Abstract
The present study was designed to investigate the antagonistic activity of Bacillus licheniformis BL350-2 against mycotoxigenic strains of Aspergillus and Penicillium. In vitro coincubation for 5 days indicated Aspergillus westerdijkiae BA1 as the most sensitive strain, with a growth inhibition of 62%, followed by A. carbonarius MG7 (60%), Penicillium verrucosum MC12 (53%), A. niger MC05 (50%), A. flavus CM5 (49%), A. parasiticus SB01 (47%), and A. ochraceus MD1 (44%). Likewise, the majority of the tested strains on exposure to bacterial volatiles showed complete inhibition of mycotoxin synthesis. In vivo assays on maize ears resulted in 88% reduction in A. flavus CM5 growth and complete inhibition of fungal sporulation and aflatoxin accumulation. The GC–MS-based volatile profile showed 3-methyl-1-butanol as the most abundant compound. The findings of the present study advocate that B. licheniformis BL350-2 is suitable as a biocontrol agent against mycotoxigenic fungi, at least during storage of cereal grains.
Introduction
Contamination of food and feed with mycotoxigenic fungi and their toxic metabolites is a persistent threat to human and animal health. The ever-increasing list of mycotoxins includes more than 400 bioactive compounds. Aflatoxins (AFs) and ochratoxins (OTs) are known as the most toxic fungal metabolites and are produced by several species of Aspergillus and Penicillium. Aspergillus flavus and A. parasiticus are two important Aspergilli, responsible for the accumulation of AFs in cereals and other food commodities.1,2 Aflatoxin B1 (AFB1), among other toxicological properties, presents strong hepatotoxic, immunosuppressive, and carcinogenic activities.3 The International Agency for the Research on Cancer has classified AFB1 among group-1 human carcinogens.4 Ochratoxin A (OTA), after AFB1, is another important mycotoxin, with nephrotoxic and possible human carcinogenic effects.5 The contamination with OTA of food and feed commodities mainly results from the pre- and post-harvest infection by filamentous fungi belonging to the species A. ochraceus, A. westerdijkiae, A. carbonarius, A. niger, and Penicillium verrucosum.6
In most countries, strict regulatory limits for the mycotoxin contamination in food and feed are enforced. The European Union has set a maximum AF limit of 20 μg/kg for complete feedstuff and 4 μg/kg for cereals and processed cereal products used for human consumption. Likewise, for OTA contamination, maximum EU limits for cereals and cereal feed products are 250 μg/kg, while for processed foodstuffs this limit is 3 μg/kg.7−9
A key to prevention and control of mycotoxins is the pre-harvest management of the cereal crops and post-harvest storage of grains, minimizing the favorable conditions (humidity, water activity, temperature) for fungal growth. Field application of synthetic fungicides for the prevention of fungal infections on cereal crops has shown promising outcomes, but their residual transfer to the food chain is being strictly regulated around the globe. Additionally, continuous and improper use of these chemicals might result in unwanted outcomes including the emergence of resistant fungal populations10 and induction of mycotoxin biosynthesis.11 To decontaminate the grains from mycotoxins, methods include physical, chemical, and biological treatments.12 Another approach is the removal of mycotoxins from contaminated food and feed that involves their adsorption on binding substances including biological cell walls or cell-wall-based formulations and activated materials.13 Apart from binding the target mycotoxins, there is always a risk of depriving the animal subjects from dietary important nutrients and antibiotics, which are sequestered in the gastrointestinal tracts by adsorbents.14 Also, the binding efficacy of the adsorbent molecule is a function of several factors, including the nature, polarity, size, and concentration of target mycotoxins and especially the pH of the medium.15 The noncovalent mycotoxin–adsorbent interaction further limits the reliable acceptability of these substances.16
In recent years, to address the problem of mycotoxin contamination in food and feed, rigorous focus is being made on the preventive inhibition of fungal growth. To this end, non-pathogenic microbes (yeast and bacteria) are being explored for their potential to be used against toxigenic fungi and their metabolites.17−19 The commercial efficacies of biocontrol agents against the mycotoxigenic and phytopathogenic fungi are primarily associated with the mode of action of each antagonist.20 The competition for space and nutrients, induced resistance, production of antifungal compounds (antibiosis), production of antagonistic volatiles, and secretion of enzymes inhibiting the key metabolic processes are potential mechanisms by which bacterial antagonists may inhibit the growth of toxigenic fungi. In these regard, some strains of Bacillus licheniformis have also been reported to synthesize the diffusible antimicrobials, which are effective against the important plant pathogenic fungi.21,22 The aim of this study was to evaluate the potential and nature of the volatiles released/produced by a new antagonistic strain of B. licheniformis BL350-2 against the growth and mycotoxin biosynthesis of AF- and OTA-producing Aspergillus and Penicillium spp.
Results and Discussion
B. licheniformis BL350-2 Inhibits the Growth and Sporulation of Toxigenic Aspergillus and Penicillium spp.
The application of biocontrol agents, especially bacteria, against phytopathogenic fungi has been established several decades ago. Initially, the antagonism was believed to be the outcome of direct bacterium–fungus interaction, and their mechanisms of action were based on (i) iron depletion of fungi,28 (ii) degradation of fungal virulence factors,29 (iii) production of a large variety of antifungal compounds,30 and/or (iv) induction of systemic resistance.31 Later, it was realized that some bacteria and yeasts can also exhibit biocontrol activities against fungi from a distance by producing volatiles. For a long time, hydrogen cyanide was the sole known bacterial volatile compound active against pathogenic fungi. Now, several bacterial volatile organic compounds have been explored for their antifungal properties.18 In the present study, attempts were made to demonstrate the antifungal features of volatiles produced by B. licheniformis BL350-2 against aflatoxin- and ochratoxin A (OTA)-producing Aspergillus and Penicillium species.
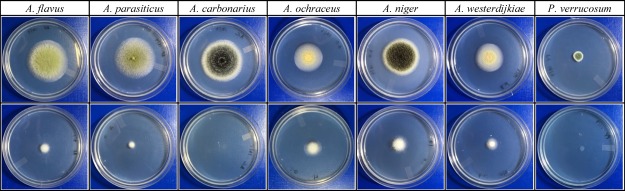
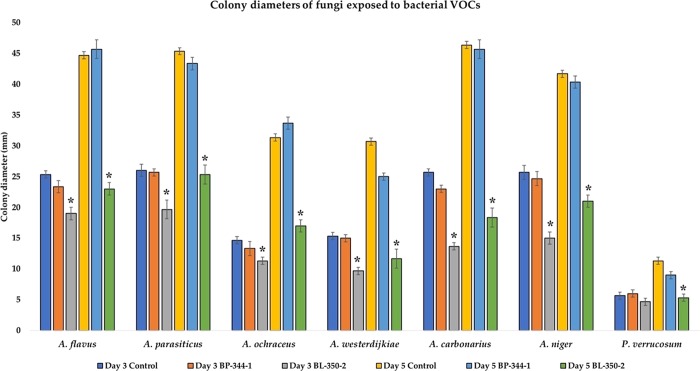
The protein profile matching was expressed on the log scale from 0 to 3 score, as per the manufacturer guidelines. The score 1.70–1.999 is designated as probable genus-level identification, and 2.00–2.299 represents confirmed genus-level identification and probable species-level identification. The score 2.30 to 3.0 is highly probable species-level identification. Our bacterial isolate (coded as BL350-2) showed a matching score of 2.32 and was identified as B. licheniformis. This score (2.32) falls in the highly probable species-level identification. The test was repeated thrice with the same score of 2.32. In vitro coincubation experiments resulted in a significant reduction of the fungal growth and complete inhibition of sporulation (Figure 1). This result was most likely associated with the volatiles produced by antagonistic B. licheniformis BL-350-2 since the experimental setup ensured the lack of physical contact between fungal and bacterial strains. At day 3 of the experiment, except for P. verrucosum MC12, all the volatiles-exposed fungal species showed significantly lower colony sizes, as compared to their respective control (Figure 2). However, at day 7 of post-exposure, the colony diameters of all the test fungi, including P. verrucosum MC12, were significantly smaller than that of unexposed control (Figure 3).
Figure 1.
Antifungal activities of B. licheniformis 350-2 volatiles against AF- and OTA-producing fungi. The fungi in the first row were not exposed to bacterial volatiles, while those in the second row, showing a significant growth inhibition, were exposed to B. licheniformis VOCs. These pictures were taken at day 3 of the experiment (after sealing the plates).
Figure 2.
Effect of bacterial VOCs on the fungal colony size (mm). For each time point, measurements were performed on nine fungal colonies. The asterisks indicate the significant (p < 0.05) difference between the colony diameter of bacterial VOCs exposed vs unexposed (control) fungi.
Figure 3.
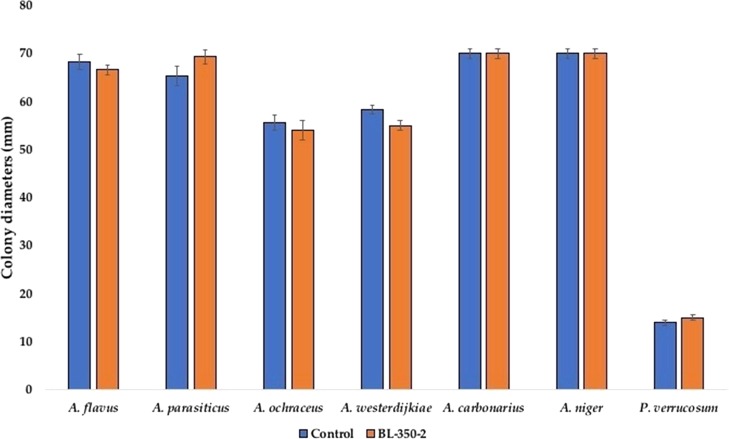
Colony diameters of B. licheniformis (BL350-2) exposed fungi on fresh PDA media vs never exposed fungi (control) fungi. Mycelial plugs were removed from the margins of fungal colonies, transferred to fresh PDA, and incubated for 10 days for the measurement of colony diameters (mm). Bars represent the means of nine observations ± SD.
In general, very low oxygen levels (0.5% or even lower in the environment) are needed to inhibit the fungal growth.32 In order to rule out the possibility that fungal growth inhibition could be due to accumulation of CO2 or deficiency of oxygen as reported earlier,6,33 we coincubated another set of fungi, replacing B. licheniformis 350-2 with B. pumilus 344-3 (known for not producing antifungal volatiles). In the presence of B. pumilus 344-3, there was no effect on fungal growth (Figure 2) and sporulation as compared to control, suggesting that the CO2 generated or oxygen consumed by bacteria has no effect on fungal growth or sporulation, rather these effects are associated with volatiles produced by B. licheniformis 350-2. In the present experiments, either the test fungi were not sensitive to low oxygen tension or the threshold levels were not reached. Additionally, dissolved oxygen concentration in the substrate has a greater impact on fungal growth, rather than atmospheric oxygen.34
In line with the present study, a significant reduction in growth, sporulation, and conidial germination of A. flavus exposed to volatiles produced by B. megaterium KU143, Microbacterium testaceum KU313, and Pseudomonas protegens AS15 has been reported.35 In a more recent investigation, the same group of researchers36 reported that VOCs produced by B. megaterium KU143 and P. protegens AS15 also exhibit antifungal activities against several other fungi belonging to the genera Aspergillus and Penicillium. GC–MS-based analysis confirmed that the biocontrol activities were associated with the production of 5-methyl-2-phenyl-1H-indole by B. megaterium and 2-butyl-1-octanal, dimethyl disulfide, 2-isopropyl-5-methyl-1-heptanol, and 4-trifluoroacetoxyhexadecane by P. protegens.36 Similarly, VOCs produced by B. subtilis, B. amyloliquefaciens, and B. cereus have been reported for their antifungal activities against Aspergillus, Penicillium, and Fusarium spp.37
B. licheniformis 350-3 Produces Reversible Effects on Fungal Morphology
The volatiles released by the B. licheniformis 350-2 resulted in a significant reduction in growth and complete inhibition of sporulation in all seven tested fungi. However, upon transfer to the fresh PDA media, in the absence of bacterial VOCs, all the fungal isolates displayed normal growth and sporulation, comparable with untreated fungi (results not shown). In line with our findings, the effects of antagonistic yeast Candida friedrichii 778 on toxigenic A. carbonarius MPVA566 and A. carbonarius AN6 were also observed as reversible.38 The reversibility in the morphological alterations highlights the need for a continuous availability of bacterial VOCs to allow effective biocontrol.
Mycotoxin Biosynthesis Is Inhibited by Bacterial Volatiles
The effects of bacterial VOCs on mycotoxin biosynthesis by toxigenic fungi were quantified at day 10 of the coincubation experiment. The UPLC-based analysis confirmed that exposure to B. licheniformis 350-2 resulted in a complete inhibition of OTA synthesis by highly toxigenic A. westerdijkiae BA1, A. carbonarius MG7, and P. verrucosum MC12. Nevertheless, in the presence of bacterial volatiles, toxigenic A. ochraceus MD1 (21.84 μg/kg) and A. niger MC5 (29.32 μg/kg) were still able to synthesize OTA, albeit the levels were significantly lower than that of unexposed control (Table 1).
Table 1. Inhibitory Effect of Bacterial VOCs on the Mycotoxin Synthesis (Mean ± SD) by Toxigenic Fungia.
| mycotoxin
production (μg/kg) |
|||
|---|---|---|---|
| fungi | produced mycotoxin | controlb | VOCs exposedc |
| A. ochraceus MD1 | OTA | 87.21 ± 6.32d | 21.84 ± 3.33e |
| A. westerdijkiae BA1 | OTA | 5141.96 ± 21.41 | nd |
| A. niger MC5 | OTA | 75.44 ± 7.90d | 29.32 ± 5.03e |
| A. carbonarius MG7 | OTA | 941.35 ± 12.54d | nd |
| P. verrucosum MC12 | OTA | 27.32 ± 2.41d | nd |
| A. flavus CM5 | AFs | 392.22 ± 15.85d | nd |
| A. parasiticus SB01 | AFs | 265.34 ± 17.22d | nd |
Effect of bacterial volatiles on mycotoxin synthesis potential of the toxigenic fungi. Each value represents the mean and SD calculated from the three replicates. The values in each row followed by different superscript letters are significantly different from each other at p ≤ 0.05. nd – not detected.
Mycotoxin contents in the media inoculated with toxigenic fungi.
Mycotoxin levels in the media inoculated with fungi as well as exposed to bacterial VOCs.
Similarly, under the influence of bacterial VOCs, A. flavus CM5 and A. parasiticus SB01 exhibited complete inhibition of AF synthesis, while high levels of toxins were demonstrated by the control cultures. In line with these findings, a significant reduction in AF synthesis by A. flavus exposed to VOCs produced by B. megaterium KU143, M. testaceum KU313, and P. protegens AS15 was recorded.35 The inhibition of mycotoxin production by the toxigenic fungi may be due to bacterial volatiles-associated downregulation in the key genes involved in the mycotoxin biosynthetic pathways in A. carbonarius and A. ochraceus exposed to yeast VOCs.23 At low levels of 2-phenylethanol (2PE), a common VOC produced by antagonistic yeast, the AF synthesis inhibition is associated with the stimulation of A. flavus growth and decreased breakdown of branched-chain amino acid, which serves as a precursor for the synthesis of AFs.39
Bacterial Volatiles Inhibit in Vivo Fungal Infection on Maize Ears
In vivo assays using maize ears were performed in order to investigate the effects of bacterial volatiles on fungal growth and sporulation. Maize ears in the control group (not inoculated with fungal spores) showed no fungal infection. Bacterial volatiles caused a significant (p ≤ 0.05) reduction in the presence of kernels infected with fungi [7.14 ± 1.97 (mean ± SD)], as compared to high infection percentage (38.77 ± 6.22) in the absence of bacterial VOCs (Figure 4). The presence of TSA media alone had no inhibitory effect on the fungal infection of kernels.
Figure 4.
In vivo fungal growth inhibition by bacterial volatiles on maize ears. Control ear-cuts (without fungal spore inoculation) showed no infection (A); meanwhile, A. flavus growth was significantly lower on the ear-cuts exposed to bacterial VOCs (C), as compared to fungal infection in the absence of bacteria (D) or in the presence of TSA media (without inoculated bacteria) alone (B).
In vivo fungal growth inhibition by B. licheniformis BL350-2 is in agreement with what was noted in the in vitro experiments. Our findings are in line with earlier reports of antifungal activities of VOCs produced by B. amyloliquefaciens SQR-9 against Ralstonia solanacearum infection on tomato roots.40 Likewise, the volatiles emitted by B. megaterium KU143, M. testaceum KU313, and P. protegens AS15 showed similar activity against A. flavus, A. candidus, A. fumigatus, P. fellutanum, and P. islandicum infections on stored rice.35,36
The AF content in maize kernels was analyzed by UPLC and showed no levels of AF in the untreated kernels and those infected with the A. flavus CM5 in the presence of bacterial VOCs. On the other hand, in the absence of bacterial VOCs, A. flavus CM5 resulted in the accumulation of high levels of AFs at 173.83 ± 13 μg/kg (mean ± SD) in the infected kernels. The presence of TSA media showed a nonsignificant difference on the AF production potential [181.22 ± 17 (mean ± SD)] of fungi.
3-Methy-1-butanol Is the Main Component of BL350-2 Volatiles
Head space (HS) bacterial volatiles analyzed through GC–MS allowed identification of 32 compounds belonging to different chemical classes, including aldehyde, hydrocarbons, alcohols, and terpenes. In total, 28 compounds detected in the BL350-2 volatiles were also recovered from the TSB media flasks (having no added bacterial) and those inoculated with B. pumilus 344-1. The three compounds (benzeneacetaldehyde, 1,5-dimethyl-2-piperidone, and dimethyl disulfide) were produced by the tested bacterial strains, B. pumilus 344-1 and BL350-2 (Table 2). The only difference between the volatile profile of B. pumilus 344-1 and BL350-1 was the production of 3-methyl-1-butanol by BL350-2, suggesting the responsible compound for its antifungal activity. Recently, the synthesis of 3-methyl-1-butanol by P. chlororaphis subsp. aureofaciens SPS-41 and its antifungal activity against Ceratocystis fimbriata infection in sweet potatoes has been published.41 Also, some Bacillus spp. including B. subtilis, B. amyloliquefaciens, and B. cereus have been reported for the production of 3-methyl-1-butanol, acting as a strong antifungal compound.37
Table 2. GC–MS Analysis of Head Space Volatiles of B. licheniformis BL350-2 and B. pumilus 344-1.
| S. No. | namea | retention time (min) | BL350-2b (area %) | BP344-1 (area %) |
|---|---|---|---|---|
| 1 | benzeneacetaldehyde | 3.327 | 2.87 | 3.02 |
| 2 | 1,5-dimethyl-2-piperidone | 4.677 | 5.45 | 3.22 |
| 3 | 3-methyl-1-butanol | 12.422 | 26.33 | 1.05 |
| 4 | dimethyl disulfide | 14.102 | 3.21 | 2.79 |
VOCs with a peak area of >1.5% and those not detected in the control (flasks having TSB without any bacterial contamination) are listed in the table.
Area (%) of a peak was calculated on the basis of total area of all the detected peaks.
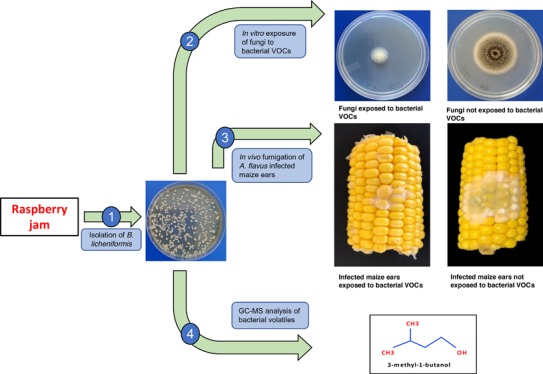
In conclusion, a new strain of B. licheniformis isolated from raspberry jam and provisionally coded as BL350-2 was tested for its antagonistic activity against representative toxigenic strains of A. flavus CM5, A. parasiticus SB01, A. carbonarius MG7, A. ochraceus MD1, A. westerdijkiae BA1, A. niger MC5, and P. verrucosum MC12. In vitro experiments on PDA media showed a significant decrease in fungal growth, sporulation, and mycotoxin biosynthesis of the fungi exposed to bacterial volatiles. Likewise, in in vivo experiments carried out on maize ears, bacterial volatiles resulted in a significant reduction of A. flavus CM5 growth and complete inhibition of sporulation and AF biosynthesis. Based on these findings, it can be concluded that the 3-methyl-1-butanol produced by B. licheniformis 350-2 confers a significant protection from fungal growth and mycotoxin accumulation, both in vitro and in vivo.
Materials and Methods
Strains and Media
All the fungal isolates used in this study were obtained from feed samples, marketed in Qatar. Morphological identification of these isolates was followed by PCR-based identification using species-specific primers. Mycotoxin production potential was tested on yeast extract sucrose (YES) agar, which contained yeast extract (20 g), sucrose (150 g), agar (20 g), and magnesium sulfate (0.5 g) in 1 L of distilled water, and by amplification of genes involved in mycotoxin biosynthesis pathways.1
The B. licheniformis BL350-2 and B. pumilus BP344-1 strains used in this study were isolated from raspberry and strawberry jams, marketed in Qatar (imported from Turkey), respectively. Pure culture was obtained by streaking isolated colonies on Luria–Bertani agar, prepared by adding tryptone (10 g), yeast extract (5 g), NaCl (10 g), and agar (15 g) in 1 L of distilled water.
Identification of Bacterial Strain
Identification of the bacterial strains was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The ethanol/formic acid procedure was adopted for protein extraction. Briefly, a loopful of bacterial cells from colonies grown overnight was suspended in 300 μL of sterile distilled water. After thorough mixing, 900 μL of absolute ethanol was added, mixed, and centrifuged. The supernatant was decanted, and to the pellets, 70 μL of formic acid was added. After thorough mixing by pipetting, 70 μL of acetonitrile was added and centrifuged. A total volume of 1 μL of supernatants was transferred to a MALDI Biotarget sample plate. The spots after drying were overlaid with 1 μL of α-cyanohydroxycinnamic acid (HCCA) matrix solution. The mass-to-charge (m/z) spectrum was obtained by analyzing the samples on a Bruker Biotype (Bruker Daltonics, Bremen, Germany), and identification was made by comparing the spectrum with those available in the database. The protein profile matching was expressed on the log scale from 0 to 3 score, as per the manufacturer guidelines. Before running the test samples, the instrument was calibrated with bacterial test standards provided by the manufacturer (Bruker Daltonics, Bremen, Germany).
Tests for in Vitro Antifungal Activity of B. licheniformis BL350-2
The antifungal activity of B. licheniformis volatiles was tested by coincubation assays23 against aflatoxigenic (A. flavus CM5 and A. parasiticus SB01) and ochratoxigenic (A. ochraceus MD1, A. westerdijkiae BA1, A. carbonarius MG7, A. niger MC5, and P. verrucosum MC12) fungi. Briefly, from an overnight pure bacterial culture on LB agar, a loopful of cells was suspended in Ringer’s solution (0.9% NaCl). To find the best activity of absolute bacterial CFU on the growth and mycotoxin synthesis potential of fungi, serial dilutions of the bacterial cell suspension were made, and from each dilution, 100 μL was evenly spread on tryptic soy agar (TSA) plates, prepared by adding pancreatic digest of casein (15 g), peptic digest of soybean meal (5 g), NaCl (5 g), agar (15 g), K2HPO4 (2.5 g), and dextrose (2.5 g) in 1 L of distilled water. This protocol resulted in 12 to more than 500 bacterial CFU/plate of TSA media. These plates were incubated without sealing for 24 h at 30 °C. In the coincubation experiment, the cover of the bacterial plate was replaced by a base PDA plate having inoculated fungal spores, sealed with parafilm, and inoculated. After 3 days of coincubation, we realized that 280–300 bacterial CFU produce enough volatile for the optimal antifungal activity. After optimization, in further experiments, we used only those plates containing 280–300 bacterial CFU each. The lid of bacterial plates was replaced by another base plate, containing a PDA medium point inoculated with 4 μL of fungal spore suspension (106 spores/mL). The two base plates were sealed together with four layers of parafilm and one additional layer of sealing tape to prevent VOC escape. B. pumilus BP344-1, tested for not producing antifungal volatiles, was used as a negative control. Plates were incubated at 26 °C for 10 days. The effects of bacterial volatiles on fungal growth and sporulation were measured by recording colony diameters and morphology at days 3 and 7 of post-sealing. The control treatment was represented by sealing fungus-inoculated plates with a sterile TSA plate. This experiment was repeated thrice, with six replicates of each test fungi. Each time, three plates of each exposed fungi were opened at day 3 of the experiment (for taking pictures and measuring colony diameters), and the rest were incubated until day 5 of the experiment.
To evaluate the reversibility of the effects on fungal growth and sporulation, at day 5, from the margin of each fungal colony, one plug (∼1 cm2) from each three replicates was removed with a sterile scalpel blade and transferred to fresh PDA plates. These plates were incubated at 26 °C for 7 days. The fungal growth and sporulation were monitored on a daily basis and compared with the control group (never exposed to bacterial volatiles).
In Vivo Antagonistic Activity of B. licheniformis 350-2 Volatiles
To test the in vivo antifungal activity of bacterial volatiles, full-grown sweet corn (Green Giant, Valencia, Spain) ears were purchased from a local market. Each maize ear was divided in two halves by making a longitudinal cut using a sterile knife. Each half was further divided into three pieces, sterilized with NaOCl (1%) for 2 min, washed twice with sterile distilled water, and dried with absorbent paper.24 One day before starting the experiment, B. licheniformis BL350-2 was streak-inoculated on 90 mm tryptic soy agar (TSA) plates and incubated at 30 °C. Each disinfected maize ear cut was placed on a sterile platform in a glass box (12.5 × 12.5 × 5.5 cm), below which an opened-lid bacteria-inoculated plate was placed to allow the dispersal of volatiles. On the surface of maize ears, 8 μL of the spore suspension of A. flavus CM5 (105 spores/mL) was placed as a point inoculum. These experiments were repeated thrice with three replicates each time. Appropriate controls were maintained by incubating maize ears, inoculated/uninoculated with fungal spores, in the absence of bacterial VOCs. The containers, upon tight sealing, were placed in an incubator at 26 °C. At day 21 of post-incubation, containers were opened and the percentage of infection was calculated as:
| 1 |
To evaluate the effect of bacterial volatiles on mycotoxin synthesis, 5 g of infected kernels was removed from each replicate and pooled to gather for the analysis of AFs as described below. The mean values of the three experiments were calculated and compared with untreated control.
Mycotoxin Extraction and Analysis
To explore the inhibitory effects of B. licheniformis BL350-2 on mycotoxin biosynthesis, another set of experiments having nine fungal plates from each test fungi were exposed to bacterial volatile by the method described above. Control fungi plates were sealed against TSA plates not inoculated with any bacteria. At day 10 of coincubation, colonized media plugs (n = 3/plate) of 7 mm diameter were removed with a sterile cork borer, weighed, and shifted to a 1.5 mL Eppendorf tube.1 In the case of smaller colony size, plugs from more than one plate of the same fungi were pooled together. Three samples from each fungus were analyzed for either ochratoxin A or aflatoxins (AFs).
OTA was extracted from A. westerdijkiae BA1, A. carbonarius MG7, P. verrucosum MC12, A. niger MC05, and A. ochraceus MD1 infected media plugs, by adding 1 mL of HPLC-grade methanol (Sigma, St. Louis, MO, USA). The sample was sonicated (Bandelin, Berlin-W. Germany) for 1 h, filtered using 0.22 μm syringe filters (Pall Corporation, MI, USA), and stored at 4 °C until analysis.25 Before analysis, samples were allowed to evaporate in a SpeedVac and resuspended in the mobile phase. OTA content was analyzed by using an ultra performance liquid chromatography (UPLC) system (Waters, MA, USA) equipped with a Nova-Pak C18 column (4 μm × 3.9 mm × 150 mm) for separation. The isocratic mobile phase was a mix of acetonitrile, water, and acetic acid (45:54:1 v/v/v, respectively). A constant flow rate of 1 mL/min was maintained. A fluorescence detector (FLD) was set at 333 and 460 nm excitation and emission wavelengths, respectively. OTA was identified by comparing the retention time (3.563 min) of the pure OTA reference material (Trilogy Analytical Laboratory Inc., Washington, MO, USA) with a target peak in samples. External calibration using five dilutions of pure OTA standards with a mean correlation coefficient of 0.999626 was used for quantification purposes.
For the quantification of AFs synthesized by A. flavus CM5 and A. parasiticus SB01, colonized media plugs were dissolved in 1 mL of solvent mixture of methanol, dichloromethane, ethyl acetate (1:2:3 v/v/v, respectively) containing 1% formic acid.26 After extraction for 60 min, a total of 0.5 mL of sample was transferred to another tube and was dried using a SpeedVac (Thermo Fisher, USA). Before analysis, samples were resuspended in the UPLC mobile phase, which was a mixture of water/methanol/acetonitrile (250/55/55, respectively), and were passed through the syringe filter (0.22 μm). The fluorescence detector was set at 440 and 360 nm emission and excitation wavelengths, respectively. A constant flow rate of 1 mL/min was maintained. Five dilutions of aflatoxin mix standard solution (AFB1, B2, G1, and G2) obtained from Trilogy Analytical Laboratory Inc. (Washington, MO, USA) were used to obtain a calibration curve.
Collection and Analysis of Bacterial Volatiles
The head space VOCs produced by B. licheniformis BL350-2 were collected on activated charcoal.27 For this purpose, BL350-2 was shake-incubated in 200 mL of TSB in 500 mL conical flasks, fitted with two-way rubber corks, with two glass tubes passing through it. To the end of one glass tube, a nitrogen supply system was attached, while its other end was placed just 1 cm above the bacterial culture to remove the head space volatiles. One end of the second tube was placed near the neck of flask, while its other end was attached to a VOC trap. The trap was made of 6 cm long glass tube of 5 mm diameter and filled with 400 mg of activated charcoal (Sigma-Aldrich, Missouri, USA). Before use, the charcoal was wrapped in aluminum paper and placed in an oven at 350 °C for 48 h for sterilization. All the junctions including the neck of the flasks were tightly sealed to avoid the leakage of volatiles. The flasks were placed in a shaking water bath at 30 °C for 48 h. Control flasks contained TSB (alone), and the others were inoculated with B. pumilus BP344-1 (not showing antifungal activity). For each treatment, three flasks were maintained for the analysis of VOCs. The nitrogen supply (200 mL/min) was introduced 10 h after inoculation of bacteria. The VOCs on the activated charcoal were eluted with 1 mL of dichloromethane (Sigma-Aldrich, MO, USA) into new glass vials.
Analysis of the volatiles was performed using gas chromatography (GC) with an MSD detector (Agilent, CA, USA). The samples were separated on fused silica column (25 m × 0.2 mm i.d., 0.11 μm). Helium was used as the carrier gas, and the flow rate was maintained at 1 mL/min. Column temperatures were programmed at 30 °C for 3 min and then increased at a rate of 4 °C/min to 210 °C. The mass spectra of the unknown compounds were compared with those of the NIST/EPA/NIH mass spectral libraries.
Statistical Analysis
Data obtained from the in vitro experiments regarding the effects of bacterial VOCs on fungal growth and mycotoxin synthesis potential was analyzed by the analysis of variance test (ANOVA). In the case of in vivo experiments data, if ANOVA showed a significant difference among the treatment groups, post hoc analysis was performed using Fisher’s Least Significant Difference test. Means were considered significant at p ≤ 0.05. SPSS statistical software (Version 23, IBM, NY, USA, 2017) was used for this purpose.
This publication was made possible by NPRP grant # NPRP8-392-4-003 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors.
The authors declare no competing financial interest.
References
- Hassan Z. U.; al-Thani R. F.; Migheli Q.; Jaoua S. Detection of toxigenic mycobiota and mycotoxins in cereal feed market. Food Control 2018, 84, 389–394. 10.1016/j.foodcont.2017.08.032. [DOI] [Google Scholar]
- el Khoury A.; Rizk T.; Lteif R.; Azouri H.; Delia M.-L.; Lebrihi A. Occurrence of ochratoxin A- and aflatoxin B1-producing fungi in Lebanese grapes and ochratoxin A content in musts and finished wines during 2004. J. Agric. Food Chem. 2006, 54, 8977–8982. 10.1021/jf062085e. [DOI] [PubMed] [Google Scholar]
- Ul-Hassan Z.; Khan M. Z.; Khan A.; Javed I. Immunological status of the progeny of breeder hens kept on ochratoxin A (OTA)- and aflatoxin B1(AFB1)-contaminated feeds. J. Immunotoxicol. 2012, 9, 381–391. 10.3109/1547691X.2012.675365. [DOI] [PubMed] [Google Scholar]
- IARC Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Vol. 56IARC: Lyon, 1993, p.245–395.8411624 [Google Scholar]
- Heussner A. H.; Bingle L. E. H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. 10.3390/toxins7104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. I.; Hocking A. D.. Fungi and food spoilage; 3rd Ed.; Springer: New York, 2009. [Google Scholar]
- European Commission . (2002). Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 (consolidated version 2013-12e27) on undesirable substances in animal feed. http://data.europa.eu/eli/dir/2002/32/2013-12-27.
- European Commission Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding; 2006, http://eur-lex.europa.eu/ legal content/EN/TXT/PDF/?uri.CELEX:32006H0576&from.EN.
- Commission regulation (EC) no. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 364 2006, 365–324. [Google Scholar]
- Zhang H.; Apaliya M. T.; Mahunu G. K.; Chen L.; Li W. Control of ochratoxin A-producing fungi in grape berry by microbial antagonists: A review. Trends Food Sci. Technol. 2016, 51, 88–97. 10.1016/j.tifs.2016.03.012. [DOI] [Google Scholar]
- Schmidt-Heydt M.; Stoll D.; Geisen R. Fungicides effectively used for growth inhibition of several fungi could induce mycotoxin biosynthesis in toxigenic species. Int. J. Food Microbiol. 2013, 166, 407–412. 10.1016/j.ijfoodmicro.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Jard G.; Liboz T.; Mathieu F.; Guyonvarc’h A.; Lebrihi A. Review of mycotoxin reduction in food and feed: from prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam., Part A 2011, 28, 1590–1609. 10.1080/19440049.2011.595377. [DOI] [PubMed] [Google Scholar]
- De Mil T.; Devreese M.; De Baere S.; Van Ranst E.; Eeckhout M.; De Backer P.; Croubels S. Characterization of 27 mycotoxin binders and the relation with in vitro Zearalenone adsorption at a single concentration. Toxins 2015, 7, 21–33. 10.3390/toxins7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mil T.; Devreese M.; De Saeger S.; Eeckhout M.; De Backer P.; Croubels S. Influence of mycotoxin binders on the oral bioavailability of doxycycline in pigs. J. Agric. Food Chem. 2016, 64, 2120–2126. 10.1021/acs.jafc.5b06084. [DOI] [PubMed] [Google Scholar]
- Faucet-Marquis V.; Joannis-Cassan C.; Hadjeba-Medjdoub K.; Ballet N.; Pfohl-Leszkowicz A. Development of an in vitro method for the prediction of mycotoxin binding on yeast-based products: case of aflatoxin B1, zearalenone and ochratoxin A. Appl. Microbiol. Biotechnol. 2014, 98, 7583–7596. 10.1007/s00253-014-5917-y. [DOI] [PubMed] [Google Scholar]
- Jouany J. P.; Yiannikouris A.; Bertin G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Arch. Zootech. 2005, 8, 26–50. [Google Scholar]
- Weisskopf L.The potential of bacterial volatiles for crop protection against phytophathogenic fungi. In Microbial pathogens and strategies for combating them: science, technology and education; Méndez-Vilas A., Ed.; Formatex: Badajoz, Spain, 2013, 1352–1363. [Google Scholar]
- Effmert U.; Kalderás J.; Warnke R.; Piechulla B. Volatile mediated interactions between bacteria and fungi in the Soil. J. Chem. Ecol. 2012, 38, 665–703. 10.1007/s10886-012-0135-5. [DOI] [PubMed] [Google Scholar]
- Pfliegler W. P.; Pusztahelyi T.; Pócsi I. Mycotoxins - prevention and decontamination by yeasts. J. Basic Microbiol. 2015, 55, 805–818. 10.1002/jobm.201400833. [DOI] [PubMed] [Google Scholar]
- Haidar R.; Fermaud M.; Calvo-Garrido C.; Roudet J.; Deschamps A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol. Mediterr. 2016, 55, 301–322. 10.14601/Phytopathol_Mediterr-18079. [DOI] [Google Scholar]
- Nigris S.; Baldan E.; Tondello A.; Zanella F.; Vitulo N.; Favaro G.; Guidolin V.; Bordin N.; Telatin A.; Barizza E.; Marcato S.; Zottini M.; Squartini A.; Valle G.; Baldan B. Biocontrol traits of Bacillus licheniformis GL174, a culturable endophyte of Vitis vinifera cv. Glera. BMC Microbiology. 2018, 18, 133. 10.1186/s12866-018-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. P.; Lee S.-W.; Kim C. S.; Son J. H.; Song J. H.; Lee K. Y.; Kim H. J.; Jung S. J.; Moon B. J. Evaluation of formulations of Bacillus licheniformis for the biological control of tomato gray mold caused by Botrytis cinerea. Biol. Control 2006, 37, 329–337. 10.1016/j.biocontrol.2006.01.001. [DOI] [Google Scholar]
- Farbo M. G.; Urgeghe P. P.; Fiori S.; Marcello A.; Oggiano S.; Balmas V.; Hassan Z. U.; Jaoua S.; Migheli Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. 10.1016/j.ijfoodmicro.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Joo H. J.; Kim H.-Y.; Kim L.-H.; Lee S.; Ryu J. G.; Lee T. A Brevibacillus sp. antagonistic to mycotoxigenic Fusarium spp. Biol. Control 2015, 87, 64–70. 10.1016/j.biocontrol.2015.04.010. [DOI] [Google Scholar]
- Bragulat M. R.; Abarca M. L.; Cabañes F. J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. 10.1016/S0168-1605(01)00581-5. [DOI] [PubMed] [Google Scholar]
- Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 1997, 760, 264–270. 10.1016/S0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- DeMilo A. B.; Lee C.-J.; Moreno D. S.; Martinez A. J Identification of volatiles derived from Citrobacter freundii fermentation of a trypticase soy broth. J. Agric. Food Chem. 1996, 44, 607–612. 10.1021/jf950525o. [DOI] [Google Scholar]
- Kloepper J. W.; Leong J.; Teintze M.; Schroth M. N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. 10.1038/286885a0. [DOI] [Google Scholar]
- Schoonbeek H.-j.; Jacquat-Bovet A.-C.; Mascher F.; Métraux J.-P. Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea. Mol. Plant-Microbe Interact. 2007, 20, 1535–1544. 10.1094/MPMI-20-12-1535. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B.; Kamilova F. Plant-growth-promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- van Loon L. C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- Nielsen P. V.; Haasum I.. Inhibition of fungal growth with extreme low oxygen levels. In Book of abstract, IFT annual meeting ;IFT, Chicago, 1998. [Google Scholar]
- Schalchli H.; Hormazabal E.; Becerra J.; Birkett M.; Alvear M.; Vidal J.; Quiroz A. Antifungal activity of volatile metabolites emitted by mycelial cultures of saprophytic fungi. Chem. Ecol. 2011, 27, 503–513. 10.1080/02757540.2011.596832. [DOI] [Google Scholar]
- Hocking A. D.Responses of fungi to modified atmospheres. In Fumigation and controlled atmosphere storage of grain; Champ B. R.; Highley E.; Banks H. J. Eds.; ACIAR; Proceedings No. 25. Canberra, Australia, 1990, pp 70–82. [Google Scholar]
- Mannaa M.; Oh J. Y.; Kim K. D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology 2017, 45, 213–219. 10.5941/MYCO.2017.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaa M.; Kim K. D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. Predominant in stored rice grains: Study II. Mycobiology 2018, 46, 52–63. 10.1080/12298093.2018.1454015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves-López C.; Serio A.; Gianotti A.; Sacchetti G.; Ndagijimana M.; Ciccarone C.; Stellarini A.; Corsetti A.; Paparella A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. 10.1111/jam.12847. [DOI] [PubMed] [Google Scholar]
- Fiori S.; Urgeghe P. P.; Hammami W.; Razzu S.; Jaoua S.; Migheli Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. 10.1016/j.ijfoodmicro.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Chang P.-K.; Hua S. S. T.; Sarreal S. B. L.; Li R. W. Suppression of aflatoxin biosynthesis in Aspergillus flavus by 2-phenylethanol is associated with stimulated growth and decreased degradation of branched-chain amino acids. Toxins 2015, 7, 3887–3902. 10.3390/toxins7103887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza W.; Ling N.; Yang L.; Huang Q.; Shen Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. 10.1038/srep24856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Tengjie L.; Yuanfang L.; Xiaoyan L.; Chunmei Z.; Zhaozhong F.; Xue P.; Zongyun L.; Sheng Q.; Ke X. Volatile organic compounds produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 as biological fumigants to control Ceratocystis fimbriata in postharvest sweet potatoes. J. Agric. Food Chem. 2019, 67, 3702–3710. [DOI] [PubMed] [Google Scholar]