Abstract
A new method of data interpretation based on classical nucleation theory is proposed in this work to elucidate the influence of solvents on the pre-exponential nucleation factor and interfacial energy using the induction time data for three crystallization systems, including isonicotinamide, lovastatin, and phenacetin. In this method, the pre-exponential nucleation factor is replaced by the intrinsic nucleation factor multiplied by temperature and divided by solution viscosity. The proposed method is applied to study the nucleation kinetics of isonicotinamide, lovastatin, and phenacetin among various solvents using the induction time data measured in this work. The results indicate that the intrinsic nucleation factor increases linearly with increasing square root of interfacial energy in various solvents for each system.
Introduction
Nucleation is the initial process for the formation of crystals in solutions. In classical nucleation theory (CNT),1−3 the nucleation rate is expressed in the thermally activated Arrhenius form governed by the pre-exponential nucleation factor and interfacial energy. The interfacial energy is the energy required to create a new solid liquid interface for the formation of crystals in solutions. Traditionally, the interfacial energy is determined from the induction time measurements by assuming J ∝ ti–1.1,4−7 Generally, the higher the value of interfacial energy, the more difficult it is for the solute to crystallize.
As the nucleation behavior of the same solute is greatly influenced by the choice of solvent, the study of nucleation in various solvents has long been an important research subject.8−14 Recent studies have indicated an increasing trend of the interfacial energy with the increasing corresponding solute–solvent interaction for the same solute in various solvents.15−18 Apart from the interfacial energy, nucleation should also be influenced by the pre-exponential factor based on CNT. However, few studies have been published regarding to the influence of the solvent type on the pre-exponential factor for nucleation.
Although the pre-exponential factor is related to the solute mobility in solutions, it is also implicitly dependent on the interfacial energy of a crystalline solid according to the derivation of CNT,2,3,19 which nevertheless has not been experimentally validated in the literature. Nucleation in various solvents for a system can provide important information for nucleation rate parameters. In this work, the influence of the solvent type on nucleation will be investigated based on CNT to examine the implicit relationship between the pre-exponential factor and interfacial energy in various solvents using the induction time data for three common model compounds widely studied in crystal engineering, including isonicotinamide, lovastatin, and phenacetin. The chemical structures of these compounds are given in Figure 1. Various common crystal structures of these compounds have been reported in the literature.20−23
Figure 1.
Chemical structures of (a) isonicotinamide, (b) lovastatin, and (c) phenacetin.
Theory
The nucleation rate based on CNT is expressed as1−3
| 1 |
where AJ is the nucleation pre-exponential
factor, γ is the interfacial energy, kB is the Boltzmann constant, 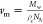 is the molecular volume,
and S = C0/Ceq is the supersaturation ratio. As the solute attachment
for small critical nucleus in a stirred solution should be interface-transfer
control, it yields based on CNT2,3,19
is the molecular volume,
and S = C0/Ceq is the supersaturation ratio. As the solute attachment
for small critical nucleus in a stirred solution should be interface-transfer
control, it yields based on CNT2,3,19
| 2 |
where DAB is the solute diffusivity in the solution.
For simplicity, the solute diffusivity is usually estimated based on the Stokes–Einstein equation as1
| 3 |
where r0 is the molecular radius of solute and η is the solution viscosity. As DAB is generally assumed to be proportional to T/η(T,S) for the same solute among various solvents,10,13,19eq 2 becomes
| 4 |
To differentiate between the effects of γ1/2 and T/η(T,S) on AJ, the intrinsic nucleation factor A0 is introduced in this work as24
| 5 |
Substituting eq 5 into eq 4 yields
| 6 |
Consequently, although AJ in eq 2 is dependent on DAB among various solvents, A0 is not related to the dependence of DAB on T/η(T,S) among various solvents. Substituting eq 5 into eq 1 yields
| 7 |
Thus, J is expressed in terms of A0 and γ, as opposed to J commonly adopted in terms of AJ and γ in eq 1.
In the induction time study, the nucleation event is usually assumed to correspond to a point at which the total number density of accumulated crystals in a vessel has reached a fixed (but unknown) value, fN.25−28 Thus, one obtains at the nucleation time ti
| 8 |
where fN depends on the measurement device and on the substance. Note that eq 8 is consistent with J ∝ ti–1 reported in the literature.1 Based on the study of 28 systems, Mersmann and Bartosch29 estimated fV = 10–4 to 10–3 with a detectable size of 10 μm. If the intermediate value, fV = 4 × 10–4, for spherical nuclei with kV = π/6 is assumed, it leads to fN = 7.64 × 1011 m–3 proposed by Shiau.24
Substituting eq 1 into eq 8 yields
| 9 |
Experimental induction time data can be evaluated by plotting ln(1/ti) versus 1/T3 ln2S for determination of γ from the slope and AJ from the intercept, respectively.
Substituting eq 7 into eq 8 yields
| 10 |
Experimental induction time data can be evaluated by plotting ln[η(T,S)/tiT] versus 1/T3 ln2S for determination of γ from the slope and A0 from the intercept, respectively.
Results and Discussion
Tables 1–3 list the experimental average induction time data of each solute in various solvents measured for various S at the specified temperature for three crystallization systems, including isonicotinamide, lovastatin, and phenacetin. The induction time measurements under each condition are repeated three times, and the deviation of the induction time is generally less than 15%. In the following, eqs 9 and 10 are applied to determine the nucleation kinetics in various solvents using the induction time data for each system.
Table 1. Experimental Induction Time Data of Isonicotinamide in Each Solvent for Various S at 303 K.
| solute | solvent | S (-) | ti (s) |
|---|---|---|---|
| isonicotinamide | methanol | 1.43 | 664 |
| 1.45 | 564 | ||
| 1.50 | 400 | ||
| 1.55 | 370 | ||
| acetone | 1.20 | 1077 | |
| 1.25 | 330 | ||
| 1.30 | 186 | ||
| 1.40 | 122 | ||
| acetonitrile | 1.10 | 2879 | |
| 1.13 | 1338 | ||
| 1.14 | 787 | ||
| 1.20 | 206 | ||
| ethyl acetate | 1.05 | 1156 | |
| 1.07 | 605 | ||
| 1.10 | 589 | ||
| 1.15 | 341 |
Table 3. Experimental Induction Time Data of Phenacetin in Each Solvent for Various S at 298 K.
| solute | solvent | S (-) | ti (s) |
|---|---|---|---|
| phenacetin | ethanol | 1.10 | 3507 |
| 1.15 | 1223 | ||
| 1.18 | 638 | ||
| 1.20 | 530 | ||
| acetonitrile | 1.04 | 3602 | |
| 1.07 | 842 | ||
| 1.10 | 377 | ||
| 1.113 | 279 | ||
| ethyl acetate | 1.05 | 1799 | |
| 1.07 | 1114 | ||
| 1.09 | 737 | ||
| 1.12 | 504 |
Table 2. Experimental Induction Time Data of Lovastatin in Each Solvent for Various S at 303 K.
| solute | solvent | S (-) | ti (s) |
|---|---|---|---|
| lovastatin | ethyl acetate | 1.45 | 1139 |
| 1.50 | 970 | ||
| 1.60 | 573 | ||
| 1.70 | 275 | ||
| ethanol | 1.40 | 1998 | |
| 1.50 | 1240 | ||
| 1.70 | 633 | ||
| 1.90 | 357 | ||
| butyl acetate | 1.40 | 1156 | |
| 1.45 | 788 | ||
| 1.50 | 531 | ||
| 1.70 | 363 | ||
| methanol | 1.30 | 1389 | |
| 1.40 | 889 | ||
| 1.50 | 378 | ||
| 1.70 | 278 | ||
| acetone | 1.25 | 846 | |
| 1.30 | 545 | ||
| 1.40 | 447 | ||
| 1.50 | 321 |
In the application of eq 10, the solution viscosities η(T,S) in various solvents for each system are experimentally measured in this work using a rotational viscometer (Brookfield DV2T). The measurements under each condition are repeated three times, and the deviation of the viscosity value is generally less than 6%.
Figure 2a shows the measured supersaturation dependence of solution viscosity for isonicotinamide in various solvents at 303 K, where Ceq for isonicotinamide in each solvent at 303 K is taken from a report by Hansen et al.22 (Ceq = 210 mg solute/g solvent for methanol, Ceq = 11 mg solute/g solvent for ethyl acetate, Ceq = 23 mg solute/g solvent for acetonitrile, and Ceq = 37 mg solute/g solvent for acetone). Figure 2b shows the measured induction time data fitted to eq 10 for isonicotinamide in various solvents at 303 K, where the induction time data are experimentally obtained in this work for various initial concentrations cooled to 303 K. Figure 2c shows that A0 increases linearly with increasing γ1/2 for isonicotinamide in various solvents at 303 K, where A0 and γ in each solvent are determined using the corresponding induction time data fitted to eq 10. On the other hand, Figure 2d shows that no clear relationship is observed between AJ and γ1/2 for isonicotinamide in various solvents at 303 K, where AJ and γ in each solvent are determined using the corresponding induction time data fitted to eq 9.
Figure 2.
Isonicotinamide in various solvents: (a) dependence of η on supersaturation at 303 K; (b) induction time data fitted to eq 10 at 303 K; (c) linear relationship between A0 and γ1/2 at 303 K; and (d) AJ vs γ1/2 at 303 K.
As shown in Figure 2a, η increases in the order: acetone < acetonitrile < ethyl acetate < methanol. Although Figure 2c shows that A0 increases in the order: ethyl acetate < acetonitrile < acetone < methanol, AJ in Figure 2d increases in the order: ethyl acetate < methanol < acetonitrile < acetone, which is different from the increasing order of A0. It should be noted that η in methanol is significantly greater than that in other solvents. Consequently, although A0 in methanol is the greatest among various solvents, AJ in methanol becomes smaller than that in acetone or acetonitrile because of eq 5.
Figure 3a shows the measured supersaturation dependence of solution viscosity for lovastatin in various solvents at 303 K, where Ceq for lovastatin in each solvent at 303 K is taken from a report by Sun et al.30 (Ceq = 38 mg solute/g solvent for ethanol, Ceq = 22 mg solute/g solvent for butyl acetate, Ceq = 52 mg solute/g solvent for methanol, Ceq = 31 mg solute/g solvent for ethyl acetate, and Ceq = 105 mg solute/g solvent for acetone). Figure 3b shows the measured induction time data fitted to eq 10 for lovastatin in various solvents at 303 K, where the induction time data are experimentally obtained in this work for various initial concentrations cooled to 303 K. Figure 3c shows that A0 increases linearly with increasing γ1/2 for lovastatin in various solvents at 303 K, where A0 and γ in each solvent are determined using the corresponding induction time data fitted to eq 10. On the other hand, Figure 3d shows that no clear relationship is observed between AJ and γ1/2 for lovastatin in various solvents at 303 K, where AJ and γ in each solvent are determined using the corresponding induction time data fitted to eq 9.
Figure 3.
Lovastatin in various solvents: (a) dependence of η on supersaturation at 303 K; (b) induction time data fitted to eq 10 at 303 K; (c) linear relationship between A0 and γ1/2 at 303 K; and (d) AJ vs γ1/2 at 303 K.
Figure 4a shows the measured supersaturation dependence of solution viscosity for phenacetin in various solvents at 298 K, where Ceq for phenacetin in each solvent at 298 K is taken from a report by Croker et al.21 (Ceq = 72 mg solute/g solvent for ethanol, Ceq = 24 mg solute/g solvent for ethyl acetate, and Ceq = 48 mg solute/g solvent for acetonitrile). Figure 4b shows the measured induction time data fitted to eq 10 for phenacetin in various solvents at 298 K, where the induction time data are experimentally obtained in this work for various initial concentrations cooled to 298 K. Figure 4c shows that A0 increases linearly with increasing γ1/2 for phenacetin in various solvents at 298 K, where A0 and γ in each solvent are determined using the corresponding induction time data fitted to eq 10. On the other hand, Figure 4d shows that no clear relationship is observed between AJ and γ1/2 for phenacetin in various solvents at 298 K, where AJ and γ in each solvent are determined using the corresponding induction time data fitted to eq 9.
Figure 4.
Phenacetin in various solvents: (a) dependence of η on supersaturation at 298 K; (b) induction time data fitted to eq 10 at 298 K; (c) linear relationship between A0 and γ1/2 at 298 K; and (d) AJ vs γ1/2 at 298 K.
As shown in Figures 2a,3a, and 4a, the supersaturation dependence of solution viscosity in these systems is nearly negligible because of the narrow concentration range associated with the varied supersaturations. Table 4 lists the value of γ and the correlation coefficient R2 for each line in Figures 2b, 3b, and 4b. The value of γ in each solvent for these systems agrees with the reported literature value.27,28 Note that the correlation coefficient in each solvent for these systems exceeds the critical value of 0.900 for the 90% confidence interval and 4 points (i.e., degree of freedom = 2).
Table 4. Value of γ and the Correlation Coefficient for Each Line in Figures 2b, 3b, and 4b.
| solute | solvent | γ (mJ/m2) | R2 (-) |
|---|---|---|---|
| isonicotinamide | methanol | 3.32 | 0.973 |
| acetone | 2.53 | 0.992 | |
| acetonitrile | 1.72 | 0.951 | |
| ethyl acetate | 0.77 | 0.900 | |
| lovastatin | ethyl acetate | 1.94 | 0.915 |
| ethanol | 1.72 | 0.959 | |
| butyl acetate | 1.62 | 0.974 | |
| methanol | 1.44 | 0.926 | |
| acetone | 1.08 | 0.965 | |
| phenacetin | ethanol | 1.17 | 0.964 |
| acetonitrile | 0.674 | 0.960 | |
| ethyl acetate | 0.632 | 0.943 |
Table 5 lists comparison between the correlation coefficient for each line in Figures 2c, 3c, and 4c and the corresponding critical value based on the 95% confidence interval. As the correlation coefficient for these systems exceeds the corresponding critical value based on the 95% confidence interval, it is concluded that A0 increases linearly with increasing γ1/2 in various solvents for each system. As an increasing trend of the interfacial energy with the increasing corresponding solute–solvent interaction for the same solute in various solvents has been reported in the literature,15−18 it is speculated that the effect of this interaction on γ is also strongly correlated with that on A0 for the same system. Consequently, if the choice of solvent results in a greater γ because of a stronger solute–solvent interaction, it simultaneously results in a greater A0. On the other hand, if the choice of solvent results in a smaller γ because of a weaker solute–solvent interaction, it simultaneously results in a smaller A0.
Table 5. Comparison between the Correlation Coefficient for Each Line in Figures 2c, 3c, and 4c and the Corresponding Critical Value Based on 95% Confidence Interval.
| solute | number of solvents (-) | degree of freedom (-)a | critical value (-) | R2 (-) |
|---|---|---|---|---|
| isonicotinamide | 4 | 2 | 0.950 | 0.957 |
| lovastatin | 5 | 3 | 0.878 | 0.986 |
| phenacetin | 3 | 1 | 0.997 | 0.997 |
Degree of freedom = number of solvents – 2.
Conclusions
According to CNT, 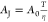 is proposed in this work. Equation 10 is derived to investigate the nucleation
kinetics in various solvents using the induction time data for isonicotinamide,
lovastatin, and phenacetin. Although no clear relationship is observed
between AJ and γ1/2 among
various solvents for each system, A0 increases
linearly with increasing γ1/2 among various solvents
for each system, which is consistent with eq 6 derived based on CNT. Based on the analyzed
results of nucleation kinetics in these systems, it is proposed that AJ consists of two parts: the first part T/η is proportional to DAB, and the other part A0 is proportional
to γ1/2. Although AJ is
dependent on DAB among various solvents, A0 is not related to the dependence of DAB on T/η(T,S) among various solvents. It is speculated that
both γ and A0 are proportional to
the solute–solvent interaction for the corresponding solvent.
is proposed in this work. Equation 10 is derived to investigate the nucleation
kinetics in various solvents using the induction time data for isonicotinamide,
lovastatin, and phenacetin. Although no clear relationship is observed
between AJ and γ1/2 among
various solvents for each system, A0 increases
linearly with increasing γ1/2 among various solvents
for each system, which is consistent with eq 6 derived based on CNT. Based on the analyzed
results of nucleation kinetics in these systems, it is proposed that AJ consists of two parts: the first part T/η is proportional to DAB, and the other part A0 is proportional
to γ1/2. Although AJ is
dependent on DAB among various solvents, A0 is not related to the dependence of DAB on T/η(T,S) among various solvents. It is speculated that
both γ and A0 are proportional to
the solute–solvent interaction for the corresponding solvent.
Experimental Section
The experimental apparatus consists of a 250 mL crystallizer immersed in a programmable thermostatic water bath shown in Figure 5. The crystallizer is equipped with a magnetic stirrer at a constant stirring rate 350 rpm. The turbidity probe (Crystal Eyes manufactured by HEL limited) is used to detect the nucleation event during the induction time study.
Figure 5.
Schematic diagram of the experimental apparatus: (1) 250 mL crystallizer, (2) magnetic stirrer, (3) constant temperature water bath, (4) turbidity probe, (5) temperature probe, and (6) computer.
The induction times for three crystallization systems, including isonicotinamide (Alfa Aesar, purity 99%), lovastatin (Acros, purity 98%), and phenacetin (Acros, purity 78%) are measured in this work. Analytical grade solvents (purity 99.9%) are used to prepare the supersaturated solution. In each experiment, a 200 mL solution with the desired supersaturation is loaded into the crystallizer. The solution is held at 3 °C above the saturated temperature for 5–10 min to ensure a complete dissolution at the beginning of the experiment, which is also confirmed by the turbidity measurement. Then, the supersaturated solution is rapidly cooled to the desired temperature for the induction time measurements.
Acknowledgments
The author would like to thank Chang Gung Memorial Hospital (CMRPD2G0242) and Ministry of Science and Technology of Taiwan (MOST107-2221-E-182-031) for financial support of this research. The author also expresses his gratitude to Tai-Jyun Chen, Wen-Chi Chang and Chen-I Lai for their experimental work.
Glossary
Notation
- AJ
pre-exponential nucleation factor (m–3 s–1)
- A0
intrinsic nucleation factor (Pa m–3 K–1)
- C0
initial concentration of solute molecules (m–3)
- Ceq
equilibrium concentration of solute molecules (m–3)
- DAB
solute diffusivity (m2/s)
- fN
minimum detectable number density of accumulated crystals (m–3)
- fV
minimum detectable volume fraction of accumulated crystals (-)
- J
nucleation rate (m–3 s–1)
- kB
Boltzmann constant (=1.38 × 10–23 J/K)
- kV
volume shape factor (-)
- MW
molar mass (kg/mol)
- NA
Avogadro number (=6.02 × 1023 mol–1)
- r0
molecular radius of solute (m)
- S
supersaturation ratio (-)
- T
temperature (K)
- t
time (s)
- ti
induction time (s)
- vm
volume of the solute molecule (m3)
Glossary
Greek letters
- γ
interfacial energy (J/m2)
- ρC
crystal density (kg/m3)
- η
solution viscosity (Pa s)
The author declares no competing financial interest.
References
- Mullin J. W.Crystallization; Butterworth-Heinemann: Oxford, 1993. [Google Scholar]
- Kashchiev D.Nucleation: Basic Theory with Applications; Butterworth-Heinemann: Oxford, 2000. [Google Scholar]
- Kashchiev D.; van Rosmalen G. M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. 10.1002/crat.200310070. [DOI] [Google Scholar]
- Du W.; Yin Q.; Bao Y.; Xie C.; Hou B.; Hao H.; Chen W.; Wang J.; Gong J. Concomitant polymorphism of prasugrel hydrochloride in reactive crystallization. Ind. Eng. Chem. Res. 2013, 52, 16182–16189. 10.1021/ie4020815. [DOI] [Google Scholar]
- You S.; Zhang Y.; Zhang Y. Nucleation of ammonium aluminum sulfate dodecahydrate from unseeded aqueous solution. J. Cryst. Growth 2015, 411, 24–29. 10.1016/j.jcrysgro.2014.11.006. [DOI] [Google Scholar]
- Yang H. Relation between metastable zone width and induction time of butyl paraben in ethanol. CrystEngComm 2015, 17, 577–586. 10.1039/c4ce01625d. [DOI] [Google Scholar]
- Yang L.; Cao J.; Luo T. Effect of Mg2+, Al3+, and Fe3+ ions on crystallization of type α hemi-hydrated calcium sulfate under simulated conditions of hemi-hydrate process of phosphoric acid. J. Cryst. Growth 2018, 486, 30–37. 10.1016/j.jcrysgro.2018.01.014. [DOI] [Google Scholar]
- Granberg R. A.; Ducreux C.; Gracin S.; Rasmuson Å. C. Primary nucleation of paracetamol in acetone-water mixtures. Chem. Eng. Sci. 2001, 56, 2305–2313. 10.1016/s0009-2509(00)00439-5. [DOI] [Google Scholar]
- Omar W.; Mohnicke M.; Ulrich J. Determination of the solid liquid interfacial energy and thereby the critical nucleus size of paracetamol in different solvents. Cryst. Res. Technol. 2006, 41, 337–343. 10.1002/crat.200510584. [DOI] [Google Scholar]
- Nordström F. L.; Svärd M.; Rasmuson Å. C. Primary nucleation of salicylamide: the influence of process conditions and solvent on the metastable zone width. CrystEngComm 2013, 15, 7285–7297. 10.1039/c3ce40619a. [DOI] [Google Scholar]
- Yang H.; Rasmuson Å. C. Nucleation of butyl paraben in different solvents. Cryst. Growth Des. 2013, 13, 4226–4238. 10.1021/cg400177u. [DOI] [Google Scholar]
- Yang H.; Svärd M.; Zeglinski J.; Rasmuson Å. C. Influence of solvent and solid-state structure on nucleation of parabens. Cryst. Growth Des. 2014, 14, 3890–3902. 10.1021/cg500449d. [DOI] [Google Scholar]
- Mealey D.; Croker D. M.; Rasmuson Å. C. Crystal nucleation of salicylic acid in organic solvents. CrystEngComm 2015, 17, 3961–3973. 10.1039/c4ce01428f. [DOI] [Google Scholar]
- Zou F.; Zhuang W.; Chen Q.; Yang P.; Lin C.; Jiao P.; Zhou J.; Wu J.; Ying H. Solvent effects on nucleation of disodium guanosine 5′-monophosphate in anti-solvent/water mixtures. CrystEngComm 2016, 18, 6653–6663. 10.1039/c6ce00867d. [DOI] [Google Scholar]
- Khamar D.; Zeglinski J.; Mealey D.; Rasmuson Å. C. Investigating the role of solvent–solute interaction in crystal nucleation of salicylic acid from organic solvents. J. Am. Chem. Soc. 2014, 136, 11664–11673. 10.1021/ja503131w. [DOI] [PubMed] [Google Scholar]
- Mealey D.; Zeglinski J.; Khamar D.; Rasmuson Å. C. Influence of solvent on crystal nucleation of risperidone. Faraday Discuss. 2015, 179, 309–328. 10.1039/c4fd00223g. [DOI] [PubMed] [Google Scholar]
- Zeglinski J.; Kuhs M.; Devi K. R.; Khamar D.; Hegarty A. C.; Thompson D.; Rasmuson Å. C. Probing crystal nucleation of fenoxycarb from solution through the effect of solvent. Cryst. Growth Des. 2019, 19, 2037–2049. 10.1021/acs.cgd.8b01387. [DOI] [Google Scholar]
- Yang Y.; Zhou L.; Zhang X.; Yang W.; Zhang S.; Xiong L.; Wei Y.; Zhang M.; Hou B.; Yin Q. Influence of solvent properties and intermolecular interaction between solute and solvent on nucleation kinetics of hmbtad. J. Cryst. Growth 2018, 498, 77–84. 10.1016/j.jcrysgro.2018.06.006. [DOI] [Google Scholar]
- Lindenberg C.; Mazzotti M. Effect of temperature on the nucleation kinetics of α l-glutamic acid. J. Cryst. Growth 2009, 311, 1178–1184. 10.1016/j.jcrysgro.2008.12.010. [DOI] [Google Scholar]
- Sato S.; Hata T.; Tsujita Y.; Terahara A.; Tamura C. The structure of monacolin K, C24H26O5. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1984, 40, 195–198. 10.1107/s0108270184003565. [DOI] [Google Scholar]
- Croker D. M.; Kelly D. M.; Horgan D. E.; Hodnett B. K.; Lawrence S. E.; Moynihan H. A.; Rasmuson Å. C. Demonstrating the influence of solvent choice and crystallization conditions on phenacetin crystal habit and particle size distribution. Org. Process Res. Dev. 2015, 19, 1826–1836. 10.1021/op500308x. [DOI] [Google Scholar]
- Hansen T. B.; Taris A.; Rong B.-G.; Grosso M.; Qu H. Polymorphic behavior of isonicotinamide in cooling crystallization from various solvents. J. Cryst. Growth 2016, 450, 81–90. 10.1016/j.jcrysgro.2016.06.030. [DOI] [Google Scholar]
- Turner T. D.; Hatcher L. E.; Wilson C. C.; Roberts K. J. Habit modification of the active pharmaceutical ingredient lovastatin through a predictive solvent selection approach. J. Pharm. Sci. 2019, 108, 1779–1787. 10.1016/j.xphs.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Shiau L.-D. The influence of solvent on the pre-exponential factor and interfacial energy based on the metastable zone width data. CrystEngComm 2016, 18, 6358–6364. 10.1039/c6ce00895j. [DOI] [Google Scholar]
- Kubota N. A new interpretation of metastable zone widths measured for unseeded solutions. J. Cryst. Growth 2008, 310, 629–634. 10.1016/j.jcrysgro.2007.11.123. [DOI] [Google Scholar]
- Kobari M.; Kubota N.; Hirasawa I. Deducing primary nucleation parameters from metastable zone width and induction time data determined with simulation. CrystEngComm 2013, 15, 1199–1209. 10.1039/c2ce26679b. [DOI] [Google Scholar]
- Shiau L.-D.; Lu T.-S. A model for determination of the interfacial energy from the measured metastable zone width by the polythermal method. J. Cryst. Growth 2014, 402, 267–272. 10.1016/j.jcrysgro.2014.06.024. [DOI] [Google Scholar]
- Shiau L.-D.; Lu T.-S. A model for determination of the interfacial energy from the induction time or metastable zone width data based on turbidity measurements. CrystEngComm 2014, 16, 9743–9752. 10.1039/c4ce01245c. [DOI] [Google Scholar]
- Mersmann A.; Bartosch K. How to predict the metastable zone width. J. Cryst. Growth 1998, 183, 240–250. 10.1016/s0022-0248(97)00417-x. [DOI] [Google Scholar]
- Sun H.; Gong J.-b.; Wang J.-k. Solubility of Lovastatin in Acetone, Methanol, Ethanol, Ethyl Acetate, and Butyl Acetate between 283 K and 323 K. J. Chem. Eng. Data 2005, 50, 1389–1391. 10.1021/je0500781. [DOI] [Google Scholar]








