Abstract
Samples of diaphragm were collected from 53 sika deer from Gifu Prefecture, Japan; 220 sarcocysts were isolated, examined in wet mounts and classified according to their cyst wall protrusions. The sarcocysts were then examined molecularly in order to assign them to different species. All but 11 of the 220 sarcocysts were initially identified by means of a multiplex PCR assay targeting cox1 of five species, whereas the remaining 11 sarcocysts were identified by standard PCR and sequencing. DNA from selected sarcocysts was used for PCR amplification and sequencing of cox1 (59 sequences) and 18S rDNA (23 sequences). The 220 sarcocysts comprised seven major cox1 sequence types or species. Types 4 and 7 were assigned to the known species Sarcocystis pilosa and Sarcocystis ovalis, whereas types 1, 3 and 5 were considered to represent three new species, for which the names Sarcocystis japonica, Sarcocystis matsuoae and Sarcocystis gjerdei have been proposed. Types 2 and 6 were most similar to Sarcocystis tarandi and Sarcocystis taeniata, respectively, but could not be unequivocally assigned to these species. Sarcocysts belonging to S. japonica were macroscopic with fairly thick finger-like protrusions, whereas most sarcocysts of the six other species were microscopic. Sarcocysts of S. cf. tarandi and S. matsuoae were spindle-shaped and possessed thin finger-like cyst-wall protrusions. Sarcocysts of S. pilosa and S. gjerdei had similar hair-like protrusions, whereas those of S. cf. taeniata had a smooth surface. Sarcocysts of S. japonica, S. pilosa, S. cf. tarandi, S. gjerdei, S. matsuoae, S. cf. taeniata and S. ovalis were found in 50 (94.3%), 29 (54.7%), 22 (41.5%), 10 (18.9%), 8 (15.1%), 6 (11.3%) and 1 (1.9%) of the 53 sika deer examined, respectively. An improved multiplex PCR assay targeting cox1 was developed, through which the seven Sarcocystis spp. found in the present study could be identified.
Keywords: Sarcocystis japonica, Sarcocystis matsuoae, Sarcocystis gjerdei, Cervus nippon centralis, 18S ribosomal RNA gene, Cytochrome c oxidase subunit I gene, Japan
Graphical abstract
Highlights
-
•
220 sarcocysts from 53 sika deer in Japan were examined morphologically and genetically.
-
•
The sarcocysts belonged to seven cox1 sequence types or species.
-
•
Three types represented new species, Sarcocystis japonica, S. matsuoae and S. gjerdei.
-
•
Two types represented the known species, Sarcocystis pilosa and S. ovalis.
-
•
Two types were closely similar to S. tarandi and S. taeniata, but could not be assigned to both species.
1. Introduction
The genus Sarcocystis comprises intracellular protozoan parasites that require two hosts, usually in a prey-predator relationship, to maintain their life cycle. More than 200 species in various mammals, birds and reptiles have been reported as valid based on their definitive or intermediate hosts, cross transmission studies, sarcocyst morphology and/or comparative molecular studies (Prakas et al., 2016, 2017; Gjerde, 2016b; Gjerde et al., 2017a, b, 2018). Prior to the molecular era, sarcocysts in intermediate hosts were assigned to species mainly on the basis of their morphology as seen in wet mounts or histological sections by light microscopy (LM) or in ultrathin sections by transmission electron microscopy (TEM). Moreover, it was assumed that Sarcocystis spp. were intermediate host specific and that morphologically indistinguishable sarcocysts in different intermediate hosts therefore belonged to separate species. Molecular studies in recent years have shown, however, that some Sarcocystis spp. may infect more than one intermediate host (e.g., Sarcocystis ovalis in moose, red deer and sika deer) (Dahlgren and Gjerde, 2008, 2010; Abe, 2014; Rudaitytė-Lukošienė et al., 2018), and that morphologically indistinguishable sarcocysts in a given host might belong to more than one species (e.g., Sarcocystis cervicanis and Sarcocystis linearis in red deer (Gjerde et al., 2017b). Therefore, molecular analysis using appropriate markers has become an essential tool in order to make an accurate identification of sarcocysts in the muscles of different intermediate hosts and for identification of Sarcocystis oocysts/sporocysts in the intestine or feces of definitive hosts. Moreover, molecular data make it possible to establish the phylogenetic relationships between different species, which again makes it possible to predict the likely definitive hosts of species for which the complete life cycle has not yet been established.
As regards Sarcocystis species in cervids, nucleotide sequences of the nuclear 18S ribosomal RNA gene (18S rDNA) and/or the mitochondrial cytochrome c oxidase subunit I gene (cox1) of about 30 named species are currently available for comparative molecular studies in public nucleotide databases (Gjerde et al., 2017b; Rudaitytė-Lukošienė et al., 2018). The cox1 marker has been shown to be superior to the 18S rDNA for many Sarcocystis spp. of cervids and other ruminant intermediate hosts, since cox1 is better able to resolve unclear species boundaries of closely related species (Gjerde, 2013; Gjerde et al., 2017a, b; Rudaitytė-Lukošienė et al., 2018). However, neither the 18S rDNA nor the cox1 marker is able to discriminate between some Sarcocystis spp. using birds or carnivores as definitive hosts, and therefore the internal transcribed spacer 1 region (ITS1) has become the marker of choice for identification of the latter taxa (Gjerde et al., 2018).
Seven Sarcocystis spp. have been characterized both morphologically and molecularly (18S rDNA, cox1) from farmed sika deer in Lithuania (Prakas et al., 2016; Rudaitytė-Lukošienė et al., 2018). Those species were Sarcocystis entzerothi, Sarcocystis frondea, Sarcocystis nipponi, Sarcocystis ovalis, Sarcocystis taeniata, Sarcocystis truncata and Sarcocystis pilosa. The sika deer in those studies descended from animals imported to Lithuania from the Caucasus region (Chechnya) and the Far East (Vladivostok) of Russia.
In Japan, several surveys of Sarcocystis infection in sika deer have been performed and they have all shown a high prevalence (Saito et al., 1998; Narisawa et al., 2008; Saito and Hagiwara, 2013; Matsuo et al., 2014). However, in these studies, the isolates from sika deer were only tentatively identified on the basis of their morphological similarity to other named species, and no comparative molecular studies with already known Sarcocystis species were performed. Thus, Arai et al. (2010) and Saito and Hagiwara (2013), reporting in part from the same study, identified their isolates as Sarcocystis sybillensis and Sarcocystis wapiti on the basis of the sarcocyst morphology as seen by LM and TEM and a successful experimental transmission to dogs. More recently, Irie et al. (2017) identified Sarcocystis ovalis in sika deer on the basis of an 18S rDNA sequence originally obtained from an unnamed Sarcocystis sp. in the study by Takano et al. (2006). In addition, about 20 nucleotide sequences (of 18S rDNA) generated from sarcocysts in sika deer in Japan have been deposited in GenBank without an accompanying description of the sarcocyst morphology. Most of these sequences derive from unpublished studies, but five sequences are from the recent study by Sugita-Konishi et al. (2019), focusing on food poisoning. Based on their phylogenetic position, the majority of these 18S rDNA sequences from sika deer have been found to be closely related to those of S. truncata from red deer (Dahlgren and Gjerde, 2010; Gjerde, 2014a; Ota et al., 2019; Sugita-Konishi et al., 2019).
As regards the use of the cox1 marker for identification of Sarcocystis spp. in sika deer in Japan, we recently obtained cox1 sequences from 21 sarcocysts from nine sika deer in Gifu prefecture, and identified five Sarcocystis species, of which three species (Types 1, 3 and 5) seemed to be new, that is, not previously well characterized and named (Abe et al., 2019). However, the sarcocysts used in that study, which were obtained from the study by Matsuo et al. (2016), were only cursorily examined under a stereo microscope in connection with their isolation, and hence no detailed morphological data were recorded for each sarcocyst. Therefore, the new species were not named. Similarly, Irie et al. (2019), identified three sarcocyst types in histological sections of the diaphragm of 65 Hokkaido sika deer (Cervus nippon yesoensis) from Hokkaido, Japan, and characterized six DNA isolates of each morphological type at 18S rDNA and cox1 following laser microdissection of sections of formalin fixed sarcocysts. They identified three sequence types at both markers used (Sarcocystis truncata-like, S. tarandi-like, S. pilosa), which corresponded to Types 1, 2 and 4 of our recent study (Abe et al., 2019), but they did not name the species with type 1 sarcocysts and sequences.
When describing and naming new Sarcocystis spp., it is desirable and useful to link molecular data to data on sarcocyst morphology, since this will make it possible to identify tentatively to species sarcocysts found in newly collected material, as well as sarcocysts previously described in the literature. Therefore, in the present study, 220 sarcocysts from 53 sika deer in Gifu prefecture were isolated and examined both morphologically by light microscopy and molecularly by species specific multiplex PCR targeting cox1 followed by standard PCR amplification and sequencing of cox1 and 18S rDNA of selected isolates. This approach made it possible to identify and characterize both morphologically and molecularly seven Sarcocystis species, of which three have been described and named as new species in the following sections.
2. Materials and methods
2.1. Isolation and microscopic examination of sarcocysts; DNA extraction
Samples of the diaphragm (approximately 50–100 g) were collected from 53 sika deer (Cervus nippon centralis) killed during 2017 in Gifu Prefecture (20 for damage control and academic research before the hunting period and 33 for hunting during the regular hunting period in November). The samples were collected during the processing of the carcasses, mostly for human consumption, and placed separately from each animal into labelled plastic bags. The samples were stored at −20 °C until examined at the Department of Veterinary Parasitological Diseases, Gifu University. There, the frozen samples were thawed in a refrigerator (4 °C), and subsamples containing sarcocysts were identified under a stereomicroscope. Frozen positive samples were then transferred to the Division of Microbiology, Osaka Institute of Public Health, for further examination. Following thawing, one to six sarcocysts per sample (animal) were excised under a stereomicroscope (model NSZ-405, Shodensha Co., Ltd., Osaka) using disposable fine needles and tweezers. From each sample, sarcocysts displaying different morphological features (macroscopic or microscopic; thread-like, fusiform or nearly oval) were isolated whenever possible. A total of 220 sarcocysts were collected and mounted separately in a drop of water on a glass slides for morphological examination under a stereomicroscope and a biological microscope (ECLIPS E800, Nikon Corp., Tokyo). Measurements of length and maximum diameter were made of all sarcocysts and some of their protrusions, and digital photographs of some sarcocysts were recorded. Following the microscopic examination, the sarcocysts were carefully lifted with a needle from the wet mount and transferred to labelled micro-centrifuge tubes containing 70% ethanol. The tubes were stored in a refrigerator (4 °C) until extraction of genomic DNA from the sarcocysts using the DNeasy Blood and Tissue Kit (Qiagen GmbH, Hilden, Germany). Extracted DNA was then stored at −20 °C.
2.2. Multiplex PCR
Genomic DNA from all 220 sarcocysts was initially tested separately using the previously described multiplex PCR assay (Abe et al., 2019) with a slight modification of the reaction conditions. This assay was able to detect and discriminate between five Sarcocystis spp. with types 1 to 5 cox1 sequences in a single reaction. From 11 sarcocysts no amplification products were obtained with this assay. DNA from these samples were therefore amplified and sequenced with primer pair SF1/SR9 as described for the standard PCR in the next section, and two additional cox1 sequence types (6 and 7) were obtained. Based on the new sequences, two new primer sets for identification of Sarcocystis species with types 6 and 7 cox1 sequences were designed with the Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Sequences of the new primers are listed in Table S1 in Supplementary material along with those of the previously developed specific primers.
The specificity of each of the new primer pairs was tested against templates containing DNA of the targeted species only, or a mixture of DNA from either two or four of the six other species. The ability of the expanded multiplex PCR assay to detect and discriminate between several Sarcocystis spp. simultaneously in templates containing DNA from multiple species was also tested. In one assay, specific primers targeting Sarcocystis spp. with type 1, 3, 4 and 5 sequences, respectively, were tested against DNA from these species in a single PCR tube, while in a second assay primers targeting Sarcocystis spp. with type 2, 6 and 7 sequences, respectively, were tested against DNA from these species in another PCR tube. The PCR amplifications were performed in a final volume of 25 μl containing 1.25 units of TaKaRa Ex Taq Hot Start Version (TaKaRa Shuzo Co. Ltd., Otsu, Japan), 1 × PCR buffer, 2 mM MgCl2, 250 μM of each dNTP (these reagents were supplied with the polymerase), 0.2 μM of each primer, and 2.5 μl of the DNA sample. The PCR reactions were conducted under the following conditions: samples were denatured at 94 °C for 5 min and then subjected to 40 cycles of 94 °C for 30 s, 65 °C (64 °C for Sarcocystis species with types 2, 6 and 7 sequences) for 30 s, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. Reactions were performed using a GeneAmp PCR System 9700 thermocycler (Applied Biosystems, CA, USA). The PCR products were separated on 3% agarose gels and visualized under UV light after staining with ethidium bromide.
2.3. Molecular examination of sarcocysts using standard PCR and cloning
The selection of isolates for amplification at cox1 (59 isolates) and/or 18S rDNA (23 isolates) was based on the preliminary identification of 209 sarcocysts by multiplex PCR (types 1–5) and of 11 sarcocysts (types 6 and 7) by sequencing of cox1 (Section 2.2). For comparative purposes, an aliquot of a DNA sample extracted from the intestinal mucosa of a Japanese jungle crow (Corvus macrorhynchos) in Hokkaido (Irie et al., 2017) in which DNA of S. ovalis had been detected through amplification of the 18S rDNA (sequence LC184601), was also included for amplification of cox1.
A 1085-bp-long portion of cox1 was PCR-amplified using primers SF1 and SR9, whereas the complete 18S rDNA (approximately 1900 bp) was amplified using primers ERIB1 and Primer BSarc as described previously (Gjerde, 2013, 2014a, 2014b; Gjerde et al., 2017a, b). The PCR amplification was performed in a final volume of 25 μl containing the same reagents as described for the multiplex PCR. After denaturation at 94 °C for 5 min, the PCR samples were subjected to 40 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. Aliquots of the PCR products were separated and visualized as for multiplex PCR.
The PCR products were purified using either the QIAquick Gel Extraction Kit or the QIAquick PCR Purification Kit (Qiagen GmbH, Hilden, Germany). The amplicons were cloned into JM109-competent cells (Competent high JM109; Toyobo Co. Ltd., Osaka, Japan) using the Target Clone (Toyobo Co. Ltd., Osaka, Japan) according to the manufacturer's instructions. Transformed cells were plated onto LB plates (LB Broth Base; Invitrogen Corp., Carlsbad, CA, USA) containing 100 μg/ml of ampicillin. The colonies were grown overnight at 37 °C. The insertion was confirmed using the premix colony direct PCR kit (Insert Check Ready; Toyobo Co. Ltd., Osaka, Japan), and one to three positive colonies from each isolate were processed and sequenced using primers SF1, SR9, SF6 and SR66 (Gjerde, 2016b) for cox1, and primers ERIB1, Primer BSarc, S5f and S4r (Barta et al., 1997; Fischer and Odening, 1998; Gjerde, 2014b) for 18S rDNA. In addition, primers M13-P7 and M13-P8, targeting areas near the multi-cloning sites of plasmid vector pTA 2, were used to confirm the sequences of the PCR primer regions. Sequences of primers used for PCR amplification and/or sequencing of cox1 and 18S rDNA are listed in Table S2 in Supplementary material. DNA sequencing was performed using BigDye Terminator v3.1 Cycle Sequencing kit on an automated ABI3130 sequencer (Applied Biosystems, Foster City, CA, USA).
2.4. Sequence analysis
Sequence chromatograms from each strand were inspected using Sequencher Version 4.1 (Gene Codes Corp., Ann Arbor, MI, USA). Nucleotide similarity searches with cox1 and 18S rDNA sequences obtained in the present study were performed using the BLAST program of the National Center for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The software package DnaSP (DNA Sequence polymorphism) version 5.10.01 (Librado and Rozas, 2009) was used for the analysis of nucleotide polymorphisms among the new cox1 sequences and those of the previous study (Abe et al., 2019) and for comparison of these sequences with those of some closely related Sarcocystis species. The nucleotide sequence data reported herein were deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers LC481011–LC481033 (18S rDNA) and LC481034–LC481093 (cox1).
2.5. Phylogenetic analyses
Phylogenetic analyses were conducted separately on nucleotide sequences of cox1 and 18S rDNA using MEGA7 (Kumar et al., 2016). In both analyses, the phylogeny was tested with the bootstrap method, using 1000 bootstrap replications, and Toxoplasma gondii was used as outgroup species to root the trees. Alignments of both loci were obtained using the ClustalW program integrated in MEGA7 as described previously (Gjerde, 2013; Gjerde et al., 2017b). As regards cox1, a total of 322 sequences from 61 taxa were used in the analysis, including 60 new sequences from the present study (Table S3 in Supplementary material). The final alignment comprised 1020 positions with no gaps. The phylogenetic tree was reconstructed using the neighbour-joining method with the p-distance algorithm and the pairwise deletion option. All codon positions were used.
As regards 18S rDNA, a total of 247 sequences from 60 taxa were used in the analysis including 23 new sequences from the present study (Table S3). The final alignment comprised 2041 aligned positions, including gaps. The phylogenetic tree was reconstructed using the maximum parsimony (MP) method with the subtree-pruning-regrafting (SPR) algorithm. All sites were used.
3. Results
3.1. Cox1 sequence typing by multiplex PCR
Sarcocysts were found and isolated from the diaphragm of all 53 sika deer sampled, and thus the overall prevalence of Sarcocystis infection was 100%. In the initial screening of the 220 sarcocysts with the previously developed multiplex PCR assay targeting five cox1 sequence types (Abe et al., 2019), 209 sarcocysts could be identified as Sarcocystis spp. with types 1–5 cox1 sequences, while 11 sarcocysts were negative for these types (no amplification). DNA from the latter sarcocysts were then subjected to amplification and sequencing by standard PCR. Ten of these sequences, designated type 6, were most similar to sequences of S. taeniata, whereas the single type 7 sequences belonged to S. ovalis as will be further described in the next section. Hence, based on the screening by multiplex PCR and the additional sequencing of 11 isolates, the 220 sarcocysts from 53 sika deer were found to belong to seven major sequence types, which, based on additional data obtained through standard PCR, were considered to represent seven distinct Sarcocystis spp. (Table 1).
Table 1.
Frequency of different cox1 sequence types/species among 220 sarcocysts from 53 sika deer based on type-specific multiplex PCR.
| Types of cox1 sequences | Type distribution (%) in 220 sarcocysts | Occurrence of each type (%) in 53 sika deer |
|---|---|---|
| Type 1 (Sarcocystis japonica n. sp.) |
45.9 (101/220) | 94.3 (50/53) |
| Type 2 (Sarcocystis cf. tarandi) |
14.1 (31/220) | 41.5 (22/53) |
| Type 3 (Sarcocystis matsuoae n. sp.) |
5.9 (13/220) | 15.1 (8/53) |
| Type 4 (Sarcocystis pilosa) |
23.2 (51/220) | 54.7 (29/53) |
| Type 5 (Sarcocystis gjerdei n. sp.) |
5.9 (13/220) | 18.9 (10/53) |
| Type 6 (Sarcocystis cf. taeniata) |
4.5 (10/220) | 11.3 (6/53) |
| Type 7 (Sarcocystis ovalis) |
0.5 (1/220) | 1.9 (1/53) |
Sarcocystis sp. type 1 was the most frequently found type/species among the 220 sarcocysts identified (45.9%, 101/220), and almost all sika deer (94.3%, 50/53) were infected with this type (Table 1). Sarcocystis sp. type 4 was also common, being found in 23.2% (51/220) of the isolated sarcocysts and in 54.7% (29/53) of the sika deer examined. Sarcocystis sp. type 2 was the third most common type, being found in 14.1% (31/220) of sarcocysts and in 41.5% (22/53) of the sika deer. The remaining four sequence types were each found in only a few sarcocysts and sika deer (Table 1). Mixed infections with sarcocysts of more than one type/species were found in 45 sika deer (84.9%). Thus, 20 (37.7%), 22 (41.5%) and 3 (5.7%) sika deer were infected with two, three and four types, respectively (data not shown).
The two newly designed primer pairs targeting type 6 and type 7 sequences, respectively, were found to amplify the target species only, and not any of the other five species (Fig. S1a). Likewise, the two new primer sets, as well as the five previously designed specific primer sets (Abe et al., 2019), were found to selectively amplify their target species when used together in multiplex PCR assays containing DNA of three or four species (Fig. S1b).
3.2. Characteristics of cox1 and 18S rDNA sequences
A total of 59 cox1 and 23 18S rDNA sequences from 38 to 19 sika deer, respectively, were obtained in the present study (Table S4). All cox1 sequences were 1085-bp-long, including the sequences of both primers, while those of 18S rDNA varied from 1845 to 1921 bp in length, depending on species, including the sequences of both primers.
The cox1 sequences could be classified into seven types (types 1–7), with each type possessing a high sequence identity (>99% in types 1–5 and 7; >98% in type 6) (Table 2), even when the recently typed isolates belonging to types 1–5 (LC349938–LC349976) (Abe et al., 2019) were included. The identities of the new sequences with those of the most similar taxa are shown in Table 2. Sequences of types 1, 3 and 5 were clearly different (>3%) from those of all previously characterized named species, those of types 2 and 6 differed by 1.3–2.4% and 1.7–2.8% from their most similar taxa, S. tarandi and S. taeniata, respectively, whereas sequences of types 4 differed by 0.7–1.6% from those of S. pilosa, and could therefore be assigned to this species. The single sequence of type 7 from a sarcocyst in sika deer was 100% identical with the new cox1 sequence obtained in this study from the DNA sample from the intestinal mucosa of a Japanese jungle crow, which had previously been assumed to harbor oocysts of S. ovalis based on sequencing of 18S rDNA (Irie et al., 2017). Both new sequences shared 97.8–98.9% identity with cox1 sequences of S. ovalis from cervids in Norway, Lithuania and Canada. A comparison of the three sequence types derived from Hokkaido sika deer in the recent study by Irie et al. (2019) showed that their types 1, 2 and 3 sequences corresponded to our types 1, 2 and 4 sequences, respectively (Table 2).
Table 2.
Identity of the seven types of cox1 sequences from the present study with those from other taxa, including sequences obtained from sika deer in Japan.
| Types of cox1 sequences | Sarcocystis spp. closely related to the present types | Sequence identity (%)a |
|---|---|---|
| Type 1 (Sarcocystis japonica n. sp.) |
Sarcocystis sp. Type 1b | 98.5-100 |
| Sarcocystis silva | 95.7-97.6 | |
| Sarcocystis truncata | 96.0-97.0 | |
| Type 2 (Sarcocystis cf. tarandi) |
Sarcocystis sp. Type 2b | 98.5-99.5 |
| Sarcocystis tarandi | 97.6-98.7 | |
| Sarcocystis elongata | 96.5-97.8 | |
| Type 3 (Sarcocystis matsuoae n. sp.) |
Sarcocystis entzerothi | 94.3-94.4 |
| Type 4 (Sarcocystis pilosa) |
Sarcocystis sp. Type 3b | 99.0-99.5 |
| Sarcocystis pilosa | 98.4-99.3 | |
| Sarcocystis hjorti | 95.5-96.3 | |
| Type 5 (Sarcocystis gjerdei n. sp.) |
Sarcocystis iberica | 92.4-92.5 |
| Sarcocystis venatoria | 92.7-92.8 | |
| Type 6 (Sarcocystis cf. taeniata) |
Sarcocystis taeniata | 97.2-98.3 |
| Sarcocystis linearis | 95.1-96.6 | |
| Type 7 (Sarcocystis ovalis) |
Sarcocystis ovalis | 97.8-98.9 |
| Sarcocystis hardangeri | 92.5-92.8 |
The identity with previous cox1 sequences was determined on the basis of a comparison of 1020 or 906 (Sarcocystis entzerothi) overlapping nucleotides.
Three sequence types derived from Hokkaido sika deer in the recent study by Irie et al. (2019).
The 18S rDNA sequences of the isolates examined could also be classified into seven types, with each type possessing a high sequence identity (>99%). The identities of the new sequences with those of the most similar taxa are shown in Table S5. As found for cox1, the 18S rDNA sequences of types 1, 2 and 3 in the study by Irie et al. (2019), showed a high identity with our sequences of types 1, 2 and 4, respectively. Moreover, sequences of Sarcocystis sp. HM050622 from sika deer in Hokkaido (AB151926; AB257085–86; AB257154–62) from an unpublished study, and from an unnamed Sarcocystis sp. from sika deer in Yamanashi Prefecture (LC405947–50) (Sugita-Konishi et al., 2019), all showed a high identity with our new type 1 sequences. Additionally, sequences LC405951 and LC405946 from the latter study showed a high identity with our type 2 and 6 sequences, respectively (Table S5). The single sequence of Sarcocystis sp. type 7 showed 99.1–99.5% identity with sequences of S. ovalis, including the sequences from sika deer and a jungle crow in Hokkaido (Irie et al., 2017).
3.3. Phylogenetic analysis
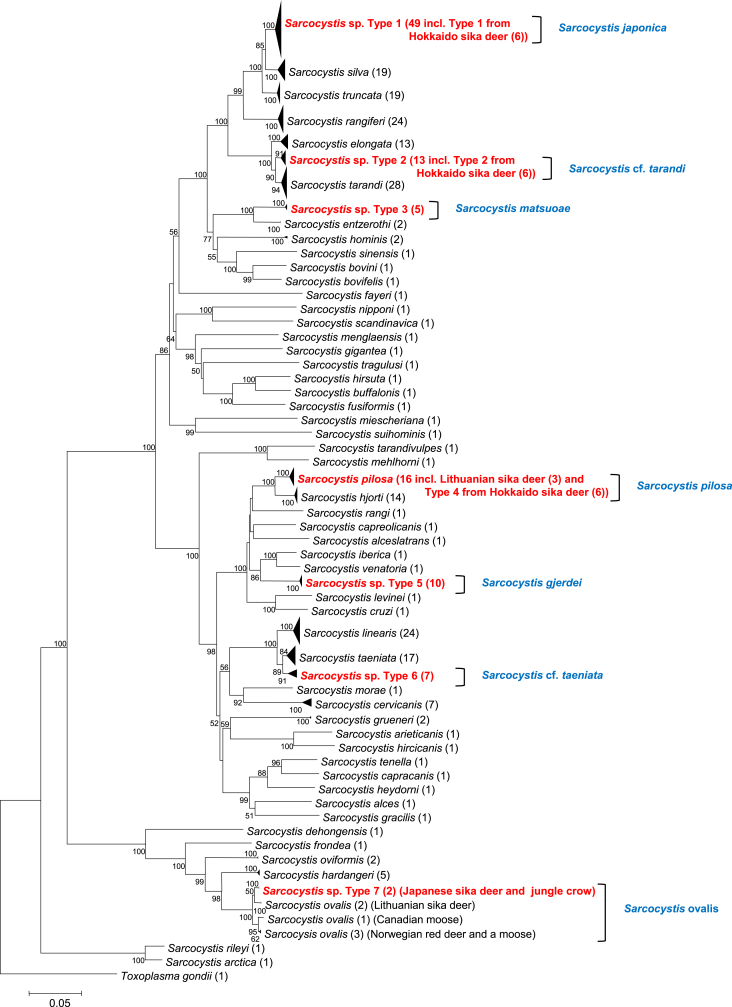
In the phylogenetic analysis based on cox1, the species that shared the highest sequence identities with each cox1 sequence type from sika deer (Table 2) were also placed closest to the seven types in the phylogram (Fig. 1). The 49 sequences of type 1, comprising 33 new sequences from the present study, 10 sequences from our previous study (Abe et al., 2019) and six sequences from the study by Irie et al. (2019) formed a separate clade, which with near maximum bootstrap support was sister to a clade comprising S. silva and S. truncata. Likewise, the five sequences of type 3, including one from our previous study (Abe et al., 2019), was separated with near maximum support from sequences of S. entzerothi. Similarly, the 10 sequences of type 5 including five from our previous study (Abe et al., 2019), were separated with high support from sequences of S. iberica and S. venatoria. The seven sequences of type 4, including two from our previous study (Abe et al., 2019), as well as six new sequences designated type 3 from the recent study by Irie et al. (2019) were placed among, or in close association with, previous sequences of S. pilosa from sika deer in Lithuania. The two new sequences of type 7 were placed close to previous sequences of S. ovalis from moose, red deer and Lithuanian sika deer.
Fig. 1.
Phylogenetic tree based on 322 partial sequences of cox1 of 61 taxa, including the seven Sarcocystis species (types 1–7) from this study and inferred using the neighbour-joining method and with evolutionary distances computed using the p-distance method. Bootstrap support (1000 replicates) is shown at each node. Subtrees formed by two or more haplotypes of the same species have been collapsed. The number of haplotypes included is given in parentheses. The number of sequences of each Sarcocystis species used in this analysis and their GenBank accession numbers are shown in Table S3.
The thirteen sequences of type 2, including six new sequences from the present study, one from our previous study (Abe et al., 2019) and six type 2 sequences from the study by Irie et al. (2019) formed a separate cluster, which was sister to a clade comprising sequences of S. tarandi, whereas S. elongata was a sister clade to both taxa. Sequences of type 6 formed a separate cluster, which was a sister clade to sequences of S. taeniata, whereas both of these taxa were sister to the clade comprising sequences of S. linearis.
The phylogenetic analysis based on18S rDNA using MP (Fig. S2) placed all Sarcocystis spp. with ruminant intermediate hosts into three major clades according to their known or presumed definitive hosts (birds; canids; felids, humans, unknown), as in the analysis using cox1 sequences. Sequences of Sarcocystis spp. type 1, 2 and 6 did not form monophyletic groups, but were interleaved or mixed with sequences of two or three other taxa. Thus, sequences of Sarcocystis sp. type 1 from our study were mixed with those of Sarcocystis sp. type 1 from the study by Irie et al. (2019), with those of Sarcocystis sp. HM00560 from Hokkaido sika deer, those of a Sarcocystis sp. from sika deer in Yamanashi Prefecture, as well as those of S. truncata and S. rangiferi. Likewise, our sequences of type 2 were mixed with those of S. tarandi, S. elongata, S. silva, a second Sarcocystis sp. from sika deer in Yamanashi Prefecture, and type 2 sequences from the study by Irie et al. (2019) (Fig. S3). Moreover, sequences of type 6 were interleaved with sequences of S. taeniata, S. linearis and a third unnamed Sarcocystis sp. from sika deer in Yamanashi Prefecture (Fig. S4). By contrast, sequences of types 3 and 5 formed monophyletic groups and were clearly separated from those of their most closely related species, that is, S. entzerothi concerning type 3 and S. iberica/S. venatoria concerning type 5. Sequences of types 4 and 7, on the other hand, clustered with sequences of S. pilosa and S. ovalis, respectively.
3.4. Sarcocyst morphology
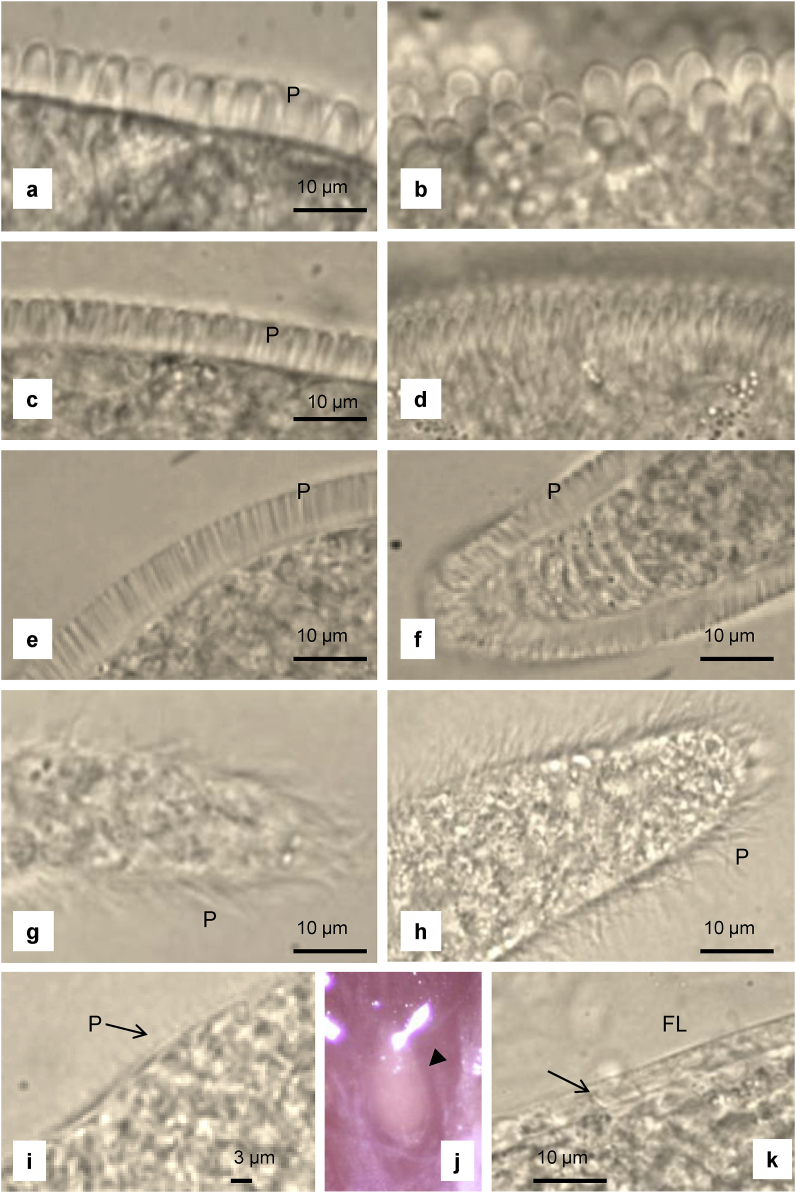
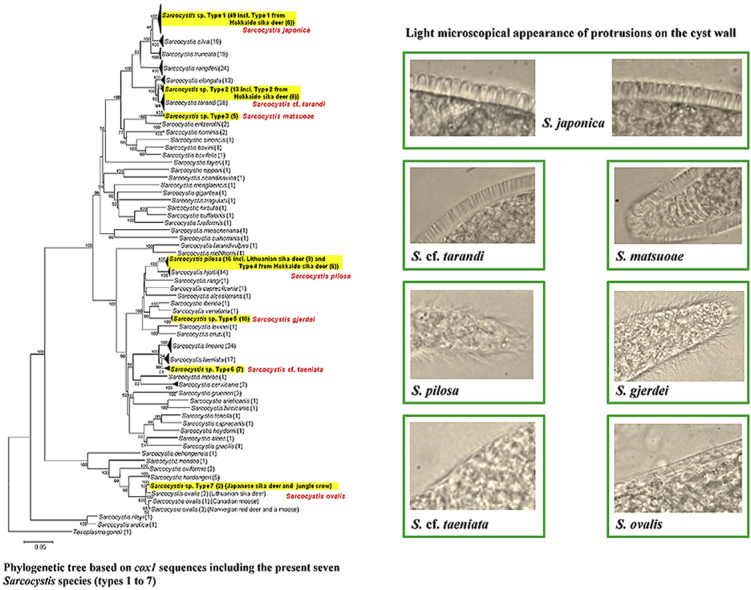
Following the identification of sarcocysts based on the cox1 sequences derived from each of them, various morphological features could retrospectively be attributed to sarcocysts of particular species. The single sarcocyst of S. ovalis was oval (Fig. 2j), whereas the sarcocysts belonging to the six other species were cigar-like, spindle-shaped or slender and thread-like and about 0.2–4 mm long. Most of the sarcocysts examined were microscopic (detected only under the stereo microscope), but some of the sarcocysts belonging to Sarcocystis sp. type 1 were grossly visible (macroscopic). Details about the sarcocyst morphology of each of the seven species found are given in Table 3. Sarcocysts of Sarcocystis spp. types 1, 2 and 3 had a thick cyst wall due to tightly packed, upright finger-like cyst wall protrusions. These protrusions were thicker in sarcocysts of Sarcocystis sp. type 1 (Fig. 2a–d), than in sarcocysts of type 2 and 3, which were indistinguishable by light microscopy (Fig. 2e and f). However, the larger macroscopic sarcocysts of Sarcocystis sp. type 1 had thicker thumb-like protrusions (Fig. 2a and b) than the smaller sarcocysts of this species, which had more slender protrusions (Fig. 2c and d).
Fig. 2.
Light microscopic appearance of sarcocysts isolated from sika deer from Gifu Prefecture, Central Japan. a-d Thumb-like (a, b) and elongated finger-like (c, d) protrusions (P) of S. japonica. e, f Finger-like protrusions in S. cf. tarandi (e) and S. matsuoae (f). g, h Hair-like protrusions in S. pilosa (g) and S. gjerdei (h). i Indistinct protrusions on cyst S. cf. taeniata.j, k Oval sarcocyst of S. ovalis (j); cyst surrounded by fibrous layer (FL), making the slanting tongue-like protrusions (arrow) nearly invisible (k).
Table 3.
Taxonomic summary of three new Sarcocystis species in sika deer. Comparable data on four other Sarcocystis spp. identified in the present study are also included for comparison.
| Species | Sarcocystis japonica n. sp. | Sarcocystis cf. tarandi | Sarcocystis matsuoae n. sp. | Sarcocystis pilosa | Sarcocystis gjerdei n. sp. | Sarcocystis cf. taeniata | Sarcocystis ovalis |
|---|---|---|---|---|---|---|---|
| Intermediate host | Sika deer, Cervus nippon centralis (type host) | Sika deer, Cervus nippon centralis | Sika deer, Cervus nippon centralis (type host) | Sika deer, Cervus nippon centralis | Sika deer, Cervus nippon centralis (type host) | Sika deer, Cervus nippon centralis | Sika deer, Cervus nippon centralis |
| cox1 sequence types | Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 | Type 7 |
| Definitive hosts | Unknown, but probably felids | As for S. japonica | As for S. japonica | Unknown, but probably canids | As for S. pilosa | As for S. pilosa | Corvid birds |
| Locality | Gifu, Japan | Gifu, Japan | Gifu, Japan | Gifu, Japan | Gifu, Japan | Gifu, Japan | Gifu, Japan |
| Sarcocyst morphology | Macroscopic or microscopic; 1238.51 × 225.96 μm (363–4000 × 49–620, n = 101); cigar-like sarcocysts in diaphragm; thick thumb-like (Fig. 2a, b) or slender finger-like protrusions (Fig. 2c, d), 5.67 μm (3.20–9.00, n = 101) | Microscopic, 679.29 × 81.21 μm (330.0–1155.0 × 33.00–144.00, n = 31); spindle-shaped sarcocysts in diaphragm; thin finger-like protrusions, 7.92 μm (5.00–10.20, n = 31) (Fig. 2e) | Microscopic, 1115.69 × 112.19 μm (396.0–2970.0 × 66.00–192.00, n = 13); spindle-shaped sarcocysts in diaphragm; thin finger-like protrusions, 6.09 μm (4.27–9.50, n = 13) (Fig. 2f) | Microscopic, 954.45 × 71.29 μm (330–2310.0 × 33.00–144.00, n = 51); spindle-shaped sarcocysts in diaphragm; hair-like protrusions, 7.94 μm (4.67–11.59, n = 46) (Fig. 2g) | Microscopic, 1107.31–89.62 μm (396.0–1840.0 × 49.00–166.00, n = 13); spindle-shaped sarcocysts in diaphragm; hair-like protrusions, 8.48 μm (6.70–13.50, n = 12) (Fig. 2h) | Microscopic, 444.10–71.70 μm (217.00–825.00 × 33.00–148.00, n = 10); spindle-shaped sarcocysts with a smooth or slightly fuzzy surface by LM (Fig. 2i) | Microscopic oval sarcocyst, 825 × 363 μm, in diaphragm; slanting tongue-like protrusions, 6.20 μm, n = 1, (Fig. 2j, k) |
| Molecular characteristics (GenBank) | LC349445, LC349447, LC349450, LC349452, LC349454, LC349456, LC349458, LC349460, LC349462, LC349464, LC349466, LC481012-LC481017, LC349943, LC349946, LC349948, LC349955, LC349957, LC349958, LC349963, LC349965, LC349968, LC349969, LC349974, LC349975, LC481036-LC481066 | LC349468, LC481018-LC481021, LC349960, LC481067-LC481072 | LC349471, LC481022-LC481024, LC349971, LC481073-LC481076 | LC349474, LC481025-LC481027, LC349942, LC349966, LC481077-LC481081 | LC349475-LC349477, LC349479, LC481028-LC481031, LC349938, LC349940, LC349950, LC349952, LC349954, LC481082-LC481086 | LC481032, LC481033, LC481087-LC481093 | LC481011, LC481034-LC481035 |
| Deposited material | Nucleotide sequences submitted to DDBJ; genomic DNA stored at the Department of Microbiology, Osaka Institute of Public Health | As for S. japonica | As for S. japonica | As for S. japonica | As for S. japonica | As for S. japonica | As for S. japonica |
| Etymology | Named ‘japonica’ to reflect its seemingly wide geographical distribution in Japan | Named in honor of Dr. Kayoko Matsuo for her great contributions concerning the study of Sarcocystis infections in Japan | Named in honor of Prof. Bjørn Gjerde for his great contributions concerning morphological and molecular characterization of numerous Sarcocystis spp. in ruminant intermediate hosts, including the establishment of the cox1 gene as a marker for delimitation of these species |
Sarcocysts belonging to Sarcocystis spp. with type 4 and type 5 sequences possessed similar delicate hair-like protrusions (Fig. 2g and h), and were thus indistinguishable by light microscopy. The sarcocysts of Sarcocystis sp. type 6 were microscopic and five of the six sarcocysts examined had a smooth surface with no visible protrusions on the cyst wall. However, in one sarcocyst, the surface had a fuzzy appearance, which seemed to be due to very delicate short and thin protrusions (Fig. 2i, arrow). The single sarcocyst of type 7 (S. ovalis) was microscopic and oval (Fig. 2j). This cyst was surrounded by a fibrous layer (about 5 μm thick), which to some extent obscured the protrusions, but they seemed to be tongue-like and slanting (Fig. 2k, arrow).
3.5. Taxonomic summary of the three new species
Based on the cox1 sequence comparisons and the results of the phylogenetic analysis using this marker, the sarcocysts examined belonged to seven different species (Table 1, Table 2, Fig. 1), of which types 1, 3 and 5 are considered to represent three new species, whereas sarcocysts associated with types 4 and 7 sequences are considered to belong the known species S. pilosa and S. ovalis, respectively. Type 2 and type 6 cox1 sequences, on the other hand, could not be unambiguously assigned to or separated from the known species S. tarandi and S. taeniata, and these taxa will therefore be preliminary referred to as S. cf. tarandi and S. cf. taeniata. Regarding the three new species with cox1 sequences of types 1, 3 and 5, respectively, we propose the names Sarcocystis japonica n. sp., Sarcocystis matsuoae n. sp. and Sarcocystis gjerdei n. sp. Important taxonomic features of the three new species, as well as the four other species, have been summarized in Table 3. The species S. japonica was so named to reflect its putative wide distribution in Japan (see Discussion). The species S. matsuoae was named in honor of Dr. Kayoko Matsuo for her contributions concerning the study of Sarcocystis infections in Japan. Likewise, the species S. gjerdei was named in honor of Prof. Bjørn Gjerde for his contributions concerning morphological and molecular characterization of numerous Sarcocystis spp. in ruminant intermediate hosts, including the establishment of the cox1 gene as a marker for delimitation of these species.
4. Discussion
Using the cox1 marker, the present study showed that the 53 sika deer examined were intermediate hosts for seven Sarcocystis spp., of which three species have been named as new. Moreover, this study again showed that the cox1 marker was superior to the 18S rDNA in separating closely related species in ruminants (Gjerde, 2013; Prakas et al., 2016; Gjerde et al., 2017a; Rudaitytė-Lukošienė et al., 2018). Thus, based on cox1 (Fig. 1), S. japonica was clearly distinct from S. silva and S. truncata, whereas at 18S rDNA (Figs. S2 and S3), it was not clearly separated from these species, and in particular not from S. truncata. Hence, the recent putative identification of S. truncata as the possible causative agent of food poisoning in a man after consumption of sika dear meat (Ota et al., 2019) might not be correct, since that identification was based on 18S rDNA sequence comparisons. In the present study, S. truncata was not identified among any of the 220 sarcocysts examined, and hence we consider it likely that the species identified and described by Ota et al. (2019) actually was S. japonica. The presence of S. truncata in sika deer in Lithuania (Rudaitytė-Lukošienė et al., 2018), might be the result of recent infections with isolates derived from red deer in that country (via definitive hosts), rather than a continuous presence of the species in sika deer since their introduction to Lithuania from Central Asia about 60 years ago (Pūraitė and Paulauskas, 2016). Moreover, S. japonica seems to be widespread in sika deer in Japan. Thus, the six cox1 sequences of the Type 1 species from Hokkaido sika deer (Irie et al., 2019) clearly belonged to this species. Moreover, we believe that the twelve 18S rDNA sequences in GenBank from Sarcocystis sp. HM050622 from sika deer in Hokkaido (AB251926, AB257085–86, AB257154–62), as well as the four sequences of a Sarcocystis sp. from sika deer in Yamanashi Prefecture (LC405947–50) (see Figs. S2 and S3) belong to S. japonica. Thus, we recommend that the cox1 marker is used in future studies trying to identify Sarcocystis spp. in sika deer meat suspected of having caused food poisoning, since this will provide a more accurate identification of the species involved.
In the phylogenetic tree based on cox1 sequences, our type 2 sequences clustered with the type 2 sequences derived from the study by Irie et al. (2019) within a clade that was sister to sequences of S. tarandi from reindeer, and both of these clades were sister to sequences of S. elongata from red deer, which was also the case in previous analyses using fewer sequences (Abe et al., 2019; Irie et al., 2019). In the phylogeny based on 18S rRNA sequences (Figs. S2 and S3), on the other hand, the associated type 2 sequences from this study and that of Irie et al. (2019), clustered in between sequences of S. tarandi and S. elongata. Based on the sequence identities and the phylogenetic placement, the cox1 sequences of type 2 seem to be more closely related to S. tarandi than to S. elongata. The type 2 sequences formed a monophyletic cluster and thus represented a lineage separate from S. tarandi in reindeer. However, based on the available type 2 sequences, it is still difficult to decide whether they represent a separate species or just a subgroup within S. tarandi. To resolve this question, it will be necessary to examine more isolates of this species from sika deer at cox1 or other markers, in order to determine whether there are consistent differences between these isolates and those of S. tarandi. For the time being, we will refer to this taxon as S. tarandi-like or Sarcocystis cf. tarandi.
In the phylogenetic trees for both molecular markers (Fig. 1, Fig. S2), sequences of type 3 were clearly separated from those of S. entzerothi from roe deer and farmed sika deer in Lithuania (Prakas et al., 2017; Rudaitytė-Lukošienė et al., 2018). Likewise, the cox1 and 18S rDNA sequences of type 3 shared an identity of only 94.3–94.4% and 96.9–97.6% respectively, with those of S. entzerothi. Hence, the type 3 sequences are considered to belong to a separate species, which we have named S. matsuoae. The sarcocysts of S. matsuoae possessed slender finger-like protrusions (Fig. 2f) similar to those of S. entzerothi (Prakas et al., 2017). The size of their sarcocysts were also nearly the same, being 1115 × 112 μm in S. matsuoae (Table 3) and 1139 × 108 μm in S. entzerothi (Prakas et al., 2017).
The cox1 sequences of type 4 could be assigned to S. pilosa since they shared a high identity with sequences previously attributed to this species (Abe et al., 2019; this study). Likewise, the Type 3 sequences from Hokkaido sika deer (Irie et al., 2019) belonged to S. pilosa. Hence, this species seems to be widespread in sika deer in Japan. Moreover, S. pilosa was first described from farmed sika deer in Lithuania (Prakas et al., 2016), that is, in animals descending from sika deer imported from the Gorno-Altaysk area in Russia in 1954 (Pūraitė and Paulauskas, 2016). It is likely that S. pilosa was imported to Lithuania with infected sika deer and therefore might occur even in sika deer in Central Asia.
In our recent study (Abe et al., 2019), the Sarcocystis sp. with type 5 sequences was suspected to be morphologically similar to S. pilosa based on its phylogenetic placement. The present study showed that this was indeed the case, since both species possessed hair-like cyst wall protrusions (Fig. 2g and h). The addition of five more cox1 sequences of type 5 in the phylogenetic analysis again showed that these sequences were clearly separated from those of other known Sarcocystis spp. (Fig. 1). The same was true in the phylogenetic analyses based on 18S rDNA sequences (Fig. S2). Hence, we consider the species with type 5 sequences to belong to a previously unrecognized and unnamed species, for which the name S. gjerdei has been proposed. This species was found in fewer of the sika deer examined than S. pilosa, which suggests that it is a less common species. However, only samples of the diaphragm were examined, and the two species might have different predilection sites. In the study of Hokkaido sika deer by Irie et al. (2019) all the thin-walled sarcocysts identified by molecular methods belonged to S. pilosa, but again, samples were only collected from the diaphragm.
At 18S rDNA (Figs. S2 and S4), the Sarcocystis sp. with type 6 sequences could not be separated from S. taeniata and S. linearis, whereas at cox1 (Fig. 1), type 6 sequences formed a sister clade to sequences of S. taeniata, and both of these taxa were sister to S. linearis. Hence, Sarcocystis sp. type 6 seems either to be a separate lineage of S. taeniata or to represent a new species. Characterization of additional isolates from sika deer at cox1 or other markers are necessary to resolve this question. For the time being, we will refer to this taxon as S. cf. taeniata. The species S. taeniata was first described from moose in Canada (Gjerde, 2014b), but has later been found in farmed sika deer in Lithuania (Prakas et al., 2016). The species S. linearis was first described from roe deer in Italy (Gjerde et al., 2017a), but has later been found in red deer in Spain (Gjerde et al., 2017b) and in moose in Lithuania (Prakas et al., 2019). By light microscopy, sarcocysts of S. cf. taeniata were similar to those of S. taeniata and S. linearis in having a smooth surface with barely visible protrusions (Fig. 2i). Examination of sarcocysts of the latter two species by SEM (Gjerde, 2014b; Gjerde et al., 2017a), have shown that these species have delicate ribbon-like protrusions running along the surface of the sarcocysts, and one may expect the same to be the case for S. cf. taeniata. However, the sarcocysts of S. cf. taeniata seem to be smaller than those of the two other species (217–825 × 33–148 μm in S. cf. taeniata, Table 3; 1000–1100 × 60–80 μm in S. taeniata (Gjerde, 2014b); and 500–1000 × 100 μm in S. linearis (Gjerde et al., 2017a), but only six sarcocysts of S. cf. taeniata were examined.
In this study we found one sarcocyst that could be assigned to S. ovalis based on cox1 and 18S rDNA sequences. This finding confirms the presence of this species in sika deer in Japan, which was first reported as an unnamed species from Hokkaido sika deer by Takano et al. (2006), but the 18S rDNA sequence generated in that study was later identified as belonging to S. ovalis (Abe, 2014; Irie et al., 2017). Moreover, S. ovalis was identified in the intestine of a Japanese jungle crow based on sequencing of 18S rDNA (Irie et al., 2017). The latter identification was confirmed in this study through sequencing of cox1 from the jungle crow isolate. S. ovalis has previously been identified in sika deer in Lithuania (Rudaitytė-Lukošienė et al., 2018), but the species was originally reported from moose from Norway and Canada (Dahlgren and Gjerde, 2008) and then from red deer in Norway (Dahlgren and Gjerde, 2010). Recently, S. ovalis was also reported from moose in Lithuania (Prakas et al., 2019).
As regards the relationship of the six species other than S. ovalis found in this study to species previously described from sika deer in Japan by means of LM, TEM or SEM of sarcocysts, no definite conclusions can be made, since more than one species may share the same or a closely similar sarcocyst morphology. However, the type 1 sarcocysts described by LM and TEM from wild Hokkaido sika deer by Narisawa et al. (2008) probably represent sarcocysts of S. japonica. These large and thick sarcocysts measured 920–2100 × 264–328 μm and had thick tombstone-like protrusions, which are consistent with our findings regarding S. japonica. Moreover, the Type 1 cysts in the study of Irie et al. (2019), which belong to S. japonica, had similar tombstone-like protrusions in histological sections. Alternatively, such large cysts might represent sarcocysts of S. truncata (Dahlgren and Gjerde, 2010; Gjerde, 2014a), but as noted above, this species might not occur in sika deer in Japan. The species with more slender fusiform sarcocysts with finger-like protrusions, which were designated Sarcocystis sp. Type 2 by Narisawa et al. (2008), Sarcocystis sp. by Saito et al. (1995), Sarcocystis sp. 2 by Saito et al. (1996), Sarcocystis sp. 1 by Saito et al. (1998) and Sarcocystis sybillensis by Arai et al. (2010) and Saito and Hagiwara (2013), might belong to the S. tarandi-like species or S. matsuoae. It should be noted that the TEM micrographs used to depict this cyst type in the five last-mentioned papers (Figs. 3, 3, 5, 5 and 17, respectively), reporting from different studies, are from the same portion of a single sarcocyst from the first study, four of them being different versions (cropped, inverted) of a single micrograph apparently taken at high magnification (Saito et al., 1995, 1996, 1998; Saito and Hagiwara, 2013), and one being a micrograph of a larger area taken at low magnification (Fig. 3 in Arai et al., 2010). Hence, this cyst type may not be as prevalent as suggested by its repeated reporting, and the sarcocysts examined by LM or SEM in some of these studies might belong to a different species. The finger-like cyst-wall protrusions of the single cyst depicted in the TEM micrographs in these papers seem to have a broad round base and a thinner, more flattened distal end, causing the tip of the protrusions to bend over, which are features known from sarcocysts of S. entzerothi (Prakas et al., 2017), Sarcocystis bovifelis, Sarcocystis bovini and Sarcocystis sinensis (Gjerde, 2016a), whereas sarcocysts of S. tarandi have erect columnar/cylindrical protrusions (Gjerde, 1985). Moreover, since S. matsuoae was placed as a sister species to S. entzerothi, it seems likely that the sarcocyst depicted in the abovementioned TEM micrographs belong to S. matsuoae. The same is probably also true for the single sarcocyst shown in a wet mount by LM in Fig. 1 of Saito et al. (1995), since these TEM micrographs originated from that study of a single farmed sika deer. This sarcocyst is shown again in Fig. 1 of Saito et al. (1998). The type 2 sarcocyst examined by TEM by Narisawa et al. (2008), on the other hand, is consistent with sarcocysts of S. tarandi (Gjerde, 1985). Additional studies of the sarcocyst morphology of S. japonica, S. tarandi and S. matsuoae by TEM and SEM should be carried out in order to establish which features might separate these species at the ultrastructural level.
The previously reported sarcocysts with hair-like protrusions might belong to either S. pilosa or S. gjerdei, or a mixture of both species. Previously they have been assigned to Sarcocystis sp. Type 3 (Narisawa et al., 2008), Sarcocystis sp. 2 (Saito et al., 1998), and S. wapiti (Arai et al., 2010; Saito and Hagiwara, 2013). The identification of the species with hair-like protrusions in sika deer as S. wapiti in the latter papers is clearly incorrect, since S. wapiti as described from the North-American elk does not have hair-like, but ribbon-like protrusions, which are invisible by LM (see Table 1 in Gjerde et al., 2017b). This is the same type of protrusions that have been found on sarcocysts of S. taeniata and S. linearis (Gjerde, 2014b; Gjerde et al., 2017a) and which we expect to find in sarcocysts of S. cf. taeniata if examined by TEM or SEM, but sarcocysts with this type of protrusions have not been reported in any of the previous studies of sika deer in Japan. However, there seems to be two species with this type of sarcocyst morphology in Japanese sika deer. Thus, in phylogenetic analyses, an 18S rDNA sequence (GenBank: AB698065) of the unnamed Sarcocystis sp. T18, derived from sarcocysts in cardiac muscle of sika deer in Hyogo prefecture, has been placed either in between sequences of Sarcocystis cervicanis (Fig. S3 in Supplementary material of Gjerde et al., 2017b), or as a sister taxon to this species (Fig. S2, this study). Hence, Sarcocystis sp. T18 is either identical to or a sister species to S. cervicanis, which has ribbon-like cyst wall protrusions (Fig. 2 in Gjerde et al., 2017b). Moreover, data in the GenBank entry of AB698065 suggest that this sequence was derived from a study that was briefly described in a congress abstract by Kimoto et al. (2011). In this abstract, the sarcocysts are reported to have thin walls with no protrusions (by LM), and in phylogenetic analyses based on 18S rDNA, the species clustered among species with canine definitive hosts, which is what might be expected for a S. cervicanis-like species. Thus, there is at least one more Sarcocystis sp. in sika deer in Japan than the seven species found in this study.
As regards the transmission of the various Sarcocystis spp. to sika deer, the jungle crow has been shown to act as definitive host of S. ovalis (Irie et al., 2017; this study). The definitive hosts of the six other Sarcocystis spp. identified in the present study have not been definitely established. However, based on their phylogenetic positions, it is likely that canids act as definitive hosts for S. pilosa, S. gjerdei and S. cf. taeniata, while felids act as definitive hosts for S. japonica, S. cf. tarandi and S. matsuoae. According to Saito et al. (1995, 1998), Arai et al. (2010) and Saito and Hagiwara (2013), dogs fed sarcocysts with either hair-like protrusions (presumably cysts of S. pilosa and/or S. gjerdei), or thin finger-like protrusions (presumably cysts of S. cf. tarandi and/or S. matsuoae) became infected and shed sporocysts. The outcome regarding the cysts with hair-like protrusions are thus as expected, whereas the sporocyst shedding by dogs following ingestion of sarcocysts with finger-like protrusions is at odds with the phylogenetic position of the species with such protrusions. It is not clear from the abovementioned papers how meticulously the sarcocysts fed to dogs were isolated from the surrounding muscle tissue, but we think that the material containing sarcocysts with finger-like protrusions might also have contained some sarcocysts of species actually transmitted by dogs, that is, of S. pilosa, S. gjerdei, S. cf. taeniata and/or the S. cervicanis-like species. Nowadays, molecular methods may be used to identify the oocysts/sporocysts in the intestine or feces of either naturally or experimentally infected definitive hosts.
In the previous study (Abe et al., 2019), the newly developed multiplex PCR assay using type-specific primers was found to be capable of identifying DNA from individual sarcocysts of five Sarcocystis spp., but its ability to identify more than one species simultaneously from mixed templates was not tested. In the present study, the multiplex PCR assay was expanded with two additional species-specific primer pairs, allowing the identification of all seven Sarcocystis spp. found in this study. Moreover, the expanded assay was shown to be able to identify different species simultaneously when a mixture of DNA from several species was used as template. Hence, this assay may be useful for identification of all the species present when DNA has been extracted from small pieces of muscle, which may contain sarcocysts of more than one species. Similarly, it may be useful for simultaneous identification of oocysts/sporocysts of several Sarcocystis spp. in samples of the intestinal mucosa of potential definitive hosts of these species.
5. Conclusion
The present study has provided molecular and morphological evidence for the presence of seven Sarcocystis spp., including three new species, in sika deer in Japan, that is, S. japonica n. sp., S. cf. tarandi, S. matsuoae n. sp., S. pilosa, S. gjerdei n. sp., S. cf. taeniata and S. ovalis. In addition, a S. cervicanis-like species seems to be present based on an 18S rDNA sequence in GenBank. A multiplex PCR assay capable of identifying the seven species found in this study has been developed and might become useful in future studies aimed at identifying the definitive hosts of these species.
Compliance with ethical standards
The diaphragm samples used in the present study were collected from legally hunted sika deer for reasons of damage control, academic research or human consumption.
Declaration of competing interest
The authors declare that they have no competing interests. The proposal and decision to name S. matsuoae and S. gjerdei in honor of Dr. Matsuo and Prof. Gjerde, respectively, was made by the first author, and the other coauthors agreed to such naming.
Acknowledgement
The authors would like to thank Mr. Alexey Takashima for his valuable help regarding the initial examination of muscle samples and identifying those containing sarcocysts. We would also like to thank the wild game meat processing facilities in Gifu prefecture, for kindly providing the muscle samples from sika deer examined in the present study. This study was supported by a Grant-in-Aid for Scientific Research (C) (17K09185) from the Japan Society for the Promotion of Sciences (JSPS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.10.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abe N. Current topics and future issues in parasitic food poisoning in Japan. Jpn. J. Food Microbiol. 2014;31:129–137. [Google Scholar]
- Abe N., Matsuo K., Moribe J., Takashima Y., Baba T., Gjerde B. Molecular differences of five Sarcocystis species in sika deer (Cervus nippon centralis) in Japan based on mitochondrial cytochrome c oxidase subunit I gene (cox1) sequences. Parasitol. Res. 2019;118:1975–1979. doi: 10.1007/s00436-019-06319-5. [DOI] [PubMed] [Google Scholar]
- Arai Y., Tanaka M., Saito M. Sarcocystis sybillensis and S. wapiti from sika deer, Cervus nippon centralis, in Japan. J. Anim. Protozooses. 2010;25:13–16. [Google Scholar]
- Barta J.R., Martin D.S., Liberator P.A., Dashkevicz M., Anderson J.W., Feighner S.D., Elbrecht A., Perkins-Barrow A., Jenkins M.C., Danforth H.D., Ruff M.D., Profous-Juchelka H. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997;83:262–271. [PubMed] [Google Scholar]
- Dahlgren S.S., Gjerde B. Sarcocystis in moose (Alces alces): molecular identification and phylogeny of six Sarcocystis species in moose, and a morphological description of three new species. Parasitol. Res. 2008;103:93–110. doi: 10.1007/s00436-008-0936-1. [DOI] [PubMed] [Google Scholar]
- Dahlgren S.S., Gjerde B. Molecular characterization of five Sarcocystis species in red deer (Cervus elaphus), including Sarcocystis hjorti n. sp., reveals that these species are not intermediate host specific. Parasitology. 2010;137:815–840. doi: 10.1017/S0031182009991569. [DOI] [PubMed] [Google Scholar]
- Fischer S., Odening K. Characterization of bovine Sarcocystis species by analysis of their 18S ribosomal DNA sequences. J. Parasitol. 1998;84:50–54. [PubMed] [Google Scholar]
- Gjerde B. Ultrastructure of the cysts of Sarcocystis tarandi from skeletal muscle of reindeer (Rangifer tarandus tarandus) Can. J. Zool. 1985;63:2913–2918. doi: 10.1186/BF03546567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013;43:579–591. doi: 10.1016/j.ijpara.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Sarcocystis species in red deer revisited: with a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology. 2014;141:441–452. doi: 10.1017/S0031182013001819. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Morphological and molecular characteristics of four Sarcocystis spp. in Canadian moose (Alces alces), including Sarcocystis taeniata n. sp. Parasitol. Res. 2014;113:1591–1604. doi: 10.1007/s00436-014-3806-z. [DOI] [PubMed] [Google Scholar]
- Gjerde B. The resurrection of a species: Sarcocystis bovifelis Heydorn et al., 1975 is distinct from the current Sarcocystis hirsuta in cattle and morphologically indistinguishable from Sarcocystis sinensis in water buffaloes. Parasitol. Res. 2016;115:1–21. doi: 10.1007/s00436-015-4785-4. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis) Parasitol. Res. 2016;115:1473–1492. doi: 10.1007/s00436-015-4881-5. [DOI] [PubMed] [Google Scholar]
- Gjerde B., Giacomelli S., Bianchi A., Bertoletti I., Mondani H., Gibelli L.R. Morphological and molecular characterization of four Sarcocystis spp., including Sarcocystis linearis n. sp., from roe deer (Capreolus capreolus) in Italy. Parasitol. Res. 2017;116:1317–1338. doi: 10.1007/s00436-017-5410-5. [DOI] [PubMed] [Google Scholar]
- Gjerde B., Luzón M., Alunda J.M., de la Fuente C. Morphological and molecular characteristics of six Sarcocystis spp. from red deer (Cervus elaphus) in Spain, including Sarcocystis cervicanis and three new species. Parasitol. Res. 2017;116:2795–2811. doi: 10.1007/s00436-017-5590-z. [DOI] [PubMed] [Google Scholar]
- Gjerde B., Vikøren T., Hamnes I.S. Molecular identification of Sarcocystis halieti n. sp., Sarcocystis lari and Sarcocystis truncata in the intestine of a white-tailed sea eagle (Haliaeetus albicilla) in Norway. Int. J. Parasitol. Parasites Wildl. 2018;7:1–11. doi: 10.1016/j.ijppaw.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T., Ikeda T., Nakamura T., Ichii O., Yamada N., Ito T., Yamazaki A., Takai S., Yagi K. First molecular detection of Sarcocystis ovalis in the intestinal mucosa of a Japanese jungle crow (Corvus macrorhynchos) in Hokkaido, Japan. Vet. Parasitol. Reg. Stud. Rep. 2017;10:54–57. doi: 10.1016/j.vprsr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Irie T., Ichii O., Nakamura T., Ikeda T., Ito T., Yamazaki A., Takai S., Yagi K. Molecular characterization of three Sarcocystis spp. from wild sika deer (Cervus nippon yesoensis) in Hokkaido, Japan. Vet. Parasitol. Reg. Stud. Rep. 2019;18 doi: 10.1016/j.vprsr.2019.100327. 100327. [DOI] [PubMed] [Google Scholar]
- Kimoto K., Matsuo S., Aoki A., Kikuta M., Morita T., Ike K., Imai S. Abstracts of the 2011 Japan Society of Protistology Meeting. 2011. Taxonomical analysis of Sarcocystis sp. from Sika deer (Cervus nippon centralis)http://protistology.jp/journal/congress_ab/44th_nara/en/37KIMOTO_E.pdf Available at: [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P., Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Matsuo S., Morita T., Imai S., Ike K. Prevalence of Sarcocystis in Japanese sika deer (Cervus nippon centralis) in Hyogo prefecture, Japan. J. Vet. Epidemiol. 2014;18:124–129. [Google Scholar]
- Matsuo K., Uetsu H., Takashima Y., Abe N. High occurrence of Sarcocystis infection in sika deer Cervus nippon centralis and Japanese wild boar Sus scrofa leucomystax and molecular characterization of Sarcocystis and Hepatozoon isolates from their muscles. Jpn. J. Zoo Wildl. Med. 2016;21:35–40. [Google Scholar]
- Narisawa A., Yokoi S., Kawai K., Sakui M., Sugawara K. Sarcocystis spp. infection in wild sika deer (Cervus nippon yesoensis) J. Jpn. Vet. Med. Assoc. 2008;61:321–323. [Google Scholar]
- Ota T., Nakano Y., Mizuno T., Shiozaki A., Hori Y., Yamanishi K., Hayakawa K., Hayakawa T., Fujimoto T., Nakamoto C., Mejima K., Wada Y., Terasoma F., Ohnishi T. First case report of possible Sarcocystis truncata-induced food poisoning in venison. Intern. Med. 2019;58:2727–2730. doi: 10.2169/internalmedicine.2817-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakas P., Butkauskas D., Rudaitytė E., Kutkienė L., Sruoga A., Pūraitė I. Morphological and molecular characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the sika deer (Cervus nippon) in Lithuania. Parasitol. Res. 2016;115:3021–3032. doi: 10.1007/s00436-016-5057-7. [DOI] [PubMed] [Google Scholar]
- Prakas P., Rudaitytė E., Butkauskas D., Kutkienė L. Sarcocystis entzerothi n. sp. from the European roe deer (Capreolus capreolus) Parasitol. Res. 2017;116:271–279. doi: 10.1007/s00436-016-5288-7. [DOI] [PubMed] [Google Scholar]
- Prakas P., Kirillova V., Calero-Bernal R., Kirjušina M., Rudaitytė-Lukošienė E., Habela M.Á., Gavarāne I., Butkauskas D. Sarcocystis species identification in the moose (Alces alces) from the Baltic States. Parasitol. Res. 2019;118:1601–1608. doi: 10.1007/s00436-019-06291-0. [DOI] [PubMed] [Google Scholar]
- Pūraitė I., Paulauskas A. Genetic diversity of the sika deer Cervus nippon in Lithuania. Balkan J. Wildl. Res. 2016;3:19–25. [Google Scholar]
- Rudaitytė-Lukošienė E., Prakas P., Butkauskas D., Kutkiene L., Vepstaite-Monstavice I., Serviene E. Morphological and molecular identification of Sarcocystis spp. from the sika deer (Cervus nippon), including two new species Sarcocystis frondea and Sarcocystis nipponi. Parasitol. Res. 2018;117:1305–1315. doi: 10.1007/s00436-018-5816-8. [DOI] [PubMed] [Google Scholar]
- Saito M., Hagiwara A. Prevalence of Sarcocystis sybillensis and S. wapiti infection in sika deer, Cervus nippon centralis in Japan. J. Anim. Protozooses. 2013;28:20–24. [Google Scholar]
- Saito M., Itagaki T., Shibata Y., Itagaki H. Morphology and experimental definitive hosts of Sarcocystis sp. from sika deer, Cervus nippon centralis, in Japan. Jpn. J. Parasitol. 1995;44:218–221. [Google Scholar]
- Saito M., Shibata Y., Kobayashi T., Kobayashi M., Kubo M., Itagaki H. Ultrastructure of the cyst wall of Sarcocystis species with canine final host in Japan. J. Vet. Med. Sci. 1996;58:861–867. doi: 10.1292/jvms.58.861. [DOI] [PubMed] [Google Scholar]
- Saito M., Shibata Y., Kubota M., Itagaki H. Sarcocystis spp. from sika deer, Cervus nippon centralis and Cervus nippon yesoensis. J. Jpn. Vet. Med. Assoc. 1998;51:683–686. [Google Scholar]
- Sugita-Konishi Y., Kobayashi N., Takasaki K., Kanno T., Itoh M., Riztyan Futo S., Asakura H., Taira K., Kawakami Y. Detection of Sarcocystis spp. and Shiga toxin-producing Escherichia coli in Japanese sika deer meat using a loop-mediated isothermal amplification-lateral flow strip. J. Vet. Med. Sci. 2019;81:586–592. doi: 10.1292/jvms.18-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K., Hamada K., Ogiwara Y., Yagi K. Phylogenetic analysis of Sarcocystis sp. isolated from muscle of sika deer in Hokkaido by partial 18S rRNA gene sequence. Rep. Hokkaido Inst. Public Health. 2006;56:41–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.