] [PubMed] [Google Scholar]
- 189. Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH. Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Arch Gen Psychiatry. 1992;49:345–353. [DOI] [PubMed] [Google Scholar]
- 190. Wolkowitz OM, Pickar D. Benzodiazepines in the treatment of schizophrenia: a review and reappraisal. Am J Psychiatry. 1991;148:714–726. [DOI] [PubMed] [Google Scholar]
- 191. Dold M, Li C, Tardy M, Khorsand V, Gillies D, Leucht S. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2012;11:CD006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Dold M, Li C, Gillies D, Leucht S. Benzodiazepine augmentation of antipsychotic drugs in schizophrenia: a meta-analysis and Cochrane review of randomized controlled trials. Eur Neuropsychopharmacol. 2013;23:1023–1033. [DOI] [PubMed] [Google Scholar]
- 193. Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Breier AF. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156:299–303. [DOI] [PubMed] [Google Scholar]
- 194. Ahn K, Gil R, Seibyl J, Sewell RA, D’Souza DC. Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharmacology. 2011;36:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93:137–143. [DOI] [PubMed] [Google Scholar]
- 196. Menza MA, Harris D. Benzodiazepines and catatonia: an overview. Biol Psychiatry. 1989;26:842–846. [DOI] [PubMed] [Google Scholar]
- 197. Northoff G, Krill W, Wenke J, et al. Major differences in subjective experience of akinetic states in catatonic and parkinsonian patients. Cogn Neuropsychiatry. 1998;3:161–178. [Google Scholar]
- 198. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry. 1990;51:357–362. [PubMed] [Google Scholar]
- 199. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophr Bull. 2018;44:1133–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lewis DA, Cho RY, Carter CS, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Buchanan RW, Keefe RS, Lieberman JA, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry. 2011;69:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Rosato-Siri MD, Zambello E, Mutinelli C, et al. A novel modulator of Kv3 potassium channels regulates the firing of parvalbumin-positive cortical interneurons. J Pharmacol Exp Ther. 2015;354:251–260. [DOI] [PubMed] [Google Scholar]
- 205. Yanagi M, Joho RH, Southcott SA, Shukla AA, Ghose S, Tamminga CA. Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs. Mol Psychiatry. 2014;19:573–579. [DOI] [PubMed] [Google Scholar]
- 206. Yung AR, Buckby JA, Cosgrave EM, et al. Association between psychotic experiences and depression in a clinical sample over 6 months. Schizophr Res. 2007;91:246–253. [DOI] [PubMed] [Google Scholar]
- 207. Fusar-Poli P, Valmaggia L, McGuire P. Can antidepressants prevent psychosis? Lancet. 2007;370:1746–1748. [DOI] [PubMed] [Google Scholar]
- 208. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic imaging and resonance study. Neuropsychopharmacology. 2019;44:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Dubin MJ, Mao X, Banerjee S, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2016;41:E37–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit Rev Neurobiol. 2000;14:23–45. [DOI] [PubMed] [Google Scholar]