Abstract
A new synthetic strategy to the ABCD ring system of the anticancer agent fredericamycin A (NSC-305263) was realized by the Diels–Alder reaction and olefin metathesis as key steps. The tactics developed here for the construction of the ABCD ring system also involve double Claisen rearrangement followed by a retro-Diels–Alder reaction and ring-closing metathesis. The metathesis approach performs a key role in the construction of A and D rings of the ABCD core unit. More importantly, ABCD fragment synthesis was accomplished without the involvement of protecting groups.
Introduction
Fredericamycin A 1 (Figure 1), a quinone-based natural product, was first isolated in 1981 from the fermentation broth of the strain Streptomyces griseus (ATCC 49344, FCRC-48).1 It comprises a hexacyclic ring containing a single chiral quaternary center (spiro[4.4]nonene ring system) conjoined with the naphthoquinone and isoquinolone moieties, which are not present in any other natural product.2 The design and synthesis of highly oxygenated polyaromatic fredericamycin A 1 are difficult tasks due to the presence of a dense functionality, and synthesis is important due to its in vitro cytotoxic and in vivo antibiotic antitumor activity.3
Figure 1.

Structure of fredericamycin A 1.
Because of its special features of the spirocyclic core (spiro[4.4]nonane ring), fredericamycin A 1 exhibits an interesting biological activity,4 is highly cytotoxic against murine leukemia KB and Du-145 prostate tumors, and also shows good activity against in vivo tumor models such as P338D1 mouse leukemia, CD8F mammary tumors, B16 melanoma, and L1210 cell lines.5,3b Fredericamycin A also acts as a potent inhibitor against ovarian tumors and also inhibits both topoisomerases I and II.6,3b Furthermore, compound 1 exhibited an irreversible inhibitor of the peptidyl-prolyl cis–trans isomerase (PPI) Pin 1 with a Ki of 0.82 μM and also inhibited the DNA processing enzymes.7,6,3b In this context, several synthetic routes have been reported in the literature.8 The first successful total synthesis of compound 1 was completed in racemic form by Kelly and co-workers in 1986.9 Afterward, several research groups like Boger et al.,5 Clive et al.,8c Kita et al.,8e Bach et al.,11 and Rama Rao et al.8d reported the total synthesis of fredericamycin.
Limited approaches are available for the synthesis of the spirocyclic indene framework of fredericamycin A 1.10 These includes the rearrangement reactions,11 cycloadditions,12 intermolecular alkyne-chromium carbene complex cyclization,13 metal-mediated 1,2-carbonyl shift,14 radical pathways,15 photochemical approach,16 Diels–Alder reactions (DA),17 and palladium-catalyzed cross-coupling acylation.18 The construction of a spirocyclic ring system is indeed a synthetic challenge. To the best of our knowledge, there are no reports available for the construction of the spirocyclic core with a functionalized BCD ring system of fredericamycin A 1 through double Claisen rearrangement (CR) and ring-closing metathesis (RCM) as key steps. Here, the RCM protocol was identified as a key step to create a spirocyclic core, the A and D ring system of fredericamycin A 1. Several metathesis catalysts are now available for ring closure of olefinic precursors. Here, we used G-I and G-II catalysts to realize the metathesis step. The key synthon to fredericamycin A 1, consisting of the ABCD ring system, was assembled by adopting the DA reaction,19 Claisen rearrangement,20 and RCM protocol.21 The key steps in our synthetic strategy involving RCM for CD ring construction, ceric ammonium nitrate (CAN) oxidation, and DA reaction along with the aromatization sequence have been considered to assemble a fully functionalized BCD ring system. Additionally, one-pot Claisen rearrangement (CR) with the retro-Diels–Alder reaction (rDA)22 followed by RCM provides a new entry to the ABCD framework of fredericamycin A 1.
Results and Discussion
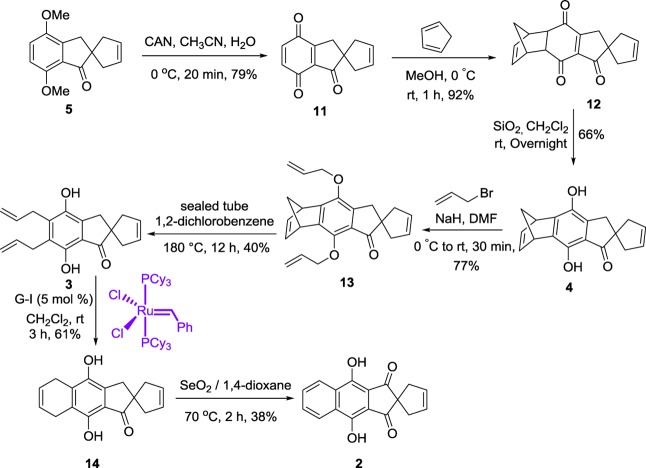
Our retrosynthetic approach to the ABCD ring system 2 of fredericamycin A 1 is shown in Scheme 1. The target molecule 2 may be assembled from bis-hydroxy derivative 3 via the RCM and oxidation sequence. Interestingly, the bis-hydroxy derivative 3 could be derived from the aromatized compound 4 through O-allylation and one-pot double Claisen rearrangement (CR) followed by the rDA reaction. The dihydroxy ketone 4 may be generated from 5 via oxidation and DA reactions followed by aromatization. The spiro derivative 5 can be obtained from dimethoxy indanone 6 by C-allylation followed by RCM. Indanone 6 was prepared from the commercially available starting material such as 2,5-dimethoxybenzaldehyde 7 based on the known procedures.23
Scheme 1. Retrosynthetic Approach toward ABCD Core 2 of Fredericamycin A 1.
Our journey toward the target molecule 2 started with the synthesis of key building block dimethoxy indanone 6.23 In this regard, 2,5-dimethoxybenzaldehyde 7 reacted with malonic acid under Knoevenagel conditions to produce the acid 8(23a) in 88% yield. Next, acid derivative 8 was subjected to hydrogenation with 10% Pd/C and gave the saturated acid 9 in 97% yield. Further, acid-catalyzed cyclization of 9 in the presence of phosphorous pentoxide (P2O5) and methanesulfonic acid (MeSO3H) produced the dimethoxy indanone derivative 6 (69%).23c−23g Having prepared the known indanone derivative 6, our next target was the spiro[4.4]nonene ring system, which constitutes the CD ring fragment of fredericamycin A. Next, the indanone 6 was subjected to allylation with allyl bromide in the presence of NaH in THF to provide the diallyl indanone 10 in excellent yield. Subsequently, the indanone derivative 10 proceeded for RCM via the G-II catalyst to generate the ring-closure product 5 (89%). Here, we observed that spiro ring formation with a quaternary center in the presence of the G-I catalyst is slower and the RCM product has less yield (60–65%). The structure of the RCM product 5 (Scheme 2) has been proven by spectral parameters (1H, 13C NMR, and HRMS data).
Scheme 2. Synthesis of Spirocyclic Compound 5.
Having prepared the tricyclic spiro compound 5, the attention was then directed toward its expansion to the ABCD ring system. The spiro ketone 5 was subjected for CAN oxidation at 0 °C to deliver the quinone derivative 11, which acts as a powerful dienophile in the DA reaction with cyclopentadiene. Thermal cycloaddition of quinone 11 with a freshly cracked cyclopentadiene gave the DA adduct 12 in excellent yield (92%). Armed with the DA adduct 12 in hand, we focused our efforts to assemble the ABCD core of fredericamycin A 1. In this regard, the Diels–Alder adduct 12 was further treated for aromatization24 with 60–120 mesh silica gel to generate the corresponding annulated hydroquinone-fused norbornadiene 4 in moderate yield (Scheme 3). The aromatized product structure 4 was supported by spectral data (1H, 13C NMR DEPT 135, and HRMS). Next, the hydroquinone 4 was O-allylated under basic conditions using allyl bromide in dry DMF to produce the diallyl compound 13 in 77% yield. To introduce the A ring of the ABCD core of fredericamycin A 1, we utilized a one-pot rDA reaction followed by double CR. To this end, the O-allyl derivative 13 was heated at 180 °C in a sealed tube for 12 h to produce the diallyl hydroquinone 3 in 40% yield (based on the starting material recovered, 11%). Also, several minor products were formed during the rearrangement sequence, and they could not be isolated in pure form (TLC monitoring).
Scheme 3. Synthetic Approach to ABCD Core 2 of Fredericamycin A 1.
The formation of unexpected hydroquinone derivative 3 can be explained on the basis of initially formed quinone intermediate 3A from compound 13 (via Claisen/Retro), which undergoes rapid transformation to hydroquinone 3 due to the presence of the active −CH2 group adjacent to the spiro system, which acts as a hydrogen source and is further involved in the aromatization process (Figure 2). The highly conjugated quinone 3A participated in the generation of carbocation intermediate 3C via vinylogous enol 3B. Later on, the formed carbocation 3C further rearranged to 3D, and elimination of proton followed to produce 3E. Finally, the quinone intermediate 3A was involved in the virtual disproportionation to give the major product 3 along with the oxidized product 3F. The by-product 3F was confirmed by its mass spectral (HRMS) data (Supporting Information).
Figure 2.
Suggested mechanism for hydroquinone 3 formation.
To install the A ring by the RCM strategy, compound 3 was reacted with the Grubbs (G-I) catalyst to generate the tetracyclic compound (RCM product) 14. The RCM product 14 was fully established with spectral parameters such as IR, 1H NMR, and 13C NMR and further supported by HRMS data (Scheme 3). To complete the target compound, the ABCD ring analogue of fredericamycin A 1, the RCM product 14 was subjected to SeO2 oxidation in dioxane for 2 h at 70 °C to produce compound 2 in 38% yield. There are some other minor products (observed by TLC) that were formed during this process, and they could not be isolated in pure form by column chromatography. The structure of compound 2 was supported with 1H, 13C NMR, and mass spectroscopic data (Scheme 3).
Conclusions
To conclude, we have established a useful synthetic strategy to assemble the ABCD ring system of antitumor agent fredericamycin A starting with commercially available 2,5-dimethoxybenzaldehyde 7 through the CR and two-fold RCM sequence. The metathesis sequence provided an opportunity for the construction of A and D rings of the ABCD framework of fredericamycin A. So far, there are no reports available in the literature for the construction of the ABCD core of fredericamycin A. Here, a tactful combination of RCM, one-pot rDA reaction, and double CR has been used to provide access to the target molecule. Moreover, construction of the ABCD ring system of fredericamycin A 1 was successfully established with protecting-group-free synthesis.25
Experimental Section
General Experimental Details
All of the essential reagents, chemicals, and required solvents were used as such directly obtained from commercial suppliers. Thin-layer chromatography (TLC) plates were made on 10 × 5 glass plates layered with commercial grade Acme silica gel (GF-254) containing 13% CaSO4, which acts as a binder. All the reaction progress was analyzed by the chromatographic technique (TLC analysis) with suitable solvent systems (EtOAc/Pet ether), and observation was done by UV, iodine spray, and immersion in KMnO4 solution. Moisture-sensitive (dry/anhydrous) reactions were performed by oven-dried glassware under a nitrogen/argon atmosphere by using syringe-septum techniques. Column purification was done by 100–200 mesh silica gel in all cases with suitable solvent systems. Dimethylformamide (DMF) and CH2Cl2 were distilled over calcium hydride (CaH2), and EtOAc was dried with anhydrous K2CO3.
All IR samples were recorded with DCM and chloroform as solvents on a Nicolet Impact-400 FTIR spectrometer. Nuclear magnetic resonance (NMR) spectra (1H, 13C, and DEPT 135) were recorded on 400 and 500 MHz spectrometers (Bruker) with a CDCl3 solvent, and chemical shifts (δ ppm) are reported relative to the internal standard such as TMS. The J values (coupling constants) are given in hertz. Mass spectra (HRMS) were recorded under positive ion electrospray ionization (ESI, Q-TOF) mode.
Synthesis of (E)-3-(2,5-Dimethoxyphenyl)acrylic Acid (8)23a,23b,23e
2,5-Dimethoxybenzaldehyde 7 (5 g, 30 mmol), malonic acid (6 g, 60 mmol), and pyridine (10 mL) were placed in a two-neck RB, the mixture was heated at 80 °C, and then piperidine (1 mL) was added. Later on, the reaction mixture was heated at 80 °C for 1 h, and slowly, the temperature was increased to 110–115 °C for 3 h. Upon completion, the reaction mixture was allowed to cool, and then cold water was poured. Acidification by HCl (pH 5) led to the formation of crude acid 8 as a yellow precipitate. Afterward, crude (E)-3-(2,5-dimethoxyphenyl)acrylic acid was recrystallized from EtOAc/hexane and yielded the pure cinnamic acid 8 as yellow crystalline needles.
Yield: 88% (5.5 g); mp: 145–147 °C; 1H NMR (400 MHz, CDCl3): δ = 8.07 (d, J= 16.2 Hz, 1H), 7.07 (d, J= 3.04 Hz, 1H), 6.95 (d, J = 3.0 Hz, 1H), 6.93–6.85 (m, 1H), 6.52 (d, J= 16.1 Hz, 1H), 3.86 (s, 3H), 3.80 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ = 173.1, 153.5, 153.1, 142.3, 123.6, 118.0, 117.8, 113.5, 112.5, 56.1, 55.8 ppm.
Synthesis of 3-(2,5-Dimethoxyphenyl)propanoic Acid (9)23a,23b
To a stirred solution of unsaturated acid 8 (5 g, 24 mmol) in dry EtOAc (50 mL), Pd/C (250 mg, 10% palladium on carbon) was added, and then the reaction mixture was stirred at room temperature for 5 h under hydrogen gas (balloon pressure). Progress of the reaction was monitored by TLC, and the crude mixture was passed through a Celite pad and washed with ethyl acetate (3 times, 20 mL). The filtrate was evaporated at reduced pressure, and then the crude product was recrystallized from the EtOAC/hexane mixture to furnish the saturated derivative 9 as a colorless crystalline solid.
Yield: 97% (4.85 g); mp: 67–69 °C; 1H NMR (400 MHz, CDCl3): δ = 6.77–6.70 (m, 3H), 3.78 (s, 3H), 3.75 (s, 3H), 2.91 (d, J = 7.6 Hz, 2H), 2.65 (d, J = 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ = 179.7, 153.5, 151.8, 129.8, 116.4, 111.8, 111.2, 55.8, 55.8, 34.1, 26.1 ppm.
4,7-Dimethoxy-2,3-dihydro-1H-inden-1-one (6)23c
To a stirred solution of CH3SO3H (10.0 mL, 153 mmol), anhydrous P2O5 (2.00 g, 13.7 mmol) was added, and the resulting mixture was stirred at 50 °C for 0.5 h. Later on, a solution of acid 9 (800 mg, 3.80 mmol) in CH2Cl2 (20 mL) was added to the reaction mixture, and it was stirred at rt for 19 h. On completion of the reaction (based on TLC monitoring), the crude product was poured onto ice (50 mL), added with 10% NaOH dropwise to basify (pH 10), and was extracted with DCM (3 × 50 mL). The organic layers were combined, washed with saturated sodium bicarbonate solution (1 × 70 mL), H2O (1 × 70 mL), and brine (1 × 70 mL), and dried over sodium sulfate. The solvent was evaporated, and further purification of the crude mixture with (100–200 mesh silica gel) column chromatography by using 40% ethyl acetate in pet ether as the eluent system afforded 6 as a colorless crystalline needles.
Yield: 69% (507 mg); mp: 127–129 °C (lit. 123–125 °C); 1H NMR (500 MHz, CDCl3): δ = 6.96 (d, J = 8.6 Hz, 1H), 6.71 (d, J = 8.6 Hz, 1H), 3.88 (s, 3H), 3.83 (s, 3H), 2.97–2.95 (m, 2H), 2.65–2.63 (m, 2H) ppm; 13C NMR (125 MHz, CDCl3): δ = 205.1, 151.8, 150.5, 146.1, 126.4, 116.6, 56.1, 55.9, 36.8, 22.3 ppm.
2,2-Diallyl-4,7-dimethoxy-2,3-dihydro-1H-inden-1-one (10)
To a stirred solution of NaH (2.87 g, 6 equiv.) in THF, dimethoxy indanone 6 (4 g, 20.81 mmol) in 50 mL of THF was added at 0 °C under a N2 atmosphere. Further, the reaction mixture was kept under stirring at rt for 10 min. Later on, allyl bromide (10 mL, 124.8 mmol) was added, and then the reaction mixture was allowed to stir at rt for 6 h. Progress of the reaction was monitored based on TLC, and the reaction mixture was quenched with saturated NH4Cl solution (15 mL) and extracted with EtOAc. The organic layers were washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo. The reaction mixture was purified by column chromatography using 100–200 mesh silica gel with 10% EtOAc/pet ether as the solvent system to afford the diallylated compound 10 obtained as a colorless crystalline solid.
Yield: 96% (5.4 g); mp: 96–98 °C; IR (neat, cm–1): 3071, 2928, 2840, 1707, 1596, 1496, 1265, 1070; 1H NMR (500 MHz, CDCl3): δ = 6.94 (d, J = 8.7 Hz, 1H), 6.68 (d, J = 8.5 Hz, 1H), 5.64–5.56 (m, 2H), 5.03 (dd, J = 17.0 Hz, 1.7 Hz, 2H), 4.96–4.93 (m, 2H), 3.85 (s, 3H), 3.80 (s, 3H), 2.84 (s, 2H), 2.38 (dd, J = 13.6, 6.5 Hz, 2H), 2.25 (dd, J = 13.6, 8.2 Hz, 2H) ppm; 13C NMR (125 MHz, CDCl3): δ = 208.0, 151.6, 150.3, 143.6, 133.5, 125.8, 118.5, 116.8, 109.4, 56.0, 55.8, 52.2, 41.9, 32.3 ppm; HRMS (ESI, Q-TOF): m/z calcd for C17H21O3 [M + H]+: 273.1485, found: 273.1487.
4′,7′-Dimethoxyspiro[cyclopentane-1,2′-inden]-3-en-1′(3′H)-one (5)
A solution of 10 (5.8 g, 21.3 mmol) in dry CH2Cl2 (100 mL) was degasified with nitrogen for 0.5 h, then the G-II catalyst (5 mol %) was added, and the resulting reaction mixture was allowed to stir at rt for 16 h. Progress of the reaction was monitored based on TLC. The solvent was evaporated under reduced pressure, and the crude mixture was purified by silica gel column chromatography in 20% EtOAc/pet ether as the eluent to afford the desired spirocyclic compound 5 as a colorless crystalline solid.
Yield: 89% (4.62 g); mp: 93–95 °C; IR (neat, cm–1): 3061, 2940, 2840, 1707, 1596, 1496, 1265, 1065. 1H NMR (400 MHz, CDCl3): δ = 6.98 (d, J = 9.0 Hz, 1H), 6.73 (d, J = 8.7 Hz, 1H), 5.68 (s, 2H), 3.89 (s, 3H), 3.82 (s, 3H), 3.00 (s, 2H), 2.87 (d, J = 15.0 Hz, 2H), 2.28 (d, J = 14.1 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ = 208.5, 151.9, 150.3, 143.4, 128.7, 125.4, 116.7, 109.6, 56.0, 55.9, 55.6, 45.7, 42.0 ppm; HRMS (ESI, Q-TOF): m/z calcd for C15H17O3 [M + H]+: 245.1172, found: 245.1171.
Spiro[cyclopentane-1,2′-inden]-3-ene-1′,4′,7′(3′H)-trione (11)
The compound 5 (250 mg, 1.0 mmol) was dissolved in CH3CN (10 mL) and further stirred under an ice bath for 5 min. Next, cerium (IV) ammonium nitrate (1.4 g, 2.5 mmol) was dissolved in 10 mL of cold water and further added to the reaction mixture in a dropwise manner. After 20 min (TLC monitoring), water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. Further, the organic layer was washed with water and brine solution and dried over anhydrous Na2SO4. Solvent was removed, and the crude product was used for the DA reaction without any further purification due to its instability.
Yellow liquid (173 mg, 79% yield); IR (neat, cm–1): 3027, 2830, 1730, 1722, 1664, 1429, 1051; 1H NMR (400 MHz, CDCl3): δ = 6.89 (d, J = 10.4 Hz, 1H), 6.81 (d, J = 10.1 Hz, 1H), 5.69 (s, 2H), 2.93 (s, 2H), 2.86 (d, J = 14.6 Hz, 2H), 2.32 (d, J = 14.6 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ = 207.3, 186.9, 182.8, 166.4, 137.9, 136.9, 135.3, 128.4, 55.3, 45.5, 42.4 ppm; HRMS (ESI, Q-TOF): m/z calcd for C13H10NaO3 [M + Na]+: 237.05272, found 245.0524.
Diels–Alder Adduct (12)
To a solution of trione 11 (200 mg, 0.9 mmol) in methanol (10 mL), freshly cracked cyclopentadiene (3 mL) was added at 0 °C. Afterward, the reaction mixture was allowed to stir for 1 h until the completion of reaction (TLC). Later on, the solvent was evaporated under reduced pressure, and the crude product was purified by recrystallization from EtOAc/pet ether to afford the desired DA product 12 as a yellow crystalline solid in quantitative yield.
Yield: 92% (240 mg); mp: 127–129 °C; IR (neat, cm–1): 2962, 1732, 1678, 1657, 1269, 1031.1H NMR (500 MHz, CDCl3): δ = 6.12 (dd, J = 5.4, 2.6 Hz, 1H), 6.04 (dd, J = 5.5, 2.7 Hz, 1H), 5.66–5.63 (m, 2H), 3.60 (d, J = 12.0 Hz, 2H), 3.43–3.37 (m, 2H), 2.83–2.68 (m, 4H), 2.24–2.21 (m, 2H),1.60–1.58 (m, 1H), 1.48 (d, J = 8.7 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ = 208.0, 199.5, 194.1, 170.9, 139.8, 136.2, 135.0, 128.4, 128.2, 55.6, 51.9, 51.4, 49.5, 49.3, 49.0, 45.6, 45.1, 42.0 ppm; HRMS (ESI, Q-TOF): m/z calcd for C18H16KO3 [M + K]+: 319.0731, found: 319.0731.
Synthesis of Aromatized Compound (4)
The Diels–Alder [4 + 2] product 12 (500 mg, 1.78 mmol) in CH2Cl2 (20 mL) and silica gel (60-mesh, 5.0 g) was added. The reaction mixture was allowed to stir at rt for 6 h with silica gel in order to promote its adsorption onto the surface of silica gel. Later on, the mixture was allowed to stand on the silica gel column overnight. After column chromatography (10% EtOAc/PE), the desired aromatized hydroquinone derivative 4 was obtained as a pure colorless solid.
Yield: 66% (330 mg); mp: 218–220 °C; IR (neat, cm–1): 3413, 2924, 1737, 1642, 1664, 1020; 1H NMR (400 MHz, CDCl3): δ = 8.55 (s, 1H), 6.88 (dd, J = 5.21, 3.08 Hz, 1H), 6.74 (dd, J = 5.04, 3.12 Hz, 1H), 5.71 (s, 2H), 4.65 (bs, 1H), 4.20 (s, 1H), 4.10 (s, 1H), 2.97 (s, 2H), 2.86 (t, J = 15.0 Hz, 2H), 2.38–2.30 (m, 3H), 2.22 (d, J = 7.0 Hz, 1 H) ppm; 13C NMR (125 MHz, CDCl3): δ = 212.9, 149.0, 145.0, 143.8, 141.2, 140.4, 138.0, 136.5, 128.8, 121.0, 69.5, 56.4, 47.0, 46.0, 45.6, 45.5, 42.1 ppm; HRMS (ESI, Q-TOF): m/z calcd for C18H16NaO3 [M + Na]+ 303.0992, found 303.0995.
O-Allylated Derivative (13)
In a suspension of NaH (660 mg, 27.5 mmol) in dry DMF (2 mL), the aromatized derivative 4 (1.1 g, 3.9 mmol) in 15 mL of anhydrous DMF and allyl bromide (1.7 mL, 19.6 mmol) were added at 0 °C in N2 and stirred for 0.5 h at room temperature. On completion of the reaction (based on TLC), the resulting mixture was quenched by aqueous NH4Cl and extracted with EtOAc. The organic layer was washed with water and brine and dried over anhydrous Na2SO4. Column chromatography by 8% EtOAc/PE gave the desired diallylated product 13 obtained with a pale yellow liquid.
Yield: 77% (1.08 g); IR (neat, cm–1): 3055, 1701, 1609, 1023; 1H NMR (500 MHz, CDCl3): δ = 6.82–6.81 (m, 1H), 6.74–6.73 (m, 1H), 6.12–6.01 (m, 2H), 5.68 (s, 2H), 5.38–5.33 (m, 2H), 5.26 (d, J = 10.4 Hz, 1H), 5.21 (d, J = 10.4 Hz, 1H), 4.62–4.60 (m, 2H), 4.54–4.51 (m, 1H), 4.46–4.43 (m, 1H), 4.17 ( brs, 2H), 3.00 (q, J = 17.3 Hz, 2H), 2.87–2.82 (m, 2H), 2.31–2.25 (m, 3H), 2.18 (d, J = 7.0, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ = 207.2, 151.4, 146.4, 146.3, 146.2, 143.9,143.8, 141.3 (d), 134.5 (d), 133.9 (d), 128.8 (d), 127.2, 118.0, 117.8 (d), 117.9, 75.4, 74.4, 68.3, 56.3, 48.3, 46.9, 45.7, 45.6, 42.3 ppm; HRMS (ESI, Q-TOF): m/z calcd for C24H24NaO3 [M + Na]+ 383.1618, found 383.1613.
5′,6′-Diallyl-4′,7′-dihydroxyspiro[cyclopentane-1,2′-inden]-3-en-1′(3′H)-one (3)
Compound 13 (440 mg, 1.22 mmol) in 1,2-dichlorobenzene (4 mL) in a sealed tube was degassed with N2 gas for 5 min with stirring. Then the solution was kept at 180 °C for 12 h. After the completion of the reaction, the crude product was directly loaded on a 100–200 mesh silica gel, and the column was eluted with pet ether (200 mL) until 1,2-dichlorobenzene was removed. Further, 2–3% ethyl acetate/petroleum ether elution furnished the rDA derivative 3 as a dark brown liquid along with the starting material in 11% yield.
Yield: 40% (145 mg); IR (neat, cm–1): 3412, 2926, 1678, 1046; 1H NMR (400 MHz, CDCl3): δ = 8.81 (s, 1H), 5.98–5.89 (m, 2H), 5.72 (s, 2H), 5.15–4.93 (m, 4H), 4.8 (s, 1H), 3.47 (dt, J = 5.6, 2.0 Hz, 2H), 3.44 (dt, J = 5.4, 1.6 Hz, 2H), 3.0 (s, 2H), 2.8 (d, J = 14.8 Hz, 2H), 2.37 (d, J = 14.4 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ = 213.0, 149.6, 144.1, 135.9 (d), 135.2, 135.1, 135.0 (d), 128.8 (d), 124.6, 120.3 (d), 116.7 (t), 115.3 (t), 55.9, 45.6 (t), 42.0 (t), 31.6 (t), 29.4 ppm; HRMS (ESI, Q-TOF): m/z calcd for C19H20NaO3 [M + Na]+ 319.1305, found 319.1302.
4′,9′-Dihydroxy-5′,8′-dihydrospiro[cyclopentane-1,2′-cyclopenta[b]naphthalen]-3-en-1′(3′H)-one (14)
A magnetically stirred solution of compound 3 (120 mg, 0.40 mmol) in dry DCM (30 mL) was degasified for 5 min with nitrogen. Later on, the G-I catalyst (16 mg, 5 mol %) was added, and then stirring was continued at rt for 3 h. Then the solvent was evaporated, and the crude reaction mixture was subjected to column chromatography (100–200 mesh silica gel, 20% EtOAc/PE) to give the ring-closure product 14 as a yellow crystalline solid.
Yield: 61% (66 mg); mp: 165–167 °C; IR (neat, cm–1): 3464, 2925, 1669, 1456, 1265, 1047; 1H NMR (500 MHz, CDCl3): δ = 8.74 (s, 1H), 5.95–5.86 (m, 2H), 5.73 (s, 2H), 3.32 (s, 4H), 3.02 (s, 2H), 2.89 (d, J = 15 Hz, 2H), 2.37 (d, J = 15.0 Hz, 2H) ppm; 13C NMR (125 MHz, CDCl3): δ = 212.7, 148.9, 142.6, 132.2, 131.7, 128.8, 123.9, 122.3, 121.4, 119.0, 56.0, 45.6, 41.8, 25.1, 23.4 ppm; HRMS (ESI, Q-TOF): m/z calcd for C17H16NaO3 [M + Na]+ 291.0992, found 291.0996.
Synthesis of ABCD Core (2) of Fredericamycin A (1)
A solution of SeO2 (124 mg, 1.11 mmol) in 1,4-dioxane (20 mL) was added with HCOOH (0.5 mL). Then the solution of compound 14 (50 mg, 0.186) in 1,4-dioxane (10 mL) was added, and the resulting mixture was allowed to heat slowly from rt to 70 °C for 2 h. Afterward, the solvent was removed, extracted with EtOAc, washed with brine solution, and dried over anhydrous Na2SO4. The crude mixture further proceeded to silica gel column purification (20% EtOAc/PE) to obtain the oxidized product 2 as a yellow crystalline solid.
Yield: 38% (20 mg); mp: 267–269 °C; IR (neat, cm–1): 3371, 3216, 3037, 2947, 1698, 1490, 1249, 870, 775; 1H NMR (500 MHz, CDCl3): δ = 8.99 (s, 2H), 8.41 (dd, J = 3.2, 6.3 Hz, 2H), 7.76 (dd, J = 3.5, 6.5 Hz, 2H), 5.7 (s, 2H), 2.8 (s, 4H) ppm; 13C NMR (125 MHz, CDCl3): δ = 206.1, 148.3, 130.0, 129.7, 128.5, 124.7, 113.9, 59.7, 41.9 ppm; HRMS (ESI, Q-TOF): m/z calcd for C17H12NaO4 [M + Na]+ 303.0628, found 303.0627.
Acknowledgments
The authors are thankful to DRDO (Defence Research and Development Organisation) (Grant: ARDB/01/1041849/M/1), New Delhi, for the research grant. S.K. is indebted to the DST (Grant: SR/S2/JCB-33/2010) award of J. C. Bose fellowship and Praj industries for Chair Professorship (Green Chemistry). A.F. and S.R.C. gratefully acknowledge UGC (University Grants Commission), New Delhi, for the doctoral fellowships.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01178.
All spectral copies of 1H NMR, 13C NMR, and DEPT-135 NMR of new compounds along with HRMS spectra of compound 3F (PDF)
Author Contributions
† S.R.C. and A.F. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Chen Y.; Wendt-Pienkoski E.; Rajski S. R.; Shen B. In Vivo Investigation of the Roles of FdmM and FdmM1 in Fredericamycin Biosynthesis Unveiling a New Family of Oxygenases. J. Biol. Chem. 2009, 284, 24735–24743. 10.1074/jbc.M109.014191. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pandey R. C.; Toussaint M. W.; Stroshane R. M.; Kalita C. C.; Aszalos A. A.; Garretson A. L.; Wei T. T.; Byrne K. M.; Stroshane R. M.; White R. J. Fredericamycin A, a new antitumor antibiotic. I. Production, isolation and physicochemical properties. J. Antibiot. 1981, 34, 1389–1401. 10.7164/antibiotics.34.1389. [DOI] [PubMed] [Google Scholar]; c Misra R.; Pandey R. C.; Silverton J. V. Fredericamycin A, an Antitumor Antibiotic of a Novel Skeletal Type. J. Am. Chem. Soc. 1982, 104, 4478–4479. 10.1021/ja00380a025. [DOI] [Google Scholar]; d Byrne K. M.; Hilton B. D.; White R. J.; Misra R.; Pandey R. C. Biosynthesis of Fredericamycin A, a New Antitumor Antibiotic. Biochemistry 1985, 24, 478–486. 10.1021/bi00323a035. [DOI] [PubMed] [Google Scholar]; (e) Sontag B. Patent Purification Method for Fredericamycin A. WO2004024696, 2004.
- a Chen Y.; Luo Y.; Ju J.; Wendt-Pienkowski E.; Rajski S. R.; Shen B. Identification of Fredericamycin E from Streptomyces griseus: Insights into Fredericamycin A Biosynthesis Highlighting Carbaspirocycle Formation. J. Nat. Prod. 2008, 71, 431–437. 10.1021/np070664n. [DOI] [PubMed] [Google Scholar]; b Miosra R.; Pandey R. C.; Hilton B. D.; Roller P. P.; Silverton J. V. Structure of Fredericamycin A, an antitumor antibiotic of a novel skeletal type; spectroscopic and mass spectral characterization. J. Antibiot. 1987, 40, 786–802. 10.7164/antibiotics.40.786. [DOI] [PubMed] [Google Scholar]; c Clive D. L. J.; Sedgeworth J. Synthesis of Heterocyclic Compounds Related to Fredericamycin A-The Cyclopent[g] isoquinoline System. J. Heterocycl. Chem. 1987, 24, 509. 10.1002/jhet.5570240240. [DOI] [Google Scholar]
- a Warnick-Pickle D. J.; Byrne K. M.; Pandey R. C.; White R. J. Fredericamycin A, a new antitumor antibiotic. II. Biological properties. J. Antibiot. 1981, 34, 1402–1407. 10.7164/antibiotics.34.1402. [DOI] [PubMed] [Google Scholar]; b Latham M. D.; King C. K.; Gorycki P.; Macdonald T. L.; Ross W. E. Inhibition of topoisomerases by Fredericamycin A. Cancer Chemother. Pharmacol. 1989, 24, 167–171. 10.1007/BF00300237. [DOI] [PubMed] [Google Scholar]; c Dalal N. S.; Shi X. On the Formation of Oxygenated Radicals by Fredericamycin A and Implications to Its Anticancer Activity: An ESR Investigation. Biochemistry 1989, 28, 748–750. 10.1021/bi00428a050. [DOI] [PubMed] [Google Scholar]; (d) Simon W.; Abel U.. Fredericamycin Derivatives as Medicaments for Treating Tumours, Patent WO2004004713, 2004.
- a Hilton B. D.; Misra R.; Zweier J. L. Magnetic Resonance Studies of Fredericamycin A: Evidence for oxygen-dependent Free-Radical Formation. Biochemistry 2002, 25, 5533–5539. 10.1021/bi00367a028. [DOI] [PubMed] [Google Scholar]; b Misra R. Water soluble salts of Fredericamycin A: Preparation and biological activity. J. Antibiot. 1988, 41, 976–981. 10.7164/antibiotics.41.976. [DOI] [PubMed] [Google Scholar]; (c) Koichi Y.; Hiroshi H.; Tadashi N.; Takemitsu A.; Kenichi K.; Seiji I.; Toshiaki N.. Novel Fredericamycin A derivatives. US Patent US4584377, 1986.
- Boger D. L.; Hueter O.; Mbiya K.; Zhang M. Total Synthesis of Natural and ent-Fredericamycin A. J. Am. Chem. Soc. 1995, 117, 11839. 10.1021/ja00153a004. [DOI] [Google Scholar]
- Lu K. P.; Fischer G.. Methods of Inhibiting pin1-Associated states using a Fredericamycin a compound. Int. Pat. Appl. WO 2004002429, 2004.
- Abel U.; Simon W.; Eckard P.; Hansske F. G. Design and Semisynthesis of Novel Fredericamycin A Derivatives with an Improved Antitumor Profile. Bioorg. Med. Chem. Lett. 2006, 16, 3292–3297. 10.1016/j.bmcl.2006.03.029. [DOI] [PubMed] [Google Scholar]
- (a) Kelly T.R.; Li Q.; Lohray V.B.. Fredericamycin A Derivatives. US Patent US5166208, 1992;; b Kelly T. R.; Bell S. H.; Ohashi N.; Armstrong-Chong R. J. Synthesis of (±)-fredericamycin A. J. Am. Chem. Soc. 1988, 110, 6471–6480. 10.1021/ja00227a030. [DOI] [Google Scholar]; c Clive D. L. J.; Tao Y.; Khodabocus A.; Wu Y.-J.; Angoh A. G.; Bennett S. M.; Boddy C. N.; Bordeleau L.; Kellner D. Total Synthesis of Crystalline (±)-Fredericamycin A. Use of Radical Spirocyclization. J. Am. Chem. Soc. 1994, 116, 11275. 10.1021/ja00104a009. [DOI] [Google Scholar]; d Rama Rao A. V.; Singh A. K.; Rao B. V.; Reddy K. M. Synthesis of (±)-Fredericamycin A. Heterocycles 1994, 37, 1893. 10.3987/COM-93-S163. [DOI] [Google Scholar]; e Kita Y.; Higuchi K.; Yoshida Y.; Iio K.; Kitagaki S.; Akai S.; Fujioka H. Asymmetric Total Synthesis of Fredericamycin A. Angew. Chem., Int. Ed. Engl. 1999, 38, 683.. [DOI] [PubMed] [Google Scholar]
- Kelly T. R.; Ohashi N.; Armstrong-Chong R. J.; Bell S. H. Synthesis of (±)-fredericamycin A. J. Am. Chem. Soc. 1986, 108, 7100. 10.1021/ja00282a042. [DOI] [Google Scholar]
- a Sánchez-Larios E.; Holmes J. M.; Daschner C. L.; Gravel M. NHC-Catalyzed Spiro Bis-Indane Formation via Domino Stetter-Aldol-Michael and Stetter-Aldol-Aldol Reactions. Org. Lett. 2010, 12, 5772–5775. 10.1021/ol102685u. [DOI] [PubMed] [Google Scholar]; b Rama Rao A. V.; Reddeppa Reddy D.; Deshpande V. H. Methodology for the synthesis of the spiro[4.4]nonane system: an approach for the total synthesis of fredericamycin A. J. Chem. Soc., Chem. Commun. 1984, 1119–1120. 10.1039/C39840001119. [DOI] [Google Scholar]; c Eck G.; Julia M.; Pfeiffer B.; Rolando C. Access to the Spiro hydrindandione ring system of Fredericamycin A through a Friedel-Crafts reaction. Tetrahedron Lett. 1985, 26, 4723–4724. 10.1016/S0040-4039(00)94933-5. [DOI] [Google Scholar]
- Wendt J. A.; Gauvreau P. J.; Bach R. D. Synthesis of (±)-Fredericamycin A. J. Am. Chem. Soc. 1994, 116, 9921–9926. 10.1021/ja00101a013. [DOI] [Google Scholar]
- a Rama Rao A. V.; Reddy D. R.; Annapurna G. S.; Deshpande V. H. Synthesis of (±) Fredericamycin A. Tetrahedron Lett. 1987, 28, 451–454. [Google Scholar]; (b) Akai S.; Tsujino T.; Fukuda N.; Iio K.; Takeda Y.; Kawaguchi K.; Naka T.; Higuchi K.; Kita Y. Enantiodivergent Synthesis of Either Enantiomer of ABCDE-Ring Analogue of Antitumor Antibiotic Fredericamycin A via Intramolecular [4 + 2] Cycloaddition Approach. Org. Lett. 2001, 3, 4015–4018. [DOI] [PubMed] [Google Scholar]
- a Boger D. L.; Jacobson I. C. Tetrahedron Lett. 1989, 30, 2037. [Google Scholar]; b Boger D. L.; Jacobson I. C. Studies of the total synthesis of fredericamycin A. Preparation of key partial structures and development of an intermolecular alkyne-chromium carbene complex benzannulation cyclization approach to the ABCD(E) ring system. J. Org. Chem. 1990, 55, 1998–1928. [Google Scholar]
- Bach R. D.; Klix R. C. A Mercury-Mediated Acyl Migration in a Pinacol-Type Rearrangement. Model Studies toward the Synthesis of Fredericamycin A. J. Org. Chem. 1986, 51, 749–752. [Google Scholar]
- a Clive D. L. J.; Tao Y.; Khodabocus A.; Wu Y.-J.; Angoh A. G.; Bennett S. M.; Boddy C. N.; Bordeleau L.; Kellner D.; Kleiner G.; Middleton D. S.; Nichols C. J.; Richardson S. R.; Vernon P. G. Total Synthesis of (±)-Fredericamycin A. Use of Radical Spirocyclization. J. Chem. Soc., Chem. Commun. 1992, 1489–1490. [Google Scholar]; b Kende A. S.; Ebetino F. H.; Ohta T. Synthesis of the Spirocyclic Centre of Fredericamycin A by Phenoxy-Enoxy Radical Coupling. Tetrahedron Lett. 1985, 26, 3063–3066. [Google Scholar]; c Clive D. L. J.; Angoh A. G.; Bennett S. M. Radical Spirocyclization: Synthesis of an Appropriately Oxygenated Spiro Compound Related to the Antitumor Antibiotic Fredericamycin A. J. Org. Chem. 1987, 52, 1339–1342. [Google Scholar]; d Rama Rao A. V.; Singh A. K.; Rao B. V.; Reddy K. M. Synthesis of (±) Fredericamycin A. Tetrahedron Lett. 1993, 34, 2665–2668. [Google Scholar]
- Mehta G.; Subrahmanyam D. Model Studies Towards Fredericaycin A. Protocol for the Rapid Creation of the Spirocyclic Centre. Tetrahedron Lett. 1987, 28, 479. [Google Scholar]
- a Toyota M.; Terashima S. A Novel Synthesis of the Basic Carbon Framework of Fredericamycin A. Promising Routes for the Spiro Chiral Centre Construction of the CD-Ring System. Tetrahedron Lett. 1989, 30, 829–832. [Google Scholar]; b Bach R. D.; Klix R. C. Model Studies Aimed at the Fredericamycin A. A Simple O-Quinodimethane Route to the Spiro Napthalene Portion. Tetrahedron Lett. 1986, 27, 1983–1986. [Google Scholar]; c Boger D. L. Azadiene Diels-Alder Reactions. Total Synthesis of Natural and ent- Fredericamycin A. J. Heterocyclic Chem. 1996, 33, 1519–1531. [Google Scholar]
- a Evans P. A.; Brandt T. A. Palladium Catalyzed Cross-Coupling Acylation Approach to the Antitumor Antibiotic Fredericamycin A. J. Org. Chem. 1995, 60, 2298. [Google Scholar]
- a Nicolaou K. C.; Snyder S. A.; Montagnon T.; Vassilikogiannakis G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [DOI] [PubMed] [Google Scholar]; b Kotha S.; Chavan A. S.; Goyal D. Diversity-Oriented Approaches to Polycyclics and Bioinspired Molecules via the Diels–Alder Strategy: Green Chemistry, Synthetic Economy, and Beyond. ACS Comb. Sci. 2015, 17, 253–302. [DOI] [PubMed] [Google Scholar]; c Kotha S.; Ravikumar O. Design and synthesis of oxa-bowls via Diels–Alder reaction and ring-rearrangement metathesis as key steps. Tetrahedron Lett. 2014, 55, 5781–5784. [Google Scholar]; d Corey E. J. Catalytic Enantioselective Diels–Alder Reactions: Methods, Mechanistic Fundamentals, Pathways, and Applications. Angew. Chem. Int. Ed. 2002, 41, 1650–1667. [DOI] [PubMed] [Google Scholar]
- a Hiersemann M.; Nubbemeyer U.. The Claisen Rearrangement: Methods and Applications, Eds.; Wiley-VCH, 2007; p. 591; [Google Scholar]; b Kotha S.; Krishna N. G.; Halder S.; Misra S. A synergistic approach to polycyclics via a strategic utilization of Claisen rearrangement and olefin metathesis. Org. Biomol. Chem. 2011, 9, 5597–5624. [DOI] [PubMed] [Google Scholar]; c Martín Castro A. M. Claisen Rearrangement over the Past Nine Decades. Chem. Rev. 2004, 104, 2939–3002. 10.1021/cr020703u. [DOI] [PubMed] [Google Scholar]; d Kotha S.; Mandal K.; Deb A. C.; Banerjee S. Microwave-assisted Claisen Rearrangement on a Silica gel Support. Tetrahedron Lett. 2004, 45, 9603–9605. 10.1016/j.tetlet.2004.11.012. [DOI] [Google Scholar]; e Kotha S.; Mandal K. A new protocol for benzoannulation by double Claisen rearrangement and ring-closing metathesis reactions as key steps. Tetrahedron Lett. 2004, 45, 2585–2588. 10.1016/j.tetlet.2004.01.149. [DOI] [Google Scholar]
- a Grubbs R. H.; Wenzel A. G.. Handbook of Metathesis; Wiley-VCH: Weinheim, 2015; Vol.1; [Google Scholar]; b Kotha S.; Lahiri K. Synthesis of Diverse Polycyclic Compounds via Catalytic Metathesis. Synlett 2007, 2767–2784. 10.1055/s-2007-990954. [DOI] [Google Scholar]; c Kotha S.; Dipak M. K. Strategies and tactics in olefin metathesis. Tetrahedron 2012, 68, 397–421. 10.1016/j.tet.2011.10.018. [DOI] [Google Scholar]; d Kotha S.; Brahmachary E. Synthesis of conformationally constrained α-amino acid derivatives using ethyl isocyanoacetate as glycine equivalent. Bioorg. Med. Chem. Lett. 1997, 7, 2719–2722. 10.1016/S0960-894X(97)10075-0. [DOI] [Google Scholar]; e Kotha S.; Manivannan E.; Sreenivasachary N.; Ganesh T.; Deb A. Spiro-annulation via ring-closing metathesis reaction. Synlett 1999, 1618–1620. [Google Scholar]
- a Ichihara A. Retro-Diels-Alder Strategy in Natural Product Synthesis. Synthesis 1987, 207–222. 10.1055/s-1987-27894. [DOI] [Google Scholar]; b Kotha S.; Gunta R. A new synthetic strategy to 2,3-diallyl-1,4-quinones via one-pot double Claisen rearrangement and retro Diels–Alder reaction. Tetrahedron Lett. 2016, 57, 3021–3023. 10.1016/j.tetlet.2016.05.101. [DOI] [Google Scholar]; c Kotha S.; Banerjee S. Recent developments in the retro-Diels–Alder reaction. RSC Adv. 2013, 3, 7642–7666. 10.1039/c3ra22762f. [DOI] [Google Scholar]
- a Anliker R.; Lindsey A. S.; Nettleton D. E. Jr.; Turner R. B. A Synthetic Approach to Polycyclic Hydroaromatic Systems Related to the 19-Norsteroids. J. Am. Chem. Soc. 1957, 79, 220–226. 10.1021/ja01558a058. [DOI] [Google Scholar]; b Kitani Y.; Morita A.; Kumamoto T.; Ishikawa T. Synthetic Studies on Kinamycin Antibiotics: Synthesis of a Trioxygenated Benz[f]indenone and its Diels ± Alder Reaction to a Kinamycin Skeleton. Helv. Chim. Acta 2002, 85, 1186–1195. . [DOI] [Google Scholar]; c Etomi N.; Kumamoto T.; Nakanishi W.; Ishikawa T. Diels–Alder reactions using 4,7-dioxygenated indanones as dienophiles for regioselective construction of oxygenated 2,3-dihydrobenz[f]indenone skeleton. J. Org. Chem. 2008, 4, 15. 10.3762/bjoc.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Coutts R.; Malicky J. L. The Synthesis of Analogs of the Hallucinogen 1-(2,5-Dimethoxy-4-methylphenyl)-2-aminopropane (DOM). II. Some Ring-methoxylated 1-Amino-and 2-Aminoindanes. Can. J. Chem. 1974, 52, 381–389. 10.1139/v74-061. [DOI] [Google Scholar]; e Kotha S.; Cheekatla S. R.; Mandal B. Synthesis and Rearrangement of Cage [4.3.2]Propellanes that Contain a Spiro Linkage. Eur. J. Org. Chem. 2017, 4277–4282. 10.1002/ejoc.201700617. [DOI] [Google Scholar]; f Kotha S.; Cheekatla S. R.; Milind M. Synthetic approach to Oxa-cage systems via Ring-Closing Metathesis. Heterocycles 2018, 97, 1008. 10.3987/COM-18-S(T)83. [DOI] [Google Scholar]; g Koo J.; Fish M. S.; Walker G. N.; Blake 2,3-Dimethoxycinnamic acid. J. Org Synth. 1963, 4, 327. [Google Scholar]
- Essiz S.; Dalkilic E.; Sari O.; Dastan A.; Balci M. Unexpected regioselectivity observed in the bromination and epoxidation reactions of p-benzoquinone-fused norbornadiene: An experimental and computational study. Tetrahedron 2017, 73, 1640–1649. 10.1016/j.tet.2017.02.017. [DOI] [Google Scholar]
- Hoffmann R. W. Protecting-Group-Free-Synthesis. Synthesis 2006, 3531–3541. 10.1055/s-2006-950311. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.