Abstract
Network analysis was used to examine how densely interconnected individual negative symptom domains are, whether some domains are more central than others, and whether sex influenced network structure. Participants included outpatients with schizophrenia (SZ; n = 201), a bipolar disorder (BD; n = 46) clinical comparison group, and healthy controls (CN; n = 27) who were rated on the Brief Negative Symptom Scale. The mutual information measure was used to construct negative symptom networks. Groups were compared on macroscopic network properties to evaluate overall network connectedness, and microscopic properties to determine which domains were most central. Macroscopic analyses indicated that patients with SZ had a less densely connected negative symptom network than BD or CN groups, and that males with SZ had less densely connected networks than females. Microscopic analyses indicated that alogia and avolition were most central in the SZ group, whereas anhedonia was most central in BD and CN groups. In addition, blunted affect, alogia, and asociality were most central in females with SZ, and alogia and avolition were most central in males with SZ. These findings suggest that negative symptoms may be highly treatment resistant in SZ because they are not very densely connected. Less densely connected networks may make treatments less likely to achieve global reductions in negative symptoms because individual domains function in isolation with little interaction. Sex differences in centralities suggest that the search for pathophysiological mechanisms and targeted treatment development should be focused on different sets of symptoms in males and females.
Keywords: negative symptoms, network analysis, psychosis, schizophrenia, bipolar disorder
Introduction
In his seminal conceptualization of “dementia praecox”, Kraepelin1 described individuals with psychotic disorders as demonstrating: “a weakening of those emotional activities which permanently form the mainsprings of volition …”. He ascribed considerable importance to the role of avolition in contributing to the characteristic decline in functional outcome that occurs in schizophrenia (SZ). Bleuler2 proposed a similarly central role for avolition in SZ, observing that “Indifference seems to be the external sign of their state …. The will … disturbed in a number of ways, but above all by the breakdown of the emotions … The patients appear lazy and negligent because they no longer have the urge to do anything either of their own initiative or at the bidding of another.” More modern conceptualizations also propose that avolition is the key symptom that leads to the emergence of all other negative symptoms.3 For example, reductions in the quantity of speech (alogia), outward expression of emotion (blunted affect), seeking out pleasurable activities (anhedonia), and engaging in social interactions (asociality) may all stem from reductions in motivation that prevent the initiation of these behaviors. The capacity to perform each of these behaviors may be spared in SZ; however, the level of motivation needed to initiate them may not cross the threshold necessary for behavioral initiation. Although conceptualizations of negative symptoms postulating a central role of avolition are intuitively appealing, they have never been formally tested using sophisticated mathematical approaches capable of addressing the question empirically. Determining whether one individual negative symptom domain (eg, avolition) is most central and drives global increases in the other domains has critical treatment implications. If this notion is correct, regardless of which symptom is most central, it would suggest that the search for pathophysiological mechanisms and targeted treatment development should be focused on that most central symptom, with the expectation of improvement in the entire constellation of negative symptoms if it is effectively treated.
In this study, we used network analysis to evaluate which of the 5 domains (anhedonia, avolition, asociality, alogia, and blunted affect) identified in the 2005 NIMH Consensus Conference on Negative Symptoms4 were most central to the negative symptom construct. Network analysis is a mathematical approach from the field of complex systems science that evaluates interconnections among variables and how they influence each other.5 Recently, there has been increasing interest in taking a network approach to evaluating psychiatric symptoms.6 The underlying theory behind this approach is that psychiatric disorders arise from the interactions among symptoms in a network.7 Disorders are thought to result from a spreading activation within the symptom network, whereby the emergence of one symptom increases the probability that an interconnected set of symptoms also arises. Symptom networks can be characterized in terms of their density.8 Highly dense networks are more interconnected. Once active, dense networks tend to coactivate, forming tightly coupled clusters of psychopathology that maintain each other and become self-sustaining. Sometimes one individual symptom is more “central” to a network than others, having very strong interconnections with the other symptoms in the network that cause those symptoms to emerge whenever the central symptom is present. In strongly connected networks, the activation of a central symptom may lead to the continued activation of other symptoms, even after the factors that triggered that symptom have disappeared.7 This may be how symptoms become chronic, forming self-sustaining feedback loops because a central symptom is driving a global increase in psychopathology. There is debate as to whether more or less densely connected symptom networks are more responsive to treatment, with evidence for both outcomes in the literature.7,9,10 One possibility is that densely connected networks are difficult to treat because symptoms are tightly coupled. In this instance, a treatment must effectively target the most central symptom and lead to a spreading effect on the global symptom network that yields improvement across other domains to be effective. Alternatively, less dense networks may be very difficult to treat because symptoms have little interaction with one another. In this instance, even if a central symptom is successfully impacted, the lack of interconnection among symptoms makes it difficult for spreading effects to occur because symptoms function as their own islands of pathology with little interaction.
Taking a network approach to evaluating negative symptoms offers a novel means of addressing questions about density and centrality. We adopted a standard approach of evaluating symptom networks by calculating macroscopic and microscopic network properties. Macroscopic properties (eg, network density, average clustering coefficient, and average shortest path length) provide information about the overall connectedness of the network as a whole (see table 2 for descriptions). Networks with a higher density, average clustering coefficient, and lower average shortest path length are tightly connected (ie, symptoms are highly interdependent). In contrast, microscopic properties (eg, degree centrality and betweenness centrality) provide information about which individual symptoms are most influential and interconnected with other symptoms in the network. Networks were evaluated using a cross-sectional dataset of outpatients with SZ and a clinical comparison group consisting of euthymic outpatients with bipolar disorder (BD) who display negative symptoms of clinical significance that are comparable to SZ in some domains.11 We also compared these groups to a sample of healthy controls (CN) who do not show severe levels of negative symptoms, but do show some variability in negative symptoms across participants, even in the absence of psychiatric diagnosis.11,12 All participants were rated on a common measure, the Brief Negative Symptom Scale (BNSS),13,14 which was designed to assess the 5 domains identified in the 2005 NIMH Consensus Conference according to modern conceptualizations. The following specific aims and hypotheses were evaluated:
Table 2.
Summary of the Network Measures for Weighted Networks
| Type | Measure | Definition | Clinical meaning | Equation |
|---|---|---|---|---|
| Macroscopic | Density | Average of all the edge weights in the network | To what extent symptoms in the network are interconnected | |
| Harmonic mean shortest path length | Average shortest path length between all nodes | Level of information efficiency in the network | ||
| Average clustering coefficient | Overall clustering in the network | To what extent symptoms tend to cluster together |
|
|
| Modularity | Partitioning networks into a collection of modules (groups) | To what extent symptoms can be separated into distinct groups | ||
| Microscopic | Degree centrality | Sum of the edge weights connected to a node | Level of connectivity of a symptom in the network | |
| Betweenness centrality | # of shortest paths between pairs of nodes that pass-through node i | How frequently a symptom emerges as part of interactions among other symptoms | ||
| Clustering coefficient | How well the neighborhoods of a node connect to each other | To what extent symptoms tend to cluster together |
Note: = Node (Brief Negative Symptom Scale symptom) Index; N = total number of nodes; V is the set of all nodes in the network; , , = weight between nodes i and j, i and u,i and v; = 1/= distance between nodes i and j; and = degrees of nodes i and j; = the number of shortest paths from node j to node u that go through node i; and = the total number of shortest paths from node j to node u.
(1) To determine whether patients with SZ differ from CN and BD on global network connectivity by evaluating multiple macroscopic properties. We hypothesized that SZ patients’ global negative symptom networks would be less densely connected than CN and BD, as indicated by lower scores on measures of network density, modularity, and average clustering coefficient, as well as higher average shortest path length. This hypothesis was based on evidence indicating that negative symptoms are highly resistant to treatment,15 and that treatment resistant networks in psychotic disorder patients are characterized by lower density of connections.9,16
(2) To identify negative symptom domains that are most central within the networks of SZ, BD, and CN by evaluating multiple microscopic network properties. In line with clinical conceptualizations proposing a key role for motivational deficits,1–3 we hypothesized that avolition would be the most central negative symptom domain in SZ and that this would be evident across a range of microscopic measures of centrality, including degree centrality, closeness centrality, betweenness centrality, and clustering coefficient. Given that anhedonia tends to be a prevalent symptom of mood disorders and a trait that occurs in the general nonclinical population with some frequency,17,18 we hypothesized that anhedonia items would be most central in BD and CN.
(3) To evaluate sex differences in macroscopic and microscopic properties in individuals with SZ. Given that men are more likely to have greater severity of negative symptoms and to be more resistant to treatments than women with SZ,15 we also evaluated sex differences in macroscopic and microscopic network properties within the SZ sample. We hypothesized that males would display less densely connected networks than females given that males are less responsive to treatment and less dense networks are more treatment resistant in SZ.9,16 Exploratory analyses were also conducted to evaluate sex differences in centrality measures.
Methods
Participants
Participants included 201 outpatients meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition19 criteria for SZ or schizoaffective disorder, 46 individuals with BD in an euthymic mood state (ie, no current manic, mixed, or depressive episode at the time of interview and had symptoms below cutoffs on the Young Mania20 and Hamilton Depression21 scales), and 27 psychiatrically CN. Patients with SZ were recruited from two sites: (1) the outpatient research clinics at the Maryland Psychiatric Research Center and community mental health clinics in the Baltimore metropolitan area (n = 146); (2) the State University of New York at Binghamton, including community outpatient mental health clinics in upstate New York (n = 55). Patients with BD were recruited from outpatient psychiatric clinics in southern Nevada (n = 46). All patients were evaluated during periods of clinical stability as determined by a minimum of 4 weeks of consistent medication dose and type. Consensus diagnosis was established via a best estimate approach based on psychiatric history and subsequently confirmed using the Structured Clinical Interview for DSM-IV (SCID).22 The study was approved by the ethics committees of each institution.
CN subjects were recruited at the University of Nevada through online/print advertisements and word of mouth among enrolled participants. All CNs underwent a screening interview, including the SCID-I and SCID-II23 and did not meet lifetime criteria for a psychotic disorder or any current Axis I or II disorder. CN also had no family history of psychosis.
The SCID was used to determine that both SZ and CN participants did not meet DSM-IV criteria for substance abuse or dependence over the past 6 months, and lack of recent substance use was confirmed by urine toxicology at the time of testing. All participants were also screened for lifetime neurological disorders and were free from neurological conditions (eg, traumatic brain injury and epilepsy).
SZ, BD, and CN groups differed significantly in sex and ethnicity but not in age or parental education (table 1).
Table 1.
Participant Demographic and Clinical Characteristics
| SZ (n = 201) | BD (n = 46) | CN (n =27) | Test statistic, P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 41.59 (12.07) | 38.89 (12.69) | 36.67 (15.26) | F(2, 273) = 2.39, .09 |
| Parent education | 13.20 (2.71) | 13.52 (2.50) | 13.58 (2.58) | F(2, 249) = 0.42, .66 |
| % Male (n) | 72.6 | 37.0 | 48.1 | χ2 = 24.12, <.001 |
| Race % (n) | χ2 = 41.97, <.001 | |||
| Caucasian | 60.7 | 78.3 | 48.1 | |
| African American | 31.8 | 6.5 | 25.9 | |
| American Indian | 1.0 | 0 | 0 | |
| Asian | 1.5 | 2.2 | 3.7 | |
| Hispanic/Latino | 0.5 | 6.5 | 7.4 | |
| Mixed-race | 4.5 | 6.5 | 7.4 | |
| Hawaiian/Pacific Islander | 0 | 0 | 7.4 | |
| BNSS Item Severity Scores | ||||
| 1. Intensity of pleasurable activities | 1.68 (1.61) | 1.15 (1.48) | 0.19 (0.56) | F(2, 273) = 12.54, <.001 |
| 2. Frequency of pleasurable activities | 2.28 (1.69) | 1.54 (1.72) | 0.26 (0.76) | F(2, 273) = 20.12, <.001 |
| 3. Intensity of future pleasure | 1.01 (1.54) | 0.89 (1.43) | 0.19 (0.48) | F(2, 273) = 3.82, <.02 |
| 4. Lack of normal distress | 0.79 (1.62) | 0.35 (0.74) | 0.00 (0.00) | F(2, 273) = 4.84, < .01 |
| 5. Asociality behavior | 2.20 (1.73) | 1.65 (1.91) | 0.56 (1.16) | F(2, 273) = 11.77, <.001 |
| 6. Asociality internal experience | 1.66 (1.72) | 1.27 (1.50) | 0.19 (0.56) | F(2, 272) = 10.36, <.001 |
| 7. Avolition behavior | 2.40 (1.80) | 1.30 (1.62) | 0.19 (0.48) | F(2, 273) = 25.37, <.001 |
| 8. Avolition internal experience | 2.13 (1.84) | 1.24 (1.65) | 0.04 (0.19) | F(2, 272) = 20.40, < .001 |
| 9. Facial expression | 2.28 (1.79) | 0.89 (1.32) | 0.26 (0.66) | F(2, 272) = 27.44, <.001 |
| 10. Vocal expression | 1.88 (1.93) | 0.89 (1.37) | 0.19 (0.62) | F(2, 273) = 14.92, <.001 |
| 11. Body gestures | 1.99 (1.83) | 0.74 (1.16) | 0.19 (0.62) | F(2, 273) = 21.77, <.001 |
| 12. Quantity of speech | 1.08 (1.56) | 0.30 (0.84) | 0.07 (0.27) | F(2, 273) = 10.52, <.001 |
| 13. Spontaneous elaboration | 1.26 (1.67) | 0.30 (0.87) | 0.11 (0.42) | F(2, 273) = 12.98, <.001 |
Note: Eighty-nine percent of the schizophrenia sample was prescribed an antipsychotic medication (5% first generation, 75% second generation, 9% both, 11% stably unmedicated). BD, bipolar disorder; SZ, schizophrenia, CN, healthy controls; BNSS, Brief Negative Symptom Scale.
Procedures
The BNSS13 is a 13-item negative symptom rating scale designed in response to recommendations from the 2005 NIMH Consensus Development Conference.4 The BNSS includes 6 subscales: anhedonia, asociality, avolition, blunted affect, alogia, and lack of normal distress. All items are rated on a 7-point (0–6) scale, with anchors generally ranging from absent (0) to severe (6). It has demonstrated excellent psychometric properties in the original and translated versions.13,14,24–27
In both studies, the BNSS13 was administered as part of larger protocols. Before the start of the study, raters at each site were trained via an in-depth review of the BNSS manual, workbook, and scoring procedures. Raters were required to meet minimum reliability standards (inter-rater agreement ≥ 0.80) on gold-standard training videos before conducting assessments. All raters had a bachelor’s degree or higher and 1 or more years of clinical experience.
Measures
Data Analysis
Analyses were conducted using the NetworkX package in Python. The BNSS symptom network was constructed using the association between BNSS variables calculated using mutual information (MI). MI is a simple method to calculate both linear and nonlinear relationships between variables using the following equation:
where X and Y are BNSS variables, H(Y) is the entropy of Y, is the entropy of Y conditioned on X, and and are the marginal probability distributions of X and Y, respectively.
The MI values were normalized between 0 and 1 as follows:
where 0 represents no mutual information between variables and 1 represents the perfect correlation.
Details regarding macroscopic and microscopic properties are presented in supplementary materials and summarized in table 2. Groups were compared on microscopic properties using ANOVA. Inclusion of sex and ethnicity as covariates did not alter the pattern of results.
Results
Aim 1: Macroscopic Properties
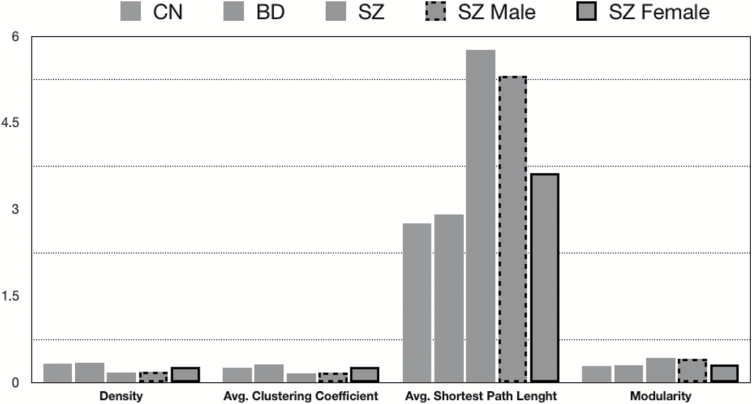
Consistent with hypothesis 1, patients with SZ had lower scores on measures of network density, and average clustering coefficient, as well as higher scores on harmonic mean shortest path length and modularity compared with CN and BD groups. These results support the hypothesis that negative symptom networks are less densely connected in SZ (figure 1).
Fig. 1.
Macroscopic properties of Brief Negative Symptom Scale network in schizophrenia (SZ) males vs females.
Aim 2: Microscopic Properties
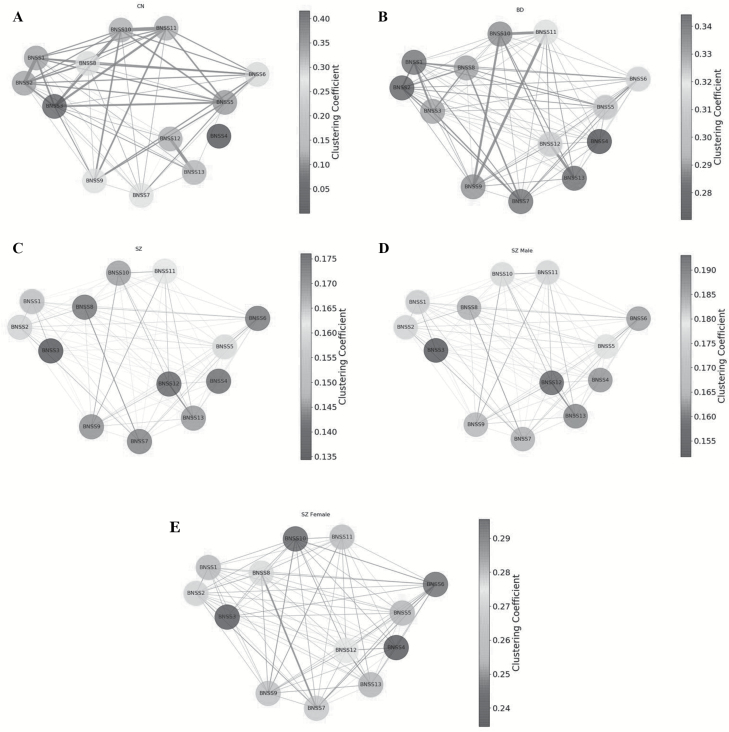
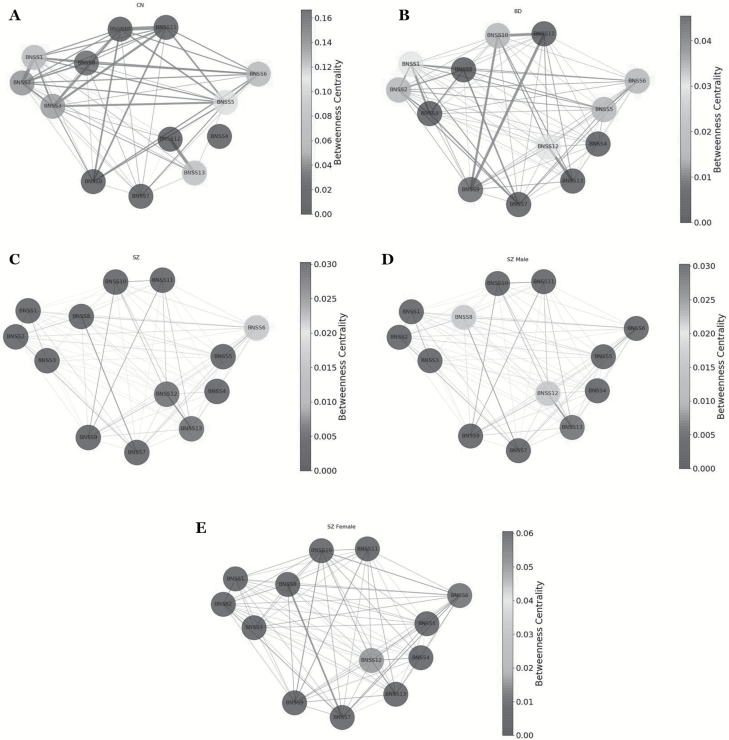
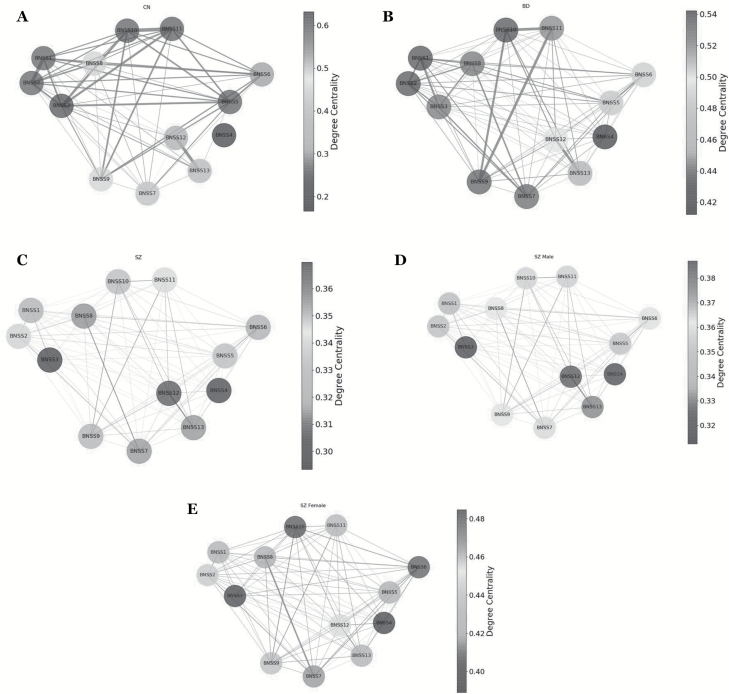
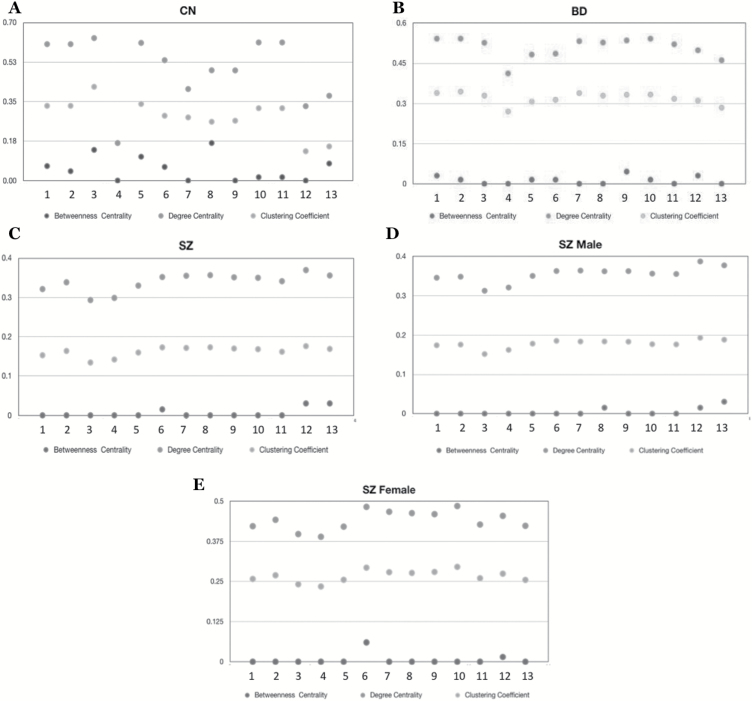
Clustering coefficient (figure 2), betweenness centrality (figure 3), and degree centrality (figure 4) were evaluated to determine which negative symptom domains are most central to the negative symptom networks of CN (A panels), BD (B panels), and SZ (C panels) (see figure 5 for summary). Statistical comparisons between SZ, BD, and CN on microscopic properties are presented in table 3. There were significant group differences in degree centrality and clustering coefficient, but not on betweenness centrality.
Fig. 2.
(A) Clustering coefficient results for controls. The nodes in the network represent the Brief Negative Symptom Scale (BNSS) symptoms; the node shades represent clustering coefficients; the edges represent connection between nodes. (B) Clustering coefficient results for bipolar disorder. The nodes in the network represent the BNSS symptoms; the node shades represent clustering coefficients; the edges represent connection between nodes. (C) Clustering coefficient results for schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent clustering coefficients; the edges represent connection between nodes. (D) Clustering coefficient results for male patients. The nodes in the network represent the BNSS symptoms; the node shades represent clustering coefficients; the edges represent connection between nodes. (E) Clustering coefficient results for female patients. The nodes in the network represent the BNSS symptoms; the node shades represent clustering coefficients; the edges represent connection between nodes.
Fig. 3.
(A) Betweenness centrality results for controls. The nodes in the network represent the Brief Negative Symptom Scale (BNSS) symptoms; the node shades represent betweenness centrality; the edges represent connection between nodes. (B) Betweenness centrality results for bipolar disorder. The nodes in the network represent the BNSS symptoms; the node shades represent betweenness centrality; the edges represent connection between nodes. (C) Betweenness centrality results for patients with schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent betweenness centrality; the edges represent connection between nodes. (D) Betweenness centrality results for male patients with schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent betweenness centrality; the edges represent connection between nodes. (E) Betweenness centrality results for female patients with schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent betweenness centrality; the edges represent connection between nodes.
Fig. 4.
(A) Degree centrality results for controls. The nodes in the network represent the Brief Negative Symptom Scale (BNSS) symptoms; the node shades represent degree centrality; the edges represent connection between nodes. (B) Degree centrality results for bipolar disorder. The nodes in the network represent the BNSS Symptoms; the node shades represent degree centrality; the edges represent connection between nodes. (C) Degree centrality results for patients with schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent degree centrality; the edges represent connection between nodes. (D) Degree centrality results for male patients with schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent degree centrality; the edges represent connection between nodes. (E) Degree centrality results for female patients with schizophrenia. The nodes in the network represent the BNSS symptoms; the node shades represent degree centrality; the edges represent connection between nodes.
Fig. 5.
(A) Microscopic network properties of controls. (B) Microscopic network properties of bipolar disorder. (C) Microscopic network properties of patients with schizophrenia. (D) Microscopic network properties of male patients with schizophrenia. (E) Microscopic network properties of female patients with schizophrenia. 1 = Intensity of past week pleasure; 2 = frequency of past week pleasure; 3 = intensity of expected future pleasure; 4 = lack of normal distress; 5 = asociality behavior; 6 = asociality inner experience; 7 = avolition behavior; 8 = avolition inner experience; 9 = blunted facial affect; 10 = blunted affect vocal expression; 11 = blunted affect body gestures; 12 = alogia quantity of speech; 13 = alogia spontaneous elaboration.
Table 3.
ANOVA Results for Sex Differences in Microscopic Properties
| SZ vs CN | SZ vs BD | BD vs CN | SZ male vs female | |
|---|---|---|---|---|
| Betweenness centrality | 0.54* | 0.31 | 0.46 | 0.07 |
| Clustering coefficient | 0.77** | 1** | 0.38 | 1.0** |
| Degree centrality | 0.85** | 1** | 0.46 | 1.0** |
Note: Values represent K–S values.
*P < .05; *** P < .001
Clustering Coefficient.
The most central items in SZ were quantity of speech, avolition internal experience, avolition behavior, and asociality inner experience, with low scores for intensity of expected pleasure from future activities and lack of normal distress. In BD, intensity of pleasure during activities and frequency of pleasurable activities were the most central items and lack of normal distress and spontaneous elaboration had the lowest clustering coefficient values. In CN, intensity of pleasure during activities, frequency of pleasurable activities, and intensity of expected pleasure from future activities (anhedonia items) had the highest centrality values and lack of normal distress had the lowest centrality values.
Betweenness Centrality.
The most central items for SZ were quantity of speech and spontaneous elaboration (alogia). All other items had low centralities. In BD, facial expression had the highest betweenness centrality value. In CN, intensity of expected pleasure from future activities and avolition inner experience had the highest centrality values.
Degree Centrality.
The most central item for SZ was quantity of speech (alogia), with low centralities for lack of normal distress and intensity of expected pleasure from future activities (anhedonia). In BD, intensity of pleasure during activities, frequency of pleasurable activities, and vocal expression were the most central items, whereas lack of normal distress had the lowest degree centrality score. In CN, intensity of expected pleasure from future activities, frequency of pleasurable activities, and intensity of pleasurable activities, vocal expression, and expressive gestures were the most central items, whereas lack of normal distress had the lowest degree centrality value.
Collectively, these findings provide modest support for Hypothesis 2, which proposed that avolition would be the most central variable in SZ. Rather, across microscopic properties, alogia appears to be most central. Anhedonia items tended to be most central in BD and CN groups, as hypothesized.
Aim 3: Sex Differences in Macroscopic and Microscopic Properties
Macroscopic and microscopic properties were compared in males and females with SZ to evaluate sex differences in network structure. For macroscopic properties, females had lower scores in average shortest path length and modularity, as well as higher scores in density and average clustering coefficient (figure 1). Collectively, these results indicate that females have more dense (ie, interconnected) negative symptom networks than males.
For microscopic properties, there were significant sex differences in degree centrality and clustering coefficient; males and females with SZ did not differ on betweenness centrality (see table 3 and figures 2–5 panels D and E). For men, the most central items fell within the domains of: alogia (betweenness centrality, clustering coefficient, degree centrality). In females, the most central items were asociality inner experience and blunted facial affect. Collectively, these results indicate differences in BNSS centralities in women (blunted affect, asociality) and men (alogia).
Discussion
This study examined negative symptoms from a network perspective by evaluating macroscopic and microscopic network properties. The study had 3 primary aims: (1) To determine whether patients with SZ differ from CN and patients with BD in global network density; (2) To identify negative symptom domains that are most central within the networks of SZ patients compared to BD and CN; (3) To evaluate sex differences in macroscopic and microscopic properties in SZ.
Macroscopic properties that were analyzed indicated that compared to CN and patients with BD, patients with SZ had negative symptom networks that were less densely connected. Less dense networks have been shown to be more treatment resistant in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study trial,9,16 which is consistent with the limited progress on effectively treating negative symptoms to date.15 From a macroscopic level, limited treatment effects may result from individual negative symptom domains not being tightly coupled, allowing individual symptoms to function in isolation and become self-sustaining. Less dense networks have little interaction among domains, making spreading effects that are necessary for global improvement within the network less tenable. To achieve an effect on the overarching negative symptom construct in SZ, it may be necessary to apply a series of treatments that target mechanisms underlying each of the 5 domains, rather than a treatment focusing on the construct as a whole. Indeed, there is growing evidence that negative symptoms are not a unitary construct, but rather 5 separate domains that may have distinct pathophysiological mechanisms.28,29 Global improvements in the broad construct may therefore not be a realistic goal given the loosely interconnected network structure of negative symptoms and the relative independence of the individual domains in the construct. Clinical trials may therefore benefit from shifting their approach from targeting the broad construct, to targeting one of the individual domains specifically.
Early and modern conceptualizations of negative symptoms2,3,30 posited that avolition may be the most core domain of negative symptoms, such that deficits in motivation give rise to each of the other negative symptoms. Although clinically intuitive, this postulation had not previously been empirically tested. Our network approach provided an objective and systematic comparison of which negative symptom domains are most central to test competing possibilities. Although there were slight differences across individual microscopic network indices, the global picture indicated modest support for avolition being most central in SZ. Specifically, avolition was highly central in only 1/3 of the microscopic properties evaluated. This is consistent with Foussias and Remington’s3 conceptualization and may suggest that the psychological (eg, effort-cost computation, reinforcement learning) and neural (eg, corticostriatal activation and connectivity) processes associated with avolition are important to the global negative symptom construct in SZ.31,32 Interestingly, alogia was an even more highly central negative symptom, with high values on all 3 microscopic indices. Mechanisms underlying alogia are perhaps the least understood in terms of all of the negative symptoms. Shaffer et al33 found evidence for differential neural correlates of negative symptom domains, with alogia associated with decreased activity in the bilateral thalamus, right caudate, and left pallidum. Reduced activation of these regions, which include key parts of the basal ganglia, may suggest that alogia stems from problems generating voluntary motor behavior. Seen from this perspective, the high centrality for alogia in patients with SZ that was observed in this study may result from deficits in initiating motor behavior, which are not only core to speech production, but also initiating other motor behaviors needed for seeking out goal-directed activities, social behaviors, pleasurable activities, and outward expression of emotion. These findings are consistent with what behavioral neuroscientists have referred to as a deficit in “behavioral initiation.” Patients with SZ are abnormal in that they do not initiate motor behaviors (in relation to facial, speech, social, pleasurable, or volitional behaviors) as often as other people. They are usually capable of all of these, but initiate them less frequently than is normal.
In contrast, anhedonia was the domain that generally had the highest centrality in BD and CN groups. Anhedonia is a fundamental and frequent symptom of mood disorders, which also occurs in the general population.17 It is thought to reflect a latent vulnerability for developing a range of psychiatric disorders.18 From a practical standpoint, it is intuitive as to why anhedonia might be central in CN and BD groups: individuals may not perform goal-directed activities, socialize, express emotion, or produce large quantities of speech because they do not anticipate that these behaviors will be rewarding or experience them to be rewarding in the moment. Dysfunction within corticostriatal circuitry is thought to be key to anhedonia, resulting in dysfunction in a range of reward processing domains (eg, hedonic response, reinforcement learning, and effort-cost computation) that are critical to decision-making processes needed to initiate pleasurable activities.34
Macroscopic and microscopic properties were also compared in males and females with SZ. Analyses examining macroscopic properties indicated that males had higher scores in average shortest path length and modularity, as well as lower scores in density and average clustering coefficient. Consistent with our hypotheses, these results suggest that females have more dense (ie, interconnected) negative symptom networks than males. Our hypotheses were based on evidence indicating that less dense networks are more treatment resistant in SZ9,16 and that males tend be less treatment responsive than females.15 Highly dense networks observed in females could explain why females are typically more treatment responsive.
Exploratory analyses of microscopic properties also indicated that there were significant sex differences in the centrality of negative symptom domains in SZ. The most central variables in females were items from blunted affect and asociality subscales. From an evolutionary perspective, high centralities among these symptoms would be functional. Each of these variables involves social communication, which was very instrumental to the survival of females evolutionarily. In contrast, alogia was most central in males. Following the motor circuitry explanation earlier, these findings may suggest that mechanisms underlying behavioral initiation are key to negative symptom pathology in males. From an evolutionary perspective, the ability to initiate a range of motor behaviors would be highly adaptive to males, perhaps influencing general levels of behavioral activation needed to promote survival. Future studies should attempt to map neural circuitry onto symptom networks to evaluate this hypothesis in men vs women.
Certain limitations should be considered. First, only a single negative symptom measure was evaluated. It is unclear whether these findings generalize to other measures that evaluate negative symptom domains differently than the BNSS, or whether alternative approaches to symptom ratings, such as Ecological Momentary Assessment, might produce different results. Second, our patients were in the chronic phase of illness, and it is unclear whether these results generalize to earlier phases of psychosis. Third, negative symptoms can result from both primary and secondary factors.35 Future studies should attempt to categorize patients based on clinical or mathematical approaches36,37 and test whether network structures differ between primary and secondary negative symptom patients. Finally, in his symptom network theory, Borsboom7 proposed that there are “environmental” factors that influence symptom networks. These environmental factors are external to the symptom network and can reflect aspects internal to the individual (eg, brain function and inflammation) or those from the environment itself (eg, early life stress) that act on the interconnections among symptoms. We were unable to address the influence of such environmental factors in this study, although this is a critically important future direction.
Despite these limitations, these findings provide valuable insight into negative symptoms from a network perspective. Like other symptom constellations,7 negative symptoms have distinct patterns of interactions that allow them to become self-sustaining and chronic. In SZ, negative symptom networks are characterized by low density, which may make them difficult to treat. There are also sex differences in network density in SZ, with greater density in females than males, which may facilitate greater treatment response in females. The sexes also differ regarding which negative symptoms are most central, potentially suggesting grounds for differential treatment targets in males (alogia) vs females (asociality, blunted affect). Taking a macroscopic network approach to analyzing treatment effects could be a promising future direction, as existing treatments may not result in an overall net reduction in symptom severity, but could still plausibly have an effect on network structure that is clinically meaningful by shifting density of symptom connections. In addition, evaluating microscopic properties at baseline to determine which domain is most central may provide a valuable tool for clinical trials aiming to take a personalized medicine approach to targeting specific symptoms (eg, cognitive-behavioral therapy).38 Centrality measures may offer a mathematical tool for selecting among available treatments once these exist for the individual domains defined in the 2005 NIMH consensus conference.4 Given that the link between negative symptoms and poor functional outcomes is a key reason why negative symptoms are important treatment targets, it will be important for future studies that take a network approach to determine which negative symptom domains are most central to driving poor functional outcome, as this would further highlight their relevance as treatment targets.
Funding
The study was supported by National Institute of Mental Health grant (K23MH092530 to G.P.S.).
Conflict of Interest
G.P.S. and B.K. are original developers of the Brief Negative Symptom Scale (BNSS) and receive royalties and consultation fees from ProPhase LLC in connection with commercial use of the BNSS and other professional activities. All other authors have no relevant conflicts of interest to report.
Supplementary Material
References
- 1. Kraepelin E. Dementia Praecox and Paraphrenia. New York, NY: Robert E Krieger; 1919. [Google Scholar]
- 2. Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press; 1911. [Google Scholar]
- 3. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sayama H. Introduction to the Modeling and Analysis of Complex Systems. Open SUNY Textbooks; 2015. https://textbooks.opensuny.org/introduction-to-the-modeling-and-analysis-of-complex-systems/. [Google Scholar]
- 6. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. [DOI] [PubMed] [Google Scholar]
- 7. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borsboom D, Robinaugh DJ, Rhemtulla M, Cramer AOJ; Psychosystems Group Robustness and replicability of psychopathology networks. World Psychiatry. 2018;17:143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esfahlani FZ, Visser KF, Strauss GP, Sayama H. A network-based classification framework for predicting treatment response of schizophrenia patients. Expert Syst Appl. 2018;109:152–161. [Google Scholar]
- 10. Lutz W, Schwartz B, Hofmann SG, Fisher AJ, Husen K, Rubel JA. Using network analysis for the prediction of treatment dropout in patients with mood and anxiety disorders: a methodological proof-of-concept study. Sci Rep. 2018;8:7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strauss GP, Vertinski M, Vogel SJ, Ringdahl EN, Allen DN. Negative symptoms in bipolar disorder and schizophrenia: a psychometric evaluation of the brief negative symptom scale across diagnostic categories. Schizophr Res. 2016;170:285–289. [DOI] [PubMed] [Google Scholar]
- 12. Emmerson LC, Ben-Zeev D, Granholm E, Tiffany M, Golshan S, Jeste DV. Prevalence and longitudinal stability of negative symptoms in healthy participants. Int J Geriatr Psychiatry. 2009;24:1438–1444. [DOI] [PubMed] [Google Scholar]
- 13. Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strauss GP, Hong LE, Gold JM, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esfahlani FZ, Sayama H, Visser KF, Strauss GP. Sensitivity of the Positive And Negative Syndrome Scale (PANSS) in detecting treatment effects via network analysis. Innov Clin Neurosci. 2017;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 17. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. [DOI] [PubMed] [Google Scholar]
- 18. Meehl PE. Primary and secondary hypohedonia. J Abnorm Psychol. 2001;110:188–193. [DOI] [PubMed] [Google Scholar]
- 19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 20. Young RC, Biggs JT, Ziegler VE, Meyer DA.. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 21. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 23. Pfohl BM, Blum N, Zimmerman M.. Structured Interview for DSM-IV Personality. 1st ed Washington, DC: American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- 24. Mané A, García-Rizo C, Garcia-Portilla MP, et al. Spanish adaptation and validation of the Brief Negative Symptoms Scale. Compr Psychiatry. 2014;55:1726–1729. [DOI] [PubMed] [Google Scholar]
- 25. Mucci A, Galderisi S, Merlotti E, et al. ; Italian Network for Research on Psychoses The Brief Negative Symptom Scale (BNSS): independent validation in a large sample of Italian patients with schizophrenia. Eur Psychiatry. 2015;30:641–647. [DOI] [PubMed] [Google Scholar]
- 26. Chieffi M, Galderisi S, Mucci A, et al. The Brief Negative Symptom Scale: convergent/discriminant validity and factor structure in a large sample of outpatients with schizophrenia. Eur Psychiatry. 2015;30:246. [DOI] [PubMed] [Google Scholar]
- 27. Merlotti E, Mucci A, Bucci P, Nardi A, Galderisi S. Italian version of the “Brief Negative Symptom Scale”. J Psychopathol. 2014;20:199–215. [Google Scholar]
- 28. Strauss GP, Zamani Esfahlani F, Galderisi S, et al. Network analysis reveals latent dimensions of negative symptoms in schizophrenia. Schizophr Bull, sby133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmed AO, Kirkpatrick B, Galderisi S, et al. Cross-cultural validation of the five-factor model of negative symptoms in schizophrenia. Schizophr Bull. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. [DOI] [PubMed] [Google Scholar]
- 31. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(suppl 2):S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaffer JJ, Peterson MJ, McMahon MA, et al. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol Neuropsychiatry. 2015;1:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. [DOI] [PubMed] [Google Scholar]
- 36. Ahmed AO, Strauss GP, Buchanan RW, Kirkpatrick B, Carpenter WT. Are negative symptoms dimensional or categorical? Detection and validation of deficit schizophrenia with taxometric and latent variable mixture models. Schizophr Bull. 2015;41:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed AO, Strauss GP, Buchanan RW, Kirkpatrick B, Carpenter WT. Schizophrenia heterogeneity revisited: clinical, cognitive, and psychosocial correlates of statistically-derived negative symptoms subgroups. J Psychiatr Res. 2018;97:8–15. [DOI] [PubMed] [Google Scholar]
- 38. Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69:121–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.