Abstract
Whether aberrant cerebral blood flow (CBF) in schizophrenia is affected by genetic influences, and consequently a potential marker for genetic susceptibility, is unknown. Our aims were to determine the heritability of CBF in thalamic, frontal, and striatal areas, and to ascertain if associations with disease were under genetic influence. Monozygotic (MZ) twin pairs concordant (n = 2) or discordant (n = 20) for schizophrenia spectrum disorders (ICD-10 F2x.x), matched on sex and age with dizygotic (DZ; n = 20) and healthy control pairs (MZ: n = 27; DZ: n = 18; total: n = 181 individuals), were recruited via the National Danish Twin Register. CBF in thalamus, frontal lobes, and putamen was measured with pseudo-continuous arterial spin labeling on a 3 T magnetic resonance scanner. Twin statistics were performed with structural equation modeling. CBF in the frontal lobes was heritable (h2 = 0.44, 95% CI [0.22–0.60]) but not correlated to disease. CBF correlated to schizophrenia spectrum disorders in the left thalamus (r = 0.17, [0.03–0.31]; P = 0.02), as well as in the left putamen (r = 0.19, [0.05–0.32]; P = 0.007) and the right putamen (r = 0.18, [0.03–0.32]; P = 0.02). When restricting the sample to schizophrenia (F20.x) only, shared genetic influences between CBF in the left putamen and schizophrenia liability (phenotypic correlation = 0.44, [0.28–0.58], P < 0.001) were found. Our results provide heritability estimates of CBF in the frontal lobes, and we find CBF in thalamus and putamen to be altered in schizophrenia spectrum disorders. Furthermore, shared genetic factors influence schizophrenia liability and striatal perfusion. Specifically, higher perfusion in the left putamen may constitute a marker of genetic susceptibility for schizophrenia.
Keywords: pCASL, cerebral blood flow, relatives, twins, genes
Introduction
Schizophrenia is a highly heritable disease1 with a heterogeneous clinical phenotype. The thalamus, the frontal regions, and the striatum are considered to play a central part in the pathophysiology, and dysfunction of cortico-striato-thalamocortical loops is a long-standing candidate for a disease mechanism.2–4 Regional cerebral blood flow (rCBF) and metabolism have been studied in schizophrenia by nuclear imaging techniques such as fluorodeoxyglucose positron emission tomography (FDG-PET) measuring glucose metabolism, which is closely linked to CBF,5,6 [15O] CBF-PET, and single photon emission tomography. Overall, previous findings from this type of studies suggest lower baseline glucose metabolism and rCBF in frontal and temporal regions of both antipsychotic naive7,8 and chronic patients.9–13 In the thalamus and the putamen, higher rCBF has been found in antipsychotic naive7 and chronic patients14,15 as well as in subjects at familial risk.14 Results, however, are not consistent and lower rCBF has also been reported in these regions.16,17 Furthermore, an abnormal anterior-posterior gradient of CBF that could support the hypothesis of frontal dysfunction as well as altered hemispheric laterality has been reported.11,13
Pseudo-continuous arterial spin labeling (pCASL) is a noninvasive magnetic resonance (MR) technique that uses arterial water as a diffusible tracer to form CBF images with high spatial resolution.18 Arterial spin labeling (ASL) studies in schizophrenia are generally small and with some variability in findings (reviewed in Guimarães et al19 and Théberge20). Nevertheless, frontal hypoperfusion at rest has been reported in several studies,21–27 most frequently in the anterior cingulate cortex21,26,27 and the medial frontal gyrus.23,24,26,27 Also, higher rCBF in the putamen has been consistently reported both in high-risk subjects25,28,29 and in chronic patients.23,27,30 In the thalamus, higher rCBF was found in non-medicated patients21 compared with healthy controls (HC), as well as in a large study comparing 106 patients with chronic schizophrenia to 94 HC.27 Furthermore, in this quite large study, higher rCBF was also found in the putamen and the inferior temporal gyrus; lower rCBF was found in frontal and occipital areas whereas rCBF in the caudate nucleus was unaltered.27 Lower thalamic blood flow measured with ASL has been described in 2 smaller studies,24,30 whereas both higher30 and lower26 rCBF was reported in the caudate nucleus. In the parietal lobes unaltered22,27 or lower rCBF has been reported.21,23,26
In a study of first-degree relatives of patients with schizophrenia, altered rCBF compared with HC was found in frontal and subcortical areas suggesting a familial, and possibly genetic, influence on rCBF and disease.14 Heritability of CBF itself has to the best of our knowledge not been estimated, but cerebral glucose metabolism, which is tightly coupled to CBF,31 was found regionally heritable in a study of healthy twins.32 Because schizophrenia is highly heritable,1 the same genetic factors could potentially influence both disease and rCBF. However, it is unknown if aberrant rCBF seen in schizophrenia can be explained by genetic factors. In the first study of its kind, our twin design, including pairs concordant and discordant for schizophrenia spectrum disorders, provides a viable way to test this.
Because aberrant CBF has been described in subjects at high risk of psychosis,25,28,29 as well as in patients with schizophrenia21,22,24,27 and schizotypal disorder,23,26 we analyzed the full spectrum of schizophrenia disorders (ICD-10 F2x.x), as well as narrowly defined schizophrenia (ICD-10 F20.x), to investigate if potential differences in rCBF were consistent within the diagnostic spectrum. Additional similar analyses were performed excluding the twin pairs where one (or both) had schizophrenia (F20.x) thus only including proband pairs where one had a F21–F29 schizophrenia spectrum diagnose and HC. CBF images were obtained by pCASL, and structural equation modeling (SEM) was used to estimate environmental and genetic effects on rCBF and on the associations with the liability for disease.
We hypothesized that the rCBF in the frontal lobes, the putamen, and the thalamus would be heritable and altered in schizophrenia spectrum disorder (F2x.x), and that liability for schizophrenia spectrum disorder (F2x.x) and rCBF would be influenced by overlapping genetic factors. Further analyses were performed in narrow schizophrenia (F20.x) and F21–F29 schizophrenia spectrum subgroups, as well as on additional cortical and subcortical areas.
Methods
Recruitment
All twin pairs in Denmark where one or both twins had a diagnosis within the schizophrenia spectrum were identified by linking the Danish Civil Registration System33 with the Danish Psychiatric Central Research Register34 and the Danish Twin Register.35 The schizophrenia spectrum (F2x.x) is defined as having a main or secondary lifetime diagnosis in ICD-8: 295, 297, 298.29, 298.39, 298.89, 298.99, 299.05, 299.09, 301.09, 301.29, or in ICD-10: F20.0–F29. Zygosity was confirmed by DNA testing (micro-array by Infinium PsychArray v1.1; Illumina) in 168 (93%) of the 181 included twins. For the remaining 13 twins, information on zygosity from the Danish Twin Register was used. The overall study population of 902 twin pairs was restricted according to the following criteria: all twin pairs had to be 18–60 years old; both twins had to be alive, reside in Denmark, and not have research protection (due to participation in a prior research project within 2 years). Of the final study population of 61 monozygotic (MZ) and 143 dizygotic (DZ) proband pairs eligible for study inclusion, all MZ proband pairs were contacted. DZ proband pairs, and MZ and DZ HC pairs were matched on sex and age to the MZ probands.
Participants
Participants were recruited as part of the Vulnerability Indicators of Psychosis study. The study population consisted of 18 MZ and 16 DZ twin pairs concordant or discordant for a schizophrenia spectrum diagnosis (see supplementary table S1 for proband subdiagnoses and co-twin diagnoses) and 27 MZ and 17 DZ HC pairs. Twenty-five additional twins were analyzed without their siblings. Of the 206 subjects scanned, 10 did not complete pCASL, 2 were excluded due to physical illness, 1 due to technical reasons, and 12 due to lack of hemoglobin measurements (used for correction in statistical analysis) leaving a total of 181 subjects (Table 1). Analyses were made on data from 3 samples: (1) the full schizophrenia spectrum (F2x.x) sample and all HC pairs (patients: n = 45, co-twins: n = 43, HC: n = 93); (2) the schizophrenia (F20.x) sample—including all proband pairs with F20.x schizophrenia (patients: n = 21, co-twins: n = 21) and all HC pairs, but excluding proband pairs where one had a F21–F29 schizophrenia spectrum diagnosis; and (3) the F21–F29 schizophrenia spectrum sample—including all proband pairs with F21–F29 schizophrenia spectrum disorder diagnoses (patients: n = 20, co-twins: n = 20) and all HC pairs, but excluding proband pairs where one had a F20.x schizophrenia diagnosis. The only 2 twin pairs where one twin had a F20.x and the other a F21–F29 diagnosis were excluded from both subsample analyses. See supplementary tables S2–S4 for overview of included subjects in each analysis. All DZ pairs were of the same sex. Four (10%) of the co-twins to the probands had a (non-F2x.x) psychiatric diagnosis (supplementary table S1). Inclusion criteria for proband pairs are listed under “Recruitment”. General exclusion criteria are as follows: serious physical illness, current addiction to alcohol or illicit drugs, serious head trauma (loss of consciousness of more than 5 minutes), and pregnancy. The HC were mentally healthy and had no first-degree relatives with diagnosis of major psychosis or affective disorder (F2x.x, F30, F31, and F32.3). Diagnoses were confirmed with semi-structured interviews: Schedules for Clinical Assessment in Neuropsychiatry 2.036 and Comprehensive Assessment of At Risk Mental State.37 The study was conducted in accordance with the Declaration of Helsinki II and approved by the National Committee on Health Research ethics (journal number H-2-2010-1289) and the Data Protection Agency (journal number 2010-41-5468).
Table 1.
Demographic Presentation of Participants by Group
| PR MZ | PR DZ | Co MZ | Co DZ | HC MZ | HC DZ | |
|---|---|---|---|---|---|---|
| Frequency(a)n = 181 | 25 (5) | 20 (4) | 21 (5) | 22 (6) | 56 (2) | 37 (1) |
| Age, mean (SD) | 38.6 (9.8) | 43.2 (9.5) | 39.9 (10.9) | 42.2 (10.2) | 40.7 (11.2) | 41.2 (9.7) |
| Sex, male/female (% male) | 18/7 (72) | 10/10 (50) | 13/8 (62) | 10/12 (45) | 25/31 (45) | 23/14 (62) |
| Concordant/discordant (subjects) | 4/21 | 0/20 | 0/21 | 0/22 | — | — |
| Handedness (right/non right) | 23/2 | 17/3 | 19/2 | 19/3 | 47/9 | 33/4 |
| Years of educationb, mean (SD) | 13.4 (3.1) | 13.7 (2.3) | 13.7 (3.4) | 14.3 (4.0) | 15.5 (2.9) | 16.4 (2.5) |
| Age at first diagnosis, mean (SD) | 25.5 (7.2) | 24.8 (5.4) | — | — | — | — |
| Years since diagnosis, mean (SD) | 12.2 (6.5) | 16.6 (9.5) | — | — | — | — |
| Antipsychotic medication (% of the patient group, their current mean daily dose) | ||||||
| Amisulpride | 4%, 300 mg | 5%, 300 mg | — | — | — | — |
| Aripiprazole | 8%, 12.5 mg | 5%, 20 mg | — | — | — | — |
| Chlorprothixene | 0 | 5%, 15 mg | — | — | — | — |
| Clozapine | 4%, 350 mg | 15%, 133 mg | — | — | — | — |
| Olanzapine | 16%, 26 mg | 10%, 33 mg | — | — | — | — |
| Paliperidone | 4%, 9 mg | 5%, 2.7 mg | — | — | — | — |
| Perphenazine | 0 | 5%, 8 mg | — | — | — | — |
| Quetiapine | 16%, 116 mg | 25%, 190 mg | — | — | — | — |
| Ziprasidone | 0 | 5%, 160 mg | — | — | — | — |
| Zuclopenthixol | 8%, 9 mg | 5%, 36 mg | — | — | — | — |
| Any antipsychotic drug use | 48% | 70% | 0% | 0% | 0% | 0% |
| Psychopathology assessed by the Positive and Negative Syndrome Scale (PANSS) | ||||||
| Positive, mean (SD)c | 14.4 (5.6) | 12.4 (4.5) | 8.8 (2.7) | 9.3 (3.6) | 7.3 (0.9) | 7.1 (0.4) |
| Negative, mean (SD)c | 16.6 (7.6) | 14.6 (6.3) | 9.5 (3.0) | 9.2 (3.6) | 8.2 (3.2) | 7.8 (1.2) |
| General, mean (SD)c | 31.6 (9.6) | 30.2 (9.0) | 20.1 (4.4) | 20.3 (6.2) | 17.2 (2.6) | 16.9 (1.6) |
| Total, mean (SD)c | 62.5 (20.5) | 57.1 (16.8) | 38.4 (8.1) | 38.9 (11.5) | 32.5 (6.2) | 31.8 (1.8) |
| Global assessment of function (GAF-F) | ||||||
| Mean (SD)d | 47.2 (19.8) | 52.4 (20.0) | 79.4 (15.6) | 82.3 (16.3) | 92.4 (6.8) | 91.7 (8.4) |
Note: PR, proband; MZ, monozygotic; DZ, dizygotic; Co, unaffected co-twin of proband; HC, healthy control; F2x.x, International Classification of Disease version 10 (ICD-10) definition of schizophrenia spectrum disorders (schizophrenia, schizotypal, and delusional disorders); age at first diagnosis and years since diagnosis are calculated from first psychiatric diagnosis.
Number of subjects in the group scanned without co-twin.
Significant difference (P < 0.05), one-way ANOVA followed by Hochberg post-hoc test, between
PR and Co MZ vs HC DZ.
PR vs all other groups.
PR vs all other groups, as well as Co MZ vs all other groups.
MR Acquisitions
Imaging was performed on a 3 T Philips system. For anatomical reference and gray and white matter tissue segmentation, we acquired a 3D T1-weighted scan, and rCBF data were obtained by a pCASL sequence consisting of 30 pairs of perfusion-weighted and control scans.38 See supplementary material for sequence details.
rCBF data analysis was performed with the FSL software package39 (fsl.fmrib.ox.ac.uk/fsl/fslwiki/oxford_asl; accessed on 5 February). Non-brain tissue was masked from the T1-weighted image using the brain extraction tool. pCASL data were co-registered to the skull-stripped T1-weighted image, which was nonlinearly co-registered to Montreal Neurological Institute (MNI) space. Finally, the combined transformation was applied to the rCBF maps.
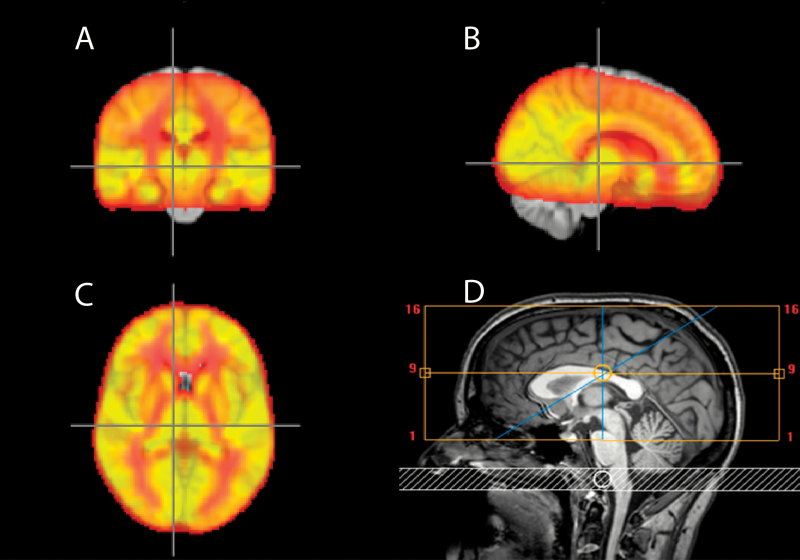
Regions of interest (ROIs) for the primary hypothesis were the thalamus, the frontal lobes, and the putamen. Secondary, exploratory analyses were performed for the temporal, parietal, and occipital lobes; the insula; the caudate nucleus; and the nucleus accumbens. The ROIs were identified using the Harvard-Oxford cortical and subcortical structural atlas and the MNI atlas from FSL. All ROIs were bilateral, and the field of view was placed to include the superior parts. Depending on head size one-third to one-half of the inferior parts of the temporal and the occipital lobes were left out due to size limitations. Only voxels that were covered by more than 90% of subjects were included in the analysis (figure 1).
Fig. 1.
Average CBF for all subjects and field of view. Averaged cerebral blood flow for all subjects. The brighter yellow the higher the flow. A: Coronal, B: Sagittal, and C: Horizontal planes. D: Field of view (yellow box) and labeling slab (white).
For each subject, rCBF was calculated as normalized rCBF (rCBF/global mean CBF of that individual) to estimate regional differences independent of differences in total CBF. The normalized results were used as primary outcome. Calibrated (absolute, in ml/100 g/min) values, calculated by considering the ASL signal in relation to the M0 images using FSL software (oxford_asl),39 were also considered and are presented in supplementary material, pp. 8–11, and supplementary tables S6 and S7.
Statistical Analyses
Demographic variables, clinical characteristics, and values of rCBF are listed as frequencies, percentage or mean values in tables 1 and 2. All CBF measurements were corrected for age, sex, gray matter (GM) fraction, scanner drift (2 piecewise linear corrections according to scanner date, one before and one after the scanner upgrade) and hemoglobin (because hemoglobin is a known determinant of perfusion and could in itself be heritable40) using a linear model, before they were entered in the genetic model. rCBF values differing more than 3 SDs from the total sample mean were excluded by outlier detection (Table 2). Twin model setup and analyses were performed using the OpenMx software package (version 2.3.141) on the R platform (version 3.1.2) with SEM. Correlations of Positive and Negative Syndrome Scale (PANSS) total as well as positive, negative, and general sub-scores with normalized rCBF in the patient group were determined using Spearman’s correlation coefficient controlling for age, gender, scanner drift, hemoglobin, and GM density.
Table 2.
Presentation of Normalized Cerebral Blood Flow by Group
| Mean (SD; n) | MZ PR | DZ PR | MZ Co | DZ Co | MZ HC | DZ HC |
|---|---|---|---|---|---|---|
| Left thalamus | 1.02 (0.17; 23) | 0.96 (0.15; 20) | 0.97 (0.14; 21) | 0.88 (0.13; 22) | 0.90 (0.14; 56) | 0.90 (0.15; 37) |
| Right thalamus | 1.01 (0.18; 23) | 0.98 (0.16; 20) | 0.97 (0.13; 21) | 0.91 (0.12; 22) | 0.93 (0.13; 56) | 0.93 (0.16; 37) |
| Frontal lobes | 0.79 (0.09; 25) | 0.92 (0.09; 20) | 0.82 (0.09; 21) | 0.86 (0.09; 22) | 0.85 (0.11; 56) | 0.86 (0.10; 36) |
| Left putamen | 0.88 (0.12; 24) | 0.94 (0.14; 20) | 0.89 (0.08; 21) | 0.87 (0.11; 22) | 0.86 (0.10; 56) | 0.89 (0.09; 37) |
| Right putamen | 0.88 (0.13; 24) | 0.93 (0.15; 20) | 0.82 (0.14; 21) | 0.86 (0.09; 22) | 0.85 (0.12; 56) | 0.91 (0.10; 37) |
| Left caudate | 0.68 (0.12; 25) | 0.76 (0.17; 20) | 0.73 (0.12; 21) | 0.77 (0.14; 22) | 0.73 (0.13; 56) | 0.72 (0.14; 36) |
| Right caudate | 0.64 (0.12; 25) | 0.70 (0.14; 20) | 0.66 (0.15; 21) | 0.73 (0.15; 22) | 0.69 (0.13; 56) | 0.68 (0.15; 37) |
| Insula (L+R) | 1.10 (0.08; 25) | 1.17 (0.12; 20) | 1.14 (0.10; 21) | 1.14 (0.11; 22) | 1.15 (0.12; 56) | 1.15 (0.08; 36) |
| Occipital lobes | 0.93 (0.13; 25) | 1.03 (0.12; 20) | 0.91 (0.10; 21) | 1.00 (0.13; 22) | 0.99 (0.14; 55) | 0.95 (0.11; 37) |
| Parietal lobes | 0.86 (0.12; 25) | 0.97 (0.13; 20) | 0.82 (0.10; 21) | 0.93 (0.10; 22) | 0.89 (0.11; 56) | 0.88 (0.08; 36) |
| Temporal lobes | 0.84 (0.09; 25) | 0.90 (0.09; 20) | 0.85 (0.07; 21) | 0.88 (0.07; 22) | 0.87 (0.10; 56) | 0.89 (0.08; 37) |
| Left accumbens | 1.09 (0.21; 24) | 1.18 (0.12; 20) | 1.03 (0.18; 21) | 1.08 (0.14; 22) | 1.07 (0.20; 56) | 1.12 (0.18; 37) |
| Right accumbens | 1.00 (0.18; 25) | 1.10 (0.16; 20) | 0.99 (0.18; 21) | 1.02 (0.12; 22) | 1.04 (0.19; 56) | 1.11 (0.16; 37) |
Note: PR, proband; MZ, monozygotic; DZ, dizygotic; Co, unaffected co-twin of proband; HC, healthy controls. Outliers of > 3 SDs were excluded, the number subjects included in each analysis is denoted by n.
Genetic Modeling
To separate how genes and environment influence a trait, the twin model exploits that (almost) 100% of genes are shared between MZ twins, whereas on average only 50% of genes are shared in DZ twins. When MZ twins are more similar than DZ twins, it indicates that genetic factors explain the variation between individuals. Common environmental factors are presumed to play a role when MZ and DZ twins are equally similar, as demonstrated by the correlations within pairs in the MZ and DZ groups being significant, but not different. These factors are usually described in what is known as an ACE model, including A (additive genetic), C (common environmental), and E (unique environmental, including measurement error) factors.42 The heritability h2 is then defined as the proportion of variance that can be attributed to additive genetic factors (ie, A/[A+C+E]). Similarly, the influence of common and unique environmental factors can be quantified as c2 = C/(A+C+E) and e2 = E/(A+C+E), respectively. To determine the significance of variance components for the rCBF measures, the –2×log-likelihood of the full model and the –2×log-likelihood of a model where h2, c2, or both for CBF measures were constrained to zero were compared, as this difference follows a mixture of chi-square distributions.43 When a familial component (h2 + c2) was significant but h2 or c2 individually were not, the best fitting reduced model was determined (AE or CE for CBF measures) according to the Akaike information criterion (AIC). Significance of the correlations was based on 95% CI. When investigating 2 traits together, the associations between the traits are estimated based on within-twin, cross-trait correlations. The cross-trait cross-twin correlations (here between disease liability and rCBF) within MZ and DZ groups can provide information on any genetic overlap between the 2 traits. Variance components in the full ACE model, correlations between disease liability and rCBF measures and significance of familial components were corrected using the false discovery rate (FDR, at q < 0.05). Subsequent analyses using AE or CE sub-models were only conducted based on a significant familial effect and thus were not corrected by FDR. Further details on genetic modeling are provided in supplementary material, p. 4.
Results
Normalized rCBF for Schizophrenia Spectrum (ICD-10 F2x.x)
These results were based on the full sample of 25 MZ probands, 20 DZ probands, 21 MZ co-twins, 22 DZ co-twins, 56 MZ HC, and 37 DZ HC (supplementary table S2).
Heritability of and Common Environmental Influences on rCBF
Significant genetic influences were found in the full ACE model for the parietal lobes (h2 = 39%), but the result did not survive FDR correction. On the basis of a significant familial variance component and the AIC, the AE model was the best fitting model for the frontal lobes (h2 = 44%), the occipital lobes (h2 = 39%), the left accumbens (h2 = 27%), and the left caudate (h2 = 29%; Table 3).
Table 3.
Genetic, Common Environmental, and Unique Environmental Influences on Normalized Regional Cerebral Blood Flow, and Correlation to Liability for Schizophrenia Spectrum Disorder (F2x.x)
| Normalized data | h 2 | c 2 | e 2 | Corr. to SZ spectrum F2x.x | p. Familial | Best. mod. | h 2 | c 2 | e 2 |
|---|---|---|---|---|---|---|---|---|---|
| Left thalamus | 0.01 [0.00 to 0.27] | 0.12 [0.00 to 0.30] | 0.87 [0.68 to 1.00] | 0.17 a ,o [0.03 to 0.31] | 0.164 | E | n.a. | n.a. | 1 |
| Right thalamus | 0.00 [0.00 to 0.25] | 0.19 [0.00 to 0.37] | 0.81 [0.63 to 1.00] | 0.10 [–0.04 to 0.24] | 0.126 | E | n.a. | n.a. | 1 |
| Frontal lobes | 0.29 [0.00 to 0.60] | 0.13 [0.00 to 0.47] | 0.58 [0.40 to 0.81] | 0.10 [–0.05 to 0.25] | 0.002 o | AE | 0.44 [0.22 to 0.60] | n.a. | 0.56 [0.40 to 0.78] |
| Left putamen | 0.04 [0.00 to 0.29] | 0.00 [0.00 to 0.17] | 0.96 [0.71 to 1.00] | 0.19 b ,o [0.05 to 0.32] | 0.057 | E | n.a. | n.a. | 1 |
| Right putamen | 0.00 [0.00 to 0.41] | 0.23 [0.00 to 0.40] | 0.77 [0.60 to 0.95] | 0.18 a ,o [0.03 to 0.32] | 0.061 | CE | n.a. | 0.22 [0.04 to 0.39] | 0.78 [0.61 to 0.96] |
| Left caudate | 0.06 [0.00 to 0.48] | 0.20 [0.00 to 0.40] | 0.74 [0.52 to 0.94] | 0.07 [–0.08 to 0.21] | 0.027 o | AE | 0.29 [0.06 to 0.49] | n.a. | 0.71 [0.51 to 0.94] |
| Right caudate | 0.00 [0.00 to 0.35] | 0.19 [0.00 to 0.35] | 0.81 [0.64 to 0.99] | 0.08 [–0.06 to 0.22] | 0.103 | CE | n.a. | 0.18 [0.00 to 0.35] | 0.82 [0.65 to 1.00] |
| Insula | 0.01 [0.00 to 0.08] | 0.26 [0.00 to 0.41] | 0.73 [0.57 to 0.90] | –0.02 [–0.16 to 0.12] | 0.012 o | CE | n.a. | 0.26 [0.09 to 0.42] | 0.74 [0.58 to 0.91] |
| Occipital lobes | 0.39 [0.00 to 0.57] | 0.00 [0.00 to 0.38] | 0.61 [0.43 to 0.83] | 0.09 [–0.06 to 0.24] | 0.01 o | AE | 0.39 [0.17 to 0.57] | n.a. | 0.61 [0.43 to 0.83] |
| Parietal lobes | 0.39 [0.01 to 0.56] | 0.00 [0.00 to 0.36] | 0.61 [0.44 to 0.83] | 0.21 b ,o [0.07 to 0.34] | 0.004 o | AE | 0.39 [0.17 to 0.56] | n.a. | 0.61 [0.44 to 0.83] |
| Temporal lobes | 0.01 [0.00 to 0.25] | 0.54 [0.30 to 0.65] | 0.44 [0.33 to 0.58] | 0.08 [–0.06 to 0.22] | < 0.001 o | CE | n.a. | 0.55 [0.41 to 0.66] | 0.45 [0.34 to 0.59] |
| Left accumbens | 0.02 [0.00 to 0.39] | 0.26 [0.00 to 0.42] | 0.72 [0.56 to 0.90] | 0.18 a ,o [0.03 to 0.32] | 0.011 o | AE | 0.27 [0.07 to 0.45] | n.a. | 0.73 [0.55 to 0.93] |
| Right accumbens | 0.00 [0.00 to 0.26] | 0.42 [0.16 to 0.56] | 0.58 [0.44 to 0.74] | 0.06 [–0.08 to 0.21] | < 0.001 o | CE | n.a. | 0.42 [0.26 to 0.56] | 0.58 [0.44 to 0.74] |
Note: MZ, monozygotic; DZ, dizygotic; Co, unaffected co-twin of proband; HC, healthy controls; F2x.x, International Classification of Disease version 10 (ICD-10) definition of schizophrenia spectrum disorders (schizophrenia, schizotypal, and delusional disorders). The left part of the table contains the model output for the bivariate model in which the full ACE model was assumed for cerebral blood flow (CBF) measures. Heritability estimates (h2), common environmental influences (c2), and unique environmental influences (e2) are reported with confidence intervals, together with the estimated association between the liability for schizophrenia spectrum disorder and CBF values. Significant variance components or correlations are displayed in bold and were corrected for false discovery rate (FDR).
Survived FDR. When a familial component (A + C, p familial) acting on the CBF value was significant (corrected for FDR), the best fitting model was determined based on the AIC. The right part of the table contains the model output for the best fitting model for CBF (not corrected for FDR). In all cases, the heritability of liability for schizophrenia spectrum disorders was fixed at 73%, and unique environmental influences were 27%. Prevalence of schizophrenia spectrum disorders was fixed at 1.85%. Outliers of > 3 SDs were excluded.
P value < 0.05.
P value < 0.01. Age, gender, scanner drift, hemoglobin, and GM density were included as covariates. The analyses were based on 18 complete MZ proband pairs (including 2 concordant pairs) and 13 twins from MZ proband pairs included without their co-twin; 16 complete DZ proband pairs and 10 twins from DZ proband pairs included without their co-twin; 27 complete MZ HC pairs and 2 twins from MZ HC pairs included without their co-twin; 17 complete DZ HC pairs and 3 twins from DZ HC pairs included without their co-twin (see supplementary table S2 for more detailed overview of included subjects).
Significant common environmental influences in the full ACE model were found for the temporal lobes (c2 = 54%) and the right accumbens (c2 = 42%), but results did not survive FDR correction. According to AIC, CE was the best fitting model for the right putamen, the right caudate, and insula showing common environmental contributions from 18% to 55% (Table 3).
Associations of Normalized rCBF With Schizophrenia Spectrum (F2x.x)
rCBF was positively correlated with liability for schizophrenia spectrum disorder in the left thalamus (r = 0.17; P = 0.02), the left putamen (r = 0.19; P = 0.007), the right putamen (r = 0.18; P = 0.02), the left accumbens (r = 0.18; P = 0.02), and the parietal lobes (r = 0.21; P = 0.004). Power was not sufficient to disentangle the genetic and environmental contributions to these correlations. No negative correlations were found (Table 3).
Correlation to PANSS Scores
PANSS total, positive, negative, or general scores did not correlate with levels of rCBF in any regions (all P values > 0.05).
Normalized rCBF for Schizophrenia (ICD-10 F20.x)
These results were based on 11 MZ probands, 10 DZ probands, 11 MZ co-twins, 10 DZ co-twins, and all HC (supplementary table S3).
Heritability of and Common Environmental Influences on rCBF
Significant genetic influences were found in the full ACE model for the left putamen (h2 = 7%) and the parietal lobes (h2 = 60%), but the results did not survive FDR correction. On the basis of a significant familial variance component and the AIC, the AE model was the best fitting model for the frontal lobes (h2 = 49%), the right putamen (h2 = 31%), the occipital lobes (h2 = 47%), and the left caudate (h2=38%).
Significant common environmental influences in the full ACE model were found for the right accumbens (c2 = 40%) and the temporal lobes (c2 = 52%), but the results did not survive FDR correction. According to the AIC, CE was the best fitting model for the right thalamus, the insula, the left and the right accumbens, and the temporal lobes showing common environmental influences from 29% to 53% (Table 4).
Table 4.
Genetic, Common Environmental, and Unique Environmental Influences on Normalized Regional Cerebral Blood Flow, and Correlation to Liability for Schizophrenia (F20.x)
| Normalized data | h2 | c2 | e2 | Corr. to SZ F20.x | p. Familial | Best. mod. | h2 | c2 | e2 |
|---|---|---|---|---|---|---|---|---|---|
| Left thalamus | 0.00 [0.00 to 0.38] | 0.20 [0.00 to 0.40] | 0.80 [0.60 to 1.00] | 0.05 [–0.13 to 0.22] | 0.142 | E | n.a. | n.a. | 1 |
| Right thalamus | 0.00 [0.00 to 0.43] | 0.29 [0.00 to 0.48] | 0.70 [0.52 to 0.92] | –0.07 [–0.24 to 0.11] | 0.034 o | CE | n.a. | 0.30 [0.09 to 0.48] | 0.70 [0.52 to 0.91] |
| Frontal lobes | 0.34 [0.00 to 0.65] | 0.14 [0.00 to 0.54] | 0.52 [0.35 to 0.77] | 0.06 [–0.12 to 0.23] | 0.002 o | AE | 0.49 [0.26 to 0.65] | n.a. | 0.51 [0.35 to 0.74] |
| Left putamen | 0.07 [0.01 to 0.35] | 0.01 [0.00 to 0.22] | 0.92 [0.70 to 0.99] | 0.44 c ,*,o[0.28 to 0.58] | 0.022 o | AE | 0.08 [0.01 to 0.35] | n.a. | 0.92 [0.65 to 0.99] |
| Right putamen | 0.17 [0.00 to 0.50] | 0.13 [0.00 to 0.43] | 0.71 [0.50 to 0.93] | 0.18 [–0.01 to 0.35] | 0.044 o | AE | 0.31 [0.08 to 0.51] | n.a. | 0.69 [0.49 to 0.92] |
| Left caudate | 0.17 [0.00 to 0.56] | 0.18 [0.00 to 0.46] | 0.65 [0.44 to 0.88] | 0.24 a [0.05 to 0.41] | 0.012 o | AE | 0.38 [0.14 to 0.57] | n.a. | 0.62 [0.43 to 0.86] |
| Right caudate | 0.15 [0.00 to 0.49] | 0.10 [0.00 to 0.40] | 0.74 [0.51 to 0.98] | 0.10 [–0.08 to 0.27] | 0.097 | E | n.a. | n.a. | 1 |
| Insula | 0.00 [0.00 to 0.53] | 0.37 [0.00 to 0.53] | 0.63 [0.47 to 0.81] | –0.07 [–0.25 to 0.11] | 0.003 o | CE | n.a. | 0.37 [0.19 to 0.53] | 0.63 [0.47 to 0.81] |
| Occipital lobes | 0.47 [0.00 to 0.66] | 0.00 [0.00 to 0.39] | 0.53 [0.34 to 0.78] | 0.07 [–0.11 to 0.25] | 0.008 o | AE | 0.47 [0.22 to 0.66] | n.a. | 0.53 [0.34 to 0.78] |
| Parietal lobes | 0.60 [0.08 to 0.74] | 0.00 [0.00 to 0.40] | 0.40 [0.26 to 0.62] | 0.30 b ,o [0.12 to 0.46] | <0.001 o | AE | 0.60 [0.38 to 0.74] | n.a. | 0.40 [0.26 to 0.62] |
| Temporal lobes | 0.02 [0.00 to 0.10] | 0.52 [0.05 to 0.65] | 0.47 [0.34 to 0.63] | 0.05 [–0.13 to 0.22] | <0.001 o | CE | n.a. | 0.53 [0.36 to 0.66] | 0.47 [0.34 to 0.64] |
| Left accumbens | 0.00 [0.00 to 0.46] | 0.30 [0.00 to 0.47] | 0.70 [0.53 to 0.91] | 0.18 a [0.01 to 0.35] | 0.03 o | CE | n.a. | 0.29 [0.09 to 0.47] | 0.71 [0.53 to 0.91] |
| Right accumbens | 0.00 [0.00 to 0.35] | 0.40 [0.06 to 0.55] | 0.59 [0.44 to 0.78] | 0.08 [–0.10 to 0.25] | 0.001 o | CE | n.a. | 0.40 [0.22 to 0.55] | 0.60 [0.45 to 0.78] |
Note: MZ, monozygotic; DZ, dizygotic; Co, unaffected co-twin of proband; HC, healthy controls. The left part of the table contains the model output for the bivariate model in which the full ACE model was assumed for regional cerebral blood flow (rCBF). Heritability estimates (h2), common environmental influences (c2) and unique environmental influences (e2) are reported with confidence intervals, together with the estimated association between the liability for schizophrenia and rCBF. Significant variance components or correlations are displayed in bold and were corrected for false discovery rate (FDR),
Survived FDR. When a familial component (A + C, p familial) acting on the CBF value was significant (corrected for FDR), the best fitting model was determined based on the AIC. The right part of the table contains the model output for the best fitting model for CBF (not corrected for FDR). In all cases, the heritability of liability for schizophrenia was fixed at 79%, and unique environmental influences were 21%. Prevalence of schizophrenia was fixed at 1.05%. Outliers of > 3 SDs were excluded.
P value < 0.05
P value < 0.01.
P value < 0.001
Significant genetic and unique environmental contribution of correlation. Age, gender, scanner drift, Hbg, and GM density were included as covariates. The analyses were based on 8 complete MZ proband pairs and 6 twins from MZ proband pairs included without their co-twin; 8 complete DZ proband pairs and 4 twins from DZ proband pairs included without their co-twin; 27 complete MZ HC pairs and 2 twins from MZ HC pairs included without their co-twin; 17 complete DZ HC pairs and 3 twins from DZ HC pairs included without their co-twin (see supplementary table S3 for more detailed overview of included subjects).
Associations of Normalized rCBF With Schizophrenia (F20.x)
rCBF was positively correlated with liability for schizophrenia in the left putamen (r = 0.44; P < 0.001). The correlation could be explained by both a significant positive genetic contribution and a significant unique environmental contribution (rph-a = 0.24 [0.05–0.42], P = 0.012; rph-e = 0.20 [0.03–0.34], P = 0.022). In addition, we found a significant positive correlation between schizophrenia liability and rCBF in the left caudate (r = 0.24; P = 0.012), the left accumbens (r = 0.18; P = 0.043), and the parietal lobes (r = 0.30; P = 0.001); of these, only the correlation in the parietal lobes survived FDR correction. It was not possible to disentangle the genetic and environmental contributions to these correlations. No negative correlations were found (Table 4).
Normalized rCBF for F21–F29 Schizophrenia Spectrum Disorders
These results were based on 10 MZ probands, 10 DZ probands, 10 MZ co-twins, 10 DZ co-twins, and all HC (supplementary table S4).
Heritability of and Common Environmental Influences on rCBF
Significant genetic influences were found in the full ACE model for the left putamen (h2 = 18%) and the frontal lobes (h2 = 54%), but the results did not survive FDR correction. On the basis of a significant familial variance component and the AIC, the AE model was the best fitting model for the left caudate (h2 = 49%), the occipital lobes (h2 = 43%), and the left accumbens (h2 = 30%). Significant common environmental influences in the full ACE model were found for the temporal lobes (c2 = 57%) and the right accumbens (c2 = 46%), but the results did not survive FDR correction. According to the AIC, CE was the best fitting model for the right putamen (c2 = 30%) and the right accumbens (c2 = 50%; supplementary table S5).
Associations of Normalized rCBF With F21–F29 Schizophrenia Spectrum Disorders
rCBF was positively correlated with liability for schizophrenia in the left thalamus (r = 0.28; P < 0.01) and right thalamus (r = 0.28; P < 0.01). For the right thalamus the correlation could be explained by a significant unique environmental contribution (rph-e = 0.21 [0.02–0.38], P = 0.03; Supplementary table S5).
Discussion
With a twin design comprising 181 participants, we found that CBF in the frontal lobes was heritable and that CBF in the left thalamus and the bilateral putamina was correlated to liability for schizophrenia spectrum disorder (F2x.x). In the reduced sample of schizophrenia (F20.x), rCBF in the putamen was heritable bilaterally and was significantly correlated to liability for schizophrenia (F20.x) in the left putamen. The correlation between schizophrenia (F20.x) and rCBF in the left putamen was in part carried by shared genetic influences. This suggests that higher rCBF in the putamen reported in patients with schizophrenia 23,25,27,30 is partly explained by genetic influences and is present in MZ and to a lesser extent in DZ unaffected co-twins of patients with schizophrenia (F20.x).
Our findings of higher rCBF in the thalamus as well as in the dorsal and ventral striatum are in accordance with ASL literature21,23,25,27,30 and support the hypothesis of disturbances of the cortico-striato-thalamocortical loops in schizophrenia.2–4 Aberrant frontal lobe rCBF was not found in this study, possibly due to alterations being a more localized effect, because other ASL studies generally found lower rCBF in smaller frontal areas.21–23,26,27,44
In analyses excluding twin pairs where one had a schizophrenia (F20.x) diagnosis, a significant positive correlation was found between F21–F29 schizophrenia spectrum disorders and rCBF in left and right thalamus. For the right thalamus, the effect could for the largest part be explained by unique environmental influences. These results suggest that thalamic blood flow is increased in F21–F29 schizophrenia spectrum, but not in schizophrenia (F20.x) and that the increase is influenced by unique environment. The unique environment could be disease itself and the difference in rCBF between the schizophrenia (F20.x) and the F21–F29 schizophrenia spectrum groups could be due to a normalizing effect of antipsychotic medication on rCBF in the thalamus, because 87% patients with schizophrenia (F20.x) were taking antipsychotic medication as opposed to 23% patients with a F21–F29 schizophrenia spectrum disorder. The significant correlation between schizophrenia (F20.x) and rCBF in left putamen was influenced by genetic factors, but an increase could be augmented by antipsychotic medication. A lowering effect of second-generation antipsychotic medication on rCBF in the thalamus has been reported,45 whereas increases in rCBF caused by antipsychotic medication have been reported in the putamen.45,46 Both increases and decreases have been reported in frontal areas.45–48 In a small meta-analysis of longitudinal studies on effects of antipsychotic medication on CBF, increases were found in the left caudate, and decreases were found in the right thalamus, the right medial frontal gyrus, and the cerebellum.49
In secondary analyses, rCBF in the parietal lobes was found heritable and positively correlated to schizophrenia spectrum disorder (F2x.x) as well as schizophrenia (F20.x). Similarly, rCBF in the left accumbens correlated to liability for schizophrenia spectrum (F2x.x). However, these results should be interpreted with caution because these regions were not part of the primary hypothesis. Previous ASL studies have found unaltered rCBF in the parietal lobes of patients with schizophrenia22,27 or lower rCBF in specific parts of the parietal lobes.21,23,26 The significant heritability found in this study is in accordance with a study in HC twin pairs finding glucose metabolism, which is closely linked to CBF, heritable in the parietal lobes.32
The current findings of genetic influence on increased rCBF in the left putamen of patients with schizophrenia suggest that rCBF in the putamen may serve as a marker of genetic susceptibility for disease, possibly related to mechanisms that control regulation of rCBF or development of microvasculature in schizophrenia.50 The difference in CBF in right putamen was not significant, we believe this was due to limited statistical power and have no knowledge of studies supporting laterality in putaminal CBF.
By using register-based inclusion, this study largely avoids inclusion bias and enables effective match on age and sex. We included the largest sample to date for this kind of study but restricting groups in sub-analyses of schizophrenia (F20.x) and F21–F29 schizophrenia spectrum reduced statistical power. This may have increased the risk for type II errors, as for the right putaminal CBF in the schizophrenia (F20.x) sample where the confidence interval barely crossed 0.00. The variability in duration of illness was partly addressed by correcting the data for age. We were not able to control for effects of treatment with antipsychotic medication due to lack of information of previous antipsychotic exposure and variability in patients’ current use of antipsychotics. Previous studies have shown an overall normalizing effect of antipsychotics on rCBF.49 Medication of the probands with antipsychotic compounds is a limitation, but studies in antipsychotic naive,7 non-medicated,16,21 and medicated27 patients suggest that rCBF abnormalities are present in both medicated and unmedicated states. An inherent limitation of SEM when using the best-fitted model based on the AIC is the risk of overestimation of heritability or common environmental effects if both influence the trait, because whichever is the most parsimonious model will absorb influences of the other. Future twin studies might combine investigations of rCBF with measures influencing brain perfusion, such as cerebral glutamate levels, to characterize possible composite markers for schizophrenia.
In conclusion, this study shows that CBF is heritable in the frontal lobes. In the thalamus and the putamen, we found that higher rCBF was correlated with schizophrenia. For the left putamen, this correlation was carried by shared genetic influences. This finding suggests that rCBF in the putamen could serve as a potential marker of genetic susceptibility for schizophrenia.
Funding
Lundbeck Foundation (grant nos. 25-A2701 and R155-2013-16337). Christian Stefan Legind was supported by a grant from The Mental Health Services—Capital Region of Denmark.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the valuable contributions of Mikkel Erlang Sørensen and Helle Schæbel. Dr Ebdrup has received lecture fees and/or is part of Advisory Boards of Bristol-Myers Squibb, Eli Lilly and Company, Janssen-Cilag, Otsuka Pharma Scandinavia, and Takeda Pharmaceutical Company. Dr Glenthøj is the leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), which is partially financed by an independent grant from the Lundbeck Foundation based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. Her group has also received a research grant from Lundbeck A/S for another independent investigator-initiated study. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administrated by them. She has no other conflicts to disclose. All other authors report no conflicts of interest.
References
- 1. Hilker R, Helenius D, Fagerlund B, et al. Heritability of schizophrenia and schizophrenia spectrum based on the nationwide Danish Twin Register. Biol Psychiatry. 2017:1–7. doi: 10.1016/j.biopsych.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 2. Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39 (suppl 1):10–14. [DOI] [PubMed] [Google Scholar]
- 3. Dandash O, Pantelis C, Fornito A. Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophr Res. 2017;180:48–57. [DOI] [PubMed] [Google Scholar]
- 4. Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16(3):391–402. [DOI] [PubMed] [Google Scholar]
- 5. Beil C. Comparative analysis of regional brain blood flow and glucose metabolism in focal cerebrovascular disease measured by dynamic positron emission tomography of fluorine-18-labelled tracers. J Neurol. 1987;234(5):315–321. [DOI] [PubMed] [Google Scholar]
- 6. Raichle ME, Grubb RL Jr, Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol. 1976;33(8):523–526. [DOI] [PubMed] [Google Scholar]
- 7. Andreasen NC, O’Leary DS, Flaum M, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet. 1997;349(9067):1730–1734. [DOI] [PubMed] [Google Scholar]
- 8. Erkwoh R, Sabri O, Steinmeyer EM, Bull U, Sass H. Psychopathological and SPECT findings in never-treated schizophrenia. Acta Psychiatr Scand. 1997;96(1):51–57. [DOI] [PubMed] [Google Scholar]
- 9. Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14(2):157–168. [DOI] [PubMed] [Google Scholar]
- 10. Buchsbaum MS, Hazlett EA. Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull. 1998;24(3):343–364. [DOI] [PubMed] [Google Scholar]
- 11. Hill K, Mann L, Laws KR, et al. Hypofrontality in schizophrenia : a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–256. [DOI] [PubMed] [Google Scholar]
- 12. Bachneff SA. Positron emission tomography and magnetic resonance imaging: a review and a local circuit neurons hypo(dys)function hypothesis of schizophrenia. Biol Psychiatry. 1991;30(9):857–886. [DOI] [PubMed] [Google Scholar]
- 13. Bachneff SA. Regional cerebral blood flow in schizophrenia and the local circuit neurons hypothesis. Schizophr Bull. 1996;22(1):163–182. [DOI] [PubMed] [Google Scholar]
- 14. Blackwood DH, Glabus MF, Dunan J, O’Carroll RE, Muir WJ, Ebmeier KP. Altered cerebral perfusion measured by SPECT in relatives of patients with schizophrenia. Correlations with memory and P300. Br J Psychiatry. 1999;175:357–366. [DOI] [PubMed] [Google Scholar]
- 15. Kim JJ, Mohamed S, Andreasen NC, et al. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157(4):542–548. [DOI] [PubMed] [Google Scholar]
- 16. Vita A, Bressi S, Perani D, et al. High-resolution SPECT study of regional cerebral blood flow in drug-free and drug-naive schizophrenic patients. Am J Psychiatry. 1995;152(6):876–882. [DOI] [PubMed] [Google Scholar]
- 17. Mori K, Teramoto K, Nagao M, Horiguchi J, Yamawaki S. Regional cerebral blood flow in schizophrenia using stable xenon-enhanced computed tomography. Neuropsychobiology. 1999;39(3):117–124. [DOI] [PubMed] [Google Scholar]
- 18. Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed. 2010;23(3):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guimarães TM, Machado-de-Sousa JP, Crippa JAS, Guimarães MRC, Hallak JEC. Arterial spin labeling in patients with schizophrenia: a systematic review. Rev Psiquiatr Clin. 2016;43(6):151–156. [Google Scholar]
- 20. Théberge J. Perfusion magnetic resonance imaging in psychiatry. Top Magn Reson Imaging. 2008;19(2):111–130. [DOI] [PubMed] [Google Scholar]
- 21. Scheef L, Manka C, Daamen M, et al. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256(1):253–260. [DOI] [PubMed] [Google Scholar]
- 22. Ota M, Ishikawa M, Sato N, et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res. 2014;154(1–3):113–118. [DOI] [PubMed] [Google Scholar]
- 23. Pinkham A, Loughead J, Ruparel K, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walther S, Federspiel A, Horn H, et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res. 2011;192(2):117–124. [DOI] [PubMed] [Google Scholar]
- 25. Kindler J, Schultze-Lutter F, Hauf M, et al. Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophr Bull. 2018;44(1):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kindler J, Jann K, Homan P, et al. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull. 2013;41(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu J, Zhuo C, Qin W, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res. 2015;63:28–35. [DOI] [PubMed] [Google Scholar]
- 28. Allen P, Chaddock CA, Egerton A, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173(4):392–399. [DOI] [PubMed] [Google Scholar]
- 29. Allen P, Azis M, Modinos G, et al. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophr Bull. 2018;44(6):1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J, Qiu M, Constable RT, Wexler BE. Does baseline cerebral blood flow affect task-related blood oxygenation level dependent response in schizophrenia? Schizophr Res. 2012;140(1–3):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe S, Kato H, Shimosegawa E, Hatazawa J. Genetic and environmental influences on regional brain uptake of 18F-FDG: a PET study on monozygotic and dizygotic twins. J Nucl Med. 2016;57(3):392–397. [DOI] [PubMed] [Google Scholar]
- 33. Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 34. Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39 (suppl 7):54–57. [DOI] [PubMed] [Google Scholar]
- 35. Skytthe A, Kyvik KO, Holm NV, Christensen K. The Danish twin registry. Scand J Public Health. 2011;39 (suppl 7):75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47(6):589–593. [DOI] [PubMed] [Google Scholar]
- 37. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39(11–12):964–971. [DOI] [PubMed] [Google Scholar]
- 38. Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian inference for a nonlinear forward model. IEEE Trans Signal Process. 2009;57(1):223–236. [Google Scholar]
- 40. Van ‘t Erve TJ, Wagner BA, Martin SM, et al. The heritability of hemolysis in stored human red blood cells. Transfusion. 2015;55(6):1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boker S, Neale M, Maes H, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3(11):872–882. [DOI] [PubMed] [Google Scholar]
- 43. Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet. 2006;36(2):331–340. [DOI] [PubMed] [Google Scholar]
- 44. Pinkham AE, Liu P, Lu H, Kriegsman M, Simpson C, Tamminga C. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784–792. [DOI] [PubMed] [Google Scholar]
- 45. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34(13):2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53(7):601–608. [DOI] [PubMed] [Google Scholar]
- 47. Miller DD, Andreasen NC, O’Leary DS, et al. Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology. 1997;17(4):230–240. [DOI] [PubMed] [Google Scholar]
- 48. Miller DD, Rezai K, Alliger R, Andreasen NC. The effect of antipsychotic medication on relative cerebral blood perfusion in schizophrenia: assessment with technetium-99m hexamethyl-propyleneamine oxime single photon emission computed tomography. Biol Psychiatry. 1997;41(5):550–559. [DOI] [PubMed] [Google Scholar]
- 49. Goozée R, Handley R, Kempton MJ, Dazzan P. A systematic review and meta-analysis of the effects of antipsychotic medications on regional cerebral blood flow (rCBF) in schizophrenia: association with response to treatment. Neurosci Biobehav Rev. 2014;43:118–136. [DOI] [PubMed] [Google Scholar]
- 50. Katsel P, Roussos P, Pletnikov M, Haroutunian V. Microvascular anomaly conditions in psychiatric disease. Schizophrenia - angiogenesis connection. Neurosci Biobehav Rev. 2017;77:327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.