Abstract
Background
Characterizing the link between childhood trauma and adult neurocognitive function in psychosis is crucial for improving the fields understanding of how early environmental risk factors impact the presentation of the disorder. To date, the literature has been inconsistent: meta-analytic synthesis is lacking, and it is unclear whether specific cognitive functions are affected.
Methods
A meta-analysis was performed on a total of 3315 subjects with a psychotic disorder. The links between childhood trauma, overall neurocognitive function, and four cognitive subdomains (working memory, executive function, verbal/visual memory, and attention/processing speed) were examined. Relevant sample characteristics and methodological moderators were tested. The strength of the association between trauma and overall neurocognition in individuals with psychotic disorders was also compared to that of healthy controls.
Results
Among individuals with psychotic disorders, there was a significant association between overall cognition and childhood trauma, r = −.055; 95% CI = −0.09, −0.02, P = .002. There was also a modest, negative relationship between childhood trauma and working memory, r = −.091; 95% CI = −0.15, −0.03, P = .002. Moderators did not have a significant effect on these analyses. Further, the association between childhood trauma and neurocognition was significantly stronger in healthy controls compared to patients with a psychotic disorder.
Conclusion
A small negative association was found between overall cognition and childhood trauma in individuals with psychotic disorders. Results suggest the association is less strong for individuals with a psychotic disorder compared to healthy populations. Findings are informative for prominent etiological models of psychosis.
Keywords: neurocognition, psychosis, childhood trauma, early life stress, working memory
Introduction
Childhood trauma, including exposure to physical, sexual, and emotional abuse, as well as to emotional and physical neglect,1 has been implicated as a risk factor for development of psychotic disorders.2–4 An increasing body of evidence suggests that exposure to early life trauma may affect the presentation of some observed symptoms as well as neurocognitive deficits in psychosis.5 Despite this increasing recognition, the relationship between early life trauma exposure and neurocognitive function in psychosis has, until recently, been grossly understudied.6,7 The literature is not conclusive on whether effects are uniform across all neurocognitive domains (global vs specific neurocognitive function), which would aid understanding of underlying neurodevelopmental mechanisms. Further, case–control comparisons have seldom been undertaken.
In both animal and healthy human population studies, exposure to trauma during periods of greater neurodevelopmental plasticity has been linked to lasting impairments in brain function.8 Trauma exposure has also been strongly linked to disturbances in critical developmental skills (ie, emotion regulation, social communication) and increased levels of chronic stress, which likely contribute to the development and progression of neurocognitive dysfunction across the life span.2,8–10 Numerous theories link the effects of trauma on affect, cognition, social, and role function to increased risk for developing psychotic symptoms.11,12 Indeed, childhood trauma has long been observed as a psychosis risk factor, with some studies supporting a causal relationship between trauma exposure and development of the disorder.3,13 For example, studies suggest individuals diagnosed with a psychotic disorder are more likely to report previous exposure to traumatic life events relative to healthy controls and unaffected siblings.10,14,15 In addition, recent studies have found that a history of trauma can predict symptom severity (ie, severity of delusions and hallucinations), as well as treatment resistance and number of hospitalizations after psychosis onset.10,13 However, despite neurocognitive deficits being a core facet of the presentation of schizophrenia, the link between childhood trauma and neurocognitive function to this date is not fully understood. Several critical questions remain regarding potential mechanisms through which neurocognitive function could relate to childhood trauma.
Neurocognitive dysfunction is a core feature of psychosis.16 Neurocognitive assessment can aid in predicting illness severity, social function, and occupational function, and is highly informative for understanding the etiology of the disorder.16–18 However, research exploring the association between childhood trauma and neurocognition in psychotic populations has produced mixed results. Previous studies of these associations have measured both overall neurocognition and specific cognitive functions with mixed outcomes.19–30 For example, Aas and colleagues2,31,32 reported associations between childhood trauma and specific cognitive domains, such as working memory, verbal memory, and executive function (EF). However, more recent well-powered studies have reported null results with regard to overall cognition.33,34 Given divergence in the types of neurocognition explored, many questions have yet to be answered. Moreover, little attention has been given to relevant moderators or methodological and sample characteristics that may be adding to the inconsistent findings. Furthermore, meta-analytic evidence suggests that childhood trauma has a robust effect on neurocognition in otherwise healthy individuals.35 Measuring whether this effect is present in individuals with a psychotic disorder, and whether it is quantifiably different to the effect found in healthy populations would inform the field’s understanding of the pathogenesis of psychosis.
Despite an excellent qualitative review by Aas and colleagues,2 there are currently no meta-analytic studies evaluating general and specific neurocognitive functions in relation to trauma exposure in psychosis. As noted, extant studies have rarely contrasted diverging neurocognitive domains, or carefully considered relevant moderators such as age, gender, and type of psychotic disorder.22,32 The present systematic review and meta-analysis sought to address these gaps by determining whether childhood trauma is associated with distinct neurocognitive domains in individuals with a psychotic disorder. In addition to examining specific neurocognitive processes, we also evaluated overall neurocognitive function. Understanding specific and global areas of dysfunction may help to shed light on mechanisms underlying the association. The review also examined whether moderating factors influence the relationship between childhood trauma and adult neurocognition. Findings may help to shed light on factors contributing to the discrepant findings in the existing literature. Finally, to supplement the review, exploratory analyses compared the strength of the association between childhood trauma and overall neurocognition in psychotic disorder patients and healthy controls. Taken together, this information will aid in developing and refining mechanism-specific theories as well as informing efforts for improving prediction, early identification, and intervention.
Methods
Search Strategy
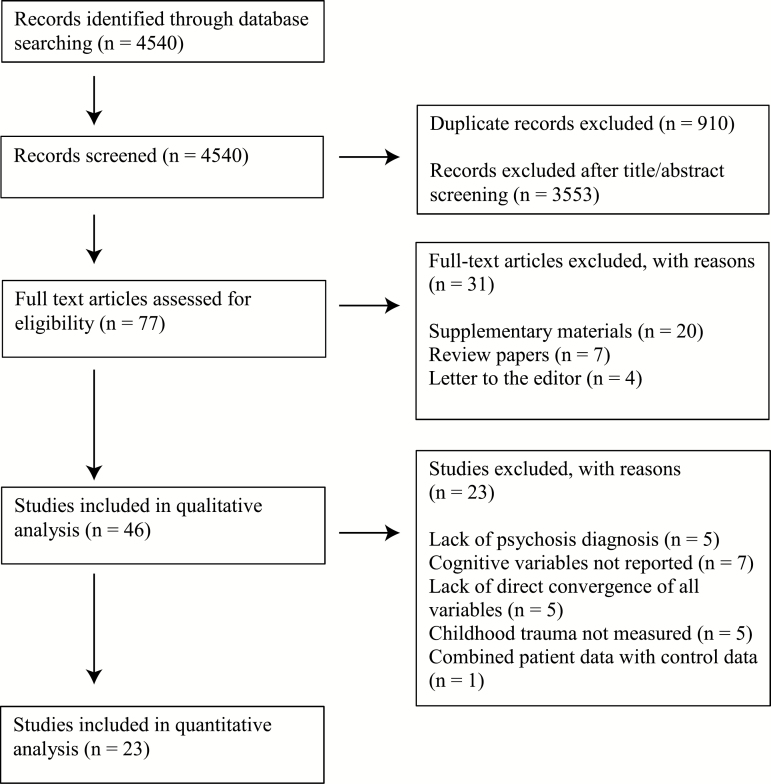
This study was conducted according to PRISMA guidelines. PubMed, Ovid Medline, and Web of Science were searched on July 28, 2018 (see figure 1). Search terms for childhood trauma (Child* abuse OR child* neglect OR child* trauma OR child* maltreatment OR child* adversity OR early life stress OR early life trauma) were combined with search terms for cognition (cognit* OR neurocognit* OR neuropsych*) and search terms for psychosis and related disorders that could have psychotic features (psychosis OR psychotic OR schizophreni* OR schizot* OR schizo* OR delusi* OR bipolar OR depressi*) with AND commands.
Fig. 1.
Flowchart of selected studies.
Study Selection and Characteristics
The studies included for overall cognition and cognitive subdomain analyses provided quantitative, published data in adults (≥18 without overlapping samples, 23 including partially overlapping samples) with a diagnosis of a psychotic disorder. Psychotic disorder diagnoses included schizophrenia, schizoaffective disorder, schizophreniform disorder, psychosis not otherwise specified/delusional disorder, bipolar disorder with psychotic features, and/or depression with psychotic features. Diagnoses were determined by standardized, validated diagnostic assessments meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Version (DSM-IV)21–25,27,28,30–34,36,37 and/or the International Classification of Diseases, Tenth Revision (ICD-10) criteria.19,20,26,29,32,38–41 To meet inclusion criteria, studies were required to provide data on (1) variables from neuropsychological testing (normed, formally validated batteries of cognitive function [see table 1]) and (2) childhood trauma, as well as data on the relationship between these two variables, such that an effect size statistic could be computed. Studies were excluded if they reported data including both control participants and psychosis groups, rather than reporting the associations of diagnostic groups separately. Two reviewers (T.V. and M.A.) independently reviewed full articles with relevant titles and abstracts with an 89.9% agreement rate, and discrepancies were resolved through discussion. When more than one published study used the same subjects, the study with the larger sample size was chosen to maximize power. The included studies for overall cognition contained 3315 diagnosed participants.
Table 1.
Study Characteristics
| First Author (Year) | N | % Female | Mean Age | Dxa Affective or FEP | Covariates | Trauma Measure | Cognitive Domains | Cognitive Measure | Nonindependent Samples (First Author [Year]) | Excluded From Main Analyses |
|---|---|---|---|---|---|---|---|---|---|---|
| Aas (2011a)32 | 138 | 47.1 | 30.6 | FEP, including affective psychosis | Education and ethnicity | CECA.Q | Overall cognition, EF, WM, memory, attention | RAVLT, WMS-R, LNS, TMT, DS, Raven’s Colored Progressive Matrices | Aas (2012a)40 | |
| Aas (2011b)39 | 30 | 33.33 | 30.1 | FEP | N/A | CECA.Q | Overall cognition, WM, memory, attention | WMS III, TMT, SWM, WAIS III | -- | |
| Aas (2012a)40 | 83 | 37.3 | 27.4 | FEP | N/A | CECA.Q | Overall cognition, EF, WM, memory, attention | WAIS-R, Raven’s Colored Progressive Matrices, TMT | Aas (2011a)32 | √ |
| Aas (2012b)31 | 406 | 47.29 | 30.7 | Including affective psychosis | Age and gender | CTQ | Overall cognition, EF, WM, memory | CVLT, LNS, DS, WASI | Aas (2012c)36 Aas (2013)37 | |
| Aas (2012c)36 | 118 | 55.1 | 32.2 | Including affective psychosis | Age, gender, and paternal education | CTQ | Overall cognition, EF, WM, memory | WASI-III, LNS, DS, CVLT | Aas (2012a)40 Aas (2013)37 | √ |
| Aas (2013)37 | 249 | 51 | 30.7 | Including affective psychosis | Age, gender, diagnosis | CTQ | Overall cognition, EF, WM, memory | WASI, D-KEFS, LNS, DS, CVLT | Aas (2012a)40 Aas (2012c)36 | √ |
| Campbell (2013)29 | 30 | 40 | 31.8 | FEP, including affective psychosis | Premorbid IQ | TEC, PDS, TREQ | IQ, overall cognition, EF, WM, memory, attention | WASI, NART, Rey- Osterieth Complex Figure, Hayling & Brixton, Corsi Block Tapping | — | |
| Garcia (2016)24 | 79 | 36.8 | 25.3 | Including affective psychosis | Age, gender, education status | CTQ | Overall cognition, WM, memory, attention | MCCB | — | |
| Green (2014)26 | 617 | 32.74 | 39.7 | Including affective psychosis | COMT genotype | CAQ | Overall cognition, EF, memory, attention | COWAT, LNS, WAIS III, WTAR, RBANS |
Green (2015)41 | |
| Green (2015)41 | 444 | 32.7 | 39.7 | Including affective psychosis | FK506 binding protein genotypes | CAQ | Overall cognition, EF, memory, attention | WTAR, LNS, COWAT, RBANS | Green (2014)26 | √ |
| Kelly (2016)22 | 80 | 30 | 32.5 | Including affective psychosis | N/A | CTQ | Overall cognition | RBANS | — | |
| Killian (2017)63 | 56 | 25 | 23.8 | FEP | N/A | CTQ | Overall cognition, WM, memory, attention | RBANS | — | |
| Li (2017)21 | 162 | 64.19 | 37.8 | Including affective psychosis | N/A | CTQ-SF | Overall cognition, EF, memory, attention | RBANS | — | |
| Lysaker (2001)28 | 43 | 0 | 45 | Including affective psychosis | N/A | Clinical interview | Overall cognition, EF, WM, memory, attention | WCST, LNS, WAIS | — | |
| Mansueto (2017)30 | 532 | 24.4 | 27.6 | N/A | Age and gender | CTQ-SF | Overall cognition, EF, memory, attention | RST, WLT, CPT | — | |
| Quide (2017)20 | 50 | 56.5 | 37.7 | Including affective psychosis | N/A | CTQ | IQ, WM | WASI, NBack | bGreen (2014, 2015), Quide (2018) | |
| Quide (2018)38 | 79 | 43.04 | 42.52 | Including affective psychosis | Age and gender | CTQ | Overall cognition, memory, attention, WM | RBANS, LNS, COWAT | bGreen (2014, 2015), Quide (2017) | |
| Ruby (2017)23 | 17 | 28.57 | 31.5 | Including affective psychosis | N/A | ETI | IQ, overall cognition, EF, memory | WMS-R, WAIS, FAS | — | |
| Schalinski (2017)19 | 168 | 33.33 | 27.9 | Including affective psychosis | N/A | MACE | Overall cognition, WM, memory, attention | MCCB | — | |
| Schenkel (2005)27 | 40 | 37.5 | 41.9 | Including affective psychosis | N/A | Clinical interview | IQ, overall cognition, EF | COWAT, Hayling & Brixton Tests, Shipley IQ | — | |
| Shannon (2011)25 | 85 | 21.18 | 41.1 | N/A | IQ and current depressive symptoms | CTQ | Overall cognition, memory | WMS-III | — | |
| Sideli (2014)34 | 134 | 35.07 | 29.4 | N/A | N/A | CECA.Q | IQ, overall cognition, EF, WM, memory, attention | NART, WAIS-III, WMS-III, TMT, DS | — | |
| Van Os (2017)33 | 698 | 23.94 | 27.6 | N/A | Age, sex, ethnic group, education level, symptom score, and cannabis use | CTQ | IQ, overall cognition | WAIS IQ | — |
Note: CECA.Q, childhood experience of care and abuse questionnaire; COWAT, Controlled Oral Word Association Test; CTQ, Childhood Trauma Questionnaire; CVLT, California Verbal Learning Test; DS, Digit Symbol; EF, executive function; FEP, first-episode psychosis; LNS, letter number sequencing; MACE, Maltreatment and Abuse Chronology of Exposure; MCCB, MATRICS Consensus Cognitive Battery; NART, National Adult Reading Test; PDS, Post-Traumatic Diagnostic Scale; RAVLT, Rey Auditory Verbal Learning Test; RST, response shifting tasks; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SWM, Spatial Working Memory (from the Cambridge Neuropsychological Test Automated Battery [CANTAB59]); TEC, Traumatic Experiences Checklist; TMT, Trail Making Test; TREQ, troubles related experiences questionnaire; WAIS, Wechsler Adult Intelligence Scale; WM, Working Memory; WMS-R, Wechsler Memory Scale-Revised; WTAR, Wechsler Test of Adult Reading.
aIndicates whether authors specified that FEP was recruited, as well as whether authors specified that sample included affective psychosis Dx individuals. N/A indicates that authors did not report either of the two.
bQuide (2017, 2018) and Green (2014 and 2015) both recruited from Schizophrenia Research Banks, therefore samples are not independent; whereas Quide reported IQ and working memory measures, Green (2014, 2015) reported overall cognition, EF, attention/processing speed, and verbal/visual memory. Because Green and colleagues’ sample size is larger, Green was chosen for overall cognition, whereas Quide et al. was designated for working memory and IQ domains.
Quality and Selective Reporting
The Downs and Black checklist42 was used to assess methodological quality and risk of bias of included studies (see supplementary table S1). This can be instructive for determining implications of the current findings, and more generally for improving understanding of the social epidemiology of psychosis. Selective reporting was assessed by marking “Outcome not reported” in cases where childhood trauma types were measured but not reported with regards to cognition (see supplementary table S2).
Outcome Variable Characteristics
Childhood Trauma.
For 12 studies included in main analyses, childhood trauma exposure was dichotomized, and for 11 it was measured on a continuous scale (see table 2). For studies that dichotomized childhood trauma, the effect size statistic was converted to a point-biserial r after correcting for potential uneven split between groups, such that increasing values indicated exposure to childhood trauma. For studies that reported childhood trauma on a continuous scale (reflecting a sum of items with more items indicating greater trauma exposure), an r statistic was calculated, with increasing values also indicating increased exposure to childhood trauma.
Table 2.
Trauma Sample Characteristics of Studies Included in Main Analyses
| Author (year) | Trauma Binary? | Coded Trauma Type(s) |
|---|---|---|
| Aas (2011a)32 | Yes | Total CTQ1 score |
| Aas (2012b)31 | No | CTQ1 Physical abuse, sexual abuse, emotional abuse, emotional neglect, physical neglect |
| Aas (2011b)39 | No | Total CECA-Q90 score |
| Campbell (2013)29 | Yes | Total trauma score from TEC,91 PDS,92 TREQ93 |
| Garcia (2016)24 | No | Total CTQ,1 emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect |
| Green (2014)26 | No | CAQ94 Physical abuse, emotional abuse, emotional neglect |
| Kelly (2016)22 | Yes | CTQ1 Physical abuse |
| Killian (2017)63 | No | CTQ1 Abuse and neglect |
| Li (2017)21 | No | Total CTQ,1 emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect |
| Lysaker (2001)28 | Yes | Sexual trauma collected from clinical interview |
| Mansueto (2017)30 | Yes | CTQ1 total |
| Quide (2018)38 | Yes | CTQ1 total |
| Ruby (2017)23 | No | ETI95 total |
| Schalinski (2017)19 | No | MACE96 abuse and neglect |
| Schenkel (2005)27 | Yes | Physical abuse, sexual abuse and neglect coded from clinical interview |
| Shannon (2011)25 | Yes | CTQ1 total |
| Sideli (2014)34 | Yes | CECA-Q90 physical abuse and sexual abuse |
| Van Os (2017)33 | Yes | CTQ1 total |
Note: CECA.Q, childhood experience of care and abuse questionnaire; CTQ, Childhood Trauma Questionnaire; ETI, Early Trauma Inventory; MACE, Maltreatment and Abuse Chronology of Exposure scale; PDS, Post-Traumatic Diagnostic Scale; TEC, Traumatic Experiences Checklist; TREQ, troubles related experiences questionnaire.
In this review, we employed a standard definition for physical, sexual, and emotional abuse, as an act causing injury or trauma in the respective domains; this approach is consistent with other work in this area.1 Emotional neglect was defined as failure by a caretaker to meet basic emotional and psychological needs, and physical neglect constituted failure to provide for a child’s basic physical needs, such as food, shelter, clothing, safety, and health care (see table 2 for details on types of trauma reported).1
Neurocognitive Variables.
Cognitive data were initially considered in 8 primary domains, including overall cognitive ability/IQ, EF, working memory, verbal/visual memory, attention/processing speed, perception/visuospatial abilities, premorbid IQ, and social cognition. To be included in analyses using a random effects model, it was determined that a domain should have at least 5 studies with reported data, as is recommended in cases when nontrivial heterogeneity is expected.43 As a result, enough data was reported to analyze 5 of the initial cognitive domains.
Overall Cognitive Ability
Overall cognitive ability (k = 17) was assessed by averaging reported Wechsler Abbreviated Scale of Intelligence (WASI) scores (k = 4),44 Wechsler Adult Intelligence Scale (WAIS)-III scores (k = 6),45 Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score (k = 2),46 MATRICS Consensus Cognitive Battery (MCCB) total score (k = 3),47 Shipley IQ (k = 1),48 Wechsler Test of Adult Reading (WTAR) (k = 1),49 and by averaging scores on the Word Learning Task (WLT), Continuous Performance Test (CPT), and response shifting tasks (RST) tests (k = 1).50–52 In the case of 4 studies, a total WAIS estimate was derived from partial reporting of specific WAIS subtests (vocabulary and comprehension subtests, similarity and vocabulary, verbal subtest questions about geography and literature, and finally digit symbol and letter number sequencing [LNS], respectively).45,53 To provide a more specific index of IQ, an additional separate subdomain was created to compare studies that reported a complete WASI or WAIS score (k = 6).
Executive Function
EF (k = 10) was measured through tasks measuring phonological and semantic fluency, including the WAIS-R category fluency (semantic [“body parts,” “fruits,” and “animals”]) and letter fluency (phonemic [F, A, and S]) tasks,53 Controlled Word Association Test (COWAT)-FAS (the most common version of the COWAT, which uses the letters F, A, and S),54 COWAT Animals total,54 the COWAT,55 the RBANS language dimension including semantic fluency,46 the Wisconsin Card Sort Test (WCST),56 RST,52 and Hayling and Brixton test errors.57
Working Memory
The working memory domain (k = 10) was derived from the LNS and/or digit span tasks, assessed via WMS, MCCB, and WAIS-III tests.45,47,58 A study using the Nback task20 as well as another using the Cambridge Neuropsychological Test Battery Spatial Working Memory Test59 were also included.
Verbal/Visual Memory
The verbal/visual memory domain (k = 14) comprised performance on the Rey Auditory Verbal Learning Test60; the visual reproduction, paired associates, recognition, and recall subtests of the Wechsler Memory Scale (WMS)-R58; the California Verbal Learning (CVLT)56;WMS-III verbal and nonverbal memory and visual reproduction58; MCCB and RBANS verbal and visual learning46,47; the WLT composite50; and the WMS-III logical memory, word lists, and visual reproduction.58
Attention and Processing Speed
The attention and processing speed domain (k = 9) included Trail Making Test (TMT)-A, WAIS-R, and WAIS-III digit symbol subtests45,53; MCCB and RBANS attention subtests46,47; and the CPT-HQ subtest.51
Moderators
Moderators examined included mean age and percentage of female, use of covariates (eg, age, gender, premorbid IQ), recruitment of samples including affective psychotic disorders, and use of first-episode psychotic populations. Mean age and gender were chosen as they have been shown to influence outcome variables.61,62 Potentially relevant methodological moderators were chosen; these consisted of use of first-episode psychosis (FEP) populations and recruitment of samples including affective psychosis, sample size, as well as use of covariates. Variables including use of covariates, recruitment of FEP, and recruitment of affective psychosis were dummy coded. Some relevant moderators (including years of illness, race, substance use, and antipsychotics dosage) could not be directly analyzed because a majority of the studies did not provide sufficient data to examine these variables.
Healthy Control Comparisons
A recent meta-analytic review found that there was a robust association between childhood trauma and cognitive function in otherwise healthy individuals.35 The current study sought to explore whether the association between childhood trauma and overall cognition differed between healthy individuals and those with a psychotic disorder. To this end, included studies that additionally reported control data (k = 7)32–34,38,39,41,63 were tested for difference of effect using a Fisher’s Z transformation. Within each diagnostic group, Fisher’s Z was calculated by averaging the r value, and summing the total number of subjects within diagnostic categories. A z value corresponding to an alpha level of 0.05 (± 1.96) was considered to be a significantly distinct effect across diagnoses.
Meta-analytical Procedure
Analyses were run using an R script (written and published by Daniel S. Quintana) for correlational meta-analyses using the robumeta64 and metafor65 packages for R.66,67 For each cognitive test, a Pearson’s r estimate was calculated. In the case of 10 studies, the associations between childhood trauma and cognition were converted to a Cohen’s d, and then to a point-biserial correlation estimate. A correlational approach was chosen given that converting r statistics to Cohen’s d for an inherently continuous variable can result in an underestimation of the effect.68 In the case of reported Spearman’s r, values were converted to a t score and then converted to Pearson’s r. Reported beta values were converted to t scores, and then converted to Pearson’s r estimates. Pearson’s r estimates were transformed to Fisher’s Z and sample variances were estimated. A random-effects model was used.
Study heterogeneity was calculated using the I2 test, which is not sensitive to number of studies included, with 25%, 50%, and 75% constituting low, moderate, and large amounts of heterogeneity, respectively.69 Homogeneity of mean weighed effect sizes was assessed using the Q test. Bias was assessed through inspection of funnel plots, and through the Eggers regression test, which is well suited for smaller meta-analyses.70 The metafor R package was used to identify potential outliers and influential cases.71 Meta-regressions were used to assess the effects of moderator variables on observed between-group differences. Meta-regression analyses were conducted with random-effects modeling, using the restricted-information maximum likelihood method with a significance level cutoff of P < .05. For 5 studies, samples were not independent.20,36,37,40,41 In this case, samples with greater sample size were chosen for inclusion as a rule for the main analyses (see table 1). Additional analyses were run including these studies using robust variance estimation, which requires less computational power and accounts for statistical dependency (k = 23 for overall cognition, see table 3).72
Table 3.
Comparison of Analyses Including Non-independent Samples Using Robust Variance Estimation, and Main Analyses Excluding Nonindependent Samples
| Exclude Nonindependent Samples | Include Nonindependent Samples | Extra Samples Included | |
|---|---|---|---|
| Overall cognition | −0.055, CI95[−0.09, −0.02] | −0.06, CI95[−0.11, −0.01] | Aas, 2012a,40 Aas, 2012c,36 Aas, 201337; Green, 201541 |
| Executive functioning | −0.07, CI95[−0.13, 0] | −0.07, CI95[−0.17, 0.02] | Quide, 201720; Quide, 201838 |
| Working memory | −0.09, CI95[−0.15, −0.03] | −0.12, CI95[−0.18, −0.05] | |
| Attention and processing speed | −0.07, CI95[−0.15, 0] | −0.08, CI95[−0.18, 0.03] | |
| Verbal/visual memory | −0.05, CI95[−0.09, 0] | −0.04, CI95[−0.11, 0.03] |
Results
Study Characteristics and Quality
Quality scores on the Downs and Black checklist ranged from 11 to 17 (out of a possible 18 for cross-sectional, nonintervention studies). The mean for included studies was 14, SD = 1.56 (see supplementary table S1 for scores for each included study). The least well-met quality criteria were (1) conducting power analyses, (2) blinding interviewers involved in collection of outcome data, (3) reporting time period of recruitment, and (4) reporting proportion of approached subjects that declined to participate. All studies contained an adequate measure of childhood trauma exposure, including sexual and physical abuse, emotional abuse, and physical and emotional neglect. However, selective reporting was common with regard to reporting all measured specific trauma types. For instance, only 22.7% of selected studies reported outcomes with regard to all trauma types indicated (ie, physical, sexual and emotional abuse as well as emotional and physical neglect), with 36.3% having reported outcomes for 2 or more trauma types (see supplementary table S2).
Meta-analysis of Childhood Trauma, Overall Cognition, and Cognitive Subdomains
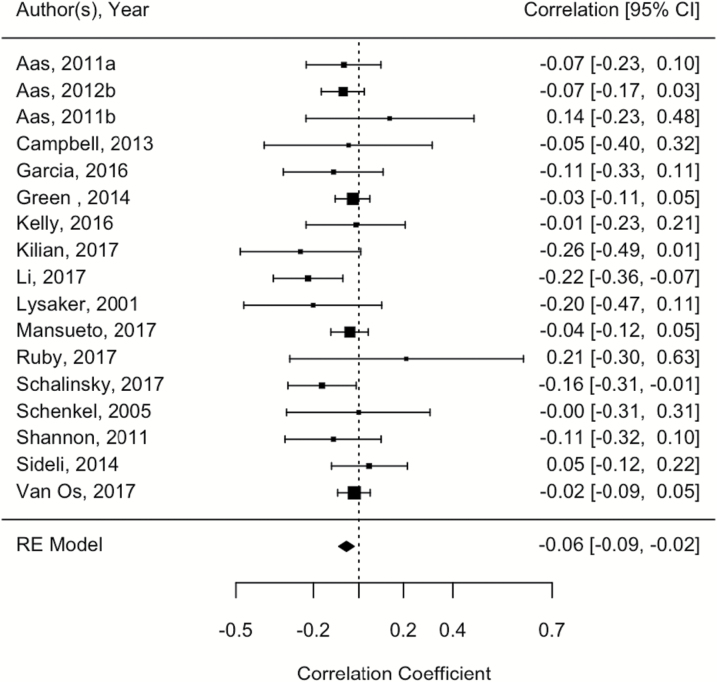
We observed an overall significant result for the association between childhood trauma and overall cognitive ability, r = −.055; 95% CI =−0.09, −0.02, P = .002; See figure 2. Heterogeneity was low (I2 = 0%, CI 0= .00, 68.01, P = .47). Egger’s coefficient bias test was not significant, suggesting an absence of small-study bias (P = .48). There were no significant moderating effects (P = .09–.71). Interestingly, although overall cognitive ability analyses were significant, analyses of studies using a WASI/WAIS complete IQ measure (k = 6) were not, r = −.008; 95% CI = −0.07, 0.06, P = .81; However, this may be due to limited power due to the substantially decreased number of included studies. Heterogeneity was low (I2 = 0%, P = .93), and no evidence of small-study bias (Egger’s coefficient bias test) was detected (P = .58).
Fig. 2.
Forest plot showing the relationship between childhood trauma and overall cognitive performance.
Analyses of the EF domain yielded significant results, r = −.065; 95% CI = −0.13, 0.00, P = .045. Heterogeneity was low to moderate (I2 = 39.06%, CI = 0.00, 90.03, P = .10). Egger’s coefficient bias test was not significant, suggesting an absence of small-study bias (P = .75). There were no significant moderating effects (P = .36–.67), asides from an effect on the border of statistical significance for the proportion of female subjects in the sample [Q (1) = 3.72, P = .05].
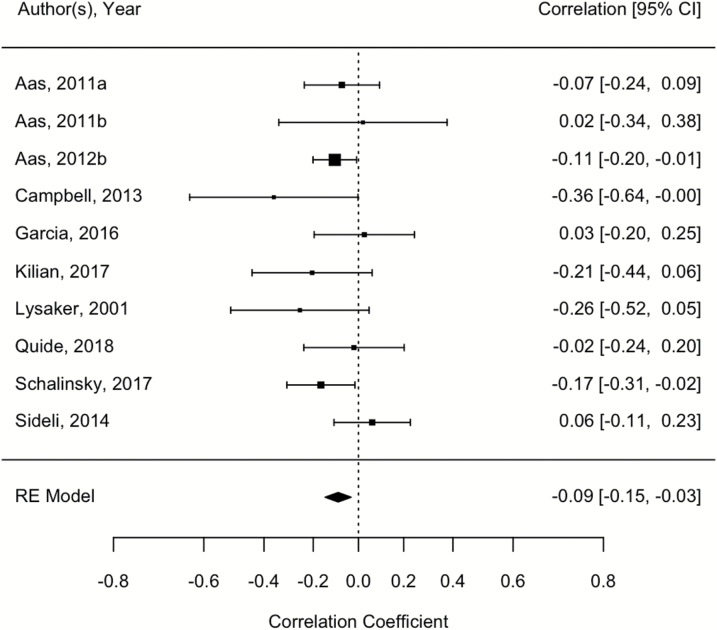
Of note, a modest significant negative relationship between childhood trauma exposure and working memory function was observed [r = −.091; 95% CI = −0.15, −0.03, P = .002; See figure 3]. Heterogeneity was low (I2 = 0.05%, CI = 0.00, 81.88, P = .35). Egger’s coefficient bias test was not significant, suggesting an absence of small-study bias (P = .55). There were no significant moderating effects (P = .36–.97).
Fig. 3.
Forest plot showing the relationship between childhood trauma and working memory performance.
In contrast, analyses of the verbal/visual memory domain yielded less strong, though significant results, r = −.047; 95% CI = −0.09, −0.00), P = .03. Heterogeneity was low (I2 = 7.61%, CI = 0.00, 76.30, P = .38). Egger’s coefficient bias test was not significant, suggesting an absence of small-study bias (P = .75). There were no significant moderating effects (P = .19–.99). Finally, analyses of the attention domain yielded significant results, r = −0.075; 95% CI =−0.15, 0.00, P = .04. Heterogeneity was moderate (I2 = 48.25%, CI = 0.00, 85.28, P = .08). Egger’s coefficient bias test was not significant, suggesting an absence of small-study bias (P = .92). There were no significant moderating effects (P = .13–.97). Finally, additional analyses using cluster-robust variance estimation (including studies with nonindependent samples) yielded results similar in magnitude to those in the main analyses (see table 3).
Case–control Comparisons
Across the 7 studies used for comparison, the average r across 1193 subjects for the healthy control comparison group was −.131. The average r across 1579 subjects for the psychotic disorders group was −.025. The calculated Fisher’s Z value was −2.77, which indicates a significant difference of effect between the two diagnostic groups (P = .006).
Discussion
This meta-analytic review detected an effect of childhood trauma on overall neurocognitive function, such that trauma exposure was associated with lower overall performance. The small effect may aid in explaining discrepancies in the literature.33,34 There are two possible interpretations. First, though sample size was not found to moderate findings, larger studies tended toward smaller effect sizes. Thus, perhaps some studies with smaller samples yielded inflated effect sizes. Second, the effect may be present, but small, and only detectable through pooling together currently available samples. Regardless, this effect size supports the broader conception that mechanisms of neurocognitive dysfunction in psychosis are complex and multifaceted, with numerous putative factors contributing in parallel to varying degrees.2,17,73–76 Notably, exploratory analyses found the association between childhood trauma and overall cognition was significantly stronger in healthy individuals compared to patients with psychotic disorders. The following sections discuss these findings in the context of the broader literature and highlight future directions and applications.
Although the effect size of the relationship between overall cognition and childhood trauma was fairly small, low heterogeneity and lack of evidence for bias lend support to the robustness of the findings (ie, “small but meaningful”). Nonetheless, a critical future direction will be to determine whether this effect is clinically meaningful. It is noteworthy that exploratory analyses yielded a significantly stronger effect of childhood trauma and neurocognition in healthy individuals in comparison to individuals with psychotic disorders. This finding is consistent with a stronger association previously observed in healthy populations,33,34,77,78 including one previous meta-analytic review.35 One possibility is that the effect of trauma is being “overpowered,” or masked, by factors intrinsic to a psychotic disorder diagnosis, such as genetic effects, current adversity, and medication use.33 However, given the vast evidence that childhood trauma increases likelihood of developing psychosis,10 and given that neurocognitive deficits are a core component of the disorder, it is also possible that a larger effect is being constrained by methodological limitations in the literature. In the present investigation, however, there was no evidence of methodological moderator effects.
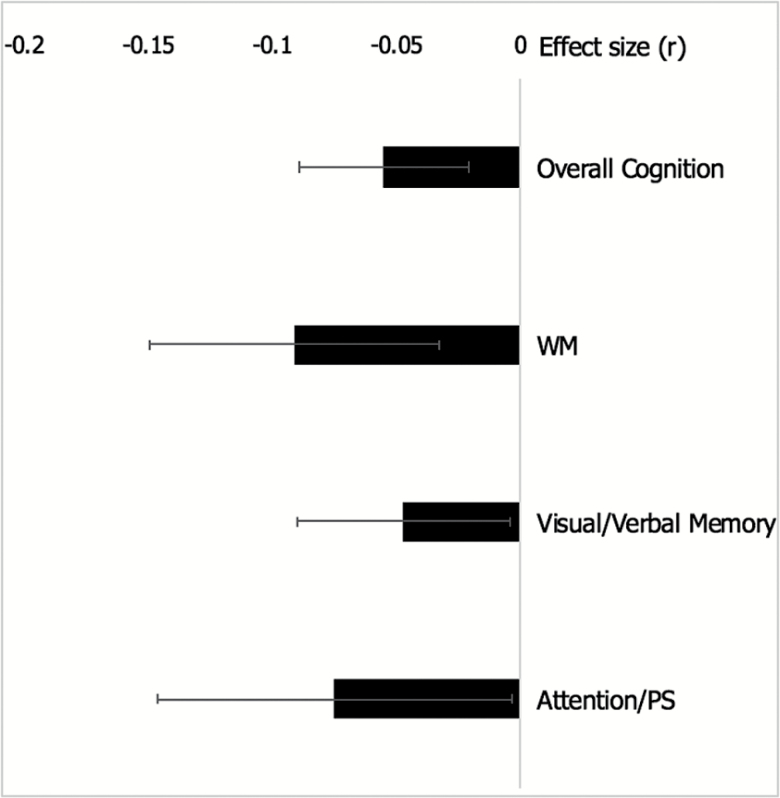
In interpreting the small effect size, another factor to consider is the perennial issue of heterogeneity of psychotic disorders. While exploring global neurocognitive function, signal may be lost with regard to specific cognitive mechanisms that may be differentially affected by childhood trauma. In the current study, the effect size for the negative association between childhood trauma and working memory, though modest, was larger than that of overall cognition (see figure 4). This is notable especially in light of the fact that working memory has long been considered a critical endophenotype for psychosis.73,79 Analyses of additional cognitive domains (EF, verbal/visual memory, and attention/processing speed) also yielded small effects in the expected direction for each respective domain. The current evidence does not allow for directly observing the mechanisms associated with these relationships. However, this is an important first step for future examinations that may allow a stronger understanding of the distinct domains and neural correlates underlying the observed associations. In addition, a marked limitation is that there were not enough studies available to examine social cognition. Given strong evidence that social cognition is critical for predicting functional outcomes in psychosis,80 and that it may be impacted by childhood trauma,38 this is an important future direction for the field going forward.
Fig. 4.
Effect sizes and 95% confidence intervals across cognitive domains.
Although the literature has not often focused on specific cognitive domains, an emphasis on isolating the effects of distinct sorts of trauma has also been sorely lacking. Neural and theoretical models of early life stress and adversity suggest that different types of exposure may negatively impact some of the same biological systems.81 Nonetheless, studying whether particular types of trauma are more impactful than others is a promising direction for research in this domain. Future investigations undertaking this aim should carefully consider the large possibility of overlap in exposure to multiple types of trauma (ie, exposure to a specific trauma may increase the likelihood that an individual will be exposed to multiple types of trauma simultaneously). Carefully mapping out various forms of trauma, timing of trauma, and severity of exposure to trauma in samples powered for modeling effects of covariation and interaction would be beneficial. This could aid in elucidating who is at greatest risk for experiencing negative outcomes due to trauma exposure. In this study, due to the limited data available, this was not possible. However, these questions will be critical to address as the literature develops further.
A better understanding of neurocognitive domain specificity may uniquely inform models of underlying mechanisms. However, the use of correlational data precluded examining causal relationships, which limited our ability to make causal inferences. Despite numerous animal models and human studies suggesting there are serious and lasting effects of stress and trauma on neurodevelopment,9,82–86 this meta-analysis was limited to cross-sectional studies and therefore cannot fully address this question. In fact, a recent prospective study on healthy populations found that cognitive deficits in victimized individuals were largely explained by childhood socioeconomic disadvantage and cognitive deficits predating childhood victimization.87 This study underscores the necessity of considering an aggregate of environmental and genetic risks when framing theoretical models of environmental effects on neurodevelopment across the life span. The present review focused on childhood trauma; however, it will be essential for future studies to create more sophisticated models of cumulative risk exposure spanning across different types of environmental risk. Incorporating risk exposure throughout the lifetime, including peri- and prenatal periods, may also yield important clues.
Furthermore, it will be important for future research to incorporate multiple types of childhood adversity beyond trauma, as well as different increments of severity. There is some promising emerging evidence that less severe exposure to childhood adversity may be associated with cognitive deficits in psychosis.88 Exploring several types of childhood adversity will further aid the field in understanding the mechanistically distinct effects of trauma and other environmental risk exposures (such as bullying, household instability, low socioeconomic status, and exposure to environments with high crime rates). Incorporating multiple types of adversity at the individual (eg, bullying) and structural (eg, exposure to poverty or low socioeconomic status) level will also be useful in gaining a more holistic understanding of how cumulative risk exposure affects global functioning in an individual and how it contributes to developing psychopathology.
This study benefitted from the wide range of pooled subjects, which constitutes a highly geographically diverse sample with low heterogeneity and absent evidence of bias. Nonetheless, there are multiple concerns that limit the generalizability of the results. Although all but two of the included studies used standardized, validated, childhood trauma batteries, there was some variability in which scales were used, and in some cases, which types of trauma were reported (see table 2). This may have introduced unexplained variance into the measurement of childhood trauma. There was also considerable variability in choice of cognitive battery used and although this study tried to introduce more homogeneity by compiling batteries into traditional cognitive subdomains, ideally there would be less heterogeneity with regard to cognitive measures used. This is especially true in the case of estimates of overall cognitive ability (see table 1 for descriptive information on cognitive measures). However, measured estimates of heterogeneity between studies were low for all major analyses. Nonetheless, it will be important for future studies to reduce the heterogeneity in cognitive batteries used once more published data becomes available. It is also critical to note that although a larger effect size was observed for working memory, the studies included in this analysis had smaller sample sizes on average than those included in the overall cognitive domain estimate. This difference in average sample size may have affected the observed effect size. However, sample size did not have a moderating effect in any analyses. The extent of the effects of these limitations will become evident as more studies examining these questions become available.
Future investigations would also benefit from preregistering studies in order to reduce the possibility of unconscious reporting and analytical biases. Illness duration and neuroleptic dosage should also be more fully considered as mediators of the strength of the observed association between childhood trauma and neurocognitive function. Despite neurodevelopmental models informing us that the timing of exposure to trauma is critically important,9 the current study was not able to explore or control for these questions, and this is an essential future direction. Finally, it is important to note the methodological limitations of retrospective life event inventories. As we gain a greater understanding of the ways in which early life trauma interacts with biology and development, the need to develop reliable and valid measures of early life event exposure will increase further.89
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
Northwestern University Society Biology and Health Cluster fellowship (to Ms. Vargas and Ms. Lam), and R01MH112545, R21/R33MH103231, and R21MH110374 (to Prof. Mittal).
Acknowledgments
The authors report no biomedical financial interests or conflicts of interest.
References
- 1. Bernstein DP, Stein JA, Newcomb MD, et al. . Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl. 2003;27:169–190. [DOI] [PubMed] [Google Scholar]
- 2. Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry. 2014;4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varese F, Smeets F, Drukker M, et al. . Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGrath JJ, Saha S, Lim CCW, et al. ; WHO World Mental Health Survey Collaborators Trauma and psychotic experiences: transnational data from the world mental health survey. Br J Psychiatry. 2017;211:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Begemann MJ, Daalman K, Heringa SM, Schutte MJ, Sommer IE. Letter to the editor: childhood trauma as a risk factor for psychosis: the confounding role of cognitive functioning. Psychol Med. 2016;46:1115–1118. [DOI] [PubMed] [Google Scholar]
- 6. Begemann MJ, Heringa SM, Sommer IE. Childhood trauma as a neglected factor in psychotic experiences and cognitive functioning. JAMA Psychiatry. 2016;73:875–876. [DOI] [PubMed] [Google Scholar]
- 7. Morgan C, Gayer-Anderson C. Childhood adversities and psychosis: evidence, challenges, implications. World Psychiatry. 2016;15:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51:1079–1095. [DOI] [PubMed] [Google Scholar]
- 9. Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl). 2011;214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibson LE, Alloy LB, Ellman LM. Trauma and the psychosis spectrum: a review of symptom specificity and explanatory mechanisms. Clin Psychol Rev. 2016;49:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hardy A. Pathways from trauma to psychotic experiences: a theoretically informed model of posttraumatic stress in psychosis. Front Psychol. 2017;8:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentall RP, de Sousa P, Varese F, et al. . From adversity to psychosis: pathways and mechanisms from specific adversities to specific symptoms. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1011–1022. [DOI] [PubMed] [Google Scholar]
- 13. Bailey T, Alvarez-Jimenez M, Garcia-Sanchez AM, Hulbert C, Barlow E, Bendall S. Childhood trauma is associated with severity of hallucinations and delusions in psychotic disorders: a systematic review and meta-analysis. Schizophr Bull. 2018;44:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Dam DS, van Nierop M, Viechtbauer W, et al. ; Genetic Risk and Outcome of Psychosis (GROUP) investigators Childhood abuse and neglect in relation to the presence and persistence of psychotic and depressive symptomatology. Psychol Med. 2015;45:1363–1377. [DOI] [PubMed] [Google Scholar]
- 15. Cannon TD, Yu C, Addington J, et al. . An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. [DOI] [PubMed] [Google Scholar]
- 17. Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. [DOI] [PubMed] [Google Scholar]
- 18. Cuesta MJ, Sánchez-Torres AM, Cabrera B, et al. ; PEPs Group Premorbid adjustment and clinical correlates of cognitive impairment in first-episode psychosis. The PEPsCog study. Schizophr Res. 2015;164:65–73. [DOI] [PubMed] [Google Scholar]
- 19. Schalinski I, Teicher MH, Carolus AM, Rockstroh B. Defining the impact of childhood adversities on cognitive deficits in psychosis: an exploratory analysis. Schizophrenia Research 2018;192:351–356. [DOI] [PubMed] [Google Scholar]
- 20. Quidé Y, O’Reilly N, Rowland JE, Carr VJ, Elzinga BM, Green MJ. Effects of childhood trauma on working memory in affective and non-affective psychotic disorders. Brain Imaging Behav. 2017;11:722–735. [DOI] [PubMed] [Google Scholar]
- 21. Li XB, Bo QJ, Zhang GP, et al. . Effect of childhood trauma on cognitive functions in a sample of Chinese patients with schizophrenia. Compr Psychiatry. 2017;76:147–152. [DOI] [PubMed] [Google Scholar]
- 22. Kelly DL, Rowland LM, Patchan KM, et al. . Schizophrenia clinical symptom differences in women vs. men with and without a history of childhood physical abuse. Child Adolesc Psychiatry Ment Health. 2016;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruby E, Rothman K, Corcoran C, Goetz RR, Malaspina D. Influence of early trauma on features of schizophrenia. Early Interv Psychiatry. 2017;11:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia M, Montalvo I, Creus M, et al. . Sex differences in the effect of childhood trauma on the clinical expression of early psychosis. Compr Psychiatry. 2016;68:86–96. [DOI] [PubMed] [Google Scholar]
- 25. Shannon C, Douse K, McCusker C, Feeney L, Barrett S, Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr Bull. 2011;37:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green MJ, Chia TY, Cairns MJ, et al. ; Australian Schizophrenia Research Bank Catechol-O-methyltransferase (COMT) genotype moderates the effects of childhood trauma on cognition and symptoms in schizophrenia. J Psychiatr Res. 2014;49:43–50. [DOI] [PubMed] [Google Scholar]
- 27. Schenkel LS, Spaulding WD, DiLillo D, Silverstein SM. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr Res. 2005;76:273–286. [DOI] [PubMed] [Google Scholar]
- 28. Lysaker PH, Meyer P, Evans JD, Marks KA. Neurocognitive and symptom correlates of self-reported childhood sexual abuse in schizophrenia spectrum disorders. Ann Clin Psychiatry. 2001;13:89–92. [DOI] [PubMed] [Google Scholar]
- 29. Campbell C, Barrett S, Shannon, et al. The relationship between childhood trauma and neuropsychological functioning in first episode psychosis. Psychosis 2013;5:48–59. [Google Scholar]
- 30. Mansueto G, van Nierop M, Schruers K, et al. ; GROUP Investigators The role of cognitive functioning in the relationship between childhood trauma and a mixed phenotype of affective-anxious-psychotic symptoms in psychotic disorders. Schizophr Res. 2018;192:262–268. [DOI] [PubMed] [Google Scholar]
- 31. Aas M, Steen NE, Agartz I, et al. . Is cognitive impairment following early life stress in severe mental disorders based on specific or general cognitive functioning?Psychiatry Res. 2012;198:495–500. [DOI] [PubMed] [Google Scholar]
- 32. Aas M, Dazzan P, Fisher HL, et al. . Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophr Res. 2011;129:12–19. [DOI] [PubMed] [Google Scholar]
- 33. van Os J, Marsman A, van Dam D, Simons CJ; GROUP Investigators Evidence that the impact of childhood trauma on IQ is substantial in controls, moderate in siblings, and absent in patients with psychotic disorder. Schizophr Bull. 2017;43:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sideli L, Fisher HL, Russo M, et al. . Failure to find association between childhood abuse and cognition in first-episode psychosis patients. Eur Psychiatry. 2014;29:32–35. [DOI] [PubMed] [Google Scholar]
- 35. Malarbi S, Abu-Rayya HM, Muscara F, Stargatt R. Neuropsychological functioning of childhood trauma and post-traumatic stress disorder: a meta-analysis. Neurosci Biobehav Rev. 2017;72:68–86. [DOI] [PubMed] [Google Scholar]
- 36. Aas M, Djurovic S, Athanasiu L, et al. . Serotonin transporter gene polymorphism, childhood trauma, and cognition in patients with psychotic disorders. Schizophr Bull. 2012;38:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aas M, Haukvik UK, Djurovic S, et al. . BDNF val66met modulates the association between childhood trauma, cognitive and brain abnormalities in psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:181–188. [DOI] [PubMed] [Google Scholar]
- 38. Quidé Y, Cohen-Woods S, O’Reilly N, Carr VJ, Elzinga BM, Green MJ. Schizotypal personality traits and social cognition are associated with childhood trauma exposure. Br J Clin Psychol. 2018;57:397–419. [DOI] [PubMed] [Google Scholar]
- 39. Aas M, Dazzan P, Mondelli V, et al. . Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med. 2011;41:463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aas M, Navari S, Gibbs A, et al. . Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis?Schizophr Res. 2012;137:73–79. [DOI] [PubMed] [Google Scholar]
- 41. Green MJ, Raudino A, Cairns MJ, et al. ; Australian Schizophrenia Research Bank Do common genotypes of FK506 binding protein 5 (FKBP5) moderate the effects of childhood maltreatment on cognition in schizophrenia and healthy controls?J Psychiatr Res. 2015;70:9–17. [DOI] [PubMed] [Google Scholar]
- 42. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh S. How to conduct and interpret systematic reviews and meta-analyses. Clin Transl Gastroenterol. 2017;8:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). Copenhagen, Denmark: Pearson Assessment; 2007. [Google Scholar]
- 45. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 46. Randolph C. RBANS repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: Psychological Corporation (Harcourt); 1998. [Google Scholar]
- 47. Nuechterlein KH, Green MF, Kern RS, et al. . The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 48. Zachary RA. Shipley Institute of Living Scale. Revised Manual. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]
- 49. Wechsler D. Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation (Harcourt Assessment); 2001. [Google Scholar]
- 50. Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol. 1985;112:201–210. [DOI] [PubMed] [Google Scholar]
- 51. Rosvold H, Mirsky AF, Sarason I, Bransome ED Jr., Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. [DOI] [PubMed] [Google Scholar]
- 52. Bilder RM, Turkel E, Lipschutz-Broch L, Lieberman JA. Antipsychotic medication effects on neuropsychological functions. Psychopharmacol Bull. 1992;28:353–366. [PubMed] [Google Scholar]
- 53. Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale—Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 54. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS.. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 55. O Spreen ALB. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA). Victoria, Canada: University of Victoria Neuropsychology Laboratory; 1969. [Google Scholar]
- 56. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G.. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 57. Burgess P, Shallice T.. The Hayling and Brixton Tests. UK: Thames Valley Test Company Bury St Edmunds; 1997. [Google Scholar]
- 58. Wechsler D. WMS-III Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 59. Gau SS, Shang CY. Executive functions as endophenotypes in ADHD: evidence from the Cambridge Neuropsychological Test Battery (CANTAB). J Child Psychol Psychiatry. 2010;51:838–849. [DOI] [PubMed] [Google Scholar]
- 60. Meyers JE, Meyers KR.. Rey Complex Figure Test and Recognition Trial: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 61. Gayer-Anderson C, Fisher HL, Fearon P, et al. . Gender differences in the association between childhood physical and sexual abuse, social support and psychosis. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fiszdon JM, Choi J, Goulet J, Bell MD. Temporal relationship between change in cognition and change in functioning in schizophrenia. Schizophr Res. 2008;105:105–113. [DOI] [PubMed] [Google Scholar]
- 63. Kilian S, Asmal L, Chiliza B, et al. . Childhood adversity and cognitive function in schizophrenia spectrum disorders and healthy controls: evidence for an association between neglect and social cognition. Psychol Med. 2017;48:1–8. [DOI] [PubMed] [Google Scholar]
- 64. Fisher Z, Tipton E.. Robumeta: Robust Variance Meta- regression. R Package Version 1.6. 2015. [Google Scholar]
- 65. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–43. [Google Scholar]
- 66. Quintana DS. From pre-registration to publication: a non-technical primer for conducting a meta-analysis to synthesize correlational data. Front Psychol. 2015;6:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Team RDC. R: A Language and Environment for Statistical Computing. The R Foundation for Statistical Computing; 2015. [Google Scholar]
- 68. Lipsey MW, Wilson DB. Practical meta-analysis. Applied Social Research Methods Series. 2001;49:63–67. [Google Scholar]
- 69. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. [DOI] [PubMed] [Google Scholar]
- 72. Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1:39–65. [DOI] [PubMed] [Google Scholar]
- 73. Ivleva EI, Morris DW, Osuji J, et al. . Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Res. 2012;196:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130:1–15. [DOI] [PubMed] [Google Scholar]
- 75. Daban C, Amado I, Baylé F, et al. . Disorganization syndrome is correlated to working memory deficits in unmedicated schizophrenic patients with recent onset schizophrenia. Schizophr Res. 2003;61:323–324. [DOI] [PubMed] [Google Scholar]
- 76. Haenschel C, Bittner RA, Waltz J, et al. . Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29:9481–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Majer M, Nater UM, Lin JM, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. 2012;46:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim D, Kim JW, Koo TH, Yun HR, Won SH. Shared and distinct neurocognitive endophenotypes of schizophrenia and psychotic bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mondragón-Maya A, Ramos-Mastache D, Román PD, Yáñez-Téllez G. Social cognition in schizophrenia, unaffected relatives and ultra- high risk for psychosis: what do we currently know?Actas Esp Psiquiatr. 2017;45:218–226. [PubMed] [Google Scholar]
- 81. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. 2016;82:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Derks NA, Krugers HJ, Hoogenraad CC, Joëls M, Sarabdjitsingh RA. Effects of early life stress on synaptic plasticity in the developing hippocampus of male and female rats. PLoS One. 2016;11:e0164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. [DOI] [PubMed] [Google Scholar]
- 85. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 86. Corcoran C, Walker E, Huot R, et al. . The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. [DOI] [PubMed] [Google Scholar]
- 87. Danese A, Moffitt TE, Arseneault L, et al. . The origins of cognitive deficits in victimized children: implications for neuroscientists and clinicians. Am J Psychiatry. 2017;174:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Morales-Munoz I, Suvisaari J, Therman S, et al. . Childhood adversities and cognitive deficits in first-episode psychosis. Schizophr Res 2018;197:596–598. [DOI] [PubMed] [Google Scholar]
- 89. Vargas T, Mittal V. Issues affecting reliable and valid assessment of early life stressors in psychosis. Schizophr Res. 2018;192:465–466. [DOI] [PubMed] [Google Scholar]
- 90. Bifulco A, Bernazzani O, Moran PM, Jacobs C. The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br J Clin Psychol. 2005;44:563–581. [DOI] [PubMed] [Google Scholar]
- 91. Nijenhuis ERS, Van Der Hart O, Kruger K. The psychometric characteristics of the traumatic experiences questionnaire (TEC): first findings among psychiatric out- patients. Clin Psy Psychotherapy. 2002;9:200–210. [Google Scholar]
- 92. Foa EB. Post Traumatic Diagnostic Scale Manual. Eden Prairie, MN: National Computer Systems; 1995. [Google Scholar]
- 93. Dorahy M, Shannon C, Maguire C. The troubles related experiences questionnaire (TREQ): a clinical and research tool for northern Irish samples. Irish J Psychol. 2007;28:185–195. [Google Scholar]
- 94. Rosenman S, Rodgers B. Childhood adversity in an Australian population. Soc Psychiatry Psychiatr Epidemiol. 2004;39:695–702. [DOI] [PubMed] [Google Scholar]
- 95. Bremner JDVE, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the ET Inventory. Depress Anxiety. 2000;12:1–12. [DOI] [PubMed] [Google Scholar]
- 96. Teicher MH, Parigger A. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One. 2015;10:e0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.