Abstract
Catatonia is a central aspect of schizophrenia spectrum disorders (SSD) and most likely associated with abnormalities in affective, motor, and sensorimotor brain regions. However, contributions of different cortical features to the pathophysiology of catatonia in SSD are poorly understood. Here, T1-weighted structural magnetic resonance imaging data at 3 T were obtained from 56 right-handed patients with SSD. Using FreeSurfer version 6.0, we calculated cortical thickness, area, and local gyrification index (LGI). Catatonic symptoms were examined on the Northoff catatonia rating scale (NCRS). Patients with catatonia (NCRS total score ≥3; n = 25) showed reduced surface area in the parietal and medial orbitofrontal gyrus and LGI in the temporal gyrus (P < .05, corrected for cluster-wise probability [CWP]) as well as hypergyrification in rostral cingulate and medial orbitofrontal gyrus when compared with patients without catatonia (n = 22; P < .05, corrected for CWP). Following a dimensional approach, a negative association between NCRS motor and behavior scores and cortical thickness in superior frontal, insular, and precentral cortex was found (34 patients with at least 1 motor and at least 1 other affective or behavioral symptom; P < .05, corrected for CWP). Positive associations were found between NCRS motor and behavior scores and surface area and LGI in superior frontal, posterior cingulate, precentral, and pericalcarine gyrus (P < .05, corrected for CWP). The data support the notion that cortical features of distinct evolutionary and genetic origin differently contribute to catatonia in SSD. Catatonia in SSD may be essentially driven by cortex variations in frontoparietal regions including regions implicated in the coordination and goal-orientation of behavior.
Keywords: catatonia, psychosis, MRI, FreeSurfer, cortical thickness, gyrification, motor and behavioral symptoms
Introduction
Catatonia occurs in 9%–17% patients with acute mental disorders.1 Catatonic symptoms are frequently present in schizophrenia spectrum disorders (SSD),2,3 affective and neurodevelopmental disorders, as well as in various medical conditions.4–6 The clinical picture of catatonia includes motor phenomena (rigor, dyskinesia, festination, counteracting, posturing, catalepsy, etc.), affective symptoms (aggression, anxiety, flat affect, affect incontinence, etc.), and disorders of behavior (autism, mutism, echolalia, etc.).7–13 In International Classification of Diseases, Tenth Revision (ICD-10), catatonia can be classified only within SSD and in the context of a medical condition.14Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR)15 definition of catatonia as a subtype of schizophrenia or major mood disorders requires the presence of at least 2 catatonic symptoms.14 In DSM-5, catatonia is defined on the basis of 3 or more of 12 specific symptoms.14,15 Further, catatonia in DSM-5 can be used as “specifier” for the characterization of several clinical phenotypes including SSD, affective, and neurodevelopmental disorders.11,16 Nevertheless, current diagnostic criteria and systems share the neglect of affective catatonic symptoms. This neglect is also well reflected in most current catatonia rating scales,17–22 except the Northoff catatonia rating scale (NCRS).7,23 NCRS considers the clinical importance of catatonic affective symptoms and extrapyramidal dyskinesias as originally emphasized by Karl Kahlbaum.7,24 NCRS includes 3 distinct categories of symptoms including hypo- and hyperkinesias, affective symptoms, and behavioral alterations.7 The neglect of the affective component in all other catatonia scales carries major clinical relevance as it may have contributed to the under-recognition of catatonia in the context of SSD, major mood disorders, and general medical conditions.25–29
In the last 2 decades, cortical motor regions have been described as important loci in the pathogenesis of catatonia in SSD. In particular, different categories of catatonic symptoms can be caused by a dysfunction of orbitofrontal-prefrontal/parietal cortical connectivity reflecting “horizontal modulation” of cortico-cortical relation.30,31 Motor symptoms, however, could mainly be explained by aberrant “top-down modulation” reflecting “vertical modulation” of caudate and other basal ganglia by gamma amino butyric acid (GABA)-ergic-mediated orbitofrontal cortical deficits.30 However, previous magnetic resonance imaging (MRI) studies have considered relatively modest sample sizes (10–15 catatonia patients) and the vast majority of MRI studies focused on aberrant activation of cortical regions neglecting the fact that catatonia in SSD is based on intertwined mosaic of genetic and environmental factors.30–38
A promising approach to study the neurodevelopmental nature of catatonia is to examine measures of cortical organization separately affected by neurodevelopment such as cortical thickness, area, and local gyrification index (LGI). The aims of this study were to determine which cortical features of distinct neurodevelopmental and genetic origin (cortical thickness, area, and LGI) are specific for SSD with catatonia as distinguished from SSD without catatonia. Specifically, we predicted that (1) there will be a difference in cortical thickness, area, and LGI in frontoparietal regions between SSD patients with and without catatonia; (2) the three dimensions of catatonia, ie, motor, behavioral, and affective, will be significantly associated with cortical alterations in distinct cortical regions, ie, areas associated with motor control, affective processing, and integration of spatiotemporal information.
Methods
Participants
This study approached and examined a total of 87 right-handed39 patients satisfying DSM-IV-TR15 for schizophrenia (n = 84; paranoid type) or schizoaffective disorder (n = 3). Patients were consecutively recruited from the Department of Psychiatry and Psychotherapy at the Central Institute of Mental Health in Mannheim, Germany. Diagnoses were made by staff psychiatrists and confirmed using the German versions of the Structured Clinical Interview for DSM axis I and II disorders and examination of the case notes by 2 experienced psychiatrists (D.H. and S.F.). Clinical evaluation included ascertainment of personal and family history and detailed physical and neurological examination. Patients were excluded if (1) they were aged <18 or >65 years, (2) they had a history of brain trauma or neurological disease (especially movement disorders), or (3) they had shown alcohol/substance use disorder within 12 months before participation. The local ethics committee (Medical Faculty at Heidelberg University, Germany) approved the study. Written informed consent was obtained from all SSD patients after all aims and procedures of the study had been fully explained.
Clinical Assessment
All patients were recruited and examined during inpatient treatment as soon as possible after partial remission of psychotic symptoms within 1 week. At the time of the psychometric assessment and MRI examination, none of the SSD patients had taken lorazepam or other benzodiazepines and all but 5 (5/87 = 5.7% antipsychotic-free) patients were on stable antipsychotic medication for at least 2 weeks. Lorazepam or other benzodiazepines were discontinued at least 72 hours before the motor assessment and MRI examination after consultation with the treating physician to avoid potential interactions between benzodiazepines, catatonic symptoms, and MRI scanning. Daily doses of antipsychotic medication were converted to olanzapine equivalents (OLZ) according to the classical mean dose method presented by Leucht and colleagues.40 The equivalence doses are based on the analyses of all antipsychotics compared with olanzapine 1 mg/d. The mean dose of each antipsychotic was weighted by the study’s sample size and finally divided by the weighted mean olanzapine dose to obtain OLZ.40 All patients were electroconvulsive-therapy-naive at the time of scanning. The duration between intake/consent into the study, motor assessment, and MRI scanning was less than 3 days. For a detailed assessment of catatonic symptoms, we used the German version of the Northoff catatonia rating scale (NCRS-dv).23 Catatonic symptoms according to NCRS-dv had to be manifest for at least half an hour on the day of assessment in the presence of the examiner (D.H. and S.F.). The scale measures the presence and severity of 40 catatonic signs, considering 3 distinct dimensions of catatonia, ie, motor (13 items), affective (12 items), and behavioral (15 items) symptoms.
This study used both a categorical and a correlation (dimensional) approach to investigate the effects of 3 different cortical features of distinct neurodevelopmental and genetic origin (cortical thickness, area, and LGI) to catatonia in an epidemiologically based sample of SSD patients. For the “categorical” approach, we used cutoffs suggested by the NCRS, ie, at least 3 catatonic symptoms, with at least 1 motor, behavioral, and affective symptom. Using these criteria, we defined the presence or absence of catatonic symptoms within the entire SSD patient group, assuming that between-group differences between the subgroups would reflect a neural signature of catatonia within SSD. Yet, acknowledging a dimensional view on catatonia, we chose to establish relationships between brain structure and catatonia symptoms in an extended sample of individuals with SSD. To establish such associations, this “dimensional” approach essentially considered the presence of at least 2 catatonic symptoms in an affected individual, without imposing psychometric cutoff constraints.
Structural MRI Data Acquisition
MRI scans were acquired at the Central Institute of Mental Health, Mannheim, Germany on a 3.0 Tesla Magnetom Tim Trio MRI scanner (Siemens Medical Systems) using T1-weigthed 3-dimensional (3D) magnetization-prepared rapid gradient-echo with following parameters: 176 sagittal slices, image matrix = 256 × 256, voxel size = 1 × 1 × 1 mm,3 repetition time (TR) = 2530 ms, echo time (TE) = 3.8 ms, inversion time (TI) = 1100 ms, flip angle = 7°. All MRI brain scans were reviewed by D.H. and S.F. No gross abnormalities (eg, tumor, space-occupying cystic lesion greater 3 mm, signs of bleeding, contusion, infarction, major grey, or white matter lesions) were found.
MRI Data Processing
Entire cortex analyses were computed with FreeSurfer version 6.0 (for detailed description of the method see http://surfer.nmr.mgh.harvard.edu/41) to explore local cortical thickness, area, and LGI in SSD patients with and without catatonic symptoms.42–48 On the basis of the pial surface reconstruction, an algorithm for measuring 3D LGI at each vertex across each hemisphere, including the default smoothing of individual LGI maps at a full-width at half-maximum (FWHM) kernel of 25 mm, was performed using FreeSurfer and MATLAB. Details of the LGI computation process can be found in the validation article,49 as well as in previous reports.50–52
MRI Data Analyses
Cortical Surface Modeling and Clustering
First, we performed a vertex-wise analysis across both study groups (categorical approach) to identify statistical maps of significant differences between SSD patients with and without catatonic symptoms using a General Linear Method (GLM) approach provided by the Query Design Estimate Contrast (QDEC) interface of FreeSurfer. All clinical and demographic measures as well as estimated Total Intracranial Volume (eTIV) and total surface area were tested as potential covariates for further analyses within the categorical approach. Measures were included as covariates when they differed significantly between patients with and without catatonia (by chi-squared tests for categorical variables and t test for continuous variables) or had an influence on the severity of catatonic symptoms, eTIV, and total surface area in SSD patients with catatonia (Pearson’s correlation; 2-tailed; see also supplementary material). According to this process, only age, gender, OLZ, Barnes Akathisia Rating Scale (BARS), Simpson and Angus Scale (SAS), and Positive and Negative Syndrome Scale (PANSS) qualified as covariates in the categorical approach. For statistical analysis, individual cortical thickness, area, and LGI maps were registered to the fsaverage template included in FreeSurfer. Additional smoothing with FWHM size of the Gaussian blurring kernel of 10 mm was applied on the statistical level when investigating cortical thickness and area.53,54 No additional smoothing was applied on the statistical level when investigating LGI.53,54 All of these analyses were performed on the right and left hemisphere separately.
Next, we used an approach that minimizes the problem of multiple comparisons, as described in previous studies54–57 to identify regions showing significant differences in cortical thickness, area, and LGI between SSD patients with and without catatonia. We performed a Monte Carlo simulation with 10 000 iterations and cluster analysis to identify significant contiguous clusters of vertex-wise differences. When investigating the between-group differences in cortical measures, an initial threshold of P < .05 for both the simulation step and the clustering step for the original data was chosen. The cluster-wise probability (CWP) resulting from the simulation and clustering is equivalent to the overall alpha significance level.
Following a dimensional (correlational) approach, we examined the relationship between the 3 catatonic dimensions (ie, motor, affective, and behavioral) and cortical thickness, area, and LGI alterations in SSD patients with at least 1 motor and at least 1 other affective or behavioral symptom according to DSM-IV-TR. Such correlations were calculated using a GLM approach provided by QDEC interface of FreeSurfer. All clinical and demographic measures as well as eTIV and total surface area were tested as potential covariates for further analyses within the dimensional (correlational) approach. Measures were included as covariates when they had an influence on the severity of catatonic symptoms, eTIV, and total surface area in SSD patients with catatonia according to DSM-IV-TR (Pearson’s correlation; 2-tailed; see also supplementary material). According to this process, age, gender, OLZ, PANSS, abnormal involuntary movement scale (AIMS), and BARS qualified as covariates in the dimensional approach.
In the next step, we also performed a Monte Carlo simulation with 10 000 iterations and cluster analysis with an initial threshold of P < .05 to identify significant contiguous clusters of significant associations between catatonic dimensions and cortical measures. Finally, to account for false-positive findings within the identified clusters, CWP values of the identified clusters (each vertex within a cluster has the same P value) were corrected for the number of tested measures in our main analysis using Bonferroni correction. To this end, α was set to P = .05/N, where N (=18) equaled the number of correlations (classical Bonferroni correction). For this reason, the corrected threshold was set to P = .0027 (α = .05/18 tests [3 measures × 3 catatonic dimensions × 2 hemispheres]).
Results
Categorical Approach—Between-Group Comparisons
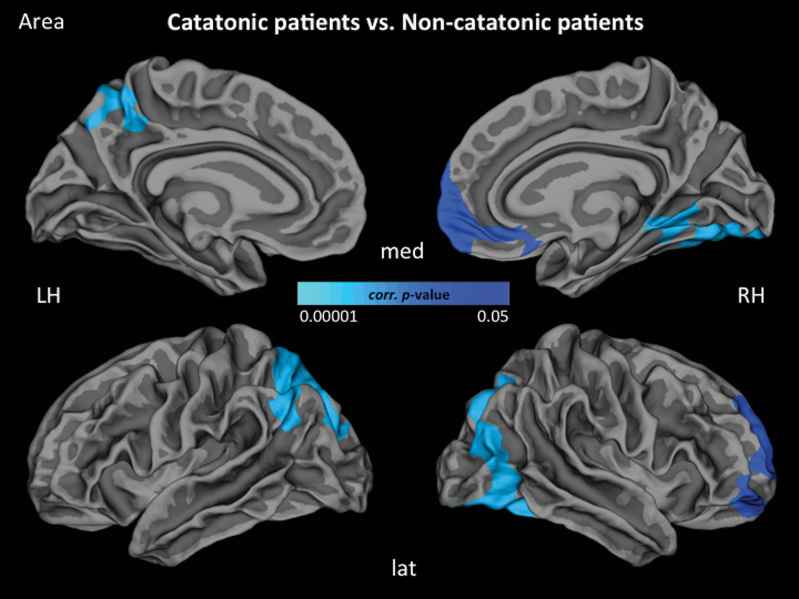
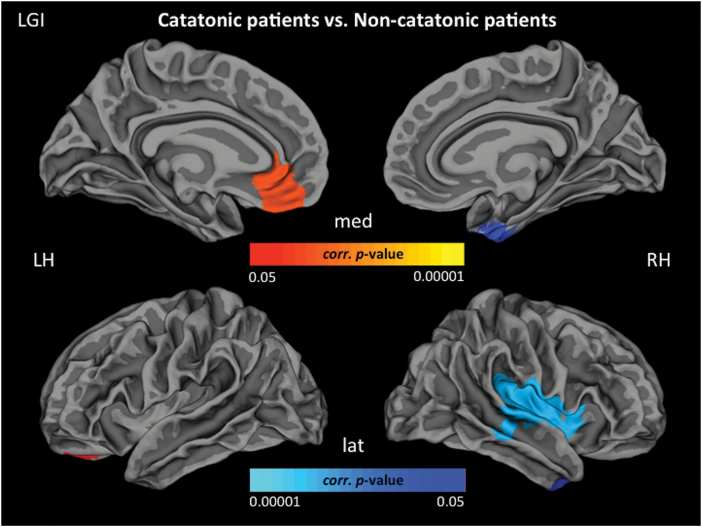
Following a categorical approach, 25 of 87 SSD patients (25/87 = 28.7%) were classified as catatonic according to NCRS criteria (NCRS total score ≥3; at least 1 point in the 3 different symptom categories, ie, motor, behavioral, affective) by 2 independent psychiatrists (D.H. and S.F.). At the time of the motor assessment and MRI examination, none of the SSD catatonic patients had taken benzodiazepines and all but 3 (3/25 = 12% antipsychotic-free) patients were on stable antipsychotic medication for at least 2 weeks. Further, the group of 25 catatonic SSD patients was matched to the group of SSD patients (control group) without catatonic symptoms (n = 22; NCRS = 0) based on age, gender, education, and OLZ. In the non-catatonic SSD group, none of the patients would qualify for any of the single DSM-IV-TR criteria of catatonia. There were no significant differences in cortical thickness between SSD patients with and without catatonia. Patients with catatonia had lower cortical area in the left superior parietal gyrus (SPG), the right medial orbitofrontal cortex (OFC) and lateral occipital gyrus (LOG) when compared with non-catatonic patients (figure 1). Regarding LGI, patients with catatonia showed hypergyrification in the left rostral anterior cingulate (ACC) and medial OFC as well as hypogyrification in the left superior temporal gyrus (STG), right inferior temporal gyrus (ITG), and right insula when compared with non-catatonic patients (figure 2). All but 2 regions hold Bonferroni correction (P = .004) for multiple testing (table 2).
Fig. 1.
Localization of reduced surface area (blue) (P < .05, cluster-wise probability-corrected) in the catatonic group compared with the non-catatonic group. Reduced surface area comprises frontoparietal and occipital regions. Lat, lateral view; med, medial view; LH, left hemisphere; RH, right hemisphere.
Fig. 2.
Localization of hypergyrification (red) and hypogyrification (blue) (P < .05, cluster-wise probability-corrected) in the catatonic-group compared with the non-catatonic-group. Hypergyrification comprises orbitofrontal, parietal and temporal regions. Lat, lateral view; med, medial view; LH, left hemisphere; RH, right hemisphere.
Table 2.
Differences Between SSD Patients With (n = 25) and Without (n = 22) Catatonia According to the Northoff Catatonia Rating Scale
| Parameter | Cluster | Area (mm2) | CWP | Peak coordinates (Tal X, TalY, TalZ) |
| Cortical area | Left superior parietal gyrus | 4619.97 | 0.0001 | –16.7, –61.9, 45.0 |
| Right lateral occipital gyrus | 6280.6 | 0.0001 | 36.8, –74.7, –7.5 | |
| Right medial orbitofrontal gyrus | 3269.11 | 0.0027 | 9.2, 54.6, –5.5 | |
| Cortical folding | Left superior temporal gyrus | 6800.13 | 0.0001 | –48.5, –20.2, –7.1 |
| Left medial orbitofrontal gyrus | 1216.21 | 0.003 | –9.3, 28.7, –13.1 | |
| Left rostral anterior cingulate gyrus | 899.64 | 0.025 | –8.8, 36.5, –7.1 | |
| Right insular gyrus | 5024.28 | 0.0001 | 40.7, –5.6, –14.9 | |
| Right inferior temporal gyrus | 937.4 | 0.009 | 41.5, –9.7, –26.7 |
Significant clusters in both hemispheres: cluster size in mm², P values from the Monte Carlo simulation (P < .05) and clustering as cluster-wise probability (CWP), resulting from the vertex-wise comparison of mean differences of cortical thickness, area, and folding (local gyrification index). Age, gender, olanzapine equivalents, Barnes Akathisia Rating Scale, Simpson and Angus Scale, and Positive and Negative Symptoms Scale scores were used as covariates. Regions holding Bonferroni correction (P = .004) for multiple testing are in bold.
Dimensional Approach—Associations Between Catatonic Symptoms and Cortical Measures
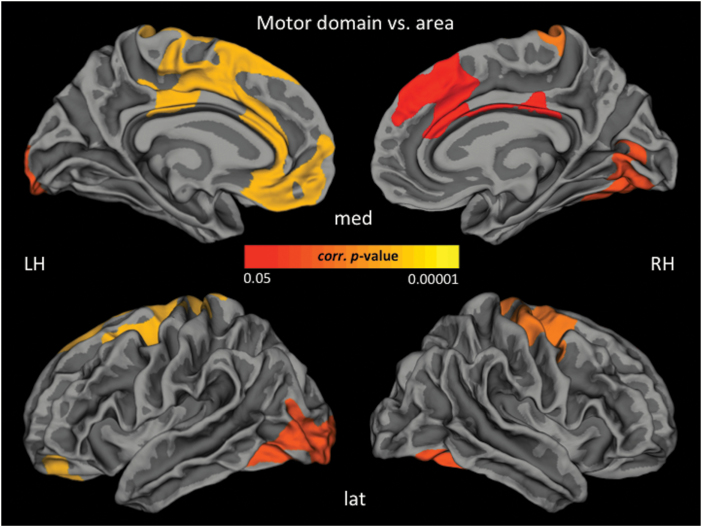
Following a dimensional (correlational) approach, 34 of 87 patients (34/87 = 39%) with 1 motor and at least 1 other symptom (behavioral or affective) were identified, resulting in a total of 34 SSD patients with catatonic syndrome according to DSM-IV-TR by 2 independent psychiatrists (D.H. and S.F.). See table 1 for further clinical details. Further, we were specifically interested in correlations between catatonic symptoms and cortical thickness, area and LGI in SSD patients with catatonia according to DSM-IV-TR (n = 34). For significant associations after CWP correction see table 3 and figure 3. All but 6 regions hold Bonferroni correction (threshold: P = .0027) for multiple testing (table 3).
Table 1.
Clinical and Demographic Variables in SSD Patients With (n = 25) and Without (n = 22) Catatonia According to the Northoff Catatonia Rating Scale
| Patients with catatonia (n = 25) | Patients without catatonia (n = 22) | T a | Df a | Sig. (2-tailed)a | |
| Age | 39.12 ± 11.42 | 40.18 ± 11.83 | –0.313 | 85 | 0.756 |
| Gender (m/f)b | 14/11 | 11/11 | — | 1 | 0.681 |
| Education (years) | 13.4 ± 2.36 | 13.32 ± 3.3 | 0.919 | 85 | 0.361 |
| Olanzapine equivalents | 17.49 ± 8.17 | 16.38 ± 10.54 | 0.406 | 45 | 0.687 |
| Duration of illness (years) | 13.68 ± 11.99 | 8.5 ± 9.54 | 1.623 | 45 | 0.112 |
| PANSS total score | 81.24 ± 21.48 | 60.09 ± 20.34 | 3.451 | 45 | <0.001 |
| PANSS positive score | 19.04 ± 8.57 | 13.95 ± 6.7 | 2.243 | 45 | 0.03 |
| PANSS negative score | 21.26 ± 9.14 | 14.59 ± 7.26 | 3.265 | 45 | 0.009 |
| PANSS global score | 40.92 ± 12.3 | 31.55 ± 10.22 | 2.817 | 45 | 0.007 |
| BPRS | 44.16 ± 14.66 | 33.73 ± 12.17 | 2.632 | 45 | 0.012 |
| GAF | 55.16 ± 14.48 | 74.55 ± 17.38 | –4.17 | 45 | <0.001 |
| SAS total score | 3.24 ± 2.18 | 1.95 ± 1.88 | 2.143 | 45 | 0.038 |
| AIMS total score | 2.2 ± 3.22 | 0.77 ± 2.2 | 1.746 | 45 | 0.088 |
| BARS global score | 1.32 ± 1.6 | 0.45 ± 0.96 | 2.208 | 45 | 0.032 |
| NCRS motor score | 1.8 ± 1.29 | 0 | 7.644 | 45 | <0.001 |
| NCRS affective score | 2.92 ± 1.75 | 0 | 4.397 | 45 | <0.001 |
| NCRS behavior score | 2.2 ± 1.11 | 0 | 11.64 | 45 | <0.001 |
| NCRS total score | 6.88 ± 2.33 | 0 | 11.06 | 45 | <0.001 |
| eTIV (cm3) | 147.21 ± 17.99 | 148.51 ± 25.12 | –0.205 | 45 | 0.838 |
| Total surface area (lh, cm2) | 850.028 ± 76.31 | 854.85 ± 81.5 | –0.199 | 45 | 0.843 |
| Total surface area (rh, cm2) | 857.62 ± 73.04 | 860.34 ± 77.93 | –0.123 | 45 | 0.902 |
Note: PANSS, Positive and Negative Symptoms Scale (p = positive, n = negative, g = global); BPRS, Brief Psychiatric Rating Scale; GAF, Global Assessment of Functioning; SAS, Simpson and Angus Scale; AIMS, Abnormal involuntary movement scale; BARS, Barnes Akathisia Rating Scale; NCRS, Northoff catatonia rating scale; eTIV, estimated Total Intracranial Volume; lh, left hemisphere; rh, right hemisphere. Data are mean ± standard deviation. Significant results are displayed in bold font.
aThe F and P values were obtained using an independent samples t test.
bThe P values for distribution of gender were obtained by chi-square test.
Table 3.
Association Between Northoff Catatonia Rating Scale (NCRS) Scores and Cortical Measurements in SSD patients with catatonia according to DSM-IV-TR (n = 34)
| NCRS domain | Cortical measure | Cluster | Size (mm2) | CWP | Peak coordinates (Tal X, TalY, TalZ) |
| Motor symptoms | Cortical thickness | Left superior frontal gyrus | 1562.63 | 0.006 | –9.0, 26.7, 31.9 |
| Left insula | 1362.78 | 0.017 | 37.2, 3.4, –0.4 | ||
| Cortical area | Left superior frontal gyrus | 6911.45 | 0.0001 | –7.0, 19.8, 51.8 | |
| Left lateral occipital gyrus | 3083.61 | 0.002 | –22.2, –96.1, –6.7 | ||
| Right precentral gyrus (M1) | 3510.3 | 0.0007 | 32.9, –19.1, 54.1 | ||
| Right fusiform gyrus | 2979.73 | 0.003 | 28.0, –73.5, –2.6 | ||
| Right posterior cingulate gyrus | 1988.46 | 0.042 | 7.9, –22.4, 27.9 | ||
| Cortical folding | Left inferior temporal gyrus | 2264.98 | 0.0001 | –52.8, –53.8, –3.3 | |
| Left pericalcarine gyrus | 1490.06 | 0.0006 | –11.7, –89.4, 1.2 | ||
| Left superior parietal gyrus | 1485.72 | 0.0006 | –22.4, –58.1, 52.0 | ||
| Left precentral gyrus (M1) | 1189.12 | 0.003 | –45.4, –7.5, 46.4 | ||
| Left lateral occipital gyrus | 819.97 | 0.045 | –43.2, –76.5, 5.8 | ||
| Right superior parietal gyrus | 6610.06 | 0.0001 | 23.9, –41.9, 59.1 | ||
| Right superior frontal gyrus | 1647.26 | 0.0002 | 20.1, –3.2, 53.8 | ||
| Right inferior frontal gyrus (po) | 962.11 | 0.018 | 47.7, 20.2, 8.1 | ||
| Affective symptoms | Cortical area | Left pericalcarine gyrus | 2496.3 | 0.014 | –14.1, –79.6, 10.5 |
| Right pericalcalcarine gyrus | 3338.78 | 0.001 | 15.4, –73.9, 14.6 | ||
| Behavioral symptoms | Cortical thickness | Right precentral gyrus (M1) | 4507.69 | 0.0001 | 46.9, 0.6, 33.0 |
| Cortical area | Left precentral gyrus (M1) | 3328.08 | 0.001 | –48.6, –7.2, 41.4 | |
| Right superior frontal gyrus | 5173.09 | 0.0001 | 22.2, 20.7, 37.7 | ||
| Cortical folding | Left supramarginal gyrus | 819.17 | 0.043 | –58.7, –31.6, 36.0 | |
| Right inferior parietal gyrus | 1384.87 | 0.0009 | 38.1, –48.2, 38.1 | ||
| Right pericalcarine gyrus | 3364.83 | 0.0001 | 12.9, –83.2, 5.1 |
Significant clusters in both hemispheres: cluster size in mm², P values from the Monte Carlo simulation (P < .05) and clustering as cluster-wise probability (CWP), resulting from the vertex-wise comparison of mean cortical thickness, area and local gyrification index and NCRS scores. Age, gender, olanzapine equivalents, Positive and Negative Symptoms Scale, Abnormal involuntary movement scale, and Barnes Akathisia Rating Scale scores were used as covariates. Significant clusters that survived the Bonferroni correction (P < .0027) are displayed in bold font.
Fig. 3.
Localization of significant positive associations between Northoff catatonia rating scale motor score and cortical area in SSD patients with catatonia according to DSM-IV-TR (n = 34) (P < .05, cluster-wise probability-corrected). Lat, lateral view; med, medial view; LH, left hemisphere; RH, right hemisphere.
Discussion
This study is the first MRI study that aimed at investigating cortical measures of distinct genetic and neurodevelopmental origin underlying catatonia in SSD patients. Three main findings emerged: First, patients with catatonia showed reduced surface area in the right medial OFC and left SPG. Second, patients with catatonia showed hypergyrification in the left medial OFC and rostral ACC. Third, motor and behavioral catatonic symptoms were significantly associated with cortex variations in frontoparietal regions.
Owing to different diagnostic criteria and rating scales, different prevalence rates of catatonia have been reported in previous studies.29,58,59 In earlier studies on catatonia, the presence of at least 4 catatonic signs was necessary to diagnose catatonia leading to a high specificity, but a low prevalence rates.59,60 According to this observation, other authors suggested the use of 3 or more symptoms to diagnose catatonia.7,58 In a recent study, Morrens and colleagues have shown that there are large differences in the prevalence of catatonia classified according to Bush-Francis Catatonia Rating Scale (BFCRS; 2 or more of the BFCRS signs; 50.8%), DSM-IV-TR (at least 2 catatonic symptoms; 28.4%) and DSM-5 (at least 3 of 12 selected catatonic symptoms; 20.9%).29 Therefore, we decided to use the NCRS in this study because it represents a clinically acceptable compromise between conservative and liberal thresholds. We found a higher number of SSD patients with catatonia classified according to DSM-IV-TR (39%) rather than SSD patients with catatonia identified according to NCRS (28.7%) criteria; this is related to the different criteria that are more liberal in DSM-IV-TR (only the presence of 2 symptoms is required) and more strict in NCRS (3 symptoms with at least 1 from each domain, eg, affective, motor, and behavioral). Because the diagnosis of catatonia according to NCRS is more strict and conservative as it requires at least 3 catatonic symptoms from each of the 3 domains, this approach may also imply lower prevalence rates of catatonia when compared with DSM-IV-TR.
Morphological Differences Between Patients With and Without Catatonia (Categorical Approach)
Consistent with previous studies and our hypothesis, SSD patients with catatonia demonstrated reduced surface area predominantly in the medial OFC, SPG, and LOG when compared with non-catatonic individuals. The OFC is a key region that subserves a variety of high-order motor control processes,61–65 and hence OFC alterations may contribute to many neuropsychiatric disorders and their typical behavioral symptoms.5 SPG plays a crucial role in execution of skillful movements predominantly at the level of sensorimotor and visuospatial control.5 Another region responsible for perception and object recognition is LOG, which functionally overlaps with a face-selective region, ie, the fusiform cortex.5 Our data are in line with previous findings from functional MRI studies that have identified alterations in prefrontal (ie, OFC) and parietal (ie, SPG) regions of catatonic patients.31,36,66,67 Furthermore, our study endorses the dysfunction of the medial OFC, SPG, and LOG that can lead to the decoupling of motor control from the spatial integration of the body and face-selective perception. Such dysfunction leads to patients’ inability to coordinate and navigate their body in space and interact with others. Therefore, our results suggest that altered structure of this frontoparietal network could lead to insufficient inhibition, disordered cognitive functioning, aberrant spatial orientation, missing temporal organization of speech as well as behavior and development of corresponding catatonic motor and behavioral symptoms such as perseveration, akinesia, catalepsy, posturing, anxiety, and impulsivity, respectively.31,68 These findings reflect what has been coined as “spatiotemporal psychopathology,”69 a novel approach to psychopathological symptoms and their underlying spatiotemporal abnormalities.
On further inspection, we also found LGI alterations in the left medial OFC, rostral ACC, and both STG and ITG in SSD patients with catatonia when compared with non-catatonic individuals. In addition to medial OFC, the ACC is crucial for integrating cognitive and emotional processes (ie, negative emotions) in support of goal-directed behavior.70–72 Whereas STG is involved in managing both object-related and space-related information, ITG is involved in spatial orientation, estimation of depth, and distance as well as stereoscopic vision.73 Overall, structural alterations in the frontotemporal network might contribute to inability to perform goal-directed bodily actions and lead to akinesia, catalepsy, and posturing in SSD.
Finally, the earlier-mentioned findings are important from a neurodevelopmental perspective.74–76 Although cortical thickness undergoes changes during adulthood that are subject to a dynamic synaptic reorganization according to environmental influences,74,77–79 cortical area undergoes a specific developmental pathway that is related to changes in cortical gyrification (ie, LGI) during pre- and perinatal brain development.34,74 In sum, our study has identified morphological differences between catatonic and non-catatonic SSD patients with consistent evidence for surface area and LGI alterations including the frontoparietal network. In particular, the categorical approach might be interpreted as supportive for the relevance of cortical surface area and LGI as correlates of vulnerability or even developmental risk phenotype for catatonia in SSD.
Morphological Substrates of Specific Catatonia Dimensions (Dimensional Approach)
In line with our predictions, we demonstrate a significant negative association between motor and behavior dimension and cortex variations in superior frontal and precentral gyrus (M1). On one side, this finding points to the specificity of cortical thickness as a neural feature that develops over time and contributes to the pathophysiology of catatonic motor symptoms. Given that catatonic symptoms are considered to be a “specifier” of psychiatric illnesses and appear as transient phenomena, this finding seems very plausible. On the other side, surface and LGI variations in a frontoparietal network once again support the developmental risk phenotype for catatonia in SSD.
Furthermore, NCRS motor score was also associated with cortical area alterations in the insula, fusiform gyrus, and ITG. The insula essentially subserves emotional processing, social cognition and behavior, sensorimotor integration, language, decision-making, awareness, and consciousness as well as orofacial motor programs.80,81 The fusiform gyrus is crucial for reading, processing of spatial information, and face recognition as well as interpretation of facial expression in SSD.82 Both regions are relevant in the pathogenesis of aberrant motor performance of the body and limbs leading to characteristic catatonic phenomena such as mannerisms, “Gegenhalten,” athetotic movements, dyskinesias, and catalepsy, respectively. Furthermore, the significant association between NCRS motor scores and surface area in the ITG corroborates previous studies83–85 showing that the temporal lobe might be involved in impulsive behavior,86 which also occurs in catatonia (ie, sudden muscular tone alterations).
We also found a cluster of pronounced association between NCRS behavior score and LGI in the pericalcarine cortex consisting of the primary visual cortex (V1) and the precuneus. Alterations in this region lead to aberrant processing of movement and spatial related stimuli.85,87 Further, relationship between behavioral symptoms and LGI in the precentral gyrus, ITG, and pericalcarine gyrus might suggest that task-focused activity requires both visuospatial perception and motor initiation/coordination. Aberrant structure of this network might lead to akinesia, hyperkinesia, and impulsive as well as repetitive behavior.
Contrary to our expectations, we did not identify any significant associations between NCRS affective score and frontoparietal cortical variations. At best, one could speculate that affective catatonic symptoms are secondary compensatory reactions to the primary sensorimotor and behavioral abnormalities. If a patient is unable to navigate in space and has lost his/her temporal organization (motor and behavioral), he/she might develop affective symptoms such as anxiety, staring, or ambivalence.36 Eventually, affective symptoms are transient phenomena that may be better reflected in the neuronal activity dynamics rather than variations of brain structure.
Strengths and Limitations
A major strength of this study involves the excellent matching of both study groups, moderate sample size of SSD patients with catatonia, and the replication of previous studies regarding frontoparietal alterations underlying catatonia in SSD. Regarding potential limitations, it appears important to acknowledge the cross-sectional design, use of antipsychotic medication, and lack of healthy controls. Another potential limitation is the fact that we did not examine first-episode SSD patients. Still, we did not find any associations between duration of illness and motor symptoms or OLZ. Finally, we focused on cortical morphology only, so that we cannot appreciate contributions of subcortical structures, particularly the basal ganglia to catatonia.88
Conclusion
This work provides first evidence for associations between markers of early cortical development disturbance and catatonia in SSD. Our results have shown specific structural alterations in a frontoparietal neural network that includes the superior frontal gyrus, lateral and medial OFC, rostral ACC, M1, and SPG.36 The data suggest that distinct dimensions of catatonia are associated with different patterns of abnormal brain structure.
Funding
German Research Foundation (DFG) (grant number DFG HI 1928/2-1 to D.H. and WO 1883/6-1 to R.C.W.). The DFG had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Supplementary Material
Acknowledgment
We are grateful to all the participants and their families for their time and interest in this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kleinhaus K, Harlap S, Perrin MC, et al. . Catatonic schizophrenia: a cohort prospective study. Schizophr Bull. 2012;38(2):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solmi M, Pigato GG, Roiter B, et al. . Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophr Bull. 2018;44:1133–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66(2):77–92. [DOI] [PubMed] [Google Scholar]
- 5. Hirjak D, Meyer-Lindenberg A, Fritze S, Sambataro F, Kubera KM, Wolf RC. Motor dysfunction as research domain across bipolar, obsessive-compulsive and neurodevelopmental disorders. Neurosci Biobehav Rev. 2018;95:315–335. [DOI] [PubMed] [Google Scholar]
- 6. Walther S, Bernard JA, Mittal VA, Shankman SA. The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol Med. 2019;49:212–216. [DOI] [PubMed] [Google Scholar]
- 7. Northoff G, Koch A, Wenke J, et al. . Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14:404–416. [DOI] [PubMed] [Google Scholar]
- 8. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013(441):1–47. [DOI] [PubMed] [Google Scholar]
- 9. Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36(2):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francis A, Fink M, Appiani F, et al. . Catatonia in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. J ECT. 2010;26(4):246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256–262. [DOI] [PubMed] [Google Scholar]
- 12. Kirby GH. The catatonic syndrome and its relation to manic-depressive insanity. J Nerv Mental Dis. 1913;40:694–704. [Google Scholar]
- 13. Lange J. Katatonische Erscheinungen im Rahmen manisch-depressiver Erkrankungen. Berlin: Springer; 1922. [Google Scholar]
- 14. Tandon R, Heckers S, Bustillo J, et al. . Catatonia in DSM-5. Schizophr Res. 2013;150:26–30. [DOI] [PubMed] [Google Scholar]
- 15. Sass H., Wittchen HU, Zaudig M, Houben I.. Diagnostisches und Statistisches Manual Psychischer Störungen DSM-IV-TR: Textrevision. Auflage 1. Göttingen, Germany: Hogrefe Verlag; 2003. [Google Scholar]
- 16. Paulzen M, Schneider F. [Schizophrenia and other psychotic disorders in DSM-5: summary of the changes compared to DSM-IV]. Nervenarzt. 2014;85:533–542. [DOI] [PubMed] [Google Scholar]
- 17. Rogers D. Catatonia: a contemporary approach. J Neuropsychiatry Clin Neurosci. 1991;3(3):334–340. [DOI] [PubMed] [Google Scholar]
- 18. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137–143. [DOI] [PubMed] [Google Scholar]
- 19. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. [DOI] [PubMed] [Google Scholar]
- 20. Braunig P, Kruger S. [Catatonia]. Psychiatrische Praxis. 2005;32(suppl 1):S7–S 24. [DOI] [PubMed] [Google Scholar]
- 21. Bräunig P, Krüger S, Shugar G, Höffler J, Börner I. The catatonia rating scale I–development, reliability, and use. Compr Psychiatry. 2000;41(2):147–158. [DOI] [PubMed] [Google Scholar]
- 22. Krüger S, Bagby RM, Höffler J, Bräunig P. Factor analysis of the catatonia rating scale and catatonic symptom distribution across four diagnostic groups. Compr Psychiatry. 2003;44:472–482. [DOI] [PubMed] [Google Scholar]
- 23. Hirjak D, Thomann PA, Northoff G, Kubera KM, Wolf RC. [German version of the Northoff catatonia rating scale (NCRS-dv): a validated instrument for measuring catatonic symptoms]. Nervenarzt. 2017;88(7):787–796. [DOI] [PubMed] [Google Scholar]
- 24.Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Verlag August Hirschwald; 1874. [Google Scholar]
- 25. Starkstein SE, Petracca G, Tesón A, et al. . Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiatry. 1996;60(3):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ungvari GS, Caroff SN, Gerevich J. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophr Bull. 2010;36(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ungvari GS. Catatonia in DSM 5: controversies regarding its psychopathology, clinical presentation and treatment response. Neuropsychopharmacol Hung. 2014;16(4):189–194. [PubMed] [Google Scholar]
- 28. van der Heijden FM, Tuinier S, Arts NJ, Hoogendoorn ML, Kahn RS, Verhoeven WM. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3–8. [DOI] [PubMed] [Google Scholar]
- 29. Stuivenga M, Morrens M. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry. 2014;5:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555–77; discussion 578. [DOI] [PubMed] [Google Scholar]
- 31. Northoff G, Kötter R, Baumgart F, et al. . Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull. 2004;30(2):405–427. [DOI] [PubMed] [Google Scholar]
- 32. Weinberger DR. Future of days past: neurodevelopment and schizophrenia. Schizophr Bull. 2017;43(6):1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitgen MM, Depping MS, Bach C, et al. . Aberrant cortical neurodevelopment in major depressive disorder. J Affect Disord. 2019;243:340–347. [DOI] [PubMed] [Google Scholar]
- 34. Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 2013;23(11):2521–2530. [DOI] [PubMed] [Google Scholar]
- 35. Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10(10):724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Northoff G. Brain imaging in catatonia: current findings and a pathophysiologic model. CNS Spectr. 2000;5(7):34–46. [DOI] [PubMed] [Google Scholar]
- 37. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62(4):404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Northoff G, Steinke R, Czcervenka C, et al. . Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 40. Leucht S, Samara M, Heres S, et al. . Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan AR, Wang L, Beg MF. FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using Large Deformation Diffeomorphic Metric Mapping. Neuroimage. 2008;41(3):735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 44. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 45. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. [DOI] [PubMed] [Google Scholar]
- 46. Ségonne F, Dale AM, Busa E, et al. . A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- 47. Fischl B, van der Kouwe A, Destrieux C, et al. . Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 48. Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 49. Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran JP, Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J Vis Exp. 2012;( 59):e3417. doi:10.3791/3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nesvåg R, Schaer M, Haukvik UK, et al. . Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr Res. 2014;152(2-3):333–338. [DOI] [PubMed] [Google Scholar]
- 51. Klein D, Rotarska-Jagiela A, Genc E, et al. . Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One. 2014;9(1):e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull. 2014;40(3):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schaer M, Ottet MC, Scariati E, et al. . Decreased frontal gyrification correlates with altered connectivity in children with autism. Front Hum Neurosci. 2013;7:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hirjak D, Kubera KM, Wolf RC, et al. . Local brain gyrification as a marker of neurological soft signs in schizophrenia. Behav Brain Res. 2015;292:19–25. [DOI] [PubMed] [Google Scholar]
- 55. Schultz CC, Koch K, Wagner G, et al. . Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116(2-3):204–209. [DOI] [PubMed] [Google Scholar]
- 56. Schultz CC, Nenadic I, Koch K, et al. . Reduced cortical thickness is associated with the glutamatergic regulatory gene risk variant DAOA Arg30Lys in schizophrenia. Neuropsychopharmacology. 2011;36(8):1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Thomann PA. Multiparametric mapping of neurological soft signs in healthy adults. Brain Struct Funct. 2016;221(3):1209–1221. [DOI] [PubMed] [Google Scholar]
- 58. Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010;118(1–3):168–175. [DOI] [PubMed] [Google Scholar]
- 59. Ungvari GS, Leung HC, Lee TS. Benzodiazepines and the psychopathology of catatonia. Pharmacopsychiatry. 1994;27(6):242–245. [DOI] [PubMed] [Google Scholar]
- 60. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry. 1990;51(9):357–362. [PubMed] [Google Scholar]
- 61. Tabibnia G, Monterosso JR, Baicy K, et al. . Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31(13):4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. [DOI] [PubMed] [Google Scholar]
- 63. Whelan R, Conrod PJ, Poline JB, et al. ; IMAGEN Consortium Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15(6):920–925. [DOI] [PubMed] [Google Scholar]
- 64. Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20(3):255–261. [DOI] [PubMed] [Google Scholar]
- 65. Swann N, Tandon N, Canolty R, et al. . Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29(40):12675–12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Northoff G, Braus DF, Sartorius A, et al. . Reduced activation and altered laterality in two neuroleptic-naive catatonic patients during a motor task in functional MRI. Psychol Med. 1999;29(4):997–1002. [DOI] [PubMed] [Google Scholar]
- 67. Northoff G, Steinke R, Nagel DCzerwenka C, et al. . Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol Med. 2000;30(3):583–596. [DOI] [PubMed] [Google Scholar]
- 68. Richter A, Grimm S, Northoff G. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol. 2010;25(1):55–62. [DOI] [PubMed] [Google Scholar]
- 69. Northoff G. The brain’s spontaneous activity and its psychopathological symptoms—“Spatiotemporal binding and integration”. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(pt B):81–90. [DOI] [PubMed] [Google Scholar]
- 70. Fornito A, Yücel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35(5):973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fornito A, Yücel M, Wood SJ, et al. . Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. Br J Psychiatry. 2009;194(5):426–433. [DOI] [PubMed] [Google Scholar]
- 72. Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. [DOI] [PubMed] [Google Scholar]
- 73. Victor M, Ropper AH, Adams RD.. Adams & Victor’s Principles of Neurology (Englisch). Vol 7th. New York: Mcgraw-Hill Professional; 2000. [Google Scholar]
- 74. Vijayakumar N, Allen NB, Youssef G, et al. . Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37:2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci USA. 1995;92(25):11323–11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rakic P. The development of the frontal lobe. A view from the rear of the brain. Adv Neurol. 1995;66:1–6; discussion 6. [PubMed] [Google Scholar]
- 77. Smith GN, Thornton AE, Lang DJ, et al. . Cortical morphology and early adverse birth events in men with first-episode psychosis. Psychol Med. 2015;45:1825–1837. [DOI] [PubMed] [Google Scholar]
- 78. Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36(5):275–284. [DOI] [PubMed] [Google Scholar]
- 79. Oostermeijer S, Whittle S, Suo C, et al. . Trajectories of adolescent conduct problems in relation to cortical thickness development: a longitudinal MRI study. Transl Psychiatry. 2016;6(6):e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. Functional organization of the insula and inner Perisylvian regions. Proc Natl Acad Sci USA. 2012;109(25):10077–10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. [DOI] [PubMed] [Google Scholar]
- 82. Lee CU, Shenton ME, Salisbury DF, et al. . Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59(9):775–781. [DOI] [PubMed] [Google Scholar]
- 83. Gardini S, Cloninger CR, Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull. 2009;79(5):265–270. [DOI] [PubMed] [Google Scholar]
- 84. Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R. Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Res. 2011;1371:32–42. [DOI] [PubMed] [Google Scholar]
- 85. Schilling C, Kühn S, Romanowski A, Schubert F, Kathmann N, Gallinat J. Cortical thickness correlates with impulsiveness in healthy adults. Neuroimage. 2012;59(1):824–830. [DOI] [PubMed] [Google Scholar]
- 86. Lyoo IK, Pollack MH, Silveri MM, et al. . Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl). 2006;184(2):139–144. [DOI] [PubMed] [Google Scholar]
- 87.Hirjak D, Thomann AK, Kubera KM, Wolf RC, Jeung H, Maier-Hein KH, Thomann PA. Cortical folding patterns are associated with impulsivity in healthy young adults. Brain Imaging Behav. 2017;11(6):1592–1603. doi:10.1007/s11682-016-9618-2. [DOI] [PubMed] [Google Scholar]
- 88. Walther S, Schäppi L, Federspiel A, et al. . Resting-State hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull. 2017;43(5):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.