Abstract
The ability to infer from uncertain information is impaired in schizophrenia and is associated with hallucinations and false beliefs. The accumulation of information is a key process for generating a predictive internal model, which statistically estimates an outcome from a specific situation. This study examines if updating the predictive model by the accumulation of information in absence of feedback is impaired in schizophrenia. We explored the implicit adaptation to the probability of being instructed to perform a movement (33%-Go, 50%-Go, or 66%-Go) in a Go/NoGo task in terms of reaction times (RTs), electromyographic activity, and corticospinal excitability (CSE) of primary motor cortex (M1). CSE was assessed at two time points to evaluate prediction of the upcoming instruction based on previously accumulated information: at rest (preceding the warning signal) and at the Go/NoGo signal onset. Three groups were compared: patients with schizophrenia (n = 20), unaffected siblings (n = 16), and healthy controls (n = 20). Controls and siblings showed earlier movement onset and increased CSE with higher Go probability. CSE adaptation seemed long-lasting, because the two CSE measures, at least 1500 ms apart, strongly correlated. Patients with schizophrenia failed to show movement onset (RT) adaptation and modulation of CSE. In contrast, all groups decreased movement duration with increasing Go probability. Modulation of CSE in the anticipatory phase of the potential movement reflected the estimation of upcoming response probability in unaffected controls and siblings. Impaired modulation of CSE supports the hypothesis that implicit adaptation to probabilistic context is altered in schizophrenia.
Keywords: internal model, schizophrenia, prediction, TMS, Go-NoGo, adaptation
Introduction
In schizophrenia, altered prediction or inference is related to symptom severity, ie, delusions and hallucinations1–4 and working memory deficits.5 In particular, patients with schizophrenia show impairment in predictions from uncertain information1,6,7 and in the ability to infer or predict from statistical information.8 Using Bayesian modeling, previous studies have shown that patients infer their decision on the basis of less information, resulting in “jump to conclusion” biases.5,9 Furthermore, patients tend to overestimate probability changes.1 Prediction deficits have also been found in the sensory domain10,11 and adaptation deficits in social context.12
Previous studies on implicit statistical learning in schizophrenia used probabilistic associative learning (ie, a cue associated with a probability of outcome) and feedbacks to reinforce the association between an input and its most probable outcome. Patients with schizophrenia showed altered associative learning related to working memory13 and feedback-driven14–17 learning potentially related to a dysfunction in evaluating reward.16,18 Dopaminergic dysfunctions in schizophrenia are associated with impaired feedback representation16,19–21 and correlate with negative symptoms.18,22 However, in the case of probabilistic spatial allocation of attention, no impairment in reward processing was found in schizophrenia.23 It, therefore, remains unclear whether feedback or associative learning should be considered as a central cause in dysfunction of statistical prediction, or whether the processing of cumulative contextual information is impaired, independently from feedback or reward.
To infer and predict from an uncertain context, the brain must rely on an internal model that relates an event or context to its most probable consequence. An adequate internal model needs to be continuously updated as new evidence is provided.24 The generation of such a model relies on implicit statistical learning–based accumulation of evidence over time to predict the outcome.24,25 This mechanism might be impaired in schizophrenia, potentially leading to the generation of an inadequate internal model that might provide impaired predictions, erroneous beliefs, and contextually inappropriate behaviors.
We addressed here the potential impairment in schizophrenia of implicit statistical adaptation due to incorrect accumulation of information over time, resulting in impaired adaptation to event probability. We used a modified Go/NoGo reaction time (RT) task, where the probability of Go trials varied implicitly (33%, 50%, and 66% from one block to the other), subjects being unaware of the changes in response (Go) probability. The adaptation to the implicit Go probability was compared between 3 groups: 20 patients with schizophrenia (PSZ), 16 unaffected siblings (SIB), and 20 healthy control subjects (HC). We examined behavioral adaptations to the probabilistic context by measuring RTs and assessed its effect on muscle activation using electromyography (EMG). Transcranial magnetic stimulation (TMS) on primary motor cortex (M1) was used to explore task-related motor cortex (corticospinal) excitability through motor-evoked potentials (MEPs). We compared patients and siblings to identify if impaired adaptation, if present, is related to genetic risk factor or disease.
As a measure of adaptation to probability, we expected RT to be shorter for a high response (Go signal) probability,26 and EMG onset to be earlier. We measured corticospinal excitability (CSE) prior to the Go/NoGo signal. These MEPs are thought to represent modulation of CSE as a function of implicit prediction of response probability. Because CSE has been observed to change with prediction of movement timing,27 we hypothesized that CSE would increase for higher Go probabilities, particularly just prior to the predictable timing of the imperative (Go or NoGo) signal. If the misevaluation of feedback in patients is the cause of impaired updating of the internal model, we expect that in the absence of any feedback behavioral and physiological adaptations should be similar in all groups. On the contrary, if the integration of information over time is altered in schizophrenia independently from any feedback, then results should show impaired adaptation for patients only.
Methods
Participants
Twenty patients, 20–44 years old (4 females, mean age ± SD: 31 ± 8 years), fulfilling Diagnostic and Statistical Manual, Fourth Edition, Text Revision (DSM-IV TR) criteria for schizophrenia28 were recruited at Sainte-Anne Hospital, Paris, France. PSZ were all medically stabilized for more than 3 months and medicated with second-generation antipsychotics for more than 1 month. In addition, 16 SIB, 20–50 years old (11 females, mean age: 35 ± 10 years), and 20 HC, 19–46 years old (6 females, mean age 31 ± 8 years), were recruited.
Patients under clozapine-based treatment (as it is reported to modulate cortical excitability)29 and participants with score <80 for approximated intelligence quotient (aIQ; using short Wechsler Adult Intelligence Scale, third edition [WAIS-III] version30) were excluded from the study. According to the modified Edinburgh handedness inventory,31 3 subjects among HC, 3 in PSZ, and 2 in SIB were left-handed. One subject in PSZ and one in SIB were ambidextrous. All subjects were assessed for psychiatric disorders with the Diagnostic Interview for Genetic Studies (version 3.0, NIMH) allowing validation of clinical status. The study received ethical approval from the regional ethics committee (Ile de France VIII) and all subjects provided written informed consent prior to the experiment.
Clinical symptoms in the PSZ group were assessed using the Positive and Negative Syndrome Scale (PANSS;32 French version33) and the Brief Psychiatric Rating Scale (BPRS) completing general psychiatric items.34 Individual patient scores are presented in table 1.
Table 1.
Clinical Evaluation of the 20 Patients With Schizophrenia
| CPZ (mg/d) | PANSS | BPRS [24–168] | NSS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total score [30–210] | Positive subscore [7–49] | Negative subscore [7–49] | General subscore [16–112] | Disorganization subscore [4–28] | Total score [0–105] | Sensory integration subscore [0–15] | Motor coordination subscore [0–21] | Motor integration subscore [0–18] | |||
| 1 | 80 | 63 | 10 | 15 | 38 | 7 | 48 | 1.5 | 0 | 0.5 | 0 |
| 2 | 35 | 54 | 13 | 11 | 30 | 10 | 46 | 18.5 | 0.5 | 9 | 2 |
| 3 | 200 | 63 | 12 | 16 | 35 | 7 | 52 | 12.5 | 0.5 | 6.5 | 0 |
| 4 | 200 | 56 | 9 | 14 | 33 | 12 | 40 | 13 | 4.5 | 5.5 | 2 |
| 5 | 300 | 44 | 9 | 12 | 23 | 6 | 37 | 6 | 0.5 | 3.5 | 0 |
| 6 | 267 | 50 | 13 | 17 | 20 | 7 | 44 | 18.5 | 3.5 | 7 | 1 |
| 7 | 75 | 44 | 9 | 9 | 26 | 6 | 36 | 5.5 | 0 | 3.5 | 0 |
| 8 | 200 | 52 | 10 | 13 | 29 | 4 | 44 | 9 | 1 | 7 | 0 |
| 9 | 200 | 63 | 9 | 21 | 33 | 6 | 45 | 7.5 | 1 | 4 | 1 |
| 10 | 200 | 56 | 9 | 11 | 36 | 9 | 41 | 18 | 0.5 | 7.5 | 1.5 |
| 11 | 300 | 81 | 14 | 28 | 39 | 11 | 49 | 19.5 | 2 | 7.5 | 3 |
| 12 | 300 | 51 | 14 | 13 | 24 | 11 | 42 | 21 | 5.5 | 6 | 4 |
| 13 | 200 | 44 | 7 | 11 | 26 | 7 | 36 | 16.5 | 1 | 7 | 0 |
| 14 | 300 | 47 | 10 | 11 | 26 | 10 | 40 | 17.5 | 4 | 8 | 1 |
| 15 | 100 | 54 | 8 | 10 | 36 | 4 | 46 | 3 | 0 | 3 | 0 |
| 16 | 1067 | 61 | 10 | 17 | 34 | 9 | 48 | 14.5 | 2.5 | 5.5 | 2 |
| 17 | 67 | 52 | 19 | 10 | 23 | 4 | 44 | 21 | 4 | 1.5 | 1 |
| 18 | 267 | 64 | 14 | 14 | 36 | 8 | 64 | 13.5 | 2 | 4 | 0.5 |
| 19 | 250 | 57 | 21 | 9 | 27 | 9 | 45 | 11.5 | 4 | 3.5 | 0 |
| 20 | 1067 | 59 | 12 | 10 | 37 | 9 | 38 | 16 | 2 | 8.5 | 1.5 |
| Mean ± SD | 284 ± 281 | 56 ± 9 | 12 ± 4 | 14 ± 5 | 31 ± 6 | 8 ± 2 | 44 ± 6 | 13.2 ± 6.1 | 2 ± 1.7 | 5.4 ± 2.4 | 1 ± 1.1 |
Note: CPZ, chlorpromazine equivalent; PANSS, Positive and Negative Syndrome Scale; BPRS, Brief Psychiatric Rating Scale; NSS, neurological soft signs; PSZ, patients with schizophrenia. Individual CPZ and scores for PANSS, BPRS, and NSS for the group of PSZ. In brackets: min–max score of the respective scale.
All 3 groups underwent clinical assessments of Neurological Soft Signs (NSS)35 and Simpson-Angus Extrapyramidal Scale (SAS).36 Neuropsychological functions, eg, inhibition, attentional processes, and working memory, were assessed with Test battery for Attentional Performance (TAP)37 and Stroop color naming test.38 An aIQ was also included with the short WAIS-III.30 Mean neuropsychological scores by group are presented in table 2. Owing to technical issues, the TAP scores of 1 subject in HC and SIB groups were missing.
Table 2.
Neuropsychological Test Scores for Each Group
| HC | SIB | PSZ | |
|---|---|---|---|
| aIQ | 116 ± 12 | 120 ± 10* | 110 ± 12 |
| TAP | |||
| Working memory (omission) | 1.7 ± 2.7* | 1 ± 2.1** | 3.8 ± 3.4 |
| Incompatibility (response) | 28.7 ± 1.2 | 29.3 ± 0.6 | 27.3 ± 4.7 |
| Incompatibility (ms) | 468 ± 127 | 499 ± 77 | 536 ± 99 |
| Divided attention (auditory-omission) | 1.1 ± 1** | 1 ± 1** | 4.9 ± 5.3 |
| Divided attention (visual-omission) | 0.2 ± 0.7* | 0.07 ± 0.3 | 2.3 ± 4.4 |
| Stroop | |||
| Interference (errors) | 0.9 ± 1.1* | 0.6 ± 0.8* | 1.9 ± 1.7 |
| Interference (total time, s) | 94 ± 39 | 89 ± 38 | 121 ± 56 |
| NSS | 7.5 ± 3.4** | 7.3 ± 3.9** | 13.2 ± 6.1 |
| SAS [0–40] | 1.5 ± 1.2* | 1.2 ± 1.3* | 3.3 ± 3.4 |
Note: PSZ, patients with schizophrenia; SIB, unaffected siblings; HC, healthy control subjects; aIQ, approximated intelligence quotient; TAP, Test battery for Attentional Performance, NSS, neurological soft signs; SAS, Simpson-Angus Extrapyramidal Scale. Mean ± SD values for the group of PSZ, SIB, and HC. Tests: aIQ, TAP, Stroop, NSS (total score), and SAS. In bracket: min–max score of the respective scale. HC and SIB scores significantly different from PSZ group: *P < .05; **P < .01, t test. None of the differences between SIB and HC scores were significantly different.
Design and Procedure
Behavioral Task.
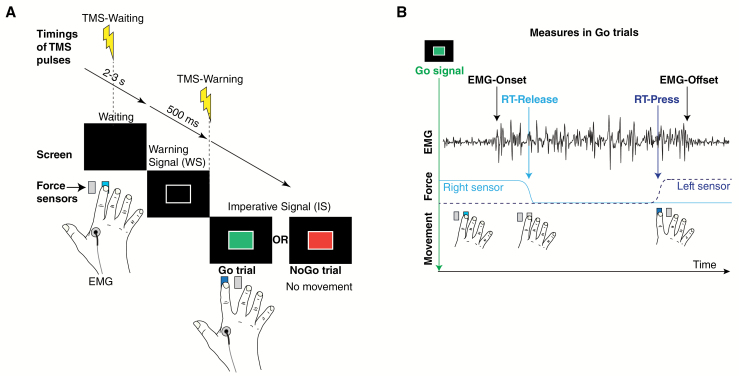
The participant sat on a chair in front of a desk on which were positioned a device with two force sensors (buttons) and a computer screen (figure 1A). The participant positioned his/her right index finger on the right force sensor. Each trial consisted of the following sequence: a waiting phase (black screen, duration randomized between 2 and 3 s), a warning signal (WS) displayed for 500 ms in the form of a white outlined rectangle (5 cm × 8 cm), and an imperative signal (IS) either as a Go (green rectangle) or a NoGo signal (red rectangle). In the case of a NoGo trial, the participant was instructed not to move the index finger. In the case of a Go trial, the subject had to move (abduct) the index finger as fast as possible from the initial position (right button) to the left button. The IS disappeared when the participant pressed the left force sensor. In the absence of a left button press, the IS disappeared after a predetermined delay (RTmax, see supplementary material). After having pressed the left button, the subject had to move the finger back to the right button, without time constraints. Reaching the initial right button was required to continue to the next trial.
Fig. 1.
Go/NoGo reaction time paradigm. (A) Time course of the different steps of a Go/NoGo trial. During the waiting period (2–3 s), the subject holds the index finger on the right button. Then a warning signal (WS) appears for 500 ms (white outlined rectangle), followed by the imperative signal (IS), which was either a Go signal (green rectangle) or a NoGo signal (red rectangle). The subject had to press the left button as fast as possible after the Go signal or keep his/her index finger on the right button in case of a NoGo signal. (B) Illustrative Go trial (without transcranial magnetic stimulation [TMS]) showing, from top to bottom: electromyography (EMG; and its relevant markers: EMG-Onset, EMG-Offset); force trace of left (stippled) and right sensor (solid) indicating behavioral events (RT-Release and RT-Press). RT, reaction time.
Experimental Conditions and Blocks.
There were 3 conditions corresponding to the 3 Go probabilities of 33%-Go, 50%-Go, and 66%-Go. Each block consisted of a constant probability of Go trials. There were 4 consecutive blocks: the first and third block corresponded to 50%-Go, and the condition of the second and fourth block (33% and 66%) was randomized. Each block lasted approximately 8 min.
In one block, the 48 Go and NoGo trials were randomized in subblocks of 6 consecutive trials. One-third of the trials had no TMS (trials randomly distributed over the block). The data retrieved from these trials were used for behavioral and EMG analyses. Behavioral (RT-Release and RT-Press) and EMG (EMG-Onset and EMG-Offset) measures are illustrated in figure 1B. The second and the last third of trials (also randomly distributed over each block) were dedicated to TMS and corresponded, respectively, to trials with TMS-Waiting or TMS-Warning. TMS never occurred twice during the same trial, and the delay between two TMS pulses was at least 4 s.
Behavioral apparatus and measures, TMS, and EMG methods are described in supplementary material.
Data Analysis
Greenhouse-Geisser corrections for repeated measures ANCOVAs were applied when assumption of sphericity was violated, and P values were Bonferroni corrected for multiple comparisons. Spearman rank correlations were used for testing the relation between TMS/behavioral measures and neuropsychological/clinical tests. Pearson correlations were applied to relate behavioral to TMS measures. P values of correlation coefficients were corrected for multiple comparisons39 and all statistical significance was set to P < .05.
Statistical analyses and MEP z-score computations are described in supplementary material.
Adaptation Score.
For the different measures, we calculated individual score corresponding to the degree of adaptation to the Go signal probability:
| (1) |
Results
Behavioral Measures
Reaction Times.
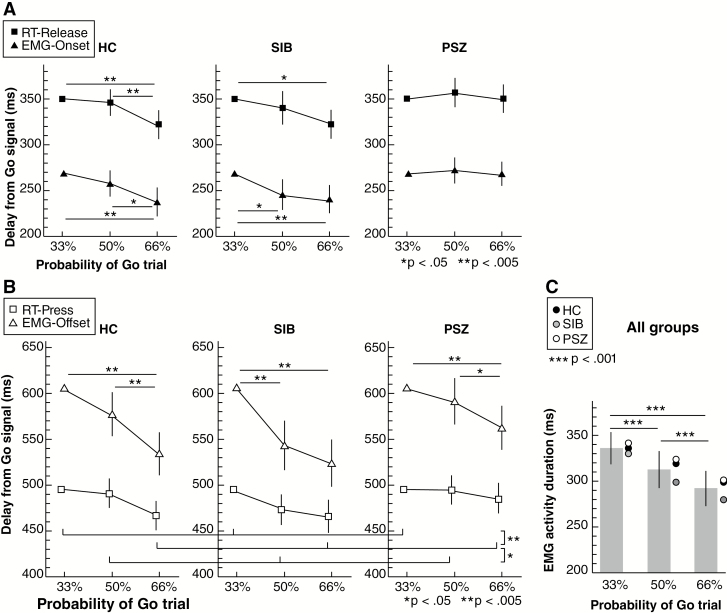
Data of RT-Release (ie, delay from Go signal to release the right button) for each group are illustrated in figure 2A. Analyses indicated a main effect of PROBABILITY (F(2,104) = 12.46, P < .001, partial η2 = 0.19, ANCOVA) and an interaction effect between GROUP and PROBABILITY (F(4,104) = 2.51, P = .046, partial η2 = 0.09, ANCOVA). Post hoc comparisons showed significant difference between 33%-Go and 66%-Go and between 50%-Go and 66% Go for HC group (P = .001 for both, threshold at 0.05) and between 33%-Go and 66%-Go for SIB group. We found no significant difference between probabilities of Go in PSZ group (P > .97 for all comparisons) and between HC and SIB groups for each condition (all Ps = 1). The RT decreased as the Go signal probability increased for the HC and SIB groups, but not for the PSZ group.
Fig. 2.
Reaction times (RTs) and electromyographic (EMG) activity for each group: healthy control subjects (HC), unaffected siblings (SIB), and patients with schizophrenia (PSZ). (A) Estimated marginal means (ANCOVA) of RT for button release at movement start (RT-Release) and EMG-Onset for each GO probability (33%, 50%, and 66%) and group. (B) Estimated marginal means (ANCOVA) of RT for button press at movement end (RT-Press) and EMG-Offset for each GO probability and group. Significant differences between probabilities across groups are indicated with horizontal lines below the graph. (C) Estimated marginal means (ANOVA) of duration of EMG activity for each Go probability over groups. Circles indicate the mean for each group. Vertical bars correspond to 95% confidence interval (CI).
Results of RT-Press for each group and condition are illustrated in figure 2B. We found a significant main effect of PROBABILITY (F(2,104) = 14.56, P < .001, partial η2 = 0.22) but no interaction effect with GROUP (F(4,104) = 1.55, P = .19). Post hoc comparisons between probabilities showed a significant difference between 33%-Go and 66%-Go and between 50%-Go and 66%-Go (P < .001 and P = .028, respectively). The time to reach the second button decreased with increased Go signal probability in all 3 groups.
Go and NoGo Errors.
For each group, we measured the percentage of erroneous movements during NoGo trials (error-NoGo). Although the error rate seemed higher in PSZ group (HC: 2.9%, SIB: 1.9%, PSZ: 6.7%), we found no significant group differences in error-NoGo (HC-PSZ: P = .12, HC-SIB: P = .72, PSZ-SIB: P = .09, Wilcoxon-Mann-Whitney test due to non-normality of data). The second type of error was to not move during Go trials (error-Go; HC: 2.7%, SIB: 3%, PSZ: 3.9%). Again, there was no significant difference between groups (HC-PSZ: P = .99, HC-SIB: P = .78, PSZ-SIB: P = .75, Wilcoxon-Mann-Whitney test due to non-normality of data).
EMG Measures.
Onset and Offset of Muscle Activity
EMG-Onset times in function of the Go probability are illustrated in figure 2A. ANCOVA showed a significant main effect of PROBABILITY (F(2,106) = 19.7, P < .001, partial η2 = 0.28) and an interaction between GROUP and PROBABILITY (F(4,106) = 3.15, P = .02, partial η2 = 0.11, ANCOVA). Post hoc comparisons showed significant differences of EMG-Onset between 33%-Go and 66%-Go and between 50%-Go and 66%-Go for HC group (P < .001 and P = .04, respectively), and between 33%-Go and 66%-Go and between 50%-Go and 50%-Go for SIB group (P = .002 and P = .013, respectively). There were no significant differences of EMG-Onset in the PSZ group (P = 1 for all comparisons) and for identical conditions between HC and SIB (all P > .88). EMG-Onset decreased as the Go signal probability increased for the HC and SIB groups, but not for the PSZ group.
EMG-Offset times are illustrated in figure 2B. ANCOVA revealed no main effect of PROBABILITY (F(2,106) = 2.88, P = .061, ANCOVA), but a significant interaction between PROBABILITY and GROUP (F(4,106) = 2.77, P = .031, partial η2 = 0.1, ANCOVA). Post hoc comparisons showed significant differences in EMG-Offset between 33%-Go and 66%-Go in all groups (HC: P < .001, SIB: P < .001, PSZ: P = .002), between 50%-Go and 66%-Go for HC (P < .001) and PSZ (P = .011), and between 33%-Go and 50%-Go for SIB (P < .001). EMG-Offset decreased with increasing Go signal probability in all 3 groups, as was the case for RT-Press.
EMG Duration
The ANOVA of the effect of probability on EMG duration (figure 1C) indicated a main effect of Go signal probability (F(2,106) = 40.35, P < .001, partial η2 = 0.43), but no interaction with GROUP (F(2,106) = 0.69, P = .60). Post hoc comparisons of EMG duration between probabilities were all significant (all Ps < .001).
Corticospinal Excitability Measures
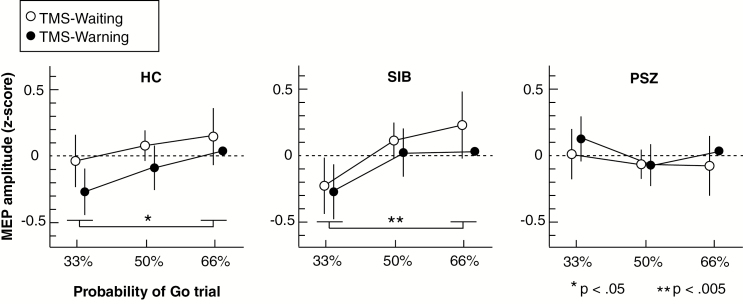
The effect of Go signal probability on MEP amplitudes for each time point is illustrated in figure 3. We found no significant effect of PROBABILITY (F(2,104) = 2.96, P = .056, ANCOVA) on MEP amplitude but a significant interaction effect between PROBABILITY and GROUP (F(4,104) = 3.19, P = .016, partial η2 = 0.11, ANCOVA). Post hoc comparisons showed a significant difference between 33%-Go and 66%-Go MEP amplitude in HC (P = .041) and SIB (P = .002). There was no significant effect of probability in PSZ group (all Ps > .76) and between HC and SIB groups for each condition (all Ps = 1). There was no main effect of TIME-POINT (F(2,104) = 3.31, P = .075, ANCOVA) or interaction effect between GROUP and TIME-POINT (F(2,104) = 2.34, P = .11). Individual differences across all groups between 66%-Go and 33%-Go MEP amplitudes for TMS-Warning and TMS-Waiting strongly correlated (r = 0.69, P < .001). CSE increased as the Go signal probability increased for the HC and SIB groups, but not for the PSZ group.
Fig. 3.
Estimated marginal means (ANCOVA) of motor-evoked potential (MEP) amplitude (z score) for each Go probability (33%, 50%, and 66%) and each group: healthy control subjects (HC), unaffected siblings (SIB), and patients with schizophrenia (PSZ). White circles correspond to MEP amplitudes during the waiting period (TMS-Waiting) and black circles correspond to MEPs at the end of the warning signal (TMS-Warning). Vertical bars represent 95% confidence interval (CI). TMS, transcranial magnetic stimulation.
Correlations Between Measures, Clinical and Neuropsychological Assessments
In order to quantify for each subject his/her degree of adaptation for each measure (RT, EMG, TMS-Warning, TMS-Waiting, Go-Error, and NoGo-Error), an adaption score was computed corresponding to the relative change between 33%-Go probability and 66%-Go probability of the respective measure (equation 1).
Correlation Between Adaptation Scores.
The degree of EMG-Onset adaptation also correlated with the number of NoGo-Errors (r = −0.37, P = .005), such that participants with a weak degree of EMG-Onset adaptation showed less inhibition of movement during NoGo trials. The two TMS adaptation scores were not correlated with RT-Release and EMG-Onset adaptation within groups (all Ps > .2).
Correlations With Clinical and Neuropsychological Assessments for Patients.
We computed correlations between adaptation scores and the neuropsychological and clinical measures (tables 1 and 2). We found a correlation between the degree of adaptation of the MEP z score with the incompatibility score of the TAP (TMS-Warning: r = −0.54, P = .021). Similar correlations were found between NoGo-Error rate and this incompatibility score (r = −0.58, P = .024). None of the measures correlated with working memory score of TAP (p>0.06), with clinical symptoms (PANSS or BPRS), NSS, SAS, or medication (chlorpromazine equivalent, all Ps > .14). Finally, aIQ correlated with the degree of EMG-Onset adaptation (r = −0.78, P < .001) and NoGo-Error rate (r = −0.54, P = .021).
Discussion
In this study, we examined the adaptation of movement production to probabilistic context, where statistical information has to be collected over time in the absence of feedback. Using behavioral and electrophysiological measures in a Go/NoGo RT task with different Go probabilities, we found that healthy control subjects and siblings of patients with schizophrenia adapted their behavior and their cortical excitability to the Go probability. When the Go probability was high, subjects in these two groups had lower RTs, earlier EMG onsets, and higher CSE. To adapt to the Go probability, subjects had to implicitly accumulate information over time, in order to predict what the next trial would be (Go or NoGo) and to adapt (optimize) their behavior accordingly. Patients with schizophrenia did not show such adaptations. This suggests that the adaptation to probabilistic context is affected in schizophrenia, independent of processing of feedback or reward, and is related to disease (not to risk) as siblings did not show impairments.
Long-Lasting Adaption of CSE and Anticipatory State
CSE was assessed at two time points: when the subject was at rest and later, concurrent with the imperative signal. At these two time points, both of them prior to processing of the imperative signal, CSE is likely to reflect the implicit anticipation of the Go probability, ie, the probability of an upcoming movement. This anticipatory state of CSE occurred well before (1000–1500 ms) the preparation for a potential movement (warning signal). In RT paradigms with warning signals, changes in CSE have been observed to increase about 100 ms after the warning signal,40 not before.
Indeed, in this study, the modulation of CSE reflected the prediction of the likelihood of the next Go instruction. Control subjects and siblings demonstrated adaptation of CSE to the Go probability, whereas persons with schizophrenia did not. For control subjects and siblings, this probability-driven modulation likely allowed more efficient movement production: when the Go probability was high, CSE increased and the movement occurred earlier, which can be interpreted as a readiness to move. Moreover, CSE at the predictable time of instruction was strongly correlated with the earlier CSE during the waiting phase, indicating that the modulation of CSE was sustained for at least 1500 ms before the instruction. This long-lasting modulation of CSE seems to reflect the estimated Go probability of the upcoming instruction based on previous information during the block (ie, over multiple trials).
In this study, short-interval intracortical inhibition at rest was lower for patients compared to controls and siblings (supplementary table S1). The lack of CSE adaptation in patients could be due to impaired modulation of M1 inhibition related to the probability of NoGo signals. Impaired inhibitory mechanisms have been generally observed in schizophrenia41,42 and particularly proactive inhibition that modulates the amplitude of cortical excitability in anticipation of stopping a movement.13 CSE modulation related to proactive inhibition could account for the facilitation43–46 of movement inhibition in the case of high NoGo probability. Basal ganglia and thalamocortical circuits are involved in inhibitory and excitatory balance of M1 and are altered in schizophrenia.47,48 fMRI studies have pointed out the role of the striatum in proactive and anticipatory inhibition of M113,26, related to slowed responses in pre-cued movements. Moreover, the thalamus has been found to play a role in the modulation of inhibition and RT for subliminally cued motor tasks49 and could account for impaired RT adaptation to probability.
Predictive Models
Previously, it has been shown by Bayesian modeling that patients with schizophrenia tend to infer future events based on scant evidence and are more inclined to change their inference on occurrence of improbable events.1,5,9 This suggests that patients accumulate evidence for inference over a shorter temporal window and, hence, extract less relevant information. This would adversely affect the generation of an adequate internal predictive model. In our task, a given response probability could have been correctly inferred from at minima 6 consecutive trials in a given condition. The use of a shorter interval in the cumulative build-up to a given response probability would hamper the generation of an adequate predictive model.
Probability-Driven Adaptation of Movement Duration
In contrast to RT (movement onset), whose adaptation to contextual cues differed between patients with schizophrenia and healthy control subjects (or unaffected siblings), movement duration shortened uniformly with increasing Go probability in the 3 groups. The fact that movement onset in patients with schizophrenia did not adapt to response probability, while movement duration did, suggests that distinct neural mechanisms determine these two movement parameters. This is in line with previous studies showing that movement duration (or velocity) can be dissociated from RT.50,51 Moreover, cortical excitability at rest has been found to reflect global movement facilitation, not specific to a particular movement parameter,40 including velocity.51
Motivation and Attention
In patients group, the lack of adaptation seemed not to be due to lesser task motivation, as the number of missed Go trials was not different from that of the control subjects and siblings. The individual level of adaptation and errors in the patients correlated with attention tests (TAP incompatibility scores). Several behavioral and physiological studies have shown that selective allocation of attention is impaired in schizophrenia52–55 and that attentional impairments modulate RTs in uncertain temporal context.56 In our task, temporal allocation of attention was predictable because the time of the imperative signal was fixed (at 500 ms after the warning signal). Temporal allocation of attention has been found to be impaired in schizophrenia.57 Additionally, other studies have reported correlations between positive symptoms and distractibility58 and predictive allocation of attention.59 Attention is known to modulate CSE,60 and consequently, the modulation of attention over the block could be reflected in CSE adaptation to probability. Although it remains unclear whether impaired allocation of attention is causally related to deficient statistical adaptation or whether impaired attention and adaptation is the consequence of another deficit in cortical processing.
Conclusions
We showed that modulation of motor cortex excitability in a Go/NoGo RT task was related to the Go probability, ie, to the statistical prediction of the upcoming movement instruction. Probability-driven adaptation of CSE at the time of the maximum expectation (concurrent with the imperative stimulus) strongly correlated with that during the waiting phase, 1500 ms before the Go/NoGo signal. Therefore, these long-lasting modulations of CSE appear to represent implicit and state-dependent anticipatory movement processing, prior to classical motor preparation. This anticipatory state was modulated by probabilistic context in healthy control subjects and siblings, but was markedly altered in schizophrenia. This suggests that impairments of behavioral adaptation to context potentially relate to impairments in the construction of predictive internal models in schizophrenia.
Funding
Fondation pour la Recherche Médicale (DPP20151033970).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kaplan CM, Saha D, Molina JL, et al. Estimating changing contexts in schizophrenia. Brain. 2016;139(7):2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hemsley DR, Garety PA. The formation of maintenance of delusions: a Bayesian analysis. Br J Psychiatry. 1986;149:51–56. [DOI] [PubMed] [Google Scholar]
- 3. Fine C, Gardner M, Craigie J, Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007;12(1):46–77. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. [DOI] [PubMed] [Google Scholar]
- 5. Garety P, Joyce E, Jolley S, et al. Neuropsychological functioning and jumping to conclusions in delusions. Schizophr Res. 2013;150(2-3):570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it’s time for a change: failures to track context in schizophrenia. Int J Psychophysiol. 2010;78(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hemsley DR. The schizophrenic experience: taken out of context?Schizophr Bull. 2005;31(1):43–53. [DOI] [PubMed] [Google Scholar]
- 8. Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J Nerv Ment Dis. 1991;179(4):194–201. [DOI] [PubMed] [Google Scholar]
- 9. Averbeck BB, Evans S, Chouhan V, Bristow E, Shergill SS. Probabilistic learning and inference in schizophrenia. Schizophr Res. 2011;127(1-3):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162(12):2384–2386. [DOI] [PubMed] [Google Scholar]
- 11. Lakatos P, Schroeder CE, Leitman DI, Javitt DC. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci. 2013;33(28):11692–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bitsch F, Berger P, Nagels A, Falkenberg I, Straube B. Impaired right temporoparietal junction–hippocampus connectivity in schizophrenia and its relevance for generating representations of other minds. Schizophr Bull. 2018. doi:10.1093/schbul/sby132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zandbelt BB, van Buuren M, Kahn RS, Vink M. Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biol Psychiatry. 2011;70(12):1151–1158. [DOI] [PubMed] [Google Scholar]
- 14. Dowd EC, Frank MJ, Collins A, Gold JM, Barch DM. Probabilistic reinforcement learning in patients with schizophrenia: relationships to anhedonia and avolition. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fervaha G, Agid O, Foussias G, Remington G. Impairments in both reward and punishment guided reinforcement learning in schizophrenia. Schizophr Res. 2013;150(2):592–593. [DOI] [PubMed] [Google Scholar]
- 16. Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25(1):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirschner M, Hager OM, Bischof M, et al. Deficits in context-dependent adaptive coding of reward in schizophrenia. NPJ Schizophr. 2016;2(1):16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36(3):472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(6):1751–1764. [DOI] [PubMed] [Google Scholar]
- 21. da Silva Alves F, Bakker G, Schmitz N, et al. Dopaminergic modulation of the reward system in schizophrenia: a placebo-controlled dopamine depletion fMRI study. Eur Neuropsychopharmacol. 2013;23(11):1577–1586. [DOI] [PubMed] [Google Scholar]
- 22. Gold JM, Waltz JA, Matveeva TM, et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bansal S, Robinson BM, Geng JJ, et al. The impact of reward on attention in schizophrenia. Schizophr Res Cogn. 2018;12:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darriba Á, Waszak F. Predictions through evidence accumulation over time. Sci Rep. 2018;8(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Körding KP, Wolpert DM. Bayesian integration in sensorimotor learning. Nature. 2004;427(6971):244–247. [DOI] [PubMed] [Google Scholar]
- 26. Zandbelt BB, Vink M. On the Role of the Striatum in Response Inhibition. PLoS One. 2010;5(11):e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19(1):121–131. [DOI] [PubMed] [Google Scholar]
- 28. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 29. Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. J Psychopharmacol. 2008;22(2):203–209. [DOI] [PubMed] [Google Scholar]
- 30. Grégoire J, Wierzbicki C. Comparison of four short forms of the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III). Rev Eur Psychol appliquée. 2009;59:17–24. [Google Scholar]
- 31. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 32. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 33. Lépine JP, Piron JJ, Chapatot E. Factor analysis of the PANSS in schizophrenia patients. In: Stefanis CN, Soltados CR, Rabavilas AD, eds. Psychiatry Today: Accomplishments and Promises. Amsterdam: Excerpta Medica; 1989; p. 828. [Google Scholar]
- 34. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812. [Google Scholar]
- 35. Krebs MO, Gut-Fayand A, Bourdel M, Dischamp J, Olié J. Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res. 2000;45(3):245–260. [DOI] [PubMed] [Google Scholar]
- 36. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 37. Zimmermann P, Fimm B.. Test for Attentional Performance (TAP). (English version 1.02). Herzogenrath: Psytest; 1995. [Google Scholar]
- 38. Stroop RJ. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–662. [Google Scholar]
- 39. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 40. Yamanaka K, Kimura T, Miyazaki M, et al. Human cortical activities during Go/NoGo tasks with opposite motor control paradigms. Exp Brain Res. 2002;142(3):301–307. [DOI] [PubMed] [Google Scholar]
- 41. Lindberg PG, Térémetz M, Charron S, et al. Altered cortical processing of motor inhibition in schizophrenia. Cortex. 2016;85:1–12. [DOI] [PubMed] [Google Scholar]
- 42. Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59(4):347–354. [DOI] [PubMed] [Google Scholar]
- 43. Hannah R, Cavanagh SE, Tremblay S, Simeoni S, Rothwell JC. Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. J Neurosci. 2018;38(5):1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greenhouse I, Sias A, Labruna L, Ivry RB. Nonspecific inhibition of the motor system during response preparation. J Neurosci. 2015;35(30):10675–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bestmann S, Duque J. Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist. 2016;22(4):392–405. [DOI] [PubMed] [Google Scholar]
- 46. Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30(10):3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017;43(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vukadinovic Z, Rosenzweig I. Abnormalities in thalamic neurophysiology in schizophrenia: could psychosis be a result of potassium channel dysfunction?Neurosci Biobehav Rev. 2012;36(2):960–968. [DOI] [PubMed] [Google Scholar]
- 49. Aron AR, Schlaghecken F, Fletcher PC, et al. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington’s disease. Brain. 2003;126(3):713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanders AF. Some effects of instructed muscle tension on choice reaction time and movement time. In: Nickerson RS, ed. Attention and Performance VIII. Hillsdale, NJ: Erlbaum; 1980:59–74. [Google Scholar]
- 51. Spijkers WAC. Programming of direction and velocity of an aiming movement: The effect of probability and response-specificity. Acta Psychol (Amst). 1987;65(3):285–304. [Google Scholar]
- 52. Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19(2):233–259. [DOI] [PubMed] [Google Scholar]
- 53. Mayer AR, Hanlon FM, Teshiba TM, et al. An fMRI study of multimodal selective attention in schizophrenia. Br J Psychiatry. 2015;207(5):420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spring BJ, Zubin J. Attention and information processing as indicators of vulnerability to schizophrenic episodes. J Psychiatr Res. 1978;14(1–4):289–301. [DOI] [PubMed] [Google Scholar]
- 55. Hemsley DR. A two-stage model of attention in schizophrenia research. Br J Soc Clin Psychol. 1975;14(1):81–89. [DOI] [PubMed] [Google Scholar]
- 56. Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977;3(3):373–428. [DOI] [PubMed] [Google Scholar]
- 57. Li CS, Lin WH, Yang YY, Huang CC, Chen TW, Chen YC. Impairment of temporal attention in patients with schizophrenia. Neuroreport. 2002;13(11):1427–1430. [DOI] [PubMed] [Google Scholar]
- 58. Cornblatt BA, Lenzenweger MF, Dworkin RH, Erlenmeyer-Kimling L. Positive and negative schizophrenic symptoms, attention, and information processing. Schizophr Bull. 1985;11(3):397–408. [DOI] [PubMed] [Google Scholar]
- 59. Morris R, Griffiths O, Le Pelley ME, Weickert TW. Attention to irrelevant cues is related to positive symptoms in schizophrenia. Schizophr Bull. 2013;39(3):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Conte A, Gilio F, Iezzi E, Frasca V, Inghilleri M, Berardelli A. Attention influences the excitability of cortical motor areas in healthy humans. Exp Brain Res. 2007;182(1):109–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.