Abstract
Unravelling the determinants of host variation in susceptibility and exposure to parasite infections, infection dynamics and the consequences of parasitism on host health is of paramount interest to understand the evolution of complex host-parasite interactions. In this study, we evaluated the determinants, temporal changes and physiological correlates of Plasmodium infections in a large natural population of mandrills (Mandrillus sphinx). Over six consecutive years, we obtained detailed parasitological and physiological data from 100 male and female mandrills of all ages. The probability of infection by Plasmodium gonderi and P. mandrilli was elevated (ca. 40%) but most infections were chronical and dynamic, with several cases of parasite switching and clearance. Positive co-infections also occurred between both parasites. Individual age and sex influenced the probability of infections with some differences between parasites: while P. mandrilli appeared to infect its hosts rather randomly, P. gonderi particularly infected middle-aged mandrills. Males were also more susceptible to P. gonderi than females and were more likely to be infected by this parasite at the beginning of an infection by the simian immunodeficiency virus. P. gonderi, and to a lesser extent P. mandrilli, influenced mandrills’ physiology: skin temperatures and neutrophil/lymphocyte ratio were both impacted, generally depending on individual age and sex. These results highlight the ecological complexity of Plasmodium infections in nonhuman primates and the efforts that need to be done to decipher the epidemiology of such parasites.
Keywords: Host susceptibility, Infection dynamics, Nonhuman primates, Plasmodium infections
Graphical abstract
Copyright: B. Quintard
Highlights
-
•
Longitudinal epidemiological and physiological data on Plasmodium infection obtained from a wild primate population.
-
•
Elevated chronical infections by two species of Plasmodium.
-
•
Contrasted dynamics of infection and physiological effects of P. gonderi and P. mandrilli.
-
•
Elevated parasitaemia (P. gonderi) in male mandrills in primo-infection by the simian immunodeficiency virus.
1. Introduction
Host susceptibility and exposure to parasites are complex traits that may vary according to external factors such as climate (Paaijmans et al., 2009) and individual characteristics such as genetics (Doeschl-Wilson et al., 2011), sex (Altizer et al., 2004; Holmes et al., 1998; Nunn et al., 2009), age (Altizer et al., 2004; De Nys et al., 2013; Mapua et al., 2015; Springer et al., 2015), social status (Altizer et al., 2003), host body conditions (Daviews et al., 1991), behavior (Hoye et al., 2012), or infections with other pathogens (Springer et al., 2015). The relative contribution of each of these factors on host susceptibility, although important for the understanding of host-parasite interactions, is often difficult to circumscribe in wild animals because of the inherent challenge to collect biological data (through invasive sampling), especially in longevous species. Another difficult aspect to evaluate in natural populations concerns the impact of infection on host health and fitness. This is particularly true when infections are endemic and chronic (McDonald et al., 2018). Harmful effects on hosts often occur, indeed, during the acute phase of infection frequently associated to elevated parasitaemia. Hosts in chronic phase, with low parasitaemia, generally suffer minor symptoms. Detecting detrimental effects of infection on hosts may require large sample sizes and long-term longitudinal data rarely available in wild animal populations (McCallum and Dobson, 1995a, McCallum and Dobson, 1995b). The typical lack of such data has sometimes led to erroneous conclusions. A longitudinal study based on regular monitoring of wild chimpanzees has demonstrated, for example, that the simian-immunodeficiency virus (SIV: (Norley et al., 1999)) dramatically affects health, reproduction and lifespan of their hosts (Keele and al., 2009) while a common early belief was that infected chimpanzees were symptomless. Highlighting the determinants of host variation in susceptibility and exposure to infections, their dynamics and effects on host health appear therefore essential to understand the evolution of host-parasite interactions.
Plasmodium spp. are protozoan organisms responsible for the vector-borne malaria disease in humans. These parasites also affect a large array of vertebrate species including mammals, birds and reptiles (Perkins, 2014). Despite their importance and large distribution worldwide, major gaps remain in our understanding of the ecology of these parasites and their impacts on their nonhuman hosts. First, risk factors of Plasmodium infections related to host characteristics are only little appreciated in natural populations due to the general lack of data in most malaria-host systems, with a few exceptions. In blue tits, for example, the probability of infection and parasitaemia both increase with host age, and individuals investing in reproduction are more heavily parasitized (Knowles et al., 2011). In humans, P. falciparum prevalence decreases with age due to the establishment of an acquired immunity (Doolan et al., 2009). In line with this, young chimpanzees and gorillas living in natural conditions are also more susceptible to infection than adults (De Nys et al., 2013; Mapua et al., 2015). Second, under the natural conditions where hosts and Plasmodium have long co-evolved, data on virulence are rare and the few studies realized so far produced conflicting results probably because of different co-evolutionary histories. For example, avian Plasmodium impacts survival and reproduction in wild birds (e.g. great tits: (Oppliger et al., 1997); blue tits: (Lachish et al., 2011); Seychelles warblers (van de Crommenacker et al., 2012)) but not in others (e.g. Tengmalm's owls: (Korpimaki et al., 1993)). There is also some evidence that Plasmodium infections in great apes have health consequences (Herbert et al., 2015; Tarello, 2005) although other studies did not reveal any clear associated symptoms (Taylor et al., 1985). Overall, there is a lack of data in nonhuman primate populations living in endemic and natural areas where Plasmodium and their hosts have long co-evolved.
Since 2012, we have been longitudinally studying a large natural population of mandrills (Mandrillus sphinx) living in Southern Gabon and habituated to human presence (e.g. (Brockmeyer et al., 2015; Poirotte et al., 2017)). Over the years, we have regularly captured and collected blood samples from individually-recognized animals and have obtained longitudinal data on Plasmodium occurrence and parasitaemia (the density of parasites within infected hosts). Wild and captive mandrills are naturally infected by two species of Plasmodium (P. sp DAJ-2004 recently named P. mandrilli and P. gonderi) and other species of the Haemosporida order such as those of the genus Hepatocystis (Ayouba et al., 2012). In addition, we have been regularly measuring various longitudinal parasitological and physiological data on these mandrills (e.g. (Beaulieu et al., 2017, 2014; Charpentier et al., 2018; Poirotte et al., 2015)).
Here, we first study how environmental (season) and host traits (sex, age, reproductive status and co-infection patterns) determine occurrence, parasitaemia, and temporal changes of Plasmodium infections. We then analyze Plasmodium-species specific physiological effects on hosts (skin temperature, blood counts and oxidative stress). We first predict sex- and age-differentiated susceptibility and responses to Plasmodium infections. The most common pattern is a greater susceptibility in males compared to females (in humans: (Pathak et al., 2012), but see: (Goselle et al., 2009)) and in young animals (in gorillas: (Mapua et al., 2015); in chimpanzees: (De Nys et al., 2013); in humans: (De Beaudrap et al., 2011); but see in Verreaux's sifakas: (Springer et al., 2015)). We further predict seasonal effects with higher probability of infection and parasitaemia observed during the long-rainy season because of increased transmission at this time of the year. Indeed, climate parameters impact mosquito abundance in the environment, thereby affecting rates of Plasmodium transmission (Altizer et al., 2006; Paaijmans et al., 2009). We also predict non-random associations between Plasmodium infections and SIV and STLV (simian T-cell leukaemia viruses) that naturally infect wild mandrills (Souquiere et al., 2001; Makuwa et al., 2004). Indeed, in humans, HIV and Plasmodium infections synergistically interact with each other (Alemu et al., 2013). Finally, we predict species-specific and sex-differentiated physiological effects of Plasmodium infections. We focused on three physiological mechanisms reflecting host defenses. First, host may increase body temperature (e.g. fever) to control Plasmodium parasitaemia (Delfini, 1973). An immune response against Plasmodium infection is also triggered and typically involves a rise in neutrophils paralleled with a characteristic lymphocytopenia, resulting in elevated N/L ratios (Kotepui et al., 2015). Because phagocytes produce pro-oxidant compounds to kill parasites (Percário et al., 2012), we expected infected mandrills to show depleted antioxidant defenses and higher oxidative damage (e.g. lipid peroxidation) than non-infected individuals. The combination of longitudinal data on this natural nonhuman primate population associated to serial blood sampling and up-to-date molecular techniques, allowing to precisely quantify parasitaemia, provides a unique approach to decipher the complex relationship between Plasmodium parasites and their nonhuman primate host.
2. Materials and methods
2.1. Ethical statement
Experimental procedures were approved by the CENAREST Institute in Gabon (permit number: AR0042/17/MESRS/CENAREST/CG/CST/CSAR). Concerning the ethical treatment of non-human primates, we followed the legal requirements of Gabon.
2.2. Study population and study site
Since the beginning of this study (April 2012), we have been monitoring the only habituated social group of free-ranging mandrills worldwide, inhabiting the Lékédi Park in Southern Gabon (Bakoumba) within the framework of the “Mandrillus Project”. At the end of this study (Dec 2017), the group was composed of ca. 180 individuals of both sexes and all ages, about 120 of them being individually-known and daily monitored (Brockmeyer et al., 2015; Poirotte et al., 2017). During everyday observations, we typically record detailed data on group living and composition as well as on social behavior. Individual ages are either known thanks to daily monitoring (for about 20% of the studied animals) or approximated using general condition and patterns of tooth wear (Galbany et al., 2014). For most approximated ages, the error made was estimated to be less than a year. Finally, we collect daily information about females’ reproductive status. The gestation period is deduced from patterns of births and from the presence of a particular pink and swollen tumescence (Setchell et al., 2006), visible about two months following impregnation. Lactating females are those females with infants younger than six months of age (Charpentier et al., 2018).
2.3. Blood collection
Over the study period, we have regularly collected blood samples from 100 individuals (53 males and 47 females aged 0.07–21.3yrs; 1–7 samples per individual; total number of collected samples N = 217) during regular trapping sessions that occurred at different periods across years, seasons and individuals (2012–2015; see Supplementary Table 1). Subsets of these blood samples were variously used for different purposes (see associated sample sizes below). During captures, animals were anesthetized by blowpipe intramuscular injections of ketamine (Imalgene® 1000; 7 mg/kg for adults, 5 mg/kg for juveniles) and xylazine (Rompun®; 3 mg/kg for adults, 5 mg/kg for juveniles). Immediately after capture and sedation (<15min), blood was sampled from the iliac vein with EDTA-coated syringes. The delay between sedation and blood sampling was short (ca. 10 min.) and presumably not long enough for blood parameters to vary due, for example, to acute stress (e.g. for leukocyte profile (Davis et al., 2008)). Following blood collection and various other biological measurements, which lasted on average 30 min, animals were anti-sedated with atipamezole (Antisedan ND, 0.5 mg/ml) to facilitate awakening. Blood samples were centrifuged (15 min, 3000 rpm) in situ the same day of collection to obtain buffy coats, plasma and blood cells, which were then stored at −20 °C until future use (Beaulieu et al., 2017). From these blood samples, we subsequently obtained various physiological measurements used in this study, including Plasmodium spp. occurrence and parasitaemia, simian retroviruses’ profiles, leukocyte profiles and markers of oxidative stress.
2.4. Plasmodium diagnosis and parasitaemia estimate
Infections were determined for 215 blood samples collected throughout the years on 53 males and 47 females (1–7 samples per individual). Total DNA was extracted from approximately 200 μl of red blood cells using the DNeasy Blood and Tissue Kit (Qiagen, France) and used as templates for the detection of Plasmodium species according to previously described protocols (Prugnolle et al., 2010). The amplification of the portion of cytochrome b (cyt-b) gene is based on a nested PCR with two sets of primers (DW2-DW4: (Perkins and Schall, 2002); Cytb1 and Cytb2: (Schwöbel et al., 2003)). All amplified products (10 μl) were run on 1.5% agarose gels in Tris-acetate-EDTA (TAE) buffer. The PCR-amplified products were used as templates for sequencing (Eurofin MWG). Only two haemosporidian species were detected in the samples: P. mandrilli and P. gonderi.
We then developed and used a qPCR for detecting co-infections within each sample and quantifying each parasite species (i.e. parasitaemia). Primers for qPCR amplification were designed to amplify two different fragments located in the cytochrome b gene, one specific of P. mandrilli (106bp) and the other of P. gonderi (188bp). Primer sequences for amplification of specific P. mandrilli fragment (106 bp) were F 5′-CATACGTCACACCAATACAG-3′ and R 5′-GTTAAAACAATTAATAAACCTGCA-3'. Primers sequences for amplification of specific P. gonderi fragment (188 bp) were F 5′-GTTATTGGGGTGCAACTGTT-3′ and R 5′-GCTACCATGTAAATGTAAAAAGAAA-3'. qPCR amplifications were performed in a Light Cycler 96 (Roche). Reaction mixtures were prepared in a 10 μl final volume containing 1.5 μl of template DNA, 2 μl of 5X Hot Firepol Evagreen qPCR Mix Plus and 5 pmol of each primer. The qPCR conditions consisted of an initial melting cycle at 95 °C for 12min, followed by 40 cycles of amplification at 95 °C for 15 s, 56 °C (P. mandrilli) or 57 °C (P. gonderi) for 20 s and 72 °C for 20 s. Dissociation curves were generated after the final amplification cycle by denaturing the amplicons at 95 °C for 15 s, 56 °C for 1 min and 95 °C for 1 s. Dissociation curves were used to estimate the specific melting temperature of both amplicon. To evaluate the efficiency and quantify parasitaemia, standard curves were generated for each species from 10-fold serial dilutions of synthesized fragments.
2.5. SIV-STLV
SIV status was determined for 209 sera collected on 53 males and 46 females (1–6 samples per individual) and by identifying specific antibodies against SIVmnd-1 and SIVmnd-2 as previously reported (Souquiere et al., 2001). Briefly, we determined the antibody reactivity against the SIV V3 loop Env protein by a specific SIVmnd peptide-based immunoassay. Positive samples were further tested by PCR and pol sequenced for phylogenetic analyses aiming to differentiate between both SIV types (Souquiere et al., 2001). Positive sera were further confirmed by western blotting and primary infection was determined according to a composite criterion including seroconversion patterns between serial samples or the increasing immunoassay reactivities combined to Western blot profiles (Onanga et al., 2006). STLV status was determined using a commercial immunoassay HTVL-1-based (ARCHITECT rHTLV-I/II immunoassay Abbott, Chigaco Ill) according to the antigenic homology between both HTLV-1 and STLV-1 (Mahieux et al., 1998). The study mandrills were exclusively infected by SIVmnd-1 and STLV-1 as expected given their range. For further analyses, we only considered males (aged 6 yrs and older) because only two adult females were found SIV-positive and one adult female was STLV-positive (unpub. data). In addition, prevalence of both SIV and STLV in young animals (≤5 and ≤7 yrs resp.; unpub. data) was null.
2.6. Skin temperature
During two trapping sessions (July 2014 and December 2015), we obtained skin temperatures on 21 and 11 (resp.) adult mandrills of both sexes that were equipped with radio-collars (6 males and 21 females including 5 females that were equipped both times; Plasmodium infections were known for all of them). Each collar included a low-power digital temperature sensor (TMP102; accuracy: ± 0.5 °C) fitted on the inner face of radio-collars, in contact with the skin of the neck (ELA Innovation, Montpellier, France). These small radio transceiver units record the external body temperature of equipped animals at intervals of 7 min (from 6:00 a.m. to 6:00p.m.) or 1 h (at night). The ID code, date, time, and temperature are stored in a non-volatile memory in the hardware of the collar devices and are downloaded daily by field observers using a portable reading device (see also: (Poirotte et al., 2017)).
In this study, we considered the temperatures collected for 30 days starting the day following the last day of each session of captures. Every day, each collar recorded an average of 107.6 data points per equipped animal, representing a total of 83,531 data points collected on all study individuals during the two study months. Individuals with less than 100 data points available for any given day were discarded from further analyses. To correct for daily variation in external temperature, we considered daily temperatures averaged over all collars. In addition, we considered the average temperature recorded each day per each collar. We studied the difference between these two means. Individuals showing daily positive temperatures indicated animals hotter than the average temperature recorded that day (and conversely). While skin temperatures may be a coarse-grained representation of core temperatures, all animals were equipped in the same conditions.
2.7. N/L ratio
Leukocyte profiles were determined for 199 blood samples collected on 53 males and 46 females (1–7 samples per individual). We deposited a drop (ca. 20 μl) of fresh whole blood on a blade and immediately performed a blood smear. The blade was colored using a kit RAL 555 with its associated protocol (derived from the May-Grünwald Giemsa method). Over a subset of 40 blood smears that were double-blind read, the global concordance reached 85% between two different observers. The total number of each of the five cell types (neutrophils, eosinophils, basophils, lymphocytes and monocytes) was counted and expressed thereafter as frequencies. For the purpose of this study, we considered the N/L (neutrophil/lymphocyte) ratio per sampled animal.
2.8. Oxidative markers
We measured oxidative markers using two methods for 209 (d-ROM test) and 212 (OXY-adsorbent test; see below) blood samples collected on 53 males and 47 females (1–7 samples per individual). After collection and centrifugation, plasma was separated from the erythrocytes and stored at −20 °C. Plasma oxidative status was then examined with (1) the d-ROM test (Diacron International, Grosseto, Italy) that measures the concentration of hydroperoxide, a reactive oxygen metabolite resulting from the attack of reactive oxygen species on organic substrates (thereby reflecting oxidative damage (Costantini, 2016)), and (2) the OXY-adsorbent test (Diacron International, Grosseto, Italy) that measures the total antioxidant capacity of the sample (i.e. its ability to cope with the pro-oxidant action of hypochlorous acid). Intra-assay coefficients of variation were 3% and 10%, respectively, and inter-assay coefficients of variation were both 8% (for details, see: (Beaulieu et al., 2014)).
2.9. Statistical tests
In the following models, Plasmodium occurrences and parasitaemia were either considered as dependent variables (determinants) or as independent variables (physiological consequences). In both cases, we performed mixed linear models for occurrences with a random effect of the individuals’ identity because of repeated blood sampling obtained from the same individuals across the years (Bolker et al., 2009). By contrast, we did not consider such a random effect when studying parasitaemia, except when indicated otherwise, because of limited sample sizes: parasitaemia was indeed considered only in infected animals. For parasitaemia, we had to remove eight outliers (four samples per Plasmodium species collected on five males and three females) to facilitate convergence of the models. These outliers showed parasitaemia at least three times higher than the next highest value.
Except when indicated otherwise, we considered all first order interactions in the following models when occurrences and parasitaemia were considered as dependent variables or interactions involving Plasmodium infection when the latter was considered as independent variable. We kept full models as final models, excluding only non-significant (P > 0.05) interaction terms and quadratic effects. We performed post-hoc tests based on differences of least squares means (“lsmeans statement”) for categorical independent variables that significantly impacted the studied dependent variables.
2.9.1. Determinants of Plasmodium spp. infections
2.9.1.1. Intrinsic host characteristics and seasonality
Considering all studied animals, we first used Generalized Linear Mixed Models (GLMM; SAS studio, “glimmix” procedure) with a binary distribution to study the relationships between Plasmodium occurrences (presence/absence of either P. mandrilli or P. gonderi) as two dependent variables and different determinants. Second, considering infected animals only, we used General Linear Models (GLM; SAS studio, “glm” procedure) with a Gaussian distribution to study the impact of the same determinants on either P. mandrilli or P. gonderi parasitaemia (two dependent variables). Parasitaemia were ln-transformed to fit to normal distributions.
In these first four models, we considered as explanatory variables the individual age and its quadratic term (continuous variable) and sex (categorical variable with two modalities). In addition, we took into account the ecological season (categorical variable with three modalities). Gabon is characterized by four climatic seasons with a pronounced long rainy season (Feb–May) and a long dry season (Jun–Sept), in addition to a short rainy season (Oct–Nov), and a short dry season (Dec–Jan; for details, see: (Nsi Akoué et al., 2017)). These two short seasons were combined in our models because two blood samples only were collected during the short rainy season (excluding these two samples did not change the results).
Restricting the dataset to females aged ≥4 yrs, we then studied whether females' reproductive status (categorical variable with three modalities) impacted Plasmodium infection. In particular, we distinguished pregnancy and lactation because these two periods of a female's life are probably the most energetically demanding ones, possibly resulting in higher parasite susceptibility (e.g. (De Nys et al., 2014)). All other females (neither pregnant nor lactating) were pooled together in a third category. In these additional models, we considered female's age as quadratic term but did not take into account the season of sampling because it was highly correlated to female's reproductive status.
2.9.1.2. Co-infections
We first tested whether being infected by a given Plasmodium species impacted the probability of infection by the other Plasmodium species using Spearman's rank correlation tests for both occurrences and parasitaemia.
Second, in males aged ≥6 yrs, we studied the relationship between P. mandrilli occurrence (dependent variable) and SIV and STLV infection statuses using GLMM. Almost all males that were infected by P. gonderi were also STLV-positive. Consequently, when studying P. gonderi occurrence, we considered males' SIV status only (GLMM). We studied the relationship between P. gonderi parasitaemia and SIV and STLV statuses using a GLM as above. In this particular case, we re-ran, however, an additional General Linear Mixed Model (LMM; SAS studio, “glimmix” procedure) with a random effect of male's identity to make sure that the marginal effect of SIV we found was not due to any confounding effect due to repeated sampling on males. For these four linear models, we considered male's age (in a quadratic form) as a confounding factor but we neither took into account the season of sampling, because this variable never influenced former models, nor interaction terms because of limited sample sizes. Finally, we studied the relationship between P. mandrilli parasitaemia and either SIV or STLV statuses using two non-parametric analyses of variance (SAS studio, “npar1way” procedure) because of limited sample sizes.
2.9.2. Physiological consequences of Plasmodium spp. infections
In these last analyses, Plasmodium occurrences and parasitaemia were considered as four independent variables. Both occurrences (presence/absence) were considered altogether in the same linear models but each parasitaemia was considered in two different models because individuals infected by one species may have not been infected by the other.
2.9.2.1. Skin temperature
We used LMM to study the relationship between skin temperature (i.e. the difference between the average daily individual's temperatures minus the temperatures averaged over all collars per day) that followed a Gaussian distribution and either Plasmodium occurrences (one model) or parasitaemia (two models). We took into account individual age and sex as explanatory variables but considered a nested random effect between the session of trapping and the individual's identity as both captures were performed at different times of the year.
2.9.2.2. N/L ratio and oxidative markers
We used LMM and GLM to study the relationship between anti-oxidant defenses (OXY), oxidative damage (ROM) and N/L ratio and either Plasmodium occurrences (one model; LMM) or parasitaemia (two models; GLM). OXY and ROM both followed Gaussian distributions and we ln-transformed N/L ratio to fit to such a distribution. We took into account the same explanatory variables and the same random effect (LMM) as above.
3. Results
In the studied population, the average prevalence was 36.7% for P. mandrilli and 41.1% for P. gonderi (all years pooled). Parasitaemia varied from 1 to 1,267,280 copies/μl of blood in individuals infected by P. mandrilli (mean ± SD: 22,023 ± 145,954) and from 1 to 2,551,395 copies in those infected by P. gonderi (41,308 ± 282,983). Across 128 positive samples (infected by at least one parasite species), we recorded 29.7% of co-infections (38 samples).
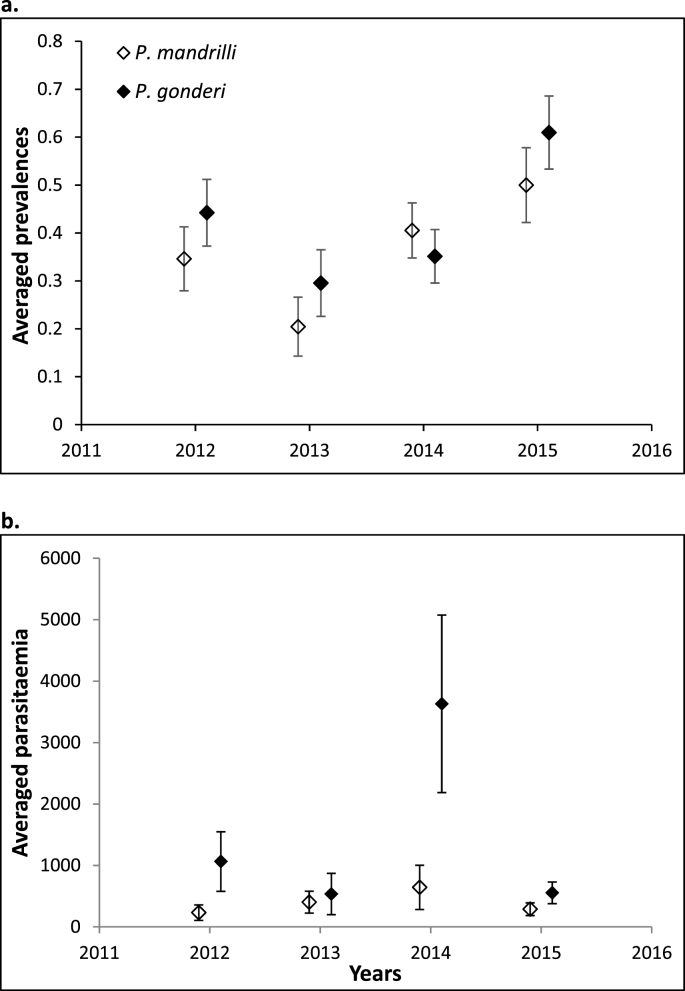
Prevalences also showed annual patterns, and interestingly, both parasite species followed similar trends (Fig. 1a). Such annual patterns were not evidenced when considering parasitaemia (Fig. 1b), although seven out of the eight most heavily infected animals, considered as outliers in our analyses, were all captured in December 2015.
Fig. 1.
Annual patterns of prevalences (a) and parasitaemia (number of copies/μl of blood; b) for the two studied Plasmodium species. Means and standard errors of the mean calculated from raw values are represented. For parasitaemia, outliers were excluded from mean calculations to allow visible inter-annual comparisons. We excluded four values for P. mandrilli (30,929, 134,387, 273,424, 1,267,280 copies) and four values for P. gonderi (78,322, 100,182, 767,718, 2,551,395 copies), all these elevated parasitaemia were measured in 2015, except one value measured in 2014 for P. mandrilli.
When examining individual temporal changes in infections (Supplementary Table 1), we found that individuals that were never infected were generally young. We further recorded 17 individuals that were infected (at least twice) by one parasite species only and 18 individuals that were infected by both species at the same time. Surprisingly, in 14 cases, infected individuals cleared their parasites and we observed four cases of parasite reversal. Finally, the only individual with an elevated parasitaemia (individual 21, P. mandrilli infection) that was captured again showed low parasitaemia a year later (all other elevated parasitaemia were recorded during the last trapping of the animals; Supplementary Table 1).
3.1. Determinants of plasmodium spp. infections
3.1.1. Intrinsic host characteristics and seasonality
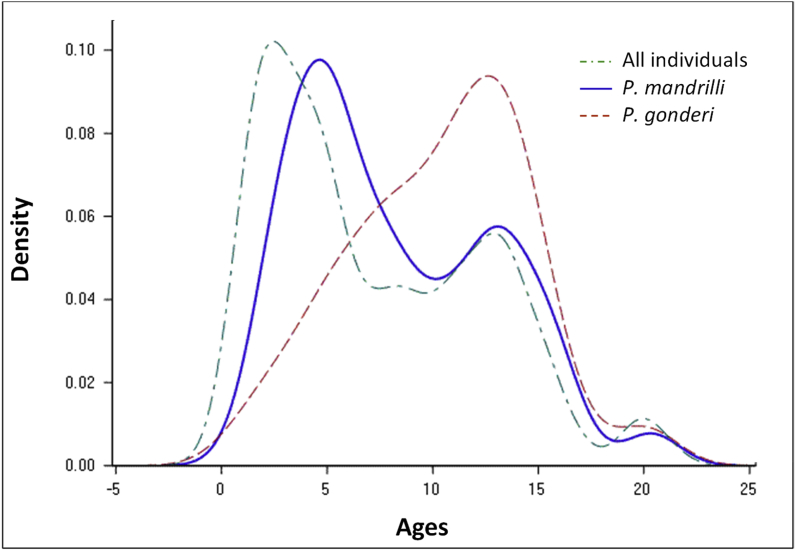
We found that individual sex significantly (P. gonderi) or marginally (P. mandrilli) influenced occurrences (Table 1): the probability of infection was higher in males (P. mandrilli and P. gonderi resp.: 40.5% and 44.1%, N = 111) than in females (32.7% and 37.9%, N = 104). In addition, the quadratic form of individual age significantly impacted the probability of both infections (Table 1). The age distribution of infected individuals (P. mandrilli) followed the age distribution of all sampled individuals, irrespective of their infection status. By contrast, the highest probability of infection for P. gonderi was around 13 yrs of age (Fig. 2). Neither female's reproductive status nor the ecological season of sampling had significant effects on occurrences, while parasitaemia were not impacted by any of the studied ecological or individual determinants (Table 1).
Table 1.
Determinants of Plasmodium occurrences and parasitaemia in mandrills.
| Sex | Age | Season | Reproductive status | |||
|---|---|---|---|---|---|---|
| P. mandrilli | All animals | Occurrence (N = 215, 100 ids) | F = 2.84 P = 0.09 |
F=7.23 P<0.01a |
F = 1.63 P = 0.20 |
|
| Parasitaemia (N = 75, 49 ids) | F = 0.03 P = 0.87 |
F = 0.09 P = 0.77 |
F = 1.25 P = 0.29 |
|||
| Females | Occurrence (N = 75, 32 ids) Parasitaemia (N = 27, 18 ids) |
F = 0.24 P = 0.63 F = 2.85 P = 0.11 |
F = 0.11 P = 0.90 - |
F = 0.77 P = 0.47 F = 0.55 P = 0.58 |
||
| P. gonderi | All animals | Occurrence (N = 214, 99 ids) |
F=4.20 P=0.04 |
F=13.04 P<0.001a |
F = 0.29 P = 0.75 |
|
| Parasitaemia (N = 84, 42 ids) | F = 0.30 P = 0.59 |
F = 0.31 P = 0.58 |
F = 0.79 P = 0.46 |
|||
| Females | Occurrence (N = 74, 31 ids) Parasitaemia (N = 35, 18 ids) |
F=6.74 P=0.01a F = 1.32 P = 0.26 |
F = 0.31 P = 0.73 - |
F = 0.62 P = 0.54 F = 2.18 P = 0.13 |
F and P values of full linear models are presented with significant P-values in bold. aQuadratic effects. “ids”: individuals.
Fig. 2.
Kernel density estimates for the distribution of ages across all studied individuals (dashed green line) and those infected by P. mandrilli (solid blue line) and P. gonderi (dashed red line). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.1.2. Co-infection patterns
The probability of being infected by one Plasmodium species was slightly higher when already infected by the other species (36.7% of individuals non-infected by P. mandrilli were infected by P. gonderi while 48.7% of individuals infected by P. mandrilli were also infected by P. gonderi). The correlation between occurrences was, however, not significant, although close to be (Spearman's rank correlation: N = 214, rs = 0.12, P = 0.09). Parasitaemia appeared non-significantly correlated (N = 214, rs = 0.09, P = 0.21; without outliers: N = 206, rs = 0.07, P = 0.34).
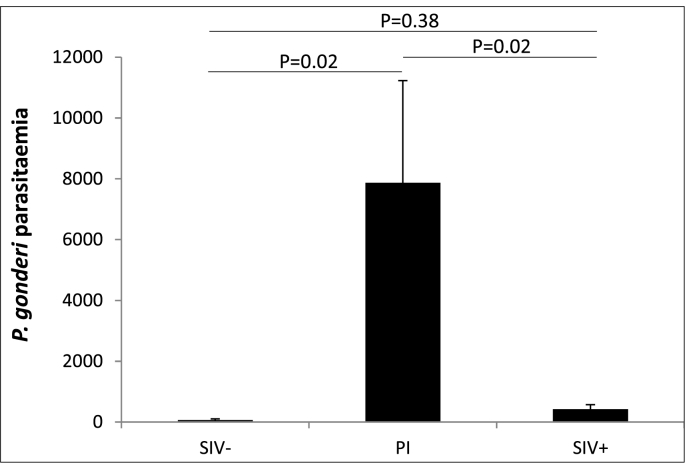
In males aged ≥6 yrs, occurrences were not influenced by retroviral infections (Table 2). By contrast, P. gonderi parasitaemia was related to SIV infection: males in primo-infection were more heavily parasitized than any other males (Fig. 3) and this effect was not due to confounding effect of the identity of the studied males (Table 2).
Table 2.
Influence of SIV and STLV infections on Plasmodium occurrences and parasitaemia in male mandrills aged 6yrs and older.
| Age | SIV status | STLV status | ||
|---|---|---|---|---|
| P. mandrilli | Occurrence (N = 42, 19 males) | F = 0.06 P = 0.81 |
F = 1.01 P = 0.38 |
F = 0.09 P = 0.76 |
| Parasitaemia (N = 21, 13 males) | F = 0.06 P = 0.37 |
F = 0.28 P = 0.60 |
||
| P. gonderi | Occurrence (N = 42, 19 males) | F = 0.01 P = 0.94 |
F = 1.67 P = 0.21 |
|
| Parasitaemia (GLM) (N = 33, 14 males) |
F = 0.08 P = 0.78 |
F = 2.71 P = 0.08 |
F = 1.22 P = 0.28 |
|
| Parasitaemia (LMM) (N = 33, 14 males) |
F = 0.01 P = 0.92 |
F=4.63 P=0.03 |
F = 1.41 P = 0.25 |
F and P values of full linear models are presented with significant P-values in bold. GLMM were used for occurrences while either non-parametric analyses of variance (P. mandrilli) or GLM (P. gonderi) were used to study parasitaemia.
Fig. 3.
P. gonderi parasitaemia across male mandrills with different SIV statuses. SIV-: SIV-negative males; SIV+: SIV-positive males; PI: males in primo-infection. Means and standard errors of the mean calculated from raw values are represented. P-values were obtained using differences of least squares means across the three categories (LMM).
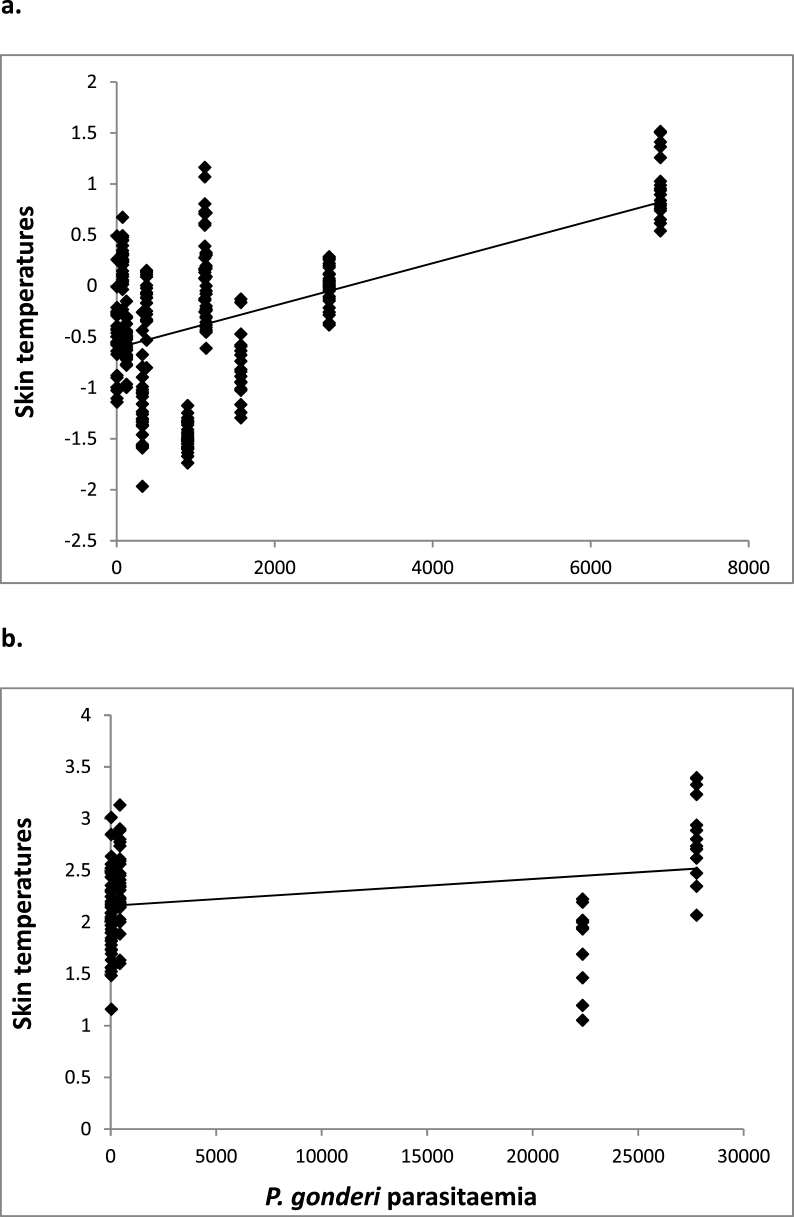
3.2. Physiological consequences of infection
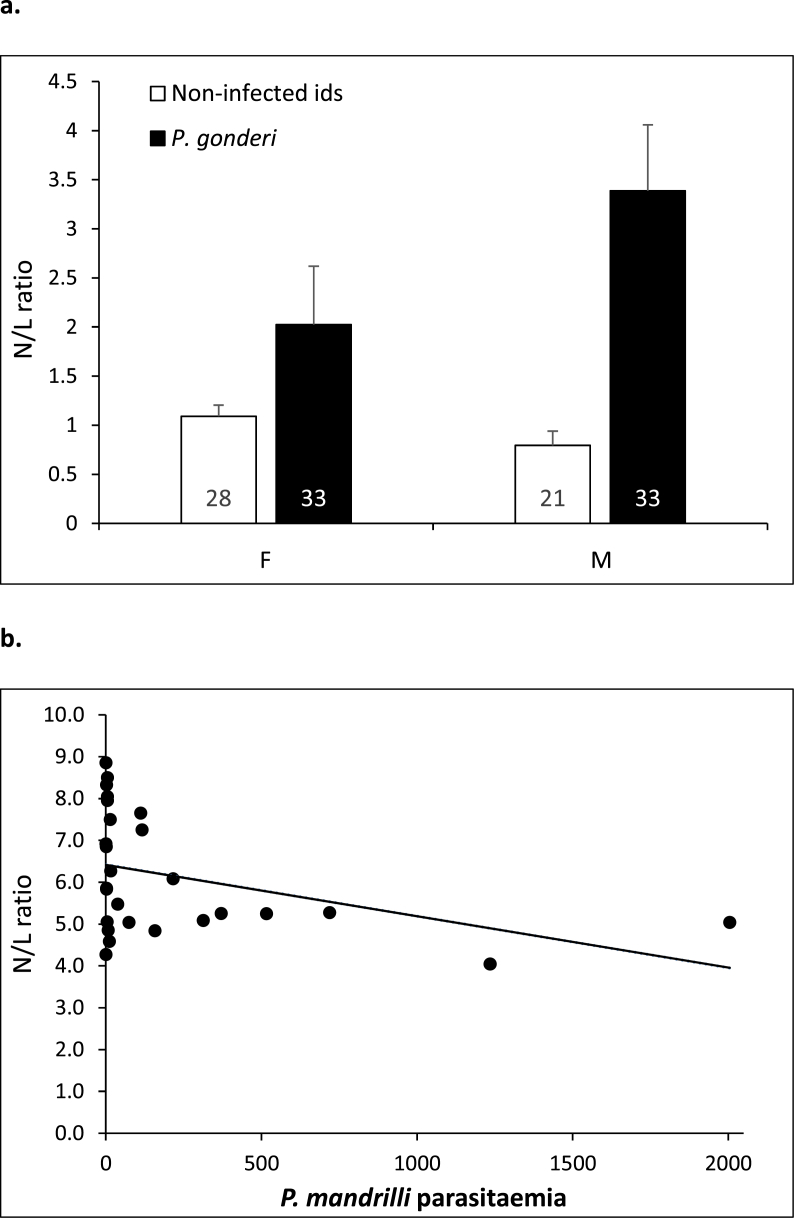
Neither Plasmodium occurrences nor P. mandrilli parasitaemia impacted skin temperatures (Table 3). Individuals with elevated P. gonderi parasitaemia were, however, hotter on average than animals with low parasitaemia. This effect was particularly pronounced in female mandrills (Fig. 4). Second, mandrills infected by P. gonderi showed elevated N/L ratio, especially males (Table 4; Fig. 5a). Intriguingly, young animals with elevated P. mandrilli parasitaemia showed a reverse tendency: they produced less neutrophils but more lymphocytes, although the sample size was highly limited (Fig. 5b). Finally, we did not find any relationship between Plasmodium occurrences and parasitaemia and markers of antioxidant defenses or of oxidative damage in individuals aged 4–16 yrs (Table 5).
Table 3.
Impact of Plasmodium occurrences (a) and parasitaemia (b: P. mandrilli, c: P. gonderi) on skin temperatures, in collared adult mandrills.
| a. | Sex | Age | P. mandrilli | P. gonderi |
|---|---|---|---|---|
| Skin temperatures (N = 726, 27 ids) | F = 60.17 P < 0.0001 |
F = 1.67 P = 0.20 |
F = 0.72 P = 0.40 |
F = 0.18 P = 0.67 |
| b. | Sex | Age | P. mandrilli |
|---|---|---|---|
| Skin temperatures (N = 319, 12 ids) | F = 8.66 P < 0.01 |
F = 3.17 P = 0.08 |
F = 0.01 P = 0.92 |
| c. | Sex | Age | P. gonderi | P. gonderi*Sex |
|---|---|---|---|---|
| Skin temperatures (N = 372, 15 ids) | F = 51.03 P < 0.0001 |
F = 2.19 P = 0.14 |
F=6.18 P=0.01 |
F=5.53 P=0.02 |
F and P values of full multivariate analyses of variance are presented with significant P-values of interest in bold. “ids”: individuals.
Fig. 4.
Influence of P. gonderi parasitaemia on female's (a) and male's (b) skin temperatures. Raw values are represented.
Table 4.
Impact of Plasmodium occurrences (a) and parasitaemia (b: P. mandrilli; c: P. gonderi) on N/L ratio, in mandrills aged 4–16yrs.
| a. | Sex | Age | Season | P. mandrilli | P. gonderi | P. gonderi*Sex |
|---|---|---|---|---|---|---|
| N/L (N = 115, 58 ids) | F = 1.36 P = 0.25 |
F = 8.71 P < 0.01 |
F = 20.50 P < 0.0001 |
F = 0.80 P = 0.37 |
F = 2.65 P = 0.11 |
F=11.44 P<0.01 |
| b. | Sex | Age | Season | P. mandrilli | P. mandrilli*Age |
|---|---|---|---|---|---|
| N/L (N = 50, 34 ids) | F = 7.05 P = 0.01 |
F = 1.81 P = 0.19 |
F = 4.69 P = 0.01 |
F=6.48 P=0.01 |
F=5.41 P=0.02 |
| c. | Sex | Age | Season | P. gonderi |
|---|---|---|---|---|
| N/L (N = 63, 34 ids) | F = 11.34 P < 0.01 |
F = 8.49 P < 0.01 |
F = 11.06 P < 0.0001 |
F = 0.12 P = 0.73 |
F and P values of full multivariate analyses of variance are presented with significant P-values of interest in bold. “ids”: individuals.
Fig. 5.
Influence of P. gonderi prevalence on N/L ratio in female (F) and male (M) mandrills aged 4–16yrs (a) and of P. mandrilli parasitaemia in young animals (b).
(a) Means and standard errors of the mean calculated from raw values are represented. Sample sizes are provided for each category.
(b) For clarity sake, we distinguished two categories of individuals: animals that were younger than the average (<
9.1 yrs) observed in the data set analyzed and those that were older (≥9.1 yrs). Preliminary observations indicated no relationship between N/L ratio and P. mandrilli parasitaemia in old individuals (not represented), we therefore chose to present visible effects in young animals only.
Table 5.
Impact of Plasmodium occurrences (a) and parasitaemia (b: P. mandrilli, c: P. gonderi) on OXY and ROM concentrations, in mandrills aged 4–16yrs.
| a. | Sex | Age | Season | P. mandrilli | P. gonderi |
|---|---|---|---|---|---|
| OXY (N = 127, 59 ids) | F = 1.47 P = 0.23 |
F = 0.19 P = 0.66 |
F = 7.48 P < 0.01 |
F = 0.60 P = 0.44 |
F = 0.03 P = 0.86 |
| ROM (N = 128, 59 ids) | F = 17.29 P < 0.0001 |
F = 0.73 P = 0.40 |
F = 2.05 P = 0.14 |
F = 0.16 P = 0.69 |
F = 1.25 P = 0.27 |
| b. | Sex | Age | Season | P. mandrilli |
|---|---|---|---|---|
| OXY (N = 56, 37 ids) | F = 0.17 P = 0.68 |
F = 0.46 P = 0.50 |
F = 5.40 P < 0.01 |
F = 0 P = 0.98 |
| ROM (N = 56, 37 ids) | F = 10.19 P < 0.01 |
F = 0.05 P = 0.82 |
F = 0.83 P = 0.44 |
F = 0.11 P = 0.74 |
| c. | Sex | Age | Season | P. gonderi |
|---|---|---|---|---|
| OXY (N = 71, 35 ids) | F = 2.39 P = 0.13 |
F = 1.70 P = 0.20 |
F = 1.61 P = 0.21 |
F = 0.19 P = 0.67 |
| ROM (N = 71, 35 ids) | F = 19.28 P < 0.0001 |
F = 0.70 P = 0.41 |
F = 2.06 P = 0.14 |
F = 0.23 P = 0.63 |
F and P values of full linear models are presented. “ids”: individuals.
4. Discussion
In this study, we adopted a broad approach to evaluate the determinants, temporal changes and physiological correlates of Plasmodium infections in a large natural primate population. In particular, we took advantage of longitudinal molecular-based measurements of occurrences and parasitaemia associated to various individually-centered biological data.
Probabilities of infections were high, and multiple infections were common in the study population, as observed in African great apes (Kaiser et al., 2010; Liu et al., 2010; Otto et al., 2018) but in contrast to captive mandrills living in Southern Gabon (Boundenga et al., 2018; Ngoubangoye et al., 2016). We found also slight evidence that the presence of one Plasmodium species facilitated the establishment of the second species. In addition, we evidenced several cases of parasite clearance, although a persistence of parasites below the detection threshold cannot be excluded. We also documented several cases of switching between both species of Plasmodium. Finally, similar determinants (individual age and sex) impacted the probability of infection for both species of parasites as well as pronounced and concomitant annual patterns suggesting that ecological factors may drive transmission, at least partly. Vector-borne parasites should, indeed, particularly depend on environmental factors (Altizer et al., 2006). Although we used a coarse-grained estimate of seasonality, we did not observe, however, any obvious effects of the season, perhaps because of persistent Plasmodium infections in the studied mandrills possibly hiding marked seasonality of transmission (see discussion in: (Springer et al., 2015)). It is interesting to note though, that in December 2015, seven out of the eight most elevated parasitaemia were recorded; this month was also characterized by the highest rainfall recorded in December, compared to previous years (Dec 2015: 223.4 mm vs. Dec 2013: 183.6 mm and Dec 2012: 145.4 mm; no data available in Dec 2014; MJEC unpub. data).
Despite these apparent similarities between both Plasmodium species, they showed, however, some marked differences. P. mandrilli appeared to infect its hosts rather randomly as suggested by Fig. 2 which depicts a distribution of P. mandrilli infections across ages very similar to the distribution of ages across randomly captured mandrills. This suggests that the probability of establishment of P. mandrilli within its host is probably high. By contrast, the distribution of P. gonderi infections was shifted towards older ages, with a peak recorded around 13–14 yrs in the studied mandrills. This result is in contrast to those obtained on humans and great apes (De Nys et al., 2013; Doolan et al., 2009; Mapua et al., 2015) where adults are generally less infected than younger animals probably because of the development of an efficient acquired immune response with age (Frölich et al., 2012). Our results may first indicate an increased duration of exposure in older animals combined with persistent chronic infections as proposed for birds (see discussion in: (Knowles et al., 2011)). Immunosenescence processes may further explain higher occurrences in adult animals (although the oldest individuals were less infected than middle-aged mandrills). Alternatively, older individuals may be more exposed than younger animals through behavioral, demographic or physiological mechanisms. In New World monkeys, for example, Plasmodium infection rate increases with individual body size (Daviews et al., 1991). Finally, different mortality rates across age classes may explain our observations, with higher pathogenicity of Plasmodium in young mandrills. While probabilities of infection were low in young mandrills, we cannot comment on the pathogenicity of Plasmodium as we never found corpses precluding further biomedical examinations. We further did not observe any conspicuous sign of sickness in infected mandrills, although we have never designed specific health monitoring of the studied animals. In this study, we found, however, some evidence that Plasmodium impacted several aspects of its hosts’ physiology and probably P. gonderi in a greater extent than P. mandrilli.
P. gonderi occurrence and parasitaemia had several consequences on its host, including: higher body temperatures, especially in females; higher probability of being in primo-infection (SIV) in males; a modified N/L ratio. The strongest effect concerned infected males that showed altered blood counts (increased N/L ratio). Intriguingly, this pattern (neutrophilia combined with lymphopenia) was reversed in young animals when considering P. mandrilli parasitaemia, although the sample sizes were limited and the most elevated values of parasitaemia were excluded from those analyses. Neutrophils are phagocytic cells produced in response to infections and stress while lymphocytes are involved in a variety of immune functions (see: (Davis et al., 2008) and references therein). An increase of the N/L ratio is a hematological response to hormonal stress reported in all vertebrate taxa. In addition, this ratio changes following a disease or an infection, perhaps reflecting these changes in hormone production during such events (for a review, see: (Davis et al., 2008)). For example, an elevated ratio is associated to higher susceptibility to diseases in newly hatched chicks (Al-Murrani et al., 2006) and parasitic infections in house finches (Davis et al., 2004) or pied flycatchers (Lobato et al., 2005). Yet, the presence of P. gonderi resulted in an increase in individual's N/L ratio while P. mandrilli parasitaemia generally decreased it. Results are also somehow contrasted in the literature: in humans, researchers have either reported a depletion in neutrophils due to Plasmodium infection (Goldstein, 1968) or an increase in patients with high parasitaemia (Kotepui et al., 2015). These contradictory results may thus reflect the complexity of Plasmodium infections and the cascade of physiological modifications they induce.
The effects of Plasmodium infection on host fitness are difficult to measure, especially in natural conditions, because the acute stage of infection is generally brief and perhaps sometimes lethal. This means that most individuals will present chronic infections following acquired immunity. P. gonderi parasitic forms are found, for example, 12 months following experimental infections in rhesus macaques although no evident sign of sickness was reported, except anemia (see for review: (Coatney et al., 2003)). Accordingly, in our study, most sampled mandrills showed low parasitaemia. This may explain why oxidative markers were not related to Plasmodium infection. These parameters presumably only vary at the time of inoculation (with higher oxidative damage levels) when the organism allocates resources towards self-maintenance but not afterwards, as such physiological changes would impair the fitness of the host in the long term (Crespi et al., 2013; Monaghan et al., 2009).
We further found a positive association between primary infection in SIV-positive males and P. gonderi parasitaemia. Primo-infected animals present an elevated number of viral copies due to the intense viral replication at this early phase of the infection. In humans, the primary infection is associated to a significant drop in CD4 T-cell number involving therefore an altered immune response. As well, rhesus macaques that are experimentally infected with SIVmnd-1 show a significant CD4 T-cell depletion (Souquière et al., 2009). The hypothesis could be that SIV-positive male mandrills may be immunologically compromised at least during this immune storm with intense SIV multiplication, facilitating Plasmodium infections, as observed in humans (Alemu et al., 2013).
Finally, we observed higher susceptibility to Plasmodium infections, although not higher parasitaemia, in males compared to females. Evidences are mixed in the human and nonhuman primate literature, from females (Goselle et al., 2009), or males (Pathak et al., 2012) being more infected than the other sex to no effect of sex on susceptibility (De Nys et al., 2013; Mapua et al., 2015). Our result in mandrills is rather logical as males’ investment in immune functions are often compromised because of an investment into competition and into immunosuppressive hormones such as testosterone (see for review: (Klein, 2004; Prall and Muehlenbein, 2014)). Male mandrills are characterized by intense male-male competition and testosterone increases when receptive females are available and when male ranks are unstable, two situations where males may compete intensely (Setchell et al., 2008). Alternatively, males may be more exposed to Plasmodium infections than females: they are on average 3.5 times heavier (Setchell et al., 2001) and most of them are generally more peripheral to the core of the group than females (Setchell, 2003), two characteristics that may increase the probability of encounter with the vectors.
Studying the determinants and health consequences of ubiquitous parasites like Plasmodium is of paramount interest to determine e.g. population sustainability. Plasmodium species are, for example, responsible for the extinction of several native Hawaiian birds (Elliott et al., 2010). This longitudinal study, based on time-series collected across six years of detailed monitoring of a natural primate population, allowed to identify individual's determinants in susceptibility to infections that also appeared highly dynamic. In addition, we demonstrated a few marked differences between both Plasmodium species suggesting different co-evolutionary histories with their primate hosts. Finally, both species induced some physiological consequences in their hosts that could impact co-infection patterns. Taken together these results highlight the ecological complexity of Plasmodium infections in primates, and the efforts that need to be done to decipher the epidemiology of parasites that are of public concern.
Acknowledgements
We are grateful to past and present field assistants of the Mandrillus Project who collect daily behavioral data on study population. This study was funded by several grants that allowed long-term collection of data used in this study. These grants were granted to MJEC: Deutsche Forschungsgemeinschaft (DFG, KA 1082-20-1), “Station d’Etudes en Ecologie Globale” (INEE-CNRS), “Laboratoire International Associé” (CIRMF and INEE-CNRS), PEPS ECOSAN (INEE-CNRS) and “Agence Nationale de la Recherche” (ANR SLEEP 17-CE02-0002). This study was approved by an authorization from the CENAREST institute (permit number: AR0060/18/MESRS/CENAREST/CG/CST/CSAR). This is a Project Mandrillus publication number 19 and ISEM 2019-202-SUD.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.09.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Al-Murrani W.K., Al-Rawi A.J., Al-Hadithi M.F., Al-Tikriti B. Association between heterophil/lymphocyte ratio, a marker of “resistance” to stress, and some production and fitness traits in chickens. Br. Poult. Sci. 2006;47:443–448. doi: 10.1080/00071660600829118. [DOI] [PubMed] [Google Scholar]
- Alemu A., Shiferaw Y., Addis Z., Mathewos B., Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasites Vectors. 2013;6:18. doi: 10.1186/1756-3305-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S., Nunn C.L., Thrall P.H., Gittleman J.L., Antonovics J., Cunningham A.A., Dobson A.P., Ezenwa V., Jones K.E., Pedersen A.B., Poss M., Pulliam J.R.C. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 2003;34:517–547. [Google Scholar]
- Altizer S., Davis A.K., Cook K.C., Cherry J.J. Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Can. J. Zool.-Rev. Can. Zool. 2004;82:755–763. [Google Scholar]
- Altizer S., Dobson A.P., Hosseini P., Hudson P., Pascual M., Rohani P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Ayouba A., Mouacha F., Learn G.H., Mpoudi‐Ngole E., Rayner J.C., Sharp P.M. Ubiquitous Hepatocystis infections, but no evidence of Plasmodium falciparum‐like malaria parasites in wild greater spot‐nosed monkeys (Cercopithecus nictitans) Int. J. Parasitol. 2012;42:709–713. doi: 10.1016/j.ijpara.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M., Mboumba S., Willaume E., Kappeler P.M., Charpentier M.J.E. The oxidative cost of unstable social dominance. J. Exp. Biol. 2014;217:2629–2632. doi: 10.1242/jeb.104851. [DOI] [PubMed] [Google Scholar]
- Beaulieu M., Benoit L., Abaga S., Kappeler P.M., Charpentier M.J.E. Mind the cell: seasonal variation in telomere length mirrors changes in leucocyte profile. Mol. Ecol. 2017;26:5603–5613. doi: 10.1111/mec.14329. [DOI] [PubMed] [Google Scholar]
- Bolker B.M., Brooks M.E., Clark C.J., Geange S.W., Poulsen J.R., Stevens M.H.H., White J.-S.S. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Boundenga L., Ngoubangoye B., Mombo I.M., Tsoubmou T.A., Renaud F., Rougeron V., Prugnolle F. Extensive diversity of malaria parasites circulating in Central African bats and monkeys. Ecol. Evol. 2018;8:10578–10586. doi: 10.1002/ece3.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer T., Kappeler P.M., Willaume E., Benoit L., Mboumba S., Charpentier M.J.E. Social organization and space use of a wild mandrill (Mandrillus sphinx) group. Am. J. Primatol. 2015;77:1036–1048. doi: 10.1002/ajp.22439. [DOI] [PubMed] [Google Scholar]
- Charpentier M.J.E., Givalois L., Faurie C., Soghessa O., Simon F., Kappeler P.M. Seasonal glucocorticoid production correlates with a suite of small-magnitude environmental, demographic, and physiological effects in mandrills. Am. J. Phys. Anthropol. 2018;165:20–33. doi: 10.1002/ajpa.23329. [DOI] [PubMed] [Google Scholar]
- Coatney G.R., Collins W.E., Warren M., Contacos P.G. CDC; Atlanta, GA: 2003. CD-ROM. The Primate Malarias [original Book Published 1971]. Version 1.0. [Google Scholar]
- Costantini D. Oxidative stress ecology and the d-ROMs test: facts, misfacts and an appraisal of a decade's work. Behav. Ecol. Sociobiol. 2016;70:809–820. doi: 10.1007/s00265-016-2091-5. [DOI] [Google Scholar]
- Crespi E.J., Williams T.D., Jessop T.S., Delehanty B. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct. Ecology. 2013;27:93–106. doi: 10.1111/1365-2435.12009. [DOI] [Google Scholar]
- Daviews C.R., Ayres J.M., Dye C., Deane L.M. Malaria infection rate of amazonian primates increases with body weight and group size. Funct. Ecol. 1991;5:655–662. doi: 10.2307/2389485. [DOI] [Google Scholar]
- Davis A.K., Cook K.C., Altizer S. Leukocyte profiles in wild house finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. EcoHealth. 2004;1:362–373. doi: 10.1007/s10393-004-0134-2. [DOI] [Google Scholar]
- Davis A.K., Maney D.L., Maerz J. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 2008;22:760–772. doi: 10.1111/j.1365-2435.2008.01467.x. [DOI] [Google Scholar]
- De Beaudrap P., Nabasumba C., Grandesso F., Turyakira E., Schramm B., Boum Y., Etard J.F. Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda. Malar. J. 2011;10:132. doi: 10.1186/1475-2875-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nys H.M., Calvignac-Spencer S., Thiesen U., Boesch C., Wittig R.M., Mundry R., Leendertz F.H. Age-related effects on malaria parasite infection in wild chimpanzees. Biol. Lett. 2013;9 doi: 10.1098/rsbl.2012.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nys H.M., Calvignac-Spencer S., Boesch C., Dorny P., Wittig R.M., Mundry R., Leendertz F.H. Malaria parasite detection increases during pregnancy in wild chimpanzees. Malar. J. 2014;13 doi: 10.1186/1475-2875-13-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini L. The relationship between body temperature and malaria parasitaemia in rural forest areas of Western Nigeria. J. Trop. Med. Hyg. 1973;76:111–114. [PubMed] [Google Scholar]
- Doeschl-Wilson A.B., Davidson R., Conington J., Roughsedge T., Hutchings M.R., Villanueva B. Implications of host genetic variation on the risk and prevalence of infectious diseases transmitted through the environment. Genetics. 2011;188:683–693. doi: 10.1534/genetics.110.125625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan D.L., Dobaño C., Baird J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G.P., Wilson P.R., Taylor R.H., Beggs J.R. Declines in common, widespread native birds in a mature temperate forest. Biol. Conserv. 2010;143:2119–2126. doi: 10.1016/j.biocon.2010.05.022. [DOI] [Google Scholar]
- Frölich S., Entzeroth R., Wallach M. Comparison of protective immune responses to apicomplexan parasites. J. Parasitol. Res. 2012 doi: 10.1155/2012/852591. [WWW Document] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbany J., Romero A., Mayo-Alesón M., Itsoma F., Gamarra B., Pérez-Pérez A., Willaume E., Kappeler P.M., Charpentier M.J.E. Age-related tooth wear differs between forest and savanna primates. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. A clinical study of falciparum and vivax malaria in Vietnam servicemen. Mil. Med. 1968;133:991–996. [PubMed] [Google Scholar]
- Goselle O.N., Onwuliri C.O.E., Onwuliri V.A. Malaria infection in HIV/AIDS patients and its correlation with packed cell volume (PCV) J. Vector Borne Dis. 2009;46:205–211. [PubMed] [Google Scholar]
- Herbert A., Boundenga L., Meyer A., Moukodoum D.N., Okouga A.P., Arnathau C., Durand P., Magnus J., Ngoubangoye B., Willaume E., Ba C.T., Rougeron V., Renaud F., Ollomo B., Prugnolle F. Malaria-like symptoms associated with a natural Plasmodium reichenowi infection in a chimpanzee. Malar. J. 2015;14 doi: 10.1186/s12936-015-0743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C.B., Hausler H., Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 1998;2:96–104. [PubMed] [Google Scholar]
- Hoye B.J., Fouchier R.A., Klaassen M. Host behaviour and physiology underpin individual variation in avian influenza virus infection in migratory Bewick's swans. Proc. Biol. Sci. 2012;279:529–534. doi: 10.1098/rspb.2011.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M., Löwa A., Ulrich M., Ellerbrok H., Goffe A.S., Blasse A., Zommers Z., Couacy-Hymann E., Babweteera F., Zuberbühler K., Metzger S., Geidel S., Boesch C., Gillespie T.R., Leendertz F.H. Wild chimpanzees infected with 5 Plasmodium species. Emerg. Infect. Dis. 2010;16:1956–1959. doi: 10.3201/eid1612.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B.F., al E. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Knowles S.C.L., Wood M.J., Alves R., Wilkin T.A., Bensch S., Sheldon B.C. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol. Ecol. 2011;20:1062–1076. doi: 10.1111/j.1365-294X.2010.04909.x. [DOI] [PubMed] [Google Scholar]
- Korpimaki E., Hakkarainen H., Bennett G.F. Blood parasites and reproductive success of Tengmalm's owls: detrimental effects on females but not on males? Funct. Ecol. 1993;7:420–426. [Google Scholar]
- Kotepui M., Piwkham D., PhunPhuech B., Phiwklam N., Chupeerach C., Duangmano S. Effects of malaria parasite density on blood cell parameters. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachish S., Knowles S.C.L., Alves R., Wood M.J., Sheldon B.C. Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J. Anim. Ecol. 2011;80:1196–1206. doi: 10.1111/j.1365-2656.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- Liu W., Li Y., Learn G.H., Rudicell R.S., Robertson J.D., Keele B.F., Ndjango J.-B.N., Sanz C.M., Morgan D.B., Locatelli S., Gonder M.K., Kranzusch P.J., Walsh P.D., Delaporte E., Mpoudi-Ngole E., Georgiev A.V., Muller M.N., Shaw G.M., Peeters M., Sharp P.M., Rayner J.C., Hahn B.H. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato E., Moreno J., Merino S., Sanz J.J., Arriero E. Haematological variables are good predictors of recruitment in nestling pied flycatchers (Ficedula hypoleuca) Ecoscience. 2005;12:27–34. doi: 10.2980/i1195-6860-12-1-27.1. [DOI] [Google Scholar]
- Mahieux R., Chappey C., Georges-Courbot M.C., Dubreuil G., Mauclere P., Georges A., Gessain A. Simian T-cell lymphotropic virus type 1 from Mandrillus sphinx as a simian counterpart of human T-cell lymphotropic virus type 1 subtype D. J. Virol. 1998;72:10316–10322. doi: 10.1128/jvi.72.12.10316-10322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuwa M., Souquiere S., Clifford S.L., Telfer P.T., Salle B., Bourry O., Onanga R., Mouinga-Ondeme A., Wickings E.J., Abernethy K.A., Rouquet P., Simon F., Roques P. Two distinct STLV-1 subtypes infecting Mandrillus sphinx follow the geographic distribution of their hosts. AIDS Res. Hum. Retrovir. 2004;20:1137–1143. doi: 10.1089/aid.2004.20.1137. [DOI] [PubMed] [Google Scholar]
- Mapua M.I., Qablan M.A., Pomajbíková K., Petrželková K.J., Hůzová Z., Rádrová J., Votýpka J., Todd A., Jirků M., Leendertz F.H., Lukeš J., Neel C., Modrý D. Ecology of malaria infections in western lowland gorillas inhabiting Dzanga Sangha Protected Areas, Central African Republic. Parasitology. 2015;142:890–900. doi: 10.1017/S0031182015000086. [DOI] [PubMed] [Google Scholar]
- McCallum H., Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- McCallum H., Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- McDonald J.L., Robertson A., Silk M.J. Wildlife disease ecology from the individual to the population: insights from a long-term study of a naturally infected European badger population. J. Anim. Ecol. 2018;87:101–112. doi: 10.1111/1365-2656.12743. [DOI] [PubMed] [Google Scholar]
- Monaghan P., Metcalfe N.B., Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Ngoubangoye B., Boundenga L., Arnathau C., Mombo I.M., Durand P., Tsoumbou T.A., Otoro B.V., Sana R., Okouga A.P., Moukodoum N., Willaume E., Herbert A., Fouchet D., Rougeron V., Bâ C.T., Ollomo B., Paupy C., Leroy E.M., Renaud F., Pontier D., Prugnolle F. The host specificity of ape malaria parasites can be broken in confined environments. Int. J. Parasitol. 2016;46:737–744. doi: 10.1016/j.ijpara.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Norley S., Beer B., Holzammer S., zur Megede J., Kurth R. Why are the natural hosts of SIV resistant to AIDS? Immunol. Lett. 1999;66:47–52. doi: 10.1016/s0165-2478(98)00184-9. [DOI] [PubMed] [Google Scholar]
- Nsi Akoué G., Mbading-Mbading W., Willaume E., Souza A., Mbatchi B., Charpentier M.J.E. Extreme diet seasonality in wild mandrills living in Equatorial forests. Ethology. 2017;123:600–613. [Google Scholar]
- Nunn C.L., Lindenfors P., Pursall E.R., Rolff J. On sexual dimorphism in immune function. Philos. Trans. R. Soc. Biol. Sci. 2009;364:61–69. doi: 10.1098/rstb.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onanga R., Souquiere S., Makuwa M., Mouinga-Ondeme A., Simon F., Apetrei C., Roques P. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx) J. Virol. 2006;80:3301–3309. doi: 10.1128/JVI.80.7.3301-3309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppliger A., Christe P., Richner H. Clutch size and malarial parasites in female great tits. Behav. Ecol. 1997;8:148–152. doi: 10.1093/beheco/8.2.148. [DOI] [Google Scholar]
- Otto T.D., Gilabert A., Crellen T., Böhme U., Arnathau C., Sanders M., Oyola S.O., Okouga A.P., Boundenga L., Willaume E., Ngoubangoye B., Moukodoum N.D., Paupy C., Durand P., Rougeron V., Ollomo B., Renaud F., Newbold C., Berriman M., Prugnolle F. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nat. Microbiol. 2018;3:687–697. doi: 10.1038/s41564-018-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans K.P., Read A.F., Thomas M.B. Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S., Rege M., Gogtay N.J., Aigal U., Sharma S.K., Valecha N., Bhanot G., Kshirsagar N.A., Sharma S. Age-dependent sex bias in clinical malarial disease in hypoendemic regions. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percário S., Moreira D.R., Gomes B.A.Q., Ferreira M.E.S., Gonçalves A.C.M., Laurindo P.S.O.C., Vilhena T.C., Dolabela M.F., Green M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012;13:16346–16372. doi: 10.3390/ijms131216346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S.L. Malaria's many mates: past, present, and future of the systematics of the order Haemosporida. J. Parasitol. 2014;100:11–25. doi: 10.1645/13-362.1. [DOI] [PubMed] [Google Scholar]
- Perkins S.L., Schall J.J. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitol. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Poirotte C., Basset D., Willaume E., Makaba F., Kappeler P.M., Charpentier M.J.E. Environmental and individual determinants of parasite richness across seasons in a wild population of mandrills (Mandrillus sphinx) Am. J. Phys. Anthropol. 2015;159:442–456. doi: 10.1002/ajpa.22888. [DOI] [PubMed] [Google Scholar]
- Poirotte C., Massol F., Herbert A., Willaume E., Bomo P.M., Kappeler P.M., Charpentier M.J.E. Mandrills use olfaction to socially avoid parasitized conspecifics. Sci. Adv. 2017 doi: 10.1126/sciadv.1601721. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall S.P., Muehlenbein M.P. Testosterone and immune function in primates: a brief summary with methodological considerations. Int. J. Primatol. 2014;35:805–824. doi: 10.1007/s10764-014-9752-x. [DOI] [Google Scholar]
- Prugnolle F., Durand P., Neel C., Ollomo B., Ayala F.J., Arnathau C., Etienne L., Mpoudi-Ngole E., Nkoghe D., Leroy E., Delaporte E., Peeters M., Renaud F. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1458–1463. doi: 10.1073/pnas.0914440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwöbel B., Alifrangis M., Salanti A., Jelinek T. Different mutation patterns of atovaquone resistance to Plasmodium falciparum in vitro and in vivo: rapid detection of codon 268 polymorphisms in the cytochrome b as potential in vivo resistance marker. Malar. J. 2003;2 doi: 10.1186/1475-2875-2-5. 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell J.M. Behavioural development in male mandrills (Mandrillus sphinx): puberty to adulthood. Behaviour. 2003;140:1053–1089. [Google Scholar]
- Setchell J.M., Lee P.C., Wickings E.J., Dixson A.F. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx) Am. J. Phys. Anthropol. 2001;115:349–360. doi: 10.1002/ajpa.1091. [DOI] [PubMed] [Google Scholar]
- Setchell J.M., Charpentier M.J.E., Bedjabaga I.B., Reed P., Wickings E.J., Knapp L.A. Secondary sexual characters and female quality in primates. Behav. Ecol. Sociobiol. 2006;61:305–315. doi: 10.1007/s00265-006-0260-7. [DOI] [Google Scholar]
- Setchell J.M., Smith T., Wickings E.J., Knapp L.A. Social correlates of testosterone and ornamentation in male mandrills. Horm. Behav. 2008;54:365–372. doi: 10.1016/j.yhbeh.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Souquiere S., Bibollet-Ruche F., Robertson D.L., Makuwa M., Apetrei C., Onanga R., Kornfeld C., Plantier J.C., Gao F., Abernethy K., White L.J.T., Karesh W., Telfer P., Wickings E.J., Mauclere P., Marx P.A., Barre-Sinoussi F., Hahn B.H., Muller-Trutwin M.C., Simon F. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 2001;75:7086–7096. doi: 10.1128/JVI.75.15.7086-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquière S., Onanga R., Makuwa M., Pandrea I., Ngari P., Rouquet P., Bourry O., Kazanji M., Apetrei C., Simon F., Roques P. Simian immunodeficiency virus types 1 and 2 (SIV mnd 1 and 2) have different pathogenic potentials in rhesus macaques upon experimental cross-species transmission. J. Gen. Virol. 2009;90:488–499. doi: 10.1099/vir.0.005181-0. [DOI] [PubMed] [Google Scholar]
- Springer A., Fichtel C., Calvignac-Spencer S., Leendertz F.H., Kappeler P.M. Hemoparasites in a wild primate: infection patterns suggest interaction of Plasmodium and Babesia in a lemur species. Int. J. Parasitol. Parasites Wildl. 2015;4:385–395. doi: 10.1016/j.ijppaw.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarello W. A fatal Plasmodium reichenowi infection in a chimpanzee? Rev. Med. Vet. 2005;156:503–505. [Google Scholar]
- Taylor D.W., Wells R.A., Vernes A., Rosenberg Y.J., Vogel S., Diggs C.L. Parasitologic and immunologic studies of experimental Plasmodium falciparum infection in nonsplenectomized chimpanzees (Pan troglodytes) Am. J. Trop. Med. Hyg. 1985;34:36–44. doi: 10.4269/ajtmh.1985.34.36. [DOI] [PubMed] [Google Scholar]
- van de Crommenacker J., Richardson D.S., Koltz A.M., Hutchings K., Komdeur J. Parasitic infection and oxidative status are associated and vary with breeding activity in the Seychelles warbler. Proc. R. Soc. Biol. Sci. 2012;279:1466–1476. doi: 10.1098/rspb.2011.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.