Abstract
Introduction
We compared peripapillary retinal nerve fiber layer and macular thickness measurements in patients with mild cognitive impairment (MCI) and control subjects using swept-source optical coherence tomography (SS-OCT). We also assessed the relationship between SS-OCT measurements and the severity of cognitive impairment.
Methods
Peripapillary retinal nerve fiber layer and macular thickness were measured in 23 patients and 24 control subjects using SS-OCT. Cognitive status was assessed using the Mini-Mental State Examination, the Montreal Cognitive Assessment, and the Pfeffer Questionnaire.
Results
Most inner retinal layer thickness parameters were significantly smaller in patients with MCI, especially macular ganglion cell complex thickness measurements. Mini-Mental State Examination and Montreal Cognitive Assessment findings were significantly correlated with most macular thickness parameters.
Discussion
The SS-OCT–measured inner retinal layers of patients with MCI displayed thinning, especially in the central macular area. SS-OCT technology can provide useful information on ocular involvement patterns and holds promise as an ocular biomarker in this patient population.
Keywords: Mild cognitive impairment, Dementia, Alzheimer's disease, Optical coherence tomography, Swept-source, Macula, Retina, Optic nerve, Retinal nerve fiber layer, Ganglion cell layer
Highlights
-
•
Inner retinal layer thickness was reduced in patients with mild cognitive impairment.
-
•
Macular ganglion cell layer complex was the most affected retinal layer.
-
•
Peripapillary retinal nerve fiber layer thickness was not significantly reduced.
-
•
Cognitive tests significantly correlated with macular parameters.
1. Introduction
Mild cognitive impairment (MCI) is a cognitive decline more accentuated than expected for the age, but not enough to compromise daily life activities [1]. One of the main concerns for patients with MCI is the potential risk of conversion to Alzheimer's disease (AD), especially the amnestic form of MCI (aMCI). The rate of conversion to dementia is 1% to 2% per year in the general population, 5% to 10% in subjects with MCI [2], and up to 50% in 30 months for aMCI [3]. Yet, many patients with MCI remain stable and do not develop dementia. Although the mechanisms responsible for the onset and progression of MCI have been subject of many studies, questions remain, especially about the transition from MCI to AD and predicting conversion.
In this scenario, the development of new biomarkers for the diagnosis and follow-up of patients with MCI is crucial. Optical coherence tomography (OCT) is a noninvasive technology, which can acquire high-resolution, in vivo cross-sectional images and quantitative and reproducible measurements of both the optic disc and the macula. As such, it has become an extremely useful diagnostic tool in many ocular conditions [4], [5]. Moreover, OCT parameters have been proposed as biomarkers in certain neurodegenerative diseases [6], [7], [8].
Over the last two decades, OCT technology has been used to evaluate changes in peripapillary retinal nerve fiber layer (pRNFL) and macular thickness parameters in AD [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Advances in OCT technology, especially the advent of Fourier-domain OCT (FD-OCT) and, more recently, swept-source OCT (SS-OCT), have increased the acquisition speed and resolution of retinal images, making accurate quantitative segmented retinal layer analyses possible [22]. In fact, several authors have concluded that the inner retinal layers, especially the ganglion cell/inner plexiform layer (GC-IPL), are affected in patients with AD [8], [23], [24], [25], [26], [27], [28]. In view of these observations, OCT assessments of peripapillary and macular parameters are likely helpful in the monitoring disease course progression in patients with AD.
If MCI represents an early stage of AD, then one would expect AD-related optic disc and retinal changes on OCT to be present in patients with MCI to a lesser extent, but the literature is inconsistent on this point. Many authors have found pRNFL thickness to be reduced in MCI [12], [13], [21], [26], [29], whereas other groups have reported macular thinning in patients with MCI mostly based on full macular thickness measurements [12], [13], [17], [21], [29], [30], [31]. Some authors have demonstrated thinning of the GC-IPL in patients with MCI [26], [27], [32], whereas others have failed to detect any significant difference in pRNFL, full macular, and GC-IPL thickness parameters [33], [34].
The recent introduction of SS-OCT technology promises to shed new light on retinal abnormalities in subjects with MCI. SS-OCT scanners use an optimized long wavelength scanning light (1050 nm) and have shorter acquisition time (100,000 A-scans/second) and higher axial resolution (2 μc) than do FD-OCT scanners. This facilitates visualization and retinal layer segmentation, with potential impact on the assessment of retinal integrity in patients with MCI.
It is also important to determine what is the role of OCT data in clinical practice and to define whether such measurements correlate with the cognitive impairment in patients with MCI. The Mini-Mental State Examination (MMSE) is the test used in most studies assessing the correlation between OCT parameters and cognitive status in AD [10], [14], [35]. Few authors have conducted similar studies on MCI, and their results have been inconsistent; some authors have found significant correlations between cognitive status and OCT parameters [8], [36], whereas others have not [15], [17], [30]. However, the use of other cognitive tests might clarify the relationship between OCT and cognitive parameters in patients with MCI. Although MMSE is the most commonly used cognitive test in dementia, the Montreal Cognitive Assessment (MoCA) seems to be more sensitive than MMSE, especially in the early stages of AD or in patients with MCI.
Thus, the purpose of this study was to evaluate the diagnostic ability of SS-OCT–measured pRNFL, total macular, and inner retinal layer thickness parameters to differentiate patients with aMCI from normal age-matched control subjects, and to evaluate the correlation between SS-OCT parameters and the level of cognitive impairment in patients with aMCI, as determined by the MMSE, the MoCA, and the Pfeffer Daily Functional Life Activities Questionnaire.
2. Methods
2.1. Study design
In this cross-sectional study, patients with aMCI and normal age-matched control subjects were recruited between February and September 2017. The study protocol followed the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee of the Federal University of Juiz de Fora, Minas Gerais, Brazil (protocol no. 1.950.667). All participants gave their written informed consent.
2.2. Participants
All patients were submitted to a complete physical examination and cognitive tests using MMSE and MoCA [37]. To be eligible, patients had to be diagnosed with MCI by a neurologist, according to previously described criteria [38]. Illiterate patients and those with <4 years of schooling were excluded. Functionality was evaluated with a daily life functional activities questionnaire administered to the patient's companion [39].
Patients underwent a complete ophthalmologic examination, performed by two experienced ophthalmologists (L.P.C. and L.V.F.C.C.). The inclusion criteria were (1) previous computed tomography or magnetic resonance imaging of the brain ruling out other causes of cognitive impairment, (2) age between 55 and 85 years, (3) best-corrected visual acuity of 20/20 in the included eye, (4) refractive error smaller than 5 spherical diopters and 3 cylindrical diopters, (5) intraocular pressure <22 mm Hg, (6) absence of ophthalmoscopic signs of diabetic retinopathy, vascular retinal occlusions, and macular disease, (7) absence of ophthalmoscopic signs of glaucomatous optic neuropathy, (8) absence of optical opacities, (9) history of ophthalmologic surgery, except for uncomplicated cataract surgery, performed at least 6 months previously, and (10) good collaboration with the OCT scan. The exclusion criteria were (1) insufficient collaboration to perform the OCT scan, (2) evidence of simultaneous noncompensated organic or metabolic brain injury, (3) history of acute myocardial infarction, stroke, or renal failure, (4) heart failure or severe cardiac arrhythmia, and (5) history of continuous use of benzodiazepines or abusive consumption of illicit drugs and alcohol.
The control group, normal individuals were recruited from the staff members of the Juiz de Fora Eye Hospital. All control subjects underwent a complete ophthalmologic examination. The inclusion criteria were (1) age match with study subject (±5 years), (2) best-corrected visual acuity of 20/20 and refraction within 5 spherical diopters and 3 cylindrical diopters, (3) intraocular pressure <22 mm Hg, (4) optic disc and macula with normal appearance, (5) no history of eye disease, and (6) absence of systemic diseases. Also, for the control group, the included participants should have no clinical signs of AD or MCI.
2.3. SS-OCT imaging
After pupil dilation with two drops of 1% tropicamide, the optic nerve head and the macula of all participants were scanned using an SS-OCT device (DRI OCT Triton Topcon Corp, Tokyo, Japan). Using a three-dimensional protocol, high-resolution (512 × 256 A-scans) images of the optic disc (6 × 6 mm) and the macula (7 × 7 mm) were acquired. The examiner reviewed the objective and subjective quality, rejecting images with a quality index less than 60. Images with abrupt eye movements causing image artifacts or black lines because of eye blinking were excluded. The SS-OCT images were automatically segmented and the pRNFL, full macula, and segmented inner retinal layer thickness in both eyes was automatically calculated.
pRNFL parameters were analyzed with the three-dimensional optic disc report protocol, based on a 3.4-mm-diameter circle around the optic disc. Thickness values were automatically calculated and divided into four quadrants (temporal, superior, inferior, and nasal).
Full-thickness retinal measurements were automatically calculated for the macula, according to the Early Treatment Diabetic Retinopathy Study (ETDRS) map and for the following nine sectors: fovea, temporal inner, superior inner, nasal inner, inferior inner, temporal outer, superior outer, nasal outer, and inferior outer. Global average macular thickness was calculated as the weighted average of sector macular thickness measurements, as described elsewhere [40].
The anatomic boundaries of the inner retinal layers were automatically defined by the built-in software. An experienced examiner (L.P.C) evaluated each scan and, if errors in the automatic segmentation were observed, repeated the acquisition process, avoiding manual correction. The following three parameters were analyzed: (1) mean macular retinal nerve fiber layer (mRNFL) thickness, (2) mean GC-IPL thickness, and (3) mean ganglion cell complex (GCC; GC-IPL plus mRNFL) thickness. The boundaries of the inner retinal layers were automatically identified by the software. mRNFL thickness was measured from the internal limiting membrane to the inner boundary of the ganglion cell layer (GCL). GC-IPL thickness was measured from the inner boundary of the GCL to the outer boundary of the IPL. GCC thickness was measured from the internal limiting membrane to the outer boundary of the IPL.
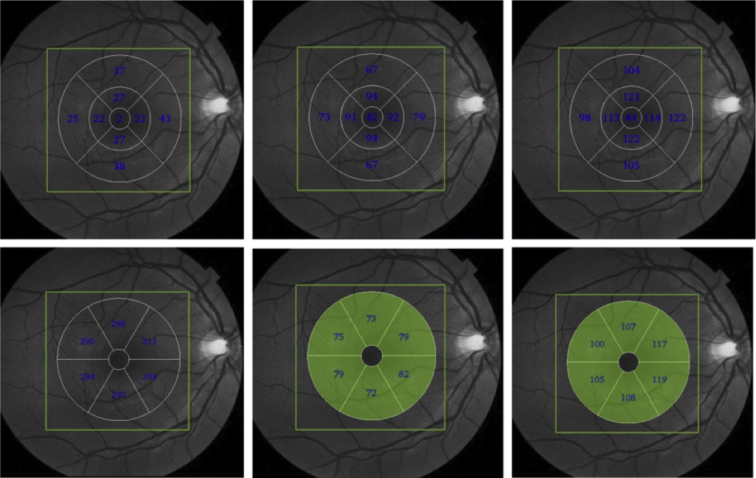
After segmentation, the mean mRNFL, GC-IPL, and GCC values were automatically calculated for three patterns of macular analysis (Fig. 1). Global macular thickness is the mean value of these three parameters within a scanning area of 6 × 6 mm. We also analyzed the mean values of these three inner retinal layers divided into nine sectors according to the ETDRS map (Fig. 1). The third form of analysis was based on a 6-mm-diameter map centered on the fovea and divided into six sectors, each measuring 60°: upper, upper temporal, upper nasal, lower, lower temporal, and lower nasal (Fig. 1). The fovea was excluded from the analysis of inner retinal layer thickness divided into six or nine sectors.
Fig. 1.
Maps used in the analysis of the inner retinal layers. Top panel: maps with inner retinal layer thickness values (μm) divided into nine sectors according to the ETDRS map: mRNFL (left), GC-IPL (center), and GCC (right). Bottom panel: maps with inner retinal layer thickness values (μm) divided into six sectors: mRNFL (left), GC-IPL (center), and GCC (right). Abbreviations: ETDRS, Early Treatment Diabetic Retinopathy Study; GCC, ganglion cell complex; GC-IPL, ganglion cell/inner plexiform layer; mRNFL, macular retinal nerve fiber layers.
2.4. Cognitive assessment
Cognitive performance was assessed with three different tests: patients with MCI were submitted to the MMSE and the MoCA, whereas their companions answered the Pfeffer Daily Functional Life Activities Questionnaire. All cognitive measurements were performed by a clinical neuropsychologist (A.L.M.A.).
2.5. Statistical methods
Findings were expressed as the mean values ± standard deviation (±SD) for normally distributed parameters and as median and quartiles for non-normally distributed parameters. Normality was assessed by histogram analysis and the Shapiro-Wilk test. OCT parameters from the two groups were compared using generalized estimating equation models to account for intereye dependencies. Receiver operating characteristic curves were used to describe the ability of OCT parameters to discriminate patients with MCI from age-matched eyes. For each parameter, sensitivities at fixed specificities of 80% and 95% were calculated. The ability of OCT parameters to differentiate MCI eyes from normal was represented by the area under the receiver operating characteristic curve. Differences between correlations of nominal variables were evaluated with the χ2 test. Pearson's correlation analysis was used to evaluate the correlation between cognitive tests and OCT parameters. P values < .05 were considered statistically significant. All statistical analyses were performed with the software IBM Statistical Package for the Social Sciences Statistics v. 24.0.
3. Results
Forty-six eyes of 23 patients with MCI and 48 eyes of 24 control subjects were included. The mean age ± SD was 67.43 ± 7.07 years for patients with MCI and 64.58 ± 9.48 years for control subjects (P = .103). There were 19 and 16 females in MCI and control groups, respectively (P = .525, χ2 test). The mean time of diagnosis was 4.33 ± 3.42 years and patients' mean level of schooling was 7.22 ± 3.82 years. The mean cognitive scores ± SD were 27.86 ± 1.98 (MMSE), 21.43 ± 5.72 (MoCA), and 2.13 ± 1.89 (Pfeffer).
Patients and control subjects did not differ significantly with regard to pRNFL thickness (Table 1). Full macular thickness was significantly smaller in patients only in the temporal and inferior inner sectors (P = .03 for both) (Table 1). Global macular thickness was smaller in patients with MCI, with significant differences observed for mRNFL (P = .04) and GCC (P = .03) (Table 2). The three inner macular thickness parameters, divided into nine sectors, were also analyzed (Table 2). Mean mRNFL thickness was significantly smaller in patients with MCI in the superior inner sector (P = .03) and the inferior outer sector (P = .01). Mean GC-IPL thickness was significantly smaller in patients with MCI in the superior inner sector (P = .02), the inferior inner sector (P = .01), and the nasal inner sector (P = .03). The macular GCC thickness was significantly smaller in patients with MCI in all sectors, except for the superior, temporal, and nasal outer sectors (Table 2).
Table 1.
SS-OCT–measured mean (±SD) pRNFL and full macular thickness values (in μc) of patients with MCI and control subjects, divided into nine sectors according to the ETDRS map, and their respective AUC (±SD) values
| Parameters | MCI (n = 46) | Control subjects (n = 48) | P | AUC | Sensitivity/specificity |
|
|---|---|---|---|---|---|---|
| Specificity ≥ 95% | Specificity ≥ 80% | |||||
| pRNFL | ||||||
| Average thickness | 103.50 (2.42) | 103.77 (2.00) | .93 | 0.53 | 10/96 | 25/80 |
| Superior | 125.52 (3.10) | 128.02 (3.38) | .59 | 0.54 | 13/96 | 33/80 |
| Temporal | 68.02 (2.26) | 72.54 (2.20) | .15 | 0.60 | 6/96 | 35/83 |
| Inferior | 135.48 (4.29) | 134.37 (2.83) | .83 | 0.58 | 6/96 | 13/80 |
| Nasal | 85.39 (2.45) | 80.15 (2.42) | .13 | 0.62 | 4/96 | 10/80 |
| Full macular thickness | ||||||
| Average thickness | 268.95 (3.08) | 275.73 (2.26) | .08 | 0.62 | 13/96 | 31/80 |
| Superior inner | 301.13 (3.77) | 309.37 (2.44) | .07 | 0.67 | 0/96 | 33/80 |
| Temporal inner | 288.83 (3.33) | 297.73 (2.35) | .03 | 0.69 | 10/96 | 38/80 |
| Inferior inner | 298.33 (3.69) | 307.69 (2.39) | .03 | 0.67 | 2/96 | 33/80 |
| Nasal inner | 301.35 (3.72) | 309.00 (2.63) | .09 | 0.66 | 0/96 | 21/80 |
| Superior outer | 264.00 (3.00) | 270.08 (2.70) | .13 | 0.61 | 17/96 | 33/83 |
| Temporal outer | 248.52 (2.81) | 254.83 (2.76) | .11 | 0.61 | 15/94 | 35/83 |
| Inferior outer | 252.61 (3.17) | 259.29 (2.20) | .08 | 0.60 | 4/98 | 33/80 |
| Nasal outer | 276.96 (3.69) | 282.94 (2.37) | .17 | 0.58 | 6/96 | 17/83 |
| Fovea | 228.54 (4.52) | 234.58 (3.35) | .28 | 0.61 | 0/96 | 31/80 |
NOTE. Statistically significant values (P < .05) are in bold.
Abbreviations: AUC, area under the receiver operating characteristic curve; ETDRS, Early Treatment Diabetic Retinopathy Study; MCI, mild cognitive impairment; P, generalized estimating equations; pRNFL, peripapillary retinal nerve fiber layer; SD, standard deviation; SS-OCT, swept-source optical coherence tomography.
Table 2.
SS-OCT–measured mean (±SD) global macular thickness (in μc) and inner retinal layer thickness (6 × 6 mm) of patients with mild cognitive impairment (MCI) and control subjects, divided into nine sectors according to the ETDRS map, and their respective AUC (±SD) values
| Inner retinal layer parameters | MCI (n = 46) | Control subjects (n = 48) | P | AUC | Sensitivity/specificity |
|
|---|---|---|---|---|---|---|
| Specificity ≥ 95% | Specificity ≥ 80% | |||||
| Global macula | ||||||
| RNFL | 36.80 (1.01) | 39.33 (0.64) | .04 | 0.70 | 4/96 | 52/80 |
| GC-IPL | 62.78 (0.95) | 64.67 (0.83) | .14 | 0.61 | 17/96 | 27/80 |
| GCC | 99.65 (1.63) | 104.00 (1.19) | .03 | 0.66 | 19/96 | 27/80 |
| mRNFL 9 ETDRS sectors | ||||||
| Average thickness | 34.21 (0.92) | 35.51 (0.50) | .22 | 0.66 | 10/93 | 44/80 |
| Superior inner | 26.83 (0.40) | 28.00 (0.36) | .03 | 0.66 | 6/96 | 38/74 |
| Temporal inner | 19.24 (0.43) | 19.19 (0.41) | .93 | 0.51 | 2/93 | 17/80 |
| Inferior inner | 27.67 (0.45) | 28.72 (0.40) | .08 | 0.66 | 4/98 | 32/80 |
| Nasal inner | 22.76 (0.39) | 23.06 (0.41) | .60 | 0.57 | 4/98 | 19/76 |
| Superior outer | 39.13 (1.08) | 40.50 (0.92) | .34 | 0.61 | 17/96 | 33/80 |
| Temporal outer | 21.33 (0.59) | 21.92 (0.36) | .39 | 0.54 | 2/96 | 25/85 |
| Inferior outer | 38.56 (1.02) | 41.75 (0.70) | .01 | 0.68 | 17/93 | 25/85 |
| Nasal outer | 46.89 (1.40) | 49.83 (1.04) | .09 | 0.63 | 2/96 | 35/82 |
| GC-IPL 9 ETDRS sectors | ||||||
| Average thickness | 68.54 (1.06) | 70.56 (0.89) | .14 | 0.61 | 17/96 | 23/80 |
| Superior inner | 86.30 (1.59) | 90.87 (1.24) | .02 | 0.70 | 2/96 | 33/80 |
| Temporal inner | 83.43 (1.44) | 87.00 (1.39) | .08 | 0.71 | 2/96 | 25/83 |
| Inferior inner | 85.87 (1.60) | 90.83 (1.12) | .01 | 0.69 | 0/96 | 21/83 |
| Nasal inner | 87.00 (1.57) | 91.46 (1.28) | .03 | 0.68 | 6/96 | 25/80 |
| Superior outer | 61.59 (1.05) | 63.27 (1.10) | .27 | 0.57 | 15/96 | 29/85 |
| Temporal outer | 66.13 (1.08) | 69.19 (1.20) | .06 | 0.64 | 10/98 | 23/83 |
| Inferior outer | 59.49 (1.04) | 60.58 (1.06) | .46 | 0.56 | 6/96 | 21/83 |
| Nasal outer | 66.67 (1.26) | 67.73 (1.13) | .53 | 0.55 | 4/96 | 23/80 |
| GCC 9 ETDRS sectors | ||||||
| Average thickness | 102.22 (1.56) | 106.35 (1.20) | .04 | 0.66 | 8/96 | 27/80 |
| Superior inner | 113.13 (1.84) | 119.62 (1.22) | <.01 | 0.72 | 0/96 | 29/80 |
| Temporal inner | 102.50 (1.60) | 107.25 (1.08) | .01 | 0.70 | 13/96 | 35/83 |
| Inferior inner | 114.09 (1.96) | 119.39 (1.30) | .02 | 0.69 | 0/96 | 25/80 |
| Nasal inner | 109.85 (1.79) | 114.48 (1.50) | .05 | 0.67 | 4/98 | 38/83 |
| Superior outer | 100.61 (1.69) | 104.04 (1.62) | .14 | 0.61 | 6/96 | 29/83 |
| Temporal outer | 87.46 (1.37) | 90.56 (1.22) | .09 | 0.62 | 10/03 | 17/85 |
| Inferior outer | 97.96 (1.71) | 102.39 (1.34) | .04 | 0.63 | 10/96 | 17/80 |
| Nasal outer | 113.76 (2.09) | 117.94 (1.50) | .11 | 0.61 | 6/96 | 33/80 |
NOTE. Statistically significant values (P < .05) are in bold.
Abbreviations: AUC, area under the receiver operating characteristic curve; ETDRS, Early Treatment Diabetic Retinopathy Study; GCC, ganglion cell complex; GC-IPL, ganglion cell layer plus inner plexiform layer; mRNFL, macular retinal nerve fiber layers; P, generalized estimating equations; SD, standard deviation; SS-OCT, swept-source optical coherence tomography.
The mRNFL, GC-IPL, and GCC were also divided into six sectors and analyzed (Table 3). mRNFL thickness was significantly smaller in patients with MCI in the inferior (P = .02) and nasal inferior (P = .04) sectors, whereas GC-IPL thickness was significantly smaller in the temporal superior (P = .04) and inferior (P = .03) sectors, and GCC thickness was significantly smaller in the inferior (P = .03) and the nasal inferior (P = .04) sectors.
Table 3.
SS-OCT–measured mean thickness ± SD (in μc) of the inner retinal layers divided into six sectors, and the corresponding AUC values ± SD of patients with MCI and control subjects
| Inner retinal layer parameters | MCI (n = 46) | Control subjects (n = 48) | P | AUC | Sensitivity/Specificity |
|
|---|---|---|---|---|---|---|
| Specificity ≥ 95% | Specificity ≥ 80% | |||||
| mRNFL 6 sectors | ||||||
| Superior | 36.74 (0.93) | 38.27 (0.69) | .18 | 0.62 | 13/96 | 33/78 |
| Temporal superior | 22.02 (0.55) | 22.25 (0.39) | .73 | 0.53 | 0/96 | 19/78 |
| Nasal superior | 41.28 (1.06) | 43.58 (0.80) | .08 | 0.65 | 11/96 | 28/85 |
| Inferior | 36.17 (0.87) | 38.69 (0.57) | .02 | 0.67 | 4/93 | 23/85 |
| Temporal inferior | 23.35 (0.54) | 23.98 (0.32) | .32 | 0.54 | 2/100 | 11/87 |
| Nasal inferior | 42.24 (1.29) | 45.52 (0.89) | .04 | 0.67 | 4/93 | 36/85 |
| GC-IPL 6 sectors | ||||||
| Superior | 62.02 (1.08) | 69.58 (1.01) | .08 | 0.62 | 23/96 | 28/83 |
| Temporal superior | 68.87 (1.09) | 71.89 (0.95) | .04 | 0.65 | 13/96 | 28/83 |
| Nasal superior | 70.85 (1.21) | 72.73 (1.05) | .24 | 0.58 | 17/93 | 25/80 |
| Inferior | 65.00 (1.13) | 66.67 (0.95) | .26 | 0.59 | 4/96 | 25/80 |
| Temporal inferior | 69.56 (1.10) | 72.69 (0.96) | .03 | 0.67 | 15/98 | 30/84 |
| Nasal inferior | 69.96 (1.25) | 71.67 (1.06) | .30 | 0.58 | 4/96 | 19/83 |
| GCC 6 sectors | ||||||
| Superior | 103.69 (1.69) | 107.83 (1.49) | .07 | 0.65 | 11/96 | 28/83 |
| Temporal superior | 90.96 (1.30) | 94.23 (1.14) | .06 | 0.64 | 8/96 | 21/80 |
| Nasal superior | 112.19 (1.83) | 116.21 (1.41) | .08 | 0.63 | 4/96 | 25/85 |
| Inferior | 101.13 (1.69) | 105.35 (1.06) | .03 | 0.64 | 13/96 | 19/83 |
| Temporal inferior | 93.39 (1.47) | 96.56 (1.04) | .08 | 0.63 | 8/96 | 19/83 |
| Nasal inferior | 111.91 (2.07) | 117.14 (1.41) | .04 | 0.65 | 8/93 | 34/80 |
NOTE. Statistically significant values (P < .05) are in bold.
Abbreviations: AUC, area under the receiver operating characteristic curve; GCC, ganglion cell complex; GC-IPL, ganglion cell layer plus inner plexiform layer; MCI, mild cognitive impairment; mRNFL, macular retinal nerve fiber layer; P, generalized estimating equations; SD, standard deviation; SS-OCT, swept-source optical coherence tomography.
Table 4 shows the correlations observed between OCT parameters and cognitive test scores. Significant coefficients were found between average pRNFL thickness and MMSE scores (r = 0.32, P = .03) or MoCA scores (r = 0.34, P = .02), between temporal and nasal sector thickness and MMSE scores (r = 0.32, P = .03), and between superior sector thickness and MoCA scores (r = 0.30, P = .04). All four inner macular thickness sectors were significantly correlated with MMSE scores (r values ranging from 0.32 to 0.38, P < .05). Correlations between full macular thickness and MoCA scores were all significant (r values ranging from 0.38 to 0.49, P ≤ .01), except for the temporal outer sector. No significant correlations were found between OCT parameters and Pfeffer scores (Table 4).
Table 4.
Correlations between cognitive scores of patients with MCI and SS-OCT–measured pRNFL, full macular thickness divided into nine sectors, global macular thickness and inner retinal layer thickness (μc) divided into nine and six sectors
| Parameters | MMSE | P | MoCA | P | Pfeffer | P |
|---|---|---|---|---|---|---|
| pRNFL | ||||||
| Average thickness | 0.32 | .03 | 0.34 | .02 | −0.11 | .45 |
| Superior | 0.11 | .47 | 0.30 | .04 | −0.01 | .92 |
| Temporal | 0.32 | .03 | 0.14 | .37 | −0.28 | .06 |
| Inferior | 0.24 | .10 | 0.29 | .05 | −0.03 | .87 |
| Full macular thickness—9 sectors | ||||||
| Average thickness | 0.24 | .10 | 0.44 | <.01 | −0.15 | .32 |
| Superior inner | 0.32 | .03 | 0.44 | <.01 | −0.17 | .27 |
| Temporal inner | 0.33 | .03 | 0.49 | <.01 | −0.21 | .17 |
| Inferior inner | 0.38 | .01 | 0.48 | <.01 | −0.12 | .43 |
| Nasal inner | 0.37 | .01 | 0.47 | <.01 | −0.17 | .27 |
| Superior outer | 0.24 | .11 | 0.42 | <.01 | −0.13 | .37 |
| Temporal outer | 0.08 | .58 | 0.28 | .06 | −0.03 | .86 |
| Inferior outer | 0.14 | .34 | 0.38 | .01 | −0.08 | .61 |
| Nasal outer | 0.27 | .08 | 0.48 | <.01 | −0.26 | .08 |
| Fovea | 0.08 | .58 | 0.38 | .01 | −0.07 | .67 |
| Global macular | ||||||
| mRNFL | 0.15 | .34 | 0.17 | .26 | 0.08 | .62 |
| GC-IPL | 0.40 | .01 | 0.41 | .01 | −0.11 | .46 |
| GCC | 0.31 | .03 | 0.33 | .03 | −0.02 | .91 |
| mRNFL 9 sectors | ||||||
| Average thickness | 0.23 | .12 | 0.24 | .11 | 0.03 | .86 |
| Superior inner | 0.19 | .21 | 0.12 | .44 | 0.07 | .66 |
| Temporal inner | 0.37 | .01 | 0.33 | .03 | −0.35 | .02 |
| Inferior inner | 0.24 | .11 | 0.24 | .12 | −0.10 | .51 |
| Nasal inner | −0.02 | .90 | −0.02 | .91 | −0.06 | .68 |
| Superior outer | 0.17 | .27 | 0.12 | .45 | 0.08 | .60 |
| Temporal outer | 0.15 | .32 | 0.12 | .42 | −0.01 | .95 |
| Inferior outer | 0.13 | .41 | 0.13 | .40 | 0.14 | .37 |
| Nasal outer | 0.07 | .64 | 0.15 | .33 | 0.01 | .96 |
| GC-IPL 9 sectors | ||||||
| Average thickness | 0.41 | <.01 | 0.38 | .01 | −0.13 | .41 |
| Superior inner | 0.43 | <.01 | 0.28 | .06 | −0.17 | .26 |
| Temporal inner | 0.50 | <.01 | 0.42 | <.01 | −0.17 | .25 |
| Inferior inner | 0.42 | <.01 | 0.32 | .03 | −0.20 | .19 |
| Nasal inner | 0.49 | <.01 | 0.25 | .09 | −0.24 | .10 |
| Superior outer | 0.44 | <.01 | 0.37 | .01 | −0.10 | .50 |
| Temporal outer | 0.11 | .49 | 0.19 | .20 | 0.18 | .23 |
| Inferior outer | 0.27 | .07 | 0.34 | .02 | −0.10 | .53 |
| Nasal outer | 0.40 | .01 | 0.38 | .01 | −0.24 | .11 |
| GCC 9 sectors | ||||||
| Average thickness | 0.36 | .02 | 0.34 | .02 | −0.06 | .70 |
| Superior inner | 0.41 | <.01 | 0.27 | .07 | −0.13 | .41 |
| Temporal inner | 0.53 | <.01 | 0.44 | <.01 | −0.26 | .09 |
| Inferior inner | 0.51 | <.01 | 0.42 | <.01 | −0.21 | .17 |
| Nasal inner | 0.45 | <.01 | 0.24 | .12 | −0.23 | .13 |
| Superior outer | 0.36 | .01 | 0.29 | .05 | −0.01 | .36 |
| Temporal outer | 0.15 | .31 | 0.20 | .19 | 0.14 | .37 |
| Inferior outer | 0.23 | .13 | 0.27 | .07 | 0.04 | .82 |
| Nasal outer | 0.31 | .04 | 0.36 | .02 | −0.14 | .36 |
| mRNFL 6 sectors | ||||||
| Superior | 0.16 | .30 | 0.12 | .41 | 0.09 | .57 |
| Temporal superior | 0.13 | .41 | 0.14 | .36 | −0.03 | .86 |
| Nasal superior | 0.08 | .62 | 0.03 | .87 | 0.08 | .61 |
| Inferior | 0.17 | .27 | 0.14 | .34 | 0.15 | .33 |
| Temporal inferior | 0.19 | .22 | 0.12 | .44 | −0.02 | .88 |
| Nasal inferior | 0.08 | .61 | 0.25 | .10 | 0.02 | .92 |
| GC-IPL 6 sectors | ||||||
| Superior | 0.43 | <.01 | 0.34 | .02 | −0.13 | .40 |
| Temporal superior | 0.31 | .04 | 0.30 | .05 | −0.03 | .83 |
| Nasal superior | 0.51 | <.01 | 0.41 | .01 | −0.27 | .07 |
| Inferior | 0.36 | .01 | 0.41 | .01 | −0.15 | .32 |
| Temporal inferior | 0.21 | .15 | 0.27 | .07 | 0.15 | .32 |
| Nasal inferior | 0.44 | <.01 | 0.39 | .01 | 0.22 | .15 |
| GCC 6 sectors | ||||||
| Superior | 0.40 | .01 | 0.29 | .05 | −0.03 | .83 |
| Temporal superior | 0.35 | .02 | 0.33 | .03 | −0.04 | .80 |
| Nasal superior | 0.37 | .01 | 0.28 | .06 | −0.14 | .37 |
| Inferior | 0.31 | .04 | 0.33 | .02 | −0.03 | .87 |
| Temporal inferior | 0.17 | .25 | 0.24 | .10 | 0.02 | .88 |
| Nasal inferior | 0.35 | .02 | 0.37 | .01 | −0.11 | .49 |
NOTE. N = 48. Pearson's correlation coefficients; statistically significant values (P < .05) are in bold.
Abbreviations: GCC, ganglion cell complex; GC-IPL, ganglion cell layer plus inner plexiform layer; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; mRNFL, macular retinal nerve fiber layer; pRNFL, peripapillary retinal nerve fiber layer; SS-OCT, swept-source optical coherence tomography.
Significant correlations were observed between global and inner macular thickness and cognitive status (MMSE and MoCA) (Table 4). Among the nine mRNFL sectors, the temporal inner sector was positively and significantly correlated with MMSE (r = 0.37, P = .01) and MoCA (r = 0.33, P = .03). The same sector was negatively and significantly correlated with Pfeffer (r = −0.35, P = .02). Most GC-IPL and GCC parameters correlated significantly with MMSE and MoCA scores (Table 4). The strongest correlation was that between MMSE scores and the inner sectors of both GC-IPL and GCC (Table 4).
No significant correlations were found between mRNFL thickness divided into six sectors and cognitive scores, regardless of the test used (Table 4). On the other hand, GC-IPL and GCC thickness divided into six sectors was significantly correlated with MMSE (r ranging from 0.31 to 0.51), except for the temporal inferior sector. Likewise, GC-IPL thickness was significantly correlated with MoCA scores, except for the temporal inferior sector, whereas GCC thickness was significantly correlated with MoCA scores in three of the six sectors (temporal superior, inferior, and nasal inferior). No SS-OCT parameter was correlated with any Pfeffer score.
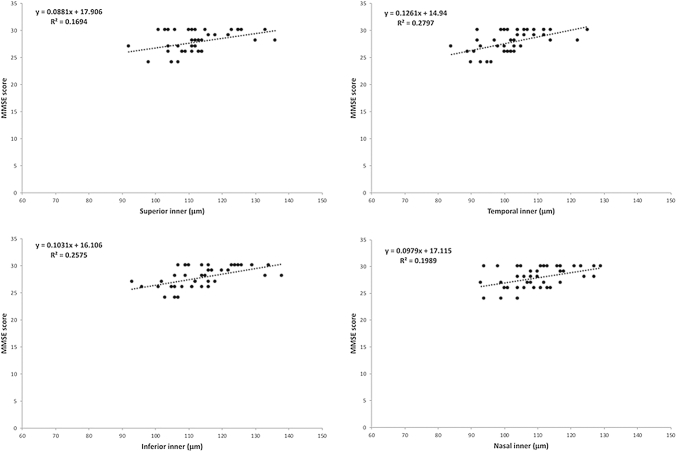
The scatterplot in Fig. 2 shows the results of the linear regression analysis of the best-performing SS-OCT parameters and MMSE scores.
Fig. 2.
MMSE scores plotted against SS-OCT–measured GCC thickness (in μc) of the four inner macular segments divided into nine sectors. Abbreviations: GCC, ganglion cell complex; MMSE, Mini-Mental State Examination; SS-OCT, swept-source optical coherence tomography.
4. Discussion
In this study, patients with aMCI and age-matched control subjects did not differ significantly with regard to SS-OCT–measured pRNFL thickness. This sheds new light on the pattern of axonal damage and neuronal loss affecting patients with MCI. Several authors have reported reductions in pRNFL thickness in patients with MCI, which, although significant, were not as severe as in AD [12], [13], [17], [21], [29], [30], [41], [42]. If we consider MCI a stage in the transition from normal cognition to dementia, then a reduction in pRNFL thickness would be expected. Thus, when Paquet et al. [12] evaluated patients with MCI and AD of different levels of severity, they found pRNFL thickness to be reduced in all patients, with a significant difference between MCI and moderate-to-severe AD, but no significant difference between MCI and mild AD, suggesting a continuum of axonal damage from MCI to AD. Choi et al. [26] conducted a prospective study on patients with AD and MCI, which showed reductions in pRNFL and GC-IPL thickness to be greater in patients with MCI progressing towards patients with AD than in patients with MCI who did not convert. Likewise, other authors observed no significant differences in pRNFL thickness or, at most, small focal reductions, between patients with MCI and healthy control subjects [30], [32]. These discrepancies may in part be ascribed to differences in sample size, the severity of cognitive impairment, and the OCT technology used.
The pRNFL is known to become thinner with aging [43], [44]. The mean age in our sample was 67.43 years. This is lower than the mean age of the patients studied by the Paquet group [12] (78.7 years), Kesler et al [17] (71.0 years), Ascaso et al. [13] (72.1 years), and Gao et al. [30] (73.42 years). The severity of cognitive impairment should also be taken into account. In our study, the reduction in MMSE scores was slight when compared with the control subjects. The mean score observed was 27.86, matching the scores reported by Paquet et al. [12] (28.8) and Kesler et al. [17] (28.1), but higher than the scores observed by Gao et al. [30] (25.77) and Ascaso et al. [13] (19.3).
Moreover, mean pRNFL and macular thickness also depends on the type of OCT device used [45]. The SS-OCT device used in this study is different from spectral-domain OCT as far as light source, acquisition speed, and resolution. The latter was used in the vast majority of earlier studies on patients with MCI. In other words, OCT thickness measurements from different studies should be compared with caution. Moreover, if MCI represents a transition towards dementia, reductions in pRNFL and macular thickness, if any, are likely subtle, perhaps even within the normal range, when compared with healthy age-matched control subjects.
In the present study, full-thickness macular measurements were reduced in all ETDRS sectors of patients with MCI, although only the superior and inferior inner sectors were significantly thinner. Using spectral-domain-OCT, other authors have found reductions in full macular thickness in patients with MCI [30], [46], but we believe the segmented inner retinal layers provide more reliable measurements. Both clinical and histopathologic studies have documented a preference for GCL impairment in AD [26], [32], [47], [48]. Thus, segmented inner retinal layer analysis is likely to reveal similar impairment patterns in MCI and AD.
Our results point to a preferential involvement of the inner retinal layers, especially GCC, in patients with MCI. As expected, in our sample full macular and pRNFL thickness displayed much smaller changes than the inner retinal layers. In patients with MCI, the macular GCC appears to yield more useful readings than the same layers when analyzed in separate. Indeed, when analyzing mRNFL, GC-IPL and GCC divided into nine sectors, thickness was significantly smaller in two of eight sectors (mRNFL), three of eight sectors (GC-IPL), and six of eight sectors (GCC). The relatively poor ability of mRNFL to detect axonal loss in patients with MCI was in part expected: the thinness of this layer can compromise OCT segmentation and potentially resulting in estimation errors.
Our study innovates by analyzing the inner retinal layers in three different ways: a global measure and divided into six or nine sectors. To our knowledge, no other study has combined these three approaches and, interestingly, diagnostic performance was different for each approach. Our results suggest a preferential involvement of the inner sectors around the fovea (3 mm) in the nine ETDRS sectors, especially for GCC, probably because of the higher concentration of ganglion cells in the 3-mm area around the fovea in healthy humans. The latter is supported by a histologic study of six retinas from healthy young adults showing a greater density of ganglion cells in the area between 0.4 and 2 mm from the center of the fovea [49]. It may be argued that this anatomic fact makes neuronal loss in MCI and AD easier to detect with OCT. Matching our findings, Lad et al. [36] found a reduction in GC-IPL thickness in the inner sector of patients with MCI. In a previous study on patients with AD conducted by our group [25], full macular thickness was preferentially affected in a 3-mm circle around the fovea, suggesting that MCI and AD have similar patterns of ganglion cell impairment and, consequently, supporting the notion that MCI and AD are part of the same spectrum of neurodegeneration.
Another relevant contribution of the present study is the analysis of the correlations between SS-OCT parameters and cognitive test values in patients with MCI. No other study has to our knowledge performed this kind of analysis using three different cognitive tests. Kesler et al. [17] correlated pRNFL thickness obtained by time-domain OCT with MMSE scores in a sample of 24 patients with MCI but found no significant associations. Gao et al. [30] found no associations either when correlating FD-OCT–measured pRNFL thickness with MMSE scores. In contrast, others have reported significant correlations between pRNFL thickness and MMSE scores in this patient population [13], [29]. Our own results suggest that macular parameters, especially GCC, allow for more meaningful correlations between OCT parameters and cognitive scores, mainly because these parameters were more efficient at discriminating eyes with MCI from control subjects. Although no correlation was found between OCT parameters and Pfeffer scores, both MMSE and MoCA scores yielded significant correlations with OCT measurements (both GC-IPL and GCC divided into nine ETDRS sectors), suggesting that these parameters do reflect the severity of cognitive impairment in patients with MCI. In support of our findings, Choi et al. [26] found a significant correlation between FD-OCT–measured GC-IPL and cognitive deficit in patients with MCI.
Our study has some limitations. The sample was relatively small, in part because of the strict sampling criteria. Many elderly patients were excluded because of concomitant diseases, such as macular disease and glaucomatous optic neuropathy, which are more prevalent in this age range. We also excluded patients with systemic diseases and history of acute myocardial infarction and stroke, all of which are prevalent in elderly patients. However, these criteria were adopted to minimize the interference of systemic and ocular diseases in pRNFL and macular thickness measurements. Other limitation is the absence of an analysis of the outer retinal layers. Currently, the SS-OCT software does not allow to perform segmentation and quantitative analysis of the outer retinal layers, but this feature is expected to be introduced in the not so distant future.
In conclusion, our results show that SS-OCT scanning may be useful in the clinical evaluation of patients with aMCI. The inner retinal layers, mainly around the fovea, were affected in patients with MCI, and many of the corresponding SS-OCT parameters were significantly correlated with cognitive test scores. In other words, SS-OCT–measured inner retinal layer thickness appears to hold promise as an ocular biomarker in patients with MCI.
Research in Context.
-
1.
Systematic review: We have compared peripapillary retinal nerve fiber layer and macular thickness measurements in patients with mild cognitive impairment and normal control subjects using swept-source optical coherence tomography and assessed the relationship between such measurements and the severity of cognitive impairment, assessed with cognitive tests (Mini-Mental State Examination and the Montreal Cognitive Assessment) and the Pfeffer Daily Functional Life Activities Questionnaire.
-
2.
Interpretation: Macular thickness parameters were assessed as global average and sectoral measurements and were evaluated both as full-thickness and segmented measurements of the inner retina.
-
3.
Future directions: Although no reduction was found in peripapillary retinal nerve fiber layer thickness measurements when compared with control subjects, they presented reduced measurements in several macular parameters, particularly the macular ganglion cell layer in sectors around the fovea. Furthermore, the Mini-Mental State Examination and Montreal Cognitive Assessment results were significantly correlated with most macular thickness parameters. We believe that swept-source optical coherence tomography holds promise as an ocular biomarker of the disease in patients with mild cognitive impairment.
Acknowledgments
A.L.M.A. was supported by a grant from PROQUALI/UFJF—Programa de Apoio à Qualificação dos servidores da Universidade Federal de Juiz de Fora (No 23071.000648/2019-66), Juiz de Fora, Brazil. M.L.R.M. was supported by a grant from CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico (No 308172/2018-3), Brasília, Brazil. The funding organizations had no role in the design or conduct of this research.
References
- 1.Burns A., Zaudig M. Mild cognitive impairment in older people. Lancet. 2002;360:1963–1965. doi: 10.1016/S0140-6736(02)11920-9. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell A.J., Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Fischer P., Jungwirth S., Zehetmayer S., Weissgram S., Hoenigschnabl S., Gelpi E. Conversion rom subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 4.Wu H., de Boer J.F., Chen T.C. Diagnostic capability of spectral-domain optical coherence tomography for glaucoma. Am J Ophthalmol. 2012;153:815–826.e2. doi: 10.1016/j.ajo.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassenstein A., Spital G., Scholz F., Henschel A., Richard G., Pauleikhoff D. Optical coherence tomography for macula diagnostics. Review of methods and standardized application concentrating on diagnostic and therapy control of age-related macula degeneration. Ophthalmologe. 2009;106:116–126. doi: 10.1007/s00347-008-1901-1. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro M.L., Fernandes D.B., Apóstolos-Pereira S.L., Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:3959–3966. doi: 10.1167/iovs.11-9324. [DOI] [PubMed] [Google Scholar]
- 7.Hajee M.E., March W.F., Lazzaro D.R., Wolintz A.H., Shrier E.M., Glazman S. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009;127:737–741. doi: 10.1001/archophthalmol.2009.106. [DOI] [PubMed] [Google Scholar]
- 8.Santos C.Y., Johnson L.N., Sinoff S.E., Festa E.K., Heindel W.C., Snyder P.J. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement (Amst) 2018;10:196–209. doi: 10.1016/j.dadm.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisi V., Restuccia R., Fattapposta F., Mina C., Bucci M.G., Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112:1860–1867. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 10.Iseri P.K., Altinaş O., Tokay T., Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 11.Berisha F., Feke G.T., Trempe C.L., McMeel J.W., Schepens C.L. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48:2285–2289. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 12.Paquet C., Boissonnot M., Roger F., Dighiero P., Gil R., Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;420:97–99. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 13.Ascaso F.J., Cruz N., Modrego P.J., Lopez-Anton R., Santabárbara J., Pascual L.F. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol. 2014;261:1522–1530. doi: 10.1007/s00415-014-7374-z. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Ramos T., Benito-León J., Villarejo A., Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis. 2013;34:659–664. doi: 10.3233/JAD-121975. [DOI] [PubMed] [Google Scholar]
- 15.Kirbas S., Turkyilmaz K., Anlar O., Tufekci A., Durmus M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol. 2013;33:58–61. doi: 10.1097/WNO.0b013e318267fd5f. [DOI] [PubMed] [Google Scholar]
- 16.Moschos M.M., Markopoulos I., Chatziralli I., Rouvas A., Papageorgiou S.G., Ladas I. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:782–788. doi: 10.2174/156720512802455340. [DOI] [PubMed] [Google Scholar]
- 17.Kesler A., Vakhapova V., Korczyn A.D., Naftaliev E., Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113:523–526. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y., Li Z., Zhang X., Ming B., Jia J., Wang J. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett. 2010;480:69–72. doi: 10.1016/j.neulet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Hedges T.R., 3rd, Perez Galves R., Speigelman D., Barbas N.R., Peli E., Yardley C.J. Retinal nerve fiber layer abnormalities in Alzheimer’s disease. Acta Ophthalmol Scand. 1996;74:271–275. doi: 10.1111/j.1600-0420.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 20.Bambo M.P., Garcia-Martin E., Pinilla J., Herrero R., Satue M., Otin S. Detection of retinal nerve fiber layer degeneration in patients with Alzheimer’s disease using optical coherence tomography: searching new biomarkers. Acta Ophthalmol. 2014;92:e581–e582. doi: 10.1111/aos.12374. [DOI] [PubMed] [Google Scholar]
- 21.Liu D., Zhang L., Li Z., Zhang X., Wu Y., Yang H. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2015;15:14. doi: 10.1186/s12883-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choma M., Sarunic M., Yang C., Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11:2183–2189. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 23.Eraslan M., Çerman E., Çekiç O., Balci S., Dericioğlu D., Sahin Ö. Neurodegeneration in ocular and central nervous systems: optical coherence tomography study in normal-tension glaucoma and Alzheimer disease. Turk J Med Sci. 2015;45:1106–1114. doi: 10.3906/sag-1406-145. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Martin E., Bambo M.P., Marques M.L., Satue M., Otin S., Larrosa J.M. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 2016;94:e454–e459. doi: 10.1111/aos.12977. [DOI] [PubMed] [Google Scholar]
- 25.Cunha L.P., Lopes L.C., Costa-Cunha L.V., Costa C.F., Pires L.A., Almeida A.L. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PLoS One. 2016;11:e0153830. doi: 10.1371/journal.pone.0153830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi S.H., Park S.J., Kim N.R. Macular ganglion cell-inner plexiform layer thickness is associated with clinical progression in mild cognitive impairment and Alzheimers disease. PLoS One. 2016;11:e0162202. doi: 10.1371/journal.pone.0162202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari L., Huang S.C., Magnani G., Ambrosi A., Comi G., Leocani L. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J Alzheimers Dis. 2017;56:1101–1107. doi: 10.3233/JAD-160886. [DOI] [PubMed] [Google Scholar]
- 28.Shao Y., Jiang H., Wei Y., Shi Y., Shi C., Wright C.B. Visualization of focal thinning of the ganglion cell-inner plexiform layer in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2018;64:1261–1273. doi: 10.3233/JAD-180070. [DOI] [PubMed] [Google Scholar]
- 29.Oktem E.O., Derle E., Kibaroglu S., Oktem C., Akkoyun I., Can U. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci. 2015;36:1141–1146. doi: 10.1007/s10072-014-2055-3. [DOI] [PubMed] [Google Scholar]
- 30.Gao L., Liu Y., Li X., Bai Q., Liu P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr. 2015;60:162–167. doi: 10.1016/j.archger.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Kwon J.Y., Yang J.H., Han J.S., Kim D.G. Analysis of the retinal nerve fiber layer thickness in Alzheimer disease and mild cognitive impairment. Korean J Ophthalmol. 2017;31:548–556. doi: 10.3341/kjo.2016.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung C.Y., Ong Y.T., Hilal S., Ikram M.K., Low S., Ong Y.L. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2015;45:45–56. doi: 10.3233/JAD-141659. [DOI] [PubMed] [Google Scholar]
- 33.Pillai J.A., Bermel R., Bonner-Jackson A., Rae-Grant A., Fernandez H., Bena J. Retinal nerve fiber layer thinning in Alzheimer’s disease: a case-control study in comparison to normal aging, Parkinson’s disease, and non-Alzheimer’s dementia. Am J Alzheimers Dis Other Demen. 2016;31:430–436. doi: 10.1177/1533317515628053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feke G.T., Hyman B.T., Stern R.A., Pasquale L.R. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement (Amst) 2015;1:144–151. doi: 10.1016/j.dadm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayhan H.A., Aslan Bayhan S., Celikbilek A., Tanık N., Gürdal C. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol. 2015;43:145–151. doi: 10.1111/ceo.12386. [DOI] [PubMed] [Google Scholar]
- 36.Lad E.M., Mukherjee D., Stinnett S.S., Cousins S.W., Potter G.G., Burke J.R. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PLoS One. 2018;13:e0192646. doi: 10.1371/journal.pone.0192646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith T., Gildeh N., Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 38.Collie A., Maruff P. An analysis of systems of classifying mild cognitive impairment in older people. Aust N Z J Psychiatry. 2002;36:133–140. doi: 10.1046/j.1440-1614.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- 39.Pfeffer R.I., Kurosaki T.T., Harrah C.H., Chance J.M., Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 40.Monteiro M.L., Afonso C.L. Macular thickness measurements with frequency domain-OCT for quantification of axonal loss in chronic papilledema from pseudotumor cerebri syndrome. Eye (Lond) 2014;28:390–398. doi: 10.1038/eye.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Y., Shi Z., Jia R., Zhu Y., Cheng Y., Feng W. The attenuation of retinal nerve fiber layer thickness and cognitive deterioration. Front Cell Neurosci. 2013;7:142. doi: 10.3389/fncel.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y., Wang X.N., Wang N., Han Y., Ma D., Lu Y. Regularity changes of the retinal nerve fiber layer and macular ganglion cell complex in patients with the amnestic mild cognitive impairment. Int J Neurosci. 2018;128:849–853. doi: 10.1080/00207454.2018.1438428. [DOI] [PubMed] [Google Scholar]
- 43.Leung C.K.S., Ye C., Weinreb R.N., Yu M., Lai G., Lam D.S. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013;120:2485–2492. doi: 10.1016/j.ophtha.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Leung C.K., Choi N., Weinreb R.N., Liu S., Ye C., Liu L. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119:731–737. doi: 10.1016/j.ophtha.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Costa-Cunha L.V., Cunha L.P., Malta R.F., Monterio M.L. Comparison of Fourier-domain and time-domain optical coherence tomography in the detection of band atrophy of the optic nerve. Am J Ophthalmol. 2009;147:56–63.e2. doi: 10.1016/j.ajo.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Giménez Castejón D., Dudekova M., Gómez Gallego M., Lajara Blesa J. Macular thickness in subjective memory complaints and mild cognitive impairment: a non-invasive biomarker. Neuroophthalmology. 2016;40:16–22. doi: 10.3109/01658107.2015.1118516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinton D.R. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 48.Koronyo Y., Biggs D., Barron E., Boyer D.S., Pearlman J.A., Au W.J. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curcio C.A., Allen K.A. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]